Abstract
Background
Papillary muscles (PMs) abnormalities may be associated with ECG repolarization abnormalities. We aimed to evaluate the relation between lateral T‐wave inversion (TWI) and PMs characteristics in a cohort of athletes with no clinically demonstrable cardiac disease.
Methods and Results
We included 53 athletes (median age, 20 years; 87% men) with lateral TWI and no evidence of heart disease on clinical and cardiac magnetic resonance evaluation. A group of healthy athletes with normal ECG served as controls. We evaluated the PMs dimensions, such as diameters, area, volume, mass, and ratio between PMs and left ventricular mass, and the prevalence of PMs apical displacement. Compared with controls, athletes with TWI showed PMs hypertrophy with significantly increased PMs diameters, area, volume, and mass. The ratio between PMs and left ventricular mass was 4.4% in athletes with TWI and 3.0% in controls (P<0.001). A PMs/left ventricular mass ratio >3.5% showed 85% sensitivity and 76% specificity for differentiating between athletes with TWI and controls. Apical displacement of PMs was found in 25 (47%) athletes with TWI versus 9 (17%) controls (P=0.001). At multivariable analysis, PMs/left ventricular mass ratio and apical displacement remained independent predictors of TWI. Clinical outcome of the athletes with TWI and PMs abnormalities was uneventful despite continuation of their sports activity.
Conclusions
PMs hypertrophy and apical displacement may underlie otherwise unexplained lateral TWI in the athlete. Lateral TWI associated with PMs abnormalities appears as a distinct anatomo‐clinical condition characterized by a favorable outcome.
Keywords: cardiac magnetic resonance, electrocardiography, imaging, preparticipation screening, sports cardiology
Subject Categories: Exercise, Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviations and Acronyms
- HCM
hypertrophic cardiomyopathy
- LGE
late gadolinium enhancement
- PMs
papillary muscles
- SCD
sudden cardiac death
- TWI
T‐wave inversion
Clinical Perspective
What Is New?
Athletes with lateral (or inferolateral) T‐wave inversion and normal cardiac magnetic resonance demonstrated a higher prevalence of papillary muscles abnormalities such as hypertrophy (increase papillary muscles/left ventricular mass ratio) and apical displacement compared with control athletes with normal ECG.
What Are the Clinical Implications?
Isolated papillary muscles abnormalities such as hypertrophy and apical displacement may underlie lateral (or inferolateral) T‐wave inversion in a sizeable proportion of athletes with otherwise structurally normal heart and appear associated with a favorable medium‐term outcome despite continuation of sports activity.
The presence of T‐wave inversion (TWI) in the athlete's ECG is a warning sign of a potential cardiovascular disease at risk of sudden cardiac death (SCD) during sports. 1 , 2 , 3 TWI in lateral leads, with or without involvement of inferior leads, is the repolarization pattern most frequently associated with structural heart diseases such as ischemic heart disease, cardiomyopathy, myocarditis, aortic valve disease, left ventricular (LV) noncompaction, and idiopathic LV fibrosis. 4 Accordingly, in the athlete with lateral TWI, any forms of heart disease need to be accurately excluded by a comprehensive clinical and imaging workup, including contrast‐enhanced cardiac magnetic resonance (CMR). 1 , 2 , 3 , 5 However, 1% to 3% of athletes have lateral TWI that remains unexplained even after an accurate clinical evaluation. 6 , 7 , 8 Whether these unexplained repolarization changes reflect the presence of a concealed or unrecognized heart muscle abnormality remains to be determined. Papillary muscles (PMs) abnormalities in isolation, that is, not associated with cardiomyopathy—particularly hypertrophic cardiomyopathy (HCM)—are considered a normal anatomic variant without clinical relevance. 9 Past clinico‐echocardiographic studies reported the association between TWI with isolated hypertrophy and/or the apical displacement of LV PMs. 10 , 11 In more recent years, CMR has become the most accurate technique for imaging study of structural heart disease in the athlete. 12 Due to its high resolution, multiplanar capabilities, and soft‐tissue contrast, CMR is the most valuable imaging modality in the evaluation of PMs, providing detailed morphologic and functional information. 9 However, systematic studies correlating lateral TWI with the anatomy and structure of PMs are lacking. Hence, the present ECG‐CMR correlation study was designed to evaluate the relation between unexplained lateral TWI and morpho‐functional PMs abnormalities, either hypertrophy or displacement, in a cohort of apparently healthy athletes with no clinically demonstrable cardiac disease.
METHODS
The data that support the findings of this study are available from the corresponding author upon reasonable request. The study included a consecutive group of athletes with TWI in lateral leads (V4–V6), with or without involvement of inferior leads (L2‐aVF), who were identified on preparticipation screening during the time interval January 2014 to December 2019 in 9 Italian sports medicine centers and referred to the University of Padova for CMR study. The study was approved by the local institutional review board and because of its retrospective nature no consent was required.
All athletes with lateral TWI underwent a routine cardiovascular evaluation including medical history, physical examination, and 12‐lead ECG. In addition, all underwent CMR imaging study for deeper investigation of a possible underlying heart muscle disease, with particular reference to HCM including the “apical” variant. Athletes older than 40 years with risk factors for coronary artery disease also performed either a coronary computed tomography or a stress imaging test to exclude an ischemic heart disease. Athletes with lateral TWI that remained unexplained after the clinical and imaging study comprised the study population. Athletes with abnormal CMR findings, such as pathological increase in wall thickness or with evidence of late gadolinium enhancement (LGE)/myocardial fibrosis other than the isolated “junctional” spotty LGE (that is normal in the athlete 5 ) were excluded from the study. Healthy athletes, matched for age class (5‐year intervals), sex, and type of sport, who had a normal CMR performed for evaluation of cardiac arrhythmias or for investigational purposes served as controls.
Molecular genetic testing was performed in a subset of athletes with lateral TWI. Indications to genetic testing were provided by each participating center according to local practice and based on family history of SCD in 2 athletes, borderline LV wall thickness (12–13 mm) in 4, and persistence of lateral TWI after detraining in 3.
Standard ECG
A standard 12‐lead ECG was recorded at the time of CMR scan. The tracing was interpreted by 2 observers who were unaware of clinical data. Lateral T‐wave inversion was defined as negative T waves ≥0.1 mV in depth in ≥2 contiguous lateral leads (V4–V6), with or without involvement of inferior leads (L2‐aVF). 1
Cardiac Magnetic Resonance
Acquisition Protocols
All images were performed on a 1.5 T scanner (Magnetom Avanto Siemens AG, Germany) using a protocol including post contrast sequences. Biventricular morpho‐functional assessment was performed by a set of steady‐state free precession sequence cine loops in sequential short‐axis views and long‐axis views as previously reported. 13 After 10 minutes since administration of gadolinium‐based contrast agent (gadobenate dimeglumine, Multihance or gadobutrol, Gadovist, typically 0.2 mmol/kg of body weight), 2‐dimensional segmented fast low‐angle short inversion recovery sequences were acquired in the same views of the cine images, covering the entire ventricles (repetition time, 5.4–8.3 ms; echo time, 1.3–3.9 ms; average in‐plane spatial resolution, 1.4–1.5×2.2–2.4 mm; 6‐mm slice thickness; 2‐mm gap; and flip angle, 20°–25°). Inversion times were adjusted to null normal myocardium using a Look‐Locker sequence, and images were repeated in 2 separate phase‐encoding directions to exclude artefacts.
Functional and LGE Analysis
Global ventricular volumes, systolic function, and maximum basal and apical wall thickness were calculated from the short‐axis cine images, excluding PMs from the myocardium, using a computer‐aided analysis package (CMR42; Circle Cardiovascular Imaging Inc, Calgary, Canada). The presence and regional distribution of LGE were visually assessed independently by 2 experienced observers (European Association of Cardiovascular Imaging third‐degree certification) who were blinded to patient clinical data. Ambiguous cases were reviewed by a third expert.
PMs Morphologic Analysis
Assessment of PMs was performed according to a previously reported protocol. 10 , 11 , 14 In brief, horizontal and vertical diameters and area of both PMs were measured on end‐diastolic midventricular short axis cine images (Figure 1A and 1B); while PMs volume and mass were calculated by manually drawing the PMs profile on each short‐axis cine slice that contained PMs (Figure 1C) using a computer‐aided analysis package (CMR42; Circle Cardiovascular Imaging Inc, Calgary, Canada). PMs volumes and mass were used in addition to linear dimensions to overcome possible errors attributable to measurements in slices of different thickness and from PMs of different geometry. In order to index the PMs volume for the different degree of LV hypertrophy of the athlete's heart, we also calculated the ratio between PMs and LV mass for each athlete. Apical displacement of PMs was identified when their implant was in the apical one‐third of the left ventricle using multiplanar views (4‐ and 3‐chamber long‐axis images and their cross section in short‐axis view [Figure 1D]). Measures were performed by 2 independent experts (M.D.L. and N.B.).
Figure 1. Papillary muscles hypertrophy quantitative analysis.
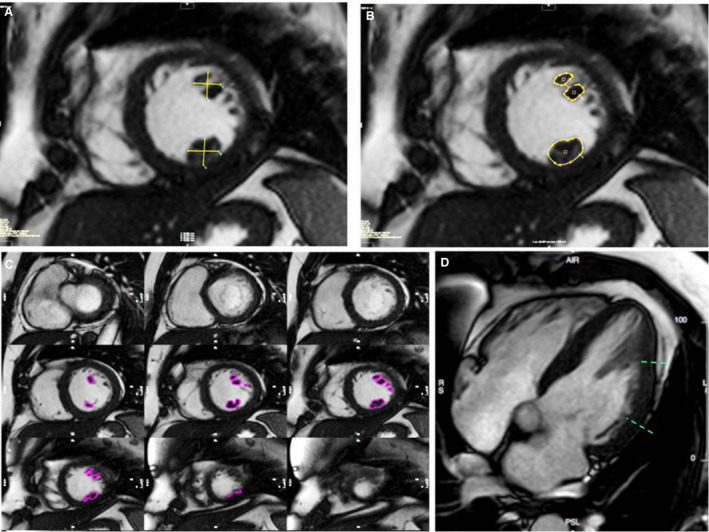
A, Linear quantification of orthogonal diameters of anterolateral and posteromedial papillary muscles. B, Papillary muscles area quantification in the same slice. C, Papillary muscles volume analysis in the entire stack of short‐axis views. D, Apical displacement assessment in long axis view.
Follow‐Up
All study athletes were followed up yearly according to the Italian guidelines for management of athletes with apparently unexplained TWI by routine cardiovascular evaluation including medical history, physical examination, 12‐lead ECG, and echocardiography. 15 Six study athletes underwent control ECG and CMR study after 3 to 6 months of detraining as a therapeutic measure after traumatic injury (N=4) or for evaluation of potential reversibility of “borderline” LV maximal wall thickness (N=3).
Statistical Analysis
Data are expressed as median with 25th to 75th percentiles because normality could not be assumed for any variable. Categorical differences among groups were evaluated by the χ2 test or the Fisher exact test, as appropriate. Differences among continuous variables were compared using the Mann‐Whitney U test. The best cutoff value to differentiate between athletes with and without TWI were calculated with the receiver operating characteristic curve. The following covariates were entered into a binary logistic regression analysis for predictors of the presence of TWI: PMs/LV mass ratio, PMs displacement, age, and sex. A 2‐tailed probability value of 0.05 was considered statistically significant. The intraobserver (M.D.L.) and interobserver (M.D.L. and N.B.) reliability analysis for quantitative measures of PMs characteristics were assessed with the Pearson correlation and the intraclass correlation coefficient. Intraobserver agreement for the presence of apical displacement was assessed with Cohen's κ coefficient for qualitative parameters. All analyses were performed using SPSS 23 (SPSS Inc, Chicago, IL).
RESULTS
Clinical and Imaging Characteristics
Of 112 athletes referred for investigation of lateral or inferolateral TWI, clinical evaluation including CMR identified an underlying heart disease in 59 (52%). Thirty‐nine athletes (35%) had overt HCM, 11 (10%) isolated nonischemic LV scar, 3 (3%) mitral valve prolapse, 2 (2%) dilated cardiomyopathy, 2 (2%) myocardial noncompaction and 2 (2%) ischemic heart disease. These 59 athletes diagnosed with a heart disease were excluded from the study.
The remaining 53 athletes (32%), with TWI and no evidence of heart disease on clinical and CMR evaluation comprised the study population. Athletes with TWI had a median age of 20 years (16–43), and 46 (87%) were males. Eighteen subjects practiced endurance sports, 2 power sports, and 33 mixed sports. Family history of SCD was ascertained in 3 athletes. Of 9 athletes undergoing molecular genetic testing, none showed pathogenic or likely pathogenic mutations of genes responsible for inherited cardiomyopathy (including HCM) and channelopathies.
In 21 athletes (40%), TWI was confined to the lateral leads and in 32 (60%) was recorded in both lateral and inferior leads.
CMR findings are summarized in Table 1. Volume and ejection fraction of both LV (median LV indexed end‐diastolic volume=85 mL/m2 and LV ejection fraction=65%) and right ventricle (median right ventricular indexed end‐diastolic volume=89 mL/m2 and right ventricular ejection fraction=60%) were within normal values. The median LV indexed mass was also normal (70 g/m2).
Table 1.
Clinical and Cardiac Magnetic Resonance Characteristics of Athletes With and Without TWI
| Characteristics | TWI (N=53) | No TWI (N=53) | P Value |
|---|---|---|---|
| Male sex | 46 (87) | 46 (87) | … |
| Age, y | 20 (16–43) | 24 (20–40) | 0.34 |
| Sport | |||
| Endurance sports | 18 (34) | 18 (34) | … |
| Power sports | 2 (4) | 2 (4) | … |
| Mixed sports | 33 (62) | 33 (62) | … |
| ECG features | |||
| LV hypertrophy (Sokolow‐Lyon Index ≥40 mm) | 32 (60) | 24 (45) | 0.173 |
| Distribution of T‐wave inversion | |||
| Lateral only (V4–V6) | 21 (40) | … | … |
| Inferolateral (V4–V6+DII and aVF) | 32 (60) | … | … |
| CMR features | |||
| LV EDVi, mL/m2 | 85 (80–103) | 96 (85–100) | 0.11 |
| LV EF (%) | 65 (61–69) | 62 (60–66) | 0.12 |
| LV mass indexed, g/m2 | 70 (63–84) | 74 (63–86) | 0.38 |
| LV mass, g | 130 (112–151) | 139 (116–158) | 0.51 |
| Basal septal maximum wall thickness, mm | 9 (8–11) | 9 (8–10) | 0.06 |
| Apical maximum wall thickness, mm | 8 (6–8) | 6 (5–6) | <0.001 |
| RV EDVi, mL/m2 | 89 (78–100) | 85 (75–101) | 0.31 |
| RV EF (%) | 60 (55–66) | 59 (56–65) | 0.74 |
Values are expressed as number/total (percentage) of subjects or median (25th–75th percentiles). EDVi indicates end‐diastolic indexed volumes; EF, ejection fraction; LV, left ventricular; RV, right ventricular; and TWI, T‐wave inversion.
Dimensions of PMs
Comparison of morpho‐functional PMs features in athletes with lateral TWI and control athletes is summarized in Table 2. Diameter, area, and volume of the anterolateral papillary muscle were greater than those of the posteromedial.
Table 2.
PMs Quantitative Analysis Relationship Between Athletes With and Without TWI
| TWI (N=53) | No TWI (N=53) | P Value | |
|---|---|---|---|
| Anterolateral papillary muscle | |||
| Orthogonal diameter 1, mm | 12.4 (10.3–13.4) | 10 (9–11.6) | 0.001 |
| Orthogonal diameter 2, mm | 9.8 (8.6–11) | 8.3 (7–9.1) | <0.001 |
| Area, cm2 | 1.1 (0.9–1.4) | 0.8 (0.7–1) | <0.001 |
| Volume, cm3 | 3.2 (2.5–3.8) | 2.1 (1.7–2.7) | <0.001 |
| Posteromedial papillary muscle | |||
| Orthogonal diameter 1, mm | 10 (8–12.2) | 9.2 (8–10.4) | 0.09 |
| Orthogonal diameter 2, mm | 8.1 (7.4–10) | 7.6 (6.7–8.9) | 0.024 |
| Area, cm2 | 0.9 (0.7–1.1) | 0.7 (0.6–0.9) | <0.001 |
| Volume, cm3 | 2.5 (1.9–3) | 1.6 (1.3–2.1) | <0.001 |
| PMs mass, g | 5.6 (4.8–7.2) | 3.9 (3.4–4.9) | <0.001 |
| PMs mass indexed, g/m2 | 3.1 (2.6–3.7) | 2.3 (1.8–2.7) | <0.001 |
| PMs mass/LV mass, % | 4.4 (3.8–5.2) | 3 (2.5–3.6) | <0.001 |
| Apical displacement | 25 (47%) | 9 (17%) | 0.001 |
| Anterolateral papillary muscle | 22 (42%) | 7 (13%) | 0.001 |
| Posteromedial papillary muscle | 15 (28%) | 7 (13%) | 0.055 |
Values are expressed as number/total (percentage) of subjects or median (25th–75th percentiles). LV indicates left ventricular; PMs, papillary muscles; and TWI, T‐wave inversion.
Compared with controls, athletes with lateral TWI showed significantly greater diameters, areas, volumes, and mass of both PMs. The ratio between the PMs mass and the LV mass was 4.4% among athletes with TWI versus 3.0% among control athletes (P<0.001) (Figure 2). Intraobserver and interobserver variability in the calculation of quantitative PMs characteristics are shown on Table 3.
Figure 2. Papillary muscles area and volume quantification in athletes with and without T‐wave inversion.
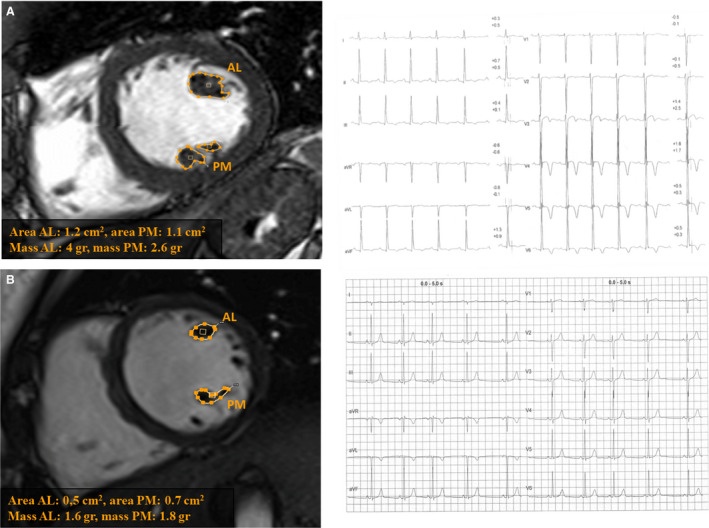
Papillary muscles area and volume quantification in an athlete with TWI in inferolateral leads (A) and in a control athlete with a normal ECG (B). AL indicates anterolateral; and PM, posteromedial.
Table 3.
Intraobserver and Interobserver Variability for PMs Quantitative Analysis in Athletes With TWI
| Interobserver Variability | Intraobserver Variability | |||
|---|---|---|---|---|
| ρ | ICC | ρ | ICC | |
| Anterolateral papillary muscle | ||||
| Orthogonal diameter 1 | 0.97 | 0.98 | 0.98 | 0.98 |
| Orthogonal diameter 2 | 0.98 | 0.98 | 0.97 | 0.98 |
| Area | 0.96 | 0.98 | 0.96 | 0.97 |
| Volume | 0.96 | 0.94 | 0.95 | 0.93 |
| Posteromedial papillary muscle | ||||
| Orthogonal diameter 1 | 0.98 | 0.98 | 0.98 | 0.98 |
| Orthogonal diameter 2 | 0.94 | 0.96 | 0.95 | 0.97 |
| Area | 0.92 | 0.94 | 0.93 | 0.94 |
| Volume | 0.89 | 0.91 | 0.90 | 0.92 |
| PMs mass indexed | 0.89 | 0.91 | 0.90 | 0.92 |
| PMs mass/LV mass | 0.88 | 0.90 | 0.91 | 0.91 |
ICC indicates intraclass correlation coefficient; LV, left ventricular; PMs, papillary muscles; and TWI, T‐wave inversion.
Apical Displacement of PMs
The prevalence of apical displacement of PMs was significantly higher among athletes with TWI than controls (47% versus 17%; P<0.001) (Figure 3). Ten (19%) athletes with TWI showed isolated displacement of the anterolateral papillary muscle, 3 (6%) an isolated displacement of the posterolateral papillary muscle, and 12 (23%) the displacement of both PMs. In control athletes, an isolated apical displacement of the anterolateral papillary muscle was found in 2 cases (4%), isolated apical displacement of posteromedial papillary muscle in 2 (4%) and apical displacement of both PMs in 5 (9%) (Figure 3). The interobserver agreement for PM apical displacement was 101 of 106 (95%, P<0.001; k=0.89).
Figure 3. Papillary muscles implantation in athletes with and without T‐wave inversion.

Normal papillary muscles implantation on the mid left ventricular wall in a control athlete with normal ECG (A). Papillary muscles apical displacement in an athlete with TWI in the inferolateral leads (B).
Correlation of Results
The relationship between apical displacement and dimensions of the PMs in athletes with lateral TWI is reported in Table 3. Normally implanted and apically displaced PMs did not differ significantly with regard to diameter, area, volume, mass, and PMs/LV mass ratio.
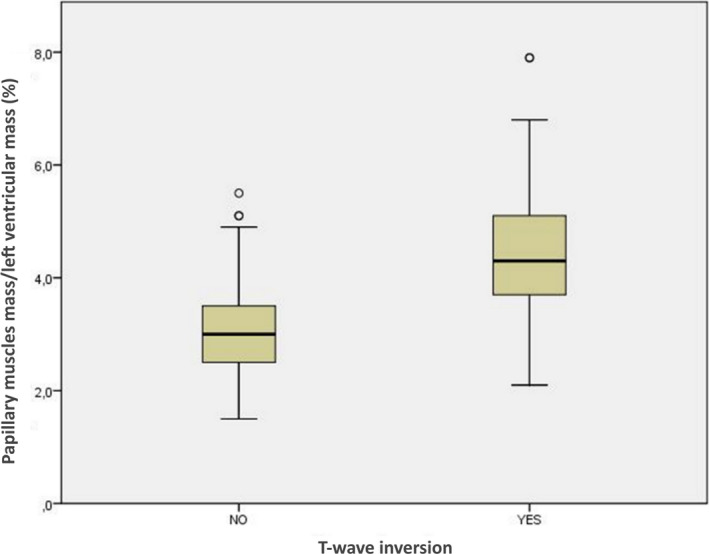
According to the receiver operating characteristic curve (area under the curve, 0.84; P<0.001), a ratio between the PMs/LV mass ratio >3.5% showed a 85% sensitivity and a 76% specificity for the presence of lateral TWI (Figure 4).
Figure 4. Box and whisker graph showing the ratio between papillary muscles mass and left ventricular mass in athletes with and without T‐wave inversion in the lateral or inferolateral leads.
Five of 8 (63%) athletes with lateral TWI, who showed a PMs/LV mass ratio <3.5%, had an apical displacement of the anterolateral papillary muscle. Three (6%) athletes with lateral TWI versus 35 (66%) control athletes showed neither a PMs/LV mass ratio <3.5% nor apical displacement (P<0.001) (Figure 5).
Figure 5. Summary of main study findings.
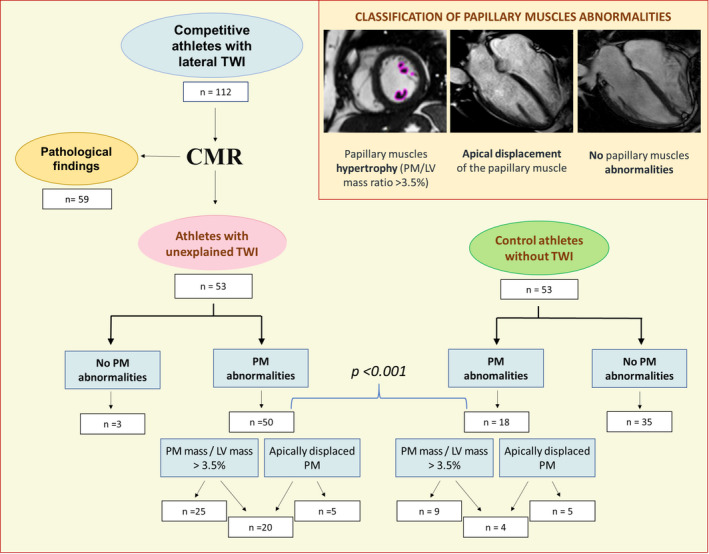
CMR indicates cardiac magnetic resonance; LV, left ventricular; PM, papillary muscles; and TWI, T‐wave inversion.
At multivariate analysis both the PMs/LV mass ratio (odds ratio [OR], 3.8 per 1% increase; 95% CI 2.1–6.7; P<0.001) and the apical displacement of the PMs (OR, 3.2; 95%, CI 1.1–9.6; P=0.03) were independent predictors of lateral TWI.
Detraining
Six athletes with lateral TWI underwent control ECG and CMR study after 3 to 6 months of detraining. All 3 athletes with persistence of lateral TWI on control ECG had an apical displacement of the PMs with unchanged hypertrophy. In 2 of 3 athletes (1 with apical displacement of the anterolateral papillary muscle), showing ECG normalization after detraining, there was a significant decrease of the PMs/LV mass ratio; 1 athlete had ECG normalization with persistence of PMs hypertrophy.
Follow‐Up
All athletes with TWI were allowed to continue the practice of competitive sports because the abnormalities of PMs in isolation were not considered a cause of disqualification by the Italian guidelines for sports eligibility. 15 During a mean follow‐up of 3.6 ± 1.2 years, the outcome of the entire study population (with and without TWI) was uneventful. During the follow‐up period, no athlete showed evidence of delayed progression toward any definite cardiac disease, with particular reference to HCM, at yearly control echocardiogram.
DISCUSSION
This case‐control observational study was designed to evaluate the relationships between lateral TWI and morpho‐functional abnormalities of PMs in a cohort of athletes who underwent CMR for exclusion of heart disease. The use of CMR imaging allowed us to obtain detailed and accurate measurements of diameters, area, volume, and mass of PMs in the study athletes.
The main study findings were the following: (1) In more than half of the athletes, the lateral TWI was explained by CMR demonstration of an underlying heart disease; (2) the remaining athletes with unexplained lateral TWI showed both a higher PMs/LV mass ratio and a higher prevalence of apical displacement of the PMs compared with controls; (3) persistence of lateral TWI after detraining was always associated with lack of PMs hypertrophy regression and/or apical displacement of the PMs, while normalization of the ECG was associated with reduction of the PMs/LV mass ratio in 2 of 3 cases; (4) over follow‐up, the clinical outcome of all athletes with lateral TWI and PMs abnormalities, who continued to participate in their sports activity, was uneventful, and there was no late occurrence of a cardiomyopathy phenotype.
Pathological Substrates of Lateral TWI in the Athlete
Previous studies consistently showed that lateral TWI in athletes is associated with a high prevalence of cardiac pathologies that may predispose to SCD. 1 , 2 , 3 , 5 Pelliccia et al 6 reported that 24 of 123 athletes with TWI had either cardiomyopathy or other heart disease diagnosed by echocardiography. In addition, 5 (6%) athletes with TWI and initial normal echocardiography, subsequently developed an overt cardiomyopathy phenotype (3 HCM, 1 arrhythmogenic right ventricular cardiomyopathy, 1 dilated cardiomyopathy) over a 9‐year follow‐up. Schnell et al 7 performed a comprehensive investigation including CMR of 155 athletes with TWI; 69 (44%) were diagnosed with structural heart disease, predominantly HCM (n=55). Of note, 88% of the athletes diagnosed with a cardiomyopathy had lateral TWI. The prevalence of disease in the present study was higher than previously reported (59 of 113 athletes; 52%), although HCM was the most common identified disease (35% of athletes) in agreement with previous studies. The use of contrast‐enhanced CMR may have accounted for our high prevalence of diagnoses of structural heart diseases, which included 11 cases (10%) of isolated nonischemic LV scar.
PMs Hypertrophy
A previous study by Kobashi et al 10 reported that patients with echocardiographic evidence of PMs hypertrophy may show TWI in the absence of other demonstrable heart disease, including HCM. Isolated PMs hypertrophy was subsequently observed on CMR scan in anecdotal young subjects and healthy athletes with TWI of unknown etiology. 16 , 17 , 18 Our study results confirm and extend previous observations by providing a systematic and accurate CMR analysis of morpho‐functional features of PMs in a cohort of athletes with otherwise unexplained lateral TWI. Our study results demonstrated a significant association between lateral TWI and increased diameters, area, volume, mass, and PMs/LV mass ratio; this latter index was used to normalize the PMs mass with the LV mass, which may be increased in athletes. The mean PMs volume and PMs/LV mass ratio among athletes with TWI were greater than those of control athletes with a normal ECG. According to the receiver operating characteristic curve analysis, a PMs/LV mass ratio >3.5% provided a sensitivity of 85% (ie, 8 of 53 athletes with lateral TWI had PMs/LV mass ratio ≤3.5%) and a specificity of 76% (ie, 13 of 53 controls without lateral TWI had PMs/LV mass ratio >3.5%).
PMs Displacement
In our study, lateral TWI was also significantly associated with apical displacement of the PMs, particularly the anterolateral. In sarcomeric gene‐related HCM, the apical displacement of PMs is a recognized accessory abnormality that can contribute to the dynamic LV outflow tract obstruction. 19 Lee et al 11 demonstrated that an apically displaced (nonhypertrophic) anterolateral papillary muscle may be associated with an hypertrophy of LV apicolateral segments (but no apicoseptal), which may mimic an apical HCM, also regarding the ECG presentation with giant TWI. This finding indicated that an abnormal implant of the anterolateral papillary muscle may cause repolarization abnormality.
In our cohort of athletes with lateral TWI, the apical displacement of the anterolateral papillary muscle was isolated and occurred in the absence of increased thickness of apical LV segments and without LV outflow tract gradient, leading to a clear exclusion of an apical HCM. It is noteworthy that PMs hypertrophy was unrelated to the PMs apical displacement, and multivariable analysis showed that both PMs abnormalities were independent predictors of lateral TWI, with an OR of 3.4 and 3.2, respectively. Of note, 5 of 8 athletes (63%) with PMs/LV mass ratio <3.5% showed an apical displacement of the PMs.
Clinical Implications
In the study of Kobashi et al, 10 one‐third of patients with isolated PMs hypertrophy had a family history of HCM, suggesting that this patient subset had a “forme frustre” of HCM (without increase of LV thickness). In our study, athletes with lateral TWI associated with PMs abnormalities had a negative family history for HCM and did not show any clinical and imaging features of HCM, such as symptoms, arrhythmias, LV hypertrophy, and LGE of LV myocardium.
Pelliccia et al 6 reported that failure to detect structural abnormalities on imaging in athletes with TWI does not conclusively exclude an underlying structural heart disease. Indeed, TWI may represent the initial phenotypic expression of a cardiomyopathy (including HCM) that may precede by years the development of structural changes detectable on cardiac imaging. In our study, athletes underwent a repeat imaging study during follow‐up with no evidence of subsequent development of LV hypertrophy consistent with late phenotypic expression of HCM. Finally, the clinical outcome of all athletes with lateral TWI and PMs abnormalities, who continued to participate in their sports activity, was uneventful.
Nine athletes (17%) underwent molecular screening of a large panel of genes associated with familial cardiomyopathies and channelopathies, which failed to identify any pathogenic or likely pathogenetic mutations, including those responsible for HCM. These findings are in agreement with those of a previous study showing the negligible added value of molecular genotyping for identification of an underlying heart disease in athletes with lateral TWI, in the absence of family history and lack of phenotypic expression of cardiomyopathy, especially HCM. 8
All these findings indicate that lateral TWI in association with PMs abnormalities, hypertrophy, and/or displacement may occur as a phenotype distinct from that of HCM and may be associated with a favorable outcome in the athlete.
Study Limitations
The study is limited by the retrospective nature, the relatively small sample size, and the short follow‐up duration. Although athletes underwent yearly echocardiography that did not show progression toward any definite cardiomyopathy, it should be recognized that the exam may be insufficiently sensitive to capture early/minor phenotypic expression because of the limitations of the technique in accurately measuring the LV wall thickness, mostly in regions such as the apex and anterior wall. These study limitations do not allow us to draw definite conclusions about the clinical meaning and outcome of isolated PMs abnormalities in athletes. However, the principal aim of our study was the correlation, previously unaddressed, between unexplained lateral TWI and isolated PMs abnormalities in the athlete, while the preliminary and short‐term follow‐up data should be regarded as a parallel but secondary study objective. Molecular genetic testing was performed in a minority of athletes with TWI and PMs abnormalities, precluding any definite conclusion about the etiology of PMs abnormalities. Likewise, findings of control ECG and CMR after detraining are inconclusive because the study was limited to few athletes, although they indicate the potential for a normalization of repolarization abnormalities in the presence of PMs hypertrophy reversion.
Conclusions
The results of our study showed that isolated hypertrophy and/or displacement of LV PMs may underlie lateral TWI in a sizeable proportion of athletes with otherwise structurally normal heart. Overall, 94% of our athletes with unexplained lateral TWI showed a PMs/LV mass ratio ≥3.5%, an apical displacement of the PMs, or both. According to our study findings, the association between lateral TWI and PMs abnormalities represents a distinct anatomo‐clinical condition characterized by a favorable medium‐term outcome even in individuals engaged in competitive sports activity. These findings have important implications for evaluation and management of athletes with repolarization abnormalities without clinically demonstrable heart disease.
Future studies on a larger population of athletes with this condition over a long‐term follow‐up are warranted to better define background, clinical meaning, and outcome. Further research should focus on systematic molecular genetic analysis and control ECG and CMR study after detraining.
Sources of Funding
None.
Disclosures
None.
Acknowledgments
The authors thank Drs Marino Tonelli, Pierluigi Russo, and Carlo Stefenelli for referring athletes with T‐wave inversion.
(J Am Heart Assoc. 2021;10:e019239. DOI: 10.1161/JAHA.120.019239.)
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Alessandro Zorzi, Email: alessandro.zorzi@unipd.it.
Domenico Corrado, Email: domenico.corrado@unipd.it.
References
- 1. Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, La Gerche A, Ackerman MJ, Borjesson M, Salerno JC, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2018;39:1466–1480. DOI: 10.1093/eurheartj/ehw631. [DOI] [PubMed] [Google Scholar]
- 2. D’Ascenzi F, Anselmi F, Adami PE, Pelliccia A. Interpretation of T‐wave inversion in physiological and pathological conditions: current state and future perspectives. Clin Cardiol. 2020;43:827–833. DOI: 10.1002/clc.23365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zorzi A, Vio R, Bettella N, Corrado D. Criteria for interpretation of the athlete’s ECG: a clinical appraisal. Pacing Clin Electrophysiol. 2020;43:882–890. [DOI] [PubMed] [Google Scholar]
- 4. Hanna EB, Glancy DL. ST‐segment depression and T‐wave inversion: classification, differential diagnosis, and caveats. Cleve Clin J Med. 2011;78:404–414. DOI: 10.3949/ccjm.78a.10077. [DOI] [PubMed] [Google Scholar]
- 5. Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, et al. Nonischemic left ventricular scar as a substrate of life‐threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol. 2016;9:e004229. DOI: 10.1161/CIRCEP.116.004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pelliccia A, Di Paolo FM, Quattrini FM, Basso C, Culasso F, Popoli G, De Luca R, Spataro A, Biffi A, Thiene G, et al. Outcomes in athletes with marked ECG repolarization abnormalities. N Engl J Med. 2008;358:152–161. DOI: 10.1056/NEJMoa060781. [DOI] [PubMed] [Google Scholar]
- 7. Schnell F, Riding N, O'Hanlon R, Axel Lentz P, Donal E, Kervio G, Matelot D, Leurent G, Doutreleau S, Chevalier L, et al. Recognition and significance of pathological T‐wave inversions in athletes. Circulation. 2015;8:e003454. [DOI] [PubMed] [Google Scholar]
- 8. Sheikh N, Papadakis M, Wilson M, Malhotra A, Adamuz C, Homfray T, Monserrat L, Behr ER, Sharma S. Diagnostic yield of genetic testing in young athletes with T‐wave inversion. Circulation. 2018;138:1184–1194. DOI: 10.1161/CIRCULATIONAHA.118.034208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rajiah P, Fulton NL, Bolen M. Magnetic resonance imaging of the papillary muscles of the left ventricle: normal anatomy, variants, and abnormalities. Insights Imaging. 2019;10:83. DOI: 10.1186/s13244-019-0761-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kobashi A, Suwa M, Ito T, Otake Y, Hirota Y, Kawamura K. Solitary papillary muscle hypertrophy as a possible form of hypertrophic cardiomyopathy. Jpn Circ J. 1998;62:811–816. DOI: 10.1253/jcj.62.811. [DOI] [PubMed] [Google Scholar]
- 11. Lee SP, Park K, Kim HK, Kim YJ, Sohn DW. Apically displaced papillary muscles mimicking apical hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2013;14:128–134. DOI: 10.1093/ehjci/jes113. [DOI] [PubMed] [Google Scholar]
- 12. Gati S, Sharma S, Pennell D. The role of cardiovascular magnetic resonance imaging in the assessment of highly trained athletes. JACC Cardiovasc Imaging. 2018;11:247–259. [DOI] [PubMed] [Google Scholar]
- 13. De Lazzari M, Zorzi A, Cipriani A, Susana A, Mastella G, Rizzo A, Rigato I, Bauce B, Giorgi B, Lacognata C, et al. Relationship between electrocardiographic findings and cardiac magnetic resonance phenotypes in arrhythmogenic cardiomyopathy. J Am Heart Assoc. 2018;7:e009855. DOI: 10.1161/JAHA.118.009855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harrigan CJ, Appelbaum E, Maron BJ, Buros JL, Gibson CM, Lesser JR, Udelson JE, Manning WJ, Maron MS. Significance of papillary muscle abnormalities identified by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. Am J Cardiol. 2008;101:668–673. DOI: 10.1016/j.amjcard.2007.10.032. [DOI] [PubMed] [Google Scholar]
- 15. Protocolli Cardiologici Per il Giudizio Dell’idoneità Allo Sport Agonistico (COCIS). Rome: Casa Editrice Scientifica Internazionale; 2017. [Google Scholar]
- 16. Correia AS, Pinho T, Madureira AJ, Araujo V, MacIel MJ. Isolated papillary muscle hypertrophy: a variant of hypertrophic cardiomyopathy? Do not miss a hypertrophic cardiomyopathy. Eur Heart J Cardiovasc Imaging. 2013;14:827–828. [DOI] [PubMed] [Google Scholar]
- 17. Ferreira C, Delgado C, Vázquez M, Trinidad C, Vilar M. Isolated papillary muscle hypertrophy: a gap in our knowledge of hypertrophic cardiomyopathy? Rev Port Cardiol. 2014;33:379.e1–379.e5. DOI: 10.1016/j.repc.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 18. Işilak Z, Uz Ö, Uzun M, Cebeci BS. An unusual cause of electrocardiographic abnormality: solitary papillary muscle hypertrophy. Turk Kardiyol Dern Ars. 2012;40:473. [DOI] [PubMed] [Google Scholar]
- 19. Kwon DH, Setser RM, Thamilarasan M, Popovic ZV, Smedira NG, Schoenhagen P, Garcia MJ, Lever HM, Desai MY. Abnormal papillary muscle morphology is independently associated with increased left ventricular outflow tract obstruction in hypertrophic cardiomyopathy. Heart. 2008;94:1295–1301. DOI: 10.1136/hrt.2007.118018. [DOI] [PubMed] [Google Scholar]


