Abstract
Background
Classical ST‐T waveform changes on standard 12‐lead ECG have limited sensitivity in detecting acute coronary syndrome (ACS) in the emergency department. Numerous novel ECG features have been previously proposed to augment clinicians' decision during patient evaluation, yet their clinical utility remains unclear.
Methods and Results
This was an observational study of consecutive patients evaluated for suspected ACS (Cohort 1 n=745, age 59±17, 42% female, 15% ACS; Cohort 2 n=499, age 59±16, 49% female, 18% ACS). Out of 554 temporal‐spatial ECG waveform features, we used domain knowledge to select a subset of 65 physiology‐driven features that are mechanistically linked to myocardial ischemia and compared their performance to a subset of 229 data‐driven features selected by multiple machine learning algorithms. We then used random forest to select a final subset of 73 most important ECG features that had both data‐ and physiology‐driven basis to ACS prediction and compared their performance to clinical experts. On testing set, a regularized logistic regression classifier based on the 73 hybrid features yielded a stable model that outperformed clinical experts in predicting ACS, with 10% to 29% of cases reclassified correctly. Metrics of nondipolar electrical dispersion (ie, circumferential ischemia), ventricular activation time (ie, transmural conduction delays), QRS and T axes and angles (ie, global remodeling), and principal component analysis ratio of ECG waveforms (ie, regional heterogeneity) played an important role in the improved reclassification performance.
Conclusions
We identified a subset of novel ECG features predictive of ACS with a fully interpretable model highly adaptable to clinical decision support applications.
Registration
URL: https://www.clinicaltrials.gov; Unique Identifier: NCT04237688.
Keywords: acute coronary syndrome, dimensionality reduction, ECG, ischemia, machine learning
Subject Categories: Biomarkers, Ischemia,
Nonstandard Abbreviations and Acronyms
- ANN
artificial neural network
- FSS
features subset selection
- LR
logistic regression
- PCA
principal component analysis
Clinical Perspective
What Is New?
This study identifies a subset of novel computational features from the standard 12‐lead ECG, other than ST‐segment and T wave changes, which would improve the detection of non‐ST elevation acute coronary syndrome at the emergency department.
While maintaining higher negative predictive value, a machine learning model based on these novel features achieved 47% gain in sensitivity compared with commercial interpretation software and 32% compared with experienced clinicians.
This model also successfully classified challenging ECGs deemed nondiagnostic for ischemia because of secondary repolarization changes (left ventricular hypertrophy, left bundle branch block, pacing, etc).
What Are the Clinical Implications?
Our machine learning model is fully interpretable and can be easily incorporated into existing ECG software or embedded into ECG interpretation platforms for decision support.
These algorithms can help clinicians in identifying non‐ST elevation acute coronary syndrome in real time, which has been a longstanding challenge in clinical practice.
Given that our model has higher sensitivity and negative predictive value compared with experienced clinicians, it is well suited as an initial screening tool (ie, rule out); this has the potential to better allocate hospital resources by avoiding prolonged observations, unnecessary admissions, or invasive testing.
The prompt identification of acute coronary syndrome (ACS) is a longstanding challenge in emergency practice. 1 , 2 , 3 The ECG is readily available during initial patient evaluation, and sensitive ECG markers of acute myocardial ischemia can expedite the current time‐consuming, biomarker‐driven approach for ACS diagnosis. 4 , 5 , 6 The electrophysiological basis of acute myocardial ischemia has been thoroughly studied over the past few decades, 7 with many studies suggesting the abundance of hidden signatures of acute myocardial ischemia in the surface ECG signal. 8 , 9 Yet, current guidelines exclusively rely on the amplitude of ST segment and T wave for ACS detection, 10 translating into a diagnostic sensitivity of ≈40% for the standard 12‐lead ECG. 11 Given that ECG waveform is among the most extensively studied signals in cardiovascular medicine, existing computational algorithms can extract hundreds of features from a single 10‐second 12‐lead ECG. Thus, recent advances in pattern recognition and machine learning could help in identifying an optimal subset of features to augment clinicians' decision in detecting ACS during initial evaluation. 12
Although it is being widely adopted in various clinical applications, machine learning is limited by the relatively small size of available clinical data sets and the difficulty of finding comparable external data sets for replication. 13 Accordingly, feature subset selection (FSS) plays a significant role in optimizing the accuracy of supervised classification systems, including improved understandability of the final classifier. In addition to available data‐driven approaches of FSS, some studies suggest the need for domain‐specific expertise to guide feature selection and model development during the learning process. 13 The electrophysiology of myocardial ischemia is well understood, and it is feasible to perform FSS based on cardiac physiology. However, there is a paucity of evidence regarding the effect of manual FSS on the performance of supervised classification systems. In fact, manual FSS is counterintuitive to the premise of machine learning—the discovery of hidden patterns in the data that might not be apparent to clinicians. Accordingly, using 2 prospective clinical cohorts, we sought to (1) compare the accuracy of supervised classifiers in detecting ACS using ECG feature subsets selected based on either data‐driven techniques or domain‐specific knowledge; and (2) whether data‐driven FSS techniques can identify ECG features indicative of ACS that were overlooked by domain‐specific human experts.
METHODS
Design and Settings
This was a prospective observational cohort study of consecutive patients with chest pain transported by emergency medical services to 1 of 3 tertiary care hospitals in the United States between 2013 and 2015. The methods of this study were previously described in detail. 14 In short, we collected the prehospital 12‐lead ECGs obtained by paramedics in the field and stored them for offline analysis. We then followed up patients to adjudicate study outcomes. Clinical data were obtained from medical charts by independent reviewers. Patients were recruited under a waiver of informed consent and the study was approved by the institutional review board of University of Pittsburgh. The data that support the findings of this study are available from the corresponding author upon reasonable request.
The primary study outcome was the presence of ACS (myocardial infarction or unstable angina) during the primary indexed admission, defined according to the fourth Universal Definition of myocardial infarction consensus statement as the presence of symptoms of ischemia (ie, diffuse discomfort in the chest, upper extremity, jaw, or epigastric area for more than 20 minutes) with the presence of biomarker, nuclear, or angiographic evidence of myocardial ischemia and/or loss of viable myocardium. 10 Study outcomes were adjudicated by 2 independent physician reviewers and disagreement was resolved by a third physician reviewer. Patients discharged from the emergency department were classified as negative for ACS if they had no 30‐day adverse events. Patients presenting ventricular tachycardia or fibrillation on prehospital ECG were excluded from this analysis.
ECG Preprocessing and Feature Extraction
Each ECG was manually overread by an independent reviewer. ECGs with excessive noise or artifact (n=24, 2%) were substituted by the next serial ECG obtained during emergency evaluation. ECGs with ventricular tachycardia or fibrillation were excluded from this analysis (n=7, 0.5%). All other available ECGs, including those with secondary repolarization changes (ie, pacing, bundle branch block, coarse atrial fibrillation, or left ventricular hypertrophy with strain, n=178, 14%) were included in the analysis. We decided to keep these ECGs because their removal had no effect on the performance of subsequent predictive models. Besides, the ability to classify these challenging ECGs would have huge clinical utility during emergency care.
Then, 10‐second, 12‐lead ECG signals (500 s/s, HeartStart MRx, Philips Healthcare) were preprocessed at Philips Healthcare Advanced Algorithm Research Center (Andover, MA). Raw ECG signals were decompressed to extract individual ECG leads. Noise, artifact, and ectopic beats were then removed, and representative average beats were computed for each ECG lead to eliminate residual baseline noise and artifacts. This technique yields high signal‐to‐noise ratio and stable average waveform signal for each of the 12 leads.
Next, fiducial points from these representative beats were identified and corresponding ECG features were extracted. The details of feature extraction from this data set was previously described in detail. 12 In short, a total of 554 features were extracted from each 12‐lead ECG. First, duration, amplitude, and area of various waveform deflections were calculated from each of the 12 leads, yielding 444 temporal ECG features (Figure 1A). Second, the 12 representative beats were superimposed, and global intervals and subintervals were computed, yielding 6 more temporal ECG features (Figure 1B). Third, principal component analysis (PCA) on time‐voltage data was performed on orthogonal leads I, II, and V1–V6 to compute PCA ratios of the eigenvalues of various ECG waveforms, yielding 13 spatial ECG features (Figure 1C). Finally, axes, angles, loops, and gradients of QRS and T vectors from xy, xz, yz, and xyz planes were computed, yielding 91 more spatial ECG features (Figure 1D).
Figure 1. Computation of ECG features.
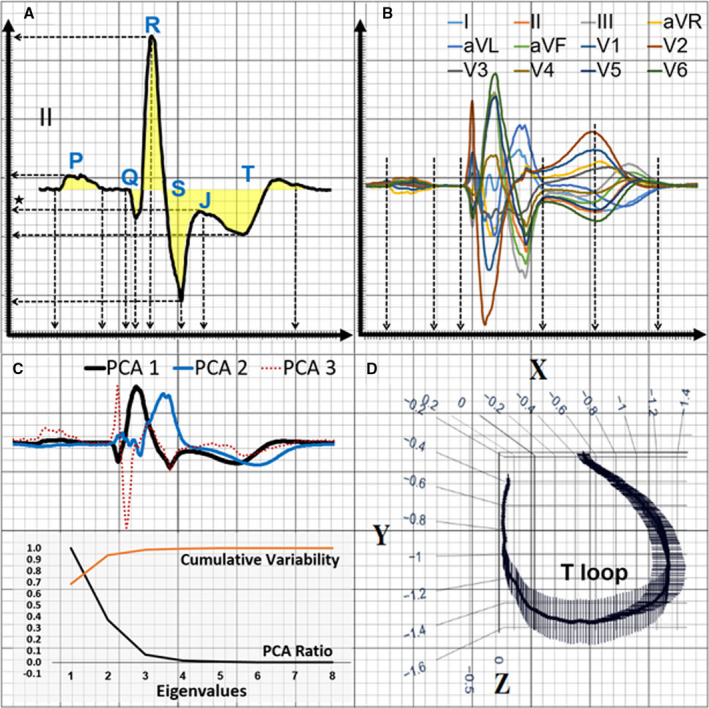
This figure shows the computation of 554 features from each 12‐lead ECG. A, Duration, amplitude, and area of various waveform deflections are calculated from the median beat of each of the 12 leads. B, The 12 median beats are superimposed, and global intervals and subintervals are computed. C, Principal component analysis (PCA) on time‐voltage data is performed on the orthogonal leads I, II, V1–V6 to compute PCA ratios of the eigenvalues of various ECG waveforms. D, Axes, angles, loops, and gradients of QRS and T vectors from xy, xz, yz, and xyz planes are computed. aVL indicates augmented vector left; and aVR, augmented vector right.
All extracted ECG features were then Z score normalized. Missing data, representing <0.2% of the total features' values available in our data set, were imputed using the mean or the mode of the corresponding feature.
FSS Using Domain‐Specific Human Expertise
Two research scientists trained in cardiac electrophysiology reviewed the 554 extracted ECG features and agreed on a reduced set of 65 features that had strong physiological basis as plausible markers of acute myocardial ischemia, including 24 classical measures (amplitude of J+80 point and T wave from each of the 12 leads), and 41 supplemental features that may correlate with acute cardiac ischemia: depolarization and repolarization times (ie, QRS duration, JTend, JTpeak, Tpeak‐end, and QT interval, k=6); depolarization and repolarization vectors (QRS and T axes and angles, k=8); repolarization velocity (ie, T wave peak inflection, amplitude, and slope, k=5); global electrical dispersion (PCA ratios between QRS, ST‐T, J, and T eigenvalues, k=13); repolarization characteristics (ie, T wave morphology and T loop features, k=7); and high frequency signal noise values (k=2). The selection of these candidate features was based on review of literature 15 and our previous work. 8 , 16 , 17
FSS Using Data‐Driven Algorithms
We used 3 different data‐driven algorithms to identify a list of features most important for optimizing the performance of the classification algorithm. First, we used Cohen's d effect size, which compares how distinguishable ACS versus non‐ACS distributions of a given feature are in terms of the distance between the means. All distributions were evaluated for normality of distributions and homogeneity of variances. Features corresponding to an effect size lower than 0.35 are assumed to fail to differentiate between the 2 populations and were excluded from our data set. Using this cutoff value, only 23 features out of 554 remained (4%). Second, we used recursive features elimination as part of logistic regression (LR). We evaluated 20 features per iteration and used F1 scores to evaluate model performance. F1 scores provide the best tradeoff between precision and recall using imbalanced data sets like ours, which had a 6:1 ratio of non‐ACS to ACS subgroups. The selection of the optimal set of features went through a 10‐fold cross‐validation process. Using this technique, 156 features out of 554 (28%) were selected. Finally, we used least absolute shrinkage and selection operator (LASSO) regression to select the most important features with non‐zero coefficients. We used the L1 norm method to penalize the least square error between the outcome and an affine function of the input variables. The regularization parameter alpha was set by the means of a 10‐fold cross‐validation. Using this technique, 96 features out of 554 (17%) were selected.
Next, given that the 3 FSS techniques described here use complementary, noncompeting approaches, we identified the features that received at least 1 vote (ie, appeared in at least 1 FSS algorithm). This yielded a total of 229 features. We used these data‐driven features in subsequent training and testing of machine learning classifiers in order to compare against the domain‐specific manually selected features. It is noteworthy that this step‐by‐step process for FSS was selected after a comprehensive evaluation of our data set. This is important to note because the performance of machine learning algorithms is dependent on the inherent properties of the data set used. Several studies have used multiple FSS procedures to tackle 1 specific disease diagnosis. 18
FSS Using a Hybrid Data‐ and Physiology‐Driven Approach
To identify any important ECG features that were missed by domain‐specific experts, we mapped the 229 data‐driven features against the major components of the 12‐lead ECG signal, identifying the overlap between the data‐driven features and the ones selected by domain‐specific experts. We identified pertinent data‐driven features that could be mechanistically linked to ischemia and yet missed by human experts. This yielded a total of 100 hybrid features that are both data driven and judged by clinicians as presumably contributing as signatures of myocardial ischemia. To reduce the apparent redundancy in these features, we used random forest to identify and keep the important features for the task of ACS detection. This yielded a final novel subset of 73 features that we used in subsequent tuning of machine learning classifiers. Figure 2 summarizes the sequential steps for ECG features selection used in this study.
Figure 2. Flow diagram of the features selection steps used in this study.
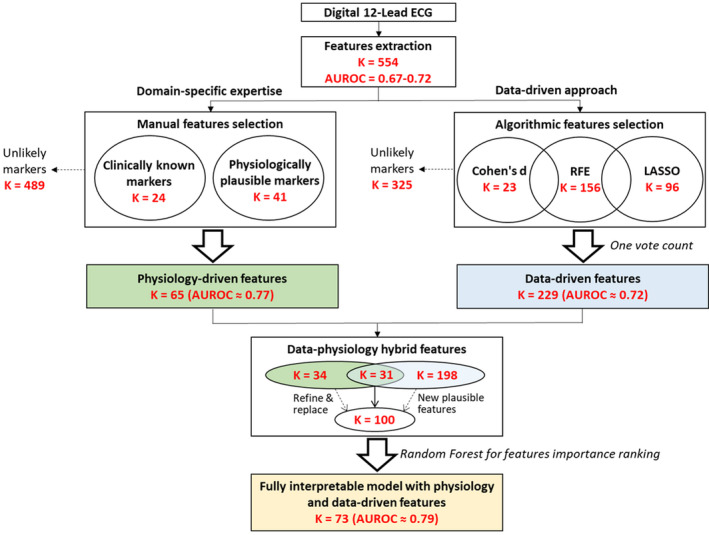
This diagram summarizes the steps used to select features using domain knowledge, data‐driven algorithms, and the hybrid combination of both approaches. AUROC indicates area under the receiver operating characteristic curve; K, number of features in each step; LASSO, least absolute shrinkage and selection operator; and RFE, recursive features elimination.
Machine Learning and Statistical Methods
LR and artificial neural networks (ANN) have been preferentially used in previous studies focusing on ECG‐based prediction of ACS. 19 , 20 , 21 Considering the size of our data set and the expected reduction of model complexity achieved through FSS, we started with LR as the machine learning classifier of choice to address the aims of our study. We then used ANN to explore whether FSS approaches would have a similar effect on more sophisticated, nonlinear machine learning classifiers.
Our LR and ANN classifiers were trained using a 10‐fold cross‐validation on Cohort 1 and, afterwards, tested on an independent Cohort 2 being completely blinded to its outcomes. We started with all 556 available features (554 ECG features with age and sex) without any FSS (ie, LR554 and ANN554). Next, we built models using the 65 manual features selected by domain‐specific human experts (ie, LR65 and ANN65), the 229 data‐driven features (ie, LR229 and ANN229), and the 73 hybrid data‐ and physiology‐driven features (ie, LR73 and ANN73). The algorithms were trained using 10‐fold cross‐validation and then evaluated on an independent testing set that was blinded to the outputs.
The classification performance of each classifier was evaluated using the area under the receiver operating characteristic (ROC) curve. This tool is powerful because it reflects the ability of binary classifiers to distinguish between 2 populations. We used DeLong's test to compare the difference between the mean area under the curve of 2 correlated ROC curves of different classifiers, 22 and we opted for pairwise comparisons. We set alpha at P<0.05 for 2‐tailed hypothesis testing.
ECG Reference Standards
We compared the performance of the final LR73 classifier against 2 current ECG reference standards: (1) clinical experts' interpretation and (2) commercial interpretation software. To get these annotations, each 12‐lead ECG was over‐read by 2 experienced clinicians. Each reviewer classified each ECG according to the likelihood of underlying ACS (yes/no) taking into account diagnostic ST‐T changes as per the fourth Universal Definition of Myocardial Infarction consensus statement, 10 and the presence of other suspicious ECG findings (ie, contiguous territorial involvement, evidence of reciprocal changes, changes beyond those caused by secondary repolarization, and lack of ECG evidence of nonischemic chest pain etiologies). Disagreements were resolved by a board‐certified cardiologist. Next, we used Philips diagnostic 12/16‐lead ECG analysis program (Philips Healthcare) for automated ECG interpretation. This software is commercially available and is used in practice to denote the diagnostic likelihood of ACS on the ECG printout (ie, “***Acute MI***”).
We computed and compared the sensitivity, specificity, and positive and negative predictive values for the final machine learning classifier and the reference standards. We also computed the net reclassification improvement index for our final machine learning classifier against each reference standard. Finally, in subsequent sensitivity analyses, we reevaluated the diagnostic performance of our final machine learning classifier in detecting patients with non−ST‐segment elevation ACS (NSTE‐ACS) after excluding patients with confirmed ST‐segment–elevation myocardial infarction on their prehospital ECG and who were sent to the catheterization laboratory emergently.
RESULTS
Baseline Characteristics
Our sample consisted of 1244 patients from 2 study cohorts: a training cohort (n=745, age 59±17, 42% female, 40% Black) and a testing cohort (n=499, age 59±16, 49% female, 40% Black). Most patients were evaluated for chest pain (90%) or shortness of breath (39%); most patients presented in normal sinus rhythm (88%) or atrial fibrillation (9%); and the rate of 30‐day cardiovascular death was 4.6%. Table 1 summarizes the baseline characteristics of each cohort. The 2 cohorts were comparable in terms of demographics, past medical history, chief complaint, baseline ECG characteristics, and clinical outcomes.
Table 1.
Baseline Study Characteristics
|
Cohort 1 (N=745) (Training Set) |
Cohort 2 (N=499) (Testing Set) |
|
|---|---|---|
| Demographics | ||
| Age in y | 59±17 | 59±16 |
| Sex (female) | 317 (42%) | 243 (49%) |
| Race (Black) | 301 (40%) | 202 (40%) |
| Past medical history | ||
| Hypertension | 519 (69%) | 329 (66%) |
| Diabetes mellitus | 196 (26%) | 132 (26%) |
| Old myocardial infarction | 205 (27%) | 122 (24%) |
| Known coronary artery disease | 248 (33%) | 179 (36%) |
| Known heart failure | 130 (17%) | 74 (15%) |
| Prior PCI/CABG | 207 (28%) | 124 (25%) |
| Clinical presentation | ||
| Chest pain | 665 (89%) | 454 (91%) |
| Shortness of breathing | 250 (34%) | 234 (47%) |
| Normal sinus rhythm | 648 (87%) | 442 (88%) |
| Atrial fibrillation | 71 (9%) | 46 (9%) |
| Course of hospitalization | ||
| Length of stay, median [interquartile range] | 2.3 [1.0–3.0] | 1.2 [0.6–2.5] |
| Confirmed ACS (all events) | 114 (15.3%) | 92 (18.4%) |
| Non−ST‐segment elevation‐ACS | 83 (11.1%) | 74 (14.8%) |
| Treated by primary PCI/CABG | 74 (10%) | 65 (13%) |
| 30‐d cardiovascular death | 33 (4.4%) | 24 (4.8%) |
ACS indicates acute coronary syndrome; CABG, coronary artery bypass graft; and PCI, primary percutaneous coronary intervention.
Performance of Machine Learning Classifiers
The primary study outcome was ACS, which occurred in 114 out of 745 patients (15.3%) in the training cohort and 92 out of 499 patients (18.4%) in the testing cohort. Figure 3 compares the area under the ROC curves of the different LR and ANN classifiers considered in this study. On training set (Figure 3A, left panel), both manual FS and data‐driven FSS techniques had better performance compared with no FSS, with the best performance (lowest bias) achieved using the data‐driven approach. However, on independent testing (Figure 3A, right panel), data‐driven FS approach generalized poorly (high variance). Manual FSS, on the other hand, generalized well to the testing set, suggesting a better bias‐variance tradeoff. Comparing the area under ROC curve of manual FSS and data‐driven FSS yielded a statistically significant difference for the LR model with a P value equal to 0.0105. The same trend was observed using ANN. The data‐driven FSS approach performed best on the training set (Figure 3B, left panel), but generalized poorly to the testing set (Figure 3B, right panel), again suggesting more overfitting compared with manual FSS approach, with a P value equal to 0.0411.
Figure 3. Classification performance using LR and ANN classifiers.
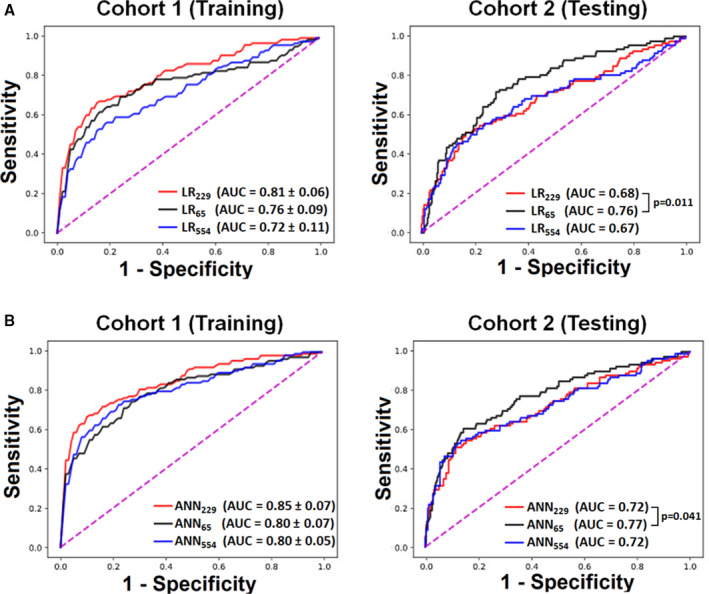
These plots show the performance of (A) logistic regression (LR) and (B) artificial neural network (ANN) classifiers on training data (Cohort 1) and testing data (Cohort 2) using all available ECG features (k=554), data‐driven subset of ECG features (k=229), or physiology‐driven subset of ECG features (k=65). P values are based on nonparametric method by Delong. AUC indicates area under the curve.
Overlap in Features Between FSS Approaches
Among the 229 data‐driven features, 31 features (14%) were among the ones manually selected by human experts. These data‐driven features with physiological plausibility for ACS classification included (1) lead‐specific ST and T wave amplitudes; (2) Tpeak–Tend interval; (3) frontal and horizontal QRS and T axes; (4) spatial QRS‐T angle and total‐cosine R‐to‐T angle; (5) T loop morphology dispersion; (6) PCA ratio of QRST waveform, ST‐T waveform, and T wave; and (7) the nondipolar component of J wave. Among these features, Tpeak–Tend was specifically selected by all 3 data‐driven FSS algorithms and was also ranked by LR classifiers as the most important feature among the ones selected by human experts. Finally, to discern which data‐driven features contributed to noise versus contributed to true prognostic value in ACS prediction, we mapped the 229 data‐driven features against the major components of the 12‐lead ECG signal (Table 2). This table highlights a potential subset of features that data‐driven algorithms ranked as important for the task of ACS detection but were not selected by domain‐specific experts.
Table 2.
Overlap in Features Between Data‐Driven and Human‐Expert Techniques
| 12‐Lead ECG Component | Number of Features Selected | Comparison Between Techniques | ||
|---|---|---|---|---|
| Human Expert | Data‐Driven | Overlap in Features | Features Overlooked by Clinicians | |
| ECG normalization (k=2) | 2 | 2 | Age and sex | … |
| P duration, amplitude, or area (k=72) | 0 | 25 | … | Lead‐specific P duration and amplitude |
| PR interval metrics (k=26) | 1 | 11 | Global PR interval | Lead‐specific PR interval |
| Q duration or amplitude (k=24) | 0 | 10 | … | Lead‐specific Q wave presence |
| R duration or amplitude (k=48) | 0 | 23 | … | Lead‐specific R amplitude |
| S duration or amplitude (k=48) | 0 | 16 | … | S amplitude in precordial leads |
| Other QRS complex metrics (k=74) | 1 | 31 | Global QRS duration | QRS notch; ventricular activation time; lead‐specific QRS duration or area |
| Selvester Score (k=19) | 1 | 0 | Total scar size | … |
| ST amplitude, duration, or slope (k=72) | 12 | 31 | Lead‐specific ST amplitude | Lead‐specific ST duration and slope |
| ST deviation morphology (k=14) | 0 | 7 | … | Presence of concaved ST deviation |
| T duration, amplitude, or area (k=76) | 14 | 33 | Lead‐specific T amplitude, T‐to‐R relative amplitude | Lead‐specific T duration or area; presence of notched T wave |
| QT interval and subintervals (k=23) | 4 | 12 | Global QTc, Tpeak−Tend | Lead‐specific QT interval |
| QRS axis (k=12) | 1 | 7 | Frontal plane QRS axis | Horizontal and spatial QRS axis |
| T axis (k=11) | 4 | 6 | T axis in frontal, horizontal, and spatial planes | … |
| QRS and T vector angles (k=5) | 2 | 3 | QRS‐T angle and TCRT | … |
| T loop morphology (k=6) | 4 | 4 | T asymmetry and dispersion | … |
| Principal components analysis (k=16) | 16 | 6 | Principal component analysis ratio of J, T, and ST‐T | … |
| Noise signal (k=8) | 3 | 2 | Noise and baseline wander | … |
Performance of Hybrid Subset of Novel Features
The final hybrid subset included 73 features that had both data‐ and physiology‐driven basis. Figure 4A compares the area under the ROC curves of the 3 LR classifiers based on data‐driven basis alone, domain‐expertise alone, and hybrid data‐ and physiology‐driven basis. As seen in this panel, the hybrid features model generalized well to the testing set, outperforming the other 2 models. Similar trends were seen with ANN algorithms, but without any additional gain compared with LR algorithms (LR73 0.79 versus ANN73 0.76). Thus, we compared the diagnostic accuracy of the final LR73 against the reference standards (Table 3). As seen in this table, our LR classifier had higher sensitivity compared with expert clinicians and the commercial software while maintaining higher negative predictive value (ie, superior rule‐out performance). Although the LR classifier had lower specificity than other reference standards, it achieved positive overall net reclassification improvement (0.10 [−0.02–0.23] and 0.21 [0.10–0.32], respectively).
Figure 4. Classification performance using different subsets of novel ECG features.
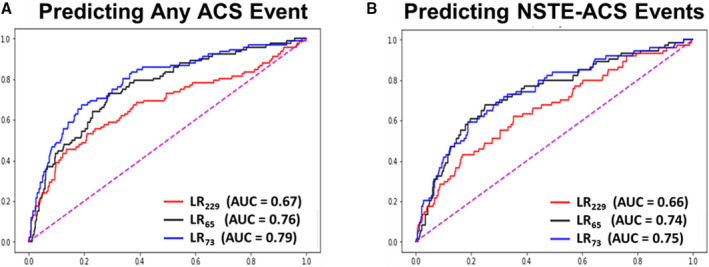
These plots show the performance of logistic regression (LR) classifiers on testing data (Cohort 2) for predicting (A) acute coronary syndrome (ACS) and (B) non–ST‐segment elevation‐acute coronary syndrome (NSTE‐ACS) using data‐driven subset of ECG features (k=229), physiology‐driven subset of ECG features (k=65), or hybrid subset with novel features (k=73). AUC indicates area under the curve.
Table 3.
Diagnostic Accuracy Measures of Machine Learning Classifiers Against Gold Standard Reference on the Testing Set (n=499)
| Clinical Experts Interpretation | Commercial Software Read | Novel ECG Features (LR73) | |
|---|---|---|---|
| Predicting Any ACS Event | |||
| Sensitivity | 0.40 (0.30–0.51) | 0.25 (0.17–0.35) | 0.72 (0.61–0.81) |
| Specificity | 0.94 (0.92–0.96) | 0.98 (0.96–0.99) | 0.73 (0.68–0.77) |
| Positive predictive value | 0.63 (0.51–0.73) | 0.79 (0.62–0.90) | 0.38 (0.33–0.42) |
| Negative predictive value | 0.88 (0.86–0.89) | 0.85 (0.83–0.87) | 0.92 (0.89–0.94) |
| NRI index | Reference | … | 0.10 (−0.02–0.23) |
| … | Reference | 0.21 (0.10–0.32) | |
| Predicting Non−ST‐segment elevation‐ACS event | |||
| Sensitivity | 0.26 (0.16–0.37) | 0.12 (0.06–0.22) | 0.72 (0.60–0.82) |
| Specificity | 0.94 (0.92–0.97) | 0.98 (0.96–0.99) | 0.68 (0.63–0.72) |
| Positive predictive value | 0.46 (0.33–0.60) | 0.60 (0.35–0.80) | 0.29 (0.25–0.33) |
| Negative predictive value | 0.87 (0.85–0.89) | 0.85 (0.84–0.87) | 0.93 (0.90–0.95) |
| NRI index | Reference | … | 0.19 (0.04–0.33) |
| … | Reference | 0.29 (0.15–0.42) | |
ACS indicates acute coronary syndrome; NRI, net reclassification improvement index; and LR73, logistic regression model based on the 73 hybrid features.
Finally, in our sensitivity analyses, we reevaluated the diagnostic performance of our final machine learning classifier in detecting patients with NSTE‐ACS. Figure 4B and Table 3 show the area under the ROC of LR73 and its corresponding diagnostic accuracy values as compared with the reference standards. Similar to previous results, our classifier had higher sensitivity compared with expert clinicians and the commercial software while maintaining higher negative predictive value (ie, superior rule‐out performance), achieving positive overall net reclassification improvement for NSTE‐ACS detection (0.19 [0.04–0.33] and 0.29 [0.15–0.42], respectively). Figure 5 displays the importance ranking of the novel ECG features for the task of NSTE‐ACS detection. Intriguingly, classical ST and T wave amplitudes had the least predictive importance, with metrics of nondipolar electrical dispersion, ventricular activation time, QRS and T axes and angles, and PCA ratio of ECG waveforms playing a more important role.
Figure 5. Importance rank of subset of novel ECG features for the task of NSTE‐ACS detection.
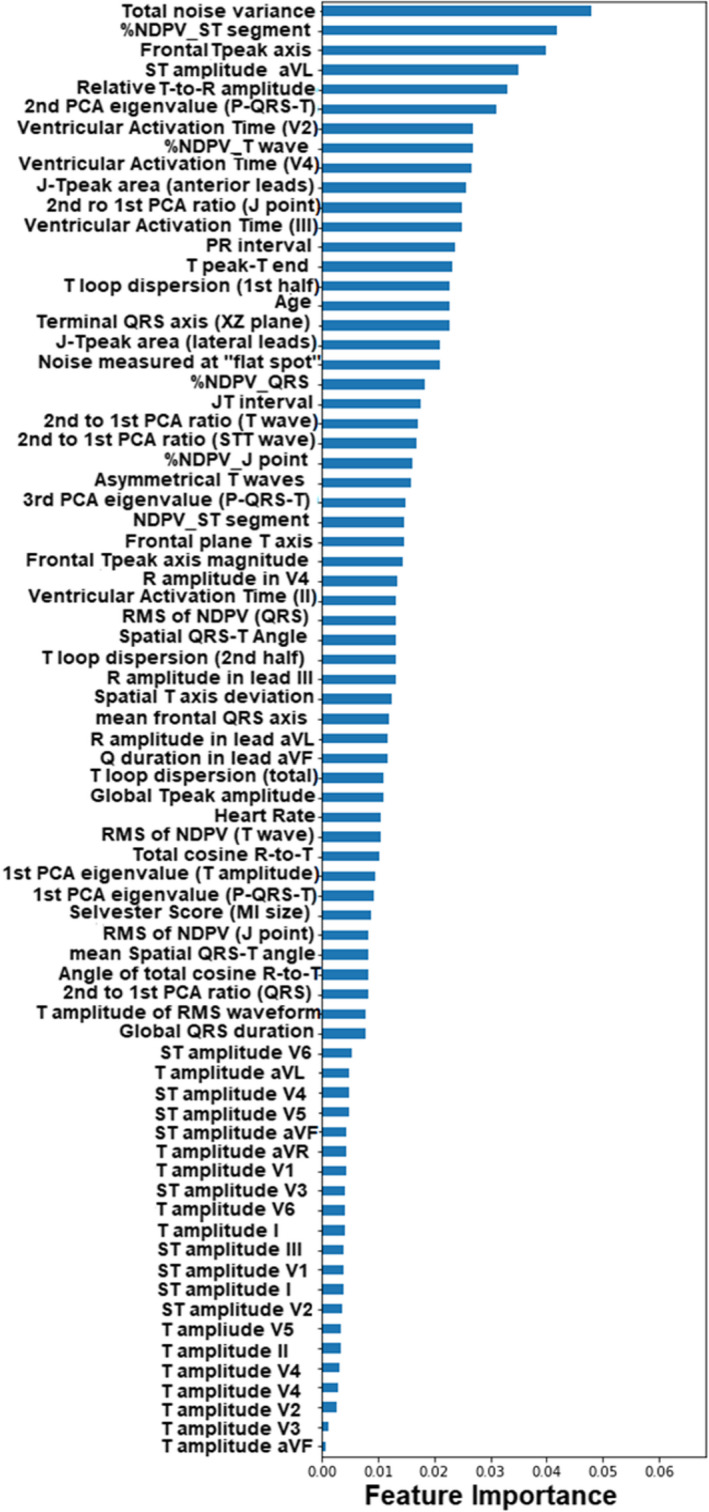
This plot shows the feature importance ranking obtained using a random forest model on a hybrid data set including novel ECG features with prehospital ECG data after excluding patients with STEMI. AUC indicates area under the curve; NDPV, nondipolar voltage; NSTE‐ACS, non–ST‐segment elevation‐acute coronary syndrome; PCA, principal component analysis; RMS, root mean square; and STEMI, ST‐segment–elevation myocardial infarction.
DISCUSSION
This study evaluated the effect of 2 FSS techniques on the accuracy of machine learning classifiers in augmenting the ECG detection of ACS. Using 2 prospective clinical cohorts, our data show that machine learning classifiers have better bias‐variance tradeoff when built based on features manually selected by human experts as compared with no FSS or using data‐driven techniques alone. On independent testing, our data show that using a hybrid subset of 73 novel ECG features based on data‐ and physiology‐driven approaches yields not only more powerful and interpretable model but also outperforms clinical experts and commercial rule‐based software in detecting any ACS event, as well as NSTE‐ACS events. More interestingly, feature importance ranking demonstrates the presence of novel and plausible markers of ischemia that are highly adaptable to clinical decision support applications.
Effect of FSS Approach on Classifiers Performance
Our data show that, compared with no FSS, physiology‐driven features optimized our LR classifier and yielded a generalizable model. This finding is expected given that using domain‐specific knowledge not only tremendously reduced the dimensionality (65 out of 556 features) but also intuitively reduced the redundancy in the data, both of which are compatible with linear classifiers. On the other hand, our data show that the initial gain observed by using data‐selected features generalized poorly to an independent unseen cohort. Our training set results are similar to the ones reported by Green et al. 20 In their work, they built the model based on 16 ECG features chosen using the PCA approach. Their cohort consisted of a comparable sample size (634 patients) and ACS prevalence (130 patients with ACS, ie, ≈20.5%). 20 However, Green et al did not have an independent testing set for validation. In our data, we showed that data‐driven FSS lacked generalizability on a new test example, indicating overfitting of training data coupled with a substantial variability of classifier performance. Although this finding was surprising, the small data set size as well as the inclusion of patients with confounders in our data sets could provide a simple rationale for this unexpected finding. Besides, some strict requirements about data nature, such as the homogeneity of variances for the Cohen's d effect size algorithm, were not satisfied, which may jeopardize the predictive performance, including its generalizability.
We observed similar trends in results when we applied ANN as a nonlinear classifier. These findings are a little bit counterintuitive given that ANN is expected to better capture the underlying characteristics of the data set when fed with more features. This divergence can be attribute to the small sample size, especially for training data, which is incompatible with learning a complex model without increasing the risk of overfitting. 23 This was observed as a significant reduction in ANN classifiers performance using all available features (k=554) or the data‐selected ones (k=229). Again, we speculate the reduced dimensionality and data redundancy when using physiology driven features reduced the complexity of the ANN classifiers, yielding a more generalizable model.
Finally, it is worth noting that using ANN classifiers consistently yielded higher classification accuracy when compared with LR classifiers, with or without any FSS (Figure 3). However, this gain in accuracy was negligible when using the physiology‐driven features (ANN65=0.77 versus LR65=0.76 [for test set]). Given that LR classifiers are easily interpretable, our results suggest that using an LR65 classifier with physiology‐driven features can yield a fully understandable decision support tool for clinical use.
Overlap Between Data‐ and Physiology‐Driven Features
The secondary aim of this study was to explore whether data‐driven FSS techniques might identify ECG features indicative of ACS that were overlooked by domain‐specific human experts. Table 2 mapped the 229 data‐driven features against the major components of the 12‐lead ECG signal, identifying the overlap between the data‐driven features and the ones selected by domain‐specific expertise. More interestingly, this table summarizes the cluster of data‐driven features that were overlooked by human‐experts. Some of these overlooked data‐driven features are contextually understandable, like ST slope, ST deviation morphology, and T wave attributes, but some other features were more challenging to classify. Upon careful annotation, we classified the overlooked data‐driven features in 1 of these 3 broad categories: (1) noise attributed to existing comorbidities or patient medications (ie, lead‐specific P duration, P amplitude, and PR interval); (2) redundant information quantified by simultaneous ECG features (ie, lead‐specific Q, R, and S wave attributes that are redundant with scar size and lead‐specific QRS duration and QT interval that are redundant with principal component analysis); and (3) features that could be mechanistically linked to myocardial ischemia and can serve as plausible features of ACS (ie, presence of fragmented QRS and lead‐specific ventricular activation time).
Novel ECG Features of Ischemia
The novel features identified in this study as plausible markers of ACS that are potentially mechanistically linked to myocardial ischemia bring a valuable addition to clinical knowledge. Intriguingly, although the classical ST and T wave amplitude measures were among the predictive features, they ranked as the least important when compared with the contribution of other novel features (Figure 5). Some of the observed patterns and clusters of the most important features can be summarized in the following major categories:
Features of the nondipolar voltage, which quantifies the spatial electrical dispersion in the fourth to eighth eigenvalues. In the context of ST, T, and J components, the nondipolar voltage would indicate the magnitude of diffusion or widespread global changes, 24 a probable measure of circumferential ischemia in ACS.
Ventricular activation time, which quantifies the time from Q onset to R peak. Whereas depolarization of the whole ventricular myocytes is assessed through global QRS duration, localized regional depolarization can be assessed using individual leads facing that myocardial region. Thus, ventricular activation time measured from anterior and inferior leads would primarily indicate transmural conduction delays in the left ventricle and apex, 25 a probable consequence of localized ischemia in these regions.
QRS and T axes and corresponding angles, which characterize the propagation direction of depolarization and repolarization signals and, hence, global electrical dispersion. In the context of ACS, these features can reflect the altered electromechanical forces in the ventricular myocardium and probably the resulting global remodeling after myocardial injury. 26
Waveform eigenvalues and corresponding ratios, which quantifies the principal components of ECG signal in perpendicular space. The altered signal propagation speed and velocity between healthy and ischemic myocardium leads to spatial heterogeneity and significantly impacts these features. 9 Thus, in the context of ACS, these eigenvalues would resemble regional myocardial ischemia (or injury vectors). 8
Other T wave metrics that quantify duration (eg, Tpeak Tend), amplitude (eg, relative R‐to‐T), area (eg, JTpeak area), morphology (eg, T asymmetry), and loop characteristics (eg, loop dispersion). Some studies have demonstrated that such simple T wave metrics may better predict early ischemia as compared with ST segment, 27 a finding that is supported by our current results.
Residual high frequency noise in the signal. Although this might be a simple incidental finding reflective of acuity of illness at the time of ECG acquisition, we previously demonstrated that such noise highly correlates with beat‐to‐beat repolarization lability. 16 This lability can resemble the alternans of intracellular Ca+2 transient in adjacent cells during acute myocardial ischemia.
Clinical Implications
Unlike the majority of previous studies that primarily used the limited, open‐source MIT‐PTB diagnostic ECG database (https://physionet.org/content/ptbdb/1.0.0/), our results are based on 2 large clinical cohorts with real‐world ECG data. Thus, our study has some immediate clinical implications. Our machine learning algorithms are fully interpretable and can be easily incorporated into existing ECG software or embedded into ECG interpretation platforms for decision support. These algorithms can help clinicians in identifying NSTE‐ACS events in real time, which constitutes a longstanding challenge in clinical practice. Given that our algorithm has higher sensitivity and negative predictive value compared with experienced clinicians, our models are well suited as an initial screening tool (ie, rule out). This has the potential to better allocate hospital resources by avoiding prolonged observations, unnecessary admissions, or invasive testing. With an average net reclassification improvement of 20%, our approach can positively affect the initial triage of 1.4 out of the 7 million Americans evaluated at the emergency department for chest pain every year. This is inclusive of the challenging group of patients whom baseline ECGs are typically deemed un‐interpretable for ischemia (eg, pacing, bundle branch block, left ventricular hypertrophy, etc). Finally, given that our machine learning models are less dependent on classical ST and T wave amplitude measures, they can be used to augment (rather than replace) commercial rule‐based ECG software that follow published recommendations by American Heart Association/American College of Cardiology guidelines. This implies that future translational research should focus on embedding these intelligent analytics in existing ECG carts and clinical workflow, with prospective evaluation on clinical decision making and patient outcomes.
Study Limitations
Strengths of our current study include the quality of our prehospital ECG data set, using 2 independent training and validation sets, the selection of features mechanistically linked to ischemia, the emphasis on the interpretability and clinical relevance, and the comparison against a reference standard. Yet, our study had some limitations. Even though the data were collected from multiple healthcare centers, both training and testing sets were still restricted to 1 region. Thus, the study may be biased by disparities inherent to the distributions of sex, race, and other factors in the community. Our algorithms need to be tested on a more diverse population including data from more geographically distant healthcare centers. Besides, the patient to feature ratio, which reaches almost 1:1 value for one of the classifiers, is low. This fact, aggregated with the unbalanced data set presenting only 15.3% prevalence of outcome, would considerably influence the performance of the classifiers, especially ANN. Future research needs to include more patients in the study while ensuring the collection of similar proportions of diseased and healthy patients with respect to the primary outcome of the study.
CONCLUSIONS
In this prospective analysis, we explored the value of different algorithms to identify an optimal subset of ECG features that can augment the diagnosis of ACS at the emergency department. In this context, we arrived at the conclusion that LR classifiers guided with domain‐specific expertise yield the most reliable classification performance and are consequently more adapted to developing clinically relevant decision support tools. However, data‐driven classifiers identified a subset of novel ECG features that would improve ACS detection by providing important insights for developing cardiac electrical biomarkers that are mechanistically linked to ischemia and can be clinically relevant.
Sources of Funding
This work was supported by National Institutes of Health grant # R01HL137761.
Disclosures
The algorithms developed in this study are associated with a US Patent no. 10820822 invented by Al‐Zaiti, Sejdić, and Callaway. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2021;10:e017871. DOI: 10.1161/JAHA.120.017871.)
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. Body R, Cook G, Burrows G, Carley S, Lewis PS. Can emergency physicians ‘rule in’and ‘rule out’acute myocardial infarction with clinical judgement? Emerg Med J. 2014;31:872–876. DOI: 10.1136/emermed-2014-203832 . [DOI] [PubMed] [Google Scholar]
- 2. Hess EP, Agarwal D, Chandra S, Murad MH, Erwin PJ, Hollander JE, Montori VM, Stiell IG. Diagnostic accuracy of the TIMI risk score in patients with chest pain in the emergency department: a meta‐analysis. CMAJ. 2010;182:1039–1044. DOI: 10.1503/cmaj.092119 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hess EP, Brison RJ, Perry JJ, Calder LA, Thiruganasambandamoorthy V, Agarwal D, Sadosty AT, Silvilotti MLA, Jaffe AS, Montori VM, et al. Development of a clinical prediction rule for 30‐day cardiac events in emergency department patients with chest pain and possible acute coronary syndrome. Ann Emerg Med. 2012;59:115–125. DOI: 10.1016/j.annemergmed.2011.07.026 . [DOI] [PubMed] [Google Scholar]
- 4. Al‐Zaiti SS, Shusterman V, Carey MG. Novel technical solutions for wireless ECG transmission & analysis in the age of the internet cloud. J Electrocardiol. 2013;46:540–545. [DOI] [PubMed] [Google Scholar]
- 5. Birnbaum Y, Wilson JM, Fiol M, de Luna AB, Eskola M, Nikus K. ECG diagnosis and classification of acute coronary syndromes. Ann Noninvasive Electrocardiol. 2014;19:4–14. DOI: 10.1111/anec.12130 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Quinn T, Johnsen S, Gale CP, Snooks H, McLean S, Woollard M, Weston C; Group MINAPS . Effects of prehospital 12‐lead ECG on processes of care and mortality in acute coronary syndrome: a linked cohort study from the Myocardial Ischaemia National Audit Project. Heart. 2014;100:944–950. DOI: 10.1136/heartjnl-2013-304599 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wagner GS, Macfarlane P, Wellens H, Josephson M, Gorgels A, Mirvis DM, Pahlm O, Surawicz B, Kligfield P, Childers R, et al. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part VI: acute ischemia/infarction a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society Endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e262–e270. DOI: 10.1161/CIRCULATIONAHA.108.191098 . [DOI] [PubMed] [Google Scholar]
- 8. Al‐Zaiti SS, Callaway CW, Kozik TM, Carey MG, Pelter MM. Clinical utility of ventricular repolarization dispersion for real‐time detection of non‐ST elevation myocardial infarction in emergency departments. J Am Heart Assoc. 2015;4:e002057. DOI: 10.1161/JAHA.115.002057 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lux RL. Non‐ST‐segment elevation myocardial infarction: a novel and robust approach for early detection of patients at risk. J Am Heart Assoc. 2015;4:e002279. DOI: 10.1161/JAHA.115.002279 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, Mickley H, Crea F, Van de Werf F. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138:e618–e651. [DOI] [PubMed] [Google Scholar]
- 11. Leisy PJ, Coeytaux RR, Wagner GS, Chung EH, McBroom AJ, Green CL, Williams JW Jr, Sanders GD. ECG‐based signal analysis technologies for evaluating patients with acute coronary syndrome: a systematic review. J Electrocardiol. 2013;46:92–97. DOI: 10.1016/j.jelectrocard.2012.11.010 . [DOI] [PubMed] [Google Scholar]
- 12. Al‐Zaiti SSBL, Bouzid Z, Faramand Z, Frisch S, Martin‐Gill C, Gregg R, Saba S, Callaway C, Sejdić E. Machine learning‐based prediction of acute coronary syndrome using only the pre‐hospital 12‐lead electrocardiogram. Nat Commun. 2020;11:3966. DOI: 10.1038/s41467-020-17804-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deo RC. Machine learning in medicine. Circulation. 2015;132:1920–1930. DOI: 10.1161/CIRCULATIONAHA.115.001593 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Al‐Zaiti SS, Martin‐Gill C, Sejdić E, Alrawashdeh M, Callaway C. Rationale, development, and implementation of the electrocardiographic methods for the prehospital identification of non‐ST Elevation Myocardial Infarction Events (EMPIRE). J Electrocardiol. 2015;48:921–926. DOI: 10.1016/j.jelectrocard.2015.08.014 . [DOI] [PubMed] [Google Scholar]
- 15. Arini PD, Baglivo FH, Martínez JP, Laguna P. Evaluation of ventricular repolarization dispersion during acute myocardial ischemia: spatial and temporal ECG indices. Med Biol Eng Comput. 2014;52:375–391. DOI: 10.1007/s11517-014-1136-z . [DOI] [PubMed] [Google Scholar]
- 16. Al‐Zaiti SS, Alrawashdeh M, Martin‐Gill C, Callaway C, Mortara D, Nemec J. Evaluation of beat‐to‐beat ventricular repolarization lability from standard 12‐lead ECG during acute myocardial ischemia. J Electrocardiol. 2017;50:717–724. DOI: 10.1016/j.jelectrocard.2017.08.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Al‐Zaiti S, Sejdić E, Nemec J, Callaway C, Soman P, Lux R. Spatial indices of repolarization correlate with non‐ST elevation myocardial ischemia in patients with chest pain. Med Biol Eng Comput. 2018;56:1–12. DOI: 10.1007/s11517-017-1659-1 . [DOI] [PubMed] [Google Scholar]
- 18. Xie J, Wang C. Using support vector machines with a novel hybrid feature selection method for diagnosis of erythemato‐squamous diseases. Expert Syst Appl. 2011;38:5809–5815. DOI: 10.1016/j.eswa.2010.10.050 . [DOI] [Google Scholar]
- 19. Forberg JL, Green M, Björk J, Ohlsson M, Edenbrandt L, Öhlin H, Ekelund U. In search of the best method to predict acute coronary syndrome using only the electrocardiogram from the emergency department. J Electrocardiol. 2009;42:58–63. DOI: 10.1016/j.jelectrocard.2008.07.010 . [DOI] [PubMed] [Google Scholar]
- 20. Green M, Björk J, Forberg J, Ekelund U, Edenbrandt L, Ohlsson M. Comparison between neural networks and multiple logistic regression to predict acute coronary syndrome in the emergency room. Artif Intell Med. 2006;38:305–318. DOI: 10.1016/j.artmed.2006.07.006 . [DOI] [PubMed] [Google Scholar]
- 21. Wu C‐C, Hsu W‐D, Islam MM, Poly TN, Yang H‐C, Nguyen P‐A, Wang Y‐C, Li Y‐C. An artificial intelligence approach to early predict non‐ST‐elevation myocardial infarction patients with chest pain. Comput Methods Programs Biomed. 2019;173:109–117. DOI: 10.1016/j.cmpb.2019.01.013 . [DOI] [PubMed] [Google Scholar]
- 22. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. DOI: 10.2307/2531595 . [DOI] [PubMed] [Google Scholar]
- 23. Myers PD, Scirica BM, Stultz CM. Machine learning improves risk stratification after acute coronary syndrome. Sci Rep. 2017;7:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abächerli R, Twerenbold R, Boeddinghaus J, Nestelberger T, Mächler P, Sassi R, Rivolta MW, Roonizi EK, Mainardi LT, Kozhuharov N, et al. Diagnostic and prognostic values of the V‐index, a novel ECG marker quantifying spatial heterogeneity of ventricular repolarization, in patients with symptoms suggestive of non‐ST‐elevation myocardial infarction. Int J Cardiol. 2017;236:23–29. DOI: 10.1016/j.ijcard.2017.01.151 . [DOI] [PubMed] [Google Scholar]
- 25. Alhamaydeh M, Gregg R, Ahmad A, Faramand Z, Saba S, Al‐Zaiti S. Identifying the most important ECG predictors of reduced ejection fraction in patients with suspected acute coronary syndrome. J Electrocardiol. 2020;61:81–85. DOI: 10.1016/j.jelectrocard.2020.06.003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Strebel I, Twerenbold R, Wussler D, Boeddinghaus J, Nestelberger T, du Fay de Lavallaz J, Abächerli R, Maechler P, Mannhart D, Kozhuharov N, et al. Incremental diagnostic and prognostic value of the QRS‐T angle, a 12‐lead ECG marker quantifying heterogeneity of depolarization and repolarization, in patients with suspected non‐ST‐elevation myocardial infarction. Int J Cardiol. 2019;277:8–15. DOI: 10.1016/j.ijcard.2018.09.040 . [DOI] [PubMed] [Google Scholar]
- 27. Lines GT, de Oliveira BL, Skavhaug O, Maleckar MM. Simple T‐wave metrics may better predict early ischemia as compared to ST segment. IEEE Trans Biomed Eng. 2016;64:1305–1309. DOI: 10.1109/TBME.2016.2600198 . [DOI] [PubMed] [Google Scholar]


