Abstract
Background
Previous reports suggest that the use of angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) may upregulate angiotensin‐converting enzyme 2 receptors and increase severe acute respiratory syndrome coronavirus 2 infectivity. We evaluated the association between ACEI or ARB use and coronavirus disease 2019 (COVID‐19) infection among patients with hypertension.
Methods and Results
We identified patients with hypertension as of March 1, 2020 (index date) from Kaiser Permanente Southern California. Patients who received ACEIs, ARBs, calcium channel blockers, beta blockers, thiazide diuretics (TD), or no therapy were identified using outpatient pharmacy data covering the index date. Outcome of interest was a positive reverse transcription polymerase chain reaction test for COVID‐19 between March 1 and May 6, 2020. Patient sociodemographic and clinical characteristics were identified within 1 year preindex date. Among 824 650 patients with hypertension, 16 898 (2.0%) were tested for COVID‐19. Of those tested, 1794 (10.6%) had a positive result. Overall, exposure to ACEIs or ARBs was not statistically significantly associated with COVID‐19 infection after propensity score adjustment (odds ratio [OR], 1.06; 95% CI, 0.90–1.25) for ACEIs versus calcium channel blockers/beta blockers/TD; OR, 1.10; 95% CI, 0.91–1.31 for ARBs versus calcium channel blockers/beta blockers/TD). The associations between ACEI use and COVID‐19 infection varied in different age groups (P‐interaction=0.03). ACEI use was associated with lower odds of COVID‐19 among those aged ≥85 years (OR, 0.30; 95% CI, 0.12–0.77). Use of no antihypertensive medication was significantly associated with increased odds of COVID‐19 infection compared with calcium channel blockers/beta blockers/TD (OR, 1.32; 95% CI, 1.11–1.56).
Conclusions
Neither ACEI nor ARB use was associated with increased likelihood of COVID‐19 infection. Decreased odds of COVID‐19 infection among adults ≥85 years using ACEIs warrants further investigation.
Keywords: angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, COVID‐19, hypertension
Subject Categories: Epidemiology, Hypertension, Quality and Outcomes
The coronavirus disease 2019 (COVID‐19) pandemic has generated concerns that use of angiotensin‐converting enzyme inhibitors (ACEIs) or angiotensin receptor blockers (ARBs) may increase risk of COVID‐19 infection or disease severity. Some animal models suggest that ACEIs or ARBs may upregulate angiotensin‐converting enzyme 2 receptors and increase severe acute respiratory syndrome coronavirus 2 infectivity. 1 However, data from human studies are largely based on the shed form of angiotensin‐converting enzyme 2 in plasma or urine and show complex results. 2 Other research suggests that ACEIs and ARBs may be protective against COVID‐19 by upregulating angiotensin‐converting enzyme 2 and mitigating the inflammatory response in the lungs of infected patients. 3
Epidemiologic studies have emerged to address this question, and these reports showed no increased risk of severity of COVID‐19 associated with ACEI or ARB exposure. 4 , 5 , 6 , 7 , 8 , 9 However, most focused on those hospitalized for COVID‐19 10 and had limited information on the susceptibility of COVID‐19. This study's purpose was to determine the risk of COVID‐19 infection among patients with hypertension taking ACEIs or ARBs compared with other frequently used antihypertensive medications (calcium channel blockers [CCB], beta‐blockers [BB], thiazide diuretics [TD]).
Methods
Anonymized data that support the findings of this study are made available from the corresponding author on reasonable request from qualified researchers with documented evidence of training for human subjects protections.
Study Cohort
The study cohort was drawn from the hypertension registry of Kaiser Permanente Southern California (KPSC), a large US integrated healthcare system. The hypertension registry consists of patients who were diagnosed with hypertension and used antihypertensive medications (Table S1). 11 Eligible individuals were identified on March 1, 2020 (index date), aged ≥18 years, and not pregnant. We required 12 months of continuous membership before the index date (baseline). The study was approved by the KPSC institutional review committee and informed consent was waived.
Antihypertensive Medication Exposure
Based on pharmacy records, a fill of antihypertensive medication covering the index date defined medication exposure allowing a 20‐day grace period. Antihypertensive medication groups were (1) any ACEIs; (2) any ARBs; (3) CCB, BB, or TD without the use of ACEIs or ARBs; (4) others (loop diuretics, potassium‐sparing diuretics, centrally acting agents, alpha‐blockers, and mineralocorticoid receptor antagonists) without the use of ACEIs or ARBs; and (5) no antihypertensive medication. Patients who had antihypertensive medication dispenses and had a gap longer than 20 days from the index date was considered as having no antihypertensive medication.
Outcomes
The primary outcome was COVID‐19 infection defined as a laboratory‐confirmed, positive reverse transcription polymerase chain reaction test for COVID‐19 between March 1 and May 6, 2020. KPSC members were tested for COVID‐19 according to guidelines from the Centers for Disease Control and Prevention. 12 Sensitivity analysis included patients with a diagnosis code for COVID‐19 but without test results at KPSC. The secondary outcome was hospitalization within 14 days after COVID‐19 infection.
Covariates
Age at index, sex, race/ethnicity, neighborhood income and education, insurance, body mass index, and smoking status were included. Outpatient blood pressure and laboratory measures closest to the index date among those before the index date were used as baseline values. We used the lowest blood pressure if there were multiple blood pressure measures during the same encounter. Baseline comorbidities and outpatient medication use were evaluated.
Statistical Analysis
Descriptive statistics compared patient and clinical characteristics by COVID‐19 testing, test results, and antihypertensive medication classes. The incidences of COVID‐19 infection and hospitalization were calculated among all patients with hypertension and among patients with hypertension who were tested.
The primary analysis was conducted among patients with hypertension and COVID‐19 test results. Propensity scores for receiving CCB/BB/TD were estimated using logistic regression considering age; sex; race/ethnicity; Elixhauser comorbidity index; body mass index; socioeconomic status; other comorbidities such as pneumonia, diabetes mellitus, heart failure, asthma, interstitial lung disease, and chronic obstructive pulmonary disease; and other medication use at baseline. Three 1:1 propensity score‐matched populations were established in comparison to patients on CCB/BB/TD: (1) ACEIs, (2) ARBs, and (3) no antihypertensive medication use. Doubly robust logistic regression models accounted for covariates with standardized mean differences >0.1 to reduce residual imbalance. Sensitivity analyses were conducted using multivariable logistic regression and inverse probability treatment weights. A priori stratified analyses were conducted by several demographic and clinical characteristics such as sex, age, race/ethnicity, presence of chronic kidney disease, heart failure, and diabetes mellitus.
The secondary analyses were conducted to determine the association between use of ACEIs or ARBs and COVID‐19 infection among all patients with hypertension. We assessed the robustness of the study findings by assessing E‐value and plausibility of magnitude of unmeasured confounders. 13 P<0.05 was considered as statistically significant with no adjustment for multiplicity.
Results
Our study included 824 650 patients with hypertension (mean age=64.4 years), of whom 16 898 (2.0%) were tested for COVID‐19 at KPSC and 1794 (10.6%) tested positive (Figure S1). A higher proportion of women were tested than men, and a higher proportion of patients who lived in lower income or education neighborhoods tested positive compared with those who lived in higher income or education neighborhoods (Table S1).
Among the 16 898 patients with hypertension and COVID‐19 testing, 28.9% were on ACEIs, 20.6% were on ARBs, 24.7% were on CCB/BB/TD, 2.2% were on other antihypertensive medications, and 23.6% were not on any antihypertensive medications (Table 1). Compared with patients on CCB/BB/TD, a higher percentage of patients on ACEIs were male and Hispanic, and a higher percentage of patients on ARBs were Asian and had diabetes mellitus.
Table 1.
Patient and Clinical Characteristics by Antihypertensive Drug Exposure Among Patients Tested for COVID‐19 (N=16 898)
| ACEI (N=4878) | ARB (N=3473) | Calcium Channel Blockers, Beta Blockers, or Thiazide diuretics (N=4177) | Other Antihypertensives* (N=377) | No Antihypertensives (N=3993) | |
|---|---|---|---|---|---|
| Row Percent=29% | 21% | 25% | 2% | 24% | |
| Age, y | 64 (13) | 66 (13) | 65 (15) | 71 (14) | 61 (16) |
| 18–39 | 159 (3%) | 76 (2%) | 195 (5%) | 8 (2%) | 335 (8%) |
| 40–64 | 2273 (47%) | 1447 (42%) | 1797 (43%) | 108 (29%) | 2032 (51%) |
| 65–85 | 2162 (44%) | 1723 (50%) | 1842 (44%) | 208 (55%) | 1322 (33%) |
| 85+ | 284 (6%) | 227 (7%) | 343 (8%) | 53 (14%) | 304 (8%) |
| Men | 2574 (53%) | 1404 (40%) | 1617 (39%) | 218 (58%) | 1777 (45%) |
| Race/ethnicity | |||||
| Asian | 528 (11%) | 699 (20%) | 566 (14%) | 27 (7%) | 495 (12%) |
| Black | 605 (12%) | 470 (14%) | 759 (18%) | 56 (15%) | 547 (14%) |
| Hispanic | 1743 (36%) | 1068 (31%) | 1138 (27%) | 107 (28%) | 1548 (39%) |
| Other † /unknown | 105 (2%) | 85 (2%) | 90 (2%) | 6 (2%) | 88 (2%) |
| White | 1897 (39%) | 1151 (33%) | 1624 (39%) | 181 (48%) | 1315 (33%) |
| Neighborhood income | |||||
| $0–49k | 976 (20%) | 635 (18%) | 753 (18%) | 75 (20%) | 922 (23%) |
| $50–79k | 1861 (38%) | 1281 (37%) | 1530 (37%) | 141 (37%) | 1536 (39%) |
| $80–99k | 984 (20%) | 772 (22%) | 856 (21%) | 77 (20%) | 749 (19%) |
| ≥$100k | 1054 (22%) | 780 (23%) | 1031 (25%) | 84 (22%) | 783 (20%) |
| Missing | 3 (0.1%) | 5 (0.1%) | 7 (0.2%) | … | 3 (0.1%) |
| Neighborhood education (% of ≥ high school graduate) | |||||
| 0%–50% | 189 (4%) | 104 (3%) | 108 (3%) | 12 (3%) | 151 (4%) |
| 51%–75% | 1299 (27%) | 865 (25%) | 989 (24%) | 95 (25%) | 1115 (28%) |
| 76%–100% | 3388 (70%) | 2499 (72%) | 3073 (74%) | 270 (72%) | 2724 (68%) |
| Missing | 2 (0.0%) | 5 (0.1%) | 7 (0.2%) | … | 3 (0.1%) |
| Insurance type | |||||
| Commercial | 2373 (49%) | 1523 (44%) | 1955 (47%) | 119 (32%) | 2219 (56%) |
| Private pay | 1581 (32%) | 1149 (33%) | 1365 (33%) | 161 (43%) | 1157 (29%) |
| Medicare | 767 (16%) | 696 (20%) | 731 (18%) | 87 (23%) | 418 (11%) |
| Medicaid | 157 (3%) | 104 (3%) | 126 (3%) | 10 (3%) | 198 (5%) |
| Other/missing | … | 1 (0.0%) | … | … | 1 (0.0%) |
| Body mass index, kg/m2 | 32 (7) | 32 (7) | 31 (7) | 31 (8) | 31 (8) |
| <25 | 783 (16%) | 555 (16%) | 888 (21%) | 79 (21%) | 852 (21%) |
| 25–29.9 | 1403 (29%) | 991 (29%) | 1272 (31%) | 124 (33%) | 1070 (27%) |
| 30–34.9 | 1242 (26%) | 925 (27%) | 924 (22%) | 84 (22%) | 827 (21%) |
| 35–39.9 | 646 (13%) | 488 (14%) | 535 (13%) | 40 (11%) | 437 (11%) |
| ≥40 | 554 (11%) | 393 (11%) | 402 (10%) | 42 (11%) | 412 (10%) |
| Missing | 250 (5%) | 121 (4%) | 156 (4%) | 8 (2%) | 395 (10%) |
| Smoking | |||||
| Current | 345 (7%) | 132 (4%) | 211 (5%) | 22 (6%) | 245 (6%) |
| Former | 1733 (36%) | 1202 (35%) | 1480 (35%) | 181 (48%) | 1277 (32%) |
| Never | 2792 (57%) | 2135 (62%) | 2475 (59%) | 174 (46%) | 2435 (61%) |
| Missing | 8 (0.2%) | 4 (0.1%) | 11 (0.3%) | … | 36 (0.9%) |
| Blood pressure, mm Hg | |||||
| Systolic BP | 128 (15) | 130 (15) | 128 (15) | 125 (16) | 129 (15) |
| Diastolic BP | 71 (12) | 71 (12) | 72 (13) | 69 (14) | 74 (13) |
| SBP/DBP <140/90 | 4006 (82%) | 2769 (80%) | 3424 (82%) | 326 (87%) | 2884 (72%) |
| SBP/DBP <130/80 | 2131 (44%) | 1371 (40%) | 1779 (43%) | 200 (53%) | 1417 (36%) |
| Laboratory tests | |||||
| Hemoglobin A1c, % | 6.8 (1.6) | 6.7 (1.4) | 6.3 (1.4) | 6.4 (1.6) | 6.6 (1.8) |
| Total cholesterol, mg/dL | 164 (46) | 164 (43) | 170 (47) | 156 (43) | 176 (47) |
| High‐density lipoprotein cholesterol, mg/dL | 48 (14) | 49 (15) | 50 (16) | 50 (16) | 49 (15) |
| Low‐density lipoprotein cholesterol, mg/dL | 91 (36) | 89 (35) | 95 (37) | 84 (34) | 102 (39) |
| Triglycerides, mg/dL | 146 (108) | 151 (157) | 139 (107) | 121 (69) | 145 (122) |
| Preexisting conditions | |||||
| Elixhauser comorbidity score | |||||
| 0 | 127 (3%) | 57 (2%) | 113 (3%) | 16 (4%) | 455 (11%) |
| 1–3 | 2079 (43%) | 1264 (36%) | 1744 (42%) | 85 (23%) | 1820 (46%) |
| 4+ | 2672 (55%) | 2152 (62%) | 2320 (56%) | 276 (73%) | 1718 (43%) |
| Diabetes mellitus | 2232 (46%) | 1710 (49%) | 1209 (29%) | 145 (39%) | 1130 (28%) |
| Heart failure | 328 (7%) | 283 (8%) | 397 (10%) | 70 (19%) | 105 (3%) |
| Coronary artery disease | 538 (11%) | 446 (13%) | 557 (13%) | 62 (16%) | 270 (7%) |
| Chronic kidney disease | 467 (10%) | 470 (14%) | 576 (14%) | 67 (18%) | 249 (6%) |
| Asthma | 619 (13%) | 621 (18%) | 721 (17%) | 76 (20%) | 511 (13%) |
| Chronic obstructive pulmonary disease | 378 (8%) | 367 (11%) | 478 (11%) | 84 (22%) | 254 (6%) |
| Arrhythmia | 842 (17%) | 658 (19%) | 1085 (26%) | 121 (32%) | 552 (14%) |
| Valvular disease | 325 (7%) | 263 (8%) | 388 (9%) | 54 (14%) | 147 (4%) |
| Pulmonary circulation disease | 154 (3%) | 148 (4%) | 213 (5%) | 41 (11%) | 106 (3%) |
| Peripheral vascular disease | 1506 (31%) | 1282 (37%) | 1565 (38%) | 205 (54%) | 1085 (27%) |
| Cancer | 101 (2%) | 82 (2%) | 101 (2%) | 8 (2%) | 80 (2%) |
| Outpatient medications before the index date | |||||
| Lipid lowering | 3417 (70%) | 2530 (73%) | 2460 (59%) | 237 (63%) | 1577 (40%) |
| Antiplatelets | 568 (12%) | 471 (14%) | 481 (12%) | 42 (11%) | 239 (6%) |
| Insulin | 892 (18%) | 643 (19%) | 452 (11%) | 57 (15%) | 386 (10%) |
| Oral hypoglycemics | 1806 (37%) | 1278 (37%) | 822 (20%) | 78 (21%) | 739 (19%) |
Mean (SD) or N (%) are reported. ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; BP, blood pressure; COVID‐19, coronavirus disease 2019; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
Other antihypertensive medications include loop diuretics, potassium‐sparing diuretics, centrally acting agents, alpha blockers, and mineralocorticoid receptor antagonists, without the use of ACEIs or ARBs.
Multi‐racial, specific races not reported, or Native American/Alaska Native.
Among all patients with hypertension, crude incidence (95% CI) of laboratory‐confirmed COVID‐19 infection per 1000 patients was similar among those on ACEIs (1.9 [1.7–2.0]), ARBs (2.2 [2.0–2.5]), and CCB/BB/TD (1.9 [1.7–2.1]) (Table 2). The crude rates (95% CI) of hospitalization per 1000 patients were also similar among those on ACEIs (274.3 [233.0–323.0]), ARBs (355.7 [299.0–423.3]), and CCB/BB/TD (315.8 [261.5–381.3]) among those tested positive for COVID‐19.
Table 2.
Incidence of COVID‐19 Infection and Hospitalization Per 1000 Patients by Antihypertensive Drug Exposure
| ACEI | ARB | Calcium Channel Blockers, Beta Blockers, or Thiazide Diuretics | Other Antihypertensives* | No Antihypertensive Medications | |
|---|---|---|---|---|---|
| Patients with hypertension, N | 279 380 | 159 248 | 184 365 | 11 457 | 190 200 |
| COVID‐19 testing per 1000 patients (95% CI) | 17.5 (17.0–18.0) | 21.8 (21.1–22.5) | 22.7 (22.0–23.4) | 32.9 (29.7–36.4) | 21.0 (20.4–21.7) |
| COVID‐19 infection (laboratory confirmed) per 1000 patients (95% CI) | 1.9 (1.7–2.0) | 2.2 (2.0–2.5) | 1.9 (1.7–2.1) | 1.9 (1.3–2.9) | 2.9 (2.6–3.1) |
| COVID‐19 infection (laboratory or clinically confirmed) per 1000 patients (95% CI) | 2.2 (2.0–2.4) | 2.5 (2.3–2.8) | 2.1 (1.9–2.3) | 2.4 (1.7–3.5) | 3.2 (3.0–3.5) |
| Patients with hypertension tested for COVID‐19, N | 4878 | 3473 | 4177 | 377 | 3993 |
| COVID‐19 infection per 1000 tested patients (95% CI) | 107.6 (98.8–117.2) | 102.8 (92.7–114.0) | 81.9 (73.6–91.0) | 58.4 (38.4–88.6) | 137.2 (126.2–149.2) |
| Patients with hypertension tested positive for COVID‐19, N | 525 | 357 | 342 | 22 | 548 |
| Hospitalization per 1000 tested positive patients (95% CI) | 274.3 (233.0–323.0) | 355.7 (299.0–423.3) | 315.8 (261.5–381.3) | 409.1 (212.9–786.2) | 295.6 (253.4–344.8) |
ACEI indicates angiotensin‐converting enzyme inhibitors; ARB, angiotensin receptor blockers; and COVID‐19, coronavirus disease 2019.
Other antihypertensive medications include loop diuretics, potassium‐sparing diuretics, centrally acting agents, alpha blockers, and mineralocorticoid receptor antagonists, without the use of ACEIs or ARBs.
Through propensity score matching (Figure S2), we balanced patient characteristics between groups (Tables S2 through S4, Figures S3 through S5). Overall, exposure to ACEIs or ARBs was not statistically significantly associated with COVID‐19 infection (odds ratio [OR] [95% CI], 1.06 [0.90–1.25] for ACEIs versus CCB/BB/TD; OR, 1.10 [0.91–1.31] for ARBs versus CCB/BB/TD) (Figure). The associations between ACEI use and COVID‐19 infection varied in different age groups (P‐interaction=0.03). The odds of COVID‐19 infection associated with ACEIs were greater among those aged 18 to 39 years (OR, 1.86 [0.73–4.79]) but became lower among those aged ≥85 years (OR, 0.30 [0.12–0.77]) when compared with CCB/BB/TD. Sensitivity analyses using multivariable logistic regression and inverse probability treatment weights showed consistent results. In all stratified analyses, ACEIs or ARBs were not associated with increased odds of COVID‐19. Secondary analyses also showed no increased odds of infection among all patients with hypertension (OR=0.92 [0.80–1.06] for ACEIs versus CCB/BB/TD; OR=1.04 [0.89–1.21] for ARBs versus CCB/BB/TD) (Table S5). Use of no antihypertensive medication was significantly associated with increased odds of infection.
Figure 1. Odds ratio (95% CI) of COVID‐19 infection associated with antihypertensive drug exposure stratified by sex, age, race/ethnicity, and comorbidities after propensity score matching.
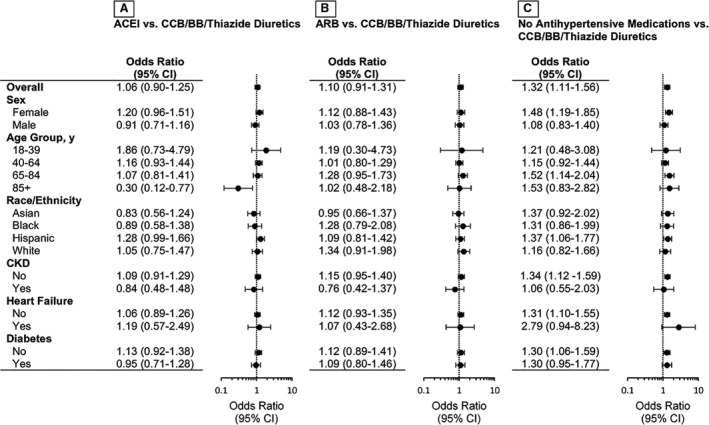
A, P‐interaction <0.05 for age‐stratified analysis. A through C, Additional variables included in the model after propensity score matching: age, sex, race/ethnicity, and covariates with standardized mean difference >0.1 (Figures S3 through S5). ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BB, beta blocker; CCB, calcium channel blocker; CKD, chronic kidney disease; and COVID‐19, coronavirus disease 2019.
Discussion
This study evaluated COVID‐19 infection among a large cohort of patients with hypertension using electronic health records with comprehensive outpatient pharmacy data. We found that ACEI or ARB use was not associated with increased risk of COVID‐19 infection compared with use of other antihypertensive medications.
Although recent epidemiologic studies reported no or lower associations between ACEI or ARB exposure and COVID‐19 infection, these studies investigated racially/ethnically homogenous populations, 4 , 5 , 6 , 9 included patients without hypertension, 7 , 14 and did not investigate patients without antihypertensive medication use separately. 5 , 7 , 8 , 14 To address these limitations, we investigated a racially/ethnically diverse cohort of patients with hypertension who had similar access to care and evaluated use of antihypertensive medications.
We found that the associations between ACEI use on COVID‐19 infection varied in different age groups. For adults ≥85 years, patients taking ACEIs, but not ARBs, had lower odds of infection compared with those with CCB/BB/TD. Although this finding is intriguing and warrants further investigation, we cannot rule out potential unmeasured confounding and potential inflation of type I errors due to multiple testing. However, this result is consistent with a previous study showing a lower risk of hospitalization associated with ACEIs but not ARBs among Medicare beneficiaries. 15 Also, the E‐value was substantially greater than accepted risk factors for COVID‐19 infection. Thus, it is unlikely that unmeasured confounders modified the conclusion of this study.
This study showed an increased likelihood of COVID‐19 infection for those without antihypertensive medications compared with those with CCB/BB/TD. These findings are reassuring that appropriate treatment with antihypertensive medications is important in patients with hypertension. However, unmeasured confounding may contribute to these findings. Before propensity score matching, a higher percentage of patients without antihypertensives had uncontrolled blood pressure, had higher cholesterol levels, and lived in a neighborhood with lower levels of income or education compared with those with CCB/BB/TD, suggesting poor health behaviors and/or adverse social determinants of health may drive these findings. Although it is likely that these patients were nonadherent to antihypertensive medications, we were not able to distinguish between patients who discontinued or did not initiate their medications and patients who were on lifestyle modifications.
This study has limitations. First, COVID‐19 testing criteria changed during the study period. From March through early April, testing was prioritized for healthcare personnel and for patients who presented signs and symptoms of COVID‐19. 12 In late April, testing was performed for all patients, symptomatic or not, who entered the hospital. Additionally, Los Angeles County expanded testing on April 29 for all residents. With testing available outside KPSC, our study did not capture all testing data. Second, <5% of patients who receive medications outside KPSC may not be captured in this study. Third, we cannot confirm medication adherence. Lastly, although we assessed hospitalization after COVID‐19, we did not have a sufficient number of patients with hypertension and who tested positive for COVID‐19 to compare hospitalization outcomes across different drug exposures or other severity levels of COVID‐19. Future studies should evaluate morbidity and mortality of COVID‐19 in this population.
Neither ACEI nor ARB use was associated with COVID‐19 infection. The decreased odds of infection among adults ≥85 years using ACEIs warrants further investigation. Our study reinforces that patients with hypertension should continue their ACEIs or ARBs as recommended by scientific communities.
Sources of Funding
This work was supported by the American Heart Association (AHA) grant #810957/An, Wei, Zhou, Harrison, Reynolds/2020. Before the AHA funding, the part of this work was supported by the Regional Research Committee of Kaiser Permanente Southern California/grant #KP‐RRC‐20200402/Wei, Creekmur, Gould/2020.
Disclosures
An reports grants from Novartis, Vital Strategies/Resolve to Save Lives, and Merck & Co. outside the submitted work. Wei and Luong report grants from Novartis and Vital Strategies/Resolve to Save Lives outside the submitted work. Zhou, Gould, Mefford, Harrison, Creekmur, Lee, Sim, Brettler, Martin, and Ong‐Su have no financial disclosures. Reynolds reports grants from Novartis, Merck & Co., Amgen, and Vital Strategies/Resolve to Save Lives outside the submitted work.
Supporting information
Tables S1–S5
Figures S1–S5
Acknowledgments
Authors acknowledge contribution of Ran Liu of her programming efforts from Kaiser Permanente Southern California. An had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2021;10:e019669. DOI: 10.1161/JAHA.120.019669.)
For Sources of Funding and Disclosures, see page 7.
Part of the findings from this study were presented at the American Heart Association Scientific Sessions, November 13‐17, 2020.
References
- 1. Ferrario CM, Jessup J, Chappell MC, Averill DB, Brosnihan KB, Tallant EA, Diz DI, Gallagher PE. Effect of angiotensin‐converting enzyme inhibition and angiotensin II receptor blockers on cardiac angiotensin‐converting enzyme 2. Circulation. 2005;111:2605–2610. DOI: 10.1161/CIRCULATIONAHA.104.510461. [DOI] [PubMed] [Google Scholar]
- 2. Kreutz R, Algharably E‐H, Azizi M, Dobrowolski P, Guzik T, Januszewicz A, Persu A, Prejbisz A, Riemer TG, Wang J‐G, et al. Hypertension, the renin‐angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID‐19. Cardiovasc Res. 2020;116:1688–1699. DOI: 10.1093/cvr/cvaa097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin‐angiotensin‐aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382:1653–1659. DOI: 10.1056/NEJMsr2005760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. de Abajo FJ, Rodríguez‐Martín S, Lerma V, Mejía‐Abril G, Aguilar M, García‐Luque A, Laredo L, Laosa O, Centeno‐Soto GA, Ángeles Gálvez M, et al. Use of renin‐angiotensin‐aldosterone system inhibitors and risk of COVID‐19 requiring admission to hospital: a case‐population study. Lancet. 2020;395:1705–1714. DOI: 10.1016/S0140-6736(20)31030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fosbøl EL, Butt JH, Østergaard L, Andersson C, Selmer C, Kragholm K, Schou M, Phelps M, Gislason GH, Gerds TA, et al. Association of angiotensin‐converting enzyme inhibitor or angiotensin receptor blocker use with COVID‐19 diagnosis and mortality. JAMA. 2020;324:168–177. DOI: 10.1001/jama.2020.11301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mancia G, Rea F, Ludergnani M, Apolone G, Corrao G. Renin‐angiotensin‐aldosterone system blockers and the risk of Covid‐19. N Engl J Med. 2020;382:2431–2440. DOI: 10.1056/NEJMoa2006923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mehta N, Kalra A, Nowacki AS, Anjewierden S, Han Z, Bhat P, Carmona‐Rubio AE, Jacob M, Procop GW, Harrington S, et al. Association of use of angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers with testing positive for coronavirus disease 2019 (COVID‐19). JAMA Cardiol. 2020;5:1020–1026. DOI: 10.1001/jamacardio.2020.1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reynolds HR, Adhikari S, Pulgarin C, Troxel AB, Iturrate E, Johnson SB, Hausvater A, Newman JD, Berger JS, Bangalore S, et al. Renin‐angiotensin‐aldosterone system inhibitors and risk of Covid‐19. N Engl J Med. 2020;382:2441–2448. DOI: 10.1056/NEJMoa2008975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vila‐Corcoles A, Satue‐Gracia E, Ochoa‐Gondar O, Torrente‐Fraga C, Gomez‐Bertomeu F, Vila‐Rovira A, Hospital‐Guardiola I, Diego‐Cabanes C, Bejarano‐Romero F, Rovira‐Veciana D, et al. Use of distinct anti‐hypertensive drugs and risk for COVID‐19 among hypertensive people: a population‐based cohort study in Southern Catalonia, Spain. J Clin Hypertens (Greenwich). 2020;22:1379–1388. DOI: 10.1111/jch.13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mackey K, King VJ, Gurley S, Kiefer M, Liederbauer E, Vela K, Sonnen P, Kansagara D. Risks and impact of angiotensin‐converting enzyme inhibitors or angiotensin‐receptor blockers on SARS‐CoV‐2 infection in adults: a living systematic review. Ann Intern Med. 2020;173:195–203. DOI: 10.7326/M20-1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sim JJ, Handler J, Jacobsen SJ, Kanter MH. Systemic implementation strategies to improve hypertension: the Kaiser Permanente Southern California experience. Can J Cardiol. 2014;30:544–552. DOI: 10.1016/j.cjca.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 12. Centers for Disease Control and Prevention . Coronavirus Disease 2019 (COVID‐19). Evaluating and testing PUI. September 16, 2020. Available at: https://www.cdc.gov/coronavirus/2019‐ncov/hcp/clinical‐criteria.html. Accessed September 16, 2020.
- 13. VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E‐value. Ann Intern Med. 2017;167:268–274. DOI: 10.7326/M16-2607. [DOI] [PubMed] [Google Scholar]
- 14. Hippisley‐Cox J, Young D, Coupland C, Channon KM, Tan PS, Harrison DA, Rowan K, Aveyard P, Pavord ID, Watkinson PJ. Risk of severe COVID‐19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106:1503–1511. DOI: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khera R, Clark C, Lu Y, Guo Y, Ren S, Truax B, Spatz ES, Murugiah K, Lin Z, Omer SB, et al. Association of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers with the risk of hospitalization and death in hypertensive patients with coronavirus disease‐19. medRxiv. 2020. May 19. DOI: 10.1101/2020.05.17.20104943. Preprint. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5
Figures S1–S5


