Abstract
Background
Previous studies have suggested a strong association of liver fibrosis scores (LFSs) with cardiovascular outcomes in patients with different cardiovascular diseases. Nonetheless, it is basically blank regarding the prognostic significance of LFSs in patients following percutaneous coronary intervention (PCI). This study sought to examine the potential role of LFSs in predicting long‐term outcomes in a large cohort of patients with stable coronary artery disease after elective PCI.
Methods and Results
In this multicenter, prospective study, we consecutively enrolled 4003 patients with stable coronary artery disease undergoing PCI. Eight currently available noninvasive LFSs were assessed for each subject. All patients were followed up for the occurrence of cardiovascular events including cardiovascular death, nonfatal myocardial infarction, and stroke. During an average follow‐up of 5.0±1.6 years, 315 (7.87%) major cardiovascular events were recorded. Subjects who developed cardiovascular events were more likely to have intermediate or high LFSs, including nonalcoholic fatty liver disease fibrosis score; fibrosis‐4 score; body mass index, aspartate aminotransferase/alanine aminotransferase ratio, diabetes mellitus score (BARD); and aspartate aminotransferase/alanine aminotransferase ratio. Furthermore, compared with subjects with low scores, those with intermediate plus high score levels had significantly increased risk of cardiovascular events (adjusted hazard ratios ranging 1.57–1.92). Moreover, the addition of non‐alcoholic fatty liver disease fibrosis score; fibrosis‐4 score; or body mass index, aspartate aminotransferase/alanine aminotransferase ratio, diabetes mellitus score into a model with established cardiovascular risk factors significantly improved the prediction ability.
Conclusions
High LFSs levels might be useful for predicting adverse prognosis in patients with stable coronary artery disease following PCI, suggesting the possibility of the application of LFSs in the risk stratification before elective PCI.
Keywords: coronary artery disease, liver fibrosis score, outcome, percutaneous coronary intervention, risk factor
Subject Categories: Clinical Studies, Coronary Artery Disease, Percutaneous Coronary Intervention, Risk Factors
Nonstandard Abbreviations and Acronyms
- APRI
aspartate aminotransferase to platelet ratio index
- BARD
body mass index, aspartate aminotransferase/alanine aminotransferase ratio, diabetes mellitus score
- CVE
cardiovascular event
- FIB‐4
fibrosis‐4 score
- LFS
liver fibrosis score
- NAFLD
nonalcoholic fatty liver disease
- NFS
nonalcoholic fatty liver disease fibrosis score
Clinical Perspective
What Is New?
Currently, no data are available regarding the prognostic significance of liver fibrosis scores in patients following percutaneous coronary intervention.
The present study first indicated the clinical impact of liver fibrosis scores on long‐term outcomes in a large cohort of patients with stable coronary artery disease following percutaneous coronary intervention.
What Are the Clinical Implications?
In patients with coronary artery disease following elective percutaneous coronary intervention, liver fibrosis scores might be novel tools for risk stratification and clinical strategy‐making.
Further studies regarding the role of liver fibrosis scores in diverse cardiovascular diseases may be of clinical interest.
Although the management of coronary artery disease (CAD) has developed and improved dramatically over the past 2 decades, CAD remains a leading cause of morbidity and mortality worldwide. 1 Percutaneous coronary intervention (PCI) has significantly reduced the rate of major adverse cardiovascular events (CVEs) in patients with CAD. 2 , 3 , 4 , 5 However, patients who are post‐PCI still constitute a high‐risk group for recurrent events and cardiovascular mortality. 6 , 7 , 8 Some screening markers and scoring systems have been demonstrated useful for patients who are post‐PCI, but they are usually expensive or difficult to evaluate in daily clinical practice. Hereby, the finding of novel economic and simple tools for further enhancement of risk stratification and identification of high‐risk patients to improve long‐term prognosis in patients after PCI is necessary. Identifying patients at high risk for CVEs may then serve as a guide to apply or sustain more aggressive therapies.
Nowadays, there is growing evidence that the degree of liver fibrosis represents the strongest predictor for cardiovascular disease, 9 composite CVEs, 10 cardiovascular and all‐cause mortality 11 , 12 , 13 in patients with nonalcoholic fatty liver disease (NAFLD), and even in the general population. 14 Liver biopsy is the golden standard for diagnosis of liver fibrosis. However, liver biopsy cannot be performed on all patients to screen for fibrosis. Noninvasive scoring systems calculated with routinely available clinical and laboratory parameters might be a safe and easily assessable alternative for initial evaluation of fibrosis, especially in subjects without symptoms or history of liver diseases. 15 The commonly used scoring systems are the NAFLD fibrosis score (NFS) 16 ; fibrosis‐4 (FIB‐4) index 17 ; body mass index, aspartate aminotransferase (AST)/alanine aminotransferase (ALT) ratio, diabetes mellitus score (BARD) 18 ; AST/ALT ratio 19 ; Forns score 20 ; gamma‐glutamyltransferase platelet ratio (GPR) 21 ; AST to platelet ratio index (APRI) 22 ; and HUI. 23 In addition to the satisfactory accuracy of liver fibrosis scores (LFSs) in detecting advanced fibrosis, recent studies have suggested an association between LFSs and adverse outcomes in various populations, not only in patients with NAFLD 24 , 25 , 26 but also in the general population. 15 , 27 , 28 , 29 However, no data are available regarding the association between LFSs and cardiovascular outcomes in patients with CAD following PCI.
Given the previous evidence, we hypothesized that LFSs might also be useful markers for worse outcomes in patients undergoing elective PCI. To test this hypothesis, we conducted the present study to comprehensively evaluate the clinical impact of LFSs on long‐term outcomes in a large cohort of patients with stable CAD following PCI and to determine whether all LFSs have similar predictive ability for CVEs in this population.
Methods
We will make the data, methods used in the analysis, and materials used to conduct the research available to any researcher for purposes of reproducing the results or replicating the procedure.
Study Design and Population
This study complied with the Declaration of Helsinki and was approved by the hospital's ethical review board (FuWai Hospital & National Center for Cardiovascular Diseases). Each patient provided written, informed consent before enrollment.
From March 2011 to March 2016, a total of 6979 subjects scheduled for coronary angiography because of angina‐like chest pain and/or significant stenosis indicated by coronary computed tomography angiography and/or positive treadmill exercise test were recruited consecutively from 3 medical centers (FuWai hospital, XuanWu hospital, and AnZhen hospital) according to the same protocol. On admission, 32 patients refused to join in. Subsequently, based on typical electrocardiogram changes, elevated myocardial enzyme levels, positive findings by coronary angiography and received treatment during hospitalization, 2687 patients with acute coronary syndrome, without PCI indication, with failed PCI, or undergoing coronary artery bypass grafting were rejected. Furthermore, 238 patients were excluded according to the exclusion criteria, including missing detailed data, hepatitis B/C virus infection, autoimmune hepatitis, hereditary liver disease, excessive alcohol consumption (>21 drinks/week in men and >14 drinks/week in women), secondary causes of fatty liver, drug‐induced liver disease, active infections, severe liver and/or renal insufficiency, or malignant disease. There were 19 patients lost to follow‐up during the study. Finally, 4003 patients with stable CAD undergoing PCI were enrolled (Figure 1). They were prescribed dual antiplatelet therapy before PCI and at least 12 months after PCI unless contraindicated and continued to take aspirin without ischemic or bleeding events.
Figure 1. Flowchart Illustrating the Study Population.
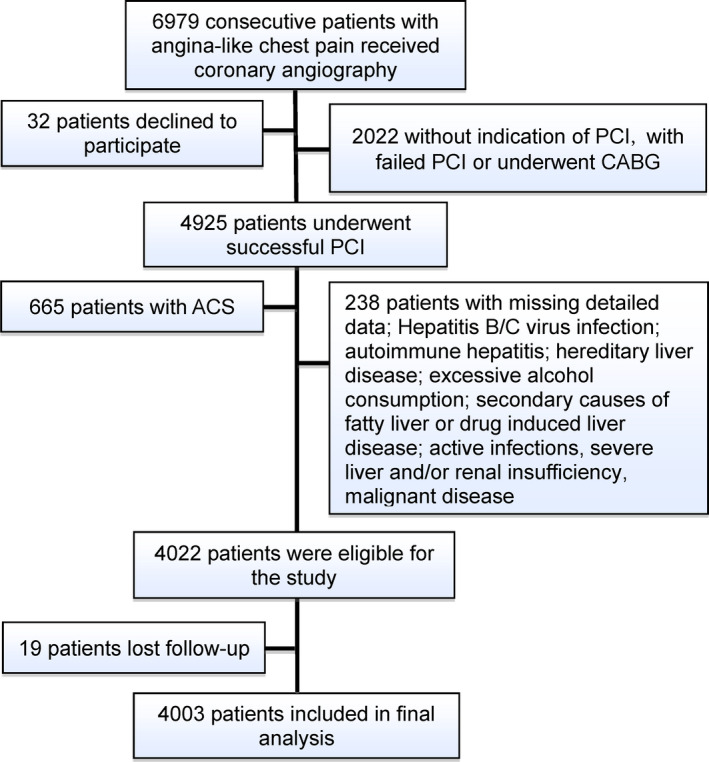
ACS indicates acute coronary syndrome; CABG, coronary artery bypass grafting; and PCI, percutaneous coronary intervention.
Clinical and Laboratory Measurements
Baseline demographic data, lifestyle characteristics, and medical history were acquired by trained cardiologists. The traditional risk factors were defined according to our previous studies. 30 , 31 Hypertension was diagnosed through a self‐reported hypertension, currently taking antihypertensive drugs, or consecutively measured systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg for 3 or more times. Diabetes mellitus was defined by fasting plasma glucose ≥7.0 mmol/L or the 2‐hour plasma glucose of the oral glucose tolerance test ≥11.1 mmol/L or current use of hypoglycemic drugs or insulin. Current smoking/drinking was defined as regular smoking/drinking within the previous 12 months. Baseline medication use indicated continuous drug use for at least 3 months before enrollment, and medications at follow‐up referred to taking drugs continuously for at least 3 months before the end of follow‐up.
Venous blood samples were collected after at least 12‐hours fasting in the morning. Plasma concentrations of ALT, AST, gamma‐glutamyltransferase, albumin, total bilirubin, lipid profiles (total cholesterol, triglyceride, low‐density lipoprotein cholesterol, and high‐density lipoprotein cholesterol), creatinine, platelet, fasting plasma glucose, glycosylated hemoglobin, and hs‐CRP (high‐sensitivity C‐reactive protein) were measured with the same standard laboratory methods in each hospital, as stated in our previous studies. 30 , 31
Liver Fibrosis Scores
Eight kinds of LFSs, including NFS, FIB‐4, BARD, AST/ALT ratio, Forns score, GPR, APRI, and HUI, were calculated as previously described (Table S1). 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 The originally described cut‐points for each score were used to categorize liver fibrosis probability as low, intermediate, and high (Table S1). 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23
Follow‐Up
All patients were actively followed up at 6‐month intervals through direct interviews or telephone calls until February 2019 by well‐trained cardiologists or nurses, who were blinded to the study aims. The primary end point was a composite of cardiovascular death, nonfatal myocardial infarction (MI), and ischemic stroke. The secondary end point incorporated the primary end point, unplanned revascularization, and hospitalized unstable angina. All available relevant data from any reported possible event were collected. Cardiovascular death indicated a death primarily caused by acute MI, congestive heart failure, malignant arrhythmia, and other structural or functional cardiac diseases. Nonfatal MI was diagnosed as positive cardiac troponins along with typical chest pain or typical electrocardiogram serial changes. Ischemic stroke referred to persistent neurological dysfunction with documentation of acute cerebral infarction on computed tomography and/or magnetic resonance imaging. Unplanned revascularization was defined as PCI or coronary artery bypass grafting >90 days later than initial revascularization. Unstable angina included resting, initial, and progressive exertional angina. The CVEs were independently adjudicated by 3 experienced cardiologists.
Statistical Analysis
Continuous variables are presented as mean±SD or median (interquartile range) as appropriate. Categorical variables are expressed as number (percentage). The differences between groups were determined with Student's t test, analysis of variance, Mann‐Whitney U test, Kruskal‐Wallis H test, χ2 test, or Fisher's exact test where appropriate. The event‐free survival rates among groups were calculated by the Kaplan‐Meier method and compared by the log‐rank test. Cox proportional hazard models were used to calculate the hazard ratios (HRs) and 95% CIs. In the multivariate Cox model, age, sex, current smoking, alcohol consumption, diabetes mellitus, hypertension, systolic blood pressure, low‐density lipoprotein cholesterol, glycosylated hemoglobin, hs‐CRP, number of lesion vessels, and baseline statin use, except for the variables included in the score formula, were adjusted. Additionally, we performed sensitivity analysis of the association between LFSs and the CVE risk by separately adjusting for each of the other significant variables in the univariate analysis. Restricted cubic spline was created to assess linearity assumptions of the association of continuous LFSs with CVEs. To assess whether adding LFSs to established cardiovascular risk factors is associated with an improvement in prediction of future CVEs, the C‐statistic was calculated in the present study. The consistency of the association between LFSs and CVEs was examined across 7 subgroups. For all analyses, 2‐tailed P values <0.05 were considered statistically significant. The statistical analyses were performed with SPSS version 24.0 software (SPSS Inc.) and R language version 3.5.2 (Feather Spray).
Results
Baseline Characteristics
The baseline and procedural characteristics of 4003 study participants are detailed in Table 1 and Table S2. The mean age of overall patients was 56.8±10.5 years old, and 76.5% were male. Compared with subjects without events, those with primary end point had significantly higher levels of LFSs including NFS, FIB‐4, BARD, AST/ALT ratio, Forns score, and HUI and were slightly older and more likely to be female and current smokers. Meanwhile, they had higher prevalence of hypertension and prior stroke and were less likely to be drinkers and statin users at baseline. Additionally, the levels of systolic blood pressure, glycosylated hemoglobin, and hs‐CRP were higher, whereas the concentrations of albumin, ALT, gamma‐glutamyltransferase, and PLT were lower in the primary end point group compared with the nonevents group. However, there was no significant difference between the 2 groups regarding the procedural characteristics except for the relatively more multivessel lesions in subjects with primary end point.
Table 1.
Clinical Characteristics of Patients With and Without Primary End Points
| Variables | Overall (n=4003) | Events (n=315) | Nonevents (n=3688) |
|---|---|---|---|
| Age, y | 56.8±10.5 | 61.6±9.4 | 56.6±10.5 |
| Male, n (%) | 3059 (76.4) | 216 (69.5) | 2843 (77.1) |
| BMI, kg/m2 | 26.02±3.18 | 25.90±3.24 | 26.03±3.18 |
| Hypertension, n (%) | 2525 (63.1) | 242 (76.8) | 2283 (61.9) |
| Diabetes mellitus, n (%) | 1141 (28.5) | 105 (33.5) | 1036 (28.1) |
| Current smokers, n (%) | 2160 (54.0) | 195 (61.9) | 1965 (53.3) |
| Alcohol consumption, n (%) | 976 (24.4) | 51 (16.3) | 925 (25.1) |
| Prior myocardial infarction, n (%) | 1281 (32.0) | 101 (32.0) | 1180 (32.0) |
| Prior percutaneous coronary intervention, n (%) | 957 (23.9) | 90 (28.6) | 867 (23.6) |
| Prior Coronary artery bypass grafting, n (%) | 85 (2.0) | 11 (3.4) | 74 (2.0) |
| Prior stroke, n (%) | 134 (3.3) | 27 (8.6) | 107 (2.9) |
| Family history of coronary artery disease, n (%) | 568 (14.2) | 33 (10.6) | 535 (14.5) |
| Systolic blood pressure, mm Hg | 127±17 | 129±18 | 127±17 |
| Diastolic blood pressure, mm Hg | 78±11 | 78±11 | 78±11 |
| Left ventricular ejection fraction, % | 63.8±7.2 | 63.3±7.0 | 63.9±7.2 |
| Total bilirubin, umol/L | 14.72±5.80 | 14.63±5.00 | 14.72±5.84 |
| Albumin, g/dL | 4.37±0.47 | 4.19±0.39 | 4.38±0.48 |
| ALT, IU/L | 25 (18–38) | 22 (16–30) | 25 (18–38) |
| AST, IU/L | 19 (16–24) | 19 (15–25) | 19 (16–24) |
| Gamma‐glutamyltransferase, IU/L | 30 (21–46) | 28 (20–42) | 30 (21‐46) |
| Fasting plasma glucose, mmol/L | 5.90±1.77 | 5.99±1.73 | 5.90±1.77 |
| Glycosylated hemoglobin, % | 6.35±1.13 | 6.58±1.13 | 6.34±1.14 |
| Total cholesterol, mmol/L | 4.10±1.09 | 4.08±1.12 | 4.10±1.11 |
| High‐density lipoprotein cholesterol, mmol/L | 1.04±0.29 | 1.05±0.30 | 1.04±0.29 |
| Low‐density lipoprotein cholesterol, mmol/L | 2.48±0.94 | 2.43±0.88 | 2.49±0.96 |
| Triglycerides, mmol/L | 1.51 (1.14–2.13) | 1.56 (1.05–2.29) | 1.51 (1.14–2.12) |
| Creatinine, umol/L | 78.56±17.36 | 80.89±19.45 | 78.45±17.25 |
| Platelet, 109/L | 212±59 | 204±52 | 213±59 |
| High‐sensitivity C‐reactive protein, mg/L | 1.46 (0.79–2.97) | 1.81 (0.97–3.90) | 1.44 (0.78–2.96) |
| Nonalcoholic fatty liver disease fibrosis score | −1.53(−2.43–[−0.72]) | −0.91(−1.63–[−0.07]) | −1.57(−2.44–[−0.75]) |
| Fibrosis‐4 | 1.06 (0.78–1.44) | 1.28 (0.93–1.66) | 1.05 (0.77–1.43) |
| BMI, AST/ALT ratio, diabetes mellitus score | 1 (0–2) | 2 (0–2) | 1 (0–2) |
| AST/ALT ratio | 0.75 (0.57–1.00) | 0.86 (0.63–1.05) | 0.74 (0.57–0.96) |
| Forns score | 5.63 (5.09–6.14) | 5.80 (5.26–6.31) | 5.62 (5.08–6.13) |
| Gamma‐glutamyltransferase platelet ratio | 0.15 (0.10–0.23) | 0.14 (0.10–0.21) | 0.15 (0.10–0.23) |
| AST to platelet ratio index | 0.27 (0.20–0.36) | 0.86 (0.63–1.05) | 0.27 (0.20–0.36) |
| HUI | 0.14 (0.06–0.31) | 0.20 (0.10–0.38) | 0.14 (0.06–0.31) |
| Baseline medications | |||
| Aspirin, n (%) | 3442 (86.0) | 267 (84.9) | 3175 (86.1) |
| ACEI/ARB, n (%) | 852 (21.3) | 48 (15.2) | 804 (21.8) |
| β‐blockers, n (%) | 2011 (50.2) | 167 (53.0) | 1844 (50.0) |
| CCB, n (%) | 785 (19.6) | 62 (19.7) | 723 (19.6) |
| Statins, n (%) | 2718 (67.9) | 184 (58.5) | 2534 (68.7) |
| Medications at follow‐up | |||
| Aspirin, n (%) | 3986 (99.6) | 313 (99.4) | 3673 (99.6) |
| ACEI/ARB, n (%) | 1966 (49.1) | 178 (56.6) | 1788 (48.5) |
| β‐blockers, n (%) | 3244 (81.0) | 246 (78.1) | 2998 (81.3) |
| CCB, n (%) | 1489 (37.2) | 121 (38.4) | 1368 (37.1) |
| Statins, n (%) | 3760 (93.9) | 293 (93.2) | 3467 (94.0) |
Continuous values are summarized as mean±SD, median (interquartile range) and categorical variables as number (percentage).
ALT indicates alanine aminotransferase; AST, aspartate aminotransferase; ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; BMI, body mass index; and CCB, calcium channel blockers.
LFSs and CVEs
During an average of 5.0±1.6 years of follow‐up, a total of 315 primary end point events were observed (16.0 events per 1000 person‐years), including 83 cardiovascular deaths (4.2 events per 1000 person‐years), 71 nonfatal MIs (3.6 events per 1000 person‐years), and 161 ischemic strokes (8.2 events per 1000 person‐years). Meanwhile, there were 697 secondary end point events (315 had primary end point, 279 underwent unplanned revascularization, 103 suffered hospitalized unstable angina) were recorded.
As shown in Figure S1A, compared with the low score groups of NFS, FIB‐4, BARD, AST/ALT ratio, and HUI, the intermediate and high score groups had significantly higher incidence of primary end point (all P<0.05). Meanwhile, patients with intermediate or high levels of NFS and HUI, intermediate levels of FIB‐4, and high levels of AST/ALT ratio also had higher incidence of secondary end point than those with low score levels (all P<0.05; Figure S1B). The Kaplan‐Meier analysis of primary end point showed that patients with intermediate or high levels of NFS, FIB‐4, BARD, and AST/ALT scores had significantly lower event‐free survival rates compared with those with low score levels (all P<0.05; Figure 2). Additionally, as shown in Figure S2, subjects with intermediate or high levels of NFS, FIB‐4, BARD, and AST/ALT ratio were also at increased risk of secondary end point compared with those with low score levels (all P<0.05). However, there was no significant difference regarding the risk of both primary and secondary end points among the 3 groups categorized according to the Forns score, GPR, APRI, and HUI scores (all P>0.05; Figure 2 and Figure S2).
Figure 2. The Cumulative Event‐Free Survival Analysis for Primary End Point According to Baseline LFSs.
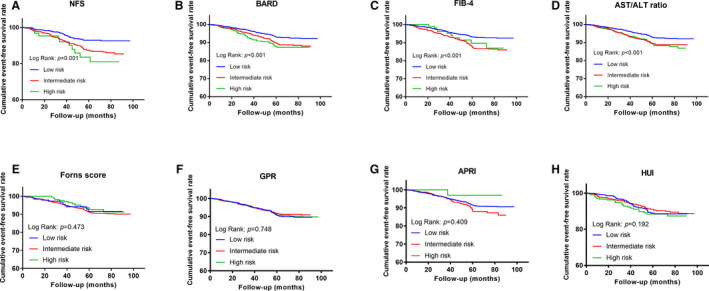
(A) NFS; (B) FIB‐4; (C) BARD; (D) AST/ALT ratio; (E) Forns score; (F) GPR; (G) APRI; (H) HUI. ALT indicates alanine aminotransferase; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; BARD, body mass index, AST/ALT ratio, diabetes mellitus score; FIB‐4, fibrosis‐4; GPR, gamma‐glutamyltransferase platelet ratio; and NFS, nonalcoholic fatty liver disease fibrosis score.
In the univariate Cox analysis, the risk of primary end point was significantly increased in patients with intermediate plus high score levels of NFS (HR, 2.27; 95% CI, 1.62–3.18), FIB‐4 (HR, 2.03; 95% CI, 1.59–2.59), BARD (HR, 1.92; 95% CI, 1.50–2.39), and AST/ALT ratio (HR, 1.91; 95% CI, 1.49–2.44), compared with those in the low score group. In addition, age, current smoking, hypertension, systolic blood pressure, glycosylated hemoglobin, hs‐CRP, and number of lesion vessels were all positively associated with the occurrence of primary CVEs, whereas male gender, alcohol consumption, and baseline statin use had a negative relationship with the incidence of primary end point (Table 2). In the multivariate Cox analysis, additional adjustment for other potential covariates did not change the association of the preceding 4 significant LFSs with primary CVEs (NFS: HR, 1.92; 95% CI, 1.28–2.87; FIB‐4: HR, 1.77; 95% CI, 1.34–2.35; BARD: HR, 1.57; 95% CI, 1.17–2.12; AST/ALT ratio: HR, 1.62, 95% CI, 1.20–2.19). Meanwhile, 1‐SD increment of NFS, FIB‐4, and AST/ALT ratio was associated with increased risk of primary end point by 59%, 7%, and 15% respectively, whereas 1‐point increase of BARD was related to a 28% increase of the risk (P<0.05, respectively). In addition, age, hypertension, hs‐CRP, number of lesion vessels, and baseline statin use were also independently related to the risk of primary end point (taking AST/ALT ratio as an example because it does not include other variables; Table S3). As shown in Figure S3, the further restricted cubic spline showed a strong trend toward nonlinear positive association of continuous NFS, FIB‐4, and AST/ALT ratio with primary outcomes. Moreover, the association between the preceding 4 LFSs and primary end point remained essentially unchanged in a sensitivity analysis, in which each of the other significant variables associated with CVEs was forced into the model with continuous LFSs (per 1‐SD or per 1‐point increment; Table S4). When the primary CVEs were analyzed separately, we observed that the association of NFS, FIB‐4, BARD, and AST/ALT ratio with cardiovascular death and nonfatal MI were obviously stronger than their relationship with ischemic stroke (Table S5). Additionally, all the preceding 4 LFSs were found to have significant associations with all‐cause death (n=157; Table S5). As to the secondary end point, we observed significant associations of intermediate plus high levels of NFS, FIB‐4, BARD, and AST/ALT ratio with the risk of events in the univariate analysis as well. However, after adjustment for potential covariates, only NFS remained significantly related to the risk of secondary end point events (for intermediate plus high NFS: HR, 1.50; 95% CI, 1.07–2.09; for per 1‐SD increment of NFS: HR, 1.34; 95% CI, 1.13–1.59; Table 3).
Table 2.
Univariate Cox Regression Analysis for the Primary End Points Among Patients Following Elective Percutaneous Coronary Intervention
| Variables | Hazard Ratio (95% CI) | P Value |
|---|---|---|
| Age | 1.05 (1.03–1.06) | <0.001 |
| Male | 0.65 (0.48–0.87) | 0.004 |
| Current smoking | 1.45 (1.09–1.93) | 0.010 |
| Alcohol consumption | 0.66 (0.45–0.96) | 0.028 |
| Diabetes mellitus | 1.28 (0.96–1.72) | 0.094 |
| Hypertension | 1.99 (1.43–2.75) | <0.001 |
| Systolic blood pressure | 1.01 (1.01–1.02) | 0.006 |
| Low‐density lipoprotein cholesterol | 0.95 (0.82–1.11) | 0.545 |
| Glycosylated hemoglobin | 1.17 (1.05–1.30) | 0.004 |
| High‐sensitivity C‐reactive protein | 1.06 (1.02–1.10) | 0.003 |
| Number of lesion vessels | 1.25 (1.04–1.50) | 0.015 |
| Baseline statin use | 0.72 (0.53–0.97) | 0.030 |
| NFS | ||
| <−1.455 | 1.00 | <0.001 |
| ≥−1.455 | 2.27 (1.62–3.18) | |
| NFS (per 1‐SD) | 1.57 (1.38–1.80)‡ | <0.001 |
| FIB‐4 | ||
| <1.3 | 1.00 | <0.001 |
| ≥1.3 | 2.03 (1.59–2.59) | |
| FIB‐4 (per 1‐SD) | 1.10 (1.05–1.15) | <0.001 |
| BARD | ||
| 0–1 | 1.00 | <0.001 |
| 2–4 | 1.92 (1.50–2.39) | |
| BARD (per 1‐point) | 1.39 (1.24–1.55) | <0.001 |
| AST/ALT ratio | ||
| <0.8 | 1.00 | <0.001 |
| ≥0.8 | 1.91 (1.49–2.44) | |
| AST/ALT ratio (per 1‐SD) | 1.18 (1.11–1.25) | 0.001 |
AST indicates aspartate aminotransferase; ALT, alanine aminotransferase; BARD, body mass index, AST/ALT ratio, diabetes mellitus score; FIB‐4, fibrosis‐4; and NFS, nonalcoholic fatty liver disease fibrosis score.
Table 3.
Adjusted Hazard Ratios of Cardiovascular Events According to Different Levels of Liver Fibrosis Scores Among Patients Following Elective Percutaneous Coronary Intervention
| Score | Adjusted Hazard Ratio (95% CI) | |
|---|---|---|
| Primary End point | Secondary End point | |
| Nonalcoholic fatty liver disease fibrosis score | ||
| <−1.455 | 1.00 | 1.00 |
| ≥−1.455 | 1.92 (1.28–2.87) † | 1.50 (1.07–2.09)* |
| Per 1‐SD | 1.59 (1.30–1.94) ‡ | 1.34 (1.13–1.59) † |
| Fibrosis‐4 | ||
| <1.3 | 1.00 | 1.00 |
| ≥1.3 | 1.77 (1.34–2.35) ‡ | 1.17 (0.94–1.47) |
| Per 1‐SD | 1.07 (1.01–1.13)* | 1.01 (0.94–1.09) |
| Body mass index, AST/ALT ratio, diabetes mellitus score | ||
| 0–1 | 1.00 | 1.00 |
| 2–4 | 1.57 (1.17–2.12) † | 1.09 (0.87–1.36) |
| Per 1‐point | 1.28 (1.12–1.47) ‡ | 1.03 (0.93–1.15) |
| AST/ALT ratio | ||
| <0.8 | 1.00 | 1.00 |
| ≥0.8 | 1.62 (1.20–2.19) † | 1.07 (0.85–1.34) |
| Per 1‐SD | 1.15 (1.04–1.27) † | 1.01 (0.90–1.13) |
The adjusted model included age, sex, current smoking, current drinking, diabetes mellitus, hypertension, systolic blood pressure, low‐density lipoprotein cholesterol, glycosylated hemoglobin, high‐sensitivity C‐reactive protein, number of lesion vessels, and baseline statin use, other than the variables included in the score formula.
ALT indicates alanine aminotransferase; and AST, aspartate aminotransferase.
P<0.05.
P<0.01.
P<0.001.
Taking FIB‐4 as an example (Figure S4), results of analyses of subgroups that were defined according to sex, age (<65 versus ≥65 years), hypertension status (yes versus no), diabetes mellitus status (yes versus no), previous MI (yes versus no), left ventricular ejection fraction(≤60% versus >60%), and the number of lesion vessels (single/double‐vessel disease versus triple‐vessel disease) reflected a relatively consistent association between this score and the risk of primary end point. There were no significant interactions between these clinical variables and FIB‐4. Finally, we assessed whether the evaluation of LFSs levels in addition to established coronary risk factors could improve risk stratification for primary CVEs in patients with stable CAD under statin treatment following PCI. The C‐statistics showed that the addition of NFS (∆C‐statistic: 0.040 [0.009–0.080], P=0.026), FIB‐4 (∆C‐statistic: 0.037 [0.004–0.067], P=0.022), or BARD (∆C‐statistic: 0.018 [0.007–0.034], P=0.008) to the original model consisting traditional risk factors showed significant improvements in the predictive ability of primary CVEs, whereas the incorporation of AST/ALT ratio into the original model brought a slight but not significant increase of C‐ statistic (∆C‐statistic: 0.013 [−0.002–0.029], P=0.094; Table S6).
Discussion
Application of a noncardiovascular scoring system to predict cardiovascular risk may present a novel strategy in cardiovascular medicine. 32 , 33 With a large cohort of patients with stable CAD treated by statins after PCI, this study first demonstrated that higher baseline LFSs, including NFS, FIB‐4, BARD, and AST/ALT ratio were significantly associated with the risk of primary end points including cardiovascular death, nonfatal MI, and ischemic stroke. Moreover, after adjusting for potential confounding variables, patients with intermediate plus high levels of the preceding 4 LFSs had a ranging from 1.57 to 1.92 fold increased risk of primary end point compared with those with low score levels, whereas 1‐SD (per 1‐point) increment of them was associated with a 7%–59% increase of CVEs risk. The positive association remained consistent in subgroup analyses according to sex, age, hypertension status, diabetes mellitus status, previous MI, left ventricular ejection fraction, and the number of lesion vessels. In addition, adding NFS, FIB‐4, and BARD to the model of established risk factors significantly improved the risk prediction of primary end point. Clinically, the present study exhibited the prognostic significance of neotactics, liver fibrosis scoring systems, in patients treated with statins with stable CAD following PCI.
The treatment of significant coronary stenosis with invasive PCI in patients with CAD has been associated with a great decrease in the rate of major adverse CVEs. 2 , 5 Meanwhile, large randomized clinical trials have already confirmed the efficacy and safety of statins for both primary and secondary prevention of cardiovascular disease. 34 However, cardiovascular risk persists despite PCI treatment and intensive lipid‐lowering therapy with statins. 6 , 7 , 8 It is reported that in the era of statin, patients receiving PCI remain at high risk of adverse outcomes over longer‐term follow‐up, with about 20% risk of death, MI, and repeat revascularization within the first year after PCI. 35 , 36 Thus, further enhancement of risk stratification and tailoring more effective risk reduction strategies are essential in this population. As is well known, on the basis of traditional cardiovascular risk factors, numerous novel biomarkers, including genetic variation, RNA, and LncRNA, have emerged in recent years. 37 Nonetheless, their predictive ability on the prognosis of patients with CAD following PCI is suboptimal because of methodological limitations, such as increased costs and complexity. 37 Hereby, the exploration of economic and simple tools for predicting long‐term outcomes in patients after PCI has become one of the focus area of cardiovascular medicine.
In spite of the prognostic importance in patients with diagnosed NAFLD, 24 , 26 noninvasive LFSs have recently been also suggested to have a predictive role of adverse outcomes in the general population and patients with cardiovascular diseases. 15 , 27 , 28 , 29 , 38 For example, Unalp‐Arida et al 27 demonstrated that NFS, FIB‐4, APRI, and Forns score were significantly associated with increased all‐cause and liver disease mortality in a US population without confirmed NAFLD. With data from the InChianti (Invecchiare in Chianti) study, an analysis of 962 older (>65 years) participants showed that NFS and FIB‐4 were related to higher all‐cause and cardiovascular mortality, whereas BARD and AST/ALT ratio were associated only with overall mortality. 38 In addition, in a study with 516 patients with chronic heart failure and 1‐year follow‐up, elevated NFS was independently associated with CVEs after adjustment for confounding factors. 28 The prognostic value of FIB‐4 index for the risk of CVEs and all‐cause mortality was also demonstrated in patients with atrial fibrillation by a multicenter Japanese registry study with about 3 years of follow‐up. 29 Recently, an analysis based on a prospective, hospital‐based GCADC (Guangdong Coronary Artery Disease Cohort) study indicated that elevated levels of NFS, FIB‐4, Forns score, GPR, and APRI were independently associated with increased risk of all‐cause and cardiovascular mortality among 3263 patients with CAD. 15 However, it is still lack of systematical investigation of the prognostic importance of LFSs in patients following elective PCI that will be of great interest and clinical relevance.
Indeed, in the present study, we observed that among the commonly used LFSs, intermediate and high levels of NFS, FIB‐4, BARD, and AST/ALT ratio were significantly associated with increased risk of cardiovascular death, nonfatal MI, and ischemic stroke. Because patients at intermediate risk for fibrosis also showed increased risk for CVEs and the high‐risk groups had small numbers of patients, we combined them in the Cox regression analysis to ensure the accuracy of the results. After adjustment for the potential covariates, the relationship between intermediate plus high levels of the preceding 4 LFSs and primary end point remained unchanged. Moreover, this association was further confirmed by subgroup and sensitivity analyses. Furthermore, we calculated C‐statistic to investigate the value of adding the significant LFSs to the original model including established cardiovascular disease risk factors and found that NFS, FIB‐4, and BARD could significantly improve risk prediction for primary end point, strongly suggesting the prognostic importance of LFSs in patients with stable CAD undergoing PCI. When the primary CVEs were considered separately, the 4 significant LFSs were more strongly tied to the risk of cardiovascular death and nonfatal MI compared with ischemic stroke. Although the exact reason for this disparity is unknown, the different pathophysiological mechanism of them may be an explanation (ischemic stroke can be induced by both atherosclerosis and atrial fibrillation). As to the secondary end point, only NFS was independently associated with the risk of events. This phenomenon suggested that LFSs might have a better prediction for hard end point. With respect to Forns score, GPR, APRI, and HUI, no significant association with CVEs was detected in this study. Future studies may be needed to further clarify their impact on the risk of CVEs in patients after elective PCI.
In line with previous studies, 13 , 38 NFS, FIB‐4 index, BARD, and AST/ALT ratio, especially the former two, performed better than the other included scoring systems in the prediction of primary CVEs. All these 4 scores made up of purely liver‐specific variables, AST and ALT, or together with other shared risk factors, suggesting the perspective that liver enzymes AST and ALT may play a significant and independent role in the pathophysiological mechanism mediating the occurrence of the studied primary outcomes.
However, several limitations must be considered in our study. First, only baseline LFSs were calculated and the related follow‐up data were not available. Some may develop increased fibrosis during the follow‐up, leading to misclassification. Nevertheless, assuming this misclassification was nondifferential among all participants, our results may be skewed toward null and underestimate the strength of the associations between LFSs and the risk of CVEs. Second, elevated LFSs were most likely caused by NAFLD in the study because we excluded subjects with excessive alcohol consumption and any other diagnosed liver diseases. However, the specific mechanisms causing elevated LFSs were uncertain because liver biopsies were not performed to evaluate liver pathology. Additionally, we cannot completely rule out the existence of unrecognized liver diseases in the study participants. Third, given the nature of observational studies, the causality of the association between baseline LFSs and primary CVEs, as well as the underlying mechanisms, could not be clearly determined in the present study and needs further deep investigation. Four, many of the scores include relatively nonspecific metrics, such as age, body mass index, platelet count, albumin, total cholesterol, and diabetes mellitus. Therefore, it is not particularly surprising to see an association of LFSs with cardiovascular outcomes.
In summary, with a real‐world large cohort of patients with stable CAD after elective PCI and long‐term follow‐up, this study fully evaluated the association between multiple LFSs and the risk of cardiovascular outcomes. Our data first indicated that LFSs including NFS, FIB‐4 index, BARD, and AST/ALT ratio might be novel tools to identify patients at high risk for primary CVEs and guide clinical strategy‐marking in patients with CAD following elective PCI.
Sources of Funding
This work was partially supported by the Capital Health Development Fund (201614035) and CAMS Major Collaborative Innovation Project (2016‐I2M‐1‐011) awarded to JJL, the Fundamental Research Funds for the Central Universities (2019‐XHQN09) and the Youth Research Fund of Peking Union Medical College (2019‐F11) awarded to HHL. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the article.
Disclosures
None.
Supporting information
Tables S1–S6
Figures S1–S4
Acknowledgments
The authors thank all the staff and participants of this study for their important contributions.
(J Am Heart Assoc. 2021;10:e018869. DOI: 10.1161/JAHA.120.018869.)
For Sources of Funding and Disclosures, see page 9.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart disease and stroke statistics‐2018 update: A report from the american heart association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Garg S, Serruys PW. Coronary stents: current status. J Am Coll Cardiol. 2010;56:S1–S42. [DOI] [PubMed] [Google Scholar]
- 3. Scandinavian Simvastatin Survival Study Group . Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian simvastatin survival study (4s). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 4. Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. [DOI] [PubMed] [Google Scholar]
- 5. Fearon WF, Nishi T, De Bruyne B, Boothroyd DB, Barbato E, Tonino P, Juni P, Pijls NHJ, Hlatky MA, FAME 2 Trial Investigators . Clinical outcomes and cost‐effectiveness of fractional flow reserve‐guided percutaneous coronary intervention in patients with stable coronary artery disease: three‐year follow‐up of the fame 2 trial (fractional flow reserve versus angiography for multivessel evaluation). Circulation. 2018;137:480–487. [DOI] [PubMed] [Google Scholar]
- 6. Kardys I, Oemrawsingh RM, Kay IP, Jones GT, McCormick SP, Daemen J, Van Geuns RJ, Boersma E, Van Domburg RT, Serruys PW. Lipoprotein(a), interleukin‐10, c‐reactive protein, and 8‐year outcome after percutaneous coronary intervention. Clin Cardiol. 2012;35:482–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mora S, Wenger NK, Demicco DA, Breazna A, Boekholdt SM, Arsenault BJ, Deedwania P, Kastelein JJ, Waters DD. Determinants of residual risk in secondary prevention patients treated with high‐ versus low‐dose statin therapy: the treating to new targets (TNT) study. Circulation. 2012;125:1979–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reith C, Armitage J. Management of residual risk after statin therapy. Atherosclerosis. 2016;245:161–170. [DOI] [PubMed] [Google Scholar]
- 9. Henson JB, Simon TG, Kaplan A, Osganian S, Masia R, Corey KE. Advanced fibrosis is associated with incident cardiovascular disease in patients with non‐alcoholic fatty liver disease. Aliment Pharmacol Ther. 2020;51:728–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Baratta F, Pastori D, Angelico F, Balla A, Paganini AM, Cocomello N, Ferro D, Violi F, Sanyal AJ, Del Ben M. Nonalcoholic fatty liver disease and fibrosis associated with increased risk of cardiovascular events in a prospective study. Clin Gastroenterol Hepatol. 2019;18:2324–2331. [DOI] [PubMed] [Google Scholar]
- 11. Adams LA, Lymp JF, St. Sauver J , Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population‐based cohort study. Gastroenterol. 2005;129:113–121. [DOI] [PubMed] [Google Scholar]
- 12. Ekstedt M, Hagstrom H, Nasr P, Fredrikson M, Stal P, Kechagias S, Hultcrantz R. Fibrosis stage is the strongest predictor for disease‐specific mortality in NAFLD after up to 33 years of follow‐up. Hepatol. 2015;61:1547–1554. [DOI] [PubMed] [Google Scholar]
- 13. Onnerhag K, Hartman H, Nilsson PM, Lindgren S. Non‐invasive fibrosis scoring systems can predict future metabolic complications and overall mortality in non‐alcoholic fatty liver disease (NAFLD). Scand J Gastroentero. 2019;54:328–334. DOI: 10.1161/CIRCIMAGING.117.007241 [DOI] [PubMed] [Google Scholar]
- 14. Ostovaneh MR, Ambale‐Venkatesh B, Fuji T, Bakhshi H, Shah R, Murthy VL, Tracy RP, Guallar E, Wu CO, Bluemke DA, et al. Association of liver fibrosis with cardiovascular diseases in the general population: the multi‐ethnic study of atherosclerosis (MESA). Circ Cardiovasc Imaging. 2018;11:e007241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chen Q, Li Q, Li D, Chen XC, Liu ZM, Hu G, Wang JF, Ling WH. Association between liver fibrosis scores and the risk of mortality among patients with coronary artery disease. Atherosclerosis. 2020;299:45–52. [DOI] [PubMed] [Google Scholar]
- 16. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, Enders F, Saksena S, Burt AD, Bida JP, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatol. 2007;45:846–854. [DOI] [PubMed] [Google Scholar]
- 17. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S. Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatol. 2006;43:1317–1325. [DOI] [PubMed] [Google Scholar]
- 18. Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander‐Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. [DOI] [PubMed] [Google Scholar]
- 19. Sheth SG, Flamm SL, Gordon FD, Chopra S. Ast/Alt ratio predicts cirrhosis in patients with chronic hepatitis c virus infection. Am J Gastroenterol. 1998;93:44–48. [DOI] [PubMed] [Google Scholar]
- 20. Forns X, Ampurdanes S, Llovet JM, Aponte J, Quinto L, Martinez‐Bauer E, Bruguera M, Sanchez‐Tapias JM, Rodes J. Identification of chronic hepatitis c patients without hepatic fibrosis by a simple predictive model. Hepatol. 2002;36:986–992. [DOI] [PubMed] [Google Scholar]
- 21. Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd JO, Goldin R, Njai H‐F, Ndow G, Taal M, et al. The gamma‐glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok ASF. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis c. Hepatol. 2003;38:518–526. [DOI] [PubMed] [Google Scholar]
- 23. Hui AY, Chan HLY, Wong VWS, Liew CT, Chim AML, Chan FKL, Sung JJY. Identification of chronic hepatitis B patients without significant liver fibrosis by a simple noninvasive predictive model. Am J Gastroenterol. 2005;100:616–623. [DOI] [PubMed] [Google Scholar]
- 24. Angulo P, Bugianesi E, Bjornsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, Haflidadottir S, Day CP, George J. Simple noninvasive systems predict long‐term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterol. 2013;145:782–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Treeprasertsuk S, Bjornsson E, Enders F, Suwanwalaikorn S, Lindor KD. NAFLD fibrosis score: a prognostic predictor for mortality and liver complications among NAFLD patients. World J Gastroentero. 2013;19:1219–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatol. 2013;57:1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Unalp‐Arida A, Ruhl CE. Liver fibrosis scores predict liver disease mortality in the United States population. Hepatol. 2017;66:84–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Takahashi T, Watanabe T, Shishido T, Watanabe K, Sugai T, Toshima T, Kinoshita D, Yokoyama M, Tamura H, Nishiyama S, et al. The impact of non‐alcoholic fatty liver disease fibrosis score on cardiac prognosis in patients with chronic heart failure. Heart Vessels. 2018;33:733–739. [DOI] [PubMed] [Google Scholar]
- 29. Saito Y, Okumura Y, Nagashima K, Fukamachi D, Yokoyama K, Matsumoto N, Tachibana E, Kuronuma K, Oiwa K, Matsumoto M, et al. Impact of the fibrosis‐4 index on risk stratification of cardiovascular events and mortality in patients with atrial fibrillation: findings from a Japanese multicenter registry. J Clin Med. 2020;9:584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu H‐H, Cao Y‐X, Li S, Guo Y‐L, Zhu C‐G, Wu N‐Q, Gao Y, Dong Q‐T, Zhao XI, Zhang Y, et al. Impacts of prediabetes mellitus alone or plus hypertension on the coronary severity and cardiovascular outcomes. Hypertension. 2018;71:1039–1046. [DOI] [PubMed] [Google Scholar]
- 31. Liu H‐H, Cao Y‐X, Sun DI, Jin J‐L, Guo Y‐L, Wu N‐Q, Zhu C‐G, Gao Y, Dong Q‐T, Zhao XI, et al. Impact of non‐alcoholic fatty liver disease on cardiovascular outcomes in patients with stable coronary artery disease: a matched case‐control study. Clin Transl Gastroenterol. 2019;10:e00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lee JK, Hung CS, Huang CC, Chen YH, Chuang PY, Yu JY, Ho YL. Use of the cha2ds2‐vasc score for risk stratification of hospital admissions among patients with cardiovascular diseases receiving a fourth‐generation synchronous telehealth program: retrospective cohort study. J Med Internet Res. 2019;21:e12790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Benjamens S, Pol RA, Glaudemans A, Wieringa I, Berger SP, Bakker SJL, Slart R. A high abdominal aortic calcification score by dual x‐ray absorptiometry is associated with cardiovascular events after kidney transplantation. Nephrol Dial Transplant. 2018;33:2253–2259. [DOI] [PubMed] [Google Scholar]
- 34. Berwanger O, Santucci EV, de Barros e Silva PGM, Jesuíno IDA, Damiani LP, Barbosa LM, Santos RHN, Laranjeira LN, Egydio FDM, Borges de Oliveira JA, et al. Effect of loading dose of atorvastatin prior to planned percutaneous coronary intervention on major adverse cardiovascular events in acute coronary syndrome: the secure‐pci randomized clinical trial. JAMA. 2018;319:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Latif F, Kleiman NS, Cohen DJ, Pencina MJ, Yen CH, Cutlip DE, Moliterno DJ, Nassif D, Lopez JJ, Saucedo JF, et al. In‐hospital and 1‐year outcomes among percutaneous coronary intervention patients with chronic kidney disease in the era of drug‐eluting stents: a report from the event (evaluation of drug eluting stents and ischemic events) registry. JACC Cardiovasc Interv. 2009;2:37–45. [DOI] [PubMed] [Google Scholar]
- 36. Stolker JM, Cohen DJ, Kennedy KF, Pencina MJ, Lindsey JB, Mauri L, Cutlip DE, Kleiman NS, Evaluation of Drug‐Eluting Stents and Ischemic Events (EVENT) Investigators . Repeat revascularization after contemporary percutaneous coronary intervention: an evaluation of staged, target lesion, and other unplanned revascularization procedures during the first year. Circ Cardiovasc Interv. 2012;5:772–782. [DOI] [PubMed] [Google Scholar]
- 37. Zheng YY, Wu TT, Chen Y, Hou XG, Yang Y, Ma X, Ma YT, Zhang JY, Xie X. Gamma‐glutamyl transferase‐to‐platelet ratio as a novel predictor of long‐term adverse outcomes in patients after undergoing percutaneous coronary intervention: a retrospective cohort study. Thromb Haemost. 2019;119:1021–1030. [DOI] [PubMed] [Google Scholar]
- 38. De Vincentis A, Costanzo L, Vespasiani‐Gentilucci U, Picardi A, Bandinelli S, Ferrucci L, Incalzi RA, Pedone C. Association between non‐invasive liver fibrosis scores and occurrence of health adverse outcomes in older people. Digest Liver Dis. 2019;51:1330–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figures S1–S4


