Abstract
Background
It is unclear whether reversion from pre–diabetes mellitus to normoglycemia reduces cardiovascular disease (CVD) and all‐cause mortality risk in a Chinese population. We aimed to fill this research gap.
Methods and Results
The current study included 14 231 Chinese participants (mean age, 58.08 years) who were free from myocardial infarction and stroke at the time of survey participation (2006–2007 and 2008–2009). Participants were divided into 3 categories according to the 2‐year changes in pre–diabetes mellitus, defined by fasting plasma glucose: those with progression to diabetes mellitus, those with reversion from pre–diabetes mellitus to normoglycemia, and those with persistent pre–diabetes mellitus. Cox proportional hazards models were used to calculate hazard ratios (HRs) and their 95% CIs for CVD and all‐cause mortality. After a median follow‐up period of 8.75 years, a total of 879 CVD events (including 180 myocardial infarction events and 713 stroke events) and 941 all‐cause mortality events were recorded. After adjustment for confounding factors, reversion from pre–diabetes mellitus to normoglycemia was associated with decreased risks of CVD (HR, 0.78; 95% CI, 0.64–0.96), myocardial infarction (HR, 0.62; 95% CI, 0.40–0.97), stroke (HR, 0.79; 95% CI, 0.63–0.98), and all‐cause mortality (HR, 0.82; 95% CI, 0.68–0.99) compared with progression to diabetes mellitus.
Conclusions
Reversion from fasting plasma glucose–defined pre–diabetes mellitus to normoglycemia was associated with a reduction in the future risk of CVD and all‐cause mortality in a Chinese population.
Registration
URL: https://www.chictr.org; Unique identifier: ChiCTRTNC‐11001489.
Keywords: all‐cause death, cardiovascular disease, diabetes mellitus, myocardial infarction, normoglycemia, pre–diabetes mellitus, stroke
Subject Categories: Cardiovascular Disease, Epidemiology, Lifestyle, Primary Prevention
Nonstandard Abbreviations and Acronyms
- DPPOS
Diabetes Prevention Program Outcomes Study
- FHS
Framingham Heart Study
- FPG
fasting plasma glucose
Clinical Perspective
What Is New?
Few studies have explored the impact of reversion from pre–diabetes mellitus to normoglycemia and cardiovascular disease (CVD) risk.
There is no evidence on whether reversion from pre–diabetes mellitus to normoglycemia is associated with CVD (distinguished as myocardial infarction and stroke) and all‐cause mortality risk in a Chinese population.
What Are the Clinical Implications?
The current findings demonstrate the importance of reversion from pre–diabetes mellitus to normoglycemia in minimizing the future risk of CVD and all‐cause mortality.
The study also highlights the need that Chinese populations with pre–diabetes mellitus may benefit from lifestyle and/or pharmacological interventions to lower their risk for CVD and all‐cause mortality.
Our finds encourage and support individuals with pre–diabetes mellitus; interventions should be implemented to prevent the development of diabetes mellitus, which is a risk factor for CVD.
Pre–diabetes mellitus is defined as an intermediate metabolic state between normoglycemia and diabetes mellitus and includes both impaired glucose tolerance and impaired fasting glucose. 1 The global prevalence of diabetes mellitus and pre–diabetes mellitus in adults has been increasing over recent decades.2, 3, 4 Recent evidence suggests that, in addition to patients with diabetes mellitus, those with pre–diabetes mellitus are also vulnerable to atherosclerotic cardiovascular diseases (CVDs).5, 6 Some,7, 8, 9 but not all, studies10, 11 showed that higher fasting blood glucose concentrations were associated with a higher CVD risk in individuals without diabetes mellitus. Even when overt diabetes mellitus is delayed or prevented, both microvascular and macrovascular diseases appear to be more prevalent in those with pre–diabetes mellitus than in their normoglycemic peers.12, 13, 14 Data from the DPPOS (Diabetes Prevention Program Outcomes Study) and Special Diabetes Program for Indians Diabetes Prevention Program both showed that patients with pre–diabetes mellitus who reverted to normoglycemia had a reduced risk of developing diabetes mellitus.15, 16 Arguably, the prevention of diabetes mellitus and its complications lies in the restoration of normoglycemia rather than in the maintenance of pre–diabetes mellitus.
Given the epidemic proportion of pre–diabetes mellitus worldwide and especially in China and the United States,17, 18 if a causal relationship exists, even a modest decrease in risk might reduce the substantial burden of CVD events. 19 In addition, reversion from pre–diabetes mellitus to normoglycemia is related to improvements in a range of cardiovascular risk factors. 20 In addition, the results from the Whitehall II cohort study showed that reversion from 2‐hour plasma glucose–defined pre–diabetes mellitus to normoglycemia was associated with a reduction in the future risk of CVD and death. 19 However, few studies have explored the impact of reversion from pre–diabetes mellitus to normoglycemia and CVD risk.16, 19, 20, 21 Notably, a previous history of CVD was not excluded in their analysis; the clinical outcomes in prior studies were comprehensive CVD outcomes; and myocardial infarction (MI) and stroke events, including stroke types, were not assessed independently. Hence, the current analysis sought to examine whether reversion from pre–diabetes mellitus to normoglycemia is also associated with a lower incidence of CVD (MI and stroke) and all‐cause mortality using data from the Kailuan study.
Methods
Study Design and Participants
The Kailuan study is a prospective cohort study that was conducted in the Kailuan community in Tangshan City, China. The data that support the findings of this study are available from the corresponding author on reasonable request. The detailed study design and characteristics of the study population have been described previously. 22 Briefly, 101 510 participants, aged 18 to 98 years, were recruited from the community from June 2006 to October 2007 (examination 1) and underwent a comprehensive biennial health examination at Kailuan General Hospital. Among the 101 510 participants from examination 1, we excluded 9489 with known diabetes mellitus at examination 1, 71 764 with normoglycemia at examination 1, 918 who were diagnosed with preexisting MI or stroke before examination 2, 5003 who did not participate in examination 2 (2008–2009), and 375 who could not be classified with normoglycemia, pre–diabetes mellitus, or diabetes mellitus on both examinations, leaving 14 231 of the participants from examination 1 for analysis. The protocol for the study was approved by the Ethics Committee of Kailuan General Hospital in compliance with the Declaration of Helsinki. All participants provided informed written consent with their signatures.
Data Collection
Demographic and clinical characteristics, including age, sex, education, income, and disease history, were collected via questionnaires. Educational attainment was categorized as illiterate or primary school, middle school, or high school or above. The average monthly income was categorized as <¥600, ¥600 to ¥800, or ≥¥800. Physical activity was classified as ≥4 times per week for ≥20 minutes at a time, <80 minutes per week, or none. Smoking status and alcohol consumption status were classified as never, former, or current, according to self‐reported information. Anthropometric parameters, such as height, weight, and waist circumference, were measured. Body mass index (BMI) was calculated as kg/m2. Systolic blood pressure and diastolic blood pressure were measured 3 times in the seated position using a mercury sphygmomanometer. All blood samples were tested using a Hitachi 747 autoanalyzer (Hitachi, Tokyo, Japan) at the central laboratory of the Kailuan General Hospital. Fasting plasma glucose (FPG), triglyceride, total cholesterol, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, hs‐CRP (high‐sensitivity C‐reactive protein), and serum creatinine levels were measured. The baseline estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation. 23
Definitions of Diabetes Mellitus, Pre–Diabetes Mellitus, Hypertension, and Dyslipidemia
Diabetes mellitus was defined as a self‐reported diabetes mellitus history, current use of insulin or oral hypoglycemic agents, or a fasting glucose concentration ≥7.0 mmol/L. 24 Pre–diabetes mellitus was defined as an absence of diabetes mellitus but an FPG level ranging from 5.6 to <7.0 mmol/L (100–126 mg/dL). Hypertension was defined as a self‐reported hypertension history, current use of antihypertensive drugs, systolic blood pressure ≥140 mm Hg, or diastolic blood pressure ≥90 mm Hg, 25 according to the JNC‐7 (The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure). Dyslipidemia was defined as any self‐reported history or use of antilipidemic agents, total cholesterol ≥6.2 mmol/L, triglyceride ≥2.3 mmol/L, low‐density lipoprotein cholesterol ≥4.1 mmol/L, or high‐density lipoprotein cholesterol <1.0 mmol/L. 26
Follow‐Up, CVDs, and All‐Cause Mortality Assessment
Participants were followed up via face‐to‐face interviews at every 2‐year routine medical examination until December 31, 2017, the event of interest, or death. The follow‐ups were performed by trained physicians who were blinded to the baseline data. The detailed MI assessment has been described previously. 27 The outcome information was further confirmed by checking discharge summaries from the 11 hospitals and medical records from medical insurance companies. For participants without face‐to‐face follow‐ups, outcome information was obtained directly by checking the death certificates from provincial vital statistics offices, discharge summaries, or medical records. The primary outcome was the first occurrence of MI or stroke. The criteria for MI were based on combinations of chest pain symptoms, ECG changes, and cardiac enzyme levels. Stroke was diagnosed according to the World Health Organization criteria, 28 combined with brain computed tomography or magnetic resonance imaging for confirmation, and was classified into 3 types: cerebral infarction, cerebral hemorrhage, and subarachnoid hemorrhage. The criteria were consistently applied across all participating hospitals. All outcomes were validated by the Data Safety Monitoring Board and the Arbitration Committee for Clinical Outcomes. Information on death was collected from the death certificates from state vital statistics offices. We used the underlying cause of death International Classification of Diseases, Tenth Revision (ICD‐10), code in the vital statistics system to group the causes of death.
Statistical Analysis
Continuous variables are presented as the mean±SD and were compared using ANOVA or the Kruskal‐Wallis test, as appropriate. Categorical variables are presented as percentages and were compared using χ2 tests. Participants were divided into 3 categories: (1) those with progression to diabetes mellitus, (2) those with reversion from pre–diabetes mellitus to normoglycemia, and (3) those with persistent pre–diabetes mellitus. Person‐years were calculated from the date the 2008 interview was conducted to the date when MI or stroke was detected, date of death, or date of participation in the last interview in this analysis, whichever came first (December 31, 2017).
Cox proportional hazards regression was used to estimate the risks for MI, stroke, and all‐cause mortality by calculating hazard ratios (HRs) and 95% CIs. We fitted 3 multivariable proportional hazards models. Model 1 was adjusted for age and sex. Model 2 was further adjusted for education level, income level, alcohol consumption, smoking status, and physical activity. Model 3 was further adjusted for hypertension, dyslipidemia, FPG, BMI, estimated glomerular filtration rate, and hs‐CRP. Statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC). All statistical tests were 2 sided, and the significance level was set at 0.05.
Results
Data from a total of 14 231 eligible participants (84.64% men) were analyzed in our study. The average age of the population was 58.08±10.69 years. As reported in Table 1, 44.92% (n=6393) of the participants regressed to normoglycemia at examination 2, 41.78% (n=5946) of the participants continued having pre–diabetes mellitus, and 13.29% (n=1892) of the participants progressed to diabetes mellitus. There was an overall significant difference among the 3 groups between age and systolic blood pressure, diastolic blood pressure, BMI, lipid level, estimated glomerular filtration rate, and hs‐CRP (P<0.001).
Table 1.
Characteristics Among Kailuan Study Participants, According to Changes in Pre–Diabetes Mellitus From 2006 to 2008
| Characteristics |
Overall Population (n=14 231) |
Progression to Diabetes Mellitus (n=1892; 13.29%) |
Persistent Pre–Diabetes Mellitus (n=5946; 41.78%) |
Normoglycemia (n=6393; 44.92%) |
P Value |
|---|---|---|---|---|---|
| Age, y | 58.08±10.69 | 61.27±9.81 | 58.54±10.41 | 56.71±10.96 | <0.001 |
| Sex, men, n (%) | 12 045 (84.64) | 1599 (84.51) | 5113 (85.99) | 5333 (83.42) | <0.001 |
| High school or above, n (%) | 2962 (20.81) | 279 (14.75) | 1200 (20.18) | 1483 (23.20) | <0.001 |
| Income >¥800/mo, n (%) | 2099 (14.75) | 264 (13.95) | 906 (15.24) | 929 (14.53) | 0.314 |
| Current smoker, n (%) | 5646 (39.67) | 686 (36.26) | 2481 (41.73) | 2479 (38.78) | <0.001 |
| Current alcohol drinker, n (%) | 6501 (45.68) | 790 (41.75) | 2900 (48.77) | 2811 (43.79) | <0.001 |
| Active physical activity, n (%) | 2265 (15.92) | 300 (15.86) | 953 (16.03) | 1012 (15.83) | 0.953 |
| Hypertension, n (%) | 6664 (46.83) | 1088 (57.51) | 2847 (47.88) | 2729 (42.69) | <0.001 |
| Dyslipidemia, n (%) | 5525 (38.83) | 895 (47.30) | 2322 (39.05) | 2308 (36.10) | <0.001 |
| Body mass index, kg/m2 | 25.59±3.41 | 26.50±3.48 | 25.74±3.40 | 25.19±3.34 | <0.001 |
| Systolic blood pressure, mm Hg | 132.44±20.60 | 138.04±20.80 | 133.13±20.70 | 130.13±20.09 | <0.001 |
| Diastolic blood pressure, mm Hg | 84.74±11.64 | 87.12±11.88 | 85.11±11.58 | 83.69±11.51 | <0.001 |
| Fasting plasma glucose, mmol/L | 6.04±0.35 | 6.27±0.39 | 6.04±0.34 | 5.96±0.32 | <0.001 |
| Total cholesterol, mmol/L | 5.09±1.13 | 5.17±1.17 | 5.17±1.07 | 5.00±1.16 | <0.001 |
| Triglycerides, mmol/L | 1.84±1.53 | 2.11±1.60 | 1.84±1.52 | 1.75±1.52 | <0.001 |
| Low‐density lipoprotein, mmol/L | 2.54±0.90 | 2.46±0.96 | 2.56±0.85 | 2.55±0.92 | <0.001 |
| High‐density lipoprotein, mmol/L | 1.53±0.39 | 1.54±0.41 | 1.52±0.37 | 1.53±0.39 | 0.629 |
| Estimated glomerular filtration rate, mL/min | 81.09±24.04 | 78.59±21.70 | 81.37±21.83 | 81.56±29.49 | <0.001 |
| hs‐CRP, mg/L | 0.81 (0.32–2.10) | 1.13 (0.41–2.80) | 0.81 (0.34–1.99) | 0.78 (0.30–2.00) | <0.001 |
Data are given as mean±SD or median (interquartile range), unless otherwise indicated. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or self‐reported use of antihypertensive medication. hs‐CRP indicates high‐sensitivity C‐reactive protein.
After a mean follow‐up period of 8.75 years, a total of 180 MI events, 713 stroke events, and 941 all‐cause mortality events were recorded, including 614 participants with ischemic stroke and 104 participants with hemorrhagic stroke (5 of those with subarachnoid hemorrhage stroke were excluded from those with total stroke). The incidence per 1000 person‐years of MI ranged from 0.78 in those with normoglycemia to 1.97 in those with progression to diabetes mellitus. The incidence per 1000 person‐years of total stroke ranged from 3.93 in those with normoglycemia to 6.47 in those with progression to diabetes mellitus. Table 2 shows the adjusted HRs of incident MI, stroke, and all‐cause mortality associated with changes in pre–diabetes mellitus from 2006 (examination 1) to 2008 (examination 2). Participants who reverted from pre–diabetes mellitus to normoglycemia had a lower MI risk (HR, 0.62; 95% CI, 0.40–0.97), stroke risk (HR, 0.79; 95% CI, 0.63–0.98), and all‐cause mortality risk (HR, 0.82; 95% CI, 0.68–0.99) than those with progression to diabetes mellitus.
Table 2.
HR for the Association Between Changes in Pre–Diabetes Mellitus From 2006 to 2008 and the Incidence of CVD and All‐Cause Mortality
| Variable | Changes in Pre–Diabetes Mellitus | ||
|---|---|---|---|
| Progression to Diabetes Mellitus | Persistent Pre–Diabetes Mellitus | Normoglycemia | |
| Myocardial infarction | |||
| No. of cases | 41 | 84 | 55 |
| Incidence rate, per 1000 person‐years | 1.97 | 1.29 | 0.78 |
| Model 1 | Reference | 0.73 (0.50–1.07) | 0.46 (0.30–0.69) |
| Model 2 | Reference | 0.74 (0.51–1.08) | 0.45 (0.30–0.69) |
| Model 3 | Reference | 0.90 (0.60–1.32) | 0.62 (0.40–0.97) |
| Stroke | |||
| No. of cases | 132 | 309 | 272 |
| Incidence rate, per 1000 person‐years | 6.47 | 4.81 | 3.93 |
| Model 1 | Reference | 0.85 (0.69–1.04) | 0.71 (0.57–0.88) |
| Model 2 | Reference | 0.85 (0.69–1.04) | 0.71 (0.58–0.88) |
| Model 3 | Reference | 0.91 (0.74–1.12) | 0.79 (0.63–0.98) |
| CVD | |||
| No. of cases | 166 | 388 | 325 |
| Incidence rate, per 1000 person‐years | 8.21 | 6.08 | 4.71 |
| Model 1 | Reference | 0.84 (0.70–1.01) | 0.67 (0.55–0.81) |
| Model 2 | Reference | 0.84 (0.70–1.01) | 0.67 (0.55–0.81) |
| Model 3 | Reference | 0.93 (0.77–1.12) | 0.78 (0.64–0.96) |
| All‐cause mortality | |||
| No. of cases | 171 | 402 | 368 |
| Incidence rate, per 1000 person‐years | 8.40 | 6.28 | 5.34 |
| Model 1 | Reference | 0.86 (0.72–1.04) | 0.78 (0.65–0.94) |
| Model 2 | Reference | 0.87 (0.72–1.04) | 0.78 (0.65–0.94) |
| Model 3 | Reference | 0.91 (0.76–1.10) | 0.82 (0.68–0.99) |
Data are given as HR (95% CI), unless otherwise indicated. Model 1, adjusted for age and sex. Model 2, adjusted for age, sex, income, education, current smoking status, current drinking status, and physical activity. Model 3, adjusted for variables in model 2 plus hypertension, dyslipidemia, fasting plasma glucose, body mass index, estimated glomerular filtration rate, and hs‐CRP (high‐sensitivity C‐reactive protein). CVD indicates cardiovascular disease; and HR, hazard ratio.
Table 3 shows the adjusted HRs of incident stroke and subtypes associated with changes in pre–diabetes mellitus from 2006 (examination 1) to 2008 (examination 2). After adjustment for covariates, participants who reverted from pre–diabetes mellitus to normoglycemia had a 28% (HR, 0.72; 95% CI, 0.56–0.91) lower risk of developing ischemic stroke than participants who progressed to diabetes mellitus. However, the association between patients who reverted from pre–diabetes mellitus to normoglycemia and those with hemorrhagic stroke was not statistically significant (HR, 0.96; 95% CI, 0.53–1.76).
Table 3.
HR for the Association Between Changes in Pre–Diabetes Mellitus From 2006 to 2008 and the Incidence of Ischemic Stroke and Hemorrhagic Stroke
| Variable | Changes in Pre–Diabetes Mellitus | ||
|---|---|---|---|
| Progression to Diabetes Mellitus | Persistent Pre–Diabetes Mellitus | Normoglycemia | |
| Ischemic stroke | |||
| No. of cases | 121 | 266 | 227 |
| Incidence rate, per 1000 person‐years | 5.91 | 4.13 | 3.26 |
| Model 1 | Reference | 0.80 (0.64–1.00) | 0.65 (0.52–0.82) |
| Model 2 | Reference | 0.80 (0.64–0.99) | 0.65 (0.52–0.82) |
| Model 3 | Reference | 0.86 (0.68–1.07) | 0.72 (0.56–0.91) |
| Hemorrhagic stroke | |||
| No. of cases | 17 | 43 | 44 |
| Incidence rate, per 1000 person‐years | 0.81 | 0.66 | 0.63 |
| Model 1 | Reference | 0.90 (0.51–1.58) | 0.82 (0.47–1.46) |
| Model 2 | Reference | 0.90 (0.51–1.59) | 0.83 (0.47–1.47) |
| Model 3 | Reference | 0.98 (0.55–1.76) | 0.96 (0.53–1.76) |
Data are given as HR (95% CI), unless otherwise indicated. Model 1, adjusted for age and sex. Model 2, adjusted for age, sex, income, education, current smoking status, current drinking status, and physical activity. Model 3, adjusted for variables in model 2 plus hypertension, dyslipidemia, fasting plasma glucose, body mass index, estimated glomerular filtration rate, and hs‐CRP (high‐sensitivity C‐reactive protein). HR indicates hazard ratio.
Figure (A through D) shows the Kaplan‐Meier cumulative risk for CVD, MI, stroke, and all‐cause mortality within groups defined by changes in pre–diabetes mellitus. Participants who reverted from pre–diabetes mellitus to normoglycemia experienced a lower risk than participants who progressed to diabetes mellitus during the 8.75‐year follow‐up period for CVD events (log‐rank test; P<0.0001; Figure [A]), MI (log‐rank test; P<0.0001; Figure [B]), stroke (log‐rank test; P<0.0001; Figure [C]), and all‐cause mortality (log‐rank test; P<0.0001; Figure [D]).
Figure 1. Kaplan‐Meier estimates of cardiovascular disease (CVD), myocardial infarction (MI), and stroke, grouped by changes in pre–diabetes mellitus.
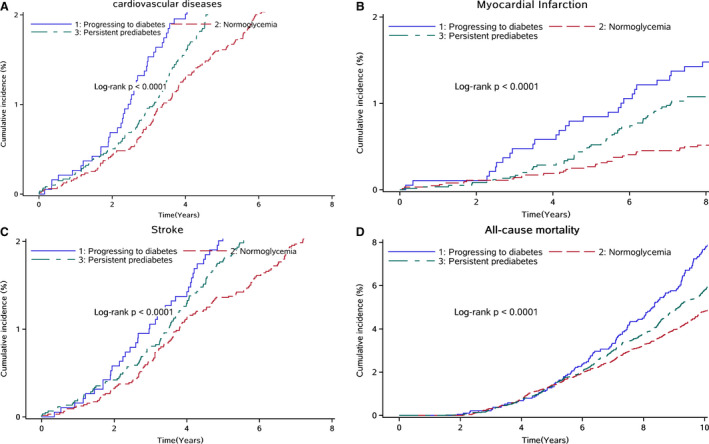
A, Kaplan‐Meier estimates of CVD, grouped by changes in pre–diabetes mellitus. Individuals who reverted from pre–diabetes mellitus to normoglycemia experienced a lower risk than participants who progressed to diabetes mellitus. B, Kaplan‐Meier estimates of MI, grouped by changes in pre–diabetes mellitus. Individuals who reverted from pre–diabetes mellitus to normoglycemia experienced a lower MI risk than participants who progressed to diabetes mellitus. C, Kaplan‐Meier estimates of stroke, grouped by changes in pre–diabetes mellitus. Individuals who reverted from pre–diabetes mellitus to normoglycemia experienced a lower stroke risk than participants who progressed to diabetes mellitus. D, Kaplan‐Meier estimates of all‐cause mortality, grouped by changes in pre–diabetes mellitus. Individuals who reverted from pre–diabetes mellitus to normoglycemia experienced a lower all‐cause mortality risk than participants who progressed to diabetes mellitus.
Discussion
In this cohort study, reversion from FPG‐defined pre–diabetes mellitus to normoglycemia was associated with an ≈22% lower risk of a CVD event and an 18% lower risk of death than progression to diabetes mellitus. The findings from the current study extend those of previous work 19 by demonstrating, for the first time, that reversion from pre–diabetes mellitus to normoglycemia is associated with a substantially lower risk for the incidence of MI and stroke than progression to diabetes mellitus. We also adjusted for BMI, blood pressure, lipid profiles, estimated glomerular filtration rate, and hs‐CRP concentrations, as well as other cardiovascular risk factors, which may have reduced the unmeasured residual confounding factors.
The results from the Whitehall II observational cohort study indicated that only reversion from 2‐hour plasma glucose–defined pre–diabetes mellitus to normoglycemia was associated with a reduction in the future risk of CVD and death. 19 No association with event rate was observed for reversion from FPG‐defined or hemoglobin A1c (HbA1c)–defined pre–diabetes mellitus to normoglycemia. Findings from the Diabetes Prevention Program also showed that individuals with 2‐hour plasma glucose–defined pre–diabetes mellitus who reverted to normoglycemia experienced a concomitant reduction in their cardiovascular risk profile. 20 Other evidence from the DPPOS also found that reversion to normoglycemia was associated with a lower prevalence of aggregate microvascular disease, nephropathy, and retinopathy, primarily caused by lower glycemic exposure over time. 16 In addition, progression from impaired glucose tolerance to diabetes mellitus is associated with mild deterioration, whereas reversion to normoglycemia is associated with improvements in risk factors. 29 Inconsistent with the above studies, in our study, reversion from FPG‐defined pre–diabetes mellitus to normoglycemia was associated with a reduction in the future risk of CVD and all‐cause mortality. However, we did not perform glucose tolerance tests or HbA1c measurements, and we may have missed subjects with impaired glucose tolerance. Differences in the age distribution, lifestyle, or socioeconomic status of the study population or the incomplete control of confounding factors may explain the different associations observed to date.
A previous study from a general Dutch population suggested that 2‐hour plasma glucose levels were more strongly associated with all‐cause and cardiovascular mortality than FPG or HbA1c levels in nondiabetic patients. 30 A recent meta‐analysis by Ford et al illustrated an ≈20% increased risk of CVD in people with pre–diabetes mellitus, irrespective of type (impaired fasting glucose versus impaired glucose tolerance) or the defining criteria. 13 In the FHS (Framingham Heart Study), impaired fasting glucose was associated with an increased CVD risk. 9 Therefore, to reduce the daunting CVD disparities borne by people with pre–diabetes mellitus, reversion from pre–diabetes mellitus to normoglycemia is an effective intervention strategy that is urgently needed to prevent CVD in this special population.
Notably, reversion from pre–diabetes mellitus to normoglycemia was associated with a more pronounced decreased risk in MI events than in stroke events in our study. Furthermore, the current study on this topic also distinguished between ischemic and hemorrhagic strokes. Interestingly, our study found that reversion from pre–diabetes mellitus to normoglycemia was only associated with a lower risk for ischemic stroke, whereas the results for hemorrhagic stroke in our study did not reach statistical significance in the multivariable‐adjusted model. The possible reason is that the sample size of the participants with hemorrhagic stroke was too small. Why reversion from pre–diabetes mellitus to normoglycemia is associated with different stroke subtypes is still unclear, and more studies are required to replicate our findings.
This study also showed that participants who developed diabetes mellitus trended toward a higher blood pressure and higher triglyceride, total cholesterol, BMI, and hs‐CRP levels than those who remained with pre–diabetes mellitus, whereas those whose pre–diabetes mellitus reverted to normal had a significantly more favorable profile. Reversion from pre–diabetes mellitus to normoglycemia was associated with reductions in blood pressure, triglycerides, and BMI. Indeed, weight loss has well‐known effects on lowering blood pressure31, 32 and triglyceride concentration, 33 which occur independently of, but in tandem with, their impact on glucose homeostasis. 34
The biological mechanisms underpinning the finding that reversion from pre–diabetes mellitus to normoglycemia decreased the CVD risk remain speculative, but several possibilities exist. First, insulin resistance in the pre–diabetes mellitus stage could promote atherogenesis and likely contribute to the elevated risk of CVD. 35 Second, impaired fasting glucose is associated with some preclinical pathological changes in CVD, such as arterial stiffness, arterial endothelial dysfunction, and intima‐media thickening. 36 Third, weight loss is associated with reversion to normoglycemia. 37 Furthermore, individuals with pre–diabetes mellitus usually have some coexisting CVD risk factors (eg, dyslipidemia, hypertension, obesity, physical inactivity, and inflammation). 38
Strengths and Limitations
The strengths of the current study include its prospective design, the large general population sample, the repeated assessment of FPG, and the validated ascertainment of CVD events and all‐cause mortality. However, there are some inherent limitations. First, the diagnoses of pre–diabetes mellitus and diabetes mellitus were based on a single measure of FPG at baseline without using an oral glucose tolerance test or HbA1c, which was caused by a lack of availability of oral glucose tolerance test and HbA1c data in such a large cohort. Second, reversion from pre–diabetes mellitus to normoglycemia may be affected by changes in blood pressure, weight, and other time‐varying lifestyle factors, such as diet, physical activity, and stress, during follow‐up. Unfortunately, the changes in BMI, blood pressure, lipid profiles, and hs‐CRP concentrations based on 2 assessments of these biomarkers were not adjusted in our analysis, which may have led to unmeasured residual confounding. Third, physical exercise and smoking status were self‐reported and thus subject to measurement error. This may partially explain the lack of association between changes in those behaviors and future CVD risk in multivariable Cox regression models. As a nonrandomized, observational cohort study, there is always a possibility of unmeasured confounding. Finally, all participants were employees of the Kailuan Coal Company, and most were men; the sex distribution of participants was unbalanced, and therefore, they cannot be viewed as a representative sample of the general Chinese population. But they have a complicated constitution from all levels of the society whose occupation may be coalminers, administrators, secretaries, accountants, as well as the supportive and service staff, such as policemen, physicians, nurses, vendors, and teachers. And studying such a geographically focused and controlled population greatly reduces residual confounding because of diverse socioeconomic factors and lifestyle patterns.
In conclusion, the current findings demonstrate the importance of reversion from pre–diabetes mellitus to normoglycemia in minimizing the future risk of CVD and all‐cause mortality. These findings suggest that Chinese populations with pre–diabetes mellitus may benefit from lifestyle and/or pharmacological interventions to lower their risk for CVD and all‐cause mortality. Among Chinese populations with pre–diabetes mellitus, interventions should be implemented to prevent the development of diabetes mellitus, which is a risk factor for CVD. These findings support the screening of abnormal glycemic metabolism in the general population. Public health campaigns that promote, encourage, and support individuals in maintaining or adopting a healthier lifestyle during early life or midlife could have significant beneficial effects on the growing prevalence of CVD.
Sources of Funding
This work was supported by National Key R&D Program of China, codes 2018YFC1312400 and 2018YFC1312402.
Disclosures
None.
Acknowledgments
We thank all study participants, their relatives, and the members of the survey teams at the 11 regional hospitals of the Kailuan Medical Group.
(J Am Heart Assoc. 2021;10:e019045. . DOI: 10.1161/JAHA.120.019045.)
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Qiaofeng Song, Email: songqiaofengxny@163.com.
Xizhu Wang, Email: tsrmyy_wxz@126.com.
References
- 1. American Diabetes Association . Diagnosis and classification of diabetes mellitus. Diabetes Care. 2011;34(suppl 1):S62–S69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103:137–149. DOI: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 3. Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res Clin Pract. 2017;128:40–50. DOI: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 4. Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. DOI: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 5. Brannick B, Dagogo‐Jack S. Prediabetes and cardiovascular disease: pathophysiology and interventions for prevention and risk reduction. Endocrinol Metab Clin North Am. 2018;47:33–50. DOI: 10.1016/j.ecl.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kleinherenbrink W, Osei E, den Hertog HM, Zandbergen AAM. Prediabetes and macrovascular disease: review of the association, influence on outcome and effect of treatment. Eur J Intern Med. 2018;55:6–11. DOI: 10.1016/j.ejim.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 7. Barr ELM, Zimmet PZ, Welborn TA, Jolley D, Magliano DJ, Dunstan DW, Cameron AJ, Dwyer T, Taylor HR, Tonkin AM, et al. Risk of cardiovascular and all‐cause mortality in individuals with diabetes mellitus, impaired fasting glucose, and impaired glucose tolerance: the Australian Diabetes, Obesity, and Lifestyle Study (AusDiab). Circulation. 2007;116:151–157. DOI: 10.1161/CIRCULATIONAHA.106.685628. [DOI] [PubMed] [Google Scholar]
- 8. Danaei G, Lawes CM, Vander Hoorn S, Murray CJ, Ezzati M. Global and regional mortality from ischaemic heart disease and stroke attributable to higher‐than‐optimum blood glucose concentration: comparative risk assessment. Lancet. 2006;368:1651–1659. DOI: 10.1016/S0140-6736(06)69700-6. [DOI] [PubMed] [Google Scholar]
- 9. Levitzky YS, Pencina MJ, D'Agostino RB, Meigs JB, Murabito JM, Vasan RS, Fox CS. Impact of impaired fasting glucose on cardiovascular disease: the Framingham Heart Study. J Am Coll Cardiol. 2008;51:264–270. DOI: 10.1016/j.jacc.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 10. Liu J, Grundy SM, Wang W, Smith SC Jr, Vega GL, Wu Z, Zeng Z, Wang W, Zhao D. Ten‐year risk of cardiovascular incidence related to diabetes, prediabetes, and the metabolic syndrome. Am Heart J. 2007;153:552–558. DOI: 10.1016/j.ahj.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 11. Rijkelijkhuizen JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD, Dekker JM. High risk of cardiovascular mortality in individuals with impaired fasting glucose is explained by conversion to diabetes: the Hoorn study. Diabetes Care. 2007;30:332–336. DOI: 10.2337/dc06-1238. [DOI] [PubMed] [Google Scholar]
- 12. Cheng YJ, Gregg EW, Geiss LS, Imperatore G, Williams DE, Zhang X, Albright AL, Cowie CC, Klein R, Saaddine JB. Association of A1C and fasting plasma glucose levels with diabetic retinopathy prevalence in the U.S. population: implications for diabetes diagnostic thresholds. Diabetes Care. 2009;32:2027–2032. DOI: 10.2337/dc09-0440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ford ES, Zhao G, Li C. Pre‐diabetes and the risk for cardiovascular disease: a systematic review of the evidence. J Am Coll Cardiol. 2010;55:1310–1317. DOI: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 14. Ziegler D, Rathmann W, Dickhaus T, Meisinger C, Mielck A; KORA Study Group . Prevalence of polyneuropathy in pre‐diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31:464–469. DOI: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]
- 15. Perreault L, Pan Q, Mather KJ, Watson KE, Hamman RF, Kahn SE; Diabetes Prevention Program Research Group . Effect of regression from prediabetes to normal glucose regulation on long‐term reduction in diabetes risk: results from the Diabetes Prevention Program Outcomes Study. Lancet. 2012;379:2243–2251. DOI: 10.1016/S0140-6736(12)60525-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pratte KA, Johnson A, Beals J, Bullock A, Manson SM, Jiang L; Special Diabetes Program for Indians Diabetes Prevention Program . Regression to normal glucose regulation in American Indians and Alaska natives of a diabetes prevention program. Diabetes Care. 2019;42:1209–1216. DOI: 10.2337/dc18-1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Selvin E, Parrinello CM, Sacks DB, Coresh J. Trends in prevalence and control of diabetes in the United States, 1988–1994 and 1999–2010. Ann Intern Med. 2014;160:517–525. DOI: 10.7326/M13-2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang L, Gao P, Zhang M, Huang Z, Zhang D, Deng Q, Li Y, Zhao Z, Qin X, Jin D, et al. Prevalence and ethnic pattern of diabetes and prediabetes in China in 2013. JAMA. 2017;317:2515–2523. DOI: 10.1001/jama.2017.7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vistisen D, Kivimaki M, Perreault L, Hulman A, Witte DR, Brunner EJ, Tabak A, Jorgensen ME, Faerch K. Reversion from prediabetes to normoglycaemia and risk of cardiovascular disease and mortality: the Whitehall II cohort study. Diabetologia. 2019;62:1385–1390. DOI: 10.1007/s00125-019-4895-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Perreault L, Temprosa M, Mather KJ, Horton ED, Kitabchi A, Larkin M, Montez MG, Thayer D, Orchard TJ, Goldberg RB; Diabetes Prevention Program Research Group . Regression from prediabetes to normal glucose regulation is associated with reduction in cardiovascular risk: results from the Diabetes Prevention Program outcomes study. Diabetes Care. 2014;37:2622–2631. DOI: 10.2337/dc14-0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Perreault L, Pan Q, Schroeder EB, Kalyani RR, Bray GA, Dagogo‐Jack S, White NH, Goldberg RB, Kahn SE, Knowler WC, et al; Diabetes Prevention Program Research Group . Regression from prediabetes to normal glucose regulation and prevalence of microvascular disease in the Diabetes Prevention Program Outcomes Study (DPPOS). Diabetes Care. 2019;42:1809–1815. DOI: 10.2337/dc19-0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhang Q, Zhou Y, Gao X, Wang C, Zhang S, Wang A, Li NA, Bian L, Wu J, Jia Q, et al. Ideal cardiovascular health metrics and the risks of ischemic and intracerebral hemorrhagic stroke. Stroke. 2013;44:2451–2456. DOI: 10.1161/STROKEAHA.113.678839. [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. DOI: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2014;37(suppl 1):S81–S90. [DOI] [PubMed] [Google Scholar]
- 25. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, et al; National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee . The seventh report of the Joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure: the JNC 7 report. JAMA. 2003;289:2560–2572. [DOI] [PubMed] [Google Scholar]
- 26. Zhu J, Gao R, Zhao S, Lu G, Zhao D, Li J. 2016 Update of the Chinese guideline on the prevention and treatment of dyslipidemia in adults. Chin Circ J. 2016;31:937–953. [Google Scholar]
- 27. Wang A, Liu X, Su Z, Chen S, Zhang N, Wang Y, Wang Y, Wu S. Two‐year changes in proteinuria and risk for myocardial infarction in patients with hypertension: a prospective cohort study. J Hypertens. 2017;35:2295–2302. DOI: 10.1097/HJH.0000000000001462. [DOI] [PubMed] [Google Scholar]
- 28. Stroke—1989: recommendations on stroke prevention, diagnosis, and therapy: report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–1431. [DOI] [PubMed] [Google Scholar]
- 29. Goldberg RB, Temprosa M, Haffner S, Orchard TJ, Ratner RE, Fowler SE, Mather K, Marcovina S, Saudek C, Matulik MJ, et al; Diabetes Prevention Program Research Group . Effect of progression from impaired glucose tolerance to diabetes on cardiovascular risk factors and its amelioration by lifestyle and metformin intervention: the Diabetes Prevention Program randomized trial by the Diabetes Prevention Program Research Group. Diabetes Care. 2009;32:726–732. DOI: 10.2337/dc08-0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de Vegt F, Dekker JM, Ruhe HG, Stehouwer CD, Nijpels G, Bouter LM, Heine RJ. Hyperglycaemia is associated with all‐cause and cardiovascular mortality in the Hoorn population: the Hoorn Study. Diabetologia. 1999;42:926–931. DOI: 10.1007/s001250051249. [DOI] [PubMed] [Google Scholar]
- 31. The effects of nonpharmacologic interventions on blood pressure of persons with high normal levels: results of the Trials of Hypertension Prevention, Phase I. JAMA. 1992;267:1213–1220. [DOI] [PubMed] [Google Scholar]
- 32. Whelton SP, Chin A, Xin X, He J. Effect of aerobic exercise on blood pressure: a meta‐analysis of randomized, controlled trials. Ann Intern Med. 2002;136:493–503. DOI: 10.7326/0003-4819-136-7-200204020-00006. [DOI] [PubMed] [Google Scholar]
- 33. Chan DC, Watts GF, Ng TW, Yamashita S, Barrett PH. Effect of weight loss on markers of triglyceride‐rich lipoprotein metabolism in the metabolic syndrome. Eur J Clin Invest. 2008;38:743–751. DOI: 10.1111/j.1365-2362.2008.02019.x. [DOI] [PubMed] [Google Scholar]
- 34. Mavros Y, Kay S, Anderberg KA, Baker MK, Wang Y, Zhao R, Meiklejohn J, Climstein M, O'Sullivan A, de Vos N, et al. Changes in insulin resistance and HbA1c are related to exercise‐mediated changes in body composition in older adults with type 2 diabetes: interim outcomes from the GREAT2DO trial. Diabetes Care. 2013;36:2372–2379. DOI: 10.2337/dc12-2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laakso M. Cardiovascular disease in type 2 diabetes from population to man to mechanisms: the Kelly West Award Lecture 2008. Diabetes Care. 2010;33:442–449. DOI: 10.2337/dc09-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Thomas GN, Chook P, Qiao M, Huang XS, Leong HC, Celermajer DS, Woo KS. Deleterious impact of "high normal" glucose levels and other metabolic syndrome components on arterial endothelial function and intima‐media thickness in apparently healthy Chinese subjects: the CATHAY study. Arterioscler Thromb Vasc Biol. 2004;24:739–743. DOI: 10.1161/01.ATV.0000118015.26978.07. [DOI] [PubMed] [Google Scholar]
- 37. Nanditha A, Ram J, Snehalatha C, Selvam S, Priscilla S, Shetty AS, Arun R, Godsland IF, Johnston DG, Ramachandran A. Early improvement predicts reduced risk of incident diabetes and improved cardiovascular risk in prediabetic Asian Indian men participating in a 2‐year lifestyle intervention program. Diabetes Care. 2014;37:3009–3015. DOI: 10.2337/dc14-0407. [DOI] [PubMed] [Google Scholar]
- 38. Grundy SM. Pre‐diabetes, metabolic syndrome, and cardiovascular risk. J Am Coll Cardiol. 2012;59:635–643. DOI: 10.1016/j.jacc.2011.08.080. [DOI] [PubMed] [Google Scholar]


