Abstract
Background
Cardiovascular disease (CVD) in women has unique features, including associations with reproductive factors that are incompletely understood. Vasomotor symptoms (VMS), the classic menopausal symptom, are linked to CVD risk factors and subclinical CVD. Evidence linking VMS to CVD events is limited. We tested whether frequent and/or persistent VMS were associated with increased risk for fatal and nonfatal CVD events in SWAN (Study of Women’s Health Across the Nation).
Methods and Results
A total of 3083 women, aged 42 to 52 years at baseline, underwent up to 16 in‐person visits over 22 years. Assessments included questionnaires on VMS frequency (0, 1–5, or ≥6 days/2 weeks), physical measures, phlebotomy, and reported CVD events (myocardial infarction, stroke, heart failure, and revascularization). A subset of events was adjudicated via medical record. Death certificates were obtained. Relationships between baseline VMS or persistent VMS over the follow‐up (proportion of visits with frequent VMS) with combined incident nonfatal and fatal CVD were tested in Cox proportional hazards models adjusted for demographics, medication use, and CVD risk factors. Participants experienced 231 CVD events over the follow‐up. Women with frequent baseline VMS had an elevated risk of subsequent CVD events (relative to no VMS; ≥6 days: hazard ratio [HR] [95% CI], 1.51 [1.05–2.17], P=0.03; 1–5 days: HR [95% CI], 1.02 [0.75–1.39], P=0.89, multivariable). Women with frequent VMS that persisted over time also had an increased CVD event risk (>33% versus ≤33% of visits: HR [95% CI], 1.77 [1.33–2.35], P<0.0001, multivariable).
Conclusions
Frequent and persistent VMS were associated with increased risk of later CVD events. VMS may represent a novel female‐specific CVD risk factor.
Keywords: cardiovascular disease, hot flashes, menopause, vasomotor symptoms
Subject Categories: Cardiovascular Disease, Risk Factors, Women
Nonstandard Abbreviations and Acronyms
- SWAN
Study of Women’s Health Across the Nation
- VMS
vasomotor symptoms
Clinical Perspective
What Is New?
In this longitudinal cohort study of midlife women followed up for >20 years, frequent or persistent menopausal vasomotor symptoms were associated with a 50% to 77% increased risk of future cardiovascular disease events.
These associations were not explained by standard cardiovascular disease risk factors or by endogenous estradiol levels.
What Are the Clinical Implications?
Vasomotor symptoms may represent a novel female‐specific cardiovascular disease risk factor.
Midlife and older women with frequent or persistent vasomotor symptoms warrant particular attention for cardiovascular disease risk reduction and prevention.
Cardiovascular disease (CVD) is the leading cause of death in women. 1 CVD in women has unique risk factors, including reproductive factors (eg, pregnancy characteristics and menopause) that remain incompletely understood. 2 It has long been observed that, on average, women experience CVD events (eg, myocardial infarction [MI]) later in life than men, primarily when women are postmenopausal. 3 The menopause transition has been identified as a time when key CVD risk factors 4 , 5 and indices of vascular health worsen, independent of the effects of aging alone. 6 , 7 Therefore, the transition through menopause is regarded as important to the development of CVD in women; however, the precise features of menopause important to cardiovascular health have not been fully delineated.
Vasomotor symptoms (VMS) are the hallmark symptom of menopause and are experienced by most women at some point during the menopause transition. 8 For up to a third of women, VMS can be frequent or severe. 8 , 9 Newer data indicate that VMS persist longer than previously thought. Frequent or severe VMS can be expected to persist for an average of 7 to 9 years, and milder VMS may be experienced for an even longer period. 10 , 11
Although VMS have typically been regarded as having adverse effects on quality of life, VMS have been assumed to have few implications for physical health. However, emerging data have indicated the potential importance of VMS to women’s cardiovascular health. A growing body of literature has demonstrated that VMS are linked to adverse CVD risk factors, including hypertension, 12 , 13 insulin resistance and diabetes mellitus, 14 , 15 and poorer lipid profiles. 12 In addition, VMS have been associated with poorer endothelial function, 16 , 17 reduced vagal control over the heart, 18 , 19 a more proinflammatory or procoagulant profile, 20 , 21 and indicators of subclinical CVD. 16 , 22 , 23 These relationships typically persist after controlling for endogenous sex hormones and traditional CVD risk factors.
Despite the literature linking VMS to indicators of CVD risk, few studies have examined associations between VMS and incident clinical CVD, and the limited available literature has key methodologic limitations. For example, most studies define VMS on the basis of a single time point, 24 and thus these studies do not adequately capture a woman’s cumulative exposure to VMS over the transition. Notably, VMS are a dynamic symptom for which the time course varies widely across women. Furthermore, in many studies, VMS are recalled from years or decades prior, 25 , 26 and thereby are limited by the pronounced recall biases characteristic of symptom reports. One longitudinal Australian study reported that VMS were associated with subsequent coronary heart disease, 27 yet key risk factors (eg, lipids and blood pressure [BP]) were not measured, and findings were based on a low disease event rate. Thus, further research on links between VMS and clinic CVD is required.
Rigorously testing whether VMS are associated with CVD events requires that prospectively‐assessed VMS over the course of the menopause transition be examined in relation to later life CVD events. Few studies have the necessary repeated VMS assessments over midlife as well as extended follow‐up into the seventh decade of life when clinical CVD events typically accumulate in women. SWAN (Study of Women’s Health Across the Nation) is a longitudinal cohort study of the menopause transition in which 3302 premenopausal or early perimenopausal women were followed up to 22 years to prospectively characterize their VMS, CVD risk factors, and incidence of CVD events. Thus, SWAN represents a unique cohort in which to examine whether VMS are associated with clinical CVD. We tested whether women with (1) more frequent VMS at baseline or (2) persistently frequent VMS over time were at increased risk for subsequent CVD events (clinical CVD or CVD mortality), taking into account a range of potential confounding or explanatory factors.
Methods
Data Access
SWAN provides access to public use data sets that include data from SWAN screening, baseline, and follow‐up visits (https://agingresearchbiobank.nia.nih.gov/, http://www.swanstudy.org/swan‐research/data‐access/). To preserve participant confidentiality, some, but not all, of the data are contained in the public use data sets. Investigators who require assistance accessing the public use data set may contact the SWAN Coordinating Center (swanaccess@edc.pitt.edu).
Study Sample
SWAN is a prospective cohort study of women conducted at 7 US sites: Boston, MA; Chicago, IL; southeast Michigan; Los Angeles, CA; Newark, NJ; Pittsburgh, PA; and Oakland, CA. 28 Each site recruited non‐Hispanic White women and one additional racial/ethnic group (Black, Chinese, Hispanic, or Japanese women). Each site implemented a sampling and recruitment strategy that was optimal for the characteristics of the local site and the specific minority population to be recruited. Primary sampling included random digit dialing supplemented by list‐based frames (eg, city census list, voter registration list, health maintenance organization enrollment list, or electric utility lists) and by acquaintance networks via snowballing at select sites to obtain adequate numbers of racial/ethnic minority women. Baseline eligibility criteria for the SWAN longitudinal cohort included being aged 42 to 52 years, having a uterus and at least one ovary, not being pregnant or lactating, not using oral contraceptives/hormone therapy (HT), and having at least one menstrual cycle in the prior 3 months. Clinic assessments, standardized across sites and that included clinical interviews, questionnaires, physical and anthropometric measurements, and phlebotomy, began in 1996 to 1997 and extended for up to 16 visits over 22 years of follow‐up. SWAN protocols were approved by the institutional review boards at each site, and each participant provided written informed consent at each visit.
A total of 3302 women were enrolled in SWAN (51% of eligible women). Women who enrolled in SWAN had somewhat higher education, were less financially strained, and were less often smokers than those who did not. 28 Furthermore, for these analyses, we excluded 219 women because of: (1) missing data on the time of CVD event/CVD mortality (n=7), (2) missing VMS data (n=18), or (3) missing all follow‐up data (n=194). Thus, 3083 women were included in these analyses. Women excluded differed from women included in this analysis in that they were more often Hispanic, were smokers, were financially strained, were less educated, were less active, had more depression, and had a poorer CVD risk factor profile (lower high‐density lipoprotein cholesterol, higher BP, triglycerides, and body mass index, and greater insulin resistance) (Table S1).
Vasomotor Symptoms
VMS were assessed via a standard questionnaire at the baseline and each follow‐up visit. At each visit, women responded to 2 questions that asked separately how often they experienced hot flashes and night sweats in the past 2 weeks (response options: not at all, 1–5 days, 6–8 days, 9–13 days, and every day). Hot flashes or night sweats were considered collectively as VMS, categorized as none (neither hot flashes nor night sweats in the prior 2 weeks), 1 to 5 days/2 weeks (either or both hot flashes or night sweats 1–5 days/2 weeks), and ≥6 days/2 weeks (either or both hot flashes or night sweats ≥6 days/2 weeks). In addition to baseline VMS, the proportion of attended visits (before a CVD event or the censoring time) in which the woman reported frequent VMS (≥6 days/2 weeks) over the follow‐up were considered in separate models.
CVD Events/CVD Mortality
Nonfatal cardiovascular events (MI, cerebrovascular accident/stroke, heart failure, or revascularization procedures [percutaneous coronary intervention or coronary artery bypass grafting]) were self‐reported at each SWAN visit. More extensive information was obtained at SWAN visits 12 and 13 and primarily during visit 15 when routine adjudication of events began. Attempts were made to obtain medical records for each self‐reported CVD event, including those events that were reported at prior study visits. On receipt of all obtainable information from medical records related to each specific event, the SWAN Coordinating Center assembled an event package with this relevant information (eg, admission history, physical examination, discharge summary, laboratory data, diagnostic test results, and operative/procedure reports). Two cardiologist reviewers blinded to VMS status reviewed this information and returned their determination of the diagnosis to the SWAN Coordinating Center. If there was agreement as to the diagnosis between the 2 members (yes, no, or indeterminate), the case was considered complete. For cases for which there was not agreement between the 2 reviewers, differences were resolved by the third member. When a participant had multiple events, the first event was designated as the incident event. Thus, there were 204 self‐reported first CVD events (MI: N=46; cerebrovascular accident: N=89; revascularization: N=27; heart failure: N=22; multiple events simultaneously: N=20). Of these 204 events, 66 were confirmed through the adjudication process (32.4%). In addition to nonfatal CVD events, fatal CVD events were considered. Fatal CVD events were identified via systematic review of death certificates, which began at the 15th annual SWAN visit. As of this visit, 161 women of the SWAN cohort of 3302 were deceased. Death certificates were obtained for 155 (cause of death available for 154) of the 161 deaths and were centrally coded for cause of death using a standardized data extraction protocol. Of these deaths, 27 of them were coded as CVD related (heart disease: n=17; heart failure: n=6; MI: n=9; stroke: n=5; categories not mutually exclusive) as the underlying cause of death in any line of the death certificate. Primary analyses evaluated the time from SWAN baseline to the first adjudicated or self‐reported nonfatal CVD event (combined outcome of MI, stroke, heart failure, and revascularization) or a fatal CVD event identified via death certificate; thus, this analysis includes a total of 231 events (204 nonfatal events and 27 fatal CVD events). A sensitivity analysis was conducted to evaluate the time to the first adjudicated nonfatal CVD event (66 events).
Covariates
At baseline, race/ethnicity and education (high school or less, some college/vocational school, or college or higher) were reported via standardized questionnaires. Age, smoking (current versus past/never), financial strain (how hard it was to pay for basics: very or somewhat hard versus not hard), physical activity, 29 and menopausal stage (on the basis of self‐reported bleeding patterns over the year) were derived from questionnaires and clinical interviews. Medication use was ascertained by self‐report and confirmed via visual inspection of pill bottles at the study visit. The therapeutic class and subclass for each medication were coded according to the Iowa Drug Information System. 30 Use of cardiovascular medications (BP lowering, lipid lowering, and antidiabetic) was classified using these classifications on the basis of clinical review by 2 study investigators to remove agents within a class not used in standard practice for the indications noted above. Height and weight were measured, and body mass index was calculated (kg/m2). Systolic BP and diastolic BP were averaged from 2 seated measurements. Phlebotomy was performed following overnight fast during the early follicular phase (days 2–5 of the menstrual cycle). EDTA‐treated plasma was separated, frozen at −20°C, and sent on dry ice to the Medical Research Laboratories (Highland Heights, KY). Baseline through the seventh follow‐up visit total cholesterol and triglyceride concentrations were determined by enzymatic methods (Hitachi 747 analyzer; Boehringer Mannheim Diagnostics, Indianapolis, IN). High‐density lipoprotein cholesterol was quantified following precipitation of low‐density lipoprotein cholesterol (LDL‐C) with heparin and manganese chloride by the modified Lipid Research Clinics procedure. Cholesterol in the supernate was measured by an automated cholesterol oxidase assay on a Hitachi 747‐200 clinical analyzer using RAICHEM reagents. 31 , 32 For subsequent visits, ADVIA assay methods were used for total cholesterol, triglycerides, and HDL‐C. 33 , 34 Calibration was based on 340 samples selected to be representative of the SWAN cohort. LDL‐C was calculated by the Friedewald equation, and values of estimated LDL‐C and triglycerides were set to missing when the triglycerides >400 mg/dL. 35 Lipid fractions with the statistically strongest associations with outcomes were considered in models (LDL‐C and triglycerides). For baseline through the seventh follow‐up visit, glucose was measured in serum by automated enzymatic assay on a Hitachi 747‐200 chemistry analyzer using the hexokinase reaction and Roche Diagnostic reagents. Insulin was measured in serum in duplicate by competitive binding radioimmunoassay using reagent from Diagnostic Products Corporation. For subsequent study visits, glucose was measured using the ADVIA Chemistry Glucose Hexokinase 3 Concentrated Reagents, 33 and insulin was measured using the ADVIA Centaur Insulin assay, which is a 2‐site sandwich immunoassay. 36 , 37 Calibration equations for glucose and insulin were developed on the basis of random samples of 565 and 400 samples, respectively. Homeostatic model assessment insulin resistance was calculated as follows: [glucose (mmol/L)*insulin (mIU/mL)]/22.5. 38 Serum estradiol levels were measured with a modified, off‐line ACS‐180 (E2‐6) chemiluminometric immunoassay (Bayer Diagnostics, Norwood, MA), 39 with a lower limit of detection of 1 to 7 pg/mL and interassay and intra‐assay coefficients of variation of 10.6% and 6.4%, respectively.
Statistical Analysis
The associations between baseline VMS category and combined fatal and nonfatal CVD events were tested in Cox proportional hazards models. In a second set of analyses, we considered the persistence of frequent VMS over time, calculated as the proportion of study visits that the woman attended (before a CVD event or censoring) in which she had frequent VMS. We examined persist frequent VMS in relation to incident CVD events/CVD mortality. The proportion of visits with persistent frequent VMS over the follow‐up was considered as both a continuous variable and a categorical variable. A range of cut points for categorical models were considered; these cut points yielded largely comparable results, and thus we chose a cut point (>33% versus ≤33% of attended visits reporting frequent VMS) broadly informed by prior work on the average duration of frequent VMS, 11 that met model assumptions, and that allowed for valid model testing. To support clinical interpretation of these cut points, we present the approximate number of visits and years with frequent VMS these cut points correspond to on the basis of the sample average number of visits and years women attended. Covariates were study site, age, race/ethnicity, education, financial strain, menopause status, smoking, physical activity, systolic BP, body mass index, lipids, homeostatic model assessment insulin resistance, and medication use (BP lowering, lipid lowering, or antidiabetic). For baseline VMS models, baseline covariate values were included. For persistence of VMS models, mean levels of the covariate over all available visits before the event or censoring for continuous variables (physical activity, systolic BP, body mass index, lipids, and homeostatic model assessment insulin resistance) and the proportion of attended visits positive for the covariate before the event/censoring for categorical variables (smoking, financial strain, medication use, and HT use) was included, with the exception of age, menopause stage, education, and race/ethnicity, which were derived from baseline. The number of attended visits was also included in persistence of VMS models. Although no women were using HT at baseline, as per entry criteria, the proportion of follow‐up visits before event/censoring with HT use was included in models. Missing covariate values at baseline were imputed; notably, the extent of missing data on covariates was minimal (ranging from systolic BP [0.2%] to LDL‐C [6.6%]). We considered 2 approaches: imputation based on that individual’s data on the next available visit and imputation based on that individual’s mean value from all other visits. The 2 approaches produced comparable results; therefore, we present findings using mean imputation. Additional sensitivity models were conducted in which the outcome was restricted to adjudicated CVD events. Endogenous estradiol concentrations were considered as an additional covariate (baseline levels for baseline VMS models and average estradiol levels before event/censoring for persistence of VMS models). Cycle day of blood draw (in or out of window of menstrual cycle days 2–5) was also included as a covariate in baseline models. Furthermore, although all women entered the study with at least one ovary, the relations between persistent VMS over the follow‐up and CVD events were also conducted censoring women at the time of bilateral oophorectomy during the study. To verify model proportional hazards assumptions, we plotted the log hazards over time (which were parallel); conducted a residual analysis of the empirical score process (plots of the observed score processes well within the simulated score processes, supremum test P values >0.50); and plotted the Schoenfeld residuals (smooths of the Schoenfeld residuals versus time flat at 0), all of which indicated no violation of the proportional hazards assumption. Analyses were performed with SAS v9.4 (SAS, Cary, NC).
Results
The analysis included 3083 women (48% White, 28% Black, 9% Japanese, 8% Chinese, and 7% Hispanic women) who were a median age of 46 (interquartile range, 44–48) years at baseline. There were no clinical CVD events (with the exception of hypertension) at baseline. Over the up to 22 years of follow‐up (median, 19 years), women reported experiencing 204 nonfatal CVD events, and 27 women had a fatal CVD event. Women attended an average of 13 of the 16 study visits, and they reported frequent VMS at an average of 20% (SD, 23%; median, 12%) of these study visits. Furthermore, 743 of the 3083 (24%) women reported frequent VMS at >33% of visits. At baseline, women with frequent VMS were more often older, perimenopausal (versus premenopausal), Black women, less educated, and more financially strained and had a poorer CVD risk factor profile than women without VMS (Table 1). Although no women were using HT at baseline, 42% of the women reported HT use at some point during the study. Women who used HT did so at 28% of the visits. Across the sample as a whole, HT use was reported at an average of 12% of the study visits.
Table 1.
Baseline Characteristics of Participants by Frequency of VMS: SWAN (N=3083)
| Characteristics |
No VMS (N=1875) |
VMS 1–5 d/2 wk (N=873) |
VMS ≥6 d/2 wk (N=335) |
|---|---|---|---|
| Age, median (Q1–Q3), y | 46.0 (44.0–48.0) | 46.0 (44.0–48.0) | 47.0 (45.0–49.0) |
| Race/ethnicity, % (n) | |||
| Black | 24.8 (465) | 32.0 (279) | 39.1 (131) |
| White | 49.7 (931) | 44.3 (387) | 43.6 (146) |
| Chinese | 9.0 (168) | 6.5 (57) | 5.1 (17) |
| Hispanic | 6.6 (123) | 9.0 (79) | 7.5 (25) |
| Japanese | 10.0 (188) | 8.1 (71) | 4.8 (16) |
| Education, % (n) | |||
| High school or less | 21.3 (395) | 27.3 (236) | 28.8 (96) |
| Vocational school/some college | 29.4 (547) | 36.3 (314) | 39.6 (132) |
| College or higher | 49.3 (916) | 36.4 (315) | 31.5 (105) |
| Financial strain, % (n) | |||
| Somewhat/very hard | 34.1 (636) | 44.6 (388) | 47.4 (158) |
| Not hard | 65.9 (1227) | 55.4 (481) | 52.6 (175) |
| Menopause stage, % (n) | |||
| Early perimenopause | 38.9 (726) | 54.5 (471) | 61.7 (205) |
| Premenopause | 61.0 (1139) | 45.4 (393) | 38.0 (126) |
| Unknown | 0.2 (3) | 0.1 (1) | 0.3 (1) |
| BMI, median (Q1–Q3), kg/m2 | 25.7 (22.4–30.9) | 27.2 (23.2–32.4) | 29.2 (24.6–36.1) |
| SBP, median (Q1–Q3), mm Hg | 113.0 (105.0–124.0) | 117.0 (107.0–129.0) | 120.0 (109.0–130.0) |
| DBP, median (Q1–Q3), mm Hg | 74.0 (68.0–80.0) | 75.0 (69.0–82.0) | 76.0 (70.0–83.0) |
| LDL‐C, median (Q1–Q3), mg/dL | 112.0 (93.0–133.0) | 115.5 (94.0–138.0) | 119.0 (99.0–141.0) |
| HDL‐C, median (Q1–Q3), mg/dL | 55.0 (47.0–65.0) | 54.0 (46.0–64.0) | 51.0 (44.0–61.0) |
| Triglycerides, median (Q1–Q3), mg/dL | 86.0 (66.0–123.0) | 92.0 (67.0–135.0) | 107.5 (75.0–161.0) |
| HOMA‐IR, median (Q1–Q3) | 1.7 (1.2–2.6) | 2.0 (1.3‐3.3) | 2.4 (1.5‐4.3) |
| Smoking status, % (n) | |||
| Past/never | 86.3 (1618) | 80.2 (699) | 74.9 (251) |
| Current | 13.7 (256) | 19.8 (173) | 25.1 (84) |
| Physical activity score, median (Q1–Q3) | 7.7 (6.5–9.0) | 7.6 (6.5–8.8) | 7.4 (6.2–8.5) |
| Medication use | |||
| BP lowering, % (n) | 11.2 (210) | 17.1 (149) | 23.9 (80) |
| Lipid lowering, % (n) | 0.9 (16) | 1.0 (9) | 1.8 (6) |
| Antidiabetics, % (n) | 2.2 (41) | 3.9 (34) | 2.7 (9) |
| Estradiol, median (Q1–Q3), pg/mL | 57.7 (34.1–89.9) | 52.1 (32.6–86.2) | 48.3 (28.6–86.0) |
BMI indicates body mass index; BP, blood pressure; DBP, diastolic BP; HDL‐C, high‐density lipoprotein cholesterol; HOMA‐IR, homeostatic model assessment insulin resistance; LDL‐C, low‐density lipoprotein cholesterol; Q1, quartile 1; Q3, quartile 3; SBP, systolic BP; SWAN, Study of Women’s Health Across the Nation; and VMS, vasomotor symptoms.
When considering baseline VMS, women with more frequent VMS (≥6 days/2 weeks) had >2‐fold higher risk of CVD events relative to women without VMS (Table 2, Figure 1). This association persisted controlling for additional demographic characteristics, established CVD risk factors, and use of HT over the study. Furthermore, more persistent frequent VMS over the study were associated with an increased risk of CVD events. These associations were apparent when we considered persistent VMS as a continuous variable (proportion of attended visits reporting frequent visits, per 12% increase: hazard ratio [95% CI], 1.11 [1.05–1.18], P = 0.0004, fully adjusted; an increase that corresponds roughly to an average of every 1.5 visits or approximately 2 years with frequent VMS). These associations were also apparent when we considered frequent VMS as a categorical variable (>33% versus ≤33% of attended visits reporting frequent VMS, a cut point that corresponds to an average of 4 visits or roughly 6 years with frequent VMS) (Table 3, Figure 2).
Table 2.
Association Between Baseline VMS and Combined Incident Fatal and Nonfatal CVD Events in SWAN (N=3083)
| Frequency of VMS |
Model 1: HR (95% CI), P Value |
Model 2: HR (95% CI), P Value |
|---|---|---|
| 1–5 d/2 wk |
1.33 (0.98–1.79), 0.065 |
1.02 (0.75–1.39), 0.89 |
| ≥6 d/2 wk |
2.16 (1.53–3.04), <0.001 |
1.51 (1.05–2.17), 0.026 |
Relative to no VMS in prior 2 weeks; missing covariate values imputed on the basis of mean levels. For 1 to 5 days/2 weeks: N=873, CVD events=73; for ≥6 days/2 weeks: N=335, CVD events=51. Model 1: adjusted for site, baseline age, and race/ethnicity. Model 2: adjusted for model 1 covariates+baseline education, financial strain, menopause stage, systolic blood pressure, body mass index, low‐density lipoprotein cholesterol, triglycerides, homeostatic model assessment insulin resistance, smoking, physical activity, medication use (blood pressure lowering, lipid lowering, or antidiabetic), and proportion of follow‐up visits using hormone therapy. CVD indicates cardiovascular disease; HR, hazard ratio; SWAN, Study of Women’s Health Across the Nation; and VMS, vasomotor symptoms.
Figure 1.
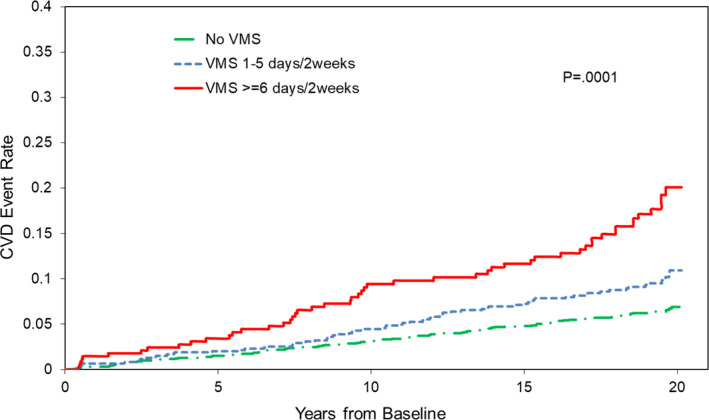
Baseline vasomotor symptoms (VMS) in relation to fatal and nonfatal cardiovascular disease (CVD) events, N=3083, 231 events.
Table 3.
Association Between VMS Over the Study and Combined Incident Fatal and Nonfatal CVD Events in SWAN (N=3083)
| Variable |
Model 1: HR (95% CI), P Value |
Model 2: HR (95% CI), P Value |
|---|---|---|
| Persistent frequent VMS (>33% of attended visits with frequent VMS)* |
1.98 (1.51–2.59), <0.0001 |
1.77 (1.33–2.35), <0.0001 |
For >33% of attended visits with frequent VMS: N=743, CVD events=99. Frequent VMS: VMS ≥6 days in the prior 2 weeks; missing covariate values imputed on the basis of mean levels. Model 1: adjusted for site, baseline age, race/ethnicity, and number of attended visits. Model 2: adjusted for model 1 covariates+baseline education, average financial strain, baseline menopause stage, average systolic blood pressure, average body mass index, average low‐density lipoprotein cholesterol, average triglycerides, average homeostatic model assessment insulin resistance, average physical activity, proportion of visits smoking, medication use (proportion of visits using blood pressure–lowering, lipid‐lowering, antidiabetic, or hormone therapy), and number of attended visits. CVD indicates cardiovascular disease; HR, hazard ratio; SWAN, Study of Women’s Health Across the Nation; and VMS, vasomotor symptoms.
Relative to ≤33% of attended visits with frequent VMS.
Figure 2.
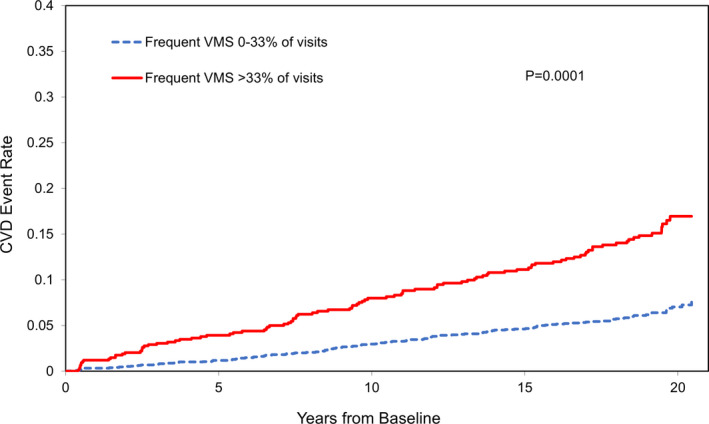
Vasomotor symptoms (VMS) over the transition in relation to fatal and nonfatal cardiovascular disease (CVD) events, N=3083, 231 events.
We considered several additional analyses to determine the robustness of results over several conditions. When we restricted analyses to adjudicated nonfatal CVD events, findings were comparable if not strengthened (Tables S2 and S3). We further adjusted for endogenous estradiol concentrations, and findings were similar (Table S4). Although all women had at least one ovary at baseline, we censored women at the time of bilateral oophorectomy during the study for models of persistent VMS, and findings were similar (data not shown).
Discussion
This study prospectively investigated associations between VMS ascertained over 22 years from baseline through 15 follow‐up visits with subsequent incident CVD events in the SWAN cohort of midlife women. This study is the largest, longitudinal study to date to address the relationships between midlife VMS and risk of subsequent incident CVD events. We found that frequent VMS at baseline as well as frequent VMS that persisted over time were associated with elevated risk of a combined outcome of incident fatal and nonfatal CVD events. Demographic factors and CVD risk factors accounted for a portion of this relationship but did not fully account for relationships between VMS and CVD. Notably, frequent VMS at baseline were associated with a 50% increased risk of later clinical CVD. Frequent VMS that persisted over time (>33% of visits, or an average of 4 visits with frequent VMS) were associated with a 77% increased risk of later clinical CVD. These findings suggest that frequent VMS early in the transition or persistent over the transition are associated with increased risk of CVD events later in life.
Menopause is an important transition for women’s cardiovascular health. This midlife period of ovarian aging occurs directly before the rates of clinical CVD events accelerate in women, and is a time also characterized by accelerated degradation in CVD risk factors and vascular health. 4 , 5 , 6 , 7 However, the precise features of menopause that influence CVD health have not been fully elucidated.
The hallmark symptom of menopause is VMS. VMS are increasingly appreciated to be frequent or severe for many women and often persist for over a decade. 10 , 11 These symptoms, previously regarded as incidental, have been linked to a poorer CVD risk factor profile, 12 , 13 , 15 , 40 to markers of endothelial dysfunction, 16 , 17 , 41 and to subclinical CVD (carotid atherosclerosis 22 , 23 and aortic calcification 16 ). However, much more limited literature has considered the associations between VMS and the onset of clinical CVD. A report from 2 combined European cohorts found VMS to be associated with incident coronary heart disease, but VMS were assessed with limited and ambiguous questions administered at a single time point. 24 An Australian study found that frequent VMS, assessed 6 times over midlife, were associated with greater risk of coronary heart disease. 27 However, in this study, the event rate was low (1.6%), precise information on the timing of events was not available, key risk factors were not measured, and the cohort was composed almost exclusively of White women. The WHI (Women’s Health Initiative) Study showed that late‐occurring VMS were associated with incident coronary heart disease, 26 whereas a report from the WISE (Women’s Ischemia Syndrome Evaluation) Study found that VMS occurring early in midlife were associated with greater subsequent CVD mortality. 25 In both of these studies, VMS reports were assessed at a single time point and relied on recalled information from many years earlier. With its repeated prospective assessments of VMS over 16 visits and 22 years and prospective collection of CVD events, the present study provides among the strongest evidence to date that frequent VMS experienced either early in the menopause transition or persistently over the course of the transition are associated with incident CVD.
Future work is required to elucidate the nature of and underlying mechanisms linking VMS to CVD. Although frequent VMS can serve as a marker of or be causally involved in poor or degrading cardiovascular health during midlife, the precise nature of this VMS‐CVD relation warrants ongoing empirical attention. Furthermore, the potential mechanisms underlying the link between VMS and subsequent CVD should be further elucidated. The investigation of these mechanisms has been limited by the somewhat uncertain etiology of VMS. Although changes in endogenous sex hormones play a role in VMS, they are not the sole determinant of VMS, 42 and most evidence indicates that VMS are hypothalamic thermoregulatory events 43 induced by the hypothalamic neuropeptide neurokinin B. 44 Further, sex hormones have typically not explained relations between VMS and indicators of CVD risk. 16 , 22 , 45 Other mechanisms may include traditional CVD risk factors. Although women with frequent VMS have a poorer CVD risk factor profile, 13 , 15 , 40 adjusting for CVD risk factors somewhat attenuated but did not explain observed associations. Notably, VMS are characterized by transient reductions in vagal influence over the heart, 18 VMS have been linked to poorer endothelial function, 16 , 17 , 41 and more limited literature links VMS to a proinflammatory or procoagulant profile 20 , 21 and deranged hypothalamic pituitary adrenal axis function. 46 The role of these factors, as well as any genetic factors that may underlie VMS‐CVD links, warrants further investigation.
These study findings should be interpreted in light of several limitations. The proportion of nonfatal events that was adjudicated was relatively low, likely due in part to the fact that collection of information required for adjudication occurred in follow‐up visits 12/13 and 15. Thus, women who did not attend these visits, who died, or who dropped out before attending these visits did not provide information required for adjudication events. Furthermore, not all women correctly identified the hospital where the event was treated or the date on which the event occurred. Other women did not provide consent to access their medical records required for adjudication. It also is possible that some of these reported CVD events were not actual CVD events. However, secondary analyses restricted to adjudicated CVD events were consistent with the main study findings. Furthermore, <7% of women were excluded from the primary analyses, but these women were more often Black women, were more often financially strained, and had a poorer CVD risk factor profile, which are also features of women with the highest burden of VMS. Women with a lower socioeconomic status were also less likely to participate in SWAN as a whole. Thus, the most high‐risk, symptomatic women may have been underrepresented in this analysis, potentially biasing our findings to the null. Several secondary models were tested that should be regarded with caution given issues of multiple testing. Women reported approximately yearly the number of days in the prior 2 weeks they had experienced VMS, but the daily frequency or severity of VMS was not assessed. Thus, women who had different patterns of VMS in the past 2 weeks from the other parts of the year would have been misclassified. Furthermore, the VMS measure was relatively crude, and women with inconsistent patterns of VMS over the 2‐week recall period (eg, frequent VMS some days and few/no VMS other days) may have been misclassified.
This study had several strengths. It was conducted in a well‐characterized cohort of women who have been observed for up to 22 years over the course of the menopause transition. Five racial/ethnic groups were represented. Prospective assessments were conducted on both exposures and outcomes. VMS were assessed repeatedly and approximately annually, and CVD events were characterized. Multiple covariates and potential confounders were assessed repeatedly over the menopause transition.
In conclusion, in this longitudinal cohort study of the menopause transition, we found that frequent VMS or persistent VMS over midlife are associated with a 50% to 77% increased risk of future CVD events. Thus, VMS may represent a novel, female‐specific CVD risk factor, as VMS may point to women at risk of future CVD events beyond those identified on the basis of traditional CVD risk factors. These findings underscore that women with frequent or persistent VMS warrant particular attention for CVD risk reduction and prevention.
Sources of Funding
SWAN (Study of Women's Health Across the Nation) has grant support from the National Institutes of Health (NIH), US Department of Health and Human Services, through the National Institute on Aging (NIA), National Institute of Nursing Research (NINR), and NIH Office of Research on Women’s Health (ORWH) (grants U01NR004061, U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, and U01AG012495). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH, or NIH.
Disclosures
Dr Thurston is a consultant/advisory board member for Astellas, Procter & Gamble, Pfizer, and Virtue Health. Dr Jackson has research support from National Institutes of Health (NIH) and Amgen, is a consultant for UpToDate and McKesson, and is an expert witness for DeBlase Brown Everly LLP. Dr Joffe is a consultant/advisory board member for NeRRe/KaNDy, Sojournix, Eisai, and Jazz, and has grant/research support from NIH, Merck, NeRRe/KaNDy, Pfizer, and QUE Oncology. Her spouse is an employee at Merck Research Lab, is a consultant and has equity in Arsenal Biosciences, and has equity in Tango. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S4
Acknowledgements
The authors acknowledge the following Clinical Centers: University of Michigan, Ann Arbor, Siobán Harlow, principal investigator (PI) 2011 to present, MaryFran Sowers, PI 1994 to 2011; Massachusetts General Hospital, Boston, MA, Joel Finkelstein, PI 1999 to present, Robert Neer, PI 1994 to 1999; Rush University, Rush University Medical Center, Chicago, IL, Howard Kravitz, PI 2009 to present, Lynda Powell, PI 1994 to 2009; University of California, Davis/Kaiser, Ellen Gold, PI; University of California, Los Angeles, Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY, Carol Derby, PI 2011 to present, Rachel Wildman, PI 2010 to 2011, Nanette Santoro, PI 2004 to 2010; University of Medicine and Dentistry–New Jersey Medical School, Newark, NJ, Gerson Weiss, PI 1994 to 2004; and the University of Pittsburgh, Pittsburgh, PA, Karen Matthews, PI. National Institutes of Health Program Office: National Institute on Aging, Bethesda, MD, Chhanda Dutta, 2016 to present, Winifred Rossi, 2012 to 2016, Sherry Sherman, 1994 to 2012, Marcia Ory, 1994 to 2001; National Institute of Nursing Research, Bethesda, MD, Program Officers. Central Laboratory: University of Michigan, Ann Arbor, MI, Daniel McConnell (Central Ligand Assay Satellite Services). Coordinating Center: University of Pittsburgh, Pittsburgh, PA, Maria Mori Brooks, PI 2012 to present; Kim Sutton‐Tyrrell, PI 2001 to 2012; New England Research Institutes, Watertown, MA, Sonja McKinlay, PI 1995 to 2001.
Steering Committee: Susan Johnson, Current Chair, and Chris Gallagher, Former Chair. We thank the study staff at each site and all the women who participated in SWAN (Study of Women’s Health Across the Nation).
(J Am Heart Assoc. 2021;10:e017416. DOI: 10.1161/JAHA.120.017416.
Supplementary material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017416
For Sources of Funding and Disclosures, see page 9.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. American Heart Association council on epidemiology and prevention statistics committee and stroke statistics subcommittee. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. Ouyang P, Wenger NK, Taylor D, Rich‐Edwards JW, Steiner M, Shaw LJ, Berga SL, Miller VM, Merz NB. Strategies and methods to study female‐specific cardiovascular health and disease: a guide for clinical scientists. Biol Sex Differ. 2016;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw LJ, Bugiardini R, Merz CN. Women and ischemic heart disease: evolving knowledge. J Am Coll Cardiol. 2009;54:1561–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Matthews KA, El Khoudary SR, Brooks MM, Derby CA, Harlow SD, Barinas‐Mitchell EJ, Thurston RC. Lipid changes around the final menstrual period predict carotid subclinical disease in postmenopausal women. Stroke. 2017;48:70–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Janssen I, Powell LH, Crawford S, Lasley B, Sutton‐Tyrrell K. Menopause and the metabolic syndrome: The Study of Women's Health Across the Nation. Arch Intern Med. 2008;168:1568–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. El Khoudary SR, Wildman RP, Matthews K, Thurston RC, Bromberger JT, Sutton‐Tyrrell K. Progression rates of carotid intima‐media thickness and adventitial diameter during the menopausal transition. Menopause. 2013;20:8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Khan ZA, Janssen I, Mazzarelli JK, Powell LH, Dumasius A, Everson‐Rose SA, Barinas‐Mitchell E, Matthews K, El Khoudary SR, Weinstock PJ, et al. Serial studies in subclinical atherosclerosis during menopausal transition (from the Study of Women's Health Across the Nation). Am J Cardiol. 2018;122:1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gold E, Colvin A, Avis N, Bromberger J, Greendale G, Powell L, Sternfeld B, Matthews K. Longitudinal analysis of vasomotor symptoms and race/ethnicity across the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Am J Public Health. 2006;96:1226–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Williams RE, Kalilani L, DiBenedetti DB, Zhou X, Granger AL, Fehnel SE, Levine KB, Jordan J, Clark RV. Frequency and severity of vasomotor symptoms among peri‐ and postmenopausal women in the United States. Climacteric. 2008;11:32–43. [DOI] [PubMed] [Google Scholar]
- 10. Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstet Gynecol. 2011;117:1095–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Avis NE, Crawford SL, Greendale G, Bromberger JT, Everson‐Rose SA, Gold EB, Hess R, Joffe H, Kravitz HM, Tepper PG, et al. Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175:531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gast GC, Grobbee DE, Pop VJ, Keyzer JJ, Wijnands‐van Gent CJ, Samsioe GN, Nilsson PM, van der Schouw YT. Menopausal complaints are associated with cardiovascular risk factors. Hypertension. 2008;51:1492–1498. [DOI] [PubMed] [Google Scholar]
- 13. Jackson EA, El Khoudary SR, Crawford SL, Matthews K, Joffe H, Chae C, Thurston RC. Hot flash frequency and blood pressure: data from the Study of Women's Health Across the Nation. J Women's Health. 2016;25:1204–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gray KE, Katon JG, LeBlanc ES, Woods NF, Bastian LA, Reiber GE, Weitlauf JC, Nelson KM, LaCroix AZ. Vasomotor symptom characteristics: are they risk factors for incident diabetes? Menopause. 2017;25:520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thurston RC, El Khoudary SR, Sutton‐Tyrrell K, Crandall CJ, Sternfeld B, Joffe H, Gold EB, Selzer F, Matthews KA. Vasomotor symptoms and insulin resistance in the Study of Women's Health Across the Nation. J Clin Endocrinol Metab. 2012;97:3487–3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thurston RC, Sutton‐Tyrrell K, Everson‐Rose SA, Hess R, Matthews KA. Hot flashes and subclinical cardiovascular disease: findings from the study of women's health across the nation heart study. Circulation. 2008;118:1234–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bechlioulis A, Kalantaridou SN, Naka KK, Chatzikyriakidou A, Calis KA, Makrigiannakis A, Papanikolaou O, Kaponis A, Katsouras C, Georgiou I, et al. Endothelial function, but not carotid intima‐media thickness, is affected early in menopause and is associated with severity of hot flushes. J Clin Endocrinol Metab. 2010;95:1199–1206. [DOI] [PubMed] [Google Scholar]
- 18. Thurston RC, Matthews KA, Chang Y, Santoro N, Barinas‐Mitchell E, von Känel R, Landsittel DP, Jennings JR. Changes in heart rate variability during vasomotor symptoms among midlife women. Menopause. 2016;23:499–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Zambotti M, Colrain IM, Sassoon SA, Nicholas CL, Trinder J, Baker FC. Vagal withdrawal during hot flashes occurring in undisturbed sleep. Menopause. 2013;20:1147–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang WY, Hsin IL, Chen DR, Chang CC, Kor CT, Chen TY, Wu HM. Circulating interleukin‐8 and tumor necrosis factor‐alpha are associated with hot flashes in healthy postmenopausal women. PLoS One. 2017;12:e0184011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Thurston RC, El Khoudary SR, Sutton‐Tyrrell K, Crandall CJ, Gold E, Sternfeld B, Selzer F, Matthews KA. Are vasomotor symptoms associated with alterations in hemostatic and inflammatory markers? Findings from the Study of Women's Health Across the Nation. Menopause. 2011;18:1044–1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thurston RC, Chang Y, Barinas‐Mitchell E, Jennings JR, Landsittel DP, Santoro N, von Kanel R, Matthews KA. Menopausal hot flashes and carotid intima media thickness among midlife women. Stroke. 2016;47:2910–2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ozkaya E, Cakir E, Kara F, Okuyan E, Cakir C, Ustun G, Kucukozkan T. Impact of hot flashes and night sweats on carotid intima‐media thickness and bone mineral density among postmenopausal women. Int J Gynaecol Obstet. 2011;113:235–238. [DOI] [PubMed] [Google Scholar]
- 24. Gast GC, Pop VJ, Samsioe GN, Grobbee DE, Nilsson PM, Keyzer JJ, Wijnands‐van Gent CJ, van der Schouw YT. Vasomotor menopausal symptoms are associated with increased risk of coronary heart disease. Menopause. 2011;18:146–151. [DOI] [PubMed] [Google Scholar]
- 25. Thurston RC, Johnson BD, Shufelt CL, Braunstein GD, Berga SL, Stanczyk FZ, Pepine CJ, Bittner V, Reis SE, Thompson DV, et al. Menopausal symptoms and cardiovascular disease mortality in the Women's Ischemia Syndrome Evaluation (WISE). Menopause. 2017;24:126–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szmuilowicz ED, Manson JE, Rossouw JE, Howard BV, Margolis KL, Greep NC, Brzyski RG, Stefanick ML, O'Sullivan MJ, Wu C, et al. Vasomotor symptoms and cardiovascular events in postmenopausal women. Menopause. 2011;18:603–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Herber‐Gast G, Brown WJ, Mishra GD. Hot flushes and night sweats are associated with coronary heart disease risk in midlife: a longitudinal study. BJOG. 2015;122:1560–1567. [DOI] [PubMed] [Google Scholar]
- 28. Sowers M, Crawford S, Sternfeld B, Morganstein D, Gold EB, Greendale GA, Evans D, Neer R, Matthews K, Sherman S, et al. SWAN: a multicenter, multiethnic, community‐based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, Lobo AR, eds. Menopause: Biology and Pathology. New York, NY: Academic Press; 2000:175–188. [Google Scholar]
- 29. Ainsworth BE, Sternfeld B, Richardson MT, Jackson K. Evaluation of the Kaiser physical activity survey in women. Med Sci Sports Exerc. 2000;32:1327–1338. [DOI] [PubMed] [Google Scholar]
- 30. Iowa Drug Information Service . IDIS Drug Vocabulary and Thesaurus Description. Coralville, Iowa: Division of Drug Information Service, College of Pharmacy, University of Iowa; 2012. [Google Scholar]
- 31. Steiner PM, Freidel J, Bremner WF, Stein EA. Standardization of micro‐methods for plasma cholesterol, triglyceride and hdl‐cholesterol with the lipid research clinics’ methodology. J Clin Chem Clin Biochem. 1981;19:850. [Google Scholar]
- 32. Warnick GR, Albers JJ. A comprehensive evaluation of the heparin‐manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 33. Siemens Healthcare Solutions Diagnostics . ADVIA 1800 chemistry system. Tarrytown, NY: Siemens Healthcare Solutions Diagnostics; 2015. [Google Scholar]
- 34. Baruch L, Agarwal S, Gupta B, Haynos A, Johnson S, Kelly‐Johnson K, Eng C. Is directly measured low‐density lipoprotein clinically equivalent to calculated low‐density lipoprotein? J Clin Lipidol. 2010;4:259–264. [DOI] [PubMed] [Google Scholar]
- 35. Friedewald W, Levy R, Fredrickson D. Estimation of the concentration of low density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 36. Siemens Medical Solutions Diagnostics . ADVIA Centaur XP Reference Manual. Tarrytown, NY: Siemens Medical Solutions Diagnostics. 2007. [Google Scholar]
- 37. El Kenz H, Bergmann P. Evaluation of immunochemiluminometric assays for the measurement of insulin and C‐peptide using the ADVIA Centaur. Clin Lab. 2004;50:171–174. [PubMed] [Google Scholar]
- 38. Matthews D, Hosker J, Rudenski A, Naylor B, Teacher D, Turner R. Homeostasis model assessment: insulin resistance and b cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 39. England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clin Chem. 2002;48:1584–1586. [PubMed] [Google Scholar]
- 40. Thurston RC, El Khoudary SR, Sutton‐Tyrrell K, Crandall CJ, Gold EB, Sternfeld B, Joffe H, Selzer F, Matthews KA. Vasomotor symptoms and lipid profiles in women transitioning through menopause. Obstet Gynecol. 2012;119:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Thurston RC, Chang Y, Barinas‐Mitchell E, Jennings JR, von Kanel R, Landsittel DP, Matthews KA. Physiologically assessed hot flashes and endothelial function among midlife women. Menopause. 2017;24:886–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Randolph JF Jr, Sowers M, Bondarenko I, Gold EB, Greendale GA, Bromberger JT, Brockwell SE, Matthews KA. The relationship of longitudinal change in reproductive hormones and vasomotor symptoms during the menopausal transition. J Clin Endocrinol Metab. 2005;90:6106–6112. [DOI] [PubMed] [Google Scholar]
- 43. Freedman RR. Menopausal hot flashes: mechanisms, endocrinology, treatment. J Steroid Biochem Mol Biol. 2014;142:115–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Rance NE, Dacks PA, Mittelman‐Smith MA, Romanovsky AA, Krajewski‐Hall SJ. Modulation of body temperature and lh secretion by hypothalamic kndy (kisspeptin, neurokinin b and dynorphin) neurons: a novel hypothesis on the mechanism of hot flushes. Front Neuroendocrinol. 2013;34:211–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Thurston RC, Sutton‐Tyrrell K, Everson‐Rose SA, Hess R, Powell LH, Matthews KA. Hot flashes and carotid intima media thickness among midlife women. Menopause. 2011;18:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gibson CJ, Thurston RC, Matthews KA. Cortisol dysregulation is associated with daily diary‐reported hot flashes among midlife women. Clin Endocrinol (Oxf). 2016;85:645–651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S4


