Abstract
Background
Soluble urokinase‐type plasminogen activator receptor (suPAR) is associated with cardiovascular risks and poor renal outcomes. However, whether elevated suPAR levels are associated with 24‐hour blood pressure patterns or kidney disease progression in patients with chronic kidney disease (CKD) is unclear.
Methods and Results
A total of 751 patients with CKD stage 1 to 5 were recruited from CMERC‐HI (Cardiovascular and Metabolic Disease Etiology Research Center–High Risk) cohort study (2013–2018). The relationship of serum suPAR levels to 24‐hour blood pressure parameters and CKD progression was analyzed. The median serum suPAR level was 1439.0 (interquartile range, 1026.2–2150.1) pg/mL, and the mean estimated glomerular filtration rate was 52.8±28.5 mL/min per 1.73 m2 at baseline. Patients with higher suPAR levels had significantly higher levels of office, 24‐hour, daytime, and nighttime systolic blood pressure and nighttime diastolic blood pressure than those with lower suPAR levels. The highest suPAR tertile was associated with an increased risk of a reverse dipping pattern (odds ratio, 2.93; 95% CI, 1.27–6.76; P=0.01). During a follow‐up of 43.2 (interquartile range, 27.0–55.6) months, the CKD progression occurred in 271 (36.1%) patients. The highest suPAR tertile was significantly associated with higher risk of CKD progression than the lowest tertile (hazard ratio [HR], 2.09; 95% CI, 1.37–3.21; P=0.001). When the relationship was reevaluated with respect to each dipping pattern (dipper, extreme dipper, nondipper, and reverse dipper), this association was consistent only in reverse dippers in whom the risk of CKD progression increased (HR, 1.43; 95% CI, 1.02–2.01; P=0.03) with every 1‐unit increase in serum suPAR levels.
Conclusions
Elevated suPAR levels are independently associated with CKD progression, and this association is prominent in reverse dippers.
Keywords: chronic kidney disease, dipping pattern, progression of kidney disease, soluble urokinase‐type plasminogen activator receptor
Subject Categories: High Blood Pressure, Hypertension, Complications
Nonstandard Abbreviations and Acronyms
- CMERC‐HI
Cardiovascular and Metabolic Disease Etiology Research Center–High Risk
- suPAR
soluble urokinase‐type plasminogen activator receptor
Clinical Perspective
What Is New?
In patients with chronic kidney disease, the elevated serum soluble urokinase‐type plasminogen activator receptor levels are associated with increased levels of office, 24‐hour, daytime, and nighttime systolic blood pressure and nighttime diastolic blood pressure and increased risk of a reverse dipping pattern.
The higher serum soluble urokinase‐type plasminogen activator receptor levels are significantly associated with higher risk of progression of chronic kidney disease.
The association of high soluble urokinase‐type plasminogen activator receptor levels and increased risk of chronic kidney disease progression is prominent in reverse dippers.
What Are the Clinical Implications?
These findings support the importance of serum soluble urokinase‐type plasminogen activator receptor levels, which can be used to predict the progression of kidney disease in patients with chronic kidney disease, especially in those with altered circadian blood pressure patterns.
Chronic kidney disease (CKD), defined as reduced kidney function or kidney damage, is one of the major public health concerns worldwide. 1 The prevalence of CKD affects >10% of the worldwide population. 2 , 3 Patients with CKD are at a high risk for adverse outcomes, including cardiovascular disease (CVD), end‐stage renal disease (ESRD), and death. 4 , 5 , 6 , 7 , 8 Approximately half of individuals with advanced CKD stages die because of CVD; thus, preventing the progression of CKD is crucial in this population. Despite the extensive resources devoted to CKD treatment, the incidence of progression to ESRD and the mortality risk remain high. Thus, it is important to identify the risk factors of kidney function decline in patients with CKD.
Soluble urokinase‐type plasminogen activator receptor (suPAR), a circulating form of a glycosyl‐phosphatidylinositol–anchored 3‐domain membrane protein, has been acknowledged to be associated with the pathogenesis of kidney disease, including focal segmental glomerulosclerosis and diabetic nephropathy, via interfering podocyte migration and apoptosis. 9 , 10 , 11 , 12 suPAR is expressed in a variety of cells, such as immune cells, endothelial cells, and podocytes. suPAR has also been reported to be involved in the pathogenesis of various inflammatory diseases, and elevated suPAR levels contribute to poor outcomes in diverse patient populations. 10 , 13 , 14 Individuals with high suPAR levels are at an increased risk for cardiovascular events, independent of traditional risk factors. 15 , 16 , 17 Recently, suPAR is suggested to be associated with kidney function decline in patients with CKD. 18 , 19 , 20 In addition, previous studies have found that elevated suPAR levels are significantly associated with endothelial dysfunction caused by chronic inflammation, which is the cornerstone of vascular complication or subclinical organ damage in both the general population and patients with CKD. 21 , 22
Although suPAR has been suggested to have a role in endothelial dysfunction and progression of CKD in patients with reduced kidney function, it is unclear whether suPAR levels, kidney function decline, and 24‐hour blood pressure (BP) patterns, which are the representatives of endothelial dysfunction, have a mutual interrelationship. Therefore, the objective of this study was to assess whether suPAR is useful for predicting CKD progression and to evaluate its association with 24‐hour BP patterns in patients with CKD.
METHODS
Study Population
Study subjects were recruited from the CMERC‐HI (Cardiovascular and Metabolic Disease Etiology Research Center–High Risk) cohort study (clinicaltrials.gov: NCT02003781). The detailed profiles and methods of how the CMERC‐HI study was designed have been previously described elsewhere and are summarized in Data S1. 23 The study cohort included 3270 subjects who met the inclusion criteria from December 2013 to June 2018. In this study, we selected patients with CKD stage 1 to 5 (defined by Kidney Disease Improving Global Outcomes guideline 24 ; presenting at least >3 months of proteinuria or estimated glomerular filtration rate [eGFR] <60 mL/min per 1.73 m2) and excluded those with ESRD requiring long‐term dialysis or those who had undergone kidney transplantation, those with inadequate samples, and those with missing ambulatory BP monitoring profiles or baseline and follow‐up creatinine data (Figure 1). Finally, 751 subjects were analyzed. All subjects voluntarily participated and provided informed consent. This study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the institutional review board of Yonsei University Health System Clinical Trial Center (4‐2013‐0581). The data that support the findings of this study are available from the corresponding author on reasonable request.
Figure 1. Study subjects.
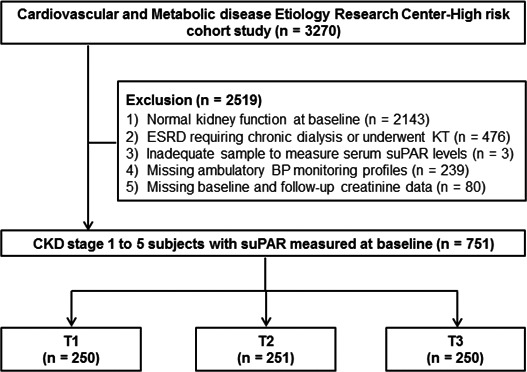
BP indicates blood pressure; CKD, chronic kidney disease; ESRD, end‐stage renal disease; KT, kidney transplantation; suPAR, soluble urokinase‐type plasminogen activator receptor; T1, tertile 1; T2, tertile 2; and T3, tertile 3.
Data Collection
Demographic and socioeconomic data, including age, sex, smoking status, alcohol intake, family history, and medical histories, were collected at enrollment. Anthropometric parameters, such as height, body weight, and body mass index, were measured by skilled evaluators. Smoking and alcohol history was assessed on the basis of the answer from survey questionnaires and classified into 2 groups: current or former versus never smoker or drinker. Hypertension was defined as a self‐reported hypertension history, antihypertensive medication use, or 24‐hour BP ≥130/80 mm Hg at the time of visit. Diabetes mellitus was defined as self‐reported diabetes mellitus history, antidiabetic medication use, fasting plasma glucose levels >126 mg/dL, or hemoglobin A1c ≥6.5%. CVD was defined as a composite of a medical history of coronary artery occlusive disease, ischemic heart disease, congestive heart failure, and cerebrovascular accident. The following biochemical data were collected by testing fasting blood samples: blood urea nitrogen, creatinine, hemoglobin, glucose, hemoglobin A1c, albumin, total cholesterol, triglyceride, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol, and CRP (C‐reactive protein). Serum creatinine level was determined using the rate‐blanked compensated Jaffe kinetic method with the Roche reagent CREA (Roche, Basel, Switzerland) and the Roche Calibrator for Automated Systems, which is traceable to an isotope dilution mass spectrometry reference method, in the Hitachi Automatic Analyzer (Hitachi, Tokyo, Japan). eGFR was calculated using the CKD–Epidemiology Collaboration equation. 25 Urine samples were collected in the morning after the first voiding. Fresh urine samples were analyzed using URISCAN Pro II (YD Diagnostics Corp, Seoul, Korea). Presence of albuminuria was assessed by urine albumin/creatinine ratio.
Measurement of Circulating suPAR Levels
Fasting venous blood samples were obtained, and serum and plasma were stored at −80°C on study enrollment. The serum levels of suPAR were measured with a commercially available ELISA kit (suPARnostic kit; ViroGates, Copenhagen, Denmark), which has a lower limit of detection of 100 pg/mL (according to the manufacturer, the intra‐assay variation is 2.75% and the interassay variation is 9.17%). The study subjects were classified into tertiles based on baseline serum suPAR levels.
BP Measurements
The 24‐hour BP measurements were obtained using the Takeda TM‐2430 instrument (A&D Medical, Tokyo, Japan), with readings taken every 30 minutes. Ambulatory BP monitoring was considered adequate if the monitor had been worn for a full 24‐hour period and if there was at least 70% of expected measurements with 20 acceptable readings between 9 am and 9 pm (daytime) and 7 acceptable readings between 1 am and 6 am (nighttime). 26 Ambulatory BP readings were averaged for the 24‐hour, daytime, and nighttime values. On the basis of nighttime fall in ambulatory systolic BP, patients were classified as dipper (nighttime BP fall, 10%–20%), extreme dipper (nighttime fall in BP, >20%), nondipper (nighttime BP fall, 0%–10%), and reverse dipper (nighttime fall in BP, <0%). 27 , 28
Office BP was obtained using a validated automatic device (HEM 7080‐IC; Omron, Kyoto, Japan), which was programmed to automatically measure the sitting BP of a person at 5, 7, and 9 minutes. 29 After positioning the participant in the sitting position and the right arm supported at heart level and setting the device, a trained nurse left the participants alone in the examination room. After a 5‐minute rest, automatic BP measurements at 2‐minute intervals were obtained in the examination room. After 3 measurements, a trained nurse recorded the BP data. The mean of the 3 BP readings was used as the office BP.
Study End Point
The study end point was the progression of CKD. The level of eGFR was assessed every 3 months, and median number of eGFR measurements was 11.7 (interquartile range, 5.0–15.0). CKD progression was defined as the composite outcome of the development of eGFR <60 mL/min per 1.73 m2 or a 30% decrease of eGFR from baseline in patients with CKD stage 1 to 2 and a 50% decrease of eGFR or progression to ESRD in those with CKD stage 3 to 5. The renal events were assessed by 2 researchers independently, and the occurrence of ESRD was decided with electronic medical records review.
Statistical Analysis
Continuous variables are expressed as mean±SD, and categorical variables are expressed as absolute numbers with percentages. All data were tested for normality before the statistical analysis. The Kolmogorov‐Smirnov test was performed to determine the distribution normality. Between‐group comparisons were performed using ANOVA or Student t test for continuous variables with a normal distribution, and the χ2 test or Fisher exact test for categorical variables. Data with a nonnormal distribution are presented as medians with interquartile ranges and compared using the Mann‐Whitney U test or Kruskal‐Wallis test. Logistic regression analysis was performed to identify the relationship between suPAR levels and 24‐hour BP patterns (dipping, extreme dipping, nondipping, and reverse dipping). Cumulative survival rates were estimated using Kaplan‐Meier analysis and the log‐rank test. Survival time was defined as the time from baseline to the onset of outcome or the last follow‐up. Subjects who were lost to follow‐up or death were censored at the last examination date. Cox proportional hazard models were constructed to determine the significant predictive values of suPAR for the study outcome. Variables that showed statistical significance in the univariable analyses or were considered to have clinical significance were included in the multivariable models, such as age, sex, smoking status, body mass index, history of hypertension, diabetes mellitus, or CVD, baseline levels of hemoglobin, total cholesterol, and CRP, albuminuria, and 24‐hour systolic BP. Results were expressed as hazard ratios and 95% CIs. To test the relationship between the risk of CKD progression and suPAR levels as a continuous variable, restricted cubic spline analyses using Cox proportional hazard model were conducted. Before investigating the interactive effect of kidney function on the impact of suPAR levels on outcomes, the multicollinearity between eGFR and serum suPAR levels was assessed on the basis of the variance inflation factor. 30 We found that the variance inflation factor was in the acceptable range (<2.0) to adjust eGFR for showing the effect of suPAR levels on the study outcome. To evaluate the effect of 24‐hour BP patterns on the association between suPAR levels and kidney function, the study subjects were classified according to the 24‐hour BP patterns (dipping, nondipping, and reverse dipping), and the predictive value of suPAR on CKD progression was analyzed. The mediation analysis was performed, and an interaction term was determined to investigate the effect of dipping patterns on the association between kidney function and suPAR levels. For all analyses, P<0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS software for Windows version 25.0 (IBM Corp, Armonk, NY) and R software version 3.3.1 (http://www.R‐project.org).
RESULTS
Baseline Characteristics
The baseline characteristics of the study subjects, according to the tertiles of baseline serum suPAR levels, are shown in Table 1. The mean age of the study subjects was 61.4±11.4 years, and 395 (52.6%) subjects were men. The median serum suPAR level was 1439.0 (interquartile range, 1026.2–2150.1) pg/mL, and the mean eGFR level was 52.8±28.5 mL/min per 1.73 m2 at baseline. The higher tertiles of suPAR showed significantly increased levels of office systolic BP, and the prevalence of diabetes mellitus was higher in tertiles with higher suPAR than in the lowest suPAR tertile. With respect to laboratory findings, the mean eGFR level was lower and the urine albumin/creatinine ratio was higher in tertiles with higher suPAR than in the lowest suPAR tertile. The levels of hemoglobin, serum albumin, and high‐density lipoprotein cholesterol were lower, and the CRP level was higher, in tertiles with higher suPAR than in the lowest suPAR tertile.
Table 1.
Baseline Characteristics, According to Tertiles of Serum suPAR Concentration
| Characteristic | Entire Cohort (n=751) | Baseline Serum suPAR Concentration, pg/mL | P Value | ||
|---|---|---|---|---|---|
| <1162 (n=250) | 1163–1863 (n=251) | ≥1874 (n=250) | |||
| Demographic data | |||||
| Age, y | 61.4±11.4 | 60.7±10.9 | 61.5±11.9 | 62.1±11.2 | 0.40 |
| Men | 395 (52.6) | 142 (56.8) | 131 (52.2) | 122 (48.8) | 0.07 |
| BMI, kg/m2 | 25.2±3.7 | 25.5±3.3 | 25.2±3.9 | 25.0±3.9 | 0.23 |
| Smoking status | 428 (57.0) | 145 (58.0) | 133 (53.0) | 150 (60.0) | 0.39 |
| Alcohol status | 272 (36.2) | 88 (35.2) | 83 (33.1) | 101 (40.4) | 0.17 |
| SBP, mm Hg | 130.1±18.2 | 125.5±14.6 | 129.0±17.4 | 135.9±20.5 | <0.001 |
| DBP, mm Hg | 75.6±11.7 | 74.9±9.3 | 76.3±10.8 | 75.5±11.7 | 0.33 |
| CKD stages | <0.001 | ||||
| I | 97 (12.9) | 74 (29.6) | 21 (8.4) | 02 (0.8) | |
| II | 180 (24.0) | 98 (39.2) | 57 (22.7) | 25 (10.0) | |
| IIIa | 161 (21.4) | 62 (24.8) | 68 (27.1) | 31 (12.4) | |
| IIIb | 116 (15.4) | 12 (4.8) | 59 (23.5) | 45 (18.0) | |
| IV | 130 (17.3) | 2 (0.8) | 40 (15.9) | 88 (35.2) | |
| V | 67 (8.9) | 2 (0.8) | 6 (2.4) | 59 (23.6) | |
| Comorbidities | |||||
| Hypertension | 669 (89.1) | 219 (87.6) | 222 (88.4) | 228 (91.2) | 0.20 |
| Diabetes mellitus | 363 (48.3) | 108 (43.2) | 105 (41.8) | 150 (60.0) | <0.001 |
| Dyslipidemia | 434 (57.8) | 157 (62.8) | 140 (55.8) | 137 (54.8) | 0.70 |
| CHF | 9 (1.2) | 3 (1.2) | 1 (0.4) | 5 (2.0) | 0.41 |
| CAD | 43 (5.7) | 21 (8.4) | 9 (3.6) | 13 (5.2) | 0.06 |
| CVA | 36 (4.8) | 13 (5.2) | 12 (4.8) | 11 (4.4) | 0.68 |
| Laboratory data | |||||
| eGFR, mL/min per 1.73 m2 | 52.8±28.5 | 75.1±23.6 | 52.2±22.7 | 31.1±19.9 | <0.001 |
| UACR, mg/g creatinine | 305.4 (63.2–953.7) | 96.0 (34.7–401.6) | 225.8 (63.8–793.0) | 774.9 (196.7–1928.8) | <0.001 |
| Hemoglobin, g/dL | 12.2±3.0 | 12.9±3.9 | 12.6±2.6 | 11.1±1.8 | <0.001 |
| Albumin, g/dL | 3.9±0.5 | 4.3±0.3 | 4.1±0.3 | 3.9±0.5 | <0.001 |
| Total cholesterol, mg/dL | 171.7±38.9 | 174.2±39.1 | 172.7±36.7 | 168.2±40.9 | 0.20 |
| LDL‐C, mg/dL | 84.1±39.5 | 82.7±39.2 | 85.1±37.7 | 84.6±41.6 | 0.78 |
| HDL‐C, mg/dL | 42.0±19.7 | 44.9±22.0 | 43.4±18.3 | 37.7±17.9 | <0.001 |
| Fasting glucose, mg/dL | 114.9±35.9 | 114.9±31.2 | 114.6±33.0 | 115.0±42.5 | 0.90 |
| HbA1c, % | 6.0±1.8 | 5.9±1.8 | 5.9±1.8 | 6.2±1.8 | 0.13 |
| CRP, mg/dL | 0.80 (0.50–1.60) | 0.70 (0.40–1.20) | 0.90 (0.50–1.70) | 0.90 (0.50–2.37) | 0.001 |
Data are presented as mean±SD, median (interquartile range), or number (percentage). CKD stages were defined on the basis of Kidney Disease: Improving Global Outcomes guidelines 2012. BMI indicates body mass index; CAD, coronary artery disease; CHF, congestive heart failure; CKD, chronic kidney disease; CRP, C‐reactive protein; CVA, cerebrovascular accident; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, hemoglobin A1c; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; suPAR, soluble urokinase‐type plasminogen activator receptor; and UACR, urine albumin/creatinine ratio.
Association of 24‐Hour BP Parameters With Serum suPAR Levels
We further evaluated the association between 24‐hour BP parameters and serum suPAR levels (Table 2). At baseline, tertiles with higher suPAR showed significantly higher levels of office, 24‐hour, daytime, and nighttime systolic BP than the lower suPAR tertiles. However, with respect to diastolic BP, only nighttime diastolic BP was significantly higher in patients in higher suPAR tertiles than in those in the lowest suPAR tertile. Furthermore, we classified the subjects according to 24‐hour BP patterns into dippers, extreme dippers, nondippers, and reverse dippers, and evaluated the association with serum suPAR levels. In patients with higher suPAR levels, the proportion of dippers was significantly lower (P=0.01) and that of reverse dippers was higher than in patients with the lowest suPAR level (P<0.001).
Table 2.
BP Parameters at 24 Hours, According to Tertiles of Serum suPAR Concentration
| Parameter | Entire Cohort (n=751) | Baseline Serum suPAR Concentration, pg/mL | P Value | ||
|---|---|---|---|---|---|
| <1162 (n=250) | 1163–1863 (n=251) | ≥1874 (n=250) | |||
| Office BP, mm Hg | |||||
| SBP | 130.1±18.2 | 125.5±14.6 | 129.0±17.4 | 135.9±20.5 | <0.001 |
| DBP | 75.6±11.7 | 74.9±9.3 | 76.3±10.8 | 75.5±11.7 | 0.33 |
| Pulse rate | 70.4±12.0 | 69.6±11.8 | 70.6±12.0 | 70.8±12.2 | 0.51 |
| Ambulatory BP, mm Hg | |||||
| 24‐h SBP | 131.3±15.1 | 127.8±13.3 | 129.7±14.4 | 136.4±16.3 | <0.001 |
| 24‐h DBP | 77.6±8.6 | 77.0±7.3 | 77.4±7.9 | 78.4±10.2 | 0.15 |
| Daytime SBP | 135.0±16.8 | 131.8±16.4 | 133.9±14.6 | 139.4±18.4 | <0.001 |
| Daytime DBP | 80.0±9.8 | 79.7±9.3 | 80.3±8.4 | 80.2±9.8 | 0.77 |
| Nighttime SBP | 123.8±18.6 | 119.1±16.8 | 122.5±16.2 | 129.8±20.8 | <0.001 |
| Nighttime DBP | 72.5±9.7 | 71.1±9.3 | 72.5±9.1 | 73.8±10.7 | 0.01 |
| Dipping patterns | |||||
| Dipper | 325 (43.3) | 120 (48.0) | 112 (44.6) | 93 (37.2) | 0.01 |
| Extreme dipper | 54 (7.2) | 23 (9.2) | 13 (5.2) | 18 (7.2) | 0.39 |
| Nondipper | 331 (44.1) | 107 (42.8) | 116 (46.2) | 108 (43.2) | 0.92 |
| Reverse dipper | 93 (12.4) | 22 (8.8) | 23 (9.2) | 48 (19.2) | <0.001 |
Data are presented as mean±SD or number (percentage). BP indicates blood pressure; DBP, diastolic BP; SBP, systolic BP; and suPAR, soluble urokinase‐type plasminogen activator receptor.
Finally, as the reverse dipping pattern was significantly associated with higher suPAR levels, we performed logistic regression analysis to further investigate the association of serum suPAR levels with dipping patterns (Table 3). In multivariable logistic regression analysis, the highest tertile of suPAR was associated with a 2.93‐fold increased risk of the reverse dipping pattern (95% CI, 1.27–6.76; P=0.01). This association was consistent when serum suPAR levels were treated as a continuous variable, in which each 1‐unit increase in log‐transformed baseline suPAR increased the risk of a reverse dipping pattern by 2.10‐fold (95% CI, 1.22–3.60; P=0.01).
Table 3.
Association Between Baseline Serum suPAR Concentration and Changes of 24‐Hour BP
| Variable | CKD Progression, % | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | ||
| Extreme dipping vs dipping | |||||||||
| suPAR* | 16.6 | 1.06 (0.66–1.72) | 0.80 | 1.06 (0.66–1.72) | 0.81 | 1.07 (0.66–1.75) | 0.78 | 1.45 (0.77–2.71) | 0.25 |
| Tertiles of suPAR | |||||||||
| Tertile 1 | 19.2 | Reference | |||||||
| Tertile 2 | 11.6 | 0.55 (0.26–1.16) | 0.16 | 0.54 (0.25–1.13) | 0.10 | 0.51 (0.24–1.09) | 0.08 | 0.67 (0.29–1.57) | 0.36 |
| Tertile 3 | 19.4 | 1.01 (0.51–2.01) | 0.90 | 1.00 (0.50–2.00) | 0.99 | 1.03 (0.51–2.10) | 0.91 | 1.71 (0.61–4.83) | 0.31 |
| Nondipping vs dipping | |||||||||
| suPAR* | 50.3 | 1.25 (0.95–1.63) | 0.11 | 1.33 (1.00–1.78) | 0.05 | 1.27 (0.97–1.67) | 0.08 | 1.47 (1.00–2.15) | 0.05 |
| Tertiles of suPAR | |||||||||
| Tertile 1 | 46.9 | Reference | |||||||
| Tertile 2 | 50.9 | 1.17 (0.81–1.69) | 0.40 | 1.19 (0.82–1.72) | 0.36 | 1.20 (0.83–1.74) | 0.33 | 1.28 (0.84–1.96) | 0.25 |
| Tertile 3 | 53.5 | 1.30 (0.89–1.90) | 0.18 | 1.30 (0.89–1.91) | 0.17 | 1.33 (0.90–1.96) | 0.15 | 1.65 (0.96–2.83) | 0.07 |
| Reverse dipping vs dipping | |||||||||
| suPAR* | 22.1 † | 2.14 (1.44–3.19) | <0.001 | 2.04 (1.35–3.09) | 0.001 | 2.10 (1.38–3.20) | 0.001 | 2.10 (1.22–3.60) | 0.01 |
| Tertiles of suPAR | |||||||||
| Tertile 1 | 15.4 ‡ | Reference | |||||||
| Tertile 2 | 17.0 ‡ | 1.13 (0.60–2.14) | 0.71 | 1.15 (0.60–2.18) | 0.68 | 1.20 (0.63–2.31) | 0.58 | 1.23 (0.60–2.52) | 0.58 |
| Tertile 3 | 33.8 ‡ | 2.81 (1.59–4.98) | <0.001 | 2.83 (1.59–5.05) | <0.001 | 2.98 (1.65–5.39) | <0.001 | 2.93 (1.27–6.76) | 0.01 |
Model 1: unadjusted. Model 2: adjusted for demographic factors (age, sex, smoking status, and body mass index). Model 3: adjusted for model 2+comorbidities (history of hypertension, diabetes mellitus, and cardiovascular disease). Model 4: adjusted for model 3+laboratory tests (hemoglobin, total cholesterol, CRP [C‐reactive protein], estimated glomerular filtration rate, and albuminuria) and mean daytime systolic BP. BP indicates blood pressure; CKD, chronic kidney disease; OR, odds ratio; and suPAR, soluble urokinase‐type plasminogen activator receptor.
OR per 1 increase of log‐transformed baseline suPAR.
P<0.001.
P for trend <0.001.
Association Between Serum suPAR Levels and CKD Progression
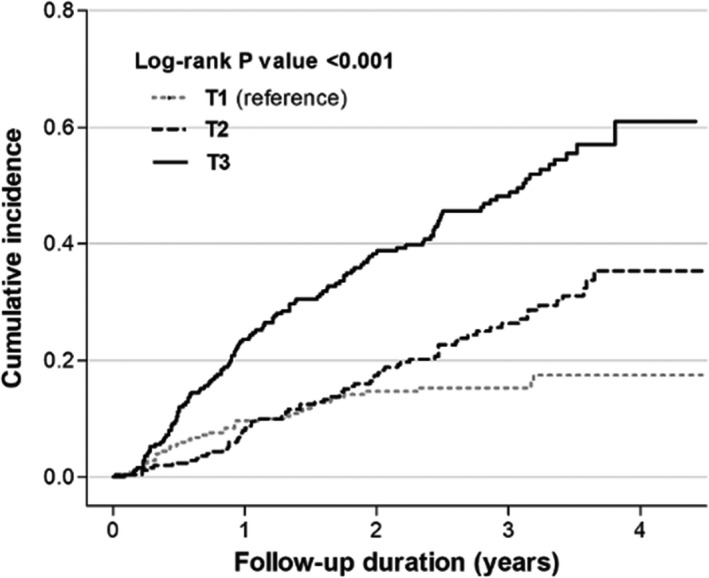
At baseline, negative correlation was observed between kidney function with eGFR as continuous variable and suPAR levels (β, −27.65; 95% CI, −30.28 to −25.01; P<0.001) (Table S1). During a median follow‐up of 43.2 (interquartile range, 27.0–55.6) months, progression of CKD occurred in 271 (36.1%) patients. The incidence of CKD progression was higher in patients in higher tertiles of suPAR than in those in the lowest suPAR tertile (15.2%, 27.1%, and 48.8% in the lowest, middle, and highest suPAR tertiles, respectively; P for trend <0.001) (Table 4). The Kaplan‐Meier curve showed that the highest tertile of suPAR was significantly associated with an increased risk of CKD progression (P<0.001; Figure 2). Furthermore, multivariable Cox proportional hazard analyses were performed to assess the association between serum suPAR levels and CKD progression. The highest tertile of suPAR was significantly associated with a 2.09‐fold increased risk of CKD progression compared with the lowest tertile (95% CI, 1.37–3.21; P=0.001). This association was consistent when the serum suPAR level was treated as a continuous variable in which each 1‐unit increase of log‐transformed serum suPAR level increased the risk of CKD progression by 1.29‐fold (95% CI, 1.02–1.62; P=0.03) (Table 4).
Table 4.
Association Between Baseline Serum suPAR Concentration and CKD Progression
| Variable | CKD Progression, %* | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | ||
| suPAR † | 36.1 | 1.73 (1.48–2.03) | <0.001 | 1.80 (1.54–2.12) | <0.001 | 1.77 (1.50–2.08) | <0.001 | 1.29 (1.02–1.62) | 0.03 |
| Tertile of suPAR | |||||||||
| Tertile 1 | 15.2 | Reference | |||||||
| Tertile 2 | 27.1 | 1.58 (1.12–2.24) | 0.01 | 1.62 (1.14–2.29) | 0.01 | 1.58 (1.12–2.24) | 0.01 | 1.30 (0.89–1.90) | 0.18 |
| Tertile 3 | 48.8 | 3.13 (2.27–4.32) | <0.001 | 3.34 (2.41–4.63) | <0.001 | 3.25 (2.34–4.52) | <0.001 | 2.09 (1.37–3.21) | 0.001 |
Model 1: unadjusted. Model 2: adjusted for demographic factors (age, sex, smoking status, and body mass index). Model 3: adjusted for model 2+comorbidities (history of hypertension, diabetes mellitus, and cardiovascular disease). Model 4: adjusted for model 3+laboratory tests (hemoglobin, total cholesterol, and CRP [C‐reactive protein]), kidney measures (estimated glomerular filtration rate and albuminuria), and 24‐hour systolic blood pressure. CKD indicates chronic kidney disease; HR, hazard ratio; and suPAR, soluble urokinase‐type plasminogen activator receptor.
P for trend <0.001.
HR per 1 increase of log‐transformed baseline suPAR.
Figure 2. Kaplan‐Meier curve of the risk for chronic kidney disease (CKD) progression, according to tertiles of soluble urokinase‐type plasminogen activator receptor levels.
Effect of Dipping Pattern on the Association Between suPAR Levels and CKD Progression
As suPAR has a role in endothelial dysfunction and CKD progression in patients with reduced kidney function, and we have confirmed that higher serum suPAR levels were associated with an increased risk for a reverse dipping pattern, we assessed whether suPAR is useful for predicting CKD progression in association with 24‐hour BP patterns. First, we performed mediation analysis to investigate the effect of dipping pattern (dipping, nondipping, and reverse dipping) on the association between CKD progression and suPAR levels; outcome variable for the analysis was CKD progression, predictor variable was suPAR levels, and mediator variable was dipping pattern. The indirect effect of suPAR on CKD progression was statistically significant (β, 0.18; P<0.001). However, the effect of suPAR levels on progression of CKD completely disappeared after adding dipping pattern as mediator (β, 0.06; P=0.07), suggesting that dipping pattern mediates between suPAR levels and kidney function decline.
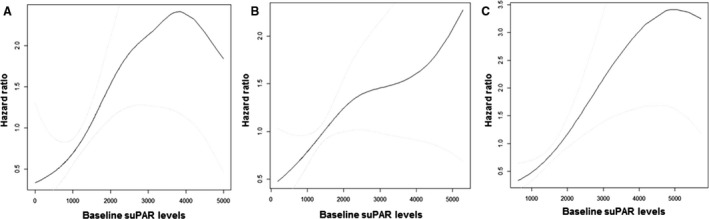
When the subjects were divided according to the dipping pattern, the association between higher suPAR levels and increased risk for CKD progression was consistent only in reverse dippers in whom the risk of CKD progression was increased by 1.43‐fold (95% CI, 1.02–2.01; P=0.03) with every 1‐unit increase in log‐transformed serum suPAR levels (P for interaction=0.004) (Figure 3).
Figure 3. Cubic spline curves for risk of chronic kidney disease progression, according to baseline serum soluble urokinase‐type plasminogen activator receptor (suPAR) concentration in dipper (A), nondipper (B), and reverse dipper (C).
DISCUSSION
In this prospective cohort of patients with CKD, we identified a mutual interrelationship between elevated serum suPAR levels, reverse dipping BP pattern, and CKD progression. In particular, higher serum suPAR levels were associated with an increased risk of CKD progression, and this association was more prominent in reverse dippers.
CKD is a public health problem that affects 10% of adults in the Western world and confers a high risk of complications, such as the development of ESRD and cardiovascular events. 2 , 6 , 31 Hypertension is a major risk factor for the development and progression of CKD. 32 In contrast, CKD is both a common cause and a sequel of uncontrolled hypertension. 32 Uncontrolled BP consequently contributes to poor outcome in patients with CKD. 33 , 34 In addition, patients with CKD show alteration in the circadian BP pattern, primarily a reduced or absent nighttime decrease in BP levels (nondipper or reverse dipper), which contributes to target organ damage and major cardiovascular events. 35 The pathophysiological features underlying the altered circadian BP patterns in patients with CKD are multifactorial and remain incompletely understood. Several factors or mechanisms, including increased sodium and fluid volume retention, impaired baroreceptor sensitivity, altered sympathetic nervous system activity, activation of the renin‐angiotensin system, endothelial dysfunction, oxidative stress, chronic inflammation, and increased arterial stiffness, have been proposed to explain the altered BP circadian rhythm in patients with CKD. 36 Among these factors, endothelial dysfunction is a common event in the development of hypertension. 37 In all stages of CKD, decreased kidney function is closely associated with abnormal circadian BP variation and a nondipping pattern. 38 , 39 In patients with hypertension, nocturnal BP is elevated and endothelial function deteriorates in parallel with decreasing kidney function. 40 , 41
suPAR is a novel marker of chronic inflammation and endothelial dysfunction. 14 It is expressed in various cells, including immune cells, endothelial cells, and podocytes. 9 , 42 The circulating and membrane‐bound forms play a direct role in regulating cell adhesion or migration, and are also involved in inflammatory processes and endothelial damage in tissues. 43 Chronic inflammation and endothelial dysfunction are the cornerstone of the development of CVD; thus, suPAR is a good prognostic marker for the development of CVD. 15 , 16 , 17 In the kidneys, suPAR regulates the permeability of the glomerular filtration barrier, and several lines of evidence about pathogenic role of suPAR in kidney disease have been mainly found in studies of focal segmental glomerulosclerosis, in which suPAR was revealed to activate integrin in podocytes, leading to foot process effacements and proteinuria. 11 Elevated suPAR levels have also been implicated in the pathogenesis of diabetic nephropathy. 44 In animal models of diabetic kidney disease, blockade of integrin was protective against kidney function decline. 45 Moreover, suPAR mediates kidney injury via several molecular mechanisms. suPAR interacts with a variety of molecules, inducing podocyte dysfunction, and mediates the development or progression of CKD. 18 , 20 , 46 Recently, Hayek et al reported that elevated suPAR levels in 3683 subjects with normal kidney function were independently associated with incident CKD and an accelerated decline in eGFR. 20 Moreover, Lv et al showed that elevated suPAR levels were independently associated with an increased risk of progression to ESRD in Chinese patients with CKD. 18
In the present study, in line with previous studies, patients with elevated suPAR levels showed a significantly increased risk of CKD progression, regardless of their baseline kidney function. Interestingly, this association was more prominent in patients showing a reverse dipping pattern. A reverse dipping pattern or increased nighttime BP and elevated suPAR levels both imply chronic inflammation and endothelial dysfunction. Patients with a reverse dipping pattern and elevated suPAR levels are at high risk of target organ damage, such as kidney injuries and destruction of vascular structures. 47 , 48 Thus, patients with both increased nighttime BP and elevated suPAR levels are prone to glomerular vasculature destruction and podocyte injuries, which eventually lead to progression of CKD. Consequently, our findings provide additional insights into the predictive role of suPAR in kidney disease by showing that altered BP patterns in patients with CKD are involved in the association between elevated suPAR levels and deterioration of kidney function.
This study had some limitations. First, serum suPAR levels may have been affected by kidney function. A previous study demonstrated a negative correlation between suPAR and eGFR levels, in which high serum suPAR levels were associated with decreased eGFR levels. 49 However, Hayek et al 46 recently reported that the negative correlation between suPAR level and baseline eGFR was weak in subjects with eGFR >90 mL/min per 1.73 m2. Moreover, they showed that a considerable proportion of participants (>30%) with normal kidney function showed elevated suPAR levels in the absence of any sepsis or cancer. Therefore, high suPAR levels are unlikely to be the consequence of decreased kidney function. In the present study, subjects with elevated suPAR levels showed decreased baseline eGFR, as observed in previous studies. Nevertheless, we adjusted the baseline eGFR levels in the prediction models and further investigated the interactive effect of kidney function on the impact of suPAR levels on outcomes. The multicollinearity, assessed using the variance inflation factor between baseline eGFR and serum suPAR levels, was acceptable in our study. Thus, we inferred that elevated serum suPAR levels are unlikely to be a simple result of decreased kidney function and may be related to the underlying pathogenic mechanism in the progression of kidney disease. Second, the study cohort was relatively small and involved a single ethnicity. Therefore, there is a limit to the generalizability of the results. Third, because of the observational study design, a clear causality between serum suPAR levels and CKD progression cannot be deduced. Finally, a single measurement of baseline suPAR level was used. Further studies with serial measurements of suPAR levels, taking into account the changes over time, are needed.
In conclusion, we identified a mutual interrelationship between elevated serum suPAR levels, a reverse dipping BP pattern, and progression of CKD. In particular, a high serum suPAR level was associated with an increased risk of CKD progression, and this association was more prominent in reverse dippers in this study. Elevated suPAR levels can be used to predict the progression of kidney disease in patients with CKD, especially in those with altered circadian BP patterns. Future studies are needed to confirm whether an elevated suPAR level serves as early treatment and intervention in patients with CKD.
Sources of Funding
This research was supported by a grant from the Korean Health Technology Research and Development (R&D) Project through the Korea Health Industry Development Institute, funded by the Ministry of Health and Welfare, Republic of Korea (grant HI13C0715; Dr S. Park) and a research grant from the Korean Centers for Disease Control and Prevention (grant 2018ER630202; Dr S. Park). Also, this study was supported by a faculty research grant of Yonsei University College of Medicine (6‐2019‐0170 and 6‐2014‐0114) and research grant from the Korean Society of Nephrology (Young Investigator Grant, 2016)..
Disclosures
None.
Supporting information
Data S1
Table S1
(J Am Heart Assoc. 2021;10:e017225. DOI: 10.1161/JAHA.120.017225.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017225
For Sources of Funding and Disclosures, see page 10.
Footnotes
Note: Progression of CKD was defined as development of estimated glomerular filtration rate (eGFR) <60 mL/min per 1.73 m2 or 30% decrease of eGFR than baseline in patients with CKD stage 1 to 2 and 50% decrease of eGFR or progression to end‐stage renal disease in those with CKD stage 3 to 5. T1 indicates tertile 1; T2, tertile 2; and T3, tertile 3.
Notes: Hazard ratio per 1 increase of log‐transformed baseline suPAR (P for interaction=0.004) and models were adjusted for demographic factors (age, sex, smoking status, and body mass index), comorbidities (history of hypertension, diabetes mellitus, and cardiovascular disease), laboratory tests (hemoglobin, total cholesterol, CRP [C‐reactive protein], and baseline estimated glomerular filtration rate), and 24‐hour systolic blood pressure.
Contributor Information
Sungha Park, Email: shpark0530@yuhs.ac.
Tae‐Hyun Yoo, Email: yoosy0316@yuhs.ac.
REFERENCES
- 1. Jha V,Garcia‐Garcia G,Iseki K,Li Z,Naicker S,Plattner B,Saran R,Wang AY,Yang CW. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382:260–272. DOI: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 2. Murphy D,McCulloch CE,Lin F,Banerjee T,Bragg‐Gresham JL,Eberhardt MS,Morgenstern H,Pavkov ME,Saran R,Powe NR, et al. Trends in prevalence of chronic kidney disease in the United States. Ann Intern Med. 2016;165:473–481. DOI: 10.7326/M16-0273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Coresh J,Selvin E,Stevens LA,Manzi J,Kusek JW,Eggers P,Van Lente F,Levey AS. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. DOI: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 4. van der Velde M,Matsushita K,Coresh J,Astor BC,Woodward M,Levey AS,de Jong PE,Gansevoort RT. Lower estimated glomerular filtration rate and higher albuminuria are associated with all‐cause and cardiovascular mortality: a collaborative meta‐analysis of high‐risk population cohorts. Kidney Int. 2011;79:1341–1352. DOI: 10.1038/ki.2010.536. [DOI] [PubMed] [Google Scholar]
- 5. Astor BC,Matsushita K,Gansevoort RT,van der Velde M,Woodward M,Levey AS,Jong PE,Coresh J,Astor BC,Matsushita K, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end‐stage renal disease: a collaborative meta‐analysis of kidney disease population cohorts. Kidney Int. 2011;79:1331–1340. DOI: 10.1038/ki.2010.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gansevoort RT,Correa‐Rotter R,Hemmelgarn BR,Jafar TH,Heerspink HJ,Mann JF,Matsushita K,Wen CP. Chronic kidney disease and cardiovascular risk: epidemiology, mechanisms, and prevention. Lancet. 2013;382:339–352. DOI: 10.1016/S0140-6736(13)60595-4. [DOI] [PubMed] [Google Scholar]
- 7. Grams ME,Yang W,Rebholz CM,Wang X,Porter AC,Inker LA,Horwitz E,Sondheimer JH,Hamm LL,He J, et al. Risks of adverse events in advanced CKD: the chronic renal insufficiency cohort (CRIC) study. Am J Kidney Dis. 2017;70:337–346. DOI: 10.1053/j.ajkd.2017.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim KM,Oh HJ,Choi HY,Lee H,Ryu D‐R. Impact of chronic kidney disease on mortality: a nationwide cohort study. Kidney Res Clin Pract. 2019;38:382–390. DOI: 10.23876/j.krcp.18.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wei C,Möller CC,Altintas MM,Li J,Schwarz K,Zacchigna S,Xie L,Henger A,Schmid H,Rastaldi MP, et al. Modification of kidney barrier function by the urokinase receptor. Nat Med. 2008;14:55–63. DOI: 10.1038/nm1696. [DOI] [PubMed] [Google Scholar]
- 10. Yoo T‐H,Pedigo CE,Guzman J,Correa‐Medina M,Wei C,Villarreal R,Mitrofanova A,Leclercq F,Faul C,Li J, et al. Sphingomyelinase‐like phosphodiesterase 3b expression levels determine podocyte injury phenotypes in glomerular disease. J Am Soc Nephrol. 2015;26:133–147. DOI: 10.1681/ASN.2013111213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei C,El Hindi S,Li J,Fornoni A,Goes N,Sageshima J,Maiguel D,Karumanchi SA,Yap H‐K,Saleem M, et al. Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med. 2011;17:952–960. DOI: 10.1038/nm.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wei C,Trachtman H,Li J,Dong C,Friedman AL,Gassman JJ,McMahan JL,Radeva M,Heil KM,Trautmann A, et al. Circulating suPAR in two cohorts of primary FSGS. J Am Soc Nephrol. 2012;23:2051–2059. DOI: 10.1681/ASN.2012030302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Backes Y,van der Sluijs KF,Mackie DP,Tacke F,Koch A,Tenhunen JJ,Schultz MJ. Usefulness of suPAR as a biological marker in patients with systemic inflammation or infection: a systematic review. Intensive Care Med. 2012;38:1418–1428. DOI: 10.1007/s00134-012-2613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Eugen‐Olsen J,Andersen O,Linneberg A,Ladelund S,Hansen TW,Langkilde A,Petersen J,Pielak T,Møller LN,Jeppesen J, et al. Circulating soluble urokinase plasminogen activator receptor predicts cancer, cardiovascular disease, diabetes and mortality in the general population. J Intern Med. 2010;268:296–308. DOI: 10.1111/j.1365-2796.2010.02252.x. [DOI] [PubMed] [Google Scholar]
- 15. Lyngbæk S,Marott JL,Sehestedt T,Hansen TW,Olsen MH,Andersen O,Linneberg A,Haugaard SB,Eugen‐Olsen J,Hansen PR, et al. Cardiovascular risk prediction in the general population with use of suPAR, CRP, and Framingham Risk Score. Int J Cardiol. 2013;167:2904–2911. DOI: 10.1016/j.ijcard.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 16. Persson M,Engstrom G,Bjorkbacka H,Hedblad B. Soluble urokinase plasminogen activator receptor in plasma is associated with incidence of CVD: results from the Malmo Diet and Cancer Study. Atherosclerosis. 2012;220:502–505. [DOI] [PubMed] [Google Scholar]
- 17. Lyngbaek S,Marott JL,Moller DV,Christiansen M,Iversen KK,Clemmensen PM,Eugen‐Olsen J,Jeppesen JL,Hansen PR. Usefulness of soluble urokinase plasminogen activator receptor to predict repeat myocardial infarction and mortality in patients with ST‐segment elevation myocardial infarction undergoing primary percutaneous intervention. Am J Cardiol. 2012;110:1756–1763. DOI: 10.1016/j.amjcard.2012.08.008. [DOI] [PubMed] [Google Scholar]
- 18. Lv LI,Wang F,Wu L,Wang J‐W,Cui Z,Hayek SS,Wei C,Reiser J,He K,Zhang L, et al. Soluble urokinase‐type plasminogen activator receptor and incident end‐stage renal disease in Chinese patients with chronic kidney disease. Nephrol Dial Transplant. 2020;35:465–470. DOI: 10.1093/ndt/gfy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo S,Coresh J,Tin A,Rebholz CM,Chen TK,Hayek SS,Tracy M,Lipkowitz MS,Appel LJ,Levey AS, et al. Soluble urokinase‐type plasminogen activator receptor in black Americans with CKD. Clin J Am Soc Nephrol. 2018;13:1013–1021. DOI: 10.2215/CJN.13631217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hayek SS,Sever S,Ko Y‐A,Trachtman H,Awad M,Wadhwani S,Altintas MM,Wei C,Hotton AL,French AL, et al. Soluble urokinase receptor and chronic kidney disease. N Engl J Med. 2015;373:1916–1925. DOI: 10.1056/NEJMoa1506362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sherif EM,El Maksood AAA,Youssef OI,Salah El‐Din NY,Khater OKM. Soluble urokinase plasminogen activator receptor in type 1 diabetic children, relation to vascular complications. J Diabetes Complications. 2019;33:628–633. DOI: 10.1016/j.jdiacomp.2019.06.001. [DOI] [PubMed] [Google Scholar]
- 22. Sehestedt T,Lyngbaek S,Eugen‐Olsen J,Jeppesen J,Andersen O,Hansen TW,Linneberg A,Jorgensen T,Haugaard SB,Olsen MH. Soluble urokinase plasminogen activator receptor is associated with subclinical organ damage and cardiovascular events. Atherosclerosis. 2011;216:237–243. DOI: 10.1016/j.atherosclerosis.2011.01.049. [DOI] [PubMed] [Google Scholar]
- 23. Shim J‐S,Song BM,Lee JH,Lee SW,Park JH,Choi DP,Lee MH,Ha KH,Kim DJ,Park S, et al. Cardiovascular and metabolic diseases etiology research center (CMERC) cohort: study protocol and results of the first 3 years of enrollment. Epidemiol Health. 2017;39:e2017016. doi: 10.4178/epih.e2017016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Group Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. [Google Scholar]
- 25. Levey AS,Stevens LA,Schmid CH,Zhang YL,Castro AF,Feldman HI,Kusek JW,Eggers P,Van Lente F,Greene T, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. DOI: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. O’Brien E,Parati G,Stergiou G,Asmar R,Beilin L,Bilo G,Clement D,de la Sierra A,de Leeuw P,Dolan E, et al. European Society of Hypertension position paper on ambulatory blood pressure monitoring. J Hypertens. 2013;31:1731–1768. DOI: 10.1097/HJH.0b013e328363e964. [DOI] [PubMed] [Google Scholar]
- 27. Lurbe E,Redon J,Kesani A,Pascual JM,Tacons J,Alvarez V,Batlle D. Increase in nocturnal blood pressure and progression to microalbuminuria in type 1 diabetes. N Engl J Med. 2002;347:797–805. DOI: 10.1056/NEJMoa013410. [DOI] [PubMed] [Google Scholar]
- 28. Parati G,Stergiou G,O’Brien E,Asmar R,Beilin L,Bilo G,Clement D,de la Sierra A,de Leeuw P,Dolan E, et al. European Society of Hypertension practice guidelines for ambulatory blood pressure monitoring. J Hypertens. 2014;32:1359–1366. DOI: 10.1097/HJH.0000000000000221. [DOI] [PubMed] [Google Scholar]
- 29. El Assaad MA,Topouchian JA,Asmar RG. Evaluation of two devices for self‐measurement of blood pressure according to the international protocol: the Omron M5‐I and the Omron 705IT. Blood Press Monit. 2003;8:127–133. doi: 10.1097/00126097-200306000-00006. [DOI] [PubMed] [Google Scholar]
- 30. Hocking RR. Methods and Applications of Linear Models; Regression and the Analysis of Variance. 2nd ed. New Jersey, U.S.A.: John Wiley and Sons; 2003. [Google Scholar]
- 31. Lee C,Yun H‐R,Joo YS,Lee S,Kim J,Nam KH,Jhee JH,Park JT,Yoo T‐H,Kang S‐W, et al. Framingham risk score and risk of incident chronic kidney disease: a community‐based prospective cohort study. Kidney Res Clin Pract. 2019;38:49–59. DOI: 10.23876/j.krcp.18.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hamrahian SM. Management of hypertension in patients with chronic kidney disease. Curr Hypertens Rep. 2017;19:43. doi: 10.1007/s11906-017-0739-9. [DOI] [PubMed] [Google Scholar]
- 33. Aggarwal R,Petrie B,Bala W,Chiu N. Mortality outcomes with intensive blood pressure targets in chronic kidney disease patients. Hypertension. 2019;73:1275–1282. DOI: 10.1161/HYPERTENSIONAHA.119.12697. [DOI] [PubMed] [Google Scholar]
- 34. Malhotra R,Nguyen HA,Benavente O,Mete M,Howard BV,Mant J,Odden MC,Peralta CA,Cheung AK,Nadkarni GN, et al. Association between more intensive vs less intensive blood pressure lowering and risk of mortality in chronic kidney disease stages 3 to 5: a systematic review and meta‐analysis. JAMA Intern Med. 2017;177:1498–1505. DOI: 10.1001/jamainternmed.2017.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Di Daniele N,Fegatelli DA,Rovella V,Castagnola V,Gabriele M,Scuteri A. Circadian blood pressure patterns and blood pressure control in patients with chronic kidney disease. Atherosclerosis. 2017;267:139–145. DOI: 10.1016/j.atherosclerosis.2017.10.031. [DOI] [PubMed] [Google Scholar]
- 36. Velasquez MT,Beddhu S,Nobakht E,Rahman M,Raj DS. Ambulatory blood pressure in chronic kidney disease: ready for prime time? Kidney Int Rep. 2016;1:94–104. DOI: 10.1016/j.ekir.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Feletou M,Vanhoutte PM. Endothelial dysfunction: a multifaceted disorder (the Wiggers Award Lecture). Am J Physiol Heart Circ Physiol. 2006;291:H985–H1002. DOI: 10.1152/ajpheart.00292.2006. [DOI] [PubMed] [Google Scholar]
- 38. Bolton CH,Downs LG,Victory JG,Dwight JF,Tomson CR,Mackness MI,Pinkney JH. Endothelial dysfunction in chronic renal failure: roles of lipoprotein oxidation and pro‐inflammatory cytokines. Nephrol Dial Transplant. 2001;16:1189–1197. DOI: 10.1093/ndt/16.6.1189. [DOI] [PubMed] [Google Scholar]
- 39. Perticone F,Maio R,Perticone M,Sciacqua A,Shehaj E,Naccarato P,Sesti G. Endothelial dysfunction and subsequent decline in glomerular filtration rate in hypertensive patients. Circulation. 2010;122:379–384. DOI: 10.1161/CIRCULATIONAHA.110.940932. [DOI] [PubMed] [Google Scholar]
- 40. Yamamoto K,Takeda Y,Yamashita S,Sugiura T,Wakamatsu Y,Fukuda M,Ohte N,Dohi Y,Kimura G. Renal dysfunction impairs circadian variation of endothelial function in patients with essential hypertension. J Am Soc Hypertens. 2010;4:265–271. DOI: 10.1016/j.jash.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 41. Zoccali C. Endothelial dysfunction and the kidney: emerging risk factors for renal insufficiency and cardiovascular outcomes in essential hypertension. J Am Soc Nephrol. 2006;17:S61–S63. [DOI] [PubMed] [Google Scholar]
- 42. Huai Q,Mazar AP,Kuo A,Parry GC,Shaw DE,Callahan J,Li Y,Yuan C,Bian C,Chen L, et al. Structure of human urokinase plasminogen activator in complex with its receptor. Science. 2006;311:656–659. [DOI] [PubMed] [Google Scholar]
- 43. Thuno M,Macho B,Eugen‐Olsen J. suPAR: the molecular crystal ball. Dis Markers. 2009;27:157–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Theilade S,Lyngbaek S,Hansen TW,Eugen‐Olsen J,Fenger M,Rossing P,Jeppesen JL. Soluble urokinase plasminogen activator receptor levels are elevated and associated with complications in patients with type 1 diabetes. J Intern Med. 2015;277:362–371. [DOI] [PubMed] [Google Scholar]
- 45. Maile LA,Busby WH,Gollahon KA,Flowers W,Garbacik N,Garbacik S,Stewart K,Nichols T,Bellinger D,Patel A, et al. Blocking ligand occupancy of the alphavbeta3 integrin inhibits the development of nephropathy in diabetic pigs. Endocrinology. 2014;155:4665–4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Hayek SS,Landsittel DP,Wei C,Zeier M,Yu ASL,Torres VE,Roth S,Pao CS,Reiser J. Soluble urokinase plasminogen activator receptor and decline in kidney function in autosomal dominant polycystic kidney disease. J Am Soc Nephrol. 2019;30:1305–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wang C,Zhang J,Deng W,Gong W,Liu X,Ye Z,Peng H,Lou T. Nighttime systolic blood‐pressure load is correlated with target‐organ damage independent of ambulatory blood‐pressure level in patients with non‐diabetic chronic kidney disease. PLoS One. 2015;10:e0131546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang C,Zhang J,Liu X,Li C,Ye Z,Peng H,Chen Z,Lou T. Reversed dipper blood‐pressure pattern is closely related to severe renal and cardiovascular damage in patients with chronic kidney disease. PLoS One. 2013;8:e55419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Spinale JM,Mariani LH,Kapoor S,Zhang J,Weyant R,Song PX,Wong HN,Troost JP,Gadegbeku CA,Gipson DS, et al. A reassessment of soluble urokinase‐type plasminogen activator receptor in glomerular disease. Kidney Int. 2015;87:564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1


