Abstract
Background
Myocardial fibrosis is an important contributor for development of diastolic dysfunction. We investigated the impact of sirolimus as primary immunosuppression on diastolic dysfunction and fibrosis progression among heart transplantation recipients.
Methods and Results
In 100 heart transplantation recipients who were either treated with a calcineurin inhibitor (CNI) (n=51) or converted from CNI to sirolimus (n=49), diastolic function parameters were assessed using serial echocardiograms and right heart catheterizations. Myocardial fibrosis was quantified on serial myocardial biopsies. After 3 years, lateral e′ increased within the sirolimus group but decreased in the CNI group (0.02±0.04 versus −0.02±0.04 m/s delta change; P=0.003, respectively). Both pulmonary capillary wedge pressure and diastolic pulmonary artery pressure significantly decreased in the sirolimus group but remained unchanged in the CNI group (−1.50±2.59 versus 0.20±2.20 mm Hg/year; P=0.02; and −1.72±3.39 versus 0.82±2.59 mm Hg/year; P=0.005, respectively). A trend for increased percentage of fibrosis was seen in the sirolimus group (8.48±3.17 to 10.10±3.0%; P=0.07) as compared with marginally significant progression in the CNI group (8.76±3.87 to 10.56±4.34%; P=0.04). The percent change in fibrosis did not differ significantly between the groups (1.62±4.67 versus 1.80±5.31%, respectively; P=0.88).
Conclusions
Early conversion to sirolimus is associated with improvement in diastolic dysfunction and filling pressures as compared with CNI therapy. Whether this could be attributed to attenuation of myocardial fibrosis progression with sirolimus treatment warrants further investigation.
Keywords: diastolic dysfunction, myocardial fibrosis, sirolimus
Subject Categories: Heart Failure, Transplantation
Nonstandard Abbreviations and Acronyms
- CNI
calcineurin inhibitor
- DD
diastolic dysfunction
- DPAP
diastolic pulmonary artery pressure
- EMB
endomyocardial biopsy
- HT
heart transplantation
- ICM
ischemic cardiomyopathy
- LVEDD
left ventricular end diastolic diameter
- LVESD
left ventricular end‐systolic diameter
- LVM
left ventricular mass
- mTOR
mammalian target of rapamycin
- RHC
right heart catheterization
Clinical Perspective
What Is New?
The present study demonstrates that among heart transplant recipients, conversion to sirolimus as primary immunosuppression, with complete withdrawal of calcineurin inhibitor therapy, was associated independently with improvement in diastolic dysfunction measures, including (1) significant increase in e′ but decrease in E/e′ using serial echocardiographic measurements and (2) significant decrease in pulmonary capillary wedge pressure and diastolic pulmonary artery pressure using invasive hemodynamic parameters obtained from serial right heart catheterizations.
A trend for more attenuated myocardial fibrosis progression was observed among heart transplant recipients converted to sirolimus as compared with continued calcineurin inhibitor therapy.
What Are the Clinical Implications?
Among heart transplant recipients, early conversion to sirolimus is associated with improvement in diastolic dysfunction and filling pressures as compared with calcineurin inhibitor therapy.
Whether this could be attributed to attenuation of myocardial fibrosis progression with sirolimus treatment warrants further investigation.
Heart transplantation (HT) is currently the definitive treatment that offers better survival and quality of life for patients with end‐stage heart failure. Advances in immunosuppressive therapy have contributed significantly to further improvement in survival following HT. 1 Diastolic dysfunction (DD) is a well‐recognized complication after HT. 2 , 3 , 4 , 5 Previous reports have demonstrated a decreasing incidence of DD over time, suggesting that after the first few weeks, the restrictive physiology of the nonrejecting allograft tends to subside, with normalization of the diastolic parameters. 2 , 3 However, the persistence of abnormal diastolic parameters by Doppler echocardiography after the first few weeks from transplant is associated with increased late mortality. 5 A recent study suggested that myocardial fibrosis is an important contributor to the development of a restrictive cardiac filling pattern based on echocardiography measurements. 6 Moreover, myocardial fibrosis has been reported to be significantly increased even during the first year following HT. 7 This has been associated with advanced cardiac allograft vasculopathy, episodes of rejection, and allograft dysfunction. 8 , 9
The mammalian target of rapamycin (mTOR) inhibitors, sirolimus and its derivative, everolimus, have potent immunosuppressive and antiproliferative properties. 10 In addition to the inhibition of lymphocyte activation and reducing the sensitivity to cytokines, mTOR inhibitors have been shown to block transforming growth factor‐β pathways and inhibit the fibrogenic activation of fibroblasts in animal kidney models. 11 Moreover, 2 previous studies from our institution showed that conversion to sirolimus‐based immunosuppression with complete calcineurin inhibitor (CNI) withdrawal in cardiac transplant recipients results in a decrease in left ventricular mass (LVM) 12 , 13 and improvement in diastolic function. 13 However, these studies were based on Doppler echocardiographic parameters and not on filling pressures measured invasively. Additionally, no pathological studies have been conducted to test the effects of mTOR inhibitors on myocardial fibrosis in HT recipients.
To extend our previous observations and correlate with the histologic data, we sought to examine whether conversion from CNI‐based to sirolimus‐based immunosuppression was associated with decreased risk of myocardial fibrosis using human myocardial biopsies and DD post‐HT using invasive hemodynamic measurements in addition to echocardiographic parameters.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. This study was a retrospective, nonrandomized, single‐center study approved by the Mayo Clinic institutional review board. Informed consent was waived because of the retrospective nature of the study.
Subject Characteristics
We identified a cohort of 100 patients who underwent HT between July 2000 and December 2015 recipients who were maintained on standard immunosuppression with CNIs (n=51) and subjects in whom CNIs were withdrawn and replaced with sirolimus (n=49). Patients in the sirolimus group were included only if they were converted to sirolimus during the first year after HT. All surviving subjects who underwent at least 2 echocardiograms and 2 endomyocardial biopsies (EMBs) over a 3‐year period and were followed up at Mayo Clinic (Rochester, MN) were included in the study. Subjects with evidence of significant acute cellular rejection more than grade 1R or any evidence of antibody‐mediated rejection during the study period were excluded. Subjects with severe mitral regurgitation or severe tricuspid regurgitation were also excluded.
Immunosuppression
In our institution, all patients received induction therapy with antithymocyte globulin. All HT recipients received maintenance immunosuppression, including a CNI (tacrolimus or cyclosporine), an antimetabolite (mycophenolate mofetil or azathioprine), and tapering doses of prednisone. Based on our new protocol, 14 we used rabbit antithymocyte globulin (dose of 1.5 mg/kg) at the time of HT and continued dosing on the basis of CD4 and CD8 T‐cell counts until tacrolimus was in the goal range of 10 to 14 ng/mL (or cyclopsporine in the old HT era), in addition to mycophenolate mofetil (goal dose of 1000–1500 mg twice daily) (or azathioprine, 1–3 mg/kg, in the old HT era) and steroid therapy. Since July 2006, a routine conversion protocol from CNI to sirolimus was introduced in our institution. Patients who were stable following HT without evidence of rejection and on stable doses of antimetabolites and prednisone received gradually increasing doses of sirolimus to achieve levels 10 to 14 ng/mL, followed by gradual down‐titration of CNI dose during conversion without changes in the antimetabolite and steroid regimens. Based on our protocol, CNI was typically used for the first 6 months after HT to avoid delayed wound healing that could occur with earlier introduction of sirolimus. Biopsies were generally performed 2 weeks following the conversion process, and a reduced dose of CNI was reintroduced if the biopsy was positive for rejection, with a second attempt to withdraw CNI therapy later if rejection subsided. Until July 2006, reasons for conversion included impaired renal function secondary to CNI (estimated glomerular filtration rate 50 mL/min per 1.73 m2 and lack of any other identifiable causes of renal dysfunction), cardiac allograft vasculopathy International Society for Heart and Lung Transplantation grade 2 or worse detected on annual coronary angiography, or intolerance of CNIs.
Echocardiographic Measurements
All echocardiograms were performed at Mayo Clinic using a standardized protocol by experienced physicians, who were unaware of treatment assignment. Left ventricular end‐diastolic diameter (LVEDD) and left ventricular end‐systolic diameter (LVESD) were measured by 2‐dimensional echocardiography using American Society of Echocardiography recommendations. Left ventricular ejection fraction (LVEF) was calculated from the apical 2‐ and 4‐chamber views using the modified biplane Simpson's method. 15 Pulsed‐wave Doppler examination of mitral inflow (the E and A waves, respectively, and mitral inflow deceleration time), Doppler tissue imaging of the mitral annulus (medial and lateral e′), as well as continuous‐wave Doppler of the tricuspid regurgitation jet systolic velocity were performed in each subject as previously described. 16 At the time of assessment, all subjects were in sinus rhythm without cardiac pacing. We did not include left atrial size for assessment of DD, as it is usually enlarged after HT due tobecause of donor‐to‐recipient atrial anastomosis. Thus, left atrial volume is not an accurate representation of diastolic function in HT recipients.
Endomyocardial Biopsy
Routine EMBs were performed according to our previously described institutional protocols to monitor for acute rejection. 17 , 18 Endomyocardial samples were analyzed using histopathology and immunofluorescence (C4d and C3d) and graded for rejection according to International Society for Heart and Lung Transplantation consensus criteria. 19 Biopsies were included if they were graded ≤1R for acute cellular rejection with the absence of histopathologic changes suggesting antibody‐mediated rejection.
Fibrosis Quantification
All biopsy specimens were originally placed in formalin and subsequently embedded in a paraffin block. For the purpose of our study, sections were stained with Masson trichrome for microscopic visualization and image analysis. Slides were scanned at ×20 magnification on the Aperio ScanScope AT2 brightfield instrument (Leica Biosystems, Richmond, IL) at a resolution of 0.50 μm per pixel. The images were 24‐bit contiguous standard pyramid tiled TIFF files compressed via JPEG with a quality setting of 70. For digital image analysis, the Aperio ImageScope Software (Leica Biosystems) was used. A minimum 85% of cardiac tissue, present on the slide, was traced with a digital pen tool to indicate the region of analysis. Care was taken, either in the tracing process or by using a negative pen tool, to eliminate tissue folds and large coronary vessels and avoid staining artifacts. A modified positive pixel count algorithm was used to analyze the annotated tissue. Positivity was calculated as the number of pixels within the blue‐staining regions divided by the total number of pixels (red‐ and blue‐stained regions). This ratio was converted to a percentage of fibrosis. To compensate for the potential trichrome stain variability in intensity of the blue stain from run to run, algorithms were developed and optimized before the study on multiple trichrome stains, performed on different runs, with manual review of the results to improve sensitivity and specificity. In addition, pixels were subclassified as “weak positive,” “positive,” and “strong positive” to account for differences in saturation and hue. Quality control review was performed by an anatomic pathologist (MCB) on a subset of the specimens to determine staining quality and resultant quantification. Ultimately, all 3 “positive” results were counted in the final analysis.
Right Heart Catheterization
Systemic arterial pressure and heart rate were measured noninvasively. Mean right atrial pressure, systolic pulmonary artery pressure, diastolic pulmonary artery pressure (DPAP), mean pulmonary artery pressure, and pulmonary capillary wedge pressure (PCWP) were measured during right heart catheterization (RHC) using a pulmonary artery catheter. Cardiac output was determined by the Fick method. Diastolic pulmonary gradient was calculated as the difference between the DPAP and PCWP. 20
Statistical Analysis
Data are displayed as mean± SD or count and percentage where appropriate. Variables with heavily skewed distribution are reported as medians with first and third quartiles in parentheses. Univariable analysis was performed using the 2‐tailed t test for numerical data and the χ2 test for categorical data. Change in the hemodynamic, echocardiographic, and fibrosis parameters was analyzed using the paired t test or Wilcoxon matched‐pairs signed‐rank test. To test the influence of sirolimus conversion on hemodynamic parameters and fibrosis progression, univariate and multivariable linear regression analysis was performed using variables known or suspected to influence the dependent variable. All changes in hemodynamic and echocardiographic parameters as well as changes in fibrosis percentages were adjusted for age at the time of HT. In the multivariable models, in addition to age at the time of HT, we included changes in mean arterial pressure (MAP) and changes in LVEF for examining the impact of conversion to sirolimus on changes in hemodynamic parameters, and changes in MAP and estimated glomerular filtration rate (eGFR) for examining the impact of conversion to sirolimus on echocardiographic parameters and myocardial fibrosis. Given heterogeneity in timing of follow‐up RHC, changes in the hemodynamic parameters were annualized. For the echocardiographic parameters and fibrosis progression, we examined absolute change in the variables given the uniform timing of tests at baseline and follow‐up. P<0.05 was considered to be statistically significant.
Results
Patients' Characteristics
The study cohort consisted of 100 HT recipients between 2000 and February 2015, of whom 49 were converted to sirolimus and 51 continued on a CNI. Conversion from a CNI to sirolimus was performed early within the first year after HT with a median time of 180 (97.5–199) days. Baseline and follow‐up echocardiograms were available for the total cohort. Seventy patients had baseline and follow‐up EMBs, and 52 patients had RHC both at baseline and at last follow‐up. All included subjects survived for the duration of the study period.
Table 1 presents baseline demographics, laboratory parameters and clinical characteristics for the overall cohort and comparing the sirolimus and CNI groups. Compared with patients maintained on a CNI, patients converted to sirolimus were significantly older (54.76±10.83 versus 46.71±15.03 years; P=0.003) and were less likely to have combined organ transplantation (22.45% versus 43.14%; P=0.03). Sirolimus converters were more likely to be treated with statins (93.88% versus 78.43%; P=0.03) with higher high‐density lipoprotein levels (72.18±22.2 versus 63.10±21.33; P=0.04) but no differences in the rest of the lipid profile were observed. Other baseline variables, including ischemic time, donor age, cytomegalovirus viremia, secondary immunosuppressants, and use of steroids, were not different between patients on CNI and those converted to sirolimus. Laboratory parameters including baseline glucose, creatinine, and eGFR were not different between the 2 groups. Finally, baseline International Society for Heart and Lung Transplantation cardiac allograft vasculopathy grades were similar between the sirolimus and CNI groups.
Table 1.
Baseline Characteristics
| Overall Cohort (n=100) | Sirolimus (n=49) | CNI (n=51) | P Value | |
|---|---|---|---|---|
| Age, y | 50.65±13.69 | 54.76±10.83 | 46.71±15.03 | 0.003 |
| Male, n (%) | 67 (67) | 31 (70.45) | 36 (73.47) | 0.75 |
| Ischemic cardiomyopathy, n (%) | 27 (27) | 14 (28.57) | 13 (25.49) | 0.73 |
| DCM, n (%) | 35 (35) | 16 (32.65) | 19 (37.25) | 0.63 |
| Body mass index, kg/m2 | 25.83±4.91 | 25.0±4.21 | 26.65±5.43 | 0.09 |
| Ischemic time, min | 158.60±51.10 | 163.73±52.10 | 153.5±50.1 | 0.32 |
| Donor age, y | 32.79±13.23 | 32.10±14.25 | 33.49±12.2 | 0.61 |
| Hypertension, n (%) | 31 (31) | 14 (28.57) | 17 (33.33) | 0.61 |
| Diabetes mellitus, n (%) | 24 (24) | 9 (18.37) | 15 (29.41) | 0.20 |
| Cytomegalovirus viremia, n (%) | 26 (26) | 11 (22.45) | 15 (29.41) | 0.43 |
| Epstein‐Barr virus viremia, n (%) | 4 (4) | 4 (8) | 4 (8) | 0.97 |
| Combined transplantation, n (%) | 33 (33) | 11 (22.45) | 22 (43.14) | 0.03 |
| Combined transplantation, n (%) | ||||
| Kidney | 19 (19) | 6 (12.24) | 13 (25.49) | 0.06 |
| Liver | 6 (6) | 4 (8.16) | 2 (3.92) | |
| Baseline primary immunosuppression, n (%) | ||||
| Cyclosporine | 44 (44) | 20 (40.82) | 24 (47.10) | 0.53 |
| Tacrolimus | 56 (56) | 29 (59.18) | 27 (53.0) | |
| Azathioprine/Mycophenolate mofetil, n (%) | ||||
| Azathioprine | 23 (23) | 14 (28.57) | 9 (18.0) | 0.21 |
| Mycophenolate mofetil | 76 (76) | 35 (71.43) | 41 (82.0) | |
| Steroids, n (%) | 98 (98) | 48 (98) | 50 (98) | 0.97 |
| Statins, n (%) | 86 (86) | 46 ( 93.88) | 40 ( 78.43) | 0.03 |
| Fibrates, n (%) | 8 (8) | 6 (12.24) | 2 ( 3.92) | 0.12 |
| Aspirin, n (%) | 15 (15) | 6 ( 12.24) | 9 ( 17.65) | 0.45 |
| Diuretics, n (%) | 68 (68) | 33 (67.35) | 35 (68.63) | 0.89 |
| Calcium channel blocker, n (%) | 56 (56) | 29 (59.18) | 27 (52.94) | 0.53 |
| Beta‐blocker, n (%) | 17 (17) | 10 (20.41) | 7 (13.73) | 0.37 |
| ACEI, n (%) | 25 (25) | 12 (24.49) | 13 (25.49) | 0.91 |
| Creatinine, mg/dL | 1.39±0.50 | 1.42±0.51 | 1.36±0.49 | 0.61 |
| eGFR, mL×min−1×1.73 m−2 | 59.22±21.04 | 57.38±20.89 | 61.03±21.23 | 0.39 |
| Total cholesterol, mg/dL | 213.53±48.18 | 216.67±47 | 210.51±49.60 | 0.53 |
| Triglycerides, mg/dL | 161.41±115.6 | 160.26±102.86 | 162.5±127.66 | 0.92 |
| HDL cholesterol, mg/dL | 67.55±22.13 | 72.18±22.20 | 63.10±21.33 | 0.04 |
| LDL cholesterol, mg/dL | 114.1±39.2 | 112.63±38.05 | 115.48±40.53 | 0.72 |
| Graft LVEF, % | 62.73±7.13 | 61.88±7.24 | 63.55±6.99 | 0.24 |
| ISHLT cardiac allograft vasculopathy grade at baseline | ||||
| Grade 0 | 76 (76) | 41 (83.67) | 35 (70.0) | 0.11 |
| Grade 1 | 24 (24) | 8 (16.33) | 15 (30.0) | |
Data expressed as mean (± SD), median (Q1, Q3) or n (%). ACEI indicates, angiotensin‐converting enzyme inhibitor; CNI, calcineurin inhibitor; DCM, dilated cardiomyopathy; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; ISHLT, International Society for Heart and Lung Transplantation; LDL, low‐density lipoprotein; and LVEF, left ventricular ejection fraction.
Differences in Echocardiographic Parameters
All HT recipients undergo routine echocardiograms during a scheduled follow‐up. Echocardiographic measurements were performed at baseline (1 month after HT, before conversion to sirolimus) and at 3 years after HT. Echocardiographic measurements are presented in Table 2. At baseline, there were no significant differences in LVEDD, LVESD, or LVEF between the CNI and sirolimus groups. Diastolic parameters including E/A ratio, medial and lateral e′, E/e′, and tricuspid regurgitation velocity were not different between patients on a CNI and sirolimus converters. During a follow‐up period of 3 years, both LVEDD (45.71±5.10 to 48.28±5.5 mm; P<0.01) and LVESD (28.41±4.8 to 30.84±4.53 mm; P<0.01) increased significantly in the sirolimus group while remaining unchanged in the CNI group (45.47±5.43 to 45.28±5.26 mm; P=0.56) and (28.10±4.50 to 27.93±3.83 mm; P=0.55), respectively. The 2 groups differed significantly in the delta change in LVEDD (2.57±4.14 in sirolimusconverters versus −0.19±6.21 in CNI group; P=0.01) and in LVESD (2.43±3.67 in sirolimusconverters versus −0.048±4.84 in CNI group; P=0.009) (Figure 1A and 1B). In univariate analysis, sirolimus conversion was found to be associated with increased LVEDD (β=+1.38, 95% CI, 1.08–4.06; P=0.01) and LVESD (β=+1.24; 95% CI, 1.1–3.7; P=0.009). After multivariable adjustment for age at HT, baseline eGFR, and the difference in MAP, sirolimus conversion remained significantly associated with increased LVEDD (β=+1.28; 95% CI, 1.02–4.00; P=0.02) and LVESD (β=+1.27; 95% CI, 1.10–3.69; P=0.008). As expected, because both LVEDD and LVESD increased in the sirolimus group, no significant change in LVEF was observed, and similarly there was no change in LVEF in the CNI group.
Table 2.
Differences in Echocardiographic Parameters Assessed in Patients Treated With Sirolimus Versus CNI at Time of Baseline (1 Month) and at 3‐Year Follow‐Up
| Sirolimus (n=49) | CNI (n=50) | Difference (Sirolimus–CNI) | P Value | Age‐Adjusted Estimates (95% CI) (Sirolimus vs CNI)* | P Value | |
|---|---|---|---|---|---|---|
| LVEF, % ±SD | ||||||
| Baseline | 62.81±7.15 | 64.66±6.25 | −1.85±2.64 | 0.17 † | ||
| Follow‐up | 60.94±6.42 | 63.61±8.20 | −2.67±2.89 | 0.07 † | ||
| Δ LVEF | −1.87±6.90 | −1.06±7.67 | −0.81±2.87 | 0.58 † | −0.34 (−1.89 to 1.2) | 0.66 |
| P value | 0.07 ‡ | 0.33 ‡ | ||||
| LVEDD, mm ±SD | ||||||
| Baseline | 45.71±5.10 | 45.47±5.43 | 0.24±2.07 | 0.82 † | ||
| Follow‐up | 48.28±5.50 | 45.28±5.26 | 3.0±2.12 | 0.007 † | ||
| Δ LVEDD | 2.57±4.14 | −0.19±6.21 | 2.76±2.07 | 0.01 † | +1.14 (0.04 to 2.24) | 0.04 |
| P value | <0.01 ‡ | 0.56 ‡ | ||||
| LVESD, mm ±SD | ||||||
| Baseline | 28.41±4.80 | 28.1±4.80 | 0.31±1.89 | 0.75 † | ||
| Follow‐up | 30.84±4.53 | 27.93±3.83 | 2.91±1.65 | 0.002 † | ||
| Δ LVESD | 2.43±3.67 | −0.048±4.84 | 2.48±1.69 | 0.009 † | +1.21 (0.25 to 2.17) | 0.014 |
| P value | <0.01 ‡ | 0.55 ‡ | ||||
| Mitral E/A ratio ±SD | ||||||
| Baseline | 2.11±0.70 | 2.01±0.69 | 0.10±0.27 | 0.60 † | ||
| Follow‐up | 2.21±0.79 | 2.13±1.06 | 0.08±0.37 | 0.77 † | ||
| Δ E/A | 0.10±0.83 | 0.13±1.1 | −0.03±0.38 | 0.92 † | +0.08 (−0.22 to 0.37) | 0.61 |
| P value | 0.58 ‡ | 0.55 ‡ | ||||
| Mitral DT, ms ±SD | ||||||
| Baseline | 166.96±24.65 | 161.65±21.46 | 5.31±9.11 | 0.39 † | ||
| Follow‐up | 168.32±32.88 | 166.13±32.31 | 2.19±12.84 | 0.80 † | ||
| Δ DT | 1.35±38.0 | 4.48±35.45 | −3.13±14.48 | 0.75 † | −0.30 (−1.02 to 0.42) | 0.40 |
| P value | 0.85 ‡ | 0.50 ‡ | ||||
| Medial e′ velocity, m/s ±SD | ||||||
| Baseline | 0.075±0.02 | 0.082±0.02 | −0.007±0.01 | 0.09 † | ||
| Follow‐up | 0.085±0.02 | 0.084±0.02 | 0.001±0.01 | 0.98 † | ||
| Δ medial e′ velocity | 0.01±0.02 | 0.002±0.02 | 0.008±0.01 | 0.15 † | +0.003 (−0.002 to 0.01) | 0.19 |
| P value | 0.01 ‡ | 0.45 ‡ | ||||
| Lateral e′ velocity, m/s ±SD | ||||||
| Baseline | 0.12±0.04 | 0.14±0.03 | −0.02±0.01 | 0.06 † | ||
| Follow‐up | 0.14±0.04 | 0.12±0.04 | 0.02±0.01 | 0.12 † | ||
| Δ lateral e′ velocity | 0.02±0.04 | −0.02±0.04 | 0.04±0.01 | 0.003 † | +0.02 (0.005 to 0.03) | 0.006 |
| P value | 0.01 ‡ | 0.06 ‡ | ||||
| E/e′ medial ±SD | ||||||
| Baseline | 12.21±3.75 | 10.96±3.32 | 1.25±1.39 | 0.08 † | ||
| Follow‐up | 10.70±4.32 | 10.77±3.95 | −0.07±1.63 | 0.92 † | ||
| Δ E/e′ medial | −1.51±5.58 | −0.19±3.53 | −1.32±1.84 | 0.15 † | −0.55 (−1.53 to 0.44) | 0.27 |
| P value | 0.06 ‡ | 0.45 ‡ | ||||
| E/e′ Lateral ±SD | ||||||
| Baseline | 8.34±4.55 | 6.84±1.75 | 1.5±1.36 | 0.17 † | ||
| Follow‐up | 6.57±2.41 | 7.82±3.70 | −1.25±1.22 | 0.16 † | ||
| Δ E/e′ lateral | −1.78±4.29 | 0.98±3.56 | −2.76±1.55 | 0.02 † | −1.35 (−2.6 to −0.12) | 0.03 |
| P value | 0.04 ‡ | 0.23 ‡ | ||||
| Avg E/e′ ±SD | ||||||
| Baseline | 9.83±2.96 | 9.17±2.16 | 0.66±1.02 | 0.40 † | ||
| Follow‐up | 9.02±2.90 | 9.73±3.53 | −0.71±1.27 | 0.45 † | ||
| Δ Avg E/e′ | −0.81±3.55 | 0.55±2.86 | −1.36±1.27 | 0.16 † | −0.69 (−1.69 to 0.32) | 0.17 |
| P value | 0.24 ‡ | 0.4 ‡ | ||||
| TR velocity, m/s ±SD | ||||||
| Baseline | 2.65±0.35 | 2.66±0.34 | −0.01±0.13 | 0.97 † | ||
| Follow‐up | 2.47±0.26 | 2.48±0.26 | −0.01±0.10 | 0.79 † | ||
| Δ TR velocity | −0.18±0.36 | −0.18±0.38 | 0 | 0.88 † | −0.001 (−0.08 to 0.08) | 0.98 |
| P value | 0.002 ‡ | 0.005 ‡ | ||||
CNI indicates calcineurin inhibitor; DT, deceleration time; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; and TR, tricuspid regurgitation.
β estimates of sirolimus conversion after adjustment for age at the time of heart transplant.
t test.
Paired t test.
Figure 1. Changes in left ventricular end systolic diameter (LVESD).
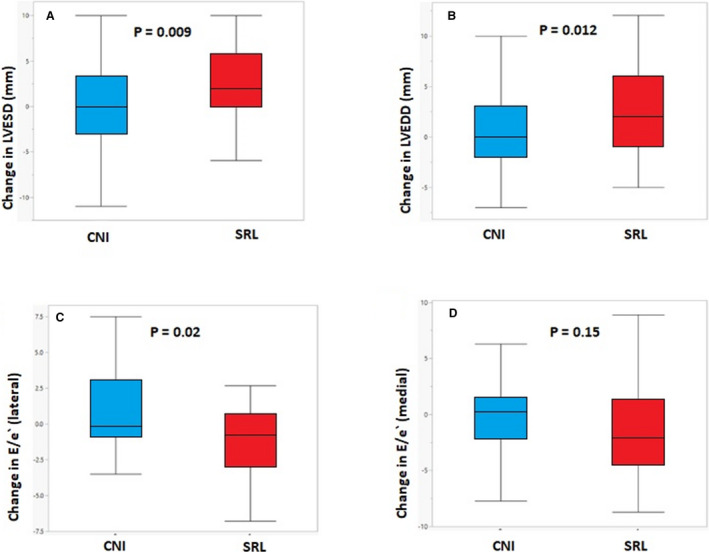
A, Left ventricular end diastolic diameter (LVEDD), (B), E/e′ lateral (C) and E/e′ medial (D) in patients treated with sirolimusvs calcineurin inhibitors (CNI).
The delta change in mean lateral e′ velocity differed significantly between the 2 groups (+0.02±0.04 in the sirolimusgroup versus −0.02±0.04 in the CNI group; P=0.003). In the univariate analysis, sirolimus was found to be significantly associated with increased lateral e′ velocity (β=+0.019; 95% CI, 0.01–0.03; P=0.003). After adjustment for age at HT, baseline eGFR, and difference in MAP, sirolimus conversion remained significantly associated with increased lateral e′ velocity (β=+0.018; 95% CI, 0.005–0.03; P=0.009). However, the delta change mean septal e′ velocity did not differ significantly between the CNI and sirolimus groups (0.01±0.025 versus 0.002±0.021; P=0.15), respectively. The delta change in lateral E/e′ differed significantly between groups (−1.78±4.29 for sirolimus versus 0.98±3.56 for CNI; P=0.02), while delta change in medial E/e′ was similar between the groups (−1.51±5.58 versus −0.19±3.53; P=0.15), respectively. (Figure 1C and 1D). Sirolimus was associated with decreased lateral E/e′ in the univariate analysis (β= −1.38; 95% CI, −2.56 to −0.20; P=0.02), and this association remained significant after adjustment (β= −1.40; 95% CI, −2.67 to −0.13; P=0.03). The delta change in tricuspid regurgitation velocity did not differ significantly between the 2 groups (−0.18±0.36 versus −0.18±0.38; P=0.88). Other diastolic parameters including average E/e′, E/A, deceleration time, were not significantly changed in the sirolimus or CNI group.
Hemodynamic Parameters
We identified 52 patients who underwent at least 2 RHCs as part of routine follow‐up, baseline RHC within 6 months from HT (before sirolimus conversion) and follow‐up RHC at 2 to 3 years after HT. Twenty‐two patients continued on a CNI, and 30 patients were converted to sirolimus. The mean time between the baseline and the follow‐up RHC was similar in both groups (2.23±0.69 versus 2.50±0.50 years in the sirolimus and CNI groups, respectively; P=0.14). The hemodynamic parameters are presented in Table 3. The baseline parameters were similar between the 2 groups.
Table 3.
Differences in Hemodynamic Parameters Assessed in Patients Treated With Sirolimus Versus CNI at Time of Baseline RHC and at Last RHC Follow‐Up
| Sirolimus (n=30) | CNI (n=22) | Difference (Sirolimus–CNI) | P Value | Age‐Adjusted Estimates (95% CI) (Sirolimus vs CNI) ‡ | P Value | |
|---|---|---|---|---|---|---|
| Time from first to last RHC, y | 2.23±0.69 | 2.50±0.50 | −0.27±0.32 | 0.14 | ||
| SBP, mm Hg ±SD | ||||||
| Baseline | 127.27±15.27 | 126.83±15.44 | 0.44±8.45 | 0.93* | ||
| Follow‐up | 116.2±22.1 | 124.61±19.46 | −8.41±11.34 | 0.2* | ||
| Δ SBP/y | −6.06±10.18 | −1.50±11.29 | −4.56±5.96 | 0.17* | −2.1 (−5.49 to 1.30) | 0.22 |
| P value | 0.02 † | 0.68 † | ||||
| DBP, mm Hg ±SD | ||||||
| Baseline | 76.65±10.63 | 81.11±13.28 | −4.46±6.72 | 0.22* | ||
| Follow‐up | 71.0±9.23 | 76.72±12.07 | −5.72±6.02 | 0.08* | ||
| Δ DBP/y | −3.34±6.13 | −3.49±14.18 | 0.15±6.31 | 0.96* | +0.35 (−2.88 to 3.58) | 0.82 |
| P value | 0.03 † | 0.37 † | ||||
| MAP, mm Hg ±SD | ||||||
| Baseline | 93.52±11.02 | 96.35±13.25 | −2.83±6.79 | 0.45* | ||
| Follow‐up | 86 .10±12.10 | 92.68±13.10 | −6.58±6.97 | 0.09* | ||
| Δ MAP/y | −4.24±6.65 | −2.82±12.23 | −1.42±5.63 | 0.62* | −0.46 (−3.42 to 2.49) | 0.75 |
| P value | 0.02 † | 0.43 † | ||||
| Heart rate, BPM ±SD | ||||||
| Baseline | 85.40±13.12 | 87.68±11.18 | −2.28±6.62 | 0.51* | ||
| Follow‐up | 83.26±11.69 | 88.04±11.50 | −4.78±6.37 | 0.15* | ||
| Δ Heart rate/y | −0.51±2.50 | −0.02±1.71 | −0.49±1.14 | 0.43* | −0.54 (−1.95 to 0.86) | 0.44 |
| P value | 0.28 † | 0.85 † | ||||
| RAP, mm Hg ±SD | ||||||
| Baseline | 7.40±4.90 | 9.20±5.50 | −1.8±2.89 | 0.22* | ||
| Follow‐up | 6.40±3.46 | 8.95±6.91 | −2.55±3.14 | 0.09* | ||
| Δ RAP/y | −0.97±5.51 | 0.31±3.56 | −1.28±2.46 | 0.34* | −0.29 (−0.93 to 0.36) | 0.39 |
| P value | 0.27 † | 0.79 † | ||||
| SPAP, mm Hg ±SD | ||||||
| Baseline | 33.10±9.72 | 35.45±10.16 | −2.35±5.48 | 0.39* | ||
| Follow‐up | 29.7±7.44 | 33.73±9.20 | −4.03±4.67 | 0.09* | ||
| Δ SPAP/y | −1.72±4.73 | −0.60±3.81 | −1.12±2.32 | 0.37* | −0.40 (−1.68 to 0.86) | 0.53 |
| P value | 0.07 † | 0.41 † | ||||
| DPAP, mm Hg ±SD | ||||||
| Baseline | 13.53±6.05 | 14.41±6.99 | −0.88±3.63 | 0.63* | ||
| Follow‐up | 10.43±3.94 | 16.36±7.11 | −5.93±3.28 | 0.0003* | ||
| Δ DPAP/y | −1.72±3.39 | 0.82±2.59 | −2.54±1.62 | 0.005* | −1.20 (−2.10 to −0.29) | 0.01 |
| P value | 0.008 † | 0.17 † | ||||
| MPAP, mm Hg ±SD | ||||||
| Baseline | 22.03±6.90 | 23.68±6.97 | −1.65±3.81 | 0.4* | ||
| Follow‐up | 19.07±4.72 | 23.77±7.32 | −4.70±3.49 | 0.007* | ||
| Δ MPAP/y | −1.51±3.23 | −0.01±2.95 | −1.5±1.68 | 0.09* | −0.69 (−1.61 to 0.22) | 0.13 |
| P value | 0.02 † | 0.95 † | ||||
| PCWP, mm Hg ±SD | ||||||
| Baseline | 12.52±5.45 | 12.27±4.88 | 0.25±2.82 | 0.87* | ||
| Follow‐up | 9.90±3.25 | 12.77±6.10 | −2.87±2.80 | 0.03* | ||
| Δ PCWP/y | −1.50±2.59 | 0.20±2.20 | −1.70±1.30 | 0.02* | −0.86 (−1.58 to −0.14) | 0.02 |
| P value | 0.02 † | 0.67 † | ||||
| Cardiac output, L/min ±SD | ||||||
| Baseline | 6.0±1.55 | 5.80±1.34 | 0.20±0.78 | 0.72* | ||
| Follow‐up | 6.40±1.18 | 6.30±1.46 | 0.10±0.74 | 0.74* | ||
| Δ Cardiac output/y | 0.14±0.96 | 0.22±0.46 | −0.08±0.39 | 0.75* | −0.05 (−0.32 to 0.20) | 0.67 |
| P value | 0.13 † | 0.09 † | ||||
| Cardiac index, L/min per m2 ±SD | ||||||
| Baseline | 3.17±0.74 | 2.93±0.57 | 0.24±0.35 | 0.23* | ||
| Follow‐up | 3.22±0.59 | 3.02±0.70 | 0.2±0.36 | 0.27* | ||
| Δ Cardiac index/y | 0.02±0.48 | 0.09±0.24 | −0.07±0.19 | 0.59* | −0.05 (−0.18 to 0.08) | 0.44 |
| P value | 0.46 † | 0.24 † | ||||
| DPG±SD | ||||||
| Baseline | 1.10±4.32 | 2.14±5.03 | −1.04±2.60 | 0.43* | ||
| Follow‐up | 0.31±3.20 | 3.59±4.43 | −3.28±2.17 | 0.003* | ||
| Δ DPG/y | −0.34±2.56 | 0.62±2.51 | −0.96±1.39 | 0.19* | −0.38 (−1.12 to 0.36) | 0.30 |
| P value | 0.42 † | 0.34 † | ||||
CNI indicates calcineurin inhibitor; DBP, diastolic blood pressure; DPAP, diastolic pulmonary artery pressure; DPG, diastolic pulmonary gradient; MAP, mean arterial pressure; MPAP, mean pulmonary artery pressure; PCWP, pulmonary capillary wedge pressure; RAP, right atrial pressure; RHC, right heart catheterization; SBP, systolic blood pressure; and SPAP, systolic pulmonary artery pressure.
t test.
Paired t test.
β estimates of sirolimus conversion after adjustment for age at the time of heart transplant.
In the sirolimus group, there was a significant decrease in the systolic blood pressure (127.27±15.27 to 116.20±22.10 mm Hg; P=0.02), diastolic blood pressure (76.65±10.63 to 71±9.23 mm Hg; P=0.03), and MAP (93.52±11.02 to 86 .10±12.10 mm Hg; P=0.02), whereas these parameters remained unchanged from baseline in the CNI group (Table 3). However, the annual change rate in all of these parameters did not differ significantly between the 2 groups; systolic blood pressure (−6.06±10.18 versus −1.50±11.29 mm Hg/year; P=0.17), diastolic blood pressure (−3.34±6.13 versus −3.49±14.18 mm Hg/year; P=0.96), and MAP (−4.24±6.65 versus −2.82±12.23 mm Hg/year; P=0.62).
Similarly, sirolimus converters had a significant decrease in DPAP (13.53±6.05 to 10.43±3.94 mm Hg; P=0.008) and mean pulmonary artery pressure (22.03±6.90 to 19.07±4.72; P=0.02), while systolic pulmonary artery pressure remained similar (33.10±9.72 to 29.70±7.44; P=0.07). All these parameters remained unchanged from baseline in the CNI group as outlined in Table 3. The change in DPAP differed significantly between the sirolimus and CNI groups (−1.72±3.39 versus 0.82±2.59 mm Hg/year; P=0.005) but not in systolic pulmonary artery pressure (−1.72±4.73 versus −0.60±3.81 mm Hg/year; P=0.37) or mean pulmonary artery pressure (−1.51±3.23 versus −0.01±2.95 mm Hg/year; P=0.09) (Figure 2B through 2D). Sirolimus was found to be significantly associated with decreased DPAP in the univariate analysis (β= −1.27; 95% CI, −2.13 to −0.40; P=0.005) and in the multivariable model after adjustment for age at HT, difference in MAP, difference in LVEF ,and time between baseline and follow‐up RHC (β= −1.36; 95% CI, −2.3 to −0.42; P=0.006).
Figure 2. Changes in pulmonary capillary wedge pressure (PCWP).
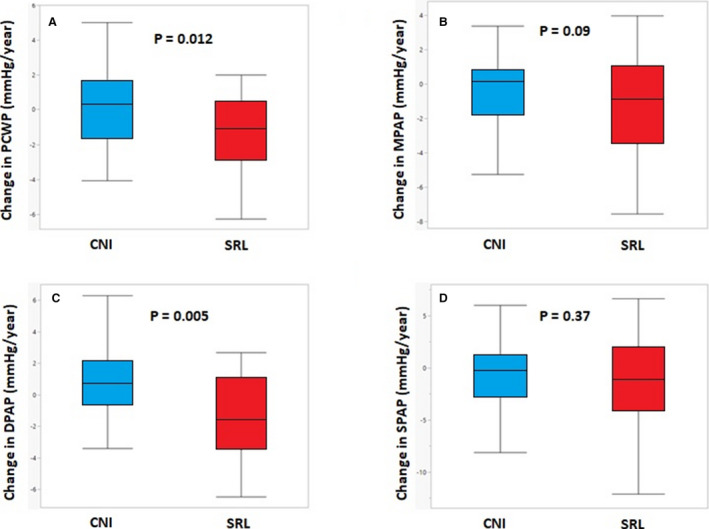
A, Mean pulmonary artery pressure (MPAP); (B), diastolic pulmonary artery pressure (DPAP); and (C) systolic pulmonary artery pressure (SPAP) (D) in patients treated with sirolimus vs calcineurin inhibitors (CNIs).
Regarding other parameters, PCWP decreased from 12.52±5.45 to 9.90±3.25 mm Hg (P=0.02) in the sirolimus group, while remaining unchanged from baseline in the CNI group (12.27±4.88 to 12.77±6.1 mm Hg; P=0.67). The annual change in PCWP differed significantly between the sirolimus and CNI groups (−1.50±2.59 mm Hg/year versus 0.2±2.2 mm Hg/year in the sirolimus and CNI groups, respectively; P=0.02) (Figure 2A). Sirolimus was associated with decrease in PCWP in both the univariate analysis (β= −0.85; 95% CI, −1.54 to −0.15; P=0.02) and the multivariable analysis (β= −0.76; 95% CI, −1.50 to −0.01; P=0.047). The differences in other hemodynamic parameters, including right atrial pressure, cardiac output, cardiac index, and diastolic pulmonary gradient did not differ between the 2 groups. Interestingly, there was a trend for increase in stroke volume in the sirolimusL group (70.88±19.23 to 78.65±15.74 mL/beat; P=0.06) but not in the CNI group (68.40±15.30 to 73.10±16.10; P=0.17).
Myocardial Fibrosis
Both baseline and follow‐up scheduled EMBs, as part of the standard follow‐up, were available for 70 patients for fibrosis quantification; 30 among those converted to sirolimus, and 40 maintained on CNI therapy. Overall, the percentage of fibrosis increased significantly over time (8.64±3.57 at baseline versus 10.36±3.82 at 3 years of follow‐up; P=0.005) (Figure 3). The fibrosis percentage at baseline did not differ significantly between the groups (Table 4). Within the sirolimus group, a trend was seen for increased fibrosis percentage during follow‐up (8.48±3.17 versus 10.1±3.04; P=0.07), as compared with marginally significant progression in myocardial fibrosis in association with CNI therapy (8.76±3.87 versus 10.56±4.34; P=0.04) (Figure 3). However, the delta change in the percentage of fibrosis was similar between the 2 groups (1.62±4.67 in the sirolimus group versus 1.80±5.31 in the CNI group; P=0.88). Sirolimus was not found to be significantly associated with fibrosis progression in the univariate analysis (β= −0.15, 95% CI: −1.31 to 1.13; P=0.88) or in the multivariable model after adjustment for age at the time of HT, differences in MAP, and eGFR (β= −0.19; 95% CI, −1.93 to 1.55; P=0.82) (Table 4).
Figure 3. Fibrosis progression from 1 to 3 years in the total cohort, CNI and sirolimus groups.
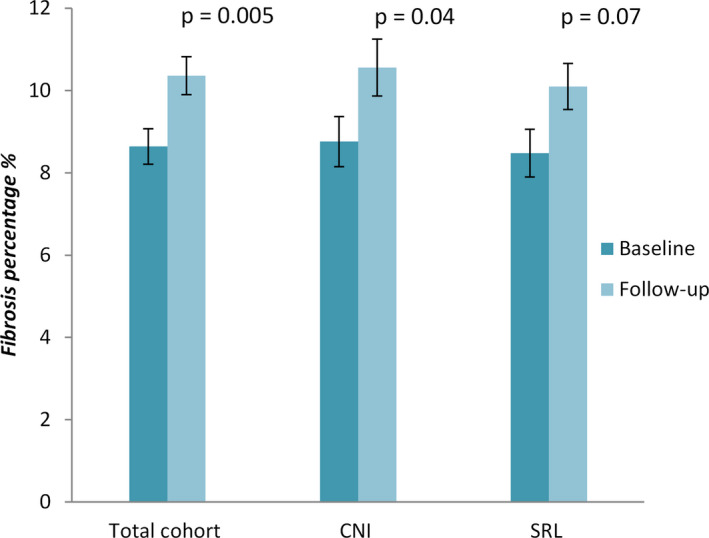
CNI indicates calcineurin inhibitors.
Table 4.
Differences in Fibrosis Percentage Assessed in Patients Treated With Sirolimus Versus CNI at Time of Baseline (1 Year) and at 3‐Year Follow‐Up
| Sirolimus (n=30) | CNI (n=40) | Difference (Sirolimus–CNI) | P Value | Age‐Adjusted Estimates (95% CI) (Sirolimus vs CNI)* | P Value | |
|---|---|---|---|---|---|---|
| % fibrosis ±SD | ||||||
| Baseline | 8.48±3.17 | 8.76±3.87 | −0.28±1.63 | 0.75 † | ||
| Follow‐up | 10.10±3.04 | 10.56±4.34 | −0.46±1.72 | 0.62 † | ||
| Δ % fibrosis | 1.62±4.67 | 1.80±5.31 | −0.18±2.33 | 0.88 † | −0.37 (−1.62 to 0.89) | 0.56 |
| P value | 0.07 ‡ | 0.04 ‡ | ||||
CNI indicates calcineurin inhibitor.
β estimates of sirolimus conversion after adjustment for age at the time of heart transplant.
t test.
Paired t test.
Discussion
The present study demonstrates for the first time that conversion to sirolimus as primary immunosuppression, with complete withdrawal of CNI therapy, was associated independently with an improvement in DD measures using different modalities, including (1) a significant increase in LVEDD and e′ but decrease in E/e′ using serial echocardiographic measurements, (2) a significant decrease in PCWP and DPAP using invasive hemodynamic parameters obtained from serial RHCs, and (3) a trend for more attenuated progression of myocardial fibrosis as assessed by quantitative digital analysis microscopy using serial EMBs, as compared with continued use of CNI after HT.
Previous studies from our group 12 , 13 and others 21 have shown that in HT recipients, conversion from CNI to sirolimus decreased LVM. One study 12 from our group, showed that the decrease in LVM was accompanied by increased p27Kip1,a cell cycle inhibitor protein that is induced by mTOR inhibition and is expressed in the myocardium. The authors postulated that this may be the mechanism by which sirolimus reduces myocardial hypertrophy and LVM. Paoletti et al 22 showed in a randomized controlled study that everolimus therapy combined with reduced CNI dose was effective in reducing LVM among renal transplant recipients, which was mainly attributed to reduced LV wall thickness. We assume that the increase in LVEDD and LVESD observed in patients receiving sirolimus in the current study may be attributable to the reduction in LV wall thickness and LVM as previously seen with sirolimus use.
Two previous studies have shown an improvement in diastolic function during mTOR inhibitor therapy. 13 , 21 Imamura et al 21 showed that an everolimus‐incorporated immunosuppressant regimen was associated with decreased E/e′ ratio and left atrial volume. However, Raichlin et al 13 showed that the improvement in DD in the sirolimus converters was indicated by a decrease in the left atrial volume with no change in the e′ velocity or E/e′ ratio. Both studies were limited, as the follow‐up time was limited to 1 year only and the echocardiographic findings were not supported by invasive hemodynamic measurements or myocardial fibrosis quantification using serial human myocardial biopsy specimens. We found that both lateral and medial e′ increased in sirolimus converters, but the increase in the medial e′ was less remarkable. Moreover, both medial and lateral E/e′ ratio decreased in sirolimus converters with a less remarkable decrease in medial E/e′. Notably, there was a trend for lower baseline e′ in the sirolimus group that is probably related to higher age compared with the tacrolimus group.The motion of the interventricular septum is often abnormal after cardiac surgery, and this might limit the reliability of medial e′ 23 compared with the lateral e′. Moreover, the left atrium is usually enlarged after HT because of donor‐to‐recipient anastomosis at the atrial level. Thus, left atrial volume may not be a true representative of diastolic function in the HT population, at least during the early stage after HT. Therefore, to overcome the limitations of echocardiography for DD evaluation in HT recipients, we further investigated the impact of sirolimus conversion on the filling pressures by RHC. We found that sirolimus conversion was independently associated with decreased PCWP and DPAP. These findings confirm the improvement in DD within sirolimus converters that was indicated by echocardiographic parameters in our study as well as in previous report from our institution. 13 We assume that the sirolimus‐related decrease in left ventricular hypertrophy and LVM likely contributes to lower left ventricular filling pressures.
Interestingly, a recent study has demonstrated that activation of the mTOR signaling cascade plays an important pathogenic role in the development of pulmonary vascular remodeling and pulmonary hypertension. This study showed that downregulation or inhibition of mTOR ameliorates experimental pulmonary hypertension in mice. 24 To investigate whether sirolimus has an isolated effect on DPAP, we calculated diastolic pulmonary gradient (DPAP minus PCWP) for both groups at baseline and follow‐up RHC. We found that the change in diastolic pulmonary gradient did not differ significantly between the sirolimus and CNI groups. Therefore, we could not confirm that sirolimus therapy is directly associated with a reduction in pulmonary artery pressures in humans undergoing HT. Given the antiproliferative properties and suggested antifibrogenic effects of mTOR inhibitors in experimental models 10 , 11 as well as the reports on the association between fibrosis and restrictive physiology in HT recipients, 6 we investigated the effect of sirolimus on progression of myocardial fibrosis. To the best of our knowledge, this is the first study to test the effect of mTOR inhibitors on myocardial fibrosis in HT recipients. We found a trend for increased fibrosis progression in the sirolimus group and marginally significant progression in fibrosis in the CNI group. Despite these discrepancies in the effect of the 2 therapeutic agents on myocardial fibrosis progression, we found that the change in fibrosis percentages did not differ significantly between the 2 groups. The blunted effects of sirolimus on fibrosis progression may be explained by a more rapid and remarkable regression in cellular hypertrophy as a result of mTOR inhibition compared with a more delayed and less remarkable attenuation of myocardial fibrosis.
Study Limitations
There were several limitations inherent to the observational, retrospective, nonrandomized design of this study. As in any observational study, we could not exclude residual confounding associations despite the adjustment for the most clinically relevant variables. Moreover, the commonly used criteria for assessing DD have not been validated in HT recipients. Finally, biopsies were taken only from the endocardial surface of the right side of the interventricular septum, in an area predisposed to repeated sampling. Fibrosis therefore might not reflect the whole myocardium and has the potential to include artifact. The main strengths of this study are the supporting findings for DD improvement by invasive hemodynamic measures, the uniform automated detection and quantification of myocardial fibrosis, and the long follow‐up period.
Conclusions
This single‐center cohort analysis provided evidence that conversion to sirolimus as primary maintenance immunosuppression therapy was associated with significant improvement in DD and left ventricular filling pressures and a trend for more attenuated myocardial fibrosis progression among HT recipients as compared with continued CNI therapy. Future prospective studies and using cardiac magnetic resonance imaging are needed to elucidate and confirm the suggested beneficial effects of sirolimus on myocardial fibrosis and DD development.
Sources of Funding
None.
Disclosures
None.
(J Am Heart Assoc. 2021;10:e018186. DOI: 10.1161/JAHA.120.018186.)
For Sources of Funding and Disclosures, see page 13.
References
- 1. Mehra MR. Heart transplantation at 50. Lancet. 2017;390:e43–e45. DOI: 10.1016/S0140-6736(17)33093-3. [DOI] [PubMed] [Google Scholar]
- 2. StGoar FG, Gibbons R, Schnittger I, Valantine HA, Popp RL. Left ventricular diastolic function. Doppler echocardiographic changes soon after cardiac transplantation. Circulation. 1990;82:872–878. DOI: 10.1161/01.CIR.82.3.872. [DOI] [PubMed] [Google Scholar]
- 3. Haussmann B, Muurling S, Stauch C, Haverich A, Hirt S, Simon R. Detection of diastolic dysfunction: acoustic quantification (AQ) in comparison to Doppler echocardiography. Int J Card Imaging. 1997;13:301–310. [DOI] [PubMed] [Google Scholar]
- 4. Ross HJ, Gullestad L, Hunt SA, Tovey DA, Puryear JB, McMillan A, Stinson EB, Valantine HA. Early Doppler echocardiographic dysfunction is associated with an increased mortality after orthotopic cardiac transplantation. Circulation. 1996;94(suppl):II289–II293. [PubMed] [Google Scholar]
- 5. Tallaj JA, Kirklin JK, Brown RN, Rayburn BK, Bourge RC, Benza RL, Pinderski L, Pamboukian S, McGiffin DC, Naftel DC. Post‐heart transplant diastolic dysfunction is a risk factor for mortality. J Am Coll Cardiol. 2007;50:1064–1069. DOI: 10.1016/j.jacc.2007.06.007. [DOI] [PubMed] [Google Scholar]
- 6. Kobashigawa JA, Itagaki BK, Razi RR, Patel JK, Chai W, Kawano MA, Goldstein Z, Kittleson MM, Fishbein MC. Correlation between myocardial fibrosis and restrictive cardiac physiology in patients undergoing retransplantation. Clin Transplant. 2013;27:E679–E684. DOI: 10.1111/ctr.12250. [DOI] [PubMed] [Google Scholar]
- 7. Armstrong AT, Binkley PF, Baker PB, Myerowitz PD, Leier CV. Quantitative investigation of cardiomyocyte hypertrophy and myocardial fibrosis over 6 years after cardiac transplantation. J Am Coll Cardiol. 1998;32:704–710. DOI: 10.1016/S0735-1097(98)00296-4. [DOI] [PubMed] [Google Scholar]
- 8. Yamani MH, Haji SA, Starling RC, Tuzcu EM, Ratliff NB, Cook DJ, Abdo A, Crowe T, Secic M, McCarthy P, et al. Myocardial ischemic‐fibrotic injury after human heart transplantation is associated with increased progression of vasculopathy, decreased cellular rejection and poor long‐term outcome. J Am Coll Cardiol. 2002;39:970–977. DOI: 10.1016/S0735-1097(02)01714-X. [DOI] [PubMed] [Google Scholar]
- 9. Feingold B, Picarsic J, Lesniak A, Popp BA, Wood‐Trageser MA, Demetris AJ. Late graft dysfunction after pediatric heart transplantation is associated with fibrosis and microvasculopathy by automated, digital whole‐slide analysis. J Heart Lung Transplant. 2017;36:1336–1343. DOI: 10.1016/j.healun.2017.09.012. [DOI] [PubMed] [Google Scholar]
- 10. Sehgal SN. Rapamune (RAPA, rapamycin, SRL): mechanism of action immunosuppressive effect results from blockade of signal transduction and inhibition of cell cycle progression. Clin Biochem. 1998;31:335–340. [DOI] [PubMed] [Google Scholar]
- 11. Chen G, Chen H, Wang C, Peng Y, Sun L, Liu H, Liu F. Rapamycin ameliorates kidney fibrosis by inhibiting the activation of mTOR signaling in interstitial macrophages and myofibroblasts. PLoS One. 2012;7:e33626. DOI: 10.1371/journal.pone.0033626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kushwaha SS, Raichlin E, Sheinin Y, Kremers WK, Chandrasekaran K, Brunn GJ, Platt JL. Sirolimus affects cardiomyocytes to reduce left ventricular mass in heart transplant recipients. Eur Heart J. 2008;29:2742–2750. DOI: 10.1093/eurheartj/ehn407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Raichlin E, Chandrasekaran K, Kremers WK, Frantz RP, Clavell AL, Pereira NL, Rodeheffer RJ, Daly RC, McGregor CGA, Edwards BS, et al. Sirolimus as primary immunosuppressant reduces left ventricular mass and improves diastolic function of the cardiac allograft. Transplantation. 2008;86:1395–1400. DOI: 10.1097/TP.0b013e318189049a. [DOI] [PubMed] [Google Scholar]
- 14. Asleh R, Briasoulis A, Kremers WK, Adigun R, Boilson BA, Pereira NL, Edwards BS, Clavell AL, Schirger JA, Rodeheffer RJ, et al. Long term sirolimus for primary immunosuppression in heart transplant recipients. J Am Coll Cardiol. 2018;71:636–650. [DOI] [PubMed] [Google Scholar]
- 15. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39.e14. DOI: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 16. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF III, Dokainish H, Edvardsen T, Flachskampf FA, Gillebert TC, Klein AL, Lancellotti P, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 17. Raichlin E, Bae J‐H, Khalpey Z, Edwards BS, Kremers WK, Clavell AL, Rodeheffer RJ, Frantz RP, Rihal C, Lerman A, et al. Conversion to sirolimus as primary immunosuppression attenuates the progression of allograft vasculopathy after cardiac transplantation. Circulation. 2007;116:2726–2733. DOI: 10.1161/CIRCULATIONAHA.107.692996. [DOI] [PubMed] [Google Scholar]
- 18. Topilsky Y, Hasin T, Raichlin E, Boilson BA, Schirger JA, Pereira NL, Edwards BS, Clavell AL, Rodeheffer RJ, Frantz RP, et al. Sirolimus as primary immunosuppression attenuates allograft vasculopathy with improved late survival and decreased cardiac events after cardiac transplantation. Circulation. 2012;125:708–720. DOI: 10.1161/CIRCULATIONAHA.111.040360. [DOI] [PubMed] [Google Scholar]
- 19. Stewart S, Winters GL, Fishbein MC, Tazelaar HD, Kobashigawa J, Abrams J, Andersen CB, Angelini A, Berry GJ, Burke MM, et al. Revision of the 1990 working formulation for the standardization of nomenclature in the diagnosis of heart rejection. J Heart Lung Transplant. 2005;24:1710–1720. DOI: 10.1016/j.healun.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 20. Alnsasra H, Asleh R, Schettle SD, Pereira NL, Frantz RP, Edwards BS, Clavell AL, Maltais S, Daly RC, Stulak JM, et al. Diastolic pulmonary gradient as a predictor of right ventricular failure after left ventricular assist device implantation. J Am Heart Assoc. 2019;8:e012073. DOI: 10.1161/JAHA.119.012073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imamura T, Kinugawa K, Nitta D, Kinoshita O, Nawata K, Ono M. Everolimus attenuates myocardial hypertrophy and improves diastolic function in heart transplant recipients. Int Heart J. 2016;57:204–210. DOI: 10.1536/ihj.15-320. [DOI] [PubMed] [Google Scholar]
- 22. Paoletti E, Marsano L, Bellino D, Cassottana P, Cannella G. Effect of everolimus on left ventricular hypertrophy of de novo kidney transplant recipients: a 1 year, randomized, controlled trial. Transplantation. 2012;93:503–508. DOI: 10.1097/TP.0b013e318242be28. [DOI] [PubMed] [Google Scholar]
- 23. Waggoner AD, Shah AA, Schuessler JS, Crawford ES, Nelson JG, Miller RR, Quinones MA. Effect of cardiac surgery on ventricular septal motion: assessment by intraoperative echocardiography and cross‐sectional two‐dimensional echocardiography. Am Heart J. 1982;104:1271–1278. DOI: 10.1016/0002-8703(82)90156-9. [DOI] [PubMed] [Google Scholar]
- 24. Tang H, Wu K, Wang J, Vinjamuri S, Gu Y, Song S, Wang Z, Zhang Q, Balistrieri A, Ayon RJ, et al. Pathogenic role of mTORC1 and mTORC2 in pulmonary hypertension. JACC Basic Transl Sci. 2018;3:744–762. DOI: 10.1016/j.jacbts.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]


