Abstract
Background
Large‐scale studies describing modern populations using an implantable cardioverter‐defibrillator (ICD) are lacking. We aimed to analyze the incidence of arrhythmia, device interventions, and mortality in a broad spectrum of real‐world ICD patients with different heart disorders.
Methods and Results
The UMBRELLA study is a prospective, multicenter, nationwide study of contemporary patients using an ICD followed up by remote monitoring, with a blinded review of arrhythmic episodes. From November 2005 to November 2017, 4296 patients were followed up. After 46.6±27.3 months, 16 067 episodes of sustained ventricular arrhythmia occurred in 1344 patients (31.3%). Appropriate ICD therapy occurred in 27.3% of study population. Patients with ischemic cardiomyopathy (hazard ratio [HR], 1.51; 95% CI, 1.29–1.78), dilated cardiomyopathy (HR, 1.28; 95% CI, 1.07–1.53), and valvular heart disease (HR, 1.94; 95% CI, 1.43–2.62) exhibited a higher risk of appropriate ICD therapies, whereas patients with hypertrophic cardiomyopathy (HR, 0.72; 95% CI, 0.54–0.96) and Brugada syndrome (HR, 0.25; 95% CI, 0.14–0.45) showed a lower risk. All‐cause death was 13.4% at follow‐up. Ischemic cardiomyopathy (HR, 3.09; 95% CI, 2.58–5.90), dilated cardiomyopathy (HR, 3.33; 95% CI, 2.18–5.10), and valvular heart disease (HR, 3.97; 95% CI, 2.25–6.99) had the worst prognoses. Delayed high‐rate detection was enabled in 39.7% of patients, and single‐zone programming occurred in 52.6% of primary prevention patients. Both parameters correlated with lower risk of first appropriate ICD therapy, with no excess risk of mortality. The rate of inappropriate shocks at follow‐up was low (6%) and did not differ among type of ICD but was lower in SmartShock‐capable devices.
Conclusions
Irrespective of the cause, contemporary ICD patients with heart failure–related disorders had a similar risk of ICD life‐saving interventions and death. Current ICD programming recommendations still need to be implemented.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NTC01561144.
Keywords: all‐cause death, appropriate implantable cardioverter‐defibrillator therapy, implantable cardioverter‐defibrillator, inappropriate shock, sustained ventricular arrhythmia
Subject Categories: Sudden Cardiac Death, Catheter Ablation and Implantable Cardioverter-Defibrillator
Nonstandard Abbreviations and Acronyms
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- BS
Brugada syndrome
- DCM
dilated cardiomyopathy
- HCM
hypertrophic cardiomyopathy
- ICM
ischemic cardiomyopathy
- IS
inappropriate shock
- LQTS
long‐QT syndrome
- PP
primary prevention
- RT
randomized trial
- SCD
sudden cardiac death
- SP
secondary prevention
- SVA
sustained ventricular arrhythmia
- VA
ventricular arrhythmia
- VHD
valvular heart disease
Clinical Perspective
What Is New?
The present study comprises a large cohort of 4296 implantable cardioverter‐defibrillation (ICD) patients with 8 different heart disorders, implanted according to current guidelines and followed up for 46.6±27.3 months.
A reliable incidence of arrhythmic events and ICD interventions by disease cause was identified by a blinded review of the stored arrhythmic episodes collected through the remote monitoring system.
What Are the Clinical Implications?
The long‐term prognosis of heart failure in patients using an ICD seems to be similar regardless of the disease cause.
Contemporary primary prevention ICD patients with channelopathies and inherited cardiomyopathies showed a low risk of life‐threatening ventricular arrhythmia and death, suggesting that a better risk stratification would be desirable in these subgroups.
Delayed high‐rate detection was enabled in <40% of patients and correlated with a lower risk of appropriate ICD interventions, showing that better implementation of current programming recommendations is required in modern‐era patients using an ICD.
A broad spectrum of cardiovascular disorders have an increased risk of life‐threatening ventricular arrhythmia (VA) and sudden cardiac death (SCD), with up to 50% of deaths related to cardiovascular disease being linked to SCD. 1 Thus, an implantable cardioverter‐defibrillator (ICD) is recommended to prevent SCD in patients at high risk of VA. In this setting, the survival benefit is achieved mainly by proper detection and subsequent treatment of life‐threatening VA. Current recommendations for ICD therapy in patients with left ventricular systolic dysfunction are robust because they were derived from randomized trials (RTs). 2 However, many cardiac diseases are not included or are underrepresented in RTs, and the risk of SCD has been inferred from small observational studies by reporting the incidence of VA and ICD interventions. 3 , 4 , 5 , 6 Large observational real‐world studies describing ICD populations implanted with modern devices are lacking. Therefore, homogeneous information derived from a wide and diverse population after long‐term follow‐up is needed to analyze the real incidence of arrhythmia, mortality, and adverse events among patients using an ICD.
The UMBRELLA observational study intended to bridge this gap and to provide further knowledge about real clinical practice and SCD risk stratification. The UMBRELLA study aims to collect a large and contemporary ICD population implanted under current clinical guidelines and followed up by remote device monitoring. The primary objective of the UMBRELLA study was to assess the incidence of arrhythmias in a cohort of patients implanted with Medtronic ICDs in Spain, as well as to analyze the arrhythmic risk and mortality prognosis according to their clinical profiles and ICD indication. The secondary objective was to investigate the existence of additional parameters that could identify ICD patients at higher risk of malignant VA.
The incidence of arrhythmias in the UMBRELLA study population after 2 years of recruitment was published previously. 7 Herein, we report the results of the entire cohort at the end of follow‐up.
METHODS
Study Design
The UMBRELLA study is a multicenter, observational, nationwide study of patients implanted with Medtronic ICDs (Medtronic, Inc) and prospectively followed up through the Medtronic remote device monitoring system (CareLink). The study, which was sponsored by Medtronic Ibérica S.A. and registered at www.clinicaltrials.gov (NTC01561144), was performed in 44 hospitals distributed among 14 of the 17 regions in Spain. Institutional ethics committee approval was acquired according to national requirements, and written informed consent was mandatory for patient enrollment. The enrollment period started in August 2011 and ended in November 2017. For each patient, the protocol allowed use of the device information from the implant date until study exit or end of follow‐up. Thus, remote transmissions stored in the CareLink system before patient enrollment could be included in the study as a contribution to the follow‐up of the patient's device.
As an observational study, ICD programming was performed according to regular clinical practice at each site.
The analytic methods and study materials that support the findings of this study will be made available to other researchers from the corresponding author on reasonable request.
Patient Selection
Patients were eligible for inclusion in the UMBRELLA study if they were at least 18 years old and implanted with a Medtronic ICD. In addition, patients were also required to be followed up by the remote monitoring system. Single‐, dual‐, and triple‐chamber ICDs were permitted. The protocol allowed the inclusion of patients at the moment of implantation (“de novo” procedure or device replacement) as well as patients implanted with the device before the moment enrollment.
Data Collection and Follow‐Up
The follow‐up period ran from the date of the first ICD implant (November 11, 2005) to the end of study follow‐up and data collection (November 30, 2017). For each patient, a baseline form was used to collected data on demographics, cardiovascular history, and implant procedure information related to the first device registered in the study database. All device information was automatically stored and collected through the remote monitoring system database. Information on adverse events, including deaths, or study exits was collected when applicable on separate forms. Data on cause‐specific mortality were further classified into SCD, non‐SCD, heart failure (HF) death, noncardiac death, or death of unknown origin.
A specific quality process was followed regularly throughout the study to ensure consistency in the collected data. Furthermore, twice a year, an analysis was performed of the patients who had not made any CareLink transmission within the previous 7 months.
Electrogram Analysis and Classification of Arrhythmic Events
A committee, composed of 6 expert electrophysiologists, analyzed all electrograms from the arrhythmic events that occurred in study patients. The electrograms were automatically stored in the CareLink network. Two committee members reviewed each event in a blinded manner, classifying the type of arrhythmia and the effectiveness of the delivered therapy. If the 2 members disagreed on the classification, the event was referred to a third reviewer. If no agreement was reached, the event was reassigned to a new pair of reviewers and, if needed, to a sixth reviewer. If consensus was not reached at this point, the event was finally classified in a joint meeting of all committee members.
Arrhythmic episodes were classified as sustained VA (SVA), non‐SVA, and nonventricular episodes. SVA was defined as ventricular episodes lasting ≥30 seconds and/or requiring ICD therapy, and was further classified into sustained monomorphic ventricular tachycardia (VT) or sustained polymorphic VT/ventricular fibrillation (VF). Non‐SVA was defined as VA lasting <30 seconds and terminated before the delivery of any ICD therapy. Nonventricular episodes were classified as atrial fibrillation (AF), sinus tachycardia, other regular supraventricular tachycardia, T‐wave oversensing, false detection, or noise.
Appropriate ICD therapy was defined as any therapy (antitachycardia pacing or high‐energy shocks) delivered to treat SVA episodes. Inappropriate shock (IS) was defined as any shock delivered to nonsustained ventricular episodes or nonventricular episodes.
All of the arrhythmic events described in this article come from the adjudication of the expert committee.
Subgroup Analysis and Outcomes
The entire cohort was clustered into subgroups according to the underlying heart disease and ICD indication. The resulting study subgroups were ischemic cardiomyopathy (ICM), nonischemic dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy (HCM), valvular heart disease (VHD), long‐QT syndrome (LQTS), Brugada syndrome (BS), arrhythmogenic right ventricular cardiomyopathy (ARVC), and adult congenital heart disease.
The study outcomes were SVA, first appropriate ICD therapy, all‐cause death, and IS.
Statistical Analysis
Continuous variables are expressed as mean±SD, and categorical data are expressed as numbers and percentages. Parametric tests were used for comparisons: Student t test and χ2 test for continuous and categorical variables, respectively. Cumulative incidence was described using the Kaplan‐Meier method, and comparisons between the estimated curves were performed using the log‐rank test.
Cox proportional hazards regression analysis was used to identify unadjusted risk factors for the study outcomes. After proportional hazard assumptions were tested, a multivariable Cox regression analysis was performed to assess independent predictors of appropriate ICD therapy, all‐cause death, and IS. The multivariate Cox regression model was adjusted for age, sex, hypertension, diabetes mellitus, hypercholesterolemia, smoking, history of AF, previous stroke, chronic kidney disease, family history of SCD, left ventricular ejection fraction (LVEF), QRS width, and heart disease, as well as the following ICD characteristics: type of device, ICD indication, detection zones enabled, and delayed high‐rate detection programming. SmartShock technology was also included in the multivariate analysis of IS outcome. Data are expressed as hazard ratios (HRs) and 95% CIs. Analyses were performed using IBM Statistical Package for Social Sciences, version 25.0 (IBM SPSS, Inc, Chicago, IL). A 2‐sided P<0.05 was considered significant for all tests.
RESULTS
Population Characteristics
A total of 4618 patients were enrolled in the study, with 74.7% (n=3449) corresponding to first implants. Figure 1 shows the patient distribution according to available follow‐up. A total of 322 patients (7%) were implanted and then included in the CareLink system but had no record of a remote transmission being made. As no follow‐up information could be obtained, these patients were excluded from the analysis. Thus, the final study sample was composed of 4296 patients (follow‐up population) in whom at least one remote transmission was performed. The mean follow‐up was 46.6±27.3 months (median, 44 months; maximum, 143 months). The baseline characteristics of the follow‐up population and study subgroups are summarized in Table 1 and Tables S1 and S2.
Figure 1. Flow diagram of patient distribution.
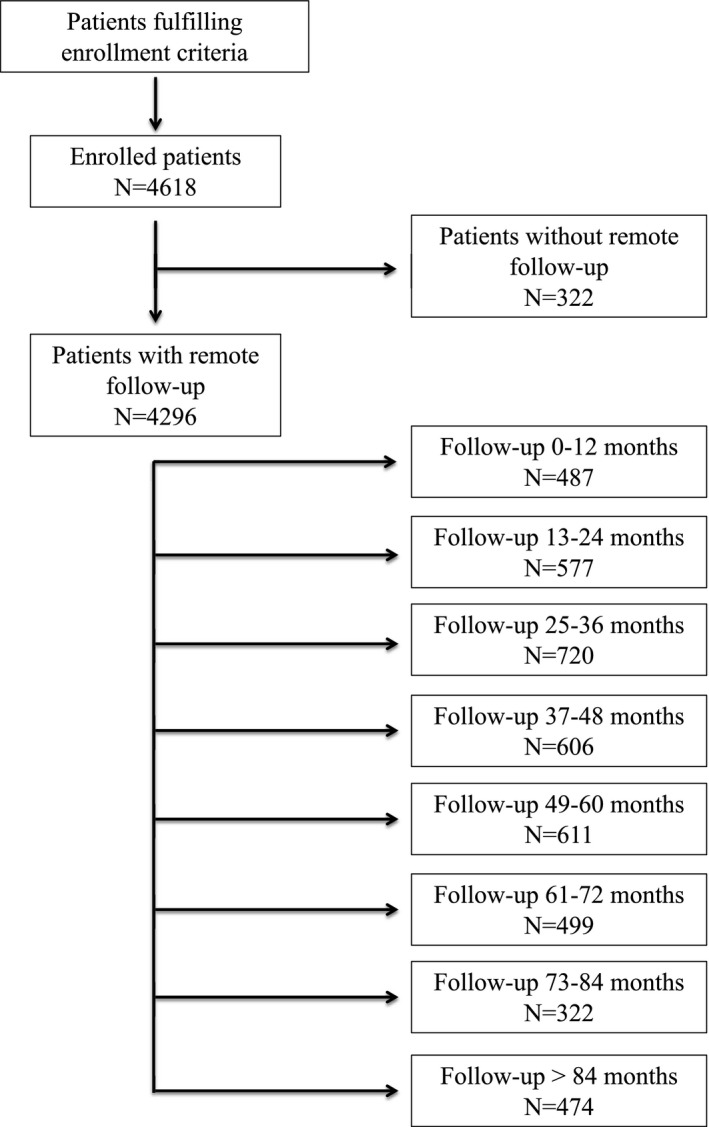
Table 1.
Baseline Characteristics at Implantation in the Follow‐Up Population and Study Subgroups
| Characteristic | Follow‐Up Population | ICM | DCM | VHD | HCM | ARVC | LQTS | BS | ACHD |
|---|---|---|---|---|---|---|---|---|---|
| No. (%) | 4296 (100) | 2150 (50) | 1166 (27.1) | 119 (2.8) | 294 (6.8) | 71 (1.7) | 43 (1) | 143 (3.3) | 60 (1.4) |
| Age, mean±SD, y | 61.9±12.9 | 65.6±10.1 | 62.1±12.1 | 63.5±12.6 | 52.6±15.1 | 47.4±11.9 | 51.1±18.5 | 50.3±12.6 | 45.6±14.2 |
| Sex (men) | 3527 (82.1) | 1938 (90.2) | 878 (75.3) | 90 (75.6) | 216 (73.5%) | 51 (71.8) | 12 (27.9) | 120 (83.9) | 34 (56.7) |
| Hypertension | 2275 (54.4) | 1358 (65) | 598 (52.4) | 60 (52.2) | 109 (37.7) | 14 (20.6) | 11 (25.6) | 24 (16.8) | 11 (18.3) |
| Diabetes mellitus | 1178 (28.3) | 758 (36.4) | 312 (27.5) | 24 (21.1) | 30 (10.3) | 0 (0) | 3 (2.3) | 9 (5.6) | 4 (6.7) |
| Hypercholesterolemia | 2040 (52.5) | 1291 (65.8) | 462 (43.8) | 40 (39.6) | 111 (41.6) | 16 (24.6) | 6 (12.5) | 33 (25.6) | 13 (22) |
| Smoking status | 1415 (37.7) | 852 (45.2) | 345 (33.8) | 21 (21.4) | 69 (26) | 10 (15.9) | 3 (7.7) | 40 (32.5) | 10 (16.9) |
| History of atrial fibrillation | 1235 (29.4) | 608 (29) | 401 (35.1) | 62 (53) | 89 (30.5) | 3 (4.3) | 7 (16.3) | 5 (3.5) | 13 (21.7) |
| Previous stroke | 252 (6.8) | 145 (7.9) | 60 (5.9) | 9 (9) | 17 (6.1) | 1 (1.7) | 3 (7.9) | 2 (1.6) | 3 (5.1) |
| Chronic kidney disease | 633 (15.3) | 380 (18.4) | 183 (16.3) | 17 (14.8) | 19 (6.6) | 3 (4.3) | 3 (7) | 4 (2.8) | 4 (6.7) |
| Family history of SCD | 356 (8.7) | 48 (2.4) | 93 (8.3) | 3 (2.7) | 104 (36.4) | 15 (22.1) | 10 (23.8) | 44 (31.9) | 14 (23.7) |
| LV ejection fraction, % | |||||||||
| ≤35 | 2841 (66.4) | 1633 (76.3) | 996 (85.6) | 64 (53.8) | 34 (11.6) | 11 (15.5) | 1 (2.3) | 1 (0.7) | 25 (41.7) |
| 36–50 | 631 (14.7) | 379 (17.7) | 131 (11.3) | 27 (22.7) | 35 (11.9) | 11 (15.5) | 5 (11.6) | 5 (3.5) | 9 (15) |
| >50 | 806 (18.9) | 128 (5.9) | 36 (3.1) | 28 (23.5) | 224 (76.5) | 49 (69) | 37 (86) | 137 (95.8) | 26 (43.3) |
| QRS width, ms | |||||||||
| <120 | 2202 (52.4) | 1154 (53.7) | 389 (34) | 42 (35.9) | 196 (68.3) | 65 (91.5) | 35 (81.4) | 127 (90.1) | 23 (40.4) |
| 120–150 | 877 (20.9) | 479 (22.3) | 249 (21.7) | 32 (27.4) | 48 (16.7) | 5 (7) | 5 (11.6) | 11 (7.8) | 9 (15.8) |
| >150 | 1119 (26.7) | 466 (21.7) | 507 (44.3) | 43 (36.8) | 43 (15) | 1 (1.4) | 3 (7) | 3 (2.1) | 25 (43.9) |
| Previous heart failure | 2973 (69.8) | 1564 (73.1) | 986 (85.4) | 89 (75.4) | 133 (45.2) | 26 (36.6) | 11 (25.6) | 23 (16.1) | 40 (66.7) |
| Type of device | |||||||||
| VR | 2027 (47.2) | 1107 (51.5) | 356 (30.5) | 46 (38.7) | 137 (46.6) | 48 (67.6) | 16 (37.2) | 133 (93) | 23 (38.3) |
| DR | 972 (22.6) | 511 (23.8) | 168 (14.4) | 20 (16.8) | 142 (48.3) | 23 (32.4) | 26 (60.5) | 10 (7) | 21 (35) |
| CRT‐D | 1297 (30.2) | 532 (24.7) | 642 (55.1) | 53 (44.5) | 15 (5.1) | 0 (0) | 1 (2.3) | 0 (0) | 16 (26.7) |
| Primary prevention | 2758 (64.2) | 1342 (62.4) | 899 (77.1) | 53 (44.5) | 228 (77.6) | 22 (31) | 4 (9.3) | 110 (76.9) | 37 (61.7) |
Data are given as number (percentage) unless otherwise noted. ACHD indicates adult congenital heart disease; ARVC, arrhythmogenic right ventricular cardiomyopathy; BS, Brugada syndrome; CRT‐D, cardiac resynchronization therapy‐defibrillator; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; ICM, ischemic cardiomyopathy; LQTS, long‐QT syndrome; LV, left ventricular; SCD, sudden cardiac death; and VHD, valvular heart disease.
ICD Type and Programming
Device models used in the UMBRELLA study are depicted in Table S3. ICD programming extracted from the first remote transmission is given in Table 2. Briefly, many primary prevention (PP) patients had a VT zone enabled (47.4%), and delayed high‐rate detection programming was only enabled in 39.7% (n=1704) of follow‐up population. Delayed high‐rate programming was defined according to current consensus 8 and comprised patients with a cutoff rate ≤320 ms and number of intervals to detect ≥30/40 within the VF zone. Nevertheless, adherence to programming recommendations increased through the study period (Table S4).
Table 2.
ICD Programming
| Variable | Follow‐Up Population (N=4296) | Primary Prevention (N=2758) | Secondary Prevention (N=1538) | P Value |
|---|---|---|---|---|
| Detection zones enabled | ||||
| Single zone* | 1853 (43.2) | 1451 (52.6) | 402 (26.2) | <0.001 |
| Multiple zones | 2440 (56.8) | 1305 (47.4) | 1135 (73.8) | |
| VF detection interval, ms | 302.7±19.4 | 302.9±18.7 | 302.4±20.4 | 0.396 |
| NID | <0.001 | |||
| <30/40 | 2490 (58) | 1508 (54.7) | 982 (63.8) | |
| ≥30/40 | 1805 (42) | 1249 (45.3) | 555 (36.2) | |
| Fast VT detection zone enabled † | 1557 (36.3) | 879 (31.9) | 678 (44.1) | <0.001 |
| Fast VT detection interval, ms † | 266.5±29.4 | 263.7±26 | 270±32.9 | <0.001 |
| VT detection interval, ms ‡ | 361.6±25.3 | 358.7±20.4 | 366.7±31.5 | <0.001 |
| NID ‡ | 0.617 | |||
| ≤16 | 1395 (57.2) | 740 (56.7) | 655 (57.7) | |
| ≥20 | 1045 (42.8) | 565 (43.3) | 480 (42.3) | |
| SmartShock technology–capable device | 2930 (72.1) | 1890 (72.6) | 1040 (71.0) | 0.267 |
Data are given as number (percentage) or mean±SD. ICD indicates implantable cardioverter‐defibrillator; NID, number of intervals to detect; VF, ventricular fibrillation; and VT, ventricular tachycardia.
Patients with fast VT zone enabled through VF zone, but without any other VT zone activated, were also considered as single‐zone detection patients.
Through VF detection zone.
Applies only to multiple‐zone detection programming patients.
SVA and Appropriate ICD Therapies
At study closure, a total of 27 472 stored arrhythmic episodes had been collected in 2048 patients (47.7%). After blinded analysis of the electrograms, 11 405 were catalogued as non‐SVA or nonventricular episodes. Therefore, 1344 patients (31.3% of follow‐up population) experienced 16 067 episodes of SVA; 1054 episodes (6.6%) were catalogued by the committee as VF/sustained polymorphic VT and 15 013 (93.4%) were catalogued as sustained monomorphic VT. The overall cumulative incidence of SVA was 23% (95% CI, 22%–24%) at 1 year, 34% (95% CI, 33%–35%) at 3 years, and 54% (95% CI, 53%–55%) at 6 years. The incidence rates of SVA, appropriate ICD therapy, and all‐cause death on the basis of the underlying heart disease and ICD indication are given in Table 3.
Table 3.
Incidence of Study End Points, According to the Underlying Heart Disease and ICD Indication
| Variable | SVA | P Value | Appropriate ICD Therapy | P Value | All‐Cause Death | P Value |
|---|---|---|---|---|---|---|
| ICM (n=2150) | 730 (34.0) | 658 (30.6) | 371 (17.3) | |||
| Primary prevention (n=1342) | 349 (26.0) | <0.001* | 310 (23.1) | <0.001* | 235 (17.5) | 0.686* |
| Secondary prevention (n=808) | 381 (47.2) | 348 (43.1) | 136 (16.8) | |||
| DCM (n=1166) | 355 (30.4) | 306 (26.2) | 167 (14.3) | |||
| Primary prevention (n=904) | 223 (24.8) | <0.001* | 187 (20.8) | <0.001* | 131 (14.6) | 0.656* |
| Secondary prevention (n=268) | 132 (49.4) | 119 (44.6) | 36 (13.4) | |||
| HCM (n=294) | 65 (22.1) | 49 (16.7) | 9 (3.1) | |||
| Primary prevention (n=228) | 38 (16.7) | <0.001* | 25 (10.1) | <0.001* | 5 (2.2) | 0.108* |
| Secondary prevention (n=66) | 27 (40.9) | 26 (39.4) | 4 (6.1) | |||
| VHD (n=119) | 49 (41.2) | 43 (36.1) | 19 (16) | |||
| Primary prevention (n=53) | 17 (33.1) | 0.071 | 16 (30.2) | 0.226* | 8 (15.1) | 0.816* |
| Secondary prevention (n=66) | 32 (48.5) | 27 (40.9) | 11 (16.7) | |||
| ARVC (n=71) | 31 (43.7) | 27 (38) | 1 (1.4) | |||
| Primary prevention (n=22) | 7 (31.8) | 0.178* | 6 (27.3) | 0.211* | 1 (4.5) | 0.310* |
| Secondary prevention (n=49) | 24 (49) | 21 (42.9) | 0 (0) | |||
| LQTS (n=43) | 8 (18.6) | 5 (11.6) | 1 (2.3) | |||
| Primary prevention (n=4) | 0 (0) | 0.424* | 0 (0) | 0.598* | 0 (0) | 0.746* |
| Secondary prevention (n=39) | 8 (20.5) | 5 (12.8) | 1 (2.6) | |||
| BS (n=143) | 13 (9.1) | 10 (7) | 1 (0.7) | |||
| Primary prevention (n=110) | 5 (4.5) | 0.002* | 3 (2.7) | 0.001* | 1 (0.9) | 0.583* |
| Secondary prevention (n=33) | 8 (24.2) | 7 (21.2) | 0 (0) | |||
| ACHD (n=60) | 23 (38.3) | 19 (31.7) | 5 (8.3) | |||
| Primary prevention (n=37) | 11 (29.7) | 0.082* | 8 (21.6) | 0.034* | 3 (8.1) | 0.936* |
| Secondary prevention (n=23) | 12 (52.2) | 11 (47.8) | 2 (8.7) |
Data are given as number (percentage). ACHD indicates adult congenital heart disease; ARVC, arrhythmogenic right ventricular cardiomyopathy; BS, Brugada syndrome; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; ICD, implantable cardioverter‐defibrillator; ICM, ischemic cardiomyopathy; LQTS, long‐QT syndrome; SVA, sustained ventricular arrhythmia; and VHD, valvular heart disease.
Comparison of the proportion of primary vs secondary prevention patients within the same cardiomyopathy subgroup.
An appropriate ICD therapy was delivered to 85.7% (n=13 767) episodes of SVA in 1173 patients (event rate, 27.3%). A total of 1334 episodes of the untreated SVA occurred in a monitor zone (58.4%). The remaining 966 events (41.6%) corresponded to episodes of SVA that terminated before the delivery of the programmed therapy. Among the treated events, 346 episodes (2.7%) of sustained monomorphic VT received high‐energy shocks, 11 074 (85.7%) received antitachycardia pacing, and 1508 (11.6%) received both therapies, whereas 615 episodes (73.3%) of sustained polymorphic VT/VF received high‐energy shocks, 63 (7.5%) received antitachycardia pacing, and 161 (19.2%) received both therapies. The overall cumulative incidence of appropriate ICD therapy was 14% (95% CI, 13%–15.2%) at 1 year, 26% (95% CI, 24.7%–27.7%) at 3 years, and 36% (95% CI, 34.4%–38.2%) at 6 years. A higher risk of first appropriate ICD therapy was observed in patients with VHD (HR, 1.94; 95% CI, 1.43–2.62; P<0.001), ARVC (HR, 1.84; 95% CI, 1.28–2.66; P=0.001), ICM (HR, 1.51; 95% CI, 1.29–1.78; P<0.001), and DCM (HR, 1.28; 95% CI, 1.07–1.53; P=0.005), whereas patients with HCM (HR, 0.72; 95% CI, 0.54–0.96; P=0.027) and BS (HR, 0.25; 95% CI, 0.14–0.45; P<0.001) were at significantly lower risk (Figure 2A). A trend toward lower risk was seen in LQTS (HR, 0.49; 95% CI, 0.22–1.09; P=0.082), whereas no differences in risk were observed in adult congenital heart disease (HR, 1.30; 95% CI, 0.85–1.99; P=0.218). Furthermore, the risk of appropriate ICD therapy was higher in secondary prevention (SP) patients than in PP patients (HR, 2.27; 95% CI, 2.02–2.54; P<0.001). The event incidence rate was 40.1% versus 20.4%, respectively (Figure 2B).
Figure 2. Kaplan‐Meier curves for first appropriate implantable cardioverter‐defibrillator (ICD) therapy according to underlying heart disease (A) and ICD indication (B); and for all‐cause death according to underlying heart disease (C) and ICD indication (D).
ACHD indicates adult congenital heart disease; ARVC, arrhythmogenic right ventricular cardiomyopathy; BS, Brugada syndrome; DCM, dilated cardiomyopathy; HCM, hypertrophic cardiomyopathy; ICM, ischemic cardiomyopathy; LQTS, long‐QT syndrome; and VHD, valvular heart disease.
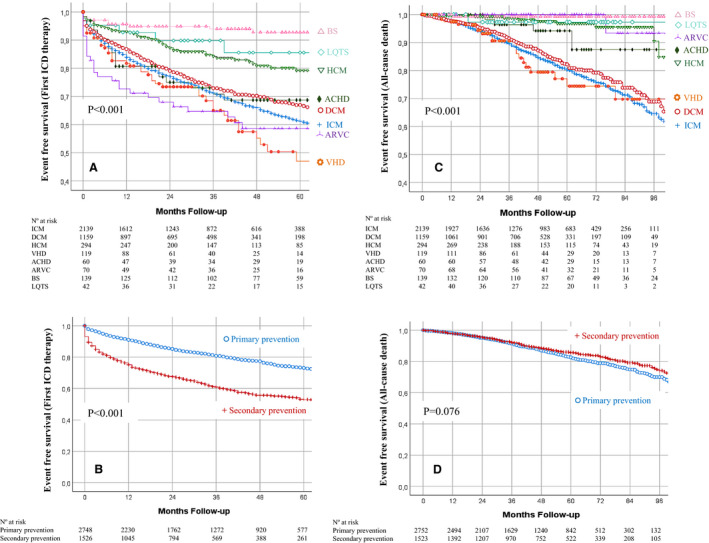
In the multivariate analysis (Table 4), age, sex, previous AF, SP, LVEF ≤35%, and QRS width (120–150 ms) emerged as clinical predictors of appropriate ICD therapy, whereas the implantation of a cardiac resynchronization therapy‐defibrillator correlated with lower risk. Among causative subgroups, an independently higher risk of appropriate ICD therapy was found in patients with DCM, VHD, and ARVC, whereas patients with BS exhibited a lower risk. Among ICD settings, delayed high‐rate detection in PP patients (Figure 3A) and single‐zone programming were associated with a lower risk of first appropriate ICD therapy.
Table 4.
Multivariate Cox Regression Analysis of the Study End Points
| End Point | Variable | HR (95% CI) | P Value |
|---|---|---|---|
| First appropriate ICD therapy | Clinical parameters | ||
| Age | 1.01 (1.00–1.01) | 0.023 | |
| Sex (men) | 1.57 (1.26–1.94) | <0.001 | |
| AF | 1.29 (1.12–1.49) | <0.001 | |
| Secondary prevention | 1.96 (1.67–2.31) | <0.001 | |
| QRS width, ms | |||
| >150 | 0.98 (0.81–1.21) | 0.896 | |
| 120–150 | 1.32 (1.09–1.61) | 0.005 | |
| <120 | REF | ||
| LVEF, % | |||
| ≤35 | 1.53 (1.18–1.97) | 0.001 | |
| 36–50 | 1.21 (0.92–1.58) | 0.168 | |
| >50 | REF | ||
| Type of device | |||
| CRT‐D | 0.76 (0.61–0.94) | 0.011 | |
| Dual‐chamber ICD | 1.03 (0.88–1.21) | 0.711 | |
| Single‐chamber ICD | REF | ||
| ICD settings | |||
| Single‐zone programming | 0.69 (0.59–0.81) | <0.001 | |
| Delayed high‐rate detection* | 0.76 (0.61–0.94) | 0.012 | |
| Causative subgroups | |||
| DCM | 1.30 (1.02–1.66) | 0.036 | |
| VHD | 1.54 (1.08–2.20) | 0.018 | |
| ARVC | 1.72 (1.14–2.61) | 0.010 | |
| BS | 0.32 (0.15–0.67) | 0.003 | |
| All‐cause death | Clinical parameters | ||
| Age | 1.05 (1.04–1.06) | <0.001 | |
| AF | 1.31 (1.09–1.59) | 0.004 | |
| Sex (men) | 1.43 (1.07–1.93) | 0.016 | |
| CKD | 1.75 (1.43–2.15) | <0.001 | |
| DM | 1.63 (1.35–1.96) | <0.001 | |
| LVEF, % | |||
| ≤35 | 2.44 (1.65–3.62) | <0.001 | |
| 36–50 | 1.79 (1.14–2.80) | 0.011 | |
| >50 | REF | ||
| Inappropriate shock | Clinical parameters | ||
| Age | 0.98 (0.97–0.99) | 0.001 | |
| AF | 2.40 (1.73–3.32) | <0.001 | |
| ICD settings | |||
| Single‐zone programming | 0.71 (0.51–0.97) | 0.035 | |
AF indicates atrial fibrillation; ARVC, arrhythmogenic right ventricular cardiomyopathy; BS, Brugada syndrome; CKD, chronic kidney disease; CRT‐D, cardiac resynchronization therapy‐defibrillator; DCM, dilated cardiomyopathy; DM, diabetes mellitus; HR, Hazard ratio; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; REF, reference group; and VHD, valvular heart disease.
Among primary prevention patients.
Figure 3. Kaplan‐Meier curves for first appropriate implantable cardioverter‐defibrillator (ICD) therapy, according to delayed high‐rate detection stratified by ICD indication (A), and for inappropriate shock, according to SmartShock technology (B).
PP indicates primary prevention; and SP, secondary prevention.
All‐Cause Death
At the end of follow‐up, 590 deaths (event rate, 13.4% of follow‐up population) had been reported. The causes of death are given in Table S5. The cumulative incidence of all‐cause death was 5% (95% CI, 4%–6%) at 1 year, 13% (95% CI, 12%–14%) at 3 years, and 25% (95% CI, 24%–26%) at 6 years. Patients with ICM (HR, 3.90; 95% CI, 2.58–5.90; P<0.001), DCM (HR, 3.33; 95% CI, 2.18–5.10; P<0.001), and VHD (HR, 3.97; 95% CI, 2.25–6.99; P<0.001) had worse prognoses (Figure 2C), and prognosis was significantly better in patients with BS (HR, 0.11; 95% CI, 0.01–0.67; P=0.017). ICD indication lacked a statistical relationship with mortality (HR, 1.19; 95% CI, 0.91–1.41; P=0.076). The event rate was 14.7% in PP patients and 13.9% in SP patients (Figure 2D).
In the multivariate analysis, sex, increasing age, previous AF, chronic kidney disease, diabetes mellitus, and impaired LVEF emerged as independent predictors of all‐cause death (Table 4). No excess risk of mortality was associated with any ICD setting.
Inappropriate Shocks
A total of 1375 patients (32% of follow‐up population) had 11 405 episodes catalogued as non‐SVA or non‐VA. Inappropriate ICD therapy was given in 13.2% (n=1503) of these episodes, but only 257 patients (6%) experienced an IS. The most frequent cause of an IS was AF and other supraventricular tachycardia (Table S6). The incidence of IS was higher in patients with HCM (9.4%; P=0.007), and a trend toward higher risk was seen in patients with ARVC (10.7%; P=0.065), but it was lower in patients with BS (1.3%; P=0.015) and ICM (5%; P=0.008). No significant differences were found in patients with DCM (6.8%; P=0.153), VHD (4.9%; P=0.477), adult congenital heart disease (3.1%; P=0.214), and LQTS (2%; P=0.232). The incidence of IS did not differ according to type of device (6.4% versus 6.2% versus 5.2% for VR, DR, and cardiac resynchronization therapy‐defibrillator, respectively; P=0.321) or ICD indication (6.8% for PP versus 5.5% for SP; P=0.081), but it was lower in patients implanted with SmartShock‐capable devices (4% versus 8.7%; P=0.002; Figure 3B).
In the multivariate analysis (Table 4), previous AF was the only clinical predictor of IS, whereas increasing age correlated with a lower risk. Among ICD settings, single‐zone programming was independently related to a lower risk of IS.
DISCUSSION
Our study comprises the largest contemporary cohort of ICD patients implanted according to current clinical guidelines and followed up over a long period of time. We had several key findings. First, left ventricular systolic dysfunction, and subsequent HF, remains the most frequent indication for ICD implantation, with PP as the leading strategy. Second, ICD patients with HF‐related disorders had a similar risk of life‐threatening VA, appropriate ICD therapies, and death, regardless of the cause (ischemic, dilated, or valvular heart disease). SP patients were more prone to VA and appropriate ICD therapies, but survival was similar to PP patients. In addition, prophylactic ICD patients with channelopathies and inherited cardiomyopathies presented a low risk of life‐threatening VA. Finally, delayed high‐rate and single‐zone detection programming were poorly implemented in patients with daily clinical ICD, and they were confirmed as essential programming strategies.
To the best of our knowledge, this is the first large‐scale study of ICD carriers including patients with no restriction on ICD indication or underlying heart disease. This point allows us to compare the evolution of different populations using an ICD in a real‐life scenario. The rigorous follow‐up allowed by the remote monitoring guarantees a reliable incidence of arrhythmia and ICD interventions and offers the opportunity to explore the impact that currently recommended programming strategies have on outcomes. A strength of the UMBRELLA study is that it provides a systematic review of stored arrhythmic events classified by a blinded committee of experts.
SVA and Appropriate ICD Therapies
The incidence of SVA and appropriate ICD interventions has declined over the past years. In major ICD trials, the incidence of appropriate therapies ranged from 17% to 31% in PP to 54% to 64% in SP studies. 9 Similar rates were reported in observational registries 10 , 11 with short‐term follow‐up that did not include channelopathies or inherited cardiomyopathies. More modern real‐world studies reported a lower rate of life‐threatening VA. In the DANISH ICD registry, 12 only 13.5% of the 1609 ischemic patients carrying a prophylactic ICD received an appropriate therapy. Nevertheless, the authors admit some underreporting of therapies, which is rare in the UMBRELLA study because of the automatic storage of all arrhythmic events. A contemporary but retrospective study from Amara et al 13 demonstrated 22.3% of appropriate therapies after a 3‐year follow‐up in a cohort of PP ICD‐only patients.
The incidence of appropriate therapies in the UMBRELLA study ranges from 7% in patients with BS to 38% in patients with ARVC. Our cohort is composed of 8 different cardiomyopathies, includes PP and SP, and has long‐term follow‐up. This kind of comparison has never been performed. VHD, ARVC, and DCM predicted a higher risk of appropriate ICD therapies, whereas BS correlated with lower risk, which indicates the importance of a pathological myocardial substrate on the risk of SCD. LVEF ≤35% was associated with a 1.5‐fold increased risk, whereas cardiac resynchronization therapy‐defibrillator patients, who have the chance for improved LVEF, were at significantly lower risk. This is somewhat striking, as concerns about the benefit of ICD in DCM are still present. 14 Our results suggest that patients with DCM, when carefully selected, are at high risk of life‐threatening VA and may obtain a similar benefit as other high‐risk populations (ICM/ARVC). In addition, the high arrhythmia risk found in patients with VHD agrees with published data on PP patients. 15 Left ventricle overload and remodeling attributable to valvular dysfunction seem to offer another pathological substrate for the occurrence of life‐threatening VA.
ICD shocks are life saving, but they are also associated with an increased risk of complications and death. We found that 14.3% of episodes of SVA did not receive an ICD intervention, which supports the existence of non–life‐threatening episodes of SVA. Current consensus recommends shock reduction strategies based on high‐rate cutoffs, delayed programming, and single‐zone detection. 8 These recommendations were derived from trials performed mainly in patients with HF. In the UMBRELLA study, high‐rate cutoffs were commonly programmed through the VF zone, but number of intervals to detect <30/40 was present in 58% of patients and VT zones were enabled in many PP patients. This may be explained by the 12‐year period of patient enrollment in which recommendations were continuously evolving.
Our results endorse the usefulness of single‐zone detection and the combination of high‐rate intervals with delayed detection. This setting was associated with 24% lower risk of appropriate ICD therapies among PP patients. Thus, despite an increase in the adoption of delayed programming throughout the study period, current programming recommendations still need to be implemented in patients using an ICD.
All‐Cause Death
Mortality was lower in the UMBRELLA study than in other observational reports, 11 , 12 , 13 , 16 which may be related to the inclusion of low‐risk populations. Our study reinforces the idea that ICM has an unfavorable prognosis. In addition, the prognosis of DCM was similar to ICM; thus, death in patients with left ventricular systolic dysfunction seems to be barely influenced by the cause of HF. Another important finding is that patients with VHD were not only at high risk of life‐threatening VA, but also death. Therefore, they probably represent a subgroup of patients with HF who may obtain a similar benefit from ICD as other patients with left ventricular systolic dysfunction. 15 However, the relationship between a high risk of malignant VA and death is not always present, as with ARVC, which is mainly an electrical disease. Taken together, the results indicate that the risk of death in patients using an ICD is influenced mainly by patient comorbidities and LVEF.
Predictors of death found in the UMBRELLA study are in agreement with the results of developed risk models. 17 , 18 , 19 Increasing age, AF, chronic kidney disease, diabetes mellitus, and impaired LVEF are well‐established predictors of death in patients using an ICD. Nevertheless, most of the models were performed with cohorts of PP and ischemic patients.
Other Subgroups
In the UMBRELLA study, the risk of arrhythmic events and death was low among HCM, LQTS, and BS carriers of a prophylactic ICD. As RTs are lacking, recommendations for ICD implantation have been derived from small observational reports. 3 , 4 , 5 , 6 Several stratification methods have been proposed to determine the minimum risk of SCD that could justify a prophylactic ICD. A 5‐year risk of SCD ≥6% supposes a class IIa recommendation for an ICD in patients with HCM, 3 and BS patients with 2 risk factors had a 5‐year risk of arrhythmic events of 10.2% in a recently developed score. 6 In the UMBRELLA study population of patients with HCM, LQTS, and BS, the risk of appropriate ICD therapy after a mean follow‐up of 46 months ranged from 0% to 10.1%, in PP setting. Therefore, in the absence of RTs, better stratification methods are necessary to select PP candidates with enough 5‐year risk of SCD to support the implantation of an ICD.
Inappropriate Shocks
Current programming recommendations led to a reduction of IS rates to <5%. 8 This tendency was confirmed in the UMBRELLA study, with an annual rate of 1.53%. Our results also agree with contemporary recommendations, as single‐zone detection correlated with 29% lower risk of IS.
AF was the leading cause of IS and correlated with a 2.4‐fold increased risk, but we also found that age was associated with a lower risk of IS. Although elderly patients are more prone to developing AF, the probability of rapid ventricular conduction may be lower. Thus, IS reduction strategies should be enhanced among younger patients using an ICD.
In theory, dual‐chamber devices have more power to discriminate between supraventricular tachycardia and VA, but several studies have failed to demonstrate this. 8 We think that our heterogeneous population supposes a barrier to identifying any potential benefit. We reported an independently lower risk of IS among dual‐chamber ICDs in the UMBRELLA study population of PP patients with HF. 20 SmartShock technology consists of 6 discrimination algorithms that are nominally activated to distinguish between true VA and other rhythms. As observed in previous reports, 21 the use of SmartShock technology in modern‐era ICD patients with real‐world programming led to a low IS rate in the UMBRELLA study.
Limitations
This study has limitations inherent to the observational design. The UMBRELLA study is a single‐brand ICD study and, as such, extrapolation of the conclusions to other brands should be made with caution. Nevertheless, characteristics of the UMBRELLA study population are similar to the characteristics of patients included in the last Spanish ICD Registry 22 (Table S7), which enrolled >90% of the devices implanted in our country. Therefore, we believe our sample might represent a real picture of the Spanish population using an ICD. ICD programming evolved throughout the 12‐year period of patient inclusion. We did not study temporal trends in programming because this topic is currently being analyzed in another study by our group. Although we included several populations that are underrepresented in RTs, the small size of these subgroups and the lack of specific predictors of death (ie, wall thickness in HCM) limit the power to extract stronger conclusions.
Conclusions
This contemporary nationwide study involved a broad ICD population with long‐term follow‐up. We observed a decrease in the incidence of SVA, appropriate ICD therapies, IS, and death compared with previous observational and randomized studies. Patients with myocardial substrate derived from an impaired LVEF (ICM, DCM, and VHD) were at higher risk of appropriate ICD interventions and death than those with channelopathies and HCM.
A better SCD risk stratification is necessary in PP ICD patients with channelopathies and inherited cardiomyopathies. A strategy based on delayed high‐rate detection and single‐zone programming still needs to be implemented in modern‐era patients using an ICD.
Appendix
Principal investigators and participating centers of the UMBRELLA study: Ricardo Pavón, Hospital Nuestra Señora De Valme (Sevilla); José María Arizón, Hospital Universitario Reina Sofía (Córdoba); Miguel Álvarez López, Hospital Universitario Virgen De Las Nieves (Granada); Javier Alzueta, Hospital Virgen De La Victoria (Málaga); Ernesto Díaz Infante, Hospital Universitario Virgen Macarena (Sevilla); David Calvo, Hospital Universitario Central De Asturias (Asturias); Juan Carlos Rodríguez, Hospital Insular De Gran Canaria (Gran Canaria); Aníbal Rodríguez, Hospital Universitario De Canarias (Tenerife); Luis Álvarez, Hospital Universitario Nuestra Señora De La Candelaria (Tenerife); Juanjo Olalla, Hospital Universitario Marqués De Valdecilla (Santander); Javier Jiménez Díaz, Hospital General De Ciudad Real (Ciudad Real); Javier Balaguer, Hospital General Y Universitario De Guadalajara (Guadalajara); Miguel Ángel Arias, Hospital Virgen De La Salud (Toledo); Jerónimo Rubio, Hospital Clinico Universitario De Valladolid (Valladolid); Marisa Fidalgo, Hospital De León (León); Francisco‐Javier García, Hospital Universitario De Burgos (Burgos); Xavier Viñolas, Hospital De La Santa Creu I Sant Pau (Barcelona); Josep Brugada, Hospital Clínico De Barcelona (Barcelona); Ignasi Anguera, Hospital De Bellvitge (Barcelona); Roger Villuendas, Hospital Germans Trias I Pujol (Barcelona); Rosa Porro, Hospital San Pedro De Alcántara (Cáceres); Joaquín Fernández de la Concha, Hospital Infanta Cristina (Badajoz); Luisa Pérez, Complejo Hospitalario Universitario A Coruña (A Coruña); Enrique García Campo, Complejo Hospitalario Universitario De Vigo (Pontevedra); Ignacio Fernández Lozano, Hospital Universitario Puerta De Hierro (Majadahonda); Adolfo Fontenla, Hospital 12 De Octubre (Madrid); Julián Villacastín, Hospital Clínico San Carlos (Madrid); Ángel Arenal, Hospital General Universitario Gregorio Marañón (Madrid); Roberto Muñoz Aguilera, Hospital Infanta Leonor (Madrid); Antonio Hernández, Hospital Universitario Ramón Y Cajal (Madrid); Rafael Peinado, Hospital Universitario La Paz (Madrid); Arcadio García Alberola, Hospital Clínico Universitario Virgen De La Arrixaca (Murcia); Fernando Pérez Lorente, Hospital General Universitario Reina Sofía (Murcia); Nuria Basterra, Hospital de Navarra (Navarra); María Fe Arcocha, Hospital de Basurto (Vizcaya); Andrés Bodegas, Hospital de Cruces (Baracaldo); Francisco Zumalde, Hospital de Galdakao‐Usansolo (Vizcaya); José B. Martínez Ferrer, Hospital Universitario De Áraba (Álava); José María Porres, Hospital Universitario Donostia (Guipúzcoa); José Moreno Arribas, Hospital Clínico San Juan (Alicante); Ricardo Ruiz, Hospital Clínico Universitario De Valencia (Valencia); Aurelio Quesada, Hospital General Universitario De Valencia (Valencia); Juan Gabriel Martínez, Hospital General Universitario De Alicante (Alicante); María José Sancho‐Tello, Hospital Universitario Y Politécnico De La Fe (Valencia).
Sources of Funding
The UMBRELLA study was supported by Medtronic Iberica.
Disclosures
Dr Briongos‐Figuero reports personal fees and nonfinancial support from Medtronic Iberica and personal fees from Boston Scientific. Dr García‐Alberola reports grants from Medtronic. Dr Rubio reports personal fees from Medtronic, St. Jude, and Boston Scientific. Dr Segura reports personal fees from Medtronic and grants from Boston Scientific. Dr Peinado reports personal fees from Medtronic and grants from Medtronic, Boston Scientific, and Abbot. Dr Martínez‐Ferrer reports personal fees and nonfinancial support from Medtronic. Drs Martín and Cerdá are employees of Medtronic.
Supporting information
Tables S1–S7
Acknowledgments
The authors appreciate the efforts made by the investigators of the UMBRELLA study and Medtronic personnel who have contributed to the success of this project.
(J Am Heart Assoc. 2021;10:e018108. DOI: 10.1161/JAHA.120.018108.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018108
For Sources of Funding and Disclosures, see page 13.
Contributor Information
Sem Briongos‐Figuero, Email: semdoc@hotmail.com.
the UMBRELLA Investigators:
Ricardo Pavón, José María Arizón, Miguel Álvarez López, Javier Alzueta, Ernesto Díaz Infante, David Calvo, Juan Carlos Rodríguez, Aníbal Rodríguez, Luis Álvarez, Juanjo Olalla, Javier Jiménez Díaz, Javier Balaguer, Miguel Ángel Arias, Jerónimo Rubio, Marisa Fidalgo, Francisco‐Javier García, Xavier Viñolas, Josep Brugada, Ignasi Anguera, Roger Villuendas, Rosa Porro, Joaquín Fernández de la Concha, Luisa Pérez, Enrique García Campo, Ignacio Fernández Lozano, Julián Villacastín, Ángel Arenal, Roberto Muñoz Aguilera, Antonio Hernández, Rafael Peinado, Arcadio García Alberola, Fernando Pérez Lorente, Nuria Basterra, María Fe Arcocha, Andrés Bodegas, Francisco Zumalde, José B Martínez Ferrer, José María Porres, José Moreno Arribas, Ricardo Ruiz, Aurelio Quesada, Juan Gabriel Martínez, and María José Sancho‐Tello
REFERENCES
- 1. Adabag AS, Luepker RV, Roger VL, Gersh BJ. Sudden cardiac death: epidemiology and risk factors. Nat Rev Cardiol. 2010;7:216–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Priori SG, Blomstrom‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, et al. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the Europe. Eur Heart J. 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 3. O’Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, et al. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk‐SCD). Eur Heart J. 2014;35:2010–2020. [DOI] [PubMed] [Google Scholar]
- 4. Orgeron GM, James CA, Te RA, Tichnell C, Murray B, Bhonsale A, Kamel IR, Zimmerman SL, Judge DP, Crosson J, et al. Implantable cardioverter‐defibrillator therapy in arrhythmogenic right ventricular dysplasia/cardiomyopathy: predictors of appropriate therapy, outcomes, and complications. J Am Heart Assoc. 2017;6:e006242. DOI: 10.1161/JAHA.117.006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schwartz PJ, Spazzolini C, Priori SG, Crotti L, Vicentini A, Landolina M, Gasparini M, Wilde AAM, Knops RE, Denjoy I, et al. Who are the long‐QT syndrome patients who receive an implantable cardioverter‐defibrillator and what happens to them? Data from the European Long‐QT Syndrome Implantable Cardioverter‐Defibrillator (LQTS ICD) Registry. Circulation. 2010;122:1272–1282. [DOI] [PubMed] [Google Scholar]
- 6. Sieira J, Conte G, Ciconte G, Chierchia G‐B, Casado‐Arroyo R, Baltogiannis G, Di GG, Saitoh Y, Julia J, Mugnai G, et al. A score model to predict risk of events in patients with Brugada syndrome. Eur Heart J. 2017;38:1756–1763. [DOI] [PubMed] [Google Scholar]
- 7. Fontenla A, Martinez‐Ferrer JB, Alzueta J, Vinolas X, Garcia‐Alberola A, Brugada J, Peinado R, Sancho‐Tello MJ, Cano A, Fernandez‐Lozano I. Incidence of arrhythmias in a large cohort of patients with current implantable cardioverter‐defibrillators in Spain: results from the UMBRELLA Registry. Europace. 2016;18:1726–1734. [DOI] [PubMed] [Google Scholar]
- 8. Wilkoff BL, Fauchier L, Stiles MK, Morillo CA, Al‐Khatib SM, Almendral J, Aguinaga L, Berger RD, Cuesta A, Daubert JP, et al. 2015 HRS/EHRA/APHRS/SOLAECE expert consensus statement on optimal implantable cardioverter‐defibrillator programming and testing. Heart Rhythm. 2016;13:e50–e86. [DOI] [PubMed] [Google Scholar]
- 9. Germano JJ, Reynolds M, Essebag V, Josephson ME. Frequency and causes of implantable cardioverter‐defibrillator therapies: is device therapy proarrhythmic? Am J Cardiol. 2006;97:1255–1261. [DOI] [PubMed] [Google Scholar]
- 10. van Welsenes GH, van Rees JB, Borleffs CJW, Cannegieter SC, Bax JJ, van Erven L, Schalij MJ. Long‐term follow‐up of primary and secondary prevention implantable cardioverter defibrillator patients. Europace. 2011;13:389–394. [DOI] [PubMed] [Google Scholar]
- 11. MacFadden DR, Crystal E, Krahn AD, Mangat I, Healey JS, Dorian P, Birnie D, Simpson CS, Khaykin Y, Pinter A, et al. Sex differences in implantable cardioverter‐defibrillator outcomes: findings from a prospective defibrillator database. Ann Intern Med. 2012;156:195–203. DOI: 10.7326/0003-4819-156-3-201202070-00007. [DOI] [PubMed] [Google Scholar]
- 12. Weeke P, Johansen JB, Jorgensen OD, Nielsen JC, Moller M, Videbaek R, Hojgaard MV, Riahi S, Jacobsen PK. Mortality and appropriate and inappropriate therapy in patients with ischaemic heart disease and implanted cardioverter‐defibrillators for primary prevention: data from the Danish ICD Register. Europace. 2013;15:1150–1157. DOI: 10.1093/europace/eut017. [DOI] [PubMed] [Google Scholar]
- 13. Amara N, Boveda S, Defaye P, Klug D, Treguer F, Amet D, Perier M‐C, Gras D, Algalarrondo V, Bouzeman A, et al. Implantable cardioverter‐defibrillator therapy among patients with non‐ischaemic vs. ischaemic cardiomyopathy for primary prevention of sudden cardiac death. Europace. 2018;20:65–72. [DOI] [PubMed] [Google Scholar]
- 14. Køber L, Thune JJ, Nielsen JC, Haarbo J, Videbæk L, Korup E, Jensen G, Hildebrandt P, Steffensen FH, Bruun NE, et al. Defibrillator implantation in patients with nonischemic systolic heart failure. N Engl J Med. 2016;375:1221–1230. DOI: 10.1056/NEJMoa1608029. [DOI] [PubMed] [Google Scholar]
- 15. Rodríguez‐Mañero M, Barrio‐López MT, Assi EA, Expósito‐García V, Bertomeu‐González V, Sánchez‐Gómez JM, González‐Torres L, García‐Bolao I, Gaztañaga L, Cabanas‐Grandío P, et al. Primary prevention of sudden death in patients with valvular cardiomyopathy. Rev Esp Cardiol (Engl Ed). 2016;69:272–278. DOI: 10.1016/j.rec.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 16. Al‐Khatib SM, Hellkamp A, Bardy GH, Hammill S, Hall WJ, Mark DB, Anstrom KJ, Curtis J, Al‐Khalidi H, Curtis LH, et al. Survival of patients receiving a primary prevention implantable cardioverter‐defibrillator in clinical practice vs clinical trials. JAMA. 2013;309:55–62. DOI: 10.1001/jama.2012.157182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. van Rees JB, Borleffs CJW, van Welsenes GH, van der Velde ET, Bax JJ, van Erven L, Putter H, van der Bom JG, Schalij MJ. Clinical prediction model for death prior to appropriate therapy in primary prevention implantable cardioverter defibrillator patients with ischaemic heart disease: the FADES risk score. Heart. 2012;98:872–877. DOI: 10.1136/heartjnl-2011-300632. [DOI] [PubMed] [Google Scholar]
- 18. Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, Maggioni AP, Anand I, Poole‐Wilson PA, Fishbein DP, et al. Maximizing survival benefit with primary prevention implantable cardioverter‐defibrillator therapy in a heart failure population. Circulation. 2009;120:835–842. DOI: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. 2012;60:1647–1655. DOI: 10.1016/j.jacc.2012.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Briongos‐Figuero S, Sánchez A, Pérez ML, Martínez‐Ferrer JB, García E, Viñolas X, Arenal Á, Alzueta J, Basterra N, Rodríguez A, et al. Single‐brand dual‐chamber discriminators to prevent inappropriate shocks in patients implanted with prophylactic implantable cardioverter defibrillators: a propensity‐weighted comparison of single‐ and dual‐chamber devices. J Interv Card Electrophysiol. 2019;54:267–275. DOI: 10.1007/s10840-018-0494-0. [DOI] [PubMed] [Google Scholar]
- 21. Auricchio A, Schloss EJ, Kurita T, Meijer A, Gerritse B, Zweibel S, AlSmadi FM, Leng CT, Sterns LD. Low inappropriate shock rates in patients with single‐ and dual/triple‐chamber implantable cardioverter‐defibrillators using a novel suite of detection algorithms: PainFree SST trial primary results. Heart Rhythm. 2015;12:926–936. DOI: 10.1016/j.hrthm.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 22. Fernandez Lozano I, Osca Asensi J, Alzueta RJ. Spanish Implantable Cardioverter‐Defibrillator Registry: 15th Official Report of the Spanish Society of Cardiology Electrophysiology and Arrhythmias Section (2018). Rev Esp Cardiol (Engl Ed). 2019;72:1054–1064. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S7


