Abstract
Background
Fine particulate matter <2.5 µm in diameter (PM2.5) has known effects on cardiovascular morbidity and mortality. However, no study has quantified and compared the risks of incident myocardial infarction, incident stroke, ischemic heart disease (IHD) mortality, and cerebrovascular mortality in relation to long‐term PM2.5 exposure.
Methods and Results
We sought to quantitatively summarize studies of long‐term PM2.5 exposure and risk of IHD and stroke events by conducting a review and meta‐analysis of studies published by December 31, 2019. The main outcomes were myocardial infarction, stroke, IHD mortality, and cerebrovascular mortality. Random effects meta‐analyses were used to estimate the combined risk of each outcome among studies. We reviewed 69 studies and included 42 studies in the meta‐analyses. In meta‐analyses, we found that a 10‐µg/m3 increase in long‐term PM2.5 exposure was associated with an increased risk of 23% for IHD mortality (95% CI, 15%–31%), 24% for cerebrovascular mortality (95% CI, 13%–36%), 13% for incident stroke (95% CI, 11%–15%), and 8% for incident myocardial infarction (95% CI, −1% to 18%). There were an insufficient number of studies of recurrent stroke and recurrent myocardial infarction to conduct meta‐analyses.
Conclusions
Long‐term PM2.5 exposure is associated with increased risks of IHD mortality, cerebrovascular mortality, and incident stroke. The relationship with incident myocardial infarction is suggestive of increased risk but not conclusive. More research is needed to understand the relationship with recurrent events.
Keywords: air pollution, cardiovascular, long‐term, mortality, particulate matter
Subject Categories: Cardiovascular Disease, Cerebrovascular Disease/Stroke, Mortality/Survival, Coronary Artery Disease
Nonstandard Abbreviations and Acronyms
- AHA
American Heart Association
- IHD
ischemic heart disease
- PM2.5
fine particulate matter <2.5 µm in diameter
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
Clinical Perspective
What Is New?
Evidence among 69 studies shows a clear relationship between long‐term particulate air pollution exposure and increased risk of cardiovascular events.
The largest risks were found for ischemic heart disease mortality and cerebrovascular mortality.
The association with incident myocardial infarction was suggestive of increased risk but not conclusive.
What Are the Clinical Implications?
Particulate air pollution exposure is a modifiable risk factor for cardiovascular events and should be considered along with other lifestyle and behavioral risk factors.
Populations at high risk for cardiovascular disease may be recommended to change behaviors to reduce personal exposure to particulate air pollution.
There is substantial evidence that exposure to fine particulate matter <2.5 µm in diameter (PM2.5) increases the risk of cardiovascular events and death, as concluded by the American Heart Association (AHA) scientific statement on particulate matter air pollution and cardiovascular disease in 2010. 1 Moreover, that report also concluded that long‐term exposures (eg, ≥1 year) posed an even greater risk to cardiovascular mortality than short‐term exposures (eg, a few days). 1 While a number of different cardiovascular disease (CVD) event end points have been studied in relation to long‐term PM2.5 exposure, there are few quantitative summaries available that synthesize and compare the magnitudes of these effects.
Early studies of long‐term PM2.5 exposure examined all‐cause mortality, lung cancer mortality, and cardiopulmonary mortality. 2 Subsequent studies of mortality end points have focused on cardiovascular mortality specifically 3 , 4 , 5 and subtypes such as ischemic heart disease (IHD) mortality 6 and stroke mortality. 7 While many studies have focused exclusively on mortality end points, some studies have examined incident cardiovascular events 8 , 9 , 10 or recurrent events among populations with preexisting CVD. 11 , 12 However, many studies of long‐term PM2.5 exposure and cardiovascular end points have published null or inconsistent findings, 8 , 13 , 14 , 15 , 16 , 17 , 18 , 19 which is a key reason to conduct a review and meta‐analysis.
Many studies have been published in the past decade, making a recent review important for understanding the current evidence. Furthermore, early studies of long‐term PM2.5 exposure focused more on the United States and Europe, 13 , 20 , 21 , 22 , 23 , 24 whereas in the past decade more studies have been published in other regions including China, Taiwan, Korea, Israel, Canada, and Australia. 7 , 12 , 41 There may also be differences in how cardiovascular end points are defined, such as using self‐reported outcomes, medical records, death certificate data, or different combinations of International Classification of Diseases (ICD) codes. While previous reviews have synthesized the overall evidence of PM2.5 and reviewed plausible mechanisms, 1 , 42 , 43 there is no recent quantitative meta‐analysis that compares the risks of IHD mortality, cerebrovascular mortality, incident stroke, and incident myocardial infarction in relation to long‐term PM2.5 exposure. Without this quantitative summary and comparison between CVD event types, it is difficult to determine which of these particular cardiovascular events have the greatest risk in relation to long‐term PM2.5 exposure. Furthermore, this information will help guide future studies needed to address research gaps.
We sought to quantitatively summarize the studies of long‐term PM2.5 exposure and risk of IHD and stroke events, including incident acute myocardial infarction (AMI), recurrent AMI, IHD mortality, incident stroke, recurrent stroke, and cerebrovascular mortality via meta‐analyses. This quantitative summary yields insight into which cardiovascular end points have the strongest associations in relation to long‐term PM2.5 exposure. Secondary objectives were to determine the consistency of the definitions used for the event end points and to identify gaps in the current knowledge.
Methods
The authors declare that all supporting data are available within the article and its supplementary files.
Search Process
To identify publications of long‐term ambient PM2.5 exposure and IHD and stroke events, SEA conducted a search of the National Library of Medicine's MEDLINE database through December 31, 2019, using PubMed. 44 Search terms included “air pollution,” “particulate matter,” “cohort,” “long‐term,” “annual,” “cardiovascular,” “CVD,” and “mortality.” X.L. independently reviewed the reference lists of several previous review articles 1 , 45 , 46 and identified relevant publications. Our inclusion criteria required articles to be peer‐reviewed, original, and empirical articles published in English.
Ischemic Heart Disease and Stroke Events
We restricted our review to studies of incident AMI, recurrent AMI, IHD mortality, incident stroke, recurrent stroke, and cerebrovascular mortality. In a supplementary analysis, we also provided an updated meta‐analysis of overall cardiovascular mortality. This review does not include more general mortality end points such as all‐cause mortality, natural‐cause mortality, cardiometabolic mortality (combining cardiovascular and diabetes mellitus mortality), or cardiopulmonary mortality (combining cardiovascular and lung disease mortality).
Statistical Analysis
We conducted a meta‐analysis for all outcomes that were analyzed in at least 4 studies. For sensitivity analyses examining subgroups of the main outcomes, we only required 3 studies to conduct meta‐analyses. When multiple studies examined the same outcome using the same cohort, we included only 1 study per cohort. We selected the largest study with the most years of follow‐up and the most recent air pollution estimates that reported the association for the main effect of long‐term average PM2.5 exposure (ie, not an interaction effect, effect modification, or an association of PM2.5 components). All study effect estimates were converted to represent a change of 10 µg/m3. We quantified heterogeneity by the I 2 statistic 47 and random effects meta‐analysis was used to account for heterogeneity. 48 We assessed outliers and publication bias using funnel plots. We also used the Newcastle‐Ottawa Scale for cohort studies to assess the risk of bias for individual studies. 49 For studies that were extreme outliers, we computed the meta‐analysis twice, where the primary meta‐analysis excluded the extreme outlier study and the secondary meta‐analysis included the extreme outlier in order to understand the sensitivity of the results on the extreme outlier study.
Results
Articles Identified
Article identification is summarized using the PRISMA flow diagram (Figure S1). The MEDLINE database search using PubMed resulted in 2138 potentially relevant publications. X.L. independently identified 29 relevant publications from reference lists of previously published review articles. 1 , 45 , 46 After record screening, 165 full‐text articles were assessed for eligibility. Our final list included 69 studies that examined the relationship between long‐term PM2.5 exposure and incident AMI, recurrent AMI, IHD mortality, incident stroke, recurrent stroke, and/or cerebrovascular mortality among 37 unique cohorts or consortia. These cohorts or consortia represented different international populations: United States (14 cohorts), Europe (9 cohorts and 1 consortia of cohorts), Asia (6 cohorts), Canada (6 cohorts), and Australia (1 cohort). The year of publication of the identified articles is illustrated in Figure S2. Notably, 62 of these 69 studies (90%) have been published in the past decade, after the 2010 AHA scientific statement on PM2.5 exposure and CVD risk.
Cardiovascular Event End Points
Our first objective was to determine which of the cardiovascular event end points have been studied the most. We found that IHD mortality was the most frequently studied cardiovascular end point (45 studies), followed by cerebrovascular mortality (27 studies), stroke (23 studies; includes incident and recurrent events), and AMI (20 studies; includes incident and recurrent events).
Study Characteristics
The Table presents characteristics of the 69 studies 4 , 8 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 38 , 39 , 40 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 examining the association between long‐term PM2.5 exposure and CVD events. All studies appropriately controlled for basic demographics such as age and sex, and most studies accounted for race and calendar time such as year of study enrollment. Nearly all studies controlled for socioeconomic status (SES) and many studies also included marital status. Education and income were the most common SES variables used and were measured on individual or neighborhood levels depending on data availability in studies. Numerous studies controlled for variables related to health and lifestyle, including body mass index, smoking, alcohol consumption, diet, and relevant comorbidities such as diabetes mellitus, hypertension, and hyperlipidemia. Additionally, some studies controlled for personal or family history of risk factors and diseases (history of myocardial infarction, stroke, or coronary heart disease), medication use (blood pressure medication, statins, or aspirin), or revascularization procedures (percutaneous coronary intervention or coronary artery bypass grafting).
Table 1.
Characteristics of 69 Studies Examining the Association Between Long‐Term PM2.5 Exposure and CVD Events
| First Author and Year | Cohort | Total Participants | Study Region | Follow‐Up Period | Covariates Adjusted for | Outcome Event (no. of Cases) | ICD‐9 and ICD‐10 Codes | Source of Outcome Data |
|---|---|---|---|---|---|---|---|---|
| Atkinson 2013 8 | CPRD | 836 557 | England | 2003–2007 | Age, sex, comorbidities,* BMI, smoking, SES † | Incident AMI (n=13 956), incident stroke (n=13 012) |
AMI: ICD‐10: I21‐I23 Stroke: ICD‐10: I61, I63 |
Incident: national database and medical records |
| Badaloni 2017 53 | RoLS | 1.2 million | Italy | 2001–2010 | Age, sex, calendar time, marital status, SES, † other covariates ‡ | IHD mortality (n=22 234) | IHD: ICD‐9: 410–414 | Mortality: national database |
| Bai 2019 25 | ONPHEC | 5.1 million | Canada | 2001–2015 | Age, sex, education, income, SES, † other covariates ‡ | Incident AMI (n=197 628) | AMI: ICD:9: 410; ICD‐10: I21 | Incident: national database and medical records |
| Beelen 2014 16 | ESCAPE | 367 383 | Europe | Varies by cohort 1985–2004 | Age, sex, calendar time, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=4992), cerebrovascular mortality (n=2484) |
IHD: ICD‐9: 410–414; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–438; ICD‐10: I60–I69 |
Mortality: national database |
| Cakmak 2016 26 | CanCHEC | 2.4 million | Canada | 1991–2006 | Age, sex, race, marital status, SES † | IHD mortality (n=57 310), cerebrovascular mortality (n=17 565) |
IHD: ICD‐9: 410–414; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–438; ICD‐10: I60–I69 |
Mortality: national database |
| Cakmak 2018 27 | CanCHEC | 2.3 million | Canada | 1991–2011 | Age, sex, race, marital status, SES † | IHD mortality (n=NL) | IHD: ICD‐9: 410–414; ICD‐10: I20–I25 | Mortality: national database |
| Carey 2013 15 | CPRD | 835 607 | England | 2003–2007 | Age, sex, BMI, smoking, SES † | IHD mortality (n=8168), cerebrovascular mortality (n=5458) |
IHD: ICD‐10: I20–I25 Cerebrovascular: ICD‐10: I61, I63 |
Mortality: national database |
| Carey 2016 50 | CPRD | 211 016 | England | 2005–2011 | Age, sex, BMI, smoking, SES † | Incident AMI (n=2582), incident stroke (n=3716) |
AMI: ICD‐10: I21–I23 Stroke: ICD‐10: I61, I63 |
Incident: medical records |
| Cesaroni 2013 51 | RoLS | 1.3 million | Italy | 2001–2010 | Age, sex, marital status, SES, † other covariates ‡ | IHD mortality (n=22 562), cerebrovascular mortality (n=13 576) |
IHD: ICD‐9: 410–414 Cerebrovascular: ICD‐9: 430–438 |
Mortality: national database |
| Cesaroni 2014 54 | ESCAPE | 100 166 | Europe | 1997–2007 | Age, sex, calendar time, smoking, marital status, SES † | Incident AMI (n=5157) | AMI: ICD‐9: 410, 411; ICD‐10: I21, I23, I20.0, I24 | Incident: national database and medical records |
| Chen 2005 13 | AHSMOG | 3239 | United States | 1977–1998 | Age, sex, calendar time, BMI, smoking, diet, § SES † | IHD mortality (n=250) | IHD: ICD‐9: 410–414 | Mortality: national database and other sources ‖ |
| Chen 2016 28 | EFFECT | 8873 | Canada | 1999–2011 | Age, sex, family history, ¶ comorbidities,* smoking, CVD history, ¶ revascularization, medications, marital status, SES, † other covariates ‡ | IHD mortality (n=1650) | IHD: ICD‐9: 410–414 | Mortality: national database |
| Chi 2016 52 | WHI study | 51 754 | United States | 1993–2005 | Age, race, comorbidities,* BMI, smoking, SES † | Incident AMI (n=NL), incident stroke (n=NL) | NL | Incident: national database, medical records, and other sources ‖ |
| Crichton 2016 55 | SLSR | 357 308 | England | 2005–2012 | Age, sex, SES † | Incident stroke (n=1800) | NL | Incident: medical records |
| Crouse 2012 29 | CanCHEC | 2.1 million | Canada | 1991–2001 | Age, sex, race, marital status, SES, † other covariates ‡ | IHD mortality (n=43 400), cerebrovascular mortality (n=13 300) |
IHD: ICD‐9: 410–414; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–434, 436–438; ICD‐10: I60–I69 |
Mortality: national database |
| Crouse 2015 30 | CanCHEC | 2.5 million | Canada | 1991–2006 | Age, sex, race, marital status, SES † | IHD mortality (n=63 050), cerebrovascular mortality (n=19 725) |
IHD: ICD‐9: 410–414; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–438; ICD‐10: I60–I69 |
Mortality: national database |
| Danesh Yazdi 2019 61 | Medicare beneficiaries | 11.1 million | United States | 2000–2012 | Age, sex, race, calendar time, SES, † other covariates ‡ | Incident AMI (n=570 668), incident stroke (n=991 077) |
Stroke: ICD‐9: 430–438 AMI: ICD‐9: 410 |
Incident: national database and medical records |
| Dirgawati 2019 31 | HIMS | 10 126 | Australia | 1996–2012 | Age, comorbidities,* BMI, smoking, SES † | Cerebrovascular mortality (n=325); incident stroke (n=1453) |
Cerebrovascular: ICD‐9: 430–431, 433, 434.x1, 435–436; ICD‐10: I60–I61, I63–I64, I66, I69 Stroke: ICD‐9: 430–431, 433.x1, 434. x1, 436; ICD‐10: I60–I61, I63–I64 |
Mortality: national database Incident: medical records |
| Gan 2011 19 | Residents in Vancouver | 452 735 | Canada | 1999–2002 | Age, sex, comorbidities*, SES † | IHD mortality (n=3104) | IHD: ICD‐9: 410–414, 429.2; ICD‐10: I20–I25 | Mortality: national database |
| Gandini 2018 62 | ILS | 74 989 | Italy | 1999–2008 | Age, sex, BMI, smoking, physical activity, marital status, SES, † other covariates ‡ | Incident AMI (n=NL), incident stroke (n=1505) |
AMI: ICD‐9: 410 Stroke: NL |
Incident: national database and medical records |
| Hart 2011 18 | Trucking industry men | 53 814 | United States | 1985–2000 | Age, race, calendar time, other covariates ‡ | IHD mortality (n=1109) | IHD: ICD‐9: 410–414; ICD‐10: I20–I25 | Mortality: national database |
| Hart 2015 56 | Nurses' Health Study | 114 537 | United States | 1989–2006 | Age, race, calendar time, family history, ¶ comorbidities,* BMI, smoking, marital status, SES, † other covariates ‡ | Incident IHD (n=3878), incident stroke (n=3295) | AMI: ICD‐9: 410–412; ICD‐10: I21–I22 Stroke: NL |
Mortality: national database and other sources ‖ Incident: medical records and other sources§ |
| Hartiala 2016 57 | Cleveland Clinic GeneBank study | 6575 | United States | 2001–2010 | Age, sex, smoking, SES † | Nonfatal AMI (n=5854), nonfatal stroke (n=5875) | NL | Incident: medical records and other sources ‖ |
| Hayes 2020 59 | NIH‐AARP | 565 477 | United States | 1995–2011 | Age, sex, race, BMI, smoking, alcohol, marital status, SES, † other covariates ‡ | IHD mortality (n=23 328), cerebrovascular mortality (n=5894) |
IHD: ICD‐10: I20–I25 Cerebrovascular: ICD‐10: I60–I69 |
Mortality: national database |
| Heritier 2019 60 | SNC | 4.4 million | Switzerland | 2000–2008 | Age, sex, race, marital status, SES † | AMI mortality (n=19 261) | AMI: ICD‐10: I21–I22 | Mortality: national database |
| Hoffmann 2015 58 | RECALL (part of ESCAPE) | 4433 | Germany | 2000–2012 | Age, sex, calendar time, BMI, smoking, alcohol, physical activity, marital status, SES † | IHD mortality (n=135); incident stroke (n=71) |
IHD: ICD‐10: I20–I25 Stroke: ICD‐10: I61, I63 |
Mortality: national database and other sources ‖ |
| Huang 2019 32 | China‐PAR | 117 575 | China | 2000–2015 | Age, sex, comorbidities,* BMI, smoking, alcohol, physical activity, SES, † other covariates ‡ | Incident stroke (n=3540) | Incident stroke: ICD‐10: I60–I69 | Incident: other sources ‖ |
| Jerrett 2005 20 | American Cancer Society CPS‐II | 22 905 | United States | 1982–2000 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=1462) | IHD: ICD‐9: 410–414 | Mortality: national database after 1989 and other sources ‖ before 1989 |
| Jerrett 2013 63 | American Cancer Society CPS‐II | 73 711 | United States | 1982–2000 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=4540), cerebrovascular mortality (n=3068) | NL | Mortality: national database after 1989 and other sources ‖ before 1989 |
| Jerrett 2017 64 | American Cancer Society CPS‐II | 668 629 | United States | 1982–2004 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=45 624) | IHD: ICD‐9: 410–414; ICD‐10: I20–I25 | Mortality: national database after 1989 and other sources ‖ before 1989 |
| Kim 2017 33 | NHIS‐NSC | 136 094 | Korea | 2007–2013 | Age, sex, comorbidities,* BMI, SES, † other covariates ‡ | Incident AMI (n=354), incident stroke (n=934) |
AMI: ICD‐10: I21–I23 Stroke: ICD‐10: I60–I63 |
Incident: national database and medical records |
| Koton 2013 12 | Israel Study of First Acute Myocardial Infarction | 1120 | Israel | 1992–2011 | Age, sex, comorbidities,* BMI, smoking, physical activity, CVD history, ¶ revascularization, SES, † other covariates ‡ | Recurrent AMI (n=341), stroke hospitalizations (n=160) | NL | Recurrent and hospitalizations: medical records and other sources ‖ |
| Lim 2019 69 | NIH‐AARP | 548 845 | United States | 1995–2011 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=22 329), cerebrovascular mortality (n=5592) |
IHD: ICD‐9: 410–414; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–438; ICD‐10: I60–I69 |
Mortality: national database |
| Lipsett 2011 65 | CTS | 73 489 | United States | 1999–2005 | Age, race, family history, ¶ BMI, smoking, alcohol, diet, § physical activity, medications, # marital status, SES, † other covariates ‡ | IHD mortality (n=773), cerebrovascular mortality (n=382); incident AMI (n=722), incident stroke (n=969) |
IHD: ICD‐9: 410–414; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–438; ICD‐10: I60–I69 AMI: ICD‐9: 410; ICD‐10: I21 Stroke: ICD‐9: 431–434, 436; ICD‐10: I61–I64 |
Mortality: national database Incident: national database, medical records, or other sources ‖ |
| Ljungman 2019 71 | Swedish cohorts (includes the Primary Prevention Study (PPS) and the Multinational Monitoring of Trends and Determinants in Cardiovascular Diseases (GOT‐MONICA)) | 114 758 | Sweden | 1990–2011 | Age, sex, calendar time, smoking, alcohol, physical activity, marital status, SES † | Incident AMI (n=5166), incident stroke (n=3119) |
AMI: ICD‐9: 410–414; ICD‐10: I20–25 Stroke: ICD‐9: 431–436; ICD‐10: I61–I65 |
Incident: national database and medical records |
| Loop 2018 70 | REGARDS | 17 126 | United States | 2003–2012 | Age, sex, race, calendar time, comorbidities,* BMI, smoking, alcohol, physical activity, medications, # SES, † other covariates ‡ | IHD mortality (n=215); nonfatal AMI (n=413) | NL |
Mortality: national database and other sources ‖ Incident: medical records and other sources ‖ |
| Madrigano 2013 66 | Worcester Heart Attack Study | 4467 | United States | 1995–2003 | Age, sex, SES, † other covariates ‡ | Incident AMI (n=4467) | NL | Incident: medical records |
| Miller 2007 21 | WHI study | 65 893 | United States | 1994–2003 | Age, race, comorbidities,* BMI, smoking, SES, † other covariates ‡ | IHD mortality (n=139), cerebrovascular mortality (n=122); incident AMI (n=584), incident stroke (n=554) |
IHD: NL Cerebrovascular: NL AMI: ICD‐9: 410 Stroke: ICD‐9: 430–434, 436.0 |
Mortality: national database and other sources ‖ Incident: national database and other sources ‖ |
| Ostro 2010 67 | CTS | 44 847 | United States | 2002–2007 | Age, race, family history, ¶ BMI, smoking, alcohol, diet, § physical activity, medications, # marital status, SES, † other covariates ‡ | IHD mortality (n=474) | IHD: ICD‐10: I20–I25 | Mortality: national database |
| Ostro 2015 68 | CTS | 101 884 | United States | 2001–2007 | Age, race, family history, ¶ BMI, smoking, alcohol, diet, § physical activity, medications, # marital status, other covariates ‡ | IHD mortality (n=1085) | IHD: ICD‐10: I20–I25 | Mortality: national database |
| Parker 2018 74 | NHIS | 657 238 | United States | 1997–2011 | Age, sex, race, calendar time, marital status, SES, † other covariates ‡ | IHD mortality (n=NL), cerebrovascular mortality (n=NL) |
IHD: NL Cerebrovascular: ICD‐10: I60–I69 |
Mortality: national database |
| Pinault 2016 34 | CCHS | 299 500 | Canada | 2000–2011 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES † | IHD mortality (n=4700), cerebrovascular mortality (n=1500) |
IHD: ICD‐10: I20–I25 Cerebrovascular: ICD‐10: I60–I69 |
Mortality: national database |
| Pinault 2017 35 | CanCHEC | 2.4 million | Canada | 2001–2011 | Age, sex, race, marital status, SES, † other covariates ‡ | IHD mortality (n=40 400), cerebrovascular mortality (n=13 300) |
IHD: ICD‐10: I20–I25 Cerebrovascular: ICD‐10: I60–I69 |
Mortality: national database |
| Pope 2004 22 | American Cancer Society CPS‐II | 500 000 | United States | 1982–1998 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=26 663), cerebrovascular mortality (n=7650) | IHD: ICD‐9: 410–414 | Mortality: national database after 1989 and other sources ‖ before 1989 |
| Pope 2009 23 | American Cancer Society CPS‐II | 500 000 | United States | 1982–1988 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=NL) | IHD: ICD‐9: 410–414 | Mortality: national database after 1989 and other sources ‖ before 1989 |
| Pope 2011 73 | American Cancer Society CPS‐II | 794 784 | United States | 1982–1988 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=11 607) | IHD: ICD‐9: 410–414 | Mortality: national database after 1989 and other sources ‖ before 1989 |
| Pope 2015 72 | American Cancer Society CPS‐II | 669 046 | United States | 1982–2004 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=45 644), cerebrovascular mortality (n=17 085) | IHD: ICD‐9: 410–414; ICD‐10: I20–I25 | Mortality: national database after 1989 and other sources ‖ before 1989 |
| Pope 2019 75 | NHIS | 635 539 | United States | 1986–2015 | Age, sex, race, calendar time, BMI, smoking, marital status, SES, † other covariates ‡ | Cerebrovascular mortality (n=6297) | Cerebrovascular: ICD‐10: I60–I69 | Mortality: national database |
| Puett 2009 24 | Nurses' Health Study | 66 250 | United States | 1992–2002 | Age, calendar time, family history, ¶ comorbidities,* BMI, smoking, physical activity, SES, † other covariates ‡ | IHD mortality (n=379), nonfatal AMI (n=854) | NL |
Mortality: national database and other sources ‖ Incident: medical records and other sources ‖ |
| Puett 2011 17 | HPFS | 17 545 | United States | 1989–2003 | Age, calendar time, family history, ¶ comorbidities,* BMI, smoking, alcohol, diet, § physical activity, other covariates ‡ | IHD mortality (n=746); nonfatal stroke (n=300), nonfatal AMI (n=646) | NL |
Mortality: national database and other sources ‖ Incident: medical records and other sources ‖ |
| Pun 2017 76 | Medicare beneficiaries | 18.9 million | United States | 2000–2008 | Age, sex, race, calendar time, SES, † other covariates ‡ | IHD mortality (n=890 806), cerebrovascular mortality (293 786) |
IHD: ICD‐10: I20–I25 Cerebrovascular: ICD‐10: I60–I69 |
Mortality: national database |
| Qiu 2017 36 | Elderly Health Centre of the Department of Health | 61 447 | China | 1998–2012 | Age, sex, BMI, smoking, alcohol, physical activity, medications, # SES, † other covariates ‡ | Incident stroke (n=6733) | Stroke: ICD‐9: 430–436 | Incident: medical records |
| Shin 2014 78 | CanCHEC | 2.1 million | Canada | 1991–2001 | Age, sex, race, marital status, SES, † other covariates ‡ | IHD mortality (n=NL) | NL | Mortality: national database |
| Shin 2019 38 | ONPHEC | 5.1 million | Canada | 2001–2015 | Age, sex, SES, † other covariates ‡ | Incident stroke (n=122 545) | Incident stroke: ICD‐9: 430–431, 434, 436; ICD‐10: I60–I61, I63.x (excluding I63.6), I64 | Incident: national database and medical records |
| Stafoggia 2014 10 | ESCAPE | 99 446 | Europe | 1992–2010 | Age, sex, calendar time, smoking, marital status, SES † | Incident stroke (n=3086) | NL | Incident: national database, medical records, other sources ‖ |
| Stockfelt 2017 77 | PPS Sweden | 5850 | Sweden | 1990–2011 | Age, calendar time, smoking, physical activity, marital status, SES † | Incident stroke (n=1139) | Stroke: ICD‐9: 431–436; ICD‐10: I61–I65 | Incident: national database and medical records |
| Stockfelt 2017 77 | GOT‐MONICA | 4500 | Sweden | 1990–2011 | Age, sex, calendar time, smoking, physical activity, marital status, SES † | Incident stroke (n=252) | Stroke: ICD‐9: 431–436; ICD‐10: I61–I65 | Incident: national database and medical records |
| Thurston 2016 4 | American Cancer Society CPS‐II | 445 860 | United States | 1982–2004 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=34 408) | IHD: ICD‐9: 410–414; ICD‐10: I20–I25 | Mortality: national database after 1989 and other sources ‖ before 1989 |
| Tonne 2016 11 | MINAP | 18 138 | London | 2003–2010 | Age, sex, calendar time, CVD history, ¶ revascularization, SES, † other covariates ‡ | Recurrent AMI (n=390) | NL | Recurrent: national database and medical records |
| Tseng 2015 14 | Civil servants | 43 227 | Taiwan | 1992–2008 | Age, sex, BMI, smoking, alcohol, marital status, SES † | IHD mortality (n=139), cerebrovascular mortality (n=141) |
IHD: ICD‐9: 410–414, 429.2, 429.7; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–438; ICD‐10: I60–I69 |
Mortality: national database |
| Turner 2016 80 | American Cancer Society CPS‐II | 669 046 | United States | 1982–2004 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=45 644), cerebrovascular mortality (n=17 085) |
IHD: ICD‐9: 410–414; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–438; ICD‐10: I60–I69 |
Mortality: national database after 1989 and other sources ‖ before 1989 |
| Turner 2017 79 | American Cancer Society CPS‐II | 429 406 | United States | 1982–2004 | Age, sex, race, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=13 478), cerebrovascular mortality (n=5582) |
IHD: ICD‐9: 410–414; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–438; ICD‐10: I60–I69 |
Mortality: national database after 1989 and other sources ‖ before 1989 |
| Villeneuve 2015 39 | CNBSS | 89 248 | Canada | 1980–2005 | Age, calendar time, BMI, smoking, marital status, SES † | IHD mortality (n=903), cerebrovascular mortality (n=434) |
IHD: ICD‐9: 410–414; ICD‐10: I20–I25 Cerebrovascular: ICD‐9: 430–438; ICD‐10: I60–I69 |
Mortality: national database |
| Weichenthal 2014 6 | AHS | 83 378 | United States | 1993–2009 | Age, sex, calendar time, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=213), cerebrovascular mortality (n=242) |
IHD: ICD‐10: I25 Cerebrovascular: ICD‐10: I60–I69 |
Mortality: national database |
| Weichenthal 2016 40 | CanCHEC | 193 300 | Canada | 1991–2009 | Age, sex, race, marital status, SES † | IHD mortality (n=8600) | IHD: ICD‐9: 410–414; ICD‐10: I20–I25 | Mortality: national database |
| Wolf 2015 9 | ESCAPE | 100 166 | Europe | 1992–2007 | Age, sex, calendar time, smoking, marital status, SES † | Incident AMI (n=5157) | AMI: ICD‐9: 410–411; ICD‐10: I20.0, I21, I23–I24 | Incident: national database and medical records |
| Wong 2015 41 | Elderly Health Centre of the Department of Health | 66 820 | China | 1998–2011 | Age, sex, BMI, smoking, physical activity, SES, † other covariates ‡ | IHD mortality (n=1810), cerebrovascular mortality (n=1621) |
IHD: ICD‐10: I20–I25 Cerebrovascular: ICD‐10: I60–I69 |
Mortality: national database |
| Yin 2017 7 | Chinese men | 189 793 | China | 1990–2006 | Age, BMI, smoking, alcohol, diet, § marital status, SES, † other covariates ‡ | IHD mortality (n=3752), cerebrovascular mortality (n=11 301) |
IHD: ICD‐9: 410–414 Cerebrovascular: ICD‐9: 431–438 |
Mortality: national database |
| Yitshak‐Sade 2018 81 | Medicare beneficiaries | 2.0 million | United States | 2001–2011 | Age, sex, race, calendar time, SES, † other covariates ‡ | Ischemic stroke hospitalizations (n=211 235) | Ischemic stroke: ICD‐9: 432–435 | Hospitalizations: medical records |
AHS indicates Agricultural Health Study; AHSMOG, Adventist Health Study on the Health Effects of Smog; American Cancer Society CPS‐II, Cancer Prevention Study‐II; AMI, acute myocardial infarction; BMI, body mass index; CanCHEC, Canadian Census Health and Environment Cohort; CCHS, Canadian Community Health Survey; China‐PAR, Prediction for Atherosclerotic Cardiovascular DiseaseRisk in China; CNBSS, Canadian National Breast Screening Study; CPRD, Clinical Practice Research Datalink; CTS, California Teachers Study; EFFECT, Enhanced Feedback For Effective Cardiac Treatment; ESCAPE, European Study of Cohorts for Air Pollution Effects; GOT‐MONICA, Multinational Monitoring of Trends and Determinants in Cardiovascular Diseases; HIMS, Health in Men Study; HPFS, Health Professionals Follow‐up Study; ICD‐9, International Classification of Diseases, Ninth Revision; ICD‐10, International Classification of Diseases, Tenth Revision; IHD, ischemic heart disease; ILS, Italian Longitudinal Study; MINAP, Myocardial Ischaemia National Audit Project; NHIS, National Health Interview Survey; NHIS‐NSC, National Health Insurance Service–National Sample Cohort; NIH‐AARP, National Institutes of Health—AARP Diet and Health Study; NL, not listed; ONPHEC, Ontario Population Health and Environment Cohort; PM2.5, fine particulate matter <2.5 µm in diameter; PPS, Primary Prevention Study; RECALL, Heinz Nixdorf Recall Study; REGARDS, Reasons for Geographic and Racial Differences in Stroke; RoLS, Rome Longitudinal Study; SLSR, South London Stroke Register; SNC, Swiss National Cohort; and WHI, Women's Health Initiative.
Comorbidities may include diabetes mellitus, hyperlipidemia, hypertension, chronic obstructive pulmonary disease, chronic renal failure, end‐stage renal disease, or cancer.
Socioeconomic status (SES) may include education, income, occupation/employment, Medicaid eligibility, or other indicators. These variables were measured on individual or neighborhood levels depending on data availability in studies.
Other covariates may include menopausal status/hormone replacement therapy use, cardiac risk scores/disease severity indices, blood measures (blood glucose, cholesterol, hemoglobin), blood pressure, thrombolysis, acute pulmonary edema, Killip class, asthma, admitting hospital/in‐hospital care, second‐hand smoke exposure, occupational/industrial exposure, household exposure, survey design, or other neighborhood factors such as population size, urban/rural area, airshed location, residential location/Census region, place of birth, percent of race/ethnicity or age group, county‐level smoking, distance to supermarket/recreation area, or season of year/temperature.
Diet may include intake of vegetables, fruit/citrus, grains/fiber, fat, and calories.
Other mortality outcome sources: interviews or reports from physicians, relatives, postal authorities, or other witnesses (often with death certificate, medical record, or autopsy confirmation or adjudication by an end point committee or physician); or church records with nosologist review. Other incident outcome sources: self‐reported questionnaires, personal interviews, next‐of‐kin/proxy reports, or postal authorities (often death certificate or medical record confirmation or adjudication by an end point committee or physician).
Family history may include history of myocardial infarction, stroke, or coronary heart disease; cardiovascular disease (CVD) history may include previous myocardial infarction (or subtype, eg, ST‐segment–elevation myocardial infarction), previous stroke, or chronic coronary heart disease.
Medications may include blood pressure medication, statins, or aspirin.
IHD Mortality
IHD mortality was the most studied outcome, analyzed in 45 studies among 24 cohorts.* Nearly all studies (37 of 45) defined IHD deaths by the same set of ICD codes (ICD, Ninth Revision [ICD‐9]: 410–414; ICD, Tenth Revision [ICD‐10]: I20–I25) with data on death obtained from a national database of death records (Table). Some studies obtained data on deaths from medical records, proxy reports, and/or church records often with death certificate review by a certified nosologist or physician adjudicator. One study described these IHD deaths as “coronary heart disease deaths,” defined by the same set of ICD codes. 39 Two studies used a slightly broader definition of IHD mortality: 1 study added deaths caused by “CVD unspecified” (ICD‐9: 429.2) and 1 study added deaths caused by “certain sequelae of myocardial infarction not elsewhere classified” (ICD‐9: 429.7). 14 , 19 Last, 3 studies used narrower definitions, with 1 study 56 excluding causes of death from “angina pectoris” and “other forms of chronic ischemic heart disease” (ICD‐9: 413–414, ICD‐10: I23–I25), 1 study 6 defining IHD deaths using a code only for “chronic ischemic heart disease” (ICD‐10: I25), and another study 60 only defining death from myocardial infarction (ICD‐10: I21–I22).
Funnel plots indicated no extreme outlier studies and no evidence of publication bias for IHD mortality (Figure S3). Figure 1 illustrates the meta‐analysis of the relative risk (RR) of IHD mortality associated with long‐term PM2.5 exposure, combining effects of 23 studies.† Fifteen of the 23 studies reported a statistically significant increased risk in IHD mortality, while 8 studies reported a CI that included the null. The combined estimate of the RR of IHD mortality among studies was 1.23 (95% CI, 1.15–1.31) per 10‐µg/m3 increase in long‐term PM2.5. There was substantial heterogeneity among studies (I 2=94.4%).
Figure 1. Meta‐analysis of the relative risk of ischemic heart disease mortality per 10‐µg/m3 increase in long‐term fine particulate matter <2.5 µm in diameter exposure, combining the effects of 23 studies. 11 .
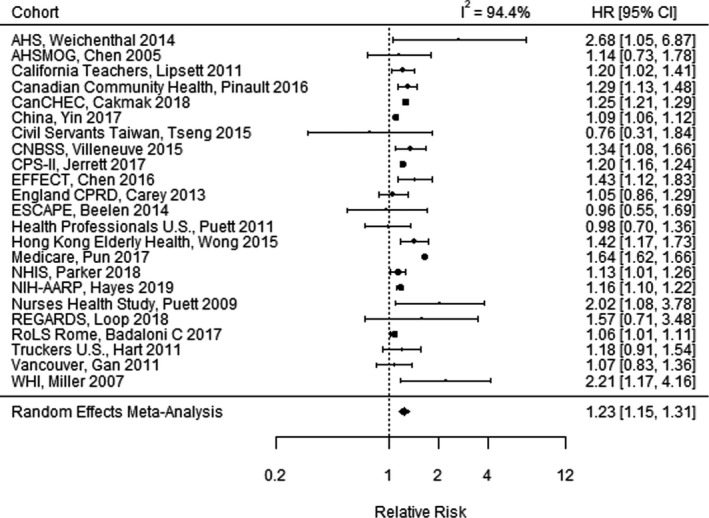
AHS indicates Agricultural Health Study; AHSMOG, Adventist Health Study on the Health Effects of Smog; CanCHEC, Canadian Census Health and Environment Cohort; CNBSS, Canadian National Breast Screening Study; CPRD, Clinical Practice Research Datalink; CPS‐II, American Cancer Society Cancer Prevention Study‐II; EFFECT, Enhanced Feedback For Effective Cardiac Treatment; ESCAPE, European Study of Cohorts for Air Pollution Effects; HR, hazard ratio; NHIS, National Health Interview Survey; NIH‐AARP, National Institutes of Health—AARP Diet and Health Study; REGARDS, Reasons for Geographic and Racial Differences in Stroke; RoLS, Rome Longitudinal Study; and WHI, Women's Health Initiative.
Incident Myocardial Infarction
We identified 17 studies‡ of long‐term PM2.5 exposure and incident AMI events in study populations of adults without a previous AMI. Most studies included incident events that may be fatal or nonfatal, while 4 studies 17 , 24 , 57 , 70 focused specifically on incident nonfatal AMI events. Of the 17 studies of AMI, five 21 , 25 , 61 , 62 , 65 restricted the definition of AMI to codes explicitly for AMI (ICD‐9: 410; ICD‐10: I21). Two studies 8 , 50 used a broader definition of AMI that also included diagnoses for “subsequent ST‐segment–elevation myocardial infarction and non–ST‐segment–elevation myocardial infarction” and “certain current complications following ST‐segment–elevation myocardial infarction and non–ST‐segment–elevation myocardial infarction” (ICD‐10: I22–I23), and 2 additional studies further broadened the definition of AMI by adding diagnoses of “unstable angina and other acute and subacute ischemic heart diseases” (ICD‐9: 410; ICD‐10: I20.0, I24). 9 , 54 Two studies did not examine AMI directly but instead examined the broader end point of incident IHD. 56 , 71 In most of these studies, ICD codes were obtained from a combination of hospitalization records, medical records, and a national database of death records. Six studies used self‐report or proxy report to define AMI with confirmation by medical records and did not list specific ICD codes used to define the outcome (Table).
Figure 2 illustrates the results of the meta‐analysis pooling results among 11 studies§ for incident AMI. Four of the 11 studies reported a statistically significant increased risk of incident AMI, while 6 studies reported a CI that included the null, and 1 study (REGARDS [Reasons for Geographic and Racial Differences in Stroke] trial, 2018) reported a decreased risk. The combined RR of incident AMI in the meta‐analysis was 1.08 (95% CI, 0.99–1.18) per 10‐µg/m3 increase in long‐term PM2.5. Funnel plots indicated no evidence of publication bias but did identify 1 extreme outlier study (Kim et al, 2017) 33 , which was excluded from the primary meta‐analysis (Figure S3). The REGARDS trial, which reported a decreased risk of AMI, did not appear to be an outlier. The extreme outlier study of patients in Seoul, Korea, reported an RR of 21.65 (95% CI, 5.49–85.36) per 10‐µg/m3 increase in long‐term PM2.5. 33 In a sensitivity analysis, we recomputed the meta‐analysis with the outlier study included, which resulted in a combined RR of 1.09 (95% CI, 0.99–1.20). Thus, the meta‐analysis results were not sensitive to the inclusion or exclusion of this extreme outlier study.
Figure 2. Meta‐analysis of the relative risk of incident acute myocardial infarction per 10‐µg/m3 increase in long‐term fine particulate matter <2.5 µm in diameter exposure, combining the effects of 11 studies. 12 .
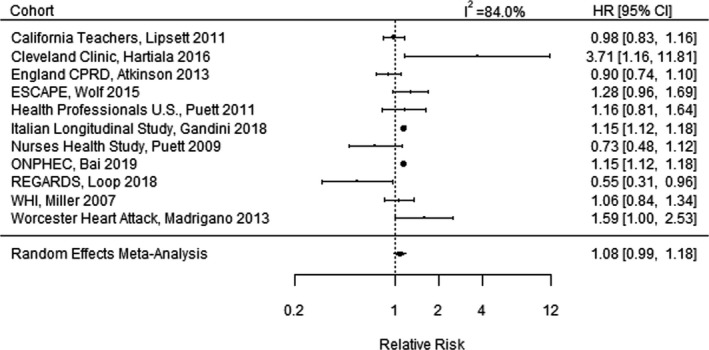
CPRD indicates Clinical Practice Research Datalink; ESCAPE, European Study of Cohorts for Air Pollution Effects; HR, hazard ratio; ONPHEC, Ontario Population Health and Environment Cohort; REGARDS, Reasons for Geographic and Racial Differences in Stroke; and WHI, Women's Health Initiative.
Recurrent Myocardial Infarction
Two studies 11 , 12 looked at recurrent AMI events in a population of adults with previous AMI. Koton et al 12 reported an RR of 1.7 (95% CI, 0.9–2.9) per 10‐µg/m3 increase in long‐term PM2.5 exposure. Tonne et al 11 measured risk for a combined outcome of recurrent AMI events with all‐cause mortality and found an RR of 1.94 (95% CI, 0.51–6.98) per 10‐µg/m3 increase in exhaust PM2.5 and 2.68 (95% CI, 0.72–13.01) for nonexhaust PM2.5. Another study examined hospital admissions for AMI, yet patient history of a previous AMI was unknown so hospitalizations included both incident and recurrent events. 61 Since there were only 2 studies of recurrent AMI events, we did not conduct a meta‐analysis for the association of long‐term PM2.5 exposure and recurrent AMI.
Cerebrovascular Mortality
The relationship between long‐term PM2.5 exposure and cerebrovascular mortality was analyzed in 27 studies among 17 cohorts.¶ Most studies (19 of 27) defined cerebrovascular deaths by the same set of ICD codes (ICD‐9: 430–438; ICD‐10: I60–I69) obtained from a national database of death records. Some studies obtained data on death from medical records, proxy reports, and/or postal officials with death certificate review by physician adjudicators. One study looked only at fatal strokes (ICD‐9: 430–431, 433.x1, 434.x1, 436; ICD‐10: I60–I61, I63–I64). 31 For consistency between ICD‐9 and ICD‐10 codes, 1 study explicitly excluded “transient cerebral ischemia” from the list of ICD‐9 codes (ICD‐9: 435), which in ICD‐10 is now listed as G45, within diseases of the nervous system. 29 Other definitions of cerebrovascular mortality were similar but excluded some causes of death such as death from “subarachnoid hemorrhage” (ICD‐9: 430, ICD‐10: I60). The narrowest definition of cerebrovascular mortality, used in 1 study, included only “nontraumatic intracerebral hemorrhage” (ICD‐10: I61) and “cerebral infarction” (ICD‐10: I63). 15 Two studies did not list specific ICD codes used to define the outcome (Table). No studies of cerebrovascular mortality examined ischemic stroke mortality separately from hemorrhagic stroke mortality.
Figure 3 illustrates the results of the meta‐analysis for cerebrovascular mortality, combining results among 17 studies.# Funnel plots indicated no extreme outlier studies and no evidence of publication bias for cerebrovascular mortality (Figure S3). Nine of the 17 studies reported a statistically significant increased risk in cerebrovascular mortality, while 8 studies reported a CI that included the null. The combined RR of cerebrovascular mortality was 1.24 (95% CI, 1.13–1.36) per 10‐µg/m3 increase in long‐term PM2.5. There was substantial heterogeneity among studies (I 2=94.4%).
Figure 3. Meta‐analysis of the relative risk of cerebrovascular mortality per 10‐µg/m3 increase in long‐term fine particulate matter <2.5 µm in diameter exposure, combining the effects of 17 studies. 13 .
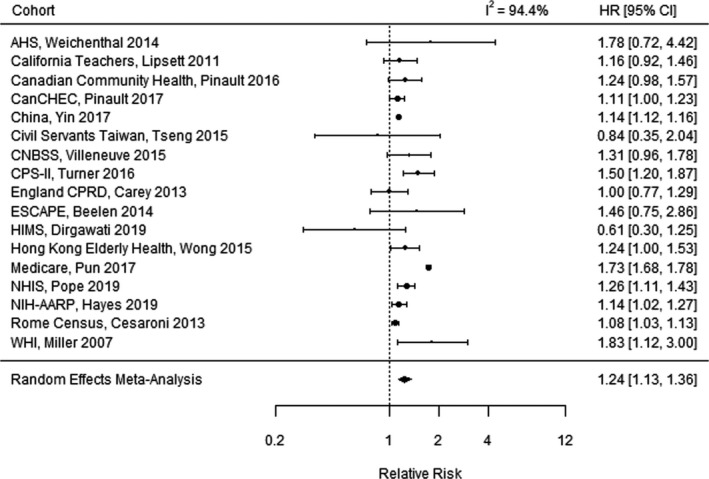
AHS indicates Agricultural Health Study; CanCHEC, Canadian Census Health and Environment Cohort; CNBSS, Canadian National Breast Screening Study; CPRD, Clinical Practice Research Datalink; CPS‐II, American Cancer Society Cancer Prevention Study‐II; ESCAPE, European Study of Cohorts for Air Pollution Effects; HR, hazard ratio; HIMS, Health in Men Study; NHIS, National Health Interview Survey; NIH‐AARP, National Institutes of Health—AARP Diet and Health Study; and WHI, Women's Health Initiative.
Incident Stroke
We identified 20 studies of long‐term PM2.5 exposure and incident stroke with study populations restricted to patients without a previous stroke.‖ Of these 20 studies, 3 studies 8 , 50 , 58 defined stroke events narrowly, only including “nontraumatic intracerebral hemorrhage” (ICD‐10: I61) and “cerebral infarction” (ICD‐10: I63) (Table). Two studies broadened this definition to also include other cerebrovascular disease diagnoses such as “other and unspecified nontraumatic intracranial hemorrhage” (ICD‐10: I62) 65 or “nontraumatic subarachnoid hemorrhage” (ICD‐10: I60). 38 Multiple studies used a wider definition, with 1 study using codes ICD‐10 I60–I69, 32 another study 36 using codes ICD‐9 430–436, and 4 studies using slightly varied versions of the latter definition: 1 study 21 excluded “transient cerebral ischemia” (ICD‐9: 435), 1 study 31 excluded “other and unspecified intracranial hemorrhage” (ICD‐9: 432), and 2 studies 71 , 77 excluded “subarachnoid hemorrhage” (ICD‐9: 430). In total, 4 studies included transient cerebral ischemia attack events (ICD‐9: 435; ICD‐10: G45) in their definition of stroke 31 , 71 , 77 and no studies examined transient cerebral ischemia attack events as a separate outcome. Two studies only included nonfatal incident strokes. 17 , 57 ICD codes were obtained from a combination of hospitalization records, medical records, and a national database of death records. Eight studies used self‐report or proxy report to define stroke events with confirmation by medical records, and 4 of those studies did not list specific ICD codes used to define the outcome.
Figure 4 illustrates the meta‐analysis for incident stroke, combining effects among 14 studies.** The RR of an incident stroke in the random effects meta‐analysis was 1.13 (95% CI, 1.11–1.15) per 10‐µg/m3 increase in long‐term PM2.5. Funnel plots indicated no evidence of publication bias but did identify 1 extreme outlier study, which was excluded from the primary meta‐analysis (Figure S3). The outlier study of patients in Seoul, Korea, reported an RR of 21.65 (95% CI, 5.49–85.36) per 10‐µg/m3 increase in long‐term PM2.5. 33 As a sensitivity analysis, we recomputed the meta‐analysis with the outlier study included, which resulted in an RR of 1.28 (95% CI, 0.92–1.78), a substantial change. Thus, the meta‐analysis results were sensitive to the inclusion or exclusion of this extreme outlier study, where inclusion of this outlier study caused both the combined effect estimate and width of the CI to increase substantially. This increase in variability was also reflected in the heterogeneity estimate, where I 2 increased from 0% to 99.4% when the extreme outlier study was included.
Figure 4. Meta‐analysis of the relative risk of incident stroke per 10‐µg/m3 increase in long‐term fine particulate matter <2.5 µm in diameter exposure, combining effects of 14 studies. 11 .
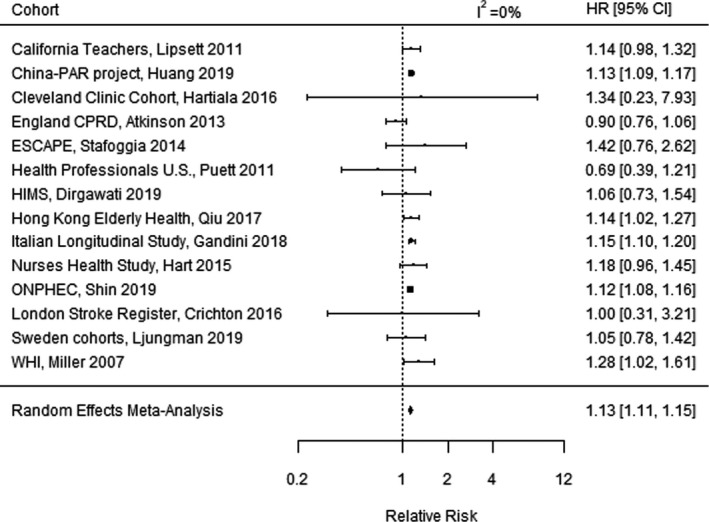
China‐PAR indicates Prediction for Atherosclerotic Cardiovascular Disease Risk in China; CPRD, Clinical Practice Research Datalink; ESCAPE, European Study of Cohorts for Air Pollution Effects; HR, hazard ratio; HIMS, Health in Men Study; ONPHEC, Ontario Population Health and Environment Cohort; and WHI, Women's Health Initiative.
Six studies of incident stroke further analyzed ischemic and hemorrhagic strokes separately. 17 , 32 , 33 , 36 , 38 , 55 Therefore, we conducted separate meta‐analyses of incident ischemic stroke and incident hemorrhagic stroke. Our meta‐analyses included 5 studies; the extreme outlier study from the overall incident stroke meta‐analysis above was excluded. The RR of an incident ischemic stroke in the random effects meta‐analysis was 1.18 (95% CI, 1.14–1.22), with low heterogeneity among studies (I 2=11.8%). The RR of an incident hemorrhagic stroke in the random effects meta‐analysis was 1.10 (95% CI, 1.05–1.16), with no heterogeneity among studies (I 2=0.0%). The excluded outlier study 33 reported an RR of 41.08 (95% CI, 14.88–117.02) for ischemic stroke and 7.30 (95% CI, 1.63–33.33) for hemorrhagic stroke per 10‐µg/m3 increase in long‐term PM2.5.
Recurrent Stroke
No studies examined recurrent stroke directly, although several studies included recurrent strokes. For example, 3 studies 12 , 61 , 81 examined stroke hospitalizations where patient history of a previous stroke was unknown; therefore, these hospitalizations could include both incident and recurrent events. One of these studies 12 examined stroke hospitalizations among a cohort of patients with a previous AMI and found an RR of 1.1 (95% CI, 0.5–2.5) per 10‐µg/m3 increase in long‐term PM2.5. The other 2 studies were among Medicare beneficiaries: 1 study 61 reported an RR of 1.36 (95% CI, 1.34–1.36) per 10‐µg/m3 increase in long‐term PM2.5, and the other study 81 specifically examined ischemic stroke hospitalizations and reported an RR of 1.04 (95% CI, 0.97–1.11). Since these stroke hospitalizations could be incident or recurrent, we did not include these 3 studies in the meta‐analysis for the association of long‐term PM2.5 exposure and incident stroke, and we were unable to conduct a meta‐analysis of recurrent stroke because of an insufficient number of studies.
Cardiovascular Mortality
In a supplementary analysis, we also provide an updated meta‐analysis of overall cardiovascular mortality by combining the effects of 28 studies.†† We found an RR of 1.14 (95% CI, 1.08–1.21) per 10‐µg/m3 increase in long‐term PM2.5. Details are provided in Figure S4.
Heterogeneity Among Studies
We found high heterogeneity in the estimated associations among studies for 3 of the study outcomes (I 2=94.4% for IHD mortality, 94.4% for cerebrovascular mortality, 84.0% for incident AMI), and no heterogeneity (I 2=0%) for incident stroke. There are many factors that may have contributed to heterogeneity among studies, including differences in the age, sex, race, and SES of study participants; adjustment of covariates; follow‐up period; country and region of exposure; and variability in the ICD codes used to define each outcome as described above and in the Table. Newcastle‐Ottawa Scale rankings and details are also provided in Table S1. Overall, studies rated from 7 to 9, which demonstrates that studies used in our meta‐analyses generally had low risk of bias according to the scale. More attention should be placed on the finer differences between studies such as sample size, covariates adjusted for, ICD codes used, and sources of outcome data provided in the Table. We analyzed which studies contributed most to the heterogeneity of each meta‐analysis. For IHD mortality and cerebrovascular mortality, Pun 76 performed a study of 18.9 million Medicare beneficiaries, which was most influential; when this study was removed, I 2 changed from 94.4% to 73.0% for IHD mortality and from 94.4% to 42.1% for cerebrovascular mortality. Pun reported an RR of 1.64 (95% CI, 1.62–1.66) for IHD mortality and 1.73 (95% CI, 1.68–1.78) for cerebrovascular mortality per 10‐µg/m3 increase in long‐term PM2.5, with a narrow CI because of the large size of the study. This study included Medicare beneficiaries among the United States who were 65 years and older, used zip code–level rather than address‐level PM2.5 exposure, and adjusted for age, sex, race, calendar time, and county‐level variables for smoking, diabetes mellitus, body mass index, alcohol consumption, asthma, and median income because of lack of data on personal health characteristics. These factors likely contributed to the heterogeneity of results found in this study.
For the incident AMI meta‐analysis, Atkinson et al 8 was most influential; when this study was removed, the I 2 changed from 84.0% to 0%, and the combined effect estimate changed from an RR of 1.08 (95% CI, 0.99–1.18) to an RR of 1.15 (95% CI, 1.13–1.17) per 10‐µg/m3 increase in long‐term PM2.5. Atkinson and colleagues included 836 557 patients aged 40 to 89 years in England; adjusted for all key covariates including age, sex, smoking, body mass index, diabetes mellitus, hypertension, and SES; had a short follow‐up period of 5 years (2003 through 2007); used a mean annual PM2.5 exposure of 12.9 µg/m3 and a small interquartile range of 1.9 µg/m3; and did not include any model performance statistics for PM2.5 exposures. The study also reported a large change in the effect estimate caused by control for SES (hazard ratio [HR] of 1.12 [95% CI, 0.92–1.36] without SES and 0.90 [95% CI, 0.74–1.10] with SES) per 10‐µg/m3 increase in long‐term PM2.5. These factors likely contributed to the heterogeneity of results found in this study.
We also considered differences in effects by world region by conducting separate meta‐analyses for IHD mortality and cerebrovascular mortality by world region: North America, Europe, and Asia. Meta‐analysis results are reported per 10‐µg/m3 increase in long‐term PM2.5 exposure. For IHD mortality, we found the highest risk in North America (combined HR, 1.27; 95% CI, 1.18–1.38 [I 2=93.2%]; 17 studies), moderately increased risk in Asia (combined HR, 1.18; 95% CI, 0.93–1.50 [I 2=73.0%]; 3 studies), and the smallest increases in risk in Europe (combined HR, 1.06; 95% CI, 1.01–1.11 [I 2=0%]; 3 studies). For cerebrovascular mortality, we also found the highest risk in North America (combined HR, 1.32; 95% CI, 1.17–1.49 [I 2=85.1%]; 10 studies), moderately increased risk in Asia (combined HR, 1.14; 95% CI, 1.12–1.16 [I 2=0%]; 3 studies), and the smallest increases in risk in Europe (combined HR, 1.08; 95% CI, 1.03–1.13 [I 2=0%]; 3 studies).
Discussion
This study reviewed 69 published articles examining the effect of long‐term PM2.5 exposure on risks of IHD and stroke events. We found that IHD mortality and cerebrovascular mortality were the most frequently studied end points and that these end points had the greatest increases in risk. In meta‐analyses, we found that a 10‐µg/m3 increase in long‐term average PM2.5 exposure was associated with a 23% increased risk of IHD mortality and a 24% increased risk of cerebrovascular mortality. We found that these 2 mortality outcomes were the most frequently studied cardiovascular end points, and that the definitions (primarily ICD codes) used to define these mortality end points have generally been consistent, although not identical among every study. Our meta‐analyses found more modest associations between long‐term PM2.5 exposure and increased risk of incident stroke and incident AMI: 13% and 8% increased risk per 10‐µg/m3 increase in long‐term average PM2.5 exposure, respectively. These outcomes have not been studied as extensively as the mortality outcomes. Furthermore, we were unable to conduct any meta‐analyses for recurrent AMI and stroke events because of an insufficient number of studies, indicating that more research is needed. Further research on recurrent events is crucial for assessing whether current PM2.5 regulatory standards are protective of populations with a history of cardiovascular events.
While there have been a number of qualitative reviews of long‐term cardiovascular effects of PM2.5, including the AHA statements, 46 , 88 the Environmental Protection Agency integrated science assessment, 89 and other journal review articles, 42 , 43 there have been relatively few meta‐analyses. To our knowledge, this is the first meta‐analysis that quantifies and compares the effect of long‐term PM2.5 on the risks of both incident AMI and IHD mortality. Notably, we found a substantial difference in these risks, with a 23% increased risk of IHD mortality and an 8% increased risk of incident AMI per 10‐µg/m3 increase in long‐term average PM2.5 exposure.
There is only 1 previous meta‐analysis of the effect of long‐term PM2.5 on the risks of nonfatal stroke 78 and 1 of incident stroke and stroke mortality. 90 Noticeably, Yuan et al 90 included only 6 studies in the meta‐analysis for stroke mortality, overlooking 9 studies‡‡ that we identified and were published by December 2018 (the cutoff for inclusion in their meta‐analysis); thus, our meta‐analysis is not only more up‐to‐date, including 3 more studies published in 2019, 31 , 59 , 75 but also much more fully representative of the published literature by including many more relevant studies. Comparing results per 10‐µg/m3 increase in long‐term average PM2.5 exposure, our finding of a 13% increased risk of incident stroke is smaller than the 23% increase in risk (95% CI, 10%–37%) reported by Yuan et al in 2019 90 and larger than the 6% increase in risk of nonfatal stroke (95% CI, 0%–13%) reported by Shin et al in 2014. 78 Notably, the stronger association in Yuan et al was largely driven by 2 studies that we excluded because they did not study incident stroke: To and colleagues 91 studied the prevalence of a previous stroke diagnosis and Lin et al 92 published a cross‐sectional association between average PM2.5 and self‐reported stroke in the past year. Additionally, the meta‐analysis pooled risk ratios for long‐term PM2.5 exposure reported by Shin et al were only among 4 studies.
Our supplementary analysis of overall cardiovascular mortality demonstrated a 14% increase in risk of cardiovascular mortality per 10‐µg/m3 increase in long‐term PM2.5. This is consistent with the AHA 2010 statement that “a 10‐µg/m3 increase in long‐term average PM2.5 exposure is associated with an ≈10% increase in all‐cause mortality and a similar or greater increase in the risk of cardiovascular death,” 88 and is slightly larger than the 2013 meta‐analysis that found a 11% increase in risk of cardiovascular mortality per 10‐µg/m3 increase in long‐term PM2.5. 45
Mechanisms
Mechanisms have been reviewed in detail in previous review articles. 1 , 42 , 43 Briefly, oxidative stress and inflammation are key mechanisms by which PM2.5 acts to increase risk of CVDs. Numerous in vitro studies have demonstrated that exposure to particulate air pollution activates pathways that generate reactive oxygen species in both cultured cells and in pulmonary and vascular tissue. 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 Activation of reactive oxygen species–dependent pathways can affect vascular inflammation, atherosclerosis, basal vasomotor balance, coagulation and thrombosis, and platelet activation. 102 Inflammatory cytokines are activated in response to air pollution exposures, with evidence of increased pulmonary inflammation and increased levels of circulating proinflammatory mediators. 103 , 104 , 105 , 106 , 107 Similarly, epidemiologic studies have demonstrated the relationship of particulate air pollution with inflammatory markers, as well as with atherosclerosis. 108 , 109 Studies in mice have also demonstrated inhibited vascular repair after PM2.5 exposure via depletion of circulating endothelial progenitor cells and functional impairment that prevents endothelial progenitor cell–mediated vascular recovery. 110 Furthermore, increasing the antioxidant capacity of the lung prevented this dysfunction, supporting the role of oxidative stress in PM2.5‐induced cardiovascular injury. 110 Another mechanism that may underly the cardiovascular responses to air pollution is altered autonomic nervous system balance. 111 Experimental studies have found that air pollution exposure is associated with rapid changes in autonomic nervous system balance, marked by sympathetic nervous system activation and parasympathetic withdrawal. 111 , 112 The mechanism of changes in autonomic nervous system balance is also supported by epidemiologic evidence demonstrating associations between air pollution exposure and changes in heart rate variability. 113 , 114 , 115 Notably, many epidemiologic studies have focused on older populations, and it has been hypothesized that the elderly are more susceptible to air pollution effects. 88 However, several epidemiologic and experimental studies have reported that PM2.5 is associated with blood pressure, 111 vasodilatation, 116 heart rate variability, 117 , 118 and ischemic stroke 119 among younger adults (aged 18–55 years).
We observed a noticeable difference in the risk of IHD mortality (23% increased risk) compared with the risk of incident AMI events (8% increased risk). These differences in risk may be related to differences in IHD and AMI survival. IHD morality is the leading cause of death worldwide, 120 and most IHD deaths occur before hospitalization and before a person is able to seek medical attention. 121 Furthermore, adults who are younger or who have no history of IHD are more likely to have an IHD death that occurs before hospitalization, although they have much less risk of IHD death overall. 121 In contrast, the AMI fatality rate among older adults who have been hospitalized has decreased substantially over the past several decades, and is now estimated to be ≈7%. 122 In addition, the increased risk observed for IHD mortality could be driven in part by an increased risk of IHD mortality among patients with a previous AMI event; however, little research has been performed examining that at‐risk population.
Notably, the differences in the strength of association in IHD mortality and incident AMI were sometimes observed within the same cohort study. For example, the REGARDS trial reported a statistically significant decreased risk of AMI (HR, 0.55; 95% CI, 0.31–0.96) and a strong but not statistically significant increased association with IHD mortality for the same cohort (HR, 1.57; 95% CI, 0.71–3.48). 70 Funnel plots in Figure S3 indicated that this study did not appear to be an outlier for incident AMI when compared with the expected distribution of effect estimates and standard errors. There is no obvious reason for the decreased HR in the REGARDS trial. The REGARDS cohort was younger and more racially and geographically diverse than many previous studies; however, the authors found no evidence that results differed by race, sex, or rural versus urban regions. The study did not account for changes in residential address during follow‐up, and instead assumed that patients remained at their baseline address for the duration of the study, which is a common study limitation.
In analyses of incident stroke types, we found that long‐term PM2.5 exposure may have a stronger association with risk of incident ischemic stroke (HR, 1.18; 95% CI, 1.14–1.22) than hemorrhagic stroke (HR, 1.10; 95% CI, 1.05–1.16). Ischemic strokes are the most common stroke subtype, while hemorrhagic strokes account for only 10% to 15% of all strokes. Ischemic and hemorrhagic strokes have different causes. Ischemic strokes occur when blood flow to the brain is blocked by a blood clot, whereas hemorrhagic strokes occur when a weak blood vessel bursts and bleeds into the brain. AMI is most often caused by decreased blood flow to a portion of the heart, typically caused by a blood clot in the epicardial artery that supplies the heart muscle. 123 Thus, ischemic strokes and AMI share a common pathway, where thrombosis is the most common underlying mechanism of AMI and ischemic stroke. There are also some differences in risk factors for ischemic and hemorrhagic strokes, where hypertension is the strongest risk factor for hemorrhagic stroke 124 and older age, cigarette smoking, hypercholesterolemia, and family history of stroke are more predictive of ischemic versus hemorrhagic stroke. 125
Gaps in Current Knowledge
Our final objective in this study was to identify gaps in the current knowledge of the relationship between long‐term exposures to PM2.5 and risk of cardiovascular morbidity and mortality. Mortality outcomes have been the most studied, and the meta‐analyses of these mortality outcomes showed clear, strong effects in relation to long‐term exposures to PM2.5. More studies of nonmortality outcomes are needed to clarify other effects, such as incident AMI events and recurrent AMI and stroke events.
Few studies have examined the effects of long‐term exposures to PM2.5 among patients with a history of CVD and measured their risk of recurrent cardiovascular events. The World Health Organization has reported that patients with a previous myocardial infarction or stroke are the groups at highest risk for further coronary and cerebral events. 126 Specifically, the annual death rate of patients who experienced an AMI is estimated to be 6 times that of same‐aged individuals without coronary heart disease. 126 Survivors of stroke have an increased risk of a subsequent stroke of 7% per year compared with those with no previous stroke. 126 Given the growing burden of CVD globally, compounded by increased survival rates among cardiac patients as a result of healthcare improvements, more research is needed to understand the health effects of long‐term air pollution exposure among these high‐risk groups.
This review article focuses on PM2.5 mass, the total exposure to PM2.5, which is the exposure measure most commonly studied and the measure that is currently regulated. 89 , 127 , 128 Notably, PM2.5 is composed of numerous elements, including organic carbon, black carbon, sulfate particles, metal oxides (aluminum, silicone, potassium, calcium, titanium, iron, and zinc), and sea salt (sodium and chorline). 129 Sources that contribute to PM2.5 exposure include regional pollution, motor vehicles, sea salt, crustal/road dust, oil combustion, and wood burning. 129 PM2.5 composition varies regionally; eg, rural areas have higher levels of crustal materials (silicone and aluminum) driven by agricultural activities and unpaved roads, urban areas have higher levels of secondary aerosol (nitrate, sulfate, and ammonium) and combustion (organic and black carbon), and industrialized areas have higher levels of trace metals (iron, palladium, and zinc). 130 Recent research has also shown differences in cardiovascular health effects related to different PM2.5 components. For example, some PM2.5 metals have been associated with increased levels of inflammatory blood markers 131 and an increased risk of coronary events. 9 Given this emerging research, differences in PM2.5 components may also contribute to differences in findings among studies, and future research is needed to better understand the role of PM2.5 components in cardiovascular health risk.
Last, more research on long‐term exposure to PM2.5 and cardiovascular health effects is needed in Asian and Middle‐Eastern countries, where ambient air pollution exposure is higher compared with the rest of the world 132 and where a large proportion of the world's population lives, such as in China and India. Populations in East Asian countries have shown higher risks of stroke mortality and lower risks of IHD mortality compared with Western countries, 133 which emphasizes the importance of studying these diverse populations who may have different underlying health risks compared with more frequently studied populations in Europe and North America. Some researchers have also suggested that air pollution effects may be greater in Asian countries. 134 However, our meta‐analysis by world region found the highest risks in North America, moderately increased risks in Asia, and the smallest increased risks in Europe. Notably, there were only 3 studies included from Asia, therefore more research is needed to verify and better understand these potential differences.
Conclusions
Our study builds on previous work that has established the causal link between PM2.5 exposure and CVDs. Using meta‐analyses, we provide quantitative evidence of the strength of associations with specific CVD event end points. We found a clear relationship between long‐term PM2.5 exposure and increased risk of cardiovascular events, with larger effects for IHD mortality and cerebrovascular mortality than incident stroke and incident AMI. The relationship with incident AMI was suggestive of a positive association but not conclusive. More research is needed to better quantify the relationships for incident AMI and for recurrent stroke and recurrent AMI events.
Sources of Funding
This study was funded by National Institute of Environmental Health Services grant R01 ES029557 Particulate Air Pollution, Cardiovascular Events, and Susceptibility Factors (PACES).
Disclosures
None.
Supporting information
Acknowledgments
Stacey E. Alexeeff, Xi Liu, and Noelle S. Liao had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2021;10:e016890. DOI: 10.1161/JAHA.120.016890.)
For Sources of Funding and Disclosures, see page 18.
Footnotes
References 4, 6, 7, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 26, 27, 28, 29, 30, 34, 35, 37, 39, 40, 41, 51, 53, 56, 58, 59, 60, 63, 64, 65, 67, 68, 69, 70, 72, 73, 74, 76, 79, 80
References 6, 7, 13, 14, 15, 16, 17, 18, 19, 21, 24, 27, 28, 34, 39, 41, 53, 59, 64, 65, 70, 74, 76.
References 6, 7, 14, 15, 16, 21, 22, 26, 29, 30, 31, 34, 35, 39, 41, 51, 58, 59, 63, 65, 69, 72, 74, 75, 76, 79, 80.
References
- 1. Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez‐Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. Particulate matter air pollution and cardiovascular disease an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. 10.1161/CIR.0b013e3181dbece1 [DOI] [PubMed] [Google Scholar]
- 2. Pope CA III, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, Heath CW Jr. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–674. 10.1164/ajrccm/151.3_Pt_1.669 [DOI] [PubMed] [Google Scholar]
- 3. Halonen JI, Blangiardo M, Toledano MB, Fecht D, Gulliver J, Ghosh R, Anderson HR, Beevers SD, Dajnak D, Kelly FJ, et al. Is long‐term exposure to traffic pollution associated with mortality? A small‐area study in London. Environ Pollut. 2016;208:25–32. 10.1016/j.envpol.2015.06.036 [DOI] [PubMed] [Google Scholar]
- 4. Thurston GD, Ahn J, Cromar KR, Shao Y, Reynolds HR, Jerrett M, Lim CC, Shanley R, Park Y, Hayes RB, et al. Ambient particulate matter air pollution exposure and mortality in the NIH‐AARP diet and health cohort. Environ Health Perspect. 2016;124:484–490. 10.1289/ehp.1509676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Beelen R, Hoek G, van den Brandt PA, Goldbohm RA, Fischer P, Schouten LJ, Jerrett M, Hughes E, Armstrong B, Brunekreef B, et al. Long‐term effects of traffic‐related air pollution on mortality in a Dutch cohort (NLCS‐AIR study). Environ Health Perspect. 2008;116:196–202. 10.1289/ehp.10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Weichenthal S, Villeneuve PJ, Burnett RT, van Donkelaar A, Martin RV, Jones RR, DellaValle CT, Sandler DP, Ward MH, Hoppin JA. Long‐term exposure to fine particulate matter: association with nonaccidental and cardiovascular mortality in the agricultural health study cohort. Environ Health Perspect. 2014;122:609–615. 10.1289/ehp.1307277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yin P, Brauer M, Cohen A, Burnett RT, Liu J, Liu Y, Liang R, Wang W, Qi J, Wang L, et al. Long‐term fine particulate matter exposure and nonaccidental and cause‐specific mortality in a large national cohort of Chinese men. Environ Health Perspect. 2017;125:117002. 10.1289/EHP1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Atkinson RW, Carey IM, Kent AJ, van Staa TP, Anderson HR, Cook DG. Long‐term exposure to outdoor air pollution and incidence of cardiovascular diseases. Epidemiology. 2013;24:44–53. 10.1097/EDE.0b013e318276ccb8 [DOI] [PubMed] [Google Scholar]
- 9. Wolf K, Stafoggia M, Cesaroni G, Andersen ZJ, Beelen R, Galassi C, Hennig F, Migliore E, Penell J, Ricceri F, et al. Long‐term exposure to particulate matter constituents and the incidence of coronary events in 11 european cohorts. Epidemiology. 2015;26:565–574. 10.1097/EDE.0000000000000300 [DOI] [PubMed] [Google Scholar]
- 10. Stafoggia M, Cesaroni G, Peters A, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, Cyrys J, de Faire U, de Hoogh K, et al. Long‐term exposure to ambient air pollution and incidence of cerebrovascular events: results from 11 European cohorts within the ESCAPE project. Environ Health Perspect. 2014;122:919–925. 10.1289/ehp.1307301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tonne C, Halonen JI, Beevers SD, Dajnak D, Gulliver J, Kelly FJ, Wilkinson P, Anderson HR. Long‐term traffic air and noise pollution in relation to mortality and hospital readmission among myocardial infarction survivors. Int J Hyg Environ Health. 2016;219:72–78. 10.1016/j.ijheh.2015.09.003 [DOI] [PubMed] [Google Scholar]
- 12. Koton S, Molshatzki N, Yuval XXX, Myers V, Broday DM, Drory Y, Steinberg DM, Gerber Y. Cumulative exposure to particulate matter air pollution and long‐term post‐myocardial infarction outcomes. Prev Med. 2013;57:339–344. 10.1016/j.ypmed.2013.06.009 [DOI] [PubMed] [Google Scholar]
- 13. Chen LH, Knutsen SF, Shavlik D, Beeson WL, Petersen F, Ghamsary M, Abbey D. The association between fatal coronary heart disease and ambient particulate air pollution: are females at greater risk? Environ Health Perspect. 2005;113:1723–1729. 10.1289/ehp.8190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tseng E, Ho WC, Lin MH, Cheng TJ, Chen PC, Lin HH. Chronic exposure to particulate matter and risk of cardiovascular mortality: cohort study from Taiwan. BMC Public Health. 2015;15:936. 10.1186/s12889-015-2272-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Carey IM, Atkinson RW, Kent AJ, van Staa T, Cook DG, Anderson HR. Mortality associations with long‐term exposure to outdoor air pollution in a national English cohort. Am J Respir Crit Care Med. 2013;187:1226–1233. 10.1164/rccm.201210-1758OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beelen R, Stafoggia M, Raaschou‐Nielsen O, Andersen ZJ, Xun WW, Katsouyanni K, Dimakopoulou K, Brunekreef B, Weinmayr G, Hoffmann B, et al. Long‐term exposure to air pollution and cardiovascular mortality: an analysis of 22 European cohorts. Epidemiology. 2014;25:368–378. 10.1097/EDE.0000000000000076 [DOI] [PubMed] [Google Scholar]
- 17. Puett RC, Hart JE, Suh H, Mittleman M, Laden F. Particulate matter exposures, mortality, and cardiovascular disease in the health professionals follow‐up study. Environ Health Perspect. 2011;119:1130–1135. 10.1289/ehp.1002921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hart JE, Garshick E, Dockery DW, Smith TJ, Ryan L, Laden F. Long‐term ambient multipollutant exposures and mortality. Am J Respir Crit Care Med. 2011;183:73–78. 10.1164/rccm.200912-1903OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gan WQ, Koehoorn M, Davies HW, Demers PA, Tamburic L, Brauer M. Long‐term exposure to traffic‐related air pollution and the risk of coronary heart disease hospitalization and mortality. Environ Health Perspect. 2011;119:501–507. 10.1289/ehp.1002511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jerrett M, Burnett RT, Ma R, Pope CA, Krewski D, Newbold KB, Thurston G, Shi Y, Finkelstein N, Calle EE, et al. Spatial analysis of air pollution and mortality in Los Angeles. Epidemiology. 2005;16:727–736. 10.1097/01.ede.0000181630.15826.7d [DOI] [PubMed] [Google Scholar]
- 21. Miller KA, Siscovick DS, Sheppard L, Shepherd K, Sullivan JH, Anderson GL, Kaufman JD. Long‐term exposure to air pollution and incidence of cardiovascular events in women. N Engl J Med. 2007;356:447–458. 10.1056/NEJMoa054409 [DOI] [PubMed] [Google Scholar]
- 22. Pope CA III, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ. Cardiovascular mortality and long‐term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation. 2004;109:71–77. 10.1161/01.CIR.0000108927.80044.7F [DOI] [PubMed] [Google Scholar]
- 23. Pope CA III, Burnett RT, Krewski D, Jerrett M, Shi Y, Calle EE, Thun MJ. Cardiovascular mortality and exposure to airborne fine particulate matter and cigarette smoke: shape of the exposure‐response relationship. Circulation. 2009;120:941–948. 10.1161/CIRCULATIONAHA.109.857888 [DOI] [PubMed] [Google Scholar]
- 24. Puett RC, Hart JE, Yanosky JD, Paciorek C, Schwartz J, Suh H, Speizer FE, Laden F. Chronic fine and coarse particulate exposure, mortality, and coronary heart disease in the Nurses' Health Study. Environ Health Perspect. 2009;117:1697–1701. 10.1289/ehp.0900572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bai LI, Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Goldberg MS, Lavigne E, Copes R, Martin RV, et al. Exposure to ambient air pollution and the incidence of congestive heart failure and acute myocardial infarction: a population‐based study of 5.1 million canadian adults living in ontario. Environ Int. 2019;132:105004. 10.1016/j.envint.2019.105004 [DOI] [PubMed] [Google Scholar]
- 26. Cakmak S, Hebbern C, Vanos J, Crouse DL, Burnett R. Ozone exposure and cardiovascular‐related mortality in the Canadian Census Health and Environment Cohort (CANCHEC) by spatial synoptic classification zone. Environ Pollut. 2016;214:589–599. 10.1016/j.envpol.2016.04.067 [DOI] [PubMed] [Google Scholar]
- 27. Cakmak S, Hebbern C, Pinault L, Lavigne E, Vanos J, Crouse DL, Tjepkema M. Associations between long‐term PM2.5 and ozone exposure and mortality in the Canadian Census Health and Environment Cohort (CANCHEC), by spatial synoptic classification zone. Environ Int. 2018;111:200–211. 10.1016/j.envint.2017.11.030 [DOI] [PubMed] [Google Scholar]
- 28. Chen H, Burnett RT, Copes R, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, et al. Ambient fine particulate matter and mortality among survivors of myocardial infarction: population‐based cohort study. Environ Health Perspect. 2016;124:1421–1428. 10.1289/EHP185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Crouse DL, Peters PA, van Donkelaar A, Goldberg MS, Villeneuve PJ, Brion O, Khan S, Atari DO, Jerrett M, Pope CA, et al. Risk of nonaccidental and cardiovascular mortality in relation to long‐term exposure to low concentrations of fine particulate matter: a Canadian national‐level cohort study. Environ Health Perspect. 2012;120:708–714. 10.1289/ehp.1104049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Crouse DL, Peters PA, Hystad P, Brook JR, van Donkelaar A, Martin RV, Villeneuve PJ, Jerrett M, Goldberg MS, Pope CA III, et al. Ambient PM2.5, O(3), and NO(2) exposures and associations with mortality over 16 years of follow‐up in the Canadian Census Health and Environment Cohort (CANCHEC). Environ Health Perspect. 2015;123:1180–1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dirgawati M, Hinwood A, Nedkoff L, Hankey GJ, Yeap BB, Flicker L, Nieuwenhuijsen M, Brunekreef B, Heyworth J. Long‐term exposure to low air pollutant concentrations and the relationship with all‐cause mortality and stroke in older men. Epidemiology. 2019;30(suppl 1):S82–S89. [DOI] [PubMed] [Google Scholar]
- 32. Huang K, Liang F, Yang X, Liu F, Li J, Xiao Q, Chen J, Liu X, Cao J, Shen C, et al. Long term exposure to ambient fine particulate matter and incidence of stroke: prospective cohort study from the China‐PAR project. BMJ. 2019;367:l6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim H, Kim J, Kim S, Kang SH, Kim HJ, Kim H, Heo J, Yi SM, Kim K, Youn TJ, et al. Cardiovascular effects of long‐term exposure to air pollution: a population‐based study with 900 845 person‐years of follow‐up. J Am Heart Assoc. 2017;6:e007170. 10.1161/JAHA.117.007170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pinault L, Tjepkema M, Crouse DL, Weichenthal S, van Donkelaar A, Martin RV, Brauer M, Chen H, Burnett RT. Risk estimates of mortality attributed to low concentrations of ambient fine particulate matter in the Canadian community health survey cohort. Environ Health. 2016;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pinault LL, Weichenthal S, Crouse DL, Brauer M, Erickson A, Donkelaar AV, Martin RV, Hystad P, Chen H, Fines P, et al. Associations between fine particulate matter and mortality in the 2001 Canadian Census Health and Environment Cohort. Environ Res. 2017;159:406–415. [DOI] [PubMed] [Google Scholar]
- 36. Qiu H, Sun S, Tsang H, Wong CM, Lee RS, Schooling CM, Tian L. Fine particulate matter exposure and incidence of stroke: a cohort study in Hong Kong. Neurology. 2017;88:1709–1717. [DOI] [PubMed] [Google Scholar]
- 37. Shin HH, Cakmak S, Brion O, Villeneuve P, Turner MC, Goldberg MS, Jerrett M, Chen H, Crouse D, Peters P, et al. Indirect adjustment for multiple missing variables applicable to environmental epidemiology. Environ Res. 2014;134:482–487. [DOI] [PubMed] [Google Scholar]
- 38. Shin S, Burnett RT, Kwong JC, Hystad P, van Donkelaar A, Brook JR, Goldberg MS, Tu K, Copes R, Martin RV, et al. Ambient air pollution and the risk of atrial fibrillation and stroke: a population‐based cohort study. Environ Health Perspect. 2019;127:87009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Villeneuve PJ, Weichenthal SA, Crouse D, Miller AB, To T, Martin RV, van Donkelaar A, Wall C, Burnett RT. Long‐term exposure to fine particulate matter air pollution and mortality among Canadian women. Epidemiology. 2015;26:536–545. [DOI] [PubMed] [Google Scholar]
- 40. Weichenthal S, Crouse DL, Pinault L, Godri‐Pollitt K, Lavigne E, Evans G, van Donkelaar A, Martin RV, Burnett RT. Oxidative burden of fine particulate air pollution and risk of cause‐specific mortality in the Canadian Census Health and Environment Cohort (CANCHEC). Environ Res. 2016;146:92–99. [DOI] [PubMed] [Google Scholar]
- 41. Wong CM, Lai HK, Tsang H, Thach TQ, Thomas GN, Lam KB, Chan KP, Yang L, Lau AK, Ayres JG, et al. Satellite‐based estimates of long‐term exposure to fine particles and association with mortality in elderly Hong Kong residents. Environ Health Perspect. 2015;123:1167–1172. 10.1289/ehp.1408264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bourdrel T, Bind MA, Bejot Y, Morel O, Argacha JF. Cardiovascular effects of air pollution. Arch Cardiovasc Dis. 2017;110:634–642. 10.1016/j.acvd.2017.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. 10.1016/S0140-6736(02)11274-8 [DOI] [PubMed] [Google Scholar]
- 44. National Center for Biotechnology Information (NCBI). 2017.
- 45. Hoek G, Krishnan RM, Beelen R, Peters A, Ostro B, Brunekreef B, Kaufman JD. Long‐term air pollution exposure and cardio‐ respiratory mortality: a review. Environ Health. 2013;12:43. 10.1186/1476-069X-12-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, Luepker R, Mittleman M, Samet J, Smith SC Jr, et al; Expert Panel on P, Prevention Science of the American Heart A . Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation. 2004;109:2655–2671. 10.1161/01.CIR.0000128587.30041.C8 [DOI] [PubMed] [Google Scholar]
- 47. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002;21:1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 48. DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986;7:177–188. 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 49. Wells GASB, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. 2019.
- 50. Carey IM, Anderson HR, Atkinson RW, Beevers S, Cook DG, Dajnak D, Gulliver J, Kelly FJ. Traffic pollution and the incidence of cardiorespiratory outcomes in an adult cohort in London. Occup Environ Med. 2016;73:849–856. 10.1136/oemed-2015-103531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Cesaroni G, Badaloni C, Gariazzo C, Stafoggia M, Sozzi R, Davoli M, Forastiere F. Long‐term exposure to urban air pollution and mortality in a cohort of more than a million adults in Rome. Environ Health Perspect. 2013;121:324–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Chi GC, Hajat A, Bird CE, Cullen MR, Griffin BA, Miller KA, Shih RA, Stefanick ML, Vedal S, Whitsel EA, et al. Individual and neighborhood socioeconomic status and the association between air pollution and cardiovascular disease. Environ Health Perspect. 2016;124:1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Badaloni C, Cesaroni G, Cerza F, Davoli M, Brunekreef B, Forastiere F. Effects of long‐term exposure to particulate matter and metal components on mortality in the Rome Longitudinal Study. Environ Int. 2017;109:146–154. [DOI] [PubMed] [Google Scholar]
- 54. Cesaroni G, Forastiere F, Stafoggia M, Andersen ZJ, Badaloni C, Beelen R, Caracciolo B, de Faire U, Erbel R, Eriksen KT, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta‐analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Crichton S, Barratt B, Spiridou A, Hoang U, Liang SF, Kovalchuk Y, Beevers SD, Kelly FJ, Delaney B, Wolfe CD. Associations between exhaust and non‐exhaust particulate matter and stroke incidence by stroke subtype in South London. Sci Total Environ. 2016;568:278–284. [DOI] [PubMed] [Google Scholar]
- 56. Hart JE, Puett RC, Rexrode KM, Albert CM, Laden F. Effect modification of long‐term air pollution exposures and the risk of incident cardiovascular disease in US women. J Am Heart Assoc. 2015;4:e002301. 10.1161/JAHA.115.002301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hartiala J, Breton CV, Tang WH, Lurmann F, Hazen SL, Gilliland FD, Allayee H. Ambient air pollution is associated with the severity of coronary atherosclerosis and incident myocardial infarction in patients undergoing elective cardiac evaluation. J Am Heart Assoc. 2016;5:e003947. 10.1161/JAHA.116.003947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Hoffmann B, Weinmayr G, Hennig F, Fuks K, Moebus S, Weimar C, Dragano N, Hermann DM, Kalsch H, Mahabadi AA, et al. Air quality, stroke, and coronary events: results of the Heinz Nixdorf Recall Study from the Ruhr Region. Dtsch Arztebl Int. 2015;112:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hayes RB, Lim C, Zhang Y, Cromar K, Shao Y, Reynolds HR, Silverman DT, Jones RR, Park Y, Jerrett M, et al. PM2.5 air pollution and cause‐specific cardiovascular disease mortality. Int J Epidemiol. 2020;49:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Heritier H, Vienneau D, Foraster M, Eze IC, Schaffner E, de Hoogh K, Thiesse L, Rudzik F, Habermacher M, Kopfli M, et al. A systematic analysis of mutual effects of transportation noise and air pollution exposure on myocardial infarction mortality: a nationwide cohort study in Switzerland. Eur Heart J. 2019;40:598–603. [DOI] [PubMed] [Google Scholar]
- 61. Danesh Yazdi M, Wang Y, Di Q, Zanobetti A, Schwartz J. Long‐term exposure to PM2.5 and ozone and hospital admissions of Medicare participants in the Southeast USA. Environ Int. 2019;130:104879. 10.1016/j.envint.2019.05.073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gandini M, Scarinzi C, Bande S, Berti G, Carnà P, Ciancarella L, Costa G, Demaria M, Ghigo S, Piersanti A, et al. Long term effect of air pollution on incident hospital admissions: results from the Italian Longitudinal Study within LIFE MED HISS project. Environ Int. 2018;121:1087–1097. 10.1016/j.envint.2018.10.020 [DOI] [PubMed] [Google Scholar]
- 63. Jerrett M, Burnett RT, Beckerman BS, Turner MC, Krewski D, Thurston G, Martin RV, van Donkelaar A, Hughes E, Shi Y, et al. Spatial analysis of air pollution and mortality in California. Am J Respir Crit Care Med. 2013;188:593–599. 10.1164/rccm.201303-0609OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jerrett M, Turner MC, Beckerman BS, Pope CA, van Donkelaar A, Martin RV, Serre M, Crouse D, Gapstur SM, Krewski D, et al. Comparing the health effects of ambient particulate matter estimated using ground‐based versus remote sensing exposure estimates. Environ Health Perspect. 2017;125:552–559. 10.1289/EHP575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lipsett MJ, Ostro BD, Reynolds P, Goldberg D, Hertz A, Jerrett M, Smith DF, Garcia C, Chang ET, Bernstein L. Long‐term exposure to air pollution and cardiorespiratory disease in the California Teachers Study cohort. Am J Respir Crit Care Med. 2011;184:828–835. 10.1164/rccm.201012-2082OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Madrigano J, Kloog I, Goldberg R, Coull BA, Mittleman MA, Schwartz J. Long‐term exposure to PM2.5 and incidence of acute myocardial infarction. Environ Health Perspect. 2013;121:192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Ostro B, Lipsett M, Reynolds P, Goldberg D, Hertz A, Garcia C, Henderson KD, Bernstein L. Long‐term exposure to constituents of fine particulate air pollution and mortality: results from the California Teachers Study. Environ Health Perspect. 2010;118:363–369. 10.1289/ehp.0901181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Ostro B, Hu J, Goldberg D, Reynolds P, Hertz A, Bernstein L, Kleeman MJ. Associations of mortality with long‐term exposures to fine and ultrafine particles, species and sources: results from the California Teachers Study cohort. Environ Health Perspect. 2015;123:549–556. 10.1289/ehp.1408565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lim CC, Hayes RB, Ahn J, Shao Y, Silverman DT, Jones RR, Thurston GD. Mediterranean diet and the association between air pollution and cardiovascular disease mortality risk. Circulation. 2019;139:1766–1775. 10.1161/CIRCULATIONAHA.118.035742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Loop MS, McClure LA, Levitan EB, Al‐Hamdan MZ, Crosson WL, Safford MM. Fine particulate matter and incident coronary heart disease in the REGARDS cohort. Am Heart J. 2018;197:94–102. 10.1016/j.ahj.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ljungman PL, Andersson N, Stockfelt L, Andersson EM, Nilsson Sommar J, Eneroth K, Gidhagen L, Johansson C, Lager A, Leander K, et al. Long‐term exposure to particulate air pollution, black carbon, and their source components in relation to ischemic heart disease and stroke. Environ Health Perspect. 2019;127:107012. 10.1289/EHP4757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Pope CA III, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D, Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116:108–115. 10.1161/CIRCRESAHA.116.305060 [DOI] [PubMed] [Google Scholar]
- 73. Pope CA III, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, Gapstur SM, Thun MJ. Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure‐response relationships. Environ Health Perspect. 2011;119:1616–1621. 10.1289/ehp.1103639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Parker JD, Kravets N, Vaidyanathan A. Particulate matter air pollution exposure and heart disease mortality risks by race and ethnicity in the United States: 1997 to 2009 National Health Interview Survey with mortality follow‐up through 2011. Circulation. 2018;137:1688–1697. 10.1161/CIRCULATIONAHA.117.029376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pope CA, Lefler JS, Ezzati M, Higbee JD, Marshall JD, Kim S Y, Bechle M, Gilliat KS, Vernon SE, Robinson AL, et al. Mortality risk and fine particulate air pollution in a large, representative cohort of U.S. adults. Environ Health Perspect. 2019;127:77007. 10.1289/EHP4438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Pun VC, Kazemiparkouhi F, Manjourides J, Suh HH. Long‐term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults. Am J Epidemiol. 2017;186:961–969. 10.1093/aje/kwx166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Stockfelt L, Andersson EM, Molnar P, Gidhagen L, Segersson D, Rosengren A, Barregard L, Sallsten G. Long‐term effects of total and source‐specific particulate air pollution on incident cardiovascular disease in Gothenburg, Sweden. Environ Res. 2017;158:61–71. 10.1016/j.envres.2017.05.036 [DOI] [PubMed] [Google Scholar]
- 78. Shin HH, Fann N, Burnett RT, Cohen A, Hubbell BJ. Outdoor fine particles and nonfatal strokes: systematic review and meta‐analysis. Epidemiology. 2014;25:835–842. 10.1097/EDE.0000000000000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Turner MC, Cohen A, Burnett RT, Jerrett M, Diver WR, Gapstur SM, Krewski D, Samet JM, Pope CA III. Interactions between cigarette smoking and ambient PM2.5 for cardiovascular mortality. Environ Res. 2017;154:304–310. 10.1016/j.envres.2017.01.024 [DOI] [PubMed] [Google Scholar]
- 80. Turner MC, Jerrett M, Pope CA, Krewski D, Gapstur SM, Diver WR, Beckerman BS, Marshall JD, Su J, Crouse DL, et al. Long‐term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med. 2016;193:1134–1142. 10.1164/rccm.201508-1633OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Yitshak‐Sade M, Bobb JF, Schwartz JD, Kloog I, Zanobetti A. The association between short and long‐term exposure to PM2.5 and temperature and hospital admissions in New England and the synergistic effect of the short‐term exposures. Sci Total Environ. 2018;639:868–875. 10.1016/j.scitotenv.2018.05.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Liang F, Xiao Q, Gu D, Xu M, Tian L, Guo Q, Wu Z, Pan X, Liu Y. Satellite‐based short‐ and long‐term exposure to PM2.5 and adult mortality in urban Beijing, China. Environ Pollut. 2018;242:492–499. 10.1016/j.envpol.2018.06.097 [DOI] [PubMed] [Google Scholar]
- 83. Sanyal S, Rochereau T, Maesano CN, Com‐Ruelle L, Annesi‐Maesano I. Long‐term effect of outdoor air pollution on mortality and morbidity: a 12‐year follow‐up study for metropolitan France. Int J Environ Res Public Health. 2018;15:2487. 10.3390/ijerph15112487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hvidtfeldt UA, Sorensen M, Geels C, Ketzel M, Khan J, Tjonneland A, Overvad K, Brandt J, Raaschou‐Nielsen O. Long‐term residential exposure to PM2.5, PM10, black carbon, NO2, and ozone and mortality in a Danish cohort. Environ Int. 2019;123:265–272. 10.1016/j.envint.2018.12.010 [DOI] [PubMed] [Google Scholar]
- 85. Lepeule J, Laden F, Dockery D, Schwartz J. Chronic exposure to fine particles and mortality: an extended follow‐up of the Harvard Six Cities study from 1974 to 2009. Environ Health Perspect. 2012;120:965–970. 10.1289/ehp.1104660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Dehbi HM, Blangiardo M, Gulliver J, Fecht D, de Hoogh K, Al‐Kanaani Z, Tillin T, Hardy R, Chaturvedi N, Hansell AL. Air pollution and cardiovascular mortality with over 25years follow‐up: a combined analysis of two British cohorts. Environ Int. 2017;99:275–281. 10.1016/j.envint.2016.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Naess O, Nafstad P, Aamodt G, Claussen B, Rosland P. Relation between concentration of air pollution and cause‐specific mortality: four‐year exposures to nitrogen dioxide and particulate matter pollutants in 470 neighborhoods in Oslo, Norway. Am J Epidemiol. 2007;165:435–443. 10.1093/aje/kwk016 [DOI] [PubMed] [Google Scholar]
- 88. Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez‐Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. 10.1161/CIR.0b013e3181dbece1 [DOI] [PubMed] [Google Scholar]
- 89. Agency USEP . U.S. EPA.Integrated Science Assessment (ISA) for particulate matter (final report, 2019).
- 90. Yuan S, Wang J, Jiang Q, He Z, Huang Y, Li Z, Cai L, Cao S. Long‐term exposure to PM2.5 and stroke: a systematic review and meta‐analysis of cohort studies. Environ Res. 2019;177:108587. 10.1016/j.envres.2019.108587 [DOI] [PubMed] [Google Scholar]
- 91. To T, Zhu J, Villeneuve PJ, Simatovic J, Feldman L, Gao C, Williams D, Chen H, Weichenthal S, Wall C, et al. Chronic disease prevalence in women and air pollution–a 30‐year longitudinal cohort study. Environ Int. 2015;80:26–32. 10.1016/j.envint.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 92. Lin H, Guo Y, Di Q, Zheng Y, Kowal P, Xiao J, Liu T, Li X, Zeng W, Howard SW, et al. Ambient PM2.5 and stroke: effect modifiers and population attributable risk in six low‐ and middle‐income countries. Stroke. 2017;48:1191–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Li Z, Hyseni X, Carter JD, Soukup JM, Dailey LA, Huang YC. Pollutant particles enhanced H2O2 production from NAD(P)H oxidase and mitochondria in human pulmonary artery endothelial cells. Am J Physiol Cell Physiol. 2006;291:C357–365. [DOI] [PubMed] [Google Scholar]
- 94. Shukla A, Timblin C, BeruBe K, Gordon T, McKinney W, Driscoll K, Vacek P, Mossman BT. Inhaled particulate matter causes expression of nuclear factor (NF)‐kappaB‐related genes and oxidant‐dependent NF‐kappaB activation in vitro. Am J Respir Cell Mol Biol. 2000;23:182–187. [DOI] [PubMed] [Google Scholar]
- 95. Han JY, Takeshita K, Utsumi H. Noninvasive detection of hydroxyl radical generation in lung by diesel exhaust particles. Free Radic Biol Med. 2001;30:516–525. 10.1016/S0891-5849(00)00501-3 [DOI] [PubMed] [Google Scholar]
- 96. Baulig A, Garlatti M, Bonvallot V, Marchand A, Barouki R, Marano F, Baeza‐Squiban A. Involvement of reactive oxygen species in the metabolic pathways triggered by diesel exhaust particles in human airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2003;285:L671–L679. 10.1152/ajplung.00419.2002 [DOI] [PubMed] [Google Scholar]
- 97. Donaldson K, Stone V, Borm PJ, Jimenez LA, Gilmour PS, Schins RPF, Knaapen AM, Rahman I, Faux SP, Brown DM, et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10). Free Radic Biol Med. 2003;34:1369–1382. 10.1016/S0891-5849(03)00150-3 [DOI] [PubMed] [Google Scholar]
- 98. Beck‐Speier I, Dayal N, Karg E, Maier KL, Schumann G, Schulz H, Semmler M, Takenaka S, Stettmaier K, Bors W, et al. Oxidative stress and lipid mediators induced in alveolar macrophages by ultrafine particles. Free Radic Biol Med. 2005;38:1080–1092. 10.1016/j.freeradbiomed.2005.01.004 [DOI] [PubMed] [Google Scholar]
- 99. Dellinger B, Pryor WA, Cueto R, Squadrito GL, Hegde V, Deutsch WA. Role of free radicals in the toxicity of airborne fine particulate matter. Chem Res Toxicol. 2001;14:1371–1377. 10.1021/tx010050x [DOI] [PubMed] [Google Scholar]
- 100. Gonzalez‐Flecha B. Oxidant mechanisms in response to ambient air particles. Mol Aspects Med. 2004;25:169–182. 10.1016/j.mam.2004.02.017 [DOI] [PubMed] [Google Scholar]
- 101. Bai Y, Suzuki AK, Sagai M. The cytotoxic effects of diesel exhaust particles on human pulmonary artery endothelial cells in vitro: role of active oxygen species. Free Radic Biol Med. 2001;30:555–562. 10.1016/S0891-5849(00)00499-8 [DOI] [PubMed] [Google Scholar]
- 102. Moller P, Jacobsen NR, Folkmann JK, Danielsen PH, Mikkelsen L, Hemmingsen JG, Vesterdal LK, Forchhammer L, Wallin H, Loft S. Role of oxidative damage in toxicity of particulates. Free Radic Res. 2010;44:1–46. 10.3109/10715760903300691 [DOI] [PubMed] [Google Scholar]
- 103. Fujii T, Hayashi S, Hogg JC, Mukae H, Suwa T, Goto Y, Vincent R, van Eeden SF. Interaction of alveolar macrophages and airway epithelial cells following exposure to particulate matter produces mediators that stimulate the bone marrow. Am J Respir Cell Mol Biol. 2002;27:34–41. 10.1165/ajrcmb.27.1.4787 [DOI] [PubMed] [Google Scholar]
- 104. Quay JL, Reed W, Samet J, Devlin RB. Air pollution particles induce IL‐6 gene expression in human airway epithelial cells via NF‐kappaB activation. Am J Respir Cell Mol Biol. 1998;19:98–106. [DOI] [PubMed] [Google Scholar]
- 105. van Eeden SF, Tan WC, Suwa T, Mukae H, Terashima T, Fujii T, Qui D, Vincent R, Hogg JC. Cytokines involved in the systemic inflammatory response induced by exposure to particulate matter air pollutants (PM(10)). Am J Respir Crit Care Med. 2001;164:826–830. [DOI] [PubMed] [Google Scholar]
- 106. Tamagawa E, Bai NI, Morimoto K, Gray C, Mui T, Yatera K, Zhang X, Xing LI, Li Y, Laher I, et al. Particulate matter exposure induces persistent lung inflammation and endothelial dysfunction. Am J Physiol Lung Cell Mol Physiol. 2008;295:L79–L85. 10.1152/ajplung.00048.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Törnqvist H, Mills NL, Gonzalez M, Miller MR, Robinson SD, Megson IL, MacNee W, Donaldson K, Söderberg S, Newby DE, et al. Persistent endothelial dysfunction in humans after diesel exhaust inhalation. Am J Respir Crit Care Med. 2007;176:395–400. 10.1164/rccm.200606-872OC [DOI] [PubMed] [Google Scholar]
- 108. Alexeeff SE, Coull BA, Gryparis A, Suh H, Sparrow D, Vokonas PS, Schwartz J. Medium‐term exposure to traffic‐related air pollution and markers of inflammation and endothelial function. Environ Health Perspect. 2011;119:481–486. 10.1289/ehp.1002560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Kaufman JD, Adar SD, Barr RG, Budoff M, Burke GL, Curl CL, Daviglus ML, Roux AVD, Gassett AJ, Jacobs DR, et al. Association between air pollution and coronary artery calcification within six metropolitan areas in the USA (the Multi‐Ethnic Study of Atherosclerosis and air pollution): a longitudinal cohort study. Lancet. 2016;388:696–704. 10.1016/S0140-6736(16)00378-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Haberzettl P, Conklin DJ, Abplanalp WT, Bhatnagar A, O'Toole TE. Inhalation of fine particulate matter impairs endothelial progenitor cell function via pulmonary oxidative stress. Arterioscler Thromb Vasc Biol. 2018;38:131–142. 10.1161/ATVBAHA.117.309971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Brook RD, Urch B, Dvonch JT, Bard RL, Speck M, Keeler G, Morishita M, Marsik FJ, Kamal AS, Kaciroti N, et al. Insights into the mechanisms and mediators of the effects of air pollution exposure on blood pressure and vascular function in healthy humans. Hypertension. 2009;54:659–667. 10.1161/HYPERTENSIONAHA.109.130237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Widdicombe J, Lee LY. Airway reflexes, autonomic function, and cardiovascular responses. Environ Health Perspect. 2001;109(suppl 4):579–584. 10.1289/ehp.01109s4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Park SK, O'Neill MS, Vokonas PS, Sparrow D, Wright RO, Coull B, Nie H, Hu H, Schwartz J. Air pollution and heart rate variability: effect modification by chronic lead exposure. Epidemiology. 2008;19:111–120. 10.1097/EDE.0b013e31815c408a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Pope CA III, Hansen ML, Long RW, Nielsen KR, Eatough NL, Wilson WE, Eatough DJ. Ambient particulate air pollution, heart rate variability, and blood markers of inflammation in a panel of elderly subjects. Environ Health Perspect. 2004;112:339–345. 10.1289/ehp.6588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Baccarelli A, Cassano PA, Litonjua A, Park SK, Suh H, Sparrow D, Vokonas P, Schwartz J. Cardiac autonomic dysfunction: effects from particulate air pollution and protection by dietary methyl nutrients and metabolic polymorphisms. Circulation. 2008;117:1802–1809. 10.1161/CIRCULATIONAHA.107.726067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Lucking AJ, Lundbäck M, Barath SL, Mills NL, Sidhu MK, Langrish JP, Boon NA, Pourazar J, Badimon JJ, Gerlofs‐Nijland ME, et al. Particle traps prevent adverse vascular and prothrombotic effects of diesel engine exhaust inhalation in men. Circulation. 2011;123:1721–1728. 10.1161/CIRCULATIONAHA.110.987263 [DOI] [PubMed] [Google Scholar]
- 117. Liu WT, Ma CM, Liu IJ, Han BC, Chuang HC, Chuang KJ. Effects of commuting mode on air pollution exposure and cardiovascular health among young adults in Taipei, Taiwan. Int J Hyg Environ Health. 2015;218:319–323. 10.1016/j.ijheh.2015.01.003 [DOI] [PubMed] [Google Scholar]
- 118. Weichenthal S, Hatzopoulou M, Goldberg MS. Exposure to traffic‐related air pollution during physical activity and acute changes in blood pressure, autonomic and micro‐vascular function in women: a cross‐over study. Part Fibre Toxicol. 2014;11:70. 10.1186/s12989-014-0070-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Yitshak Sade M, Novack V, Ifergane G, Horev A, Kloog I. Air pollution and ischemic stroke among young adults. Stroke. 2015;46:3348–3353. [DOI] [PubMed] [Google Scholar]
- 120. Organization WH . Global health estimates 2016: deaths by cause, age, sex, by country and by region, 2000–2016. 2018.
- 121. Grey C, Jackson R, Schmidt M, Ezzati M, Asaria P, Exeter DJ, Kerr AJ. One in four major ischaemic heart disease events are fatal and 60% are pre‐hospital deaths: a national data‐linkage study (ANZACS‐QI 8). Eur Heart J. 2017;38:172–180. [DOI] [PubMed] [Google Scholar]
- 122. Krumholz HM, Normand ST, Wang Y. Twenty‐year trends in outcomes for older adults with acute myocardial infarction in the United States. JAMA Netw Open. 2019;2:e191938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Saleh M, Ambrose JA. Understanding myocardial infarction. F1000Res. 2018;7:1378. DOI: 10.12688/f1000research.15096.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Gebel JM, Broderick JP. Intracerebral hemorrhage. Neurol Clin. 2000;18:419–438. [DOI] [PubMed] [Google Scholar]
- 125. Bogousslavsky J, Castillo V, Kumral E, Henriques I, Melle GV. Stroke subtypes and hypertension. Primary hemorrhage vs infarction, large‐ vs small‐artery disease. Arch Neurol. 1996;53:265–269. [DOI] [PubMed] [Google Scholar]
- 126. World Health Organization . Prevention of recurrences of myocardial infarction and stroke study.
- 127. Department for Environment F, and Rural Affairs .National statistics.Air quality statistics in the UK, 1987 to 2019—particulate matter (PM10/PM2.5). 2020.
- 128. Policy T . China: air quality standards.
- 129. Masri S, Kang CM, Koutrakis P. Composition and sources of fine and coarse particles collected during 2002–2010 in Boston, MA. J Air Waste Manag Assoc. 2015;65:287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Kundu S, Stone EA. Composition and sources of fine particulate matter across urban and rural sites in the Midwestern United States. Environ Sci Process Impacts. 2014;16:1360–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Niu J, Liberda EN, Qu S, Guo X, Li X, Zhang J, Meng J, Yan B, Li N, Zhong M, et al. The role of metal components in the cardiovascular effects of PM2.5 . PLoS One. 2013;8:e83782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R, et al. Estimates and 25‐year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet. 2017;389:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Ueshima H, Sekikawa A, Miura K, Turin TC, Takashima N, Kita Y, Watanabe M, Kadota A, Okuda N, Kadowaki T, et al. Cardiovascular disease and risk factors in Asia: a selected review. Circulation. 2008;118:2702–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Chung KF, Zhang J, Zhong N. Outdoor air pollution and respiratory health in Asia. Respirology. 2011;16:1023–1026. [DOI] [PubMed] [Google Scholar]
- 135. Moher D, Liberati A, Tetzlaff J, Altman DG; Group P . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


