Abstract
Background
The long‐term impact of new‐onset postoperative atrial fibrillation (POAF) after coronary artery bypass grafting and the benefit of early‐initiated oral anticoagulation (OAC) in patients with POAF are uncertain.
Methods and Results
All patients who underwent coronary artery bypass grafting without preoperative atrial fibrillation in Sweden from 2007 to 2015 were included in a population‐based study using data from 4 national registries: SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence‐based Care in Heart Disease Evaluated According to Recommended Therapies), National Patient Registry, Dispensed Drug Registry, and Cause of Death Registry. POAF was defined as any new‐onset atrial fibrillation during the first 30 postoperative days. Cox regression models (adjusted for age, sex, comorbidity, and medication) were used to assess long‐term outcome in patients with and without POAF, and potential associations between early‐initiated OAC and outcome. In a cohort of 24 523 patients with coronary artery bypass grafting, POAF occurred in 7368 patients (30.0%), and 1770 (24.0%) of them were prescribed OAC within 30 days after surgery. During follow‐up (median 4.5 years, range 0‒9 years), POAF was associated with increased risk of ischemic stroke (adjusted hazard ratio [aHR] 1.18 [95% CI, 1.05‒1.32]), any thromboembolism (ischemic stroke, transient ischemic attack, or peripheral arterial embolism) (aHR 1.16, 1.05‒1.28), heart failure hospitalization (aHR 1.35, 1.21‒1.51), and recurrent atrial fibrillation (aHR 4.16, 3.76‒4.60), but not with all‐cause mortality (aHR 1.08, 0.98‒1.18). Early initiation of OAC was not associated with reduced risk of ischemic stroke or any thromboembolism but with increased risk for major bleeding (aHR 1.40, 1.08‒1.82).
Conclusions
POAF after coronary artery bypass grafting is associated with negative prognostic impact. The role of early OAC therapy remains unclear. Studies aiming at reducing the occurrence of POAF and its consequences are warranted.
Keywords: CABG, oral anticoagulation therapy, postoperative atrial fibrillation
Subject Categories: Anticoagulants, Cardiovascular Surgery, Revascularization, Treatment
Nonstandard Abbreviations and Acronyms
- aHR
adjusted hazard ratio
- OAC
oral anticoagulation
- POAF
new‐onset postoperative atrial fibrillation
Clinical Perspectives
What Is New?
Postoperative atrial fibrillation after coronary artery bypass grafting was in the present large population‐based study associated with increased long‐term risk of ischemic stroke, thromboembolism, heart failure hospitalization and recurrent atrial fibrillation, but not with all‐cause mortality.
Early initiation of oral anticoagulation in patients with postoperative atrial fibrillation was not associated with reduced long‐term risk of ischemic stroke or thromboembolism, but with increased risk for major bleeding.
What Are the Clinical Implications?
Postoperative atrial fibrillation after coronary artery bypass grafting is associated with negative prognostic impact.
The role of early oral anticoagulation therapy in patients with postoperative atrial fibrillation remains unclear.
The results support an individualized anticoagulation strategy in patients with postoperative atrial fibrillation, based on stroke risk and bleeding risk.
New‐onset postoperative atrial fibrillation (POAF) is the most common arrhythmia after coronary artery bypass grafting (CABG). 1 , 2 , 3 , 4 While several studies have demonstrated that POAF is associated with an increased incidence of short‐term complications after CABG, 5 , 6 , 7 , 8 the long‐term consequences of POAF remain uncertain. Some studies have shown that POAF is associated with an increased long‐term risk for death and thromboembolic complications. 9 , 10 , 11 , 12 , 13 , 14 In contrast, Butt et al reported recently from a large Danish cohort study that patients with CABG with and without POAF had similar long‐term thromboembolic risk. 15 In that study, long‐term outcome was also compared between patients with POAF and matched nonsurgical patients with incident nonvalvular atrial fibrillation. Interestingly, patients with POAF after CABG had markedly better long‐term outcome than nonvalvular atrial fibrillation patients in terms of lower thromboembolic risk, lower mortality risk and lower risk of recurrent hospitalization for AF. These findings question if observations and treatment recommendations from studies in nonvalvular atrial fibrillation patients can be directly extrapolated to the POAF setting.
Current international guidelines recommend that oral anticoagulation (OAC) should be considered in patients who develop POAF after cardiac surgery (Class IIa, level of evidence B). 16 , 17 This recommendation is, however, based on only 1 study in cardiac surgery patients 11 and the experience from nonvalvular atrial fibrillation patients. Historically, only a limited proportion of cardiac surgery patients with POAF after CABG have been treated with OACs (8.4% to 23%). 11 , 15 , 18 , 19 Further studies investigating the role of OAC in POAF after cardiac surgery are therefore warranted.
In the present large population‐based nationwide study, we investigated if POAF after CABG is associated with increased long‐term risk of morbidity and/or mortality, which was our hypothesis. In addition, we explored if early initiation of OAC after CABG was associated with a reduced thromboembolic risk.
METHODS
Study Design and Study Population
This was an observational, nationwide, population‐based, longitudinal registry‐based cohort study complying with the Declaration of Helsinki and approved by the regional Human Research Ethics Committee in Gothenburg, Sweden (approval number 139‐16). The Ethics Committee waived the need for individual patient consent. The study population comprised all Swedish residents ≥18 years' old who underwent first‐time isolated CABG from January 1, 2007 to December 31, 2015. Patients were included in the study if they had had no previous history of AF, if they had not undergone any concomitant surgical procedures, and if they were alive on the 31th postoperative day. Patients were considered to have POAF when there was no documentation of previous AF after 1997 and they (1) were reported in the SWEDEHEART (Swedish Web System for Enhancement and Development of Evidence‐based Care in Heart Disease Evaluated According to Recommended Therapies) registry to have developed new‐onset AF during the index hospitalization, or (2) had been assigned a new AF diagnosis during the first 30 postoperative days in the Swedish National Patient Registry, or (3) had undergone electrical cardioversion during the index hospitalization. For the anticoagulation analysis, patients treated with OACs before surgery were excluded. A flow chart of the patients who were included and excluded is presented in Figure 1. All included patients were followed until death, emigration, or the end of the study period (December 31, 2015) using the unique personal identification number.
Figure 1. Flow chart of included and excluded patients.
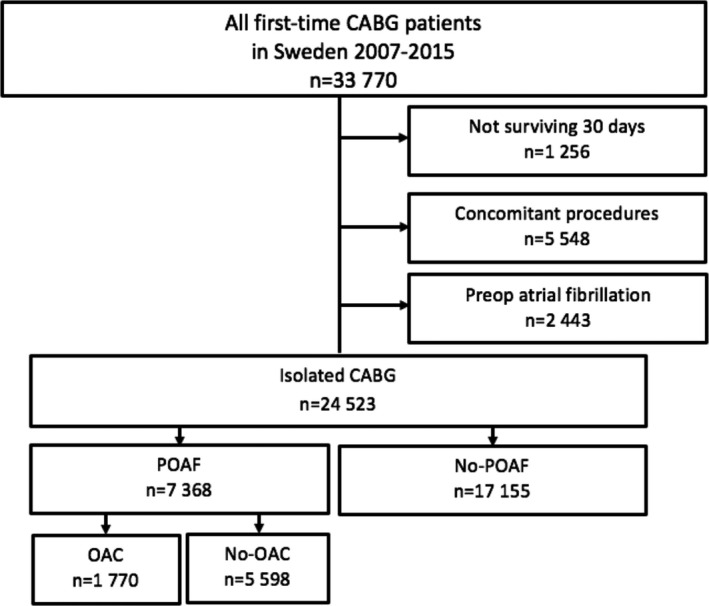
CABG indicates coronary artery bypass grafting; OAC, oral anticoagulant; and POAF, postoperative atrial fibrillation.
Data Sources
The study population was identified in the Swedish Heart Surgery Registry, which is a part of the SWEDEHEART registry. 20 All patients who have undergone cardiac surgery in Sweden since 1992 are included in the registry with detailed information on clinical and operative characteristics. In addition, data from 3 other national registries and databases were linked and combined with the information in SWEDEHEART, as previously described. 21
Information on medical history before the CABG procedure, including preoperative AF, was obtained from the SWEDEHEART registry and from the National Patient Registry. The latter is a nationwide registry established in 1987 containing detailed information on all hospitalizations and hospital‐affiliated outpatient visits including diagnoses, length of stay, and discharge dates in Sweden. The National Patient Registry is validated annually by the Swedish Board of Health and Welfare 22 ; most diagnoses have a validity of 85% to 95%. 23 Information on baseline and outcome variables was obtained using the International Classification of Diseases, Tenth Revision (ICD‐10). A list of the codes used to define conditions of interest is given in Table S1.
Information on medications dispensed after discharge was obtained from the Dispensed Drug Registry using anatomical therapeutic chemical classification codes (Table S2). This registry holds information on every dispensed prescription in Sweden since 2005. Patients were considered to be on early initiated OAC treatment if a prescription for OAC was dispensed within the first 30 days after discharge. OAC treatment was also registered after the first month by dispenses between the 31st and the 90th day following discharge (for the 3 months' time point) and by dispenses within the past 4 months before each year mark. Finally, mortality data were obtained from the national Cause of Death Registry. The authors declare that all supporting data are available within the article (and its online supplementary material).
Outcome Measures
An outcome event was accounted for when it was reported for the first time in the National Patient Registry and met all the following criteria: (1) occurred after the 30th postoperative day, (2) was associated with hospitalization, and (3) reported as the principal or secondary diagnosis for that hospitalization. The following outcome variables are reported: death from any cause, ischemic stroke, transient ischemic attack, thromboembolism (defined as ischemic stroke, or transient ischemic attack, or peripheral arterial embolism), heart failure, recurrent AF, pulmonary embolism, and major bleeding. Recurrent AF was accounted for when documented by a hospital visit, and when AF was reported in the National Patient Registry as the principal diagnosis.
Statistical Analysis
Descriptive statistics are presented as mean and SD for continuous variables and number and percentage for categorical variables. Incidence rate was estimated as the number of events per 100 person years and is presented with 95% exact Poisson CIs. Patients with and without POAF were compared before and after inverse probability treatment weighting, where the weights were defined based on propensity scores. 24 The variables used in the propensity score matching are listed in Table S3. Finally, the incidence was formally compared between patients with and without POAF using an inverse probability treatment weighting‐adjusted Cox proportional hazards regression model with POAF versus no POAF as the only independent variable. The assumption of proportional hazards was evaluated using visual inspection of the estimated survival curves. The balance between the groups was assessed with standardized mean difference. An absolute standardized difference of ≤0.10 was considered sufficient. The standardized mean difference before and after matching is presented in Figure S1.
All hypothesis testing used a P‐value of 0.05 as the significance level without any adjustment for multiple comparisons; thus, the outcome of a single test should be interpreted with caution. The tests were performed as t tests for continuous variables and chi‐square tests for categorical variables. The statistical analysis was performed using R version 3.3.0 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Study Population
Altogether, 33 770 patients underwent first‐time CABG in Sweden during the study period. A total of 24 523 patients remained, when those with preoperative AF, concomitant surgical procedures, and those who had died within 30 days were excluded (Figure 1). Among the 24 523 patients, 7368 (30.0%) had documented POAF. The patient characteristics are presented in Table 1. The mean age of the study cohort was 67 years, 80.3% were men, and 90.8% had a CHA2DS2‐VASc score ≥2. Patients in the POAF group were generally older and more likely to have heart failure, renal disease, reduced left ventricular ejection fraction, and higher CHA2DS2‐VASc score. Patients who developed POAF were more likely to be treated at discharge with OACs, anti‐arrhythmic drugs, angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, mineralocorticoid receptor antagonists, and digoxin. The use of beta‐blockers was similar in the 2 groups whereas the proportion of patients who received antiplatelet drugs was lower in the POAF group.
Table 1.
Clinical Characteristics of 24 523 Patients With First‐Time Isolated Coronary Bypass Grafting With and Without Postoperative Atrial Fibrillation
|
POAF (n=7368) |
No‐POAF (n=17 155) |
P Value | |
|---|---|---|---|
| Male sex | 6003 (81.5) | 13 691 (79.8) | 0.003 |
| Age, y | 70±8.0 | 66±9.3 | <0.001 |
| Operated with cardiopulmonary bypass | 7254 (98.5) | 16 874 (98.4) | 0.650 |
| Acute coronary syndrome | 4394 (59.6) | 10 177 (59.3) | 0.648 |
| Previous percutaneous coronary intervention | 1144 (15.5) | 2838 (16.5) | 0.048 |
| Hypertension | 5431 (73.7) | 11 556 (67.4) | <0.001 |
| Diabetes mellitus | 2115 (28.7) | 5152 (30.0) | 0.037 |
| Heart failure | 983 (13.3) | 1893 (11.0) | <0.001 |
| Left ventricular ejection fraction <50% | 2350 (32.1) | 4762 (28.0) | <0.001 |
| Previous ischemic stroke | 477 (6.5) | 975 (5.7) | 0.016 |
| Previous hemorrhagic stroke | 48 (0.7) | 96 (0.6) | 0.388 |
| Previous transient ischemic attack | 350 (4.8) | 603 (3.5) | <0.001 |
| Previous systemic embolism | 33 (0.4) | 62 (0.4) | 0.318 |
| Peripheral vascular disease | 837 (11.4) | 1497 (8.7) | <0.001 |
| CHA2DS2‐VASc | <0.001 | ||
| ≥2 | 6966 (94.6) | 15 301 (89.2) | |
| ≥4 | 3792 (51.4) | 6676 (39.0) | |
| Renal insufficiency | 249 (3.4) | 468 (2.7) | 0.006 |
| Renal replacemant therapy | 28 (0.4) | 71 (0.4) | 0.702 |
| Chronic respiratory disease | 742 (10.1) | 1596 (9.3) | 0.061 |
| Liver disease | 34 (0.5) | 94 (0.5) | 0.389 |
| History of cancer | 1070 (14.5) | 1943 (11.3) | <0.001 |
| Medications at discharge | |||
| Angiotensin‐converting enzyme inhibitors/angiotensin receptor blocker | 5247 (75.4) | 12 040 (73.1) | <0.001 |
| Beta blockers | 6346 (91.2) | 15 022 (91.2) | 0.846 |
| Mineralocorticoid receptor Antagonists | 727 (10.4) | 1172 (7.1) | <0.001 |
| Diuretics | 2920 (41.9) | 5210 (31.6) | <0.001 |
| Antiplatelets | 6245 (89.7) | 15 890 (96.5) | <0.001 |
| Vitamin K antagonist | 1527 (20.7) | 392 (2.3) | <0.001 |
| Novel oral anticoagulants | 245 (3.3) | 35 (0.2) | <0.001 |
| Lipid lowering agents | 6574 (94.4) | 15 682 (95.2) | 0.009 |
| Digoxin | 248 (3.6) | 83 (0.5) | <0.001 |
| Calcium channel blockers | 1794 (25.8) | 3947 (24.0) | 0.003 |
| Sotalol | 343 (4.7) | 121 (0.7) | <0.001 |
| Amiodarone | 623 (8.5) | 134 (0.8) | <0.001 |
Number and proportions (%) or mean±SD. POAF indicates postoperative atrial fibrillation.
The median length of follow‐up was 4.5 years (range 0‒9 years). During follow‐up, 10.6% of all patients died, 6.3% had an ischemic stroke, 3.0% had a transient ischemic attack, 9.7% suffered a thromboembolic event, 6.1% were hospitalized for heart failure, and 7.2% had an AF episode, either recurrent in patients with or new‐onset in patients without POAF. Pulmonary embolism occurred in 1.0% and major bleeding in 7.4% of the patients.
POAF and Outcome Variables
POAF was associated with an increased risk of ischemic stroke (adjusted hazard ratio [aHR] 1.18 [95% CI, 1.05‒1.32]), any thromboembolism (ischemic stroke, or transient ischemic attack, or peripheral arterial embolism [aHR 1.16 {1.05‒1.28}], heart failure hospitalization [aHR 1.35 {1.21‒1.51}], and recurrent AF [aHR 4.16 {3.76‒4.60}], but not with all‐cause mortality [aHR 1.08 {0.98‒1.18}])‐(Table 2, Figure 2). The risk of heart failure was independent of AF recurrence (incidence rate per 100 person years with recurrent AF 2.2 [1.8‒2.7] and without 2.0 [1.9‒2.2]).
Table 2.
Crude Event Rates After First‐Time Isolated Coronary Bypass Grafting With and Without Postoperative Atrial Fibrillation in Number of Events Per 100 Person‐Years and Unadjusted and Adjusted Hazard Ratios With a 95% CI, With No POAF as Reference
| Outcomes | POAF Event Rate | No POAF Event Rate | Unadjusted HR (95% CI) | Adjusted* HR (95% CI) | P Value |
|---|---|---|---|---|---|
| All‐cause mortality | 2.9 | 2.1 | 1.42 (1.31–1.54) | 1.08 (0.98–1.18) | 0.106 |
| Ischemic stroke | 1.8 | 1.3 | 1.40 (1.26–1.56) | 1.18 (1.05–1.32) | 0.004 |
| Peripheral arterial embolism | 0.1 | 0.1 | 1.00 (0.65–1.55) | 0.79 (0.50–1.23) | 0.293 |
| Transitory ischemic attack | 0.8 | 0.6 | 1.29 (1.11–1.51) | 1.10 (0.94–1.30) | 0.237 |
| Any thromboembolism | 2.4 | 1.8 | 1.37 (1.25–1.51) | 1.16 (1.05–1.28) | 0.003 |
| Heart failure | 2.1 | 1.1 | 1.80 (1.62–1.99) | 1.35 (1.21–1.51) | <0.001 |
| Recurrent AF | 4.0 | 0.8 | 4.63 (4.20–5.09) | 4.16 (3.76–4.60) | <0.001 |
| Pulmonary embolism | 0.3 | 0.2 | 1.46 (1.13–1.89) | 1.26 (0.95–1.67) | 0.106 |
| Major bleeding | 2.0 | 1.6 | 1.30 (1.18–1.43) | 1.05 (0.95–1.17) | 0.337 |
AF indicates atrial fibrillation; any thromboembolism, ischemic stroke, transitory ischemic attack, or peripheral arterial embolism; HR, hazard ratio; and POAF, postoperative atrial fibrillation.
Adjusted for patient characteristics, comorbidities, CHA2DS2‐VASc score, year of surgery, and medications. A detailed list of the variables used for adjustment is shown in Table S3.
Figure 2. The adjusted cumulative incidence of all‐cause mortality, ischemic stroke, recurrent atrial fibrillation and hospitalization for heart failure, in 24 553 patients with CABG with (blue line) and without (red line) new‐onset postoperative atrial fibrillation.
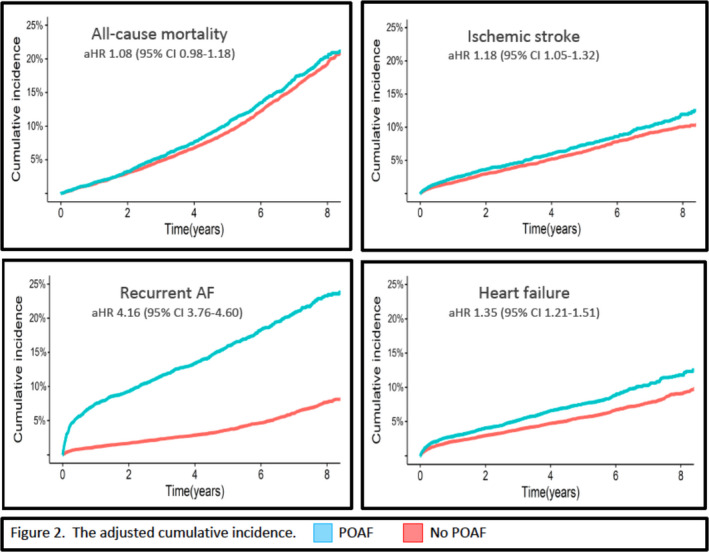
The hazard ratios are adjusted for patient characteristics, comorbidities, CHA2DS2‐VASc score, year of surgery and medications. A detailed list of the variables used for adjustment is shown in Table S3. AF indicates atrial fibrillation; aHR, adjusted hazard ratio; CABG, coronary artery bypass grafting; and POAF, postoperative atrial fibrillation.
Early Postoperative Oral Anticoagulation and Outcome in Patients With POAF
For this analysis, 135 out of 7368 patients with POAF (1.8%) were excluded due to preoperative OAC treatment unrelated to preoperative AF. Of the remaining 7233 patients with POAF, 1714 (23.7%) were dispensed OACs within the first 30 days after discharge at the discretion of the treating surgeon. The annual proportion of patients treated with OAC within 30 days increased from a plateau around 20% during 2007 to 2010 to 34% in 2015 (Figure 3). The proportion of patients with POAF treated with OAC at different time‐points after surgery is depicted in Figure 4. At 3 months 25.9 % were treated while in the period 1 to 5 years after CABG ≈15% of all patients with POAF were treated with OAC. Among patients with POAF with early initiated OAC (n=1714), 100% of the patients remained on OAC therapy at 3 months, 50% at 1 year, and ≈40% during 2 to 5 years follow‐up, Figure 5. Characteristics of patients with and without early initiated OAC treatment are presented in Table 3. Patients treated with OAC were generally older, had more often a history of heart failure, and had less diabetes. They were more often dispensed with angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, beta blockers, mineralocorticoid receptors antagonists, diuretics, digoxin, and amiodarone at discharge but less often with antiplatelet medication.
Figure 3. The annual proportion of early‐initiated oral anticoagulation during 2007 to 2015 in patients with new‐onset postoperative atrial fibrillation after coronary artery bypass grafting (CABG).

Figure 4. The proportion of patients with new‐onset postoperative atrial fibrillation treated with oral anticoagulation at different time points after coronary artery bypass grafting.

Figure 5. Oral anticoagulation therapy at different time points after discharge in all patients with new‐onset postoperative atrial fibrillation and anticoagulant therapy initiated within 30 days after coronary artery bypass grafting (n=1714 at 30 days).
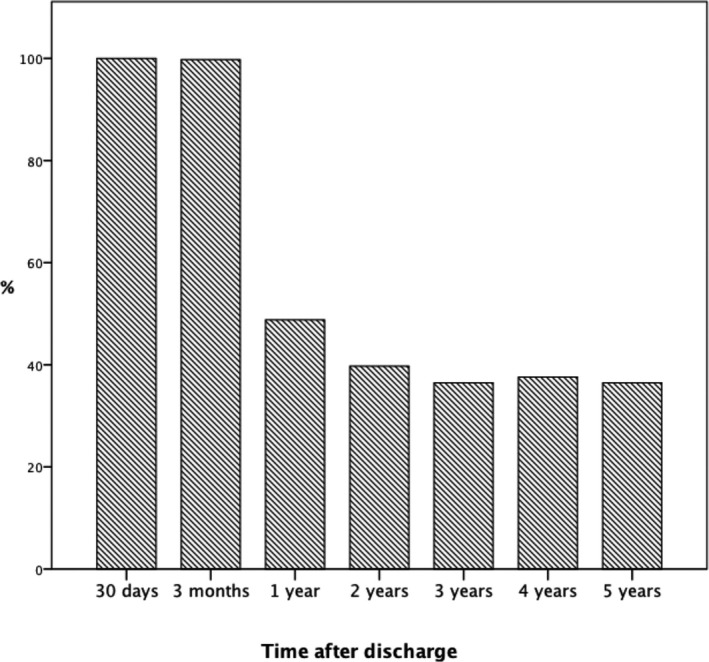
Table 3.
Patient Characteristics in Patients With Postoperative Atrial Fibrillation With and Without Oral Anticoagulation
| OACs (n=1714) | No OACs (n=5519) | P Value | |
|---|---|---|---|
| Male sex | 1412 (82.4) | 4480 (81.2) | 0.277 |
| Age, y | 71±7.4 | 69±8.0 | <0.001 |
| Acute coronary syndrome | 1018 (59.4) | 3312 (60.0) | 0.208 |
| Previous percutaneous coronary intervention | 261 (15.2) | 857 (15.5) | 0.793 |
| Hypertension | 1255 (73.2) | 4082 (74.0) | 0.563 |
| Diabetes mellitus | 425 (24.8) | 1648 (29.9) | <0.001 |
| Heart failure | 262 (15.3) | 667 (12.1) | 0.001 |
| Left ventricular ejection fraction <50% | 579 (34) | 1693 (30.9) | 0.028 |
| Previous ischemic stroke | 127 (7.4) | 337 (6.1) | 0.062 |
| Previous hemorrhagic stroke | 10 (0.6) | 38 (0.7) | 0.766 |
| Previous transitory ischemic attack | 94 (5.5) | 242 (4.4) | 0.068 |
| Previous systemic embolism | 8 (0.5) | 23 (0.4) | 0.948 |
| Peripheral vascular disease | 222 (13.0) | 589 (10.7) | 0.010 |
| CHA2DS2‐VASc score | <0.001 | ||
| ≥2 | 1637 (95.5) | 5201 (94.2) | |
| ≥4 | 925 (54.0) | 2738 (50.3) | |
| Renal insufficiency | 51 (3.0) | 191 (3.5) | 0.369 |
| Renal replacement therapy | 6 (0.4) | 21 (0.4) | 1.000 |
| Chronic respiratory disease | 146 (8.5) | 578 (10.5) | 0.021 |
| Liver disease | 5 (0.3) | 29 (0.5) | 0.301 |
| History of cancer | 262 (15.3) | 789 (14.3) | 0.329 |
| Medications at discharge | |||
| Angiotensin‐converting enzyme inhibitors/angiotensin receptor blocker | 1273 (79.8) | 3995 (76.3) | 0.004 |
| Beta blockers | 1501 (94.0) | 4844 (92.5) | 0.033 |
| Mineralocorticoid receptor antagonists | 223 (14.0) | 550 (10.5) | <0.001 |
| Diuretics | 941 (59.0) | 2534 (48.4) | <0.001 |
| Antiplatelets | 1207 (75.6) | 5127 (97.9) | <0.001 |
| Lipid lowering agents | 1538 (96.4) | 5033 (96.1) | 0.611 |
| Digoxin | 188 (11.8) | 116 (2.2) | <0.001 |
| Calcium channel blockers | 419 (26.3) | 1430 (27.3) | 0.410 |
| Sotalol | 145 (8.5) | 678 (12.3) | <0.001 |
| Amiodarone | 447 (26.1) | 665 (12.0) | <0.001 |
Mean±SD or number and proportion (%). OAC indicates oral anticoagulant.
Table 4 shows the crude event rates and HRs after 5 years of follow‐up, unadjusted and adjusted for age, sex, comorbidities, and medications. There was no significant association between early initiation of OAC and all‐cause mortality (aHR 0.89 [0.73–1.09]), ischemic stroke (aHR 1.08 [0.80–1.45]), or any thromboembolism (aHR 1.01 [0.77–1.33]) but an association with a higher risk for major bleeding (aHR 1.40 [1.08‒1.82]) at 5 years' follow‐up.
Table 4.
Crude Event Rates After Coronary Artery Bypass Grafting in Patients With Postoperative Atrial Fibrillation With (n=1714) or Without (n=5519) Early‐Initiated Oral Anticoagulants
| Outcomes | Event Rate With OAC | Event Rate Without OAC |
Unadjusted HR (95% CI) OAC vs No‐OAC |
Adjusted* HR (95% CI) OAC vs no‐OAC |
P Value |
|---|---|---|---|---|---|
| All‐cause mortality | 3.0 | 2.8 | 1.08 (0.92–1.28) | 0.89 (0.73–1.09) | 0.257 |
| Ischemic stroke | 2.0 | 1.7 | 1.13 (0.92–1.39) | 1.08 (0.80–1.45) | 0.625 |
| Any thromboembolism | 2.6 | 2.4 | 1.10 (0.92–1.32) | 1.01 (0.77–1.33) | 0.942 |
| Transitory ischemic attack | 0.9 | 0.8 | 1.09 (0.81–1.49) | 0.97 (0.58–1.63) | 0.915 |
| Pulmonary embolism | 0.2 | 0.3 | 0.75 (0.42–1.34) | 0.50 (0.22–1.13) | 0.096 |
| Major bleeding | 2.4 | 1.9 | 1.26 (1.04–1.52) | 1.40 (1.08–1.82) | 0.011 |
Event rates expressed in number of events per 100 person‐years and unadjusted and adjusted hazard ratios with a 95% CI at 5‐year follow‐up in relation to OAC status with no OAC as reference. HR indicates hazard ratio; and OAC, oral anticoagulant.
Adjusted for patient characteristics, comorbidities, CHA2DS2‐VASc score, year of surgery, and medications. A detailed list of the variables used for adjustment is shown in Table S3.
DISCUSSION
The main findings of this population‐based study were that (1) POAF after CABG was associated with increased risk of long‐term morbidity, including thromboembolic episodes, heart failure, and recurrent AF; and (2) early initiation of OAC in patients with POAF was not associated with any reduction in the risk of death or thromboembolic events, but with an increased risk for major bleeding.
In the present study, POAF after CABG was associated with a moderately increased long‐term risk of ischemic stroke and any thromboembolism. This observation corroborates the negative prognostic effects of POAF on long‐term thromboembolism risk reported previously, 10 , 13 and confirms that POAF after CABG has significant prognostic importance. At first glance the results are at odds with the recent registry study by Butt et al, which did not find any increased risk of thromboembolic events in CABG patients with POAF. 15 However, the difference in adjusted hazard ratios for thromboembolic events in the 2 studies was only marginal (1.16 in the present study versus 1.11 in Butt et al with overlapping CIs). Hence, the difference between the studies regarding statistical significance can be explained by the markedly larger study population in the present study.
POAF was associated with a 4‐fold higher risk of AF recurrence, mostly within the first few postoperative months (Figure 2). As previously mentioned in the Methods section, AF recurrence after the index hospitalization was accounted for when a diagnosis of AF was reported in the patient registry. Hence, this recurrence rate, despite being high, is most likely an underestimation of the actual rate, given the prevalence of asymptomatic (silent) AF. 25 , 26 We also found that POAF was associated with a significantly higher adjusted risk of heart failure independently of documented recurrent AF. Heart failure and AF are closely related and share common pathophysiological pathways and risk factors. 27 In addition, AF is an independent risk factor for heart failure. 28 It is, however, often difficult to determine which condition exacerbates the other, and whether both AF and heart failure are two different expressions of the same underlying pathology. 29
The American Heart Association/American College of Cardiology/Heart Rhythm Society guideline for the management of patients with AF, states that it is “reasonable” to administer OAC to patients with POAF (Class IIa recommendation, level of evidence B). 16 Similarly, the 2016 European Society of Cardiology/European Association for Cardio‐Thoracic Surgery guidelines for the management of AF recommend that long‐term use of OACs should be considered in patients with POAF who are at risk of stroke, taking into consideration the individual risk of stroke versus bleeding (Class IIa recommendation, level of evidence B). 17 These recommendations are mainly based on evidence extrapolated from studies conducted on patients with AF occurring in the nonsurgical setting, 30 , 31 and on 1 retrospective study of 613 patients with POAF treated with warfarin. 11 The adherence to these recommendations is limited, with only 8.4 to 23% of patients with POAF initially treated with OAC's in recent studies, 11 , 15 , 18 , 19 most likely due to absence of strong evidence for its efficacy and fear for bleeding complications.
In the present study, 1714 (23.7%) patients with POAF were treated with OAC within 30 days after discharge, which is a higher proportion than in most previous studies. We did not observe any overall benefit regarding risk for all‐cause mortality and thromboembolic events but an increased risk for major bleeding, when OAC treatment was initiated early. This indicates that not all patients with POAF benefit from early initiated OAC, but does not exclude that there are subgroups of patients with POAF where OAC therapy is essential for reducing the thrombo‐embolic risk. Our results should, however, be interpreted with caution because of several limitations. Most importantly, there was no randomization and no adjustment for OAC initiation or discontinuation after the first 30 postoperative days. On the other hand, the smaller study by El‐Chami et al, 11 upon which the current recommendations about OAC in patients with POAF is partly based, had a similar study design with the same inherent weaknesses as the present study. Furthermore, the treatment period in patients with early initiated OAC was limited, only 50% of the patients remained on treatment 12 months after discharge (Figure 5), which at least partially may explain the lack of association with improved outcome. On the other hand, a longer treatment period may further increase the bleeding risk. Further studies investigating the role of OACs in patients with POAF are warranted and recently, a randomized controlled trial was started (PACES; NCT04045665).
Limitations
This study has both strengths and limitations. The strengths include the large nationwide population‐based study cohort, the multicenter‐based data, and the quality of the registries. Over a period of 9 years, the POAF annual rate was stable at around 30%. This is consistent with rates published previously, 1 , 2 , 3 , 4 and indicates that the methods used to define POAF in this study were appropriate. Limitations are the observational study design, the inherent risk of bias from unregistered confounders, and the risk of selection bias. The study limitations regarding OAC treatment are discussed above. The occurrence of AF after the index hospitalization was probably underestimated, for reasons already mentioned.
Conclusions
New‐onset POAF after isolated first‐time CABG was in this large real‐world study associated with a significantly increased risk of ischemic stroke and any thromboembolism, heart failure, and recurrence of AF which corroborates its negative prognostic impact. The benefit of early OAC remains unclear. Studies aimed at reducing the occurrence and consequences of POAF, and examining the role of OACs in the POAF setting, are strongly warranted.
Sources of Funding
The study was supported by the Swedish Heart‐Lung Foundation (grant 20150587 and 20180560 to Jeppsson), the Swedish State (grant ALFGBG‐725131 to Jeppsson) under the agreement between the Swedish government and the county councils concerning economic support of research and education of doctors (ALF agreement), Region Västra Götaland (grant VGFOUREG‐847811 to Jeppsson and grant VGFOUREG‐648981 to Taha) and Wilhelm and Martina Lundgrens Foundation (grant 2019‐3110 to Taha). The supporting bodies had no influence on the analysis and interpretation of data, on the writing of the report, or on the decision to submit the paper for publication.
Disclosures
Jeppsson reports personal fees from Boehringer‐Ingelheim, XVIVO and LFB, outside the submitted work. Taha reports personal fees from Bayer outside the submitted work. Friberg and Bergfeldt reports personal fees from Bayer, Boehringer Ingelheim, and Sanofi, outside the submitted work. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figure S1
Acknowledgments
The authors thank Niklas Svensson for excellent database work.
(J Am Heart Assoc. 2021;10:e017966. DOI: 10.1161/JAHA.120.017966.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017966
For Sources of Funding and Disclosures, see page 9.
REFERENCES
- 1. Filardo G, Damiano RJ Jr, Ailawadi G, Thourani VH, Pollock BD, Sass DM, Phan TK, Nguyen H, da Graca B. Epidemiology of new‐onset atrial fibrillation following coronary artery bypass graft surgery. Heart. 2018;104:985–992. [DOI] [PubMed] [Google Scholar]
- 2. Shen J, Lall S, Zheng V, Buckley P, Damiano RJ Jr, Schuessler RB. The persistent problem of new‐onset postoperative atrial fibrillation: a single‐institution experience over two decades. J Thorac Cardiovasc Surg. 2011;141:559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zaman AG, Archbold RA, Helft G, Paul EA, Curzen NP, Mills PG. Atrial fibrillation after coronary artery bypass surgery: a model for preoperative risk stratification. Circulation. 2000;101:1403–1408. [DOI] [PubMed] [Google Scholar]
- 4. Anderson E, Dyke C, Levy JH. Anticoagulation strategies for the management of postoperative atrial fibrillation. Clin Lab Med. 2014;34:537–561. [DOI] [PubMed] [Google Scholar]
- 5. Villareal RP, Hariharan R, Liu BC, Kar B, Lee VV, Elayda M, Lopez JA, Rasekh A, Wilson JM, Massumi A. Postoperative atrial fibrillation and mortality after coronary artery bypass surgery. J Am Coll Cardiol. 2004;43:742–748. [DOI] [PubMed] [Google Scholar]
- 6. Kaw R, Hernandez AV, Masood I, Gillinov AM, Saliba W, Blackstone EH. Short‐ and long‐term mortality associated with new‐onset atrial fibrillation after coronary artery bypass grafting: a systematic review and meta‐analysis. J Thorac Cardiovasc Surg. 2011;141:1305–1312. [DOI] [PubMed] [Google Scholar]
- 7. Maisel WH, Rawn JD, Stevenson WG. Atrial fibrillation after cardiac surgery. Ann Intern Med. 2001;135:1061–1073. [DOI] [PubMed] [Google Scholar]
- 8. Greenberg JW, Lancaster TS, Schuessler RB, Melby SJ. Postoperative atrial fibrillation following cardiac surgery: a persistent complication. Eur J Cardiothorac Surg. 2017;52:665–672. [DOI] [PubMed] [Google Scholar]
- 9. Thoren E, Wernroth ML, Christersson C, Grinnemo KH, Jideus L, Stahle E. Compared with matched controls, patients with postoperative atrial fibrillation (POAF) have increased long‐term AF after CABG, and POAF is further associated with increased ischemic stroke, heart failure and mortality even after adjustment for AF. Clin Res Cardiol. 2020;109:1232–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kosmidou I, Chen S, Kappetein AP, Serruys PW, Gersh BJ, Puskas JD, Kandzari DE, Taggart DP, Morice MC, Buszman PE, et al. New‐onset atrial fibrillation after PCI or CABG for left main disease: the EXCEL trial. J Am Coll Cardiol. 2018;71:739–748. [DOI] [PubMed] [Google Scholar]
- 11. El‐Chami MF, Kilgo P, Thourani V, Lattouf OM, Delurgio DB, Guyton RA, Leon AR, Puskas JD. New‐onset atrial fibrillation predicts long‐term mortality after coronary artery bypass graft. J Am Coll Cardiol. 2010;55:1370–1376. [DOI] [PubMed] [Google Scholar]
- 12. Ahlsson A, Fengsrud E, Bodin L, Englund A. Postoperative atrial fibrillation in patients undergoing aortocoronary bypass surgery carries an eightfold risk of future atrial fibrillation and a doubled cardiovascular mortality. Eur J Cardiothorac Surg. 2010;37:1353–1359. [DOI] [PubMed] [Google Scholar]
- 13. Gialdini G, Nearing K, Bhave PD, Bonuccelli U, Iadecola C, Healey JS, Kamel H. Perioperative atrial fibrillation and the long‐term risk of ischemic stroke. JAMA. 2014;312:616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee SH, Kang DR, Uhm JS, Shim J, Sung JH, Kim JY, Pak HN, Lee MH, Joung B. New‐onset atrial fibrillation predicts long‐term newly developed atrial fibrillation after coronary artery bypass graft. Am Heart J. 2014;167:593–600.e1. [DOI] [PubMed] [Google Scholar]
- 15. Butt JH, Xian Y, Peterson ED, Olsen PS, Rorth R, Gundlund A, Olesen JB, Gislason GH, Torp‐Pedersen C, Kober L, et al. Long‐term thromboembolic risk in patients with postoperative atrial fibrillation after coronary artery bypass graft surgery and patients with nonvalvular atrial fibrillation. JAMA Cardiol. 2018;3:417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–e267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 18. Al‐Khatib SM, Hafley G, Harrington RA, Mack MJ, Ferguson TB, Peterson ED, Califf RM, Kouchoukos NT, Alexander JH. Patterns of management of atrial fibrillation complicating coronary artery bypass grafting: results from the PRoject of Ex‐vivo Vein graft ENgineering via Transfection IV (PREVENT‐IV) Trial. Am Heart J. 2009;158:792–798. [DOI] [PubMed] [Google Scholar]
- 19. Thorén E, Hellgren L, Ståhle E. High incidence of atrial fibrillation after coronary surgery. Interact Cardiovasc Thorac Surg. 2016;22:176–180. [DOI] [PubMed] [Google Scholar]
- 20. Vikholm P, Ivert T, Nilsson J, Holmgren A, Freter W, Ternstrom L, Ghaidan H, Sartipy U, Olsson C, Granfeldt H, et al. Validity of the swedish cardiac surgery registry. Interact Cardiovasc Thorac Surg. 2018;27:67–74. [DOI] [PubMed] [Google Scholar]
- 21. Bjorklund E, Nielsen SJ, Hansson EC, Karlsson M, Wallinder A, Martinsson A, Tygesen H, Romlin BS, Malm CJ, Pivodic A, et al. Secondary prevention medications after coronary artery bypass grafting and long‐term survival: a population‐based longitudinal study from the SWEDEHEART registry. Eur Heart J. 2020;41:1653–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. The Swedish National Board of Health and Welfare . Quality of data and reporting procedures. https://www.socialstyrelsen.se/en/statistics‐and‐data/registers/register‐information/the‐national‐patient‐register/. Accessed January 24, 2020.
- 23. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, Reuterwall C, Heurgren M, Olausson PO. External review and validation of the Swedish national inpatient register. BMC Public Health. 2011;11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Israel CW, Gronefeld G, Ehrlich JR, Li YG, Hohnloser SH. Long‐term risk of recurrent atrial fibrillation as documented by an implantable monitoring device: implications for optimal patient care. J Am Coll Cardiol. 2004;43:47–52. [DOI] [PubMed] [Google Scholar]
- 26. Savelieva I, Camm AJ. Clinical relevance of silent atrial fibrillation: prevalence, prognosis, quality of life, and management. J Interv Card Electrophysiol. 2000;4:369–382. [DOI] [PubMed] [Google Scholar]
- 27. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;36:3250–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stewart S, Hart CL, Hole DJ, McMurray JJ. A population‐based study of the long‐term risks associated with atrial fibrillation: 20‐year follow‐up of the Renfrew/Paisley study. Am J Med. 2002;113:359–364. [DOI] [PubMed] [Google Scholar]
- 29. Carlisle MA, Fudim M, DeVore AD, Piccini JP. Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail. 2019;7:447–456. [DOI] [PubMed] [Google Scholar]
- 30. Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor‐based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. [DOI] [PubMed] [Google Scholar]
- 31. Friberg L, Rosenqvist M, Lip GY. Evaluation of risk stratification schemes for ischaemic stroke and bleeding in 182 678 patients with atrial fibrillation: the Swedish Atrial Fibrillation cohort study. Eur Heart J. 2012;33:1500–1510. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figure S1


