Abstract
Background
In patients with obstructive hypertrophic cardiomyopathy, surgical myectomy (SM) is indicated for severe symptoms. We sought to compare long‐term outcomes of patients with obstructive hypertrophic cardiomyopathy where SM was based on guideline‐recommended Class I indication (Functional Class or FC ≥3 or angina/exertional syncope despite maximal medical therapy) versus earlier (FC 2 and/or impaired exercise capacity on exercise echocardiography with severe obstruction).
Methods and Results
We studied 2268 consecutive patients (excluding <18 years, ≥ moderate aortic stenosis and subaortic membrane, 56±14 years, 55% men), who underwent SM at our center between June 2002 and March 2018. Clinical data, including left ventricular outflow tract gradient, were recorded. Death and/or appropriate internal defibrillator discharge were primary composite end points. One thousand three hundred eighteen (58%) patients met Class I indication and 950 (42%) underwent earlier surgery; 222 (10%) had a history of obstructive coronary artery disease. Basal septal thickness, and resting and maximal left ventricular outflow tract gradient were 2.0±0.3 cm, 61±44 mm Hg, and 100±31 mm Hg, respectively. At 6.2±4 years after SM, 248 (11%) had composite events (13 [0.6%] in‐hospital deaths). Age (hazard ratio [HR], 1.61; 95% CI, 1.26–1.91), obstructive coronary artery disease (HR, 1.46; 95% CI, 1.06–1.91), and Class I versus earlier SM (HR, 1.61; 95% CI, 1.14–2.12) were associated with higher primary composite events (all P<0.001). Earlier surgery had better longer‐term survival (similar to age‐sex‐matched normal population) versus surgery for Class I indication (76 [8%] versus 193 [15%], P<0.001).
Conclusions
In patients with obstructive hypertrophic cardiomyopathy, earlier versus surgery for Class I indication had a better long‐term survival, similar to the age‐sex‐matched US population.
Keywords: earlier surgery, hypertrophic cardiomyopathy, outcomes
Subject Categories: Cardiovascular Surgery, Mortality/Survival
Nonstandard Abbreviations and Acronyms
- HCM
hypertrophic cardiomyopathy
- MR
mitral regurgitation
- NYHA
New York Heart Association
- SCD
sudden cardiac death
Clinical Perspective
What Is New?
In 2268 patients with obstructive hypertrophic cardiomyopathy undergoing surgery, where the decision was based on symptom assessment and/or objective evaluation of exercise capacity, earlier surgery had better long‐term primary outcomes versus that performed for Class I indication, with long‐term survival similar to a normal age‐sex‐matched US population.
A sizable proportion of patients needed a concomitant mitral valve/subvalvular procedure to relieve left ventricular outflow tract obstruction, with outcomes similar to isolated myectomy.
Assessment of functional capacity and dynamic left ventricular outflow tract obstruction on exercise echocardiography was effectively utilized to help guide surgical decision making.
What Are the Clinical Implications?
An earlier surgical approach to treat obstructive hypertrophic cardiomyopathy, at an experienced center, before reaching Class I indication, is associated with better longer‐term survival, reaching that of a normal age‐sex‐matched US population.
Many patients with obstructive hypertrophic cardiomyopathy need a complex mitral operation beyond surgical myectomy, and would be best (1) evaluated by experienced cardiologists using multimodality imaging and (2) operated on by experienced surgeons at an experienced center.
The perception of patients with obstructive hypertrophic cardiomyopathy regarding their asymptomatic status is often misleading, and exercise echocardiography helps elicit functional limitation in such patients, along with objective evidence of dynamic left ventricular outflow tract obstruction, which can help guide surgical decision making.
Hypertrophic cardiomyopathy (HCM) is a commonly inherited cardiomyopathy with a varied phenotypic expression ranging from asymptomatic to congestive heart failure to sudden death, which occurs in <1%/year. 1 , 2 , 3 , 4 A characteristic finding in HCM is dynamic left ventricular outflow tract (LVOT) obstruction and concomitant mitral regurgitation (MR) 5 caused by systolic anterior motion of the mitral valve, which often results in symptomatic congestive heart failure, reduced exercise capacity, and exertional syncope. LVOT obstruction, which is independently associated with adverse HCM‐related outcomes such as congestive heart failure and death, 5 , 6 is observed in ≈70% patients with HCM, when observed carefully. 7
Surgical myectomy provides excellent long‐term survival and freedom from recurrent symptoms in patients with obstructive HCM, based on prior nonrandomized observational studies. 8 , 9 , 10 , 11 , 12 , 13 On the other hand, there are no large‐scale studies demonstrating a clear survival benefit using medical therapy to relieve LVOT obstruction. As a result, currently, it is a class I indication (level of evidence C) to offer invasive therapies (surgical myectomy±mitral valve surgery or alcohol septal ablation) to patients who have severe LVOT obstruction and who are intractably symptomatic (New York Heart Association [NYHA] Class III–IV), despite maximally tolerated medical therapy. 3 , 4 However, the perception of patients about their symptoms is often misleading and in many instances, symptoms are equivocal. In such scenarios, exercise stress testing can unmask latent symptoms and help decision making. 14 , 15 Utilizing a combination of symptom assessment and impaired exercise capacity, we have demonstrated that patients with obstructed HCM have a better longer‐term survival following surgical relief of LVOT obstruction versus watchful waiting. 16 Also, there is increasing recognition that earlier surgical intervention (before meeting a Class I indication) in many valvular diseases (eg, primary mitral and chronic severe aortic regurgitation), especially if performed at an experienced center with experienced providers, is associated with significant longer‐term survival benefit. 17 , 18 , 19 , 20 Because of previously demonstrated excellent outcomes of surgical relief of LVOT obstruction in symptomatic patients with obstructive HCM at our center, 12 , 13 in a selected group of severely obstructed patients with HCM with intolerance to medications and impaired exercise capacity on stress testing, we offer surgical relief of LVOT obstruction earlier than the standard Class I indication. We sought to study whether there are differences in longer‐term outcomes in patients with obstructed HCM who underwent surgical relief of LVOT obstruction for a Class I indication versus earlier.
Methods
The authors will not make the data, analytic methods, and study materials available to other researchers.
Study Sample
The study sample consisted of 2268 consecutive patients with HCM (≥18 years) with severe dynamic LVOT obstruction, who underwent surgical relief of LVOT obstruction at our tertiary care center, between June 2002 and March 2018. These patients are part of an institutional review board–approved observational registry with waiver of individual informed consent. Because of a different pathophysiologic profile, the following patients were excluded: (1) those undergoing surgery to remove subaortic membrane (n=63) and (2) those with a mixed picture of HCM and ≥ moderate aortic stenosis requiring surgical myectomy and concomitant aortic valve replacement (n=188). By study design, patients with midventricular or apical myectomy (without LVOT obstruction) were not included. The diagnosis of HCM was made by experienced cardiologists, based on typical features, with ventricular myocardial hypertrophy (left ventricular [LV] wall thickness ≥15 mm) occurring in the absence of any other disease responsible for hypertrophy. 3 , 4 Additionally, in patients with borderline LV wall thickness (≈15 mm), the presence of resting/provocable LVOT obstruction (LVOT gradient ≥30 mm Hg) also aided in the diagnosis. 3 , 4 Because this was a surgical cohort, there were no patients with a prohibitive comorbidity (eg, cancer, advanced neurologic, pulmonary, or hepatic or renal pathologies) at the time that would preclude cardiac surgery.
Baseline clinical data were manually extracted from electronic medical records. Follow‐up information was collected by manual extraction from electronic medical records and phone calls. Presence of atrial fibrillation (AF) was recorded, based on history, ECGs, and Holter data. Nonsustained ventricular tachycardia (VT), wide complex tachycardia at ≥120 beats per minute, lasting >3 beats but <30 s or sustained VT, lasting >30 s were recorded, based on history and Holter data. The presence of implantable cardioverter defibrillator (ICD) and permanent pacemaker was ascertained.
Based on the indications for surgical relief of LVOT obstruction, the patients were divided as follows: (1) class I (intractable symptoms [NYHA class III–IV, angina, syncope]) associated with severe LVOT obstruction despite maximal medical therapy; and (2) earlier surgery (NYHA class II with drug intolerance or symptomatic impairment of exercise capacity on stress echocardiography despite maximal medical therapy 15 ). Drug intolerance consisted of significant fatigue or hypotension‐associated dizziness (typically because of β‐blockers), which significantly reduced quality of life, as perceived by the patients. Symptomatic impairment of exercise capacity was defined as development of symptoms (dyspnea, dizziness, or presyncope) in the setting of achieving <85% of age‐sex‐predicted metabolic equivalents and concomitant severe LVOT obstruction despite optimal medical therapy. The final decision regarding surgical relief of LVOT obstruction was made after a consensus between experienced cardiologists and cardiothoracic surgeons.
Echocardiography
All patients underwent comprehensive echocardiograms using commercially available instruments (Philips, WA, General Electric, WI and Siemens, PA). Maximal end‐diastolic LV wall thickness, LV dimensions, and left atrial area were measured according to guidelines. 21 Resting LVOT peak velocity was measured by continuous‐wave Doppler echocardiography, and pressure gradient was estimated by using simplified Bernoulli equation. Care was taken to avoid contamination of LVOT waveform by MR. In patients with resting LVOT gradients <30 mm Hg, provocative maneuvers, including Valsalva and amyl nitrite, were used. In patients with low resting LVOT gradients and/or NYHA Class I‐II symptoms based on clinical history, we performed exercise echocardiography to assess exercise capacity (recorded as % of age‐sex predicted metabolic equivalents), blood pressure response, and maximal postexercise LVOT gradient, as described. 15 In patients with resting peak LVOT gradient >100 mm Hg, provocative maneuvers were not used. Maximal LVOT gradient was recorded, and defined as the highest recorded gradient (either resting or provoked, including exercise) in a patient. 22 Degree of resting MR was assessed (none‐severe), using multiple criteria. 23
Outcomes Assessment
The duration of follow‐up ranged between initial surgery to event/last follow‐up. In addition to phone call (n=994) and/or outpatient follow‐up (n=1632), we queried individual state and nationally available databases in all patients (n=2268). The last query was performed in June 2018. Death notification was confirmed by observation of death certificate or verified with a family member. Out of the 248 total patients who died, we had documentation of the event and the cause in our electronic medical records in 199 patients (based on a phone call or a note from family members informing us of the event), while the remainder (n=49) were additionally identified by various database searches. The cause of death was ascertained as HCM‐related death (including sudden cardiac death [SCD]), unknown or noncardiac death, after review of records and/or discussion with family. SCD was defined as unexpected sudden collapse occurring <1 hour from symptom onset in otherwise stable patients. 24 In addition, we recorded successful resuscitation from cardiac arrest or appropriate ICD shocks (with defibrillation threshold of >200 beats on electrogram reviews at our institution). 24 For the primary composite end point, we included death (because it is more objective) and appropriate ICD discharge. The appropriateness of all ICD discharges was ascertained by a review at our center, and only ICD discharges that were deemed appropriate (n=24) were included as part of the end point. We included a secondary end point of HCM‐related death (excluding noncardiac death caused by cancer, liver failure, primary respiratory or neurologic issues, censored at the time of event), unknown cause of death, and appropriate ICD discharge. Patients with an unknown cause of death were included as part of the secondary composite outcome, unless the patients’ proximal history, just before death, strongly suggested a noncardiac cause based on chart review or family discussion. 25 In addition, presence and cause of stroke (transient or permanent) was recorded based on neurologic evaluation and neuroimaging. Arrhythmias, occurring during follow‐up (AF, VT) were recorded.
Date and type of surgical procedures performed were recorded as: (1) myectomy, (2) myectomy+mitral valve/subvalvular apparatus surgery, (3) myectomy+coronary artery bypass grafting, and (4) myectomy+coronary artery bypass grafting+mitral valve/subvalvular apparatus surgery. Details of surgical techniques by our group have been described previously. 9 , 12 , 13 , 26 The basic technique of myectomy involved muscle resection below the membranous septum, removing muscle over both papillary muscles, and extending to both trigones. In patients requiring additional mitral valve/subvalvular surgery, the following techniques were utilized: plication of A2 scallop of the anterior leaflet, resection of extra cordae tendinae, papillary muscle reorientation, papillary muscle resection, Alfieri stitch, and in a small proportion, mitral valve replacement.
Statistical Analysis
Continuous variables are expressed as mean±SD and/or median (with interquartile range) and compared using analysis of variance (normal distribution) or Mann–Whitney test (non‐normal distribution), as appropriate. Categorical data are expressed as percentage and compared using χ2. To assess the association of various predictors with longer‐term primary composite outcomes (death and appropriate ICD discharge), multivariable Cox proportional hazards analysis was utilized. Hazard ratio (HR) with 95% CI were calculated. Since longer‐term secondary composite events and non‐HCM death were competing risks, univariate and multivariable survival analysis was performed by competing risk regression analysis using the Fine‐Gray proportional subhazards model, and subdistribution HRs were calculated, along with 95% CI. 27 , 28 For univariate analysis, variables that are known to be associated with outcomes in patients with HCM were studied. Variables with a significant (P<0.05) association with events on univariate analysis were subsequently considered for the multivariable model. Additionally, Kaplan–Meier curves were generated to determine the cumulative proportion of patients with events as a function over time, and compared using log‐rank or Generalized Wilcoxon statistic, as appropriate. In addition, the survival of the 2 groups (Class I versus earlier indication) was also compared with the survival of an age‐sex‐matched US population (www.cdc.gov/nchs/products/life_tables). The discriminative ability of survival models for longer‐term composite primary events was compared using log‐likelihood ratios. Net reclassification improvement was utilized to determine whether additional risk factors provided significant reclassification of risk for primary composite events. Statistical analysis was performed using SPSS version 11.5 (SPSS Inc., Chicago, IL) and R 3.4.3 (R Foundation for Statistical Computing, Vienna, Austria). A P value of <0.05 was considered significant.
Results
The clinical and echocardiographic data, of the study sample as a whole, as well as separated on the basis of Class I indication versus earlier surgery, are shown in Tables 1 and 2. All patients had systolic anterior motion of the mitral valve and a maximal (rest or provokable) LVOT gradient ≥50 mm Hg, by study design.
Table 1.
Baseline Characteristics of Study Sample
| Variable |
Total N=2268 |
Earlier Surgery N=950 |
Class I Indication N=1318 |
|---|---|---|---|
| Age, y | 56±14 | 55±14 | 56±12 |
| Male sex | 1246 (55%) | 541 (57%) | 705 (54%) |
| Body‐surface area, m2 | 2.0±0.2 | 2.0±0.3 | 2.0±0.2 |
| Hypertension | 817 (36%) | 333 (35%) | 487 (37%) |
| Diabetes mellitus | 278 (12%) | 124 (13%) | 154 (12%) |
| Smoking history | 598 (26%) | 241 (26%) | 351 (27%) |
| Obstructive CAD | 222 (10%) | 85 (9%) | 134 (10%) |
| Family history of HCM | 408 (18%) | 162 (17%) | 246 (18%) |
| Family history of SCD | 227 (10%) | 95 (10%) | 132 (10%) |
| History SCD | 25 (1.1%) | 11 (1.2%) | 14 (1.1%) |
| History of NSVT | 223 (10%) | 93 (10%) | 130 (10%) |
| History of syncope | 295 (13%) |
76 (8%) None exertional |
219 (17%) All exertional |
| History of atrial fibrillation | 535 (24%) | 228 (24%) | 307 (23%) |
| History of stroke | 91 (4%) | 38 (4%) | 53 (4%) |
| History of prior alcohol septal ablation | 18 (0.8%) | 0 | 18 (1%) |
| Implantable defibrillator | 287 (13%) | 114 (12%) | 173 (13%) |
| Permanent pacemaker | 60 (3%) | 29 (3%) | 31 (2%) |
| β‐Blockers | 1769 (78%) | 684 (72%) | 1085 (82%) |
| Calcium channel blocker | 635 (28%) | 247 (26%) | 388 (29%) |
| Disopyramide | 91 (4%) | 38 (4%) | 53 (4%) |
| Angina | 431 (19%) | 114 (12%) | 317 (24%) |
| NYHA class | |||
| I | 209 (9%) | 209* (22%) | 0 |
| II | 1005 (44%) | 741 (78%) | 264 (20%) † |
| ≥III | 1054 (46%) | 0 | 1054 (80%) |
| ESC % 5‐y SCD risk score | 3.7±2 | 3.7±2 | 3.7±2 |
| ESC % 5‐y SCD risk categories | |||
| Low risk (<4%) | 1369 (60%) | 555 (58%) | 814 (62%) |
| Intermediate (4–6%) | 702 (31%) | 306 (32%) | 396 (30%) |
| High (>6%) | 197 (9%) | 89 (9%) | 108 (8%) |
CAD indicates coronary artery disease; ESC, European Society of Cardiology; HCM, hypertrophic cardiomyopathy; LVOT, left ventricular outflow tract; NSVT, nonsustained ventricular tachycardia; NYHA, New York Heart Association; and SCD, sudden cardiac death.
All patients deemed to be in NYHA class I at baseline had symptomatic impairment of exercise capacity on stress echocardiography along with severe LVOT obstruction.
All patients in this subgroup also had intractable angina or exertional syncope on maximally tolerated medical therapy.
Table 2.
Echocardiographic Parameters of Study Sample
| Variable |
Total N=2268 |
Earlier Surgery N=950 |
Class I Indication N=1318 |
|---|---|---|---|
| LV ejection fraction, % | 62±6 | 62±6 | 62±5 |
| Indexed LV end‐diastolic dimension, cm/m2 | 2.1±0.3 | 2.1±0.4 | 2.1±0.3 |
| Indexed LV end‐systolic dimension, cm/m2 | 1.2±0.3 | 1.2±0.3 | 1.2±0.4 |
| Maximal basal septal LV thickness, cm | 2.0±0.3 | 2.0±0.4 | 2.0±0.2 |
| Indexed left atrial dimensions, cm/m2 | 2.2±0.4 | 2.2±0.4 | 2.2±0.3 |
| Resting mitral regurgitation | |||
| Trivial‐mild | 1452 (64%) | 627 (66%) | 825 (63%) |
| ≥Moderate | 816 (36%) | 323 (34%) | 493 (37%) |
| Resting LVOT gradient, mm Hg | 61±44 | 61±43 | 61±39 |
| Maximal LVOT gradient, mm Hg* | 100±31 | 100±31 | 100±3 |
LVOT indicates left ventricular outflow tract.
Provocation used in 1214 (53%) patients in the whole group (950 [100%] in earlier surgery group and 264 [20%] in class I group whose presenting symptom was angina and not dyspnea).
The breakdown of surgical indications was as follows: (1) Class I indication (NYHA Class ≥III [n=1054] or intractable angina/exertional syncope with severe LVOT obstruction despite maximally tolerated medical therapy [n=264]), and (2) Earlier surgery (NYHA Class II with medical therapy intolerance [n=418] or impaired functional capacity [<85% age‐sex predicted metabolic equivalents] in the setting of severe dyspnea/dizziness/presyncope and severe concomitant LVOT obstruction at peak exercise echocardiography [n=532]). Further breakdown of various indications, separated by whether the operation was performed before or starting in 2010, is shown in Figure 1. Out of the 532 patients who underwent exercise stress testing, 149 (28%) patients sent for earlier surgery were seen before the year 2010, and 383 (72%) were evaluated starting in 2010. In addition, of the patients who underwent exercise stress testing, 209 (39%) claimed to have no symptoms (NYHA Class I), while 323 (61%) had minimal symptoms at rest (NYHA Class II) on initial self‐assessment of clinical history; and 148 patients (28%) had resting LVOT gradient between 30 and 60 mm Hg, while on optimal medical therapy. However, all developed a high LVOT gradient (>50 mm Hg), had severe symptoms at peak exercise, and had significantly impaired (<85%) age‐sex predicted exercise capacity (mean 72±4%). Of the patients who underwent exercise stress testing, there were 21 (4%) patients in the stress echocardiography subgroup who had an abnormal blood pressure response at peak exercise (defined as a decrease of >10 mm Hg or failure to increase >20 mm Hg at peak exercise).
Figure 1. Breakdown of various surgical indications, including the decade during which the operation was performed.
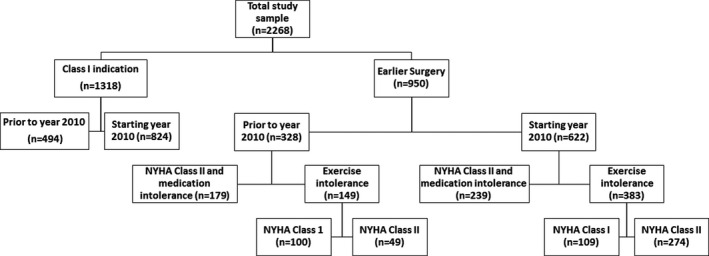
NYHA indicates New York Heart Association.
The types of cardiac surgeries were as follows: isolated myectomy (n=1248, 55%), myectomy plus mitral/subvalvular apparatus surgery (n=799, 35%), myectomy plus coronary artery bypass grafting (n=153, 7%), and myectomy plus coronary artery bypass plus mitral/subvalvular apparatus surgery (n=69, 3%). Additional surgical details are shown in Data S1. In the subgroup with concomitant mitral surgery, 109 (13%) underwent mitral valve replacement; the rest underwent various transaortic repairs as described in Data S1. During follow‐up, 45 (2%) patients needed a repeat surgical procedure to relieve LVOT obstruction (of which 85% required a mitral valve procedure). Additionally, 535 patients (24%) had invasive therapies (surgical MAZE and/or pulmonary vein isolation) for relief of AF, while 393 (17%) patients had excision/ligation of the left atrial appendage.
Four hundred sixty‐three (18%) patients had evidence of AF during long‐term follow‐up (excluding the immediate postoperative AF within 30 days of surgery) and were treated with medications (91% with amiodarone, 2% with dofetilide, and the rest using rate control). Nonsustained and sustained VT were noted in 219 (10%) and 19 (1%) patients, respectively, with no difference between groups. Also, during follow‐up, there were an additional 124 (5%) patients with pacemakers and 186 (8%) patients with implantable defibrillators, respectively, with no difference between subgroups.
During a mean follow‐up of 6.2±4 years (median 5.9 years with interquartile range of 2.7, 9.3 years), and 269 patients (12%) met the composite primary end point (248 deaths, including 150 documented sudden cardiac deaths and 24 appropriate ICD discharges). The composite secondary outcome occurred in 248 (11%) patients and included the following: 227 (10%) cardiac deaths and 24 appropriate ICD discharges (1%). In patients who developed multiple end points, time to first event was utilized as an event time cut‐off. Furthermore, out of a total of 473 (21%) patients with an ICD in the current study (287 at baseline+186 implanted during follow‐up), there were 0 documented SCD and 8 appropriate ICD discharges (an SCD event rate of 0.3%/y). There were 13 (0.6%) in‐hospital deaths and 13 (0.6%) strokes following surgery. The average length of stay in the hospital was 7±3 days and 145 (6%) patients stayed in the hospital for >14 days (88 for respiratory failure, 21 for pacemaker implantation, 13 for poststroke recovery, and 23 for multifactorial reasons including respiratory and renal failure). As shown in Figure 1, a majority of operations were performed in or after 2010 (n=1446) than before that (n=822), and the mean time of follow‐up for the group operated before 2010 was 11.9 years, while that for the group operated in or after 2010 was 5.2 years. During the mean of 11.9 years of follow‐up for the group operated before 2010, 207 patients had a primary event (2.1%/y), while for the group operated in or after 2010, there were 62 primary events (0.8%/y). Similarly, there were 174 SCD in the entire group during follow‐up (130 in the group operated before 2010 and 44 in or after 2010), and the long‐term SCD rate in the group operated before 2010 was 1.3%/y and 0.6%/y in the group operated in or after 2010.
For the entire study sample, the data on univariate survival analysis demonstrating data on association of various relevant predictors with composite primary and secondary events are shown in Tables S1 and S2. We subsequently performed multivariable Cox Proportional Hazard survival analysis for the primary composite outcome, for the entire study sample, as shown in Table 3. In addition, using the competing risk assumptions, we performed multivariable survival analysis for the secondary composite outcome, for the entire study sample, as shown in Table 4. We further demonstrate incremental prognostic utility (using log‐likelihood ratios) and improved risk reclassification (using categorical net reclassification improvement) of earlier surgery (versus Class I indication) in Table 5. The proportion of primary composite events was significantly higher in the subgroup that had a Class I indication for surgery versus those who underwent surgery earlier, as follows: (193 [15%] versus 76 [8%], log‐rank P<0.001). The Kaplan–Meier survival curves of the 2 groups, along with comparison to an age‐sex‐matched US population, are shown in Figure 2. Longer‐term survival of patients who underwent earlier surgical relief of LVOT obstruction was similar to a normal age‐sex‐matched US population; however, it was significantly worse for patients who met Class I indication.
Table 3.
Multivariable Survival Analysis for Primary and Secondary Composite Events
| Cox Proportional Hazard Survival Analysis for Primary Composite End Points | ||
|---|---|---|
| Variable | HR [95% CI] | P Value |
| Age (for every 10‐y increase) | 1.61 [1.26–1.91] | <0.001 |
| Female sex | 1.27 [0.94–1.58] | 0.102 |
| History of obstructive CAD | 1.46 [1.06–1.91] | 0.009 |
| Class I indication vs earlier indication for surgical relief of LVOT obstruction | 1.61 [1.14–2.12] | 0.005 |
| Competing Risk Assumption Survival Analysis for Secondary Composite End Points | ||
|---|---|---|
| Variable | sHR [95% CI] | P Value |
| Age (for every 10‐y increase) | 1.55 [1.36–1.74] | <0.001 |
| Female sex | 1.24 [0.96–1.64] | 0.091 |
| History of obstructive CAD | 1.47 [1.07–2.01] | 0.001 |
| Class I indication vs earlier indication for surgical relief of LVOT obstruction | 1.66 [1.21–2.31] | 0.001 |
Variables considered for multivariable analysis had P<0.05 on univariate analysis shown in Table S1. CAD indicates coronary artery disease; HR, hazard ratio; LVOT, left ventricular outflow tract; and sHR, subhazard ratio.
Table 4.
Multivariable Competing Risk Survival Analysis for Secondary Composite Events
| Competing Risk Assumption Survival Analysis for Secondary Composite End Points | ||
|---|---|---|
| Variable | sHR [95% CI] | P Value |
| Age (for every 10‐y increase) | 1.55 [1.36–1.74] | <0.001 |
| Female sex | 1.24 [0.96–1.64] | 0.091 |
| History of obstructive CAD | 1.47 [1.07–2.01] | 0.001 |
| Class I indication vs earlier indication for surgical relief of LVOT obstruction | 1.66 [1.21–2.31] | 0.001 |
Variables considered for multivariable analysis had P<0.05 on univariate analysis shown in Table S2. CAD indicates coronary artery disease; HR, hazard ratio; LVOT, left ventricular outflow tract; and sHR, subhazard ratio.
Table 5.
Incremental Prognostic Value of Various Predictors for Composite Primary and Secondary Events
| Variable | Log‐Likelihood Ratio | χ2 | P Value |
Categorical NRI [95% CI] |
P Value |
|---|---|---|---|---|---|
| For primary composite events (n=269) | |||||
| Increasing age | −1774.8 | ||||
| Increasing age+female sex | −1773.8 | 1.42 | 0.297 | 0.02 [−0.04 to 0.07] | 0.3 |
| Increasing age+female sex+obstructive CAD | −1769.3 | 10.21 | 0.001 | 0.09 [0.03 to 0.14] | 0.001 |
| Increasing age+female sex+obstructive CAD+Class I vs earlier surgical indication | −1761.6 | 28.32 | <0.001 | 0.13 [0.04 to 0.22] | 0.006 |
| For secondary composite events (n=248) | |||||
| Increasing age | 1775.3 | ||||
| Increasing age+female sex | −1774.6 | 1.38 | 0.238 | 0.02 [−0.05 to 0.08] | 0.3 |
| Increasing age+female sex+obstructive CAD | −1770.6 | 9.38 | 0.009 | 0.08 [0.03 to 0.12] | 0.001 |
| Increasing age+female sex+obstructive CAD+Class I vs earlier surgical indication | −1762.7 | 25.25 | <0.001 | 0.12 [0.03 to 0.21] | 0.008 |
CAD indicates coronary artery disease; and NRI, net reclassification improvement.
Figure 2. Kaplan–Meier survival curves demonstrating long‐term outcomes of the entire study sample (n=2268), separated on the basis of undergoing surgical relief of LVOT obstruction for Class I indication vs earlier surgery.
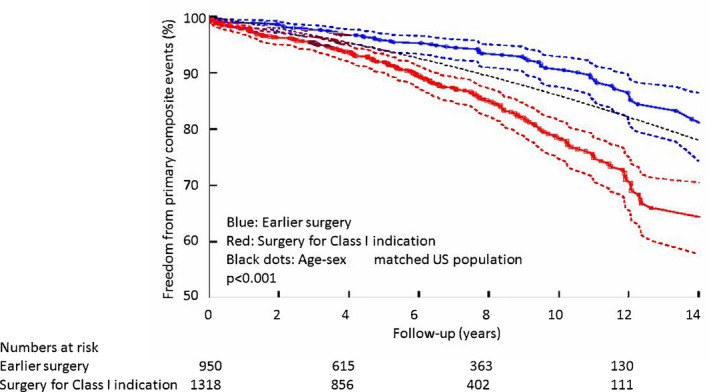
LVOT indicates left ventricular outflow tract.
The findings of Cox multivariable survival analysis for the primary composite outcome, in the subgroup excluding patients with documented obstructive coronary artery disease, were similar as follows (total n=2046, number of primary composite events=210): Age (for every 10‐year increase, HR, 1.60; 95% CI, 1.42, 1.90, P<0.001) and Class I indication versus earlier surgery (HR, 1.71; 95% CI, 1.18, 2.46, P<0.001) were associated with increased longer‐term primary composite events, while female sex was only borderline significant (HR, 1.26; 95% CI, 0.94, 1.73, P=0.089). In this subgroup, the proportion of primary composite events, separated on the basis of isolated myectomy versus myectomy+additional mitral procedures, was not significantly different as follows: (128/1248 [10%] versus 82/798 [10%], Generalized Wilcoxon statistic P=0.423). The Kaplan–Meier survival curves, separated on the basis of isolated myectomy versus additional mitral procedures, are shown in Figure 3. In the subgroup that underwent concomitant mitral procedure (in addition to myectomy), there were no significant differences in primary composite events between the subgroup that underwent mitral valve repair versus replacement (10% versus 10%, Generalized Wilcoxon statistic P=0.891).
Figure 3. Kaplan–Meier survival curves demonstrating long‐term outcomes in the subgroup excluding patients with obstructive coronary artery disease (n=2046), separated on basis of undergoing isolated myectomy vs myectomy+additional mitral valve procedure.
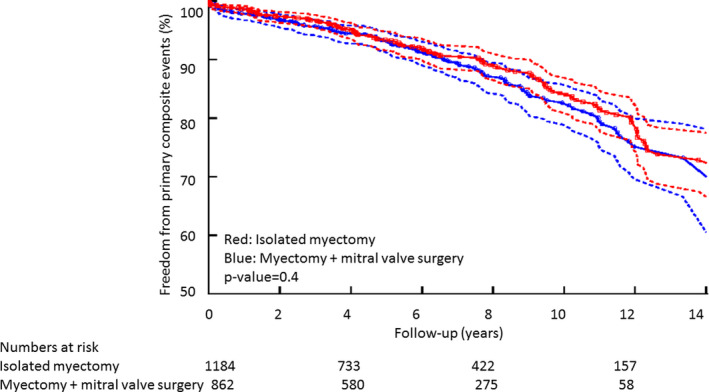
Discussion
In the current study of patients with HCM with severe resting or dynamic LVOT obstruction, we demonstrate that patients who underwent surgical relief of LVOT obstruction for a guideline‐recommended Class I indication (NYHA Class ≥III or intractable angina/exertional syncope with severe LVOT obstruction despite maximally tolerated medical therapy) had significantly higher longer‐term composite event rate versus those who underwent surgery earlier (NYHA Class II with medical therapy intolerance or impaired functional capacity in the setting of severe dyspnea/dizziness/presyncope and severe concomitant LVOT obstruction at peak exercise echocardiography). Furthermore, the longer‐term primary outcomes of the earlier surgical group were similar to a normal age‐sex‐matched US population, and much worse in the Class I indication group. This was despite no significant differences in age, sex, or baseline HCM‐related risk factors between the Class I indication versus earlier surgical groups. Furthermore, of the current cohort that underwent an additional concomitant mitral valve procedure (including the small proportion with concomitant mitral valve replacement) to relieve LVOT obstruction, there was no significant difference in longer‐term survival when compared with those who underwent an isolated myectomy. As would be expected, higher age and obstructive coronary artery disease at baseline were associated with increased longer‐term primary events, while female sex did not maintain independent significance on multivariable survival analysis (despite being significant on univariate analysis). The findings were similar even in the subgroup where obstructive coronary artery disease was excluded. In addition, the stroke risk in the overall study population was low.
It is well documented that, if evaluated diligently using various provocative maneuvers, an elevated LVOT gradient is observed in ≈70% of patients with HCM. 7 Multiple previous reports have suggested that an elevated LVOT gradient is associated with worse long‐term outcomes, including HCM‐related death, 4 , 5 , 6 with much better survival in patients with NYHA Class I versus those with Class II and III/IV (91% versus 83% and 82.6%, respectively, P<0.05). 6 However, patients commonly misjudge the intensity of their symptoms and stress echocardiography can help further clarify the true clinical picture by elucidating their functional capacity and helping to associate peak‐exercise symptoms with dynamic LVOT obstruction while also providing incremental prognostic value. 14 , 15
Currently, there are no large‐scale studies that have demonstrated that medical therapy is associated with a survival benefit in patients with symptomatic LVOT obstruction. Because surgical myectomy provides excellent long‐term survival and freedom from symptoms, based on prior retrospective observational studies (albeit without any randomized control), 8 , 9 , 10 , 11 , 12 current guidelines give it a Class I indication in patients with obstructive HCM. 3 , 4 However, based on a consensus opinion (level of evidence C) derived from smaller older studies, it is recommended only in those patients who present with advanced intractable symptoms (NYHA Class III–IV, angina and syncope). 3 Of the previous studies that reported outcomes of myectomy versus conservative therapy in patients with HCM, 8 , 11 , 16 only 1 study employed a strategy where the decision regarding surgery (versus conservative management) was based upon symptom assessment and/or objective association of symptomatic functional limitation with severe dynamic LVOT obstruction using stress echocardiography. 16 Two other reports had limitations, including (1) comparing outcomes from multiple centers with differing myectomy experiences 8 or (2) using only resting LVOT gradient in decision making. 11 Another study also reported similar findings on the positive impact of myectomy on minimizing downstream SCD events. However, that study only included patients with an ICD (including 56 patients with a myectomy). 29 Hence, the findings would be difficult to apply in a broader group of patients with myectomy who do not have an ICD. However, the effect of surgery on preventing SCD remains to be conclusively determined.
Indeed, similar findings of improved longer‐term survival with an earlier surgical referral have been demonstrated in multiple other valvular diseases, including severe chronic primary MR and aortic regurgitation. 17 , 18 , 19 , 20 While it makes intuitive sense that such findings could also be observed in patients with HCM (since they tend to be younger and relatively free of other comorbidities that could increase perioperative or even longer‐term mortality/morbidity), LVOT obstruction (and MR) in HCM is dynamic and the concept of earlier referral may not be extrapolated from these valvular disorders, which have a fixed regurgitant lesion.
Also, any suggestion of an earlier surgical intervention has to be balanced against operative risk and overall experience of the center at managing these complex patients. The current study also highlights the importance of experience in invasive management of patients who have HCM with severe LVOT obstruction. 12 , 13 At our center, we have relied on careful clinical judgment and stress echocardiography to help guide decisions regarding earlier surgery in HCM with excellent results, as demonstrated in Table 1 (≈40% such referrals before year 2010, with similar practice since then). However, even in our center, there is a subtle difference in outcomes, with the current study demonstrating a 0.6% in‐hospital mortality versus 0.38% in a previous report. 13 This is because the previous report only reported outcomes of surgeries performed by 1 surgeon and a shorter duration of patient inclusion (from 2005 onwards versus 2002 onwards for the current study). A recent analysis of the Nationwide Inpatient Sample demonstrated a 30‐day mortality of 6% in patients undergoing surgical myectomy for relief of LVOT obstruction. 30 These results strongly suggest the importance of having myectomy performed at experienced centers. 7 , 9 , 11 , 13 In fact, in our experience, a sizable proportion of symptomatic patients with HCM with severe LVOT obstruction, especially those with basal septal thickness <1.8 cm, also have mitral valve abnormalities contributing to LVOT obstruction. As a result, >50% of such patients (ie, with basal septum <1.8 cm) would require a concomitant surgical procedure (most likely anterior mitral leaflet repair and/or papillary muscle surgery) to relieve LVOT obstruction. However, we demonstrate no differences in longer‐term outcomes with addition of a concomitant mitral procedure (including mitral valve replacement versus repair). However, there are previous reports suggesting a higher national complication rate with concomitant mitral procedures. 31 These differences in outcomes further underscore the importance of an experienced team of cardiologists, imaging expert, and cardiac surgeons. 12 , 13 , 26 , 32 However, interpretation of these data, along with the findings of the current study, should not be extrapolated and compared with natural history studies in HCM, 1 , 2 , 3 , 4 given the inherent differences in populations. Also, the role of surgery in the context of patients with obstructive HCM to relieve symptoms/improve quality of life is likely to change with the advent of newer medical therapies. 33
There are many potential reasons for improvement in outcomes following surgery. An obvious reason is LVOT obstruction, ischemia, and symptomatic relief, with resultant increased exercise capacity, and improved longevity. Another potential reason (albeit to a lesser extent) could be that an extensive surgical myectomy removes a substantial amount of hypertrophied muscle. Previous reports have demonstrated that there is a significant association between LV hypertrophy and associated myocardial fibrosis. 22 Hence, septal debulking could indirectly reduce the burden of myocardial fibrosis, which could potentially be associated with reduced incidence of VT. 34 , 35 Indeed, in a recent study, we have demonstrated that patients who underwent myectomy had a lower rate of hard events versus those who were followed conservatively. 35
Limitations
This was an observational study from a single tertiary center, which could have potential selection bias. The results of all testing were available to all clinicians at the time of decision making, introducing further bias. The current study only tests associations, not causality. In order to truly understand the impact of earlier versus later surgery on outcomes, a large‐scale prospective study would have to be conducted in patients with HCM with severe LVOT obstruction. However, a prospective, randomized trial in HCM has many inherent challenges. 36 In addition, given the overall expertise involved with both the conservative and invasive management of HCM, our results might not be generalizable to other less experienced centers. 30 Also, even in our center, only with an increase in experience, there has been an evolution of practice over the decades and a gradual move towards an earlier operation in carefully selected patients. Female sex was not independently associated with outcomes in the current study, likely because of low event rates and modest longer‐term follow‐up. Ascertaining precise cause of death in an observational study can be difficult (especially classification of SCD versus other cardiac causes). Hence, we report the primary end point of all‐cause mortality and appropriate ICD discharge. However, the findings were similar for the secondary end point.
Conclusions
In a large group of patients with obstructed HCM, where decision regarding surgical relief of significant LVOT obstruction was based upon a combination of symptom assessment and objective evaluation of exercise capacity, earlier surgery performed before meeting Class I indication was associated with significantly improved long‐term freedom from composite end points of death and/or appropriate ICD discharge, similar to an age‐sex‐matched US population. A sizable proportion of patients needed a concomitant mitral valve procedure to relieve LVOT obstruction, with similar longer‐term outcomes to those who underwent isolated myectomy.
Sources of Funding
None.
Disclosures
Dr Desai is supported by the Haslam Family endowed chair in cardiovascular medicine. The remaining authors have no disclosures to report.
Supporting information
Data S1
Tables S1–S2
(J. Am. Heart Assoc. 2021;10:e016210. DOI: 10.1161/JAHA.120.016210.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016210
For Sources of Funding and Disclosures, see page 10.
See Editorial by Sherrid
References
- 1. Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA. 2002;287:1308–1320. [DOI] [PubMed] [Google Scholar]
- 2. Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004;363:1881–1891. [DOI] [PubMed] [Google Scholar]
- 3. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. [DOI] [PubMed] [Google Scholar]
- 4. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, et al. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 5. Maron MS, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F, Maron BJ. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. [DOI] [PubMed] [Google Scholar]
- 6. Elliott PM, Gimeno JR, Tome MT, Shah J, Ward D, Thaman R, Mogensen J, McKenna WJ. Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy. Eur Heart J. 2006;27:1933–1941. [DOI] [PubMed] [Google Scholar]
- 7. Maron MS, Olivotto I, Zenovich AG, Link MS, Pandian NG, Kuvin JT, Nistri S, Cecchi F, Udelson JE, Maron BJ. Hypertrophic cardiomyopathy is predominantly a disease of left ventricular outflow tract obstruction. Circulation. 2006;114:2232–2239. [DOI] [PubMed] [Google Scholar]
- 8. Ommen SR, Maron BJ, Olivotto I, Maron MS, Cecchi F, Betocchi S, Gersh BJ, Ackerman MJ, McCully RB, Dearani JA, et al. Long‐term effects of surgical septal myectomy on survival in patients with obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;46:470–476. [DOI] [PubMed] [Google Scholar]
- 9. Smedira NG, Lytle BW, Lever HM, Rajeswaran J, Krishnaswamy G, Kaple RK, Dolney DO, Blackstone EH. Current effectiveness and risks of isolated septal myectomy for hypertrophic obstructive cardiomyopathy. Ann Thorac Surg. 2008;85:127–133. [DOI] [PubMed] [Google Scholar]
- 10. Woo A, Williams WG, Choi R, Wigle ED, Rozenblyum E, Fedwick K, Siu S, Ralph‐Edwards A, Rakowski H. Clinical and echocardiographic determinants of long‐term survival after surgical myectomy in obstructive hypertrophic cardiomyopathy. Circulation. 2005;111:2033–2041. [DOI] [PubMed] [Google Scholar]
- 11. Ball W, Ivanov J, Rakowski H, Wigle ED, Linghorne M, Ralph‐Edwards A, Williams WG, Schwartz L, Guttman A, Woo A. Long‐term survival in patients with resting obstructive hypertrophic cardiomyopathy comparison of conservative versus invasive treatment. J Am Coll Cardiol. 2011;58:2313–2321. [DOI] [PubMed] [Google Scholar]
- 12. Desai MY, Bhonsale A, Smedira N, Naji P, Thamilarasan M, Lytle B, Lever H. Predictors of long term outcomes in symptomatic hypertrophic obstructive cardiomyopathy patients undergoing surgical relief of left ventricular outflow tract obstruction. Circulation. 2013;128:209–216. [DOI] [PubMed] [Google Scholar]
- 13. Hodges K, Rivas CG, Aguilera J, Borden R, Alashi A, Blackstone EH, Desai MY, Smedira NG. Surgical management of left ventricular outflow tract obstruction in a specialized hypertrophic obstructive cardiomyopathy center. J Thorac Cardiovasc Surg. 2019;157:2289–2299. [DOI] [PubMed] [Google Scholar]
- 14. Masri A, Pierson LM, Smedira NG, Agarwal S, Lytle BW, Naji P, Thamilarasan M, Lever HM, Cho LS, Desai MY. Predictors of long‐term outcomes in patients with hypertrophic cardiomyopathy undergoing cardiopulmonary stress testing and echocardiography. Am Heart J. 2015;169:684–692.e1. [DOI] [PubMed] [Google Scholar]
- 15. Desai MY, Bhonsale A, Patel P, Naji P, Smedira NG, Thamilarasan M, Lytle BW, Lever HM. Exercise echocardiography in asymptomatic HCM: exercise capacity, and not LV outflow tract gradient predicts long‐term outcomes. JACC Cardiovasc Imaging. 2014;7:26–36. [DOI] [PubMed] [Google Scholar]
- 16. Desai MY, Smedira NG, Bhonsale A, Thamilarasan M, Lytle BW, Lever HM. Symptom assessment and exercise impairment in surgical decision making in hypertrophic obstructive cardiomyopathy: relationship to outcomes. J Thorac Cardiovasc Surg. 2015;150:928–935.e1. [DOI] [PubMed] [Google Scholar]
- 17. Mentias A, Feng K, Alashi A, Rodriguez LL, Gillinov AM, Johnston DR, Sabik JF, Svensson LG, Grimm RA, Griffin BP, et al. Long‐term outcomes in patients with aortic regurgitation and preserved left ventricular ejection fraction. J Am Coll Cardiol. 2016;68:2144–2153. [DOI] [PubMed] [Google Scholar]
- 18. Suri RM, Vanoverschelde JL, Grigioni F, Schaff HV, Tribouilloy C, Avierinos JF, Barbieri A, Pasquet A, Huebner M, Rusinaru D, et al. Association between early surgical intervention vs watchful waiting and outcomes for mitral regurgitation due to flail mitral valve leaflets. JAMA. 2013;310:609–616. [DOI] [PubMed] [Google Scholar]
- 19. de Meester C, Gerber BL, Vancraeynest D, Pouleur AC, Noirhomme P, Pasquet A, de Kerchove L, El Khoury G, Vanoverschelde JL. Do guideline‐based indications result in an outcome penalty for patients with severe aortic regurgitation? JACC Cardiovasc Imaging. 2019;12:2126–2138. [DOI] [PubMed] [Google Scholar]
- 20. Yang LT, Michelena HI, Scott CG, Enriquez‐Sarano M, Pislaru SV, Schaff HV, Pellikka PA. Outcomes in chronic hemodynamically significant aortic regurgitation and limitations of current guidelines. J Am Coll Cardiol. 2019;73:1741–1752. [DOI] [PubMed] [Google Scholar]
- 21. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise JS, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 22. Kwon DH, Smedira NG, Rodriguez ER, Tan C, Setser R, Thamilarasan M, Lytle BW, Lever HM, Desai MY. Cardiac magnetic resonance detection of myocardial scarring in hypertrophic cardiomyopathy: correlation with histopathology and prevalence of ventricular tachycardia. J Am Coll Cardiol. 2009;54:242–249. [DOI] [PubMed] [Google Scholar]
- 23. Zoghbi WA, Enriquez‐Sarano M, Foster E, Grayburn PA, Kraft CD, Levine RA, Nihoyannopoulos P, Otto CM, Quinones MA, Rakowski H, et al. Recommendations for evaluation of the severity of native valvular regurgitation with two‐dimensional and Doppler echocardiography. J Am Soc Echocardiogr. 2003;16:777–802. [DOI] [PubMed] [Google Scholar]
- 24. Maron BJ, Olivotto I, Spirito P, Casey SA, Bellone P, Gohman TE, Graham KJ, Burton DA, Cecchi F. Epidemiology of hypertrophic cardiomyopathy‐related death: revisited in a large non‐referral‐based patient population. Circulation. 2000;102:858–864. [DOI] [PubMed] [Google Scholar]
- 25. Hicks KA, Tcheng JE, Bozkurt B, Chaitman BR, Cutlip DE, Farb A, Fonarow GC, Jacobs JP, Jaff MR, Lichtman JH, et al. 2014 ACC/AHA key data elements and definitions for cardiovascular endpoint events in clinical trials: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Data Standards (Writing Committee to Develop Cardiovascular Endpoints Data Standards). Circulation. 2015;132:302–361. [DOI] [PubMed] [Google Scholar]
- 26. Kwon DH, Smedira NG, Thamilarasan M, Lytle BW, Lever H, Desai MY. Characteristics and surgical outcomes of symptomatic patients with hypertrophic cardiomyopathy with abnormal papillary muscle morphology undergoing papillary muscle reorientation. J Thorac Cardiovasc Surg. 2010;140:317–324. [DOI] [PubMed] [Google Scholar]
- 27. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 28. Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. [DOI] [PubMed] [Google Scholar]
- 29. McLeod CJ, Ommen SR, Ackerman MJ, Weivoda PL, Shen WK, Dearani JA, Schaff HV, Tajik AJ, Gersh BJ. Surgical septal myectomy decreases the risk for appropriate implantable cardioverter defibrillator discharge in obstructive hypertrophic cardiomyopathy. Eur Heart J. 2007;28:2583–2588. [DOI] [PubMed] [Google Scholar]
- 30. Panaich SS, Badheka AO, Chothani A, Mehta K, Patel NJ, Deshmukh A, Singh V, Savani GT, Arora S, Patel N, et al. Results of ventricular septal myectomy and hypertrophic cardiomyopathy (from Nationwide Inpatient Sample [1998‐2010]). Am J Cardiol. 2014;114:1390–1395. [DOI] [PubMed] [Google Scholar]
- 31. Holst KA, Hanson KT, Ommen SR, Nishimura RA, Habermann EB, Schaff HV. Septal myectomy in hypertrophic cardiomyopathy: national outcomes of concomitant mitral surgery. Mayo Clin Proc. 2019;94:66–73. [DOI] [PubMed] [Google Scholar]
- 32. Patel P, Dhillon A, Popovic ZB, Smedira NG, Rizzo J, Thamilarasan M, Agler D, Lytle BW, Lever HM, Desai MY. Left ventricular outflow tract obstruction in hypertrophic cardiomyopathy patients without severe septal hypertrophy: implications of mitral valve and papillary muscle abnormalities assessed using cardiac magnetic resonance and echocardiography. Circ Cardiovasc Imaging. 2015;8:e003132. DOI: 10.1161/CIRCIMAGING.115.003132. [DOI] [PubMed] [Google Scholar]
- 33. Heitner SB, Jacoby D, Lester SJ, Owens A, Wang A, Zhang D, Lambing J, Lee J, Semigran M, Sehnert AJ. Mavacamten treatment for obstructive hypertrophic cardiomyopathy: a clinical trial. Ann Intern Med. 2019;170:741–748. [DOI] [PubMed] [Google Scholar]
- 34. Chan RH, Maron BJ, Olivotto I, Pencina MJ, Assenza GE, Haas T, Lesser JR, Gruner C, Crean AM, Rakowski H, et al. Prognostic value of quantitative contrast‐enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation. 2014;130:484–495. [DOI] [PubMed] [Google Scholar]
- 35. Mentias A, Raeisi‐Giglou P, Smedira NG, Feng K, Sato K, Wazni O, Kanj M, Flamm SD, Thamilarasan M, Popovic ZB, et al. Late gadolinium enhancement in patients with hypertrophic cardiomyopathy and preserved systolic function. J Am Coll Cardiol. 2018;72:857–870. [DOI] [PubMed] [Google Scholar]
- 36. Olivotto I, Ommen SR, Maron MS, Cecchi F, Maron BJ. Surgical myectomy versus alcohol septal ablation for obstructive hypertrophic cardiomyopathy. Will there ever be a randomized trial? J Am Coll Cardiol. 2007;50:831–834. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S2


