Abstract
Background
Collagen cross‐linking is covalent bonds among collagen fibers from catalysis of lysyl oxidase (LOX) and advanced glycation end products (AGEs). We aimed to evaluate the formation of enzymatic and nonenzymatic collagen cross‐linking and its clinical significance in patients with hypertrophic obstructive cardiomyopathy.
Methods and Results
Forty‐four patients with hypertrophic obstructive cardiomyopathy who underwent surgical myectomy were consecutively enrolled. Cardiovascular magnetic resonance parameters of left atrial/left ventricular function were measured, including peak filling rate (PFR) and early peak emptying rate (PER‐E). Total collagen was the sum of soluble and insoluble collagen, which were assessed by collagen assay. The myocardial LOX and AGEs expression were measured by molecular and biochemical methods. Compared with patients without atrial fibrillation, insoluble collagen (P=0.018), insoluble collagen fraction (P=0.017), and AGEs (P=0.039) were higher in patients with atrial fibrillation, whereas LOX expression was similar (P=0.494). The insoluble collagen fraction was correlated with PFR index (PFR normalized by left ventricular filling volume) (r=−0.44, P=0.005), left atrial diameters (r=0.36, P=0.021) and PER‐E index (PER‐E normalized by left ventricular filling volume) (r=−0.49, P=0.001).Myocardial LOX was positively correlated with total collagen (r=0.37, P=0.025) and insoluble collagen fraction (r=0.53, P < 0.001), but inversely correlated with PFR index (r=−0.43, P=0.006) and PER‐E index (r=−0.35, P=0.027). In multiple regression analysis, myocardial LOX was independently associated with PFR, while insoluble collagen fraction showed independent correlation with PER‐E after adjustment for clinical confounders.
Conclusions
Collagen cross‐linking plays an important role on heart remodeling in hypertrophic obstructive cardiomyopathy. Myocardial LOX expression is independently correlated with left ventricular stiffness, while accumulation of AGEs cross‐links might be associated with the occurrence of atrial fibrillation in patients with hypertrophic obstructive cardiomyopathy.
Keywords: atrial fibrillation, collagen cross‐linking, deformation rate, hypertrophic cardiomyopathy
Subject Categories: Biomarkers, Fibrosis, Myocardial Biology, Cardiomyopathy
Nonstandard Abbreviations and Acronyms
- AGEs
advanced glycation end products
- CCL
collagen cross‐linking
- HCM
hypertrophic cardiomyopathy
- HOCM
hypertrophic obstructive cardiomyopathy
- LATEF
total left atrial ejection fraction
- LAV
left atrial volume
- LOX
lysyl oxidase
- PER‐E
early peak emptying rate
- PFR
peak filling rate
Clinical Perspective
What Is New?
In patients with hypertrophic obstructive cardiomyopathy, collagen cross‐linking (CCL) was correlated with left atrial/left ventricular remodeling and highly expressed in patients with atrial fibrillation.
Lysyl oxidase, which enzymatically regulates CCL, was also associated with left atrial/left ventricular remodeling but did not differ between patients with and without atrial fibrillation.
Advanced glycation end products, as a nonenzymatic pathway to form CCL, were higher in patients with atrial fibrillation, although without any correlation with cardiac remodeling.
What Are the Clinical Implications?
Whereas most studies were focused on the amount of myocardial fibrosis in patients with hypertrophic cardiomyopathy, our results proposed that lysyl oxidase and CCL were also associated with cardiac remodeling in hypertrophic obstructive cardiomyopathy.
A deeper knowledge of the underlying mechanisms involved in lysyl oxidase and CCL dysregulation are required to generate both diagnostic and therapeutic strategies targeting the enzyme to identify and interfere with, respectively, its detrimental actions in patients with hypertrophic cardiomyopathy.
Hypertrophic cardiomyopathy (HCM) is a genetic disorder with a high risk for arrhythmias. Myocardial fibrosis, a process of collagen deposition, is an early and important manifestation of HCM and is associated with adverse cardiac remodeling and clinical outcomes. 1 It is usually assumed that increased collagen content is associated with worse chamber compliance and diastolic function. 2 However, the structure of the collagen network also has important effects on its mechanical properties. For example, the relative proportions of collagen type I and type III are believed to be associated with tissue stiffness because collagen I fibers provide rigidity, while type III collagen contributes to elasticity. 3
Recent studies show that the degree of collagen cross‐linking (CCL) but not collagen amount has a great influence on left ventricular (LV) stiffness in patients with heart failure. 4 , 5 CCL is the formation of intramolecular and intermolecular covalent bonds between lysine residues in collagen molecules, which greatly increases the tensile strength and stiffness of collagen fibers and makes them more resistant to degradation. 6 There are 2 major groups of cross‐links: those initiated by the enzyme lysyl oxidases (LOXs) and those derived from nonenzymatically glycated lysine and hydroxylysine residues. 7 LOX, a copper‐dependent enzyme, acts on specific lysine or hydroxylysine residues, catalyzing the formation of allysine aldehydes. Nonenzymatic cross‐links are the formation of nonenzymatic sugar‐amino adducts through a series of reactions between reducing sugar and collagen amino, which are called advanced glycation end products (AGEs). 8 It is thought that enzymatic and nonenzymatic CCLs share the same binding sites but have functional differences. 9 There are several lines of evidence indicating that LOX‐meditated CCL facilitates the increase in LV stiffness and diastolic dysfunction. 5 , 10 However, the role of CCL on cardiac function in patients with hypertrophic obstructive cardiomyopathy (HOCM) remains unknown. This study was designed to explore the formation of enzymatic and nonenzymatic CCL and its clinical significance in patients with HOCM.
METHODS
The study protocol was approved by the institutional review boards of Fuwai Hospital, and the study was conducted in accordance with the protocol. Written informed consent was issued by all participants or their parents or legal guardians. The data that support the findings of this study are available from the corresponding author upon reasonable request.
We consecutively recruited 44 patients with HOCM who underwent surgical myectomy at the Fuwai Hospital from July 2019 to September 2019. In patients with a confirmed diagnosis of HCM, the diagnosis was based on the generally used diagnostic criterion, that is, unexplained LV hypertrophy with maximal wall thickness ≥15 mm in the absence of other cardiac or systemic cause. LV outflow tract obstruction was defined as an instantaneous peak Doppler LV outflow tract (LVOT) gradient ≥30 mm Hg at rest or an exercised LVOT gradient ≥50 mm Hg. 11
The diagnosis of atrial fibrillation (AF) was based on 12‐lead electrocardiography or 24‐hour dynamic ECG recordings or an established history of paroxysmal or chronic AF. AF was defined as paroxysmal when it was either self‐terminating or successfully cardioverted to sinus rhythm; AF was considered chronic when it became established.
Patients with cardiac valve diseases, history of acute myocardial infarction, a significant stenosis (>50%) in ≥1 coronary arteries in the angiography and history of alcohol septal ablation were excluded after complete medical examination.
Control myocardial samples were obtained from autopsies of 9 accident victims (mean age, 45.4±14.3 years; 6 men/3 women), without a medical history of cardiovascular diseases and signs of macroscopic or microscopic cardiac lesions, to assess control values of the histomorphologic parameters. Blood samples were from an additional group of 29 age‐ and sex‐matched healthy subjects (mean age, 48.9±15.5 years; 14 men/15 women) for biochemical studies.
Echocardiography
Echocardiography was preoperatively performed in all patients with the use of a commercially available instrument. LV ejection fraction and maximal chamber dimensions including the left atrial (LA) diameter and end‐diastolic LV wall thickness were measured according to the standard protocol. The LVOT pressure gradient was estimated through resting LVOT peak velocity by using a simplified Bernoulli equation.
Cardiovascular Magnetic Resonance Imaging
Cardiovascular magnetic resonance was performed on 40 patients with a 1. 5‐T magnetic resonance scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany). All imaging acquisitions were captured under breath control. Cardiovascular magnetic resonance images were analyzed using standard ventricular analysis software (Medis Medical Imaging Systems BV, Leiden, The Netherlands). For all patients, wall thickness at the septal, posterior, and LV end‐diastolic dimensions were all determined in the short‐axis view (at the midpapillary level). Epicardial and endocardial borders of the LV myocardium were manually traced during the whole cardiac phase on each cine short‐axis image to obtain LV end‐diastolic and end‐systolic volumes, ejection fractions, and myocardial mass. Myocardial mass was calculated by multiplying the volume of the myocardium calculated at end diastole by the specific gravity of the myocardium (1. 05 g/mL). The end‐diastolic volume index, end‐systolic volume index, and mass index were indexed to body surface area.
Image postprocessing was performed using the Tracking Tool software (QStrain 2.0, Medis Medical Imaging Systems BV). The LV endocardial and epicardial contours were manually traced in the 2‐chamber, 3‐chamber, 4‐chamber, and short‐axis view of the end‐diastolic and end‐systolic phase. LA endocardial contour was manually traced at the phase of the maximal LA volume before mitral valve opening and at the phase of the minimum LA volume after atrial contraction on 2‐chamber and 4‐chamber view. The atrial and ventricular volume/time and dV/dt curves were obtained plotting the cavity volumes over time. From the ventricular dV/dt curves, the first positive peak was defined as the peak filling rate (PFR). PFR represents the maximum speed of LV filling. The acceleration time was defined as time from end systole to peak. The deceleration time was defined as time from peak to diastole. From atrial curves, we measured left atrial volume (LAV) at (1) the beginning of LV diastole (defined as the frame immediately before opening of the mitral leaflets [LAVmax]), (2) the end of passive LV filling (defined as the frame immediately prior to LA contraction [LAVbac]), and (3) the end of LA contraction (LAVmin). Passive left atrial ejection fraction was calculated as (LAVmax – LAVbac)/LAVmax×100; active left atrial ejection fraction as (LAVbac – LAVmin)/LAVbac×100; and total left atrial ejection fraction (LATEF) as (LAVmax – LAVmin)/LAVmax×100. Peaks of the atrial dV/dt curves were defined as follows: The first negative peak was defined as the early peak emptying rate (PER‐E), and the second peak was defined as atrial peak emptying rate A, representing maximal emptying during the conduit phase and the booster phase, respectively. To be more comparable, LAVmax and LAVmin were indexed to body surface area (BSA). PER‐E and atrial peak emptying rate A were also normalized by the LV filling volume (difference between LV end‐diastolic and end‐systolic volumes), obtaining PER‐E index, and atrial peak emptying rate A index.
Histomorphologic Studies
The surgical specimens were formalin fixed and paraffin embedded. Paraffin‐embedded sections (4 mm) of heart samples were stained with Masson's trichrome staining. Four images of every section were acquired with a projection microscope (×200). Subsequent image analysis was performed using Image‐Pro Plus 6.0 image analysis software (Media Cybernetics Inc, Buckinghamshire, UK). The myocardial fibrosis was determined by quantitative morphometry in sections stained with collagen‐specific blue. The collagen volume fraction was calculated as the ratio of collagen‐specific staining to the total area of the myocardium in each specimen. Immunohistochemical analysis of LOX (Abcam; ab174316, 1:500) was performed on formalin‐fixed and paraffin‐embedded sections. Positive staining was visualized with DAB (Solarbio, China), and tissues were counterstained with Harris hematoxylin (Solarbio, China) (Figure 1).
Figure 1. Representative histologic staining from septal biopsies in patients with HOCM.
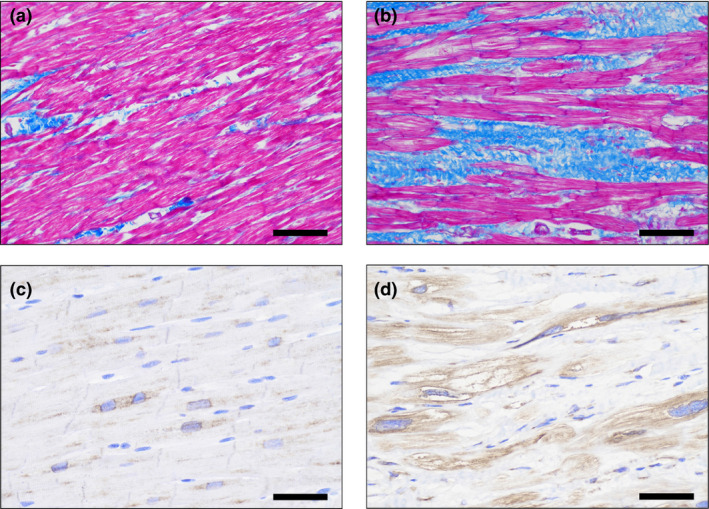
The left panel corresponded to representative pictures of Masson's trichrome staining (A) and immumohistochemical staining (C) for LOX in a control subject, respectively. The right panel corresponded to representative pictures of Masson's trichrome staining (B) and immumohistochemical staining (D) for LOX in a HOCM patient, respectively. Myocardial fibrosis was stained in blue (A, B), and the LOX expression was stained in brown (C, D). Magnification ×200. HOCM indicates obstructive hypertrophic cardiomyopathy; and LOX, lysyl oxidase.
Biochemical Studies
Circulating LOX was measured by ELISA using a commercial human LOX ELISA kit (USCN, SEC580Hu; Life Science, Wuhan, China). Western blot studies were analyzed by SDS‐PAGE and immunoblotted. Specific antibodies against LOX (Abcam; ab174316, 1:500), collagen type I (Abcam; ab88147, 1:1000), collagen type III (Abcam; ab6310, 1:1000) were used. The optical density of each band on the western blot was quantified using Image‐Pro Plus 6.0 and expressed as arbitrary densitometric units relative fold to β‐actin (Figure 2).
Figure 2. Western blot of LOX, collagen type I, and collagen type III in patients with HOCM.
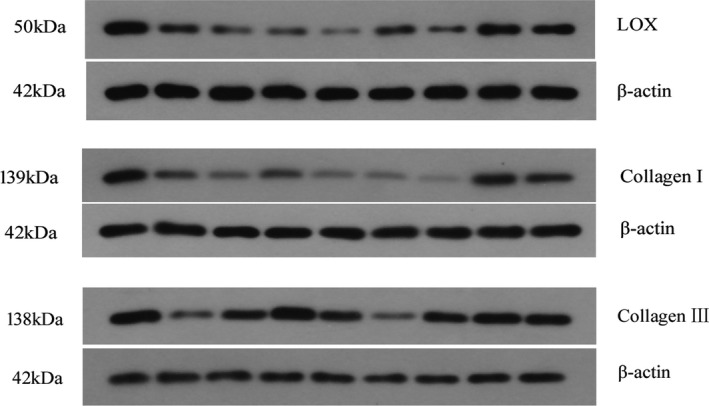
HOCM indicates obstructive hypertrophic cardiomyopathy; and LOX, lysyl oxidase.
Heart samples were homogenized, and the supernatants were collected after centrifugation. A commercial ELISA kit (Cell Biolabs Inc, San Diego, CA) was used according to the manufacturer's instructions to detect total AGEs, including Nε‐carboxymethyllysine, pentosidine, and other AGE structures. Myocardial AGE levels are standardized per 1 mg of heart tissue protein.
Collagen Assay
The soluble and insoluble collagen contents were determined by the Sircol collagen assay kit (Biocolor, Carrickfergus, UK). Briefly, heart samples were dissolved in pepsin (Sigma, St. Louis, MO) at a concentration of 1 mg/mL of 0.5 M acetic acid (Sigma) at 4°C for 72 hours, and 100 µL of supernatant was assayed for soluble collagen according to the manufacturer's instructions. The residue, after the soluble collagen extraction, was then incubated in fragmentation reagent and heated at 65°C for 2 hours, and 100 µL of supernatant was assayed for insoluble collagen according to the manufacturer's instructions. The total collagen was calculated as the sum of soluble and insoluble collagen. The fractions of soluble and insoluble collagen were also expressed as the percentage of the total collagen. The degree of CCL was determined as the ratio between the insoluble collagen and soluble collagen.
Statistical Analysis
Continuous variables are shown as mean±SD. Categorical variables are presented as frequencies (percentages). Comparisons of the groups for continuous variables were performed with the unpaired t test or Mann–Whitney U test, whereas the chi‐squared test or Fisher's exact test was used for categorical variables. Pearson's correlation test or Spearman's correlation test was used to examine correlations between 2 continuous variables when indicated. Multivariate analysis was performed with stepwise linear regression analysis to evaluate whether the variables were independently associated with PFR and PER‐E, provided they had a P value of <0.1 in univariate analysis. However, age and sex were forced to remain in the multivariate models regardless of their significance in univariate analysis. All P values were 2‐sided. Statistical analysis was performed with the SPSS software package (version 20, IBM Corp, Armonk, NY).
RESULTS
The baseline characteristics of the 44 patients with HOCM are listed in Table 1. The mean age was 48.8±14 years, and 22 (50%) were men. Six (13.6%) patients were observed to have AF (4 paroxysmal AF and 2 chronic AF).
Table 1.
Clinical Characteristics of Study Population
| Patients With HOCM | ||||
|---|---|---|---|---|
| All Patients (n=44) | Patients With AF (n=6) | Patients Without AF (n=38) | P Value | |
| Age, y | 48.8±14 | 59.2±8.4 | 47.2±14 | 0.05 |
| Male, n (%) | 22 | 4 (66.7) | 18 (47.4) | 0.66 |
| BMI, kg/m2 | 24.2±3.3 | 24.5±1.3 | 24.1±3.5 | 0.787 |
| Heart rate, beats/min | 73.3±11 | 67.5±10.6 | 74.2±10.9 | 0.165 |
| SBP, mm Hg | 124.9±13.5 | 124.3±12.3 | 125±13.9 | 0.912 |
| DBP, mm Hg | 71.1±7.8 | 69±9.5 | 71.4±7.6 | 0.485 |
| Dyspnea, n (%) | 40 | 6 (100) | 34 (89.5) | 1.000 |
| NYHA III/IV, n (%) | 14 | 2 (33.3) | 12 (31.6) | 1.000 |
| NSVT, n (%) | 7 | 2 (33.3) | 5 (13.2) | 0.238 |
| History of syncope | 14 | 1 (16.7) | 13 (34.2) | 0.700 |
| History of hypertension, n (%) | 13 | 0 (0) | 13 (34.2) | 0.22 |
| History of diabetes mellitus, n (%) | 5 | 0 (0) | 5 (13.2) | 1.000 |
| Family history of HCM, n (%) | 3 | 1 (16.7) | 2 (5.3) | 0.363 |
| Family history of SCD, n (%) | 1 | 0 (0) | 1 (2.6) | 1.000 |
| Verapamil or diltiazem, n (%) | 4 | 0 (0) | 4 (10.5) | 1.000 |
| ACEI/ARB, n (%) | 1 | 0 (0) | 1 (2.6) | 1.000 |
| Diuretics, n (%) | 5 | 1 (16.7) | 4 (10.5) | 0.538 |
| Beta blocker, n (%) | 42 | 6 (100) | 36 (94.7) | 1.000 |
ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; AF, atrial fibrillation; BMI, body mass index; DBP, diastolic blood pressure; HCM, hypertrophic cardiomyopathy; HOCM, hypertrophic obstructive cardiomyopathy; NYHA, New York Heart Association; NSVT, nonsustained ventricular tachycardia; SBP, systolic blood pressure; and SCD, sudden cardiac death.
Histomorphologic and Molecular Parameters
The collagen volume fraction was higher in HOCM patients than in controls (8.7±3.5% versus 3.7±1.2%; P<0.001). Patients with AF showed a greater extent of myocardial fibrosis than those without AF (12±2.7% versus 8.1±3.4%, P=0.015). We did not find any difference in the expression of collagen type I or type III between patients with and without AF.
Compared with patients without AF, the insoluble collagen and insoluble collagen fraction were higher in patients with AF (33.1±17.9 µg/mg versus 20.1±8.1 µg/mg; P=0.018; 75.3±7.4% versus 64.7±9.9%; P=0.017; Figure 3B). The degree of CCL was also increased in patients with AF (3.39±1.46 versus 2.09±1.05; P=0.008). Soluble collagen did not differ between patients with AF and those without AF (9.68±2.0 µg/mg1 versus 10.28±2.71 µg/mg; P=0.681). The total collagen was increased in patients with AF (42.8±19.1 µg/mg versus 30.4±8.8 µg/mg; P=0.087; Figure 3A) but without statistical significance.
Figure 3. Comparisons between patients with HOCM with AF and those without AF for molecular and cardiovascular magnetic resonance indices.
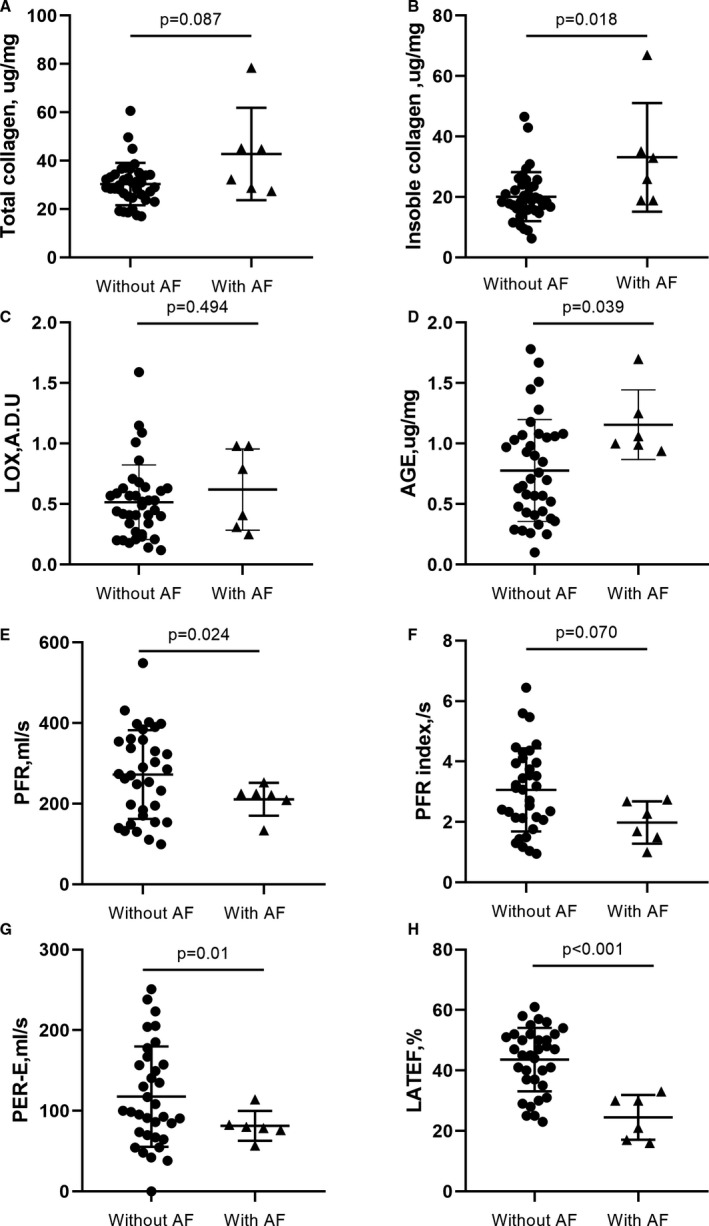
Compared with patients without AF, patients with AF showed higher myocardial total collagen (A), insoluble collagen (B), and AGE expression (D), but insignificant LOX expression (C). In addition, patients with AF also exhibited lower PFR (E), PFR index (F), PER‐E (G), and LATEF (H). ADU indicates arbitrary densitometric units; AF, atrial fibrillation; AGE, advanced glycation end‐product; HOCM, hypertrophic obstructive cardiomyopathy; LATEF, total left atrial ejection fraction; LOX, lysyl oxidase; PER‐E, early peak emptying rate; and PFR, peak filling rate.
Association analysis showed that collagen volume fraction was positively correlated with age (r=0.36, P=0.015). However, collagen volume fraction did not correlate with collagen molecular indices. No further associations were found between collagen type I or type III and insoluble collagen or CCL.
Enzymatic and Nonenzymatic CCL
Whereas the controls showed mild immunostaining for LOX, patients with HOCM exhibited moderate to severe immunostaining (Figure 1). Compared with patients with AF, the expression of myocardial LOX did not differ in those without AF (0.62±0.34 arbitrary densitometric units versus 0.52±0.31 arbitrary densitometric units; P=0.494; Figure 3C). However, serum LOX was significantly higher in patients than in controls (1.35±0.7 ng/mL versus 0.9±0.28 ng/mL; P=0.021). Patients with AF showed greater serum LOX expression than those without AF, but without statistical significance (1.9±0.7 ng/mL versus 1.27±0.67 ng/mL; P=0.075). The mean myocardial AGEs were 0.83±0.42 µg/mg in patients with HOCM. Patients with AF showed higher levels of AGEs than patients without AF (1.16±0.29 µg/mg versus 0.78±0.42 µg/mg; P=0.039; Figure 3D).
Myocardial LOX was correlated with insoluble collagen (r=0.47, P=0.001), insoluble collagen fraction (r=0.53, P < 0.001; Figure 4A), collagen type I (r=0.36, P=0.016), collagen type I/III ratio (r=0.34, P=0.026) and total collagen (r=0.34, P=0.023; Figure 4B). Serum LOX was correlated with collagen type I (r=0.32, P=0.032). It also showed a positive trend to insoluble collagen fraction (r=0.26, P=0.091). But no further correlation between myocardial and serum LOX was found (r=−0.09, P=0.579). There was also no correlation between myocardial AGEs and collagen subtypes.
Figure 4. Associations of cardiovascular magnetic resonance parameters with collagen metabolism.
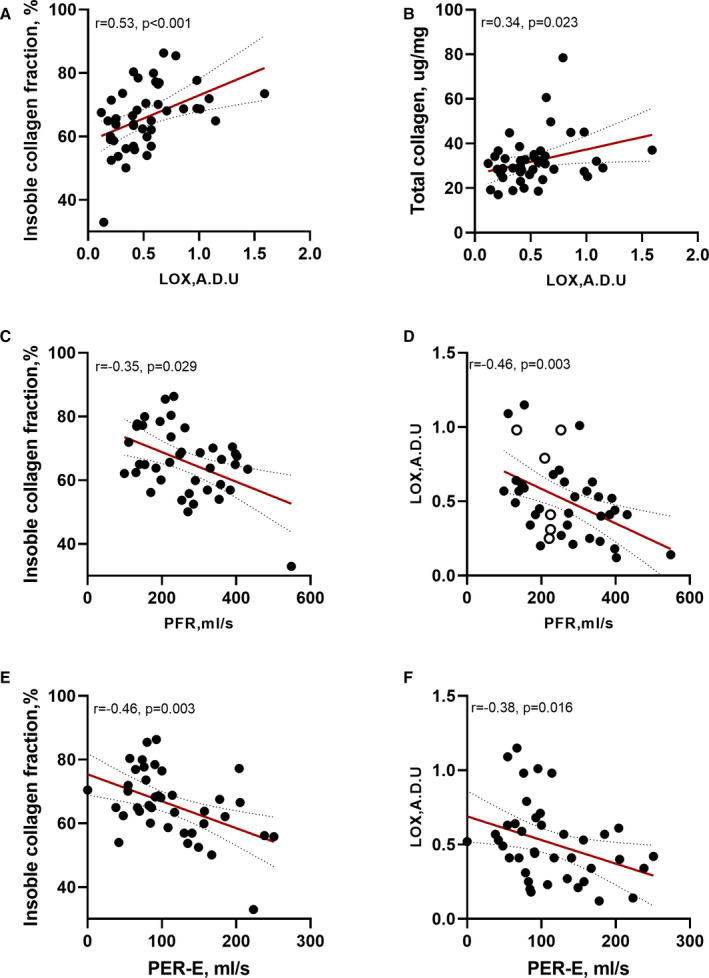
(A, B) Correlations between myocardial LOX expression and insoluble collagen fraction and total collagen. (C, D) Correlations between PFR and insoluble collagen fraction and myocardial LOX expression. (E, F) Correlations between PER‐E and insoluble collagen fraction and myocardial LOX expression. A.D.U., arbitrary densitometric units; HOCM, hypertrophic obstructive cardiomyopathy; LOX, lysyl oxidase; PER‐E, early peak emptying rate; and PFR, peak filling rate.
LV Remodeling Parameters
As shown in Table 2, the LV volume parameters, including LV end‐diastolic diameter, septal wall thickness, end‐diastolic volume index, end‐systolic volume index, and LV mass index, did not differ between patients with HOCM with and without AF. In contrast, PFR was significantly lower in patients with AF than in those without AF (211±40.6 mL/s versus 272±110 mL/s; P=0.024; Figure 3E). The PFR index was also lower in patients with AF but without statistical significance (1.98±0.7/s versus 3.06±1.37; P=0.070; Figure 3F).
Table 2.
Cardiovascular Magnetic Resonance Imaging Assessment of LA/LV Parameters in Patients With HOCM With and Without AF
| All Patients | Patients With HOCM | |||
|---|---|---|---|---|
| (n=40) | With AF | Without AF | P Value | |
| (n=6) | (n=34) | |||
| Indices of LV remodeling | ||||
| Septal wall thickness, mm | 24.7±4.2 | 25.8±1.3 | 24.7±4.5 | 0.252 |
| LV end‐diastolic diameter, mm | 45.3±3.4 | 43.7±4.7 | 45±4 | 0.478 |
| LVMI, g/m2 | 97±30.1 | 85.3±25.2 | 99.2±30.8 | 0.304 |
| LVEF, % | 64±6.6 | 63.2±10.8 | 64.5±5.8 | 0.778 |
| LVEDVi, mL/m2 | 86.9±23.3 | 90.8±18.4 | 85.7±23.7 | 0.240 |
| LVESVi, mL/m2 | 29.1±10.1 | 25.4±7.4 | 29.4±10.3 | 0.344 |
| PFR, mL/s | 264±105 | 211±40.6 | 272±110 | 0.024 |
| PFR index, s | 2.9±1.4 | 1.98±0.7 | 3.06±1.37 | 0.070 |
| AT, s | 143.6±45 | 167.3±52.2 | 140±41.9 | 0.158 |
| DT, s | 172.1±65.2 | 166.2±51.5 | 177.2±69.5 | 0.713 |
| Indices of LA remodeling | ||||
| LA diameter, mm | 41.5±8.8 | 48.8±6.4 | 40.2±8.8 | 0.028 |
| LAVmax index, mL/m2 | 65.5±20.8 | 86.6±23.9 | 61.8±18.1 | 0.005 |
| LAVmin index, mL/m2 | 40.3±19.6 | 66.5±23.7 | 35.6±14.9 | 0.004 |
| LATEF, % | 40.8±12.2 | 24.3±7.6 | 43.7±10.5 | <0.001 |
| LAPEF, % | 21.6±16.1 | 13.3±3.6 | 23.1±17 | 0.173 |
| LAAEF, % | 26.4±12 | 12.4±10.6 | 29±10.5 | 0.001 |
| PER‐E, mL | 112.1±59.1 | 81.4±18.5 | 117.5±62.3 | 0.01 |
| PER‐E index, /s | 1.3±0.6 | 1.2±0.4 | 1.3±0.6 | 0.587 |
| PER‐A, mL | 147±77.4 | 98.6±74.6 | 156.5±75.6 | 0.91 |
| PER‐A index, /s | 1.7±0.9 | 1.3±0.9 | 1.8±0.9 | 0.384 |
AT indicates acceleration time; DT, deceleration time; HOCM, hypertrophic obstructive cardiomyopathy; LA, left atrial; LAAEF, active left atrial ejection fraction; LAPEF, passive left atrial ejection fraction; LATEF, total left atrial ejection fraction; LAV, left atrial volume; LV, left ventricular; LVEDVI, left ventricular end‐diastolic volume index; LVEF, left ventricular ejection fraction; LVESVI, left ventricular end‐systolic volume index; LVMI, left ventricle mass index; PER‐A, atrial peak emptying rate A; PER‐A index, atrial peak emptying rate A normalized by left ventricular filling volume; PER‐E, early peak emptying rate; PER‐E index, early peak emptying rate normalized by left ventricular filling volume; PFR, peak filling rate; and PFR index, peak filling rate normalized by left ventricular filling volume.
Myocardial LOX was inversely correlated with PFR and PFR index (r=−0.46, P=0.003; and r=−0.43, P=0.006, respectively; Figure 4D) but positively correlated with acceleration time and LV mass index (r=0.38, P=0.016; and r=0.36; P=0.026, respectively). The soluble collagen was correlated with LV end‐diastolic diameters (r=−0.37, P=0.018). The insoluble collagen was associated with PFR index (r=−0.32, P=0.044), and the insoluble collagen fraction was correlated with PFR, PFR index, and acceleration time (r=−0.35, P=0.029; r=−0.44, P=0.005; and r=0.36, P=0.022, respectively; Figure 4C).
LA Remodeling Parameters
Compared with patients with HOCM without AF, patients with AF exhibited larger LA diameters (48.8±6.4 mm versus 40.2±8.8 mm; P=0.028), LAVmax index (86.6±23.9 mL/m2 versus 61.8±18.1 mL/m2; P=0.005) and LAVmax index (66.5±23.7 mL/m2 versus 35.6±14.9 mL/m2; P=0.004). Patients with AF also showed lower LATEF (24.3±7.6% versus 43.7±10.5%; P<0.001; Figure 3H), active left atrial ejection fraction (12.4±10.6% versus 29±10.5%; P=0.001) and PER‐E (81.4±18.5 mL versus 117.5±62.3 mL; P=0.01; Figure 3G).
In correlation analysis, collagen type III and the ratio of collagen types I and III were significantly correlated with LA diameters (r=−0.48, P=0.001; and r=0.40, P=0.010, respectively). The ratio of collagen type I and III was also correlated with LAVmax index (r=0.33, P=0.035). The total collagen was associated with PER‐E (r=−0.34, P=0.030) and PER‐E index (r=−0.39, P=0.013). The insoluble collagen and insoluble collagen fraction were also correlated with LA diameters (r=0.31, P=0.050; and r=0.36, P=0.021), PER‐E (r=−0.44, P=0.004; and r=−0.46, P=0.003; Figure 4E) and PER‐E index (r=−0.50, P=0.001; and r=−0.49, P=0.001). In addition, the insoluble collagen fraction was associated with LATEF (r=−0.40, P=0.010), LAVmax index (r=0.34, P=0.034) and LAVmin index (r=0.40, P=0.010). Furthermore, myocardial LOX expression was correlated with PER‐E (r=−0.38, P=0.016; Figure 4F) and PER‐E index (r=−0.35, P=0.027). However, the myocardial AGE levels were not correlated with LA/LV remodeling parameters.
Then multiple linear regression analysis was performed to identify whether independent determinants of LA/LV deformation rate on univariate analysis were still significant after controlling for potential confounding factors (Table 3). Only myocardial LOX expression was independently associated with PFR after adjustment for age, sex, septal wall thickness, and insoluble collagen fraction. However, insoluble collagen fraction showed independent correlation with PER‐E after adjustment for age, sex, LA diameters, LATEF, and myocardial LOX expression.
Table 3.
Univariate and Multivariate Regression for LA/LV Deformation Rate in Patients With HOCM
| Univariate | Multivariate | |||
|---|---|---|---|---|
| P Value | β (95% CI) | P Value | β (95% CI) | |
| PFR | ||||
| Age | 0.457 | 0.9 (−1.5 to 3.2) | 0.885 | −0.2 (−2.4 to 2.1) |
| Sex | 0.623 | −16.6 (−84.3 to 51.1) | 0.956 | 1.8 (−61.8 to 65.3) |
| Septal wall thickness | 0.017 | −9.4 (−17.1 to −1.8) | ||
| LOX expression | 0.003 | −185.2 (−301.3 to −69.2) | 0.005 | −188.7 (−315.6 to −61.7) |
| Insoluble collagen fraction | 0.003 | −4.6 (−7.4 to −1.7) | ||
| PER‐E | ||||
| Age | 0.246 | −0.8 (−2.1 to 0.5) | 0.406 | −0.5 (−1.7 to 0.7) |
| Sex | 0.547 | −11.5 (−49.7 to 26.7) | 0.975 | 0.6 (−36.1 to 37.2) |
| LA diameter | 0.081 | −1.9 (−4.0 to 0.2) | ||
| LOX expression | 0.023 | −80.7 (−149.9 to −11.6) | ||
| Insoluble collagen fraction | 0.002 | −2.6 (−4.3 to −1.0) | 0.005 | −2.6 (−4.3 to −0.8) |
| LATEF | 0.02 | 1.8 (0.3 to 3.3) | ||
HOCM indicates hypertrophic obstructive cardiomyopathy; LA, left atrial; LATEF, total left atrial ejection fraction; LOX, lysyl oxidase; PER‐E early peak emptying rate; and PFR, peak filling rate.
DISCUSSION
In our study, we mainly found the following: (1) The degree of CCL was correlated with the expression of the enzyme LOX rather than myocardial AGEs in patients with HOCM; (2) the degree of CCL and myocardial LOX expression were associated with LA/LV remodeling after adjusted for clinical confounders; (3) and compared with patients with HOCM without AF, patients with AF exhibited a greater degree of CCL and worse LA/LV function, even with similar amounts of total collagen.
Enzymatic and Nonenzymatic CCL in HOCM
Myocardial fibrosis is a hallmark of HCM, which is characterized as accumulation of collagen. 12 The collagen network provides the preservation of tissue architecture and chamber geometry, and its disproportionate accumulation further increases tissue stiffness. CCL is formation of covalent bonds between free collagen, and excessive CCL in the collagen network translates into tissue stiffness and more energy efficiency on deformation. 5 Previous studies reported that myocardial CCL had a close relationship with diastolic indexes rather than collagen amount in patients with heart failure. 4 , 10 Here, we showed that a lower LV deformation rate was correlated with increased abundance of insoluble collagen but not total collagen content. In addition, soluble collagen content was correlated with larger LV end‐diastolic diameters. This suggested that the relative proportion of CCL in the collagen network emerged as a critical influence of LV remodeling in HOCM.
To take this finding one step further, we analyzed the clinical significance of enzyme LOX and AGEs, which were biosynthetic pathways of enzymatic CCL and nonenzymatic CCL respectively. LOX is an extracellular, matrix‐embedded protein, which was associated with CCL and collagen expression. Animal studies showed that myocardial LOX could regulate the abundance of collagen type I and the resulting heart failure. 13 , 14 Inhibiting LOX with direct inhibitors could reduce myocardial stiffness in the left ventricle of normal adult pigs through decreasing CCL. 15 Consistent with previous studies, we found that myocardial LOX expression was higher in patients with HOCM and significantly associated with CCL, the expression of total collagen and collagen type I. LOX overexpression triggered a greater fibrotic response that was characterized by stronger collagen deposition and CCL, leading to increased LV stiffness and impaired diastolic dysfunction. In our study, patients with HOCM exhibited decreased LV deformation rate, which reflected impaired LV relaxation, progressive LV stiffness, and larger LA/LV diastolic filling gradients, 16 and a negative correlation was found between LOX expression and LV deformation rate, suggesting that LOX was associated with both formation and assembly of the collagen network and subsequent LV adverse remodeling. Although the important role of myocardial LOX was found in our study, serum LOX was not correlated with myocardial LOX or collagen subtypes, which made serum LOX not so qualified as a cardiac biomarker for myocardial LOX in HOCM.
Another pathway to form CCL is glycation‐induced nonenzymatic cross‐links (ie, AGEs). AGE cross‐links occur adventitiously and are usually stimulated by aging, hyperglycemia, and oxidative stress. 17 , 18 Enzymatic and AGE cross‐links have different effects on mechanical properties of tissue. In bone, enzymatic cross‐links are considered beneficial to bone strength. In contrast, AGE cross‐links have detrimental effects on biological functions of bone, which could make collagen fibers brittle. 19 Interestingly, no significant correlation was found between AGE levels and CCL or LV remodeling parameters in our study. This finding might indicate that AGEs had a less effective role in formation of collagen cross‐links in patients with HOCM. Results of a study by van Heerebeek et al 20 showed that AGEs were of particular influence in patients with heart failure with a reduced ejection fraction but insignificant in patients with a normal ejection fraction. This might explain the lack of clinical significance of AGEs in patients with HOCM, who had a high‐normal or elevated ejection fraction. Besides AGE cross‐links, AGEs could also mediate their effects through a receptor‐dependent pathway. AGEs bind to specific cell surface–associated receptors and accelerate the process of oxidation. 21 Thus, the role of AGEs in HOCM is not fully understood and worth further investigation.
Association Between LA Remodeling and CCL
Enlargement of the left atrium occurs frequently in patients with HCM, reflecting significant LV diastolic dysfunction, LVOT obstruction, presence of mitral regurgitation, and intrinsic atrial myopathy. 22 Recent studies also reported that patients with HCM could also exhibit impairment of LA function before LA enlargement, even in the early stage of the disease. 23 However, the mechanism remained unknown. In a study of HCM sarcomere mutation carriers, the overt HCM group had significantly lower LATEF and passive left atrial ejection fraction compared with preclinical HCM and controls, which was attributable to a greater extent of late gadolinium enhancement in subjects with overt HCM. 24 In addition, LA strain was reported as an independent predictor to LA fibrosis in patients with severe mitral regurgitation. 25 In our study, CCL and collagen type I/III were correlated with LA diameters and LA volume. In addition, CCL was associated with LA deformation rate and emptying function. Both CCL and a high proportion of collagen I are considered to be coincident with an increased in tissue stiffness. Thus, the molecular changes of the collagen network were also correlated with LA remodeling in HOCM.
AF is the most common arrhythmia observed in HCM. Roughly 20% of patients develop AF, with an annual incidence of 2%. 26 Patients with LVOT obstruction have a higher prevalence of AF. 27 A previous study showed that AF developed after the diagnosis of HCM in the majority of patients, indicating that anatomic and physiologic changes were related to AF development. 28 In our study, compared with patients without AF, patients with AF exhibited a larger left atrium but lower LA emptying function and deformation rate. Meanwhile, patients with AF also showed higher CCL than patients without AF, but the amount of total collagen was similar. Consistent with our results, Adam et al 29 previously reported that patients with AF showed higher total collagen content and 2.5‐fold increased collagen cross‐linking compared with patients with sinus rhythm. This might indicate that increased CCL was correlated with occurrence of AF in HOCM. Of interest, although LOX expression was correlated with LA deformation rate, the difference was not significant between patients with and without AF, but myocardial AGEs were higher in patients with AF. In our opinion, AF occurrence was determined by a combination of multiple factors. Lysine residues are common binding sites of enzymatic and nonenzymatic cross‐linking. 9 Accumulation of AGEs might result in competitive inhibition of enzymatic cross‐linking and deteriorate the physiologic formation of the collagen network because of their relative fragility, which might be associated with the occurrence of AF.
Several limitations must be acknowledged. First, this was a study involving a relatively small number of patients, including few patients with AF. However, because of the nature of the goals under investigation, it was adequately powered. Also, our conclusion was mainly derived from correlation analysis, which did not necessarily represent a causative relation. In addition, our study did not include patients with HCM with a normal LVOT pressure gradient or patients with systolic dysfunction. Different results could have been found by evaluating these 2 populations. Finally, because the time course was short, the follow‐up data were absent. We cannot evaluate how LOX and CCL influence clinical outcomes or longitudinal disease evolution in this study.
CONCLUSIONS
CCL plays an important role in heart remodeling in HOCM, which might be regulated mainly by LOX. Myocardial LOX expression is independently correlated with LV stiffness, while accumulation of AGE cross‐links might be associated the occurrence of AF in patients with HOCM.
Sources of Funding
This study was supported by Natural Science Foundation of China (81370327).
Disclosures
None.
(J Am Heart Assoc. 2021;10:e017752. DOI: 10.1161/JAHA.120.017752.)
For Sources of Funding and Disclosures, see page 11.
REFERENCES
- 1. Marian AJ, Braunwald E. Hypertrophic cardiomyopathy. Circ Res. 2017;121:749–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Motoyasu M, Kurita T, Onishi K, Uemura S, Tanigawa T, Okinaka T, Takeda K, Nakano T, Ito M, Sakuma H. Correlation between late gadolinium enhancement and diastolic function in hypertrophic cardiomyopathy assessed by magnetic resonance imaging. Circ J. 2008;72:378–383. [DOI] [PubMed] [Google Scholar]
- 3. Hayashi T, Shimomura H, Terasaki F, Toko H, Okabe M, Deguchi H, Hirota Y, Kitaura Y, Kawamura K. Collagen subtypes and matrix metalloproteinase in idiopathic restrictive cardiomyopathy. Int J Cardiol. 1998;64:109–116. [DOI] [PubMed] [Google Scholar]
- 4. Klotz S, Foronjy RF, Dickstein ML, Gu A, Garrelds IM, Jan Danser AH, Oz MC, Armiento DJ, Burkhoff D. Mechanical unloading during left ventricular assist device support increases left ventricular collagen cross‐linking and myocardial stiffness. Circulation. 2005;112:364–374. [DOI] [PubMed] [Google Scholar]
- 5. López B, Querejeta R, González A, Larman M, Díez J. Collagen cross‐linking but not collagen amount associates with elevated filling pressures in hypertensive patients with stage C heart failure. Hypertension. 2012;60:677–683. [DOI] [PubMed] [Google Scholar]
- 6. López B, González A, Hermida N, Valencia F, de Teresa E, Díez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol Heart C. 2010;299:H1–H9. [DOI] [PubMed] [Google Scholar]
- 7. Reiser K, McCormick RJ, Rucker RB. Enzymatic and nonenzymatic cross‐linking of collagen and elastin. FASEB J. 1992;6:2439–2449. [DOI] [PubMed] [Google Scholar]
- 8. Bailey AJ, Sims TJ, Avery NC, Halligan EP. Non‐enzymic glycation of fibrous collagen: reaction products of glucose and ribose. Biochem J. 1995;305:385–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross‐links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporosis Int. 2006;17:1514–1523. [DOI] [PubMed] [Google Scholar]
- 10. Kasner M, Westermann D, Lopez B, Gaub R, Escher F, Kühl U, Schultheiss H, Tschöpe C. Diastolic tissue Doppler indexes correlate with the degree of collagen expression and cross‐linking in heart failure and normal ejection fraction. J Am Coll Cardiol. 2011;57:977–985. [DOI] [PubMed] [Google Scholar]
- 11. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, et al. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:e783–e831. 10.1161/CIR.0b013e318223e2bd [DOI] [PubMed] [Google Scholar]
- 12. Varnava AM, Elliott PM, Sharma S, McKenna WJ, Davies MJ. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease. Heart. 2000;84:476. 10.1136/heart.84.5.476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Galán M, Varona S, Guadall A, Orriols M, Navas M, Aguiló S, Diego A, Navarro MA, García‐Dorado D, Rodríguez‐Sinovas A, et al. Lysyl oxidase overexpression accelerates cardiac remodeling and aggravates angiotensin II–induced hypertrophy. FASEB J. 2017;31:3787–3799. 10.1096/fj.201601157RR [DOI] [PubMed] [Google Scholar]
- 14. Lu M, Qin Q, Yao J, Sun L, Qin X. Induction of LOX by TGF‐β1/Smad/AP‐1 signaling aggravates rat myocardial fibrosis and heart failure. IUBMB Life. 2019;71:1729–1739. 10.1002/iub.2112 [DOI] [PubMed] [Google Scholar]
- 15. Kato S, Spinale FG, Tanaka R, Johnson W, Cooper GT, Zile MR. Inhibition of collagen cross‐linking: effects on fibrillar collagen and ventricular diastolic function. Am J Physiol. 1995;269:H863–H868. 10.1152/ajpheart.1995.269.3.H863 [DOI] [PubMed] [Google Scholar]
- 16. Thomas L, Marwick TH, Popescu BA, Donal E, Badano LP. Left atrial structure and function, and left ventricular diastolic dysfunction: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019;73:1961–1977. 10.1016/j.jacc.2019.01.059 [DOI] [PubMed] [Google Scholar]
- 17. Reiser KM. Nonenzymatic glycation of collagen in aging and diabetes. Proc Soc Exp Biol Med. 1991;196:17–29. 10.3181/00379727-196-43158C [DOI] [PubMed] [Google Scholar]
- 18. Tsukahara H, Sekine K, Uchiyama M, Kawakami H, Hata I, Todoroki Y, Hiraoka M, Kaji M, Yorifuji T, Momoi T, et al. Formation of advanced glycosylation end products and oxidative stress in young patients with type 1 diabetes. Pediatr Res. 2003;54:419–424. 10.1203/01.PDR.0000076662.72100.74 [DOI] [PubMed] [Google Scholar]
- 19. Garnero P, Borel O, Gineyts E, Duboeuf F, Solberg H, Bouxsein ML, Christiansen C, Delmas PD. Extracellular post‐translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone. 2006;38:300–309. 10.1016/j.bone.2005.09.014 [DOI] [PubMed] [Google Scholar]
- 20. van Heerebeek L, Hamdani N, Handoko ML, Falcao‐Pires I, Musters RJ, Kupreishvili K, Ijsselmuiden AJJ, Schalkwijk CG, Bronzwaer JGF, Diamant M, et al. Diastolic stiffness of the failing diabetic heart. Circulation. 2008;117:43–51. 10.1161/CIRCULATIONAHA.107.728550 [DOI] [PubMed] [Google Scholar]
- 21. Jensen LJ, Østergaard J, Flyvbjerg A. AGE‐RAGE and AGE cross‐link interaction: important players in the pathogenesis of diabetic kidney disease. Horm Metab Res. 2005;37:26–34. [DOI] [PubMed] [Google Scholar]
- 22. Abhayaratna WP, Seward JB, Appleton CP, Douglas PS, Oh JK, Tajik AJ, Tsang TS. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. [DOI] [PubMed] [Google Scholar]
- 23. Yang Y, Yin G, Jiang Y, Song L, Zhao S, Lu M. Quantification of left atrial function in patients with non‐obstructive hypertrophic cardiomyopathy by cardiovascular magnetic resonance feature tracking imaging: a feasibility and reproducibility study. J Cardiovasc Magn Reson. 2020;22:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Farhad H, Seidelmann SB, Vigneault D, Abbasi SA, Yang E, Day SM, Colan SD, Russell MW, Towbin J, Sherrid MV, et al. Left atrial structure and function in hypertrophic cardiomyopathy sarcomere mutation carriers with and without left ventricular hypertrophy. J Cardiovasc Magn R. 2017;19:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cameli M, Lisi M, Righini FM, Massoni A, Natali BM, Focardi M, Tacchini D, Geyer A, Curci V, Di Tommaso C, et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am J Cardiol. 2013;111:595–601. [DOI] [PubMed] [Google Scholar]
- 26. Olivotto I, Cecchi F, Casey SA, Dolara A, Traverse JH, Maron BJ. Impact of atrial fibrillation on the clinical course of hypertrophic cardiomyopathy. ACC Curr J Rev. 2002;11:58. [DOI] [PubMed] [Google Scholar]
- 27. Maron MS, Maron BJ, Olivotto I, Betocchi S, Casey SA, Lesser JR, Losi MA, Cecchi F. Effect of left ventricular outflow tract obstruction on clinical outcome in hypertrophic cardiomyopathy. N Engl J Med. 2003;348:295–303. [DOI] [PubMed] [Google Scholar]
- 28. Garg L, Gupta M, Sabzwari SRA, Agrawal S, Agarwal M, Nazir T, Gordon J, Bozorgnia B, Martinez MW. Atrial fibrillation in hypertrophic cardiomyopathy: prevalence, clinical impact, and management. Heart Fail Rev. 2019;24:189–197. [DOI] [PubMed] [Google Scholar]
- 29. Adam O, Theobald K, Lavall D, Grube M, Kroemer HK, Ameling S, Schäfers H, Böhm M, Laufs U. Increased lysyl oxidase expression and collagen cross‐linking during atrial fibrillation. J Mol Cell Cardiol. 2011;50:678–685. [DOI] [PubMed] [Google Scholar]


