Abstract
Background
ADRB1 (adrenergic receptor beta 1) responds to neuroendocrine stimulations, which have great implications in hypertension. GRK2 (G protein‐coupled receptor kinase 2) is an essential regulator for many G protein‐coupled receptors and subsequent cell signaling cascades, but its role as a regulator of ADRB1 and associated cardiac hypertrophy in hypertension remains to be elucidated.
Methods and Results
In this study, we found the expressions of GRK2 and ADRB1 in peripheral blood mononuclear cells were positively associated with blood pressure levels in hypertensive patients and with their expression in heart. In vitro evidence showed a direct interaction in ADRB1 and GRK2 and genetic depletion of GRK2 blocks epinephrine‐induced upregulation of hypertrophic and fibrotic genes in cardiomyocytes. Meanwhile, we discovered a selective serotonin reuptake inhibitor paroxetine specifically blockades GRK2 and ADRB1 interaction. In vivo, paroxetine treatment ameliorates hypertension‐induced cardiac hypertrophy, dysfunction, and fibrosis in animal models. We found that paroxetine suppressed sympathetic overdrive and increased the adrenergic receptor sensitivity to catecholamines. Paroxetine treatment also blocks epinephrine‐induced upregulation of hypertrophic and fibrotic genes as well as ADRB1 internalization in cardiomyocytes. Coadministration of paroxetine further potentiates metoprolol‐induced reductions in blood pressure and heart rate, further attenuating cardiac hypertrophy in spontaneously hypertensive rats. Furthermore, in patients with hypertension accompanied with depression, we observed that cardiac remodeling was less severe in those with paroxetine treatment compared with those with other types of anti‐depressive agents.
Conclusions
Paroxetine promotes ADRB1 sensitivity and attenuates cardiac hypertrophy partially via blocking GRK2‐mediated ADRB1 activation and internalization in the context of hypertension.
Keywords: adrenergic receptor beta 1, cardiac hypertrophy, G protein‐coupled receptor kinase 2, hypertension, paroxetine
Subject Categories: Animal Models of Human Disease, Basic Science Research, Hypertension, Hypertrophy
Nonstandard Abbreviations and Acronyms
- ADRB1
adrenergic receptor beta 1
- GPCR
G protein–coupled receptor
- GRKs
G protein–coupled receptor kinases
- HR
heart rate
- PBMC
peripheral blood mononuclear cell
- SBP
systolic blood pressure
- SHR
spontaneously hypertensive rats
Clinical Perspective
What Is New?
This study demonstrates that GRK2 (G protein‐coupled receptor kinase 2) inhibitor, paroxetine, attenuates cardiac remodeling and fibrosis independent of its role in regulation of hypertension.
Paroxetine blocks hypertension induced cardiac hypertrophy partially through its interaction with ADRB1 (adrenergic receptor beta 1) and subsequent ADRB1 internalization and signaling activation.
The beneficial role of paroxetine on hypertension‐induced cardiac hypertrophy may be associated with its role of reducing catecholamine and sympathetic hormone levels.
What Are the Clinical Implications?
These findings suggest the potential for GRK2 inhibition to be an alternative therapeutic target to prevent the progression of cardiac hypertrophy, dysfunction, and fibrosis in hypertension.
The effect of paroxetine in organ protection and in attenuating sympathetic hormone levels independent of the function on blood pressure control may provide a promising new avenue for neuroendocrine modulation in cardiovascular disease.
These findings propose the potential that paroxetine further potentiates metoprolol‐induced reductions in blood pressure and heart rate and attenuates hypertension‐induced cardiac hypertrophy.
Hypertension, one of the most prevalent chronic diseases, affects >20% of the global population and leads to mortality worldwide. 1 Maladaptive cardiac hypertrophy occurs as a consequence of persistent high blood pressure and ultimately leads to eccentric heart dilation and heart failure. 2 ADRB1 (adrenergic receptor beta 1), a member of the G protein–coupled receptor (GPCR) superfamily, responds to catecholamine stimulation. ADRB1 mediates a wide range of cardiovascular physiological responses and has been of great interest to researchers as a therapeutic target for cardiovascular diseases. 3 , 4 However, drug interactions, genetic variations, and epigenetic modifications have great impacts on the responsiveness of adrenergic receptors to neuroendocrine stimulators and the efficacy of individual ADRB1 antagonists used in the treatment of cardiovascular diseases such as hypertension and cardiac arrhythmia. 5 , 6 , 7 , 8
Among many essential regulators of adrenergic receptor signaling, GPCR kinases (GRKs) have been identified as a group of kinases that mediate the recruitment of β‐arrestin and the phosphorylation and internalization of receptors. 9 , 10 Such internalization limits agonist‐evoked adrenergic receptor signaling and decreases cellular responses to continuous stimulation. 10 GRK2 is also known as adrenergic receptor beta kinase 1. Inhibition of GRK2 can enhance cardiac contractility and cardiac dysfunction in ischemia and metabolic disorders related to cardiac dysfunction. 11 , 12 However, its role as a regulator of ADRB1 in hypertension‐associated cardiac dysfunction remains to be elucidated. Thus, we proposed that GRK2 is involved in the regulation of ADRB1 activity and subsequent cardiac hypertrophy in the context of hypertension. Understanding this mechanism facilitates the discovery of potent inhibitor of ADRB1‐GRK2 interaction that may result in beneficial effect on hypertension‐induced cardiac hypertrophy.
Methods
Data presented support the findings of this study and are available from the corresponding author on reasonable request. The proposal with detailed aims, statistical plans, and study materials might be required to guarantee the security of data. We constructed 3 clinical studies and the data without patient name and identifiers could be shared after reviewing and approving the proposal and related materials.
We affirm that the article is an honest, accurate, and transparent account of our study. No important aspects of the study have been omitted.
Reagents
The antibodies and reagents used in the present study and their respective commercial sources are as follows: anti‐Flag (F3165) and anti‐V5 (A7345) were purchased from Sigma (Santa Clara, CA, USA). anti‐GRK2 (13990‐1‐AP), anti‐GAPDH (10494‐AP), anti‐collagen I (14695‐1‐AP), and anti‐rabbit immunoglobulin G (H+L) (SA00001‐2) were purchased from Proteintech (Rosemont, IL, USA). Anti‐ADRB1 (ab3442) and anti‐BNP (B‐type natriuretic peptide) (ab19645) were purchased from Abcam (Cambridge, MA, USA). Anti‐MHC Class II (49525), anti‐ANP (atrial natriuretic peptide) (33030) and anti‐CTGF (connective tissue growth factor) (36807) were purchased from Signalway Antibody (College Park, MD, USA). Anti‐beta‐actin (bs‐0061R) was purchased from Bioss (Beijing, China). Anti‐Na, K ATPase (3013) was purchased from Cell Signaling Technology (Boston, MASS, USA). A BCA (bicinchoninic acid) protein assay kit was obtained from Pierce (Rockford, IL, USA). Fetal calf serum was obtained from HyClone (Logan, UT, USA). Hormone activity was measured using fluorometric activity assay kits according to the manufacturer's protocols. The kits included an aldosterone ELISA kit (ab136933) from Abcam (Cambridge, MA, USA) and a rat renin ELISA kit (CSB‐E08702r), a rat noradrenaline ELISA kit (CSB‐E07022r), and a rat epinephrine ELISA kit (CSB‐E08678r) from CUSABIO (Houston, TX, USA), a membrane protein extraction kit (P0033) from Beyotime Biotechnology (Wuhan, China).
Animals
Wistar‐Kyoto rats and spontaneously hypertensive rats (SHRs) were purchased from Charles River Laboratories (VRL) (Wilmington, MA, USA). ADRB1 knockout rats were generated by Wuhan Kangweida Gene Technology Co., Ltd. All strains were maintained under a 12‐hour light‐dark cycle in a pathogen‐free facility at the animal facility of Central South University (Changsha, Hunan, China) and were fed a standard diet with water. All animal experiments and protocols were approved by the Animal Care and Use Committee of Central South University (2018sydw0139). All mice were randomly assigned to the treatment groups by random number via invoking computer. Blinding procedures were used to prevent researcher bias. No animals were excluded from the study.
Hypertension Models and Treatment
Two hypertension models, the SHR model and the isoproterenol‐infused SHR (SHR+ isoproterenol) model (in which 5 mg isoproterenol/kg/day based on baseline weight was delivered by subcutaneous osmotic minipump for 2 weeks), were used in this study. SHRs were purchased at 6 weeks of age, and follow‐up was conducted until the rats reached 22 weeks of age. Blood pressure was measured in conscious rats by the tail‐cuff method using an electronic sphygmomanometer (FMI, Seeheim, Germany) and echocardiography evaluation were measured every other week from 10 to 22 weeks. Moreover, we harvest tissues of the SHR model when rats reached 22 weeks of age to observe the pathological changes of SHRs. Paroxetine (5 mg/kg per day), 7 metoprolol (50 mg/kg per day), and amlodipine (3 mg/kg per day) were dissolved in sterile saline and given by gavage between 10:00 and 12:00 daily and the dose is determined by body weight measured weekly.
For the SHR+isoproterenol group and its control group, isoproterenol (5 mg/kg per day based on baseline weight) dissolved in sterile saline or sterile saline only (n=6 in each group) was infused using an osmotic minipump (2002, Alzet, Cupertino, CA, USA) that was inserted subcutaneously into rats under chloral hydrate anesthesia when rats reached 12 weeks of age. 13 Blood pressure was measured in conscious rats by the tail‐cuff method using an electronic sphygmomanometer (FMI, Seeheim, Germany) from 10 to 14 weeks when the minipump stopped infusing. Echocardiography evaluation was measured from 10 to 16 weeks, which is the longest survival time of isoproterenol‐infused SHR and thus we harvest heart tissues of SHR+isoproterenol group in their 16 weeks of age.
Deletion of ADRB1 Gene in Rats
ADRB1 gene deletion was generated in the SHR rat. The Doubale‐Strand Breaks produced by Transcription Activator‐Like Effector Nuclease can be repaired in 2 ways, Homology Dependent Repair or non‐homologous end joining (NHEJ). We used the micro‐injection method to generate Doubale‐Strand Breaks using Transcription Activator‐Like Effector Nuclease technology and obtained chimeric rats by non‐homologous recombination. More details of the technical strategy are shown in Figure S1.
Tail tissues (≈0.5 cm) were taken from the rats and immersed in 20 μL of digestive juice. The tissues were digested overnight at 55°C. Rat DNA was extracted according to the instructions of the DNA extraction kit (EE101‐11, TransGen, Beijing, China) on the following day. Subsequently, forward and reverse primers were used to amplify DNA fragments through polymerase chain reaction (PCR), and 5 μL of the PCR product was used for agarose gel electrophoresis to determine whether the size of the band was correct and to determine whether the band was clear and specific. Products with a single clear target band were sent for sequencing. The sequencing primers and PCR primers are listed in Table S1. As shown in Figure S1B, PCR analysis showed that the ADRB1 knockout rats were generated successfully.
Quantitative Real‐Time PCR Analysis
Quantitative real‐time PCR analysis were performed on peripheral blood mononuclear cells (PBMCs), cultured cardiomyocytes or cardiac tissue. Briefly, total RNA was prepared from fresh‐frozen tissue using TRIzol reagent (15596026, Invitrogen, Carlsbad, CA, USA). Quantitative real‐time PCR was performed using SYBR Green (04913850001, Roche, Basel, Switzerland), and the results were normalized to GAPDH expression. The genes were amplified using the specific primers listed in Table S2.
Propensity Score‐Matched Analysis
Propensity score‐matched cohorts were created based on variables which were expected to be potential confounders associated with exposure to paroxetine among the patients with hypertension accompanied with depression, including age, sex, blood pressure, the year of hypertension, a history of smoking or drinking, and comorbidities (diabetes mellitus, liver, or kidney disease and respiratory disease). We adjusted for medications (anti‐hypertension drugs: calcium channel blockers, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, and beta‐blockers) between paroxetine versus non‐paroxetine groups. The missing data among baseline characteristics were imputed using the MissForest algorithm based on the random forest method in the R. The internally cross‐validated errors were also estimated. Paroxetine and non‐ paroxetine users were paired according to the propensity scores using exact matching with a caliper size of 0.05. The balance of covariates was evaluated by estimating standardized differences before and after matching, and a small absolute value of <0.1 was considered successful balancing between the 2 groups. From January 2017 to March 2019, outpatients with hypertension accompanied by depression who comply to our inclusion and exclusion criteria were continuously screened for our studies. Among 168 eligible patients, there were 63 patients in the paroxetine treatment and 105 patients without the paroxetine treatment. And there were 20 patients in the paroxetine treatment and 33 patients without the paroxetine treatment agreed to participate in our study and signed up for the consent form. After propensity score matching at a ratio of 1:1, matched 16 patients with paroxetine and 16 patients without paroxetine were used for analysis.
Clinical Data Collection
All procedures involving human samples complied with the principles outlined in the Declaration of Helsinki and were approved by the Ethics Committee at the Third Xiangya Hospital of Central South University. To test the correlation between ADRB1 expression in PBMCs and blood pressure levels, PBMCs and clinical information were collected from patients with hypertension (systolic blood pressure [SBP] ≥140 mm Hg or diastolic blood pressure ≥90 mm Hg), prehypertension (120 mm Hg ≤ SBP ≤139 mm Hg or 80 mm Hg ≤ diastolic blood pressure ≤89 mm Hg), and normal population (SBP <120 mm Hg and diastolic blood pressure <80 mm Hg) who were undergoing annual physical examinations at the Third Xiangya Hospital. Propensity score matching was implemented to match prehypertension and hypertension populations with the normotensive patients, all variables except SBP and diastolic blood pressure, including age, body mass index, heart rate (HR), and other relative biochemical indexes are matched at 1:1:1. The demographic characteristics of the recruited individuals undergoing health examinations are attachable and comparable which are listed in Table S3. To verify the association of the expression of ADRB1 between PBMC and heart, we harvested the heart tissue from patients with congenital heart diseases receiving heart surgery. To examine whether the cardiac remodeling attenuated in the patients with paroxetine treatment, we obtained medical history and physical exam information as well as cardiac ultrasound parameters from the patients with depression complicated with hypertension. Informed consent was obtained from the patients or the families before all procedures and sample collections.
Correlation Coefficient Analysis
In this study, the Pearson correlation coefficient analysis was applied to describe the proportion of the variance in the dependent variable that is predictable from the independent variables. Specifically, first, the association of the mRNA levels of ADRB1 and GRK2 in myocardium with those levels in PBMC was analyzed; Second, the correlation between the SBP and the mRNA levels of ADRB1 and GRK2 in PBMCs was calculated. The correlation coefficient R 2 measures the strength and direction of a linear relationship between 2 analyzed variables on a scatterplot. P<0.05 defined as statistically significant.
Plasmid Constructs
Plasmids encoding full‐length ADRB1 (pmCherry‐N1‐flag‐ADRB1) or GRK2 (pHBLV‐CMV‐MCS‐3flag‐EF1‐ZsGreen) were constructed by cloning the indicated coding regions of human ADRB1 or GRK2 cDNA into indicated vectors as listed in Table S4.
Western Blot and Immunoprecipitation Assay
Total protein was extracted from indicated cell or tissue using RIPA lysis buffer and quantified using BCA Protein Assay Kit (ab102536, Abcam, Cambridge, MA, USA). Then, the protein samples were separated by 10% SDS‐PAGE and transferred to polyvinylidene difluoride membranes, which were blocked with 5% skim milk for 90 minutes, followed by incubation overnight at 4°C with primary antibodies. The membranes were then washed before incubation with species‐appropriate secondary antibodies for 1 hour at room temperature. Membranes were analyzed using a ChemiDocTM XRS+Imaging System (Bio‐Rad, Hercules, CA, USA).
HEK293T cells were transfected with GRK2 and ADRB1 expression plasmids for 24 hours to examine the interaction between these 2 proteins. Protein concentrations were determined and volumes of lysates containing 200 μg protein were incubated with 2 μg of the indicated antibody for 1 hour at 4°C on a rotating platform. Protein A/G sepharose beads were washed in lysis buffer and 30 μL of a 50% suspension was added to each sample before incubation for a further 1 hour rotating at 4°C. Beads conjugated with the anti‐Flag or anti‐V5 antibodies for 3 hours at 4°C. Beads were washed 4 times in lysis buffer before adding 25‐μL SDS reducing buffer to each sample ready for analysis by SDS‐PAGE. Interactions between the proteins of interest were measured via immunoblotting with the indicated primary and secondary antibodies. The nucleotide sequence of tags used in this study are listed in Table S5.
Cell Culture and Infection With Recombinant Viral Vectors
The HEK293T cell line was purchased from the American Type Culture Collection (AA‐CELL‐148, ATCC, Rockefeller, MD, USA) and cultured in DMEM‐F12 medium with 10% fetal bovine serum and 1% penicillin‐streptomycin at 37°C in a humidified incubator containing 5% CO2. The medium was replaced with serum‐free medium after 72 hours, and the cells were further cultured under serum‐free conditions for 48 hours before reagents were added. AC16 adult human ventricular cardiomyocytes were purchased from Sigma‐Aldrich Inc. (SCC109, Sigma, Santa Clara, CA, USA) and cultured as previously described. To manipulate the expression of GRK2 in AC16 cardiomyocytes, we infected cells with adenoviruses expressing short hairpin RNA targeting GRK2 (shGRK2), lentivirus overexpressing GRK2 and their corresponding controls. The siRNA and shRNA sequence used in this study are listed and illustrated in Table S6.
H9C2 rat cardiomyocytes were obtained from American Type Culture Collection (CRL‐1446, ATCC, Rockefeller, MD, USA) and cultured in high glucose (4500 mg/L) DMEM. Primary ventricular myocytes were prepared from Sprague–Dawley rats. In brief, ventricles were digested with collagenase (0.4 mg/mL) and pancreatin (0.6 mg/mL) in 116 mmol/L NaCl, 20 mmol/L HEPES (pH 7.35), 0.8 mmol/L NaH2PO4, 5.6 mmol/L glucose, 5.4 mmol/L KCl, 0.8 mmol/L MgSO4. Cells were recovered by centrifugation (5 minutes, 60 g), resuspended in plating medium (70% DMEM, 15% M199, 15% fetal calf serum, 100 U/mL of penicillin and streptomycin) and pre‐plated on 60‐mm Primaria culture dishes precoated in 1% gelatin (37°C, 30 minutes) to remove non‐myocytes. The non‐adherent cardiac myocytes were plated on gelatin‐coated 60‐mm Primaria culture dishes at 1×106 cells per dish. After 18 hours, cardiomyocytes were washed and cultured in serum‐free maintenance medium (80% DMEM, 20% M199, 100 U/mL of penicillin and streptomycin) for the duration of the experiments.
Echocardiography Evaluation
Echocardiographic studies were performed between 16:00 and 20:00 on animals in the left lateral decubitus position that had been anesthetized with sodium pentobarbital (45 mg/kg intraperitoneally). A MyLab 30CV (Esaote) ultrasound system was used to perform 2‐dimensional guided M‐mode echocardiography and pulse‐wave Doppler echocardiography with a linear‐phase array probe (L15‐7io; frequency range 7–15 MHz). The following parameters were measured: interventricular septum dimensions during both diastole and systole , left ventricular end‐diastolic volume, left ventricular end‐systolic volume, and left ventricular ejection fraction. The measurements were obtained from 3 beats and averaged.
Measure of Adrenergic Receptor Sensitivity
An intra‐arterial blood pressure transducer was applied to measure the changes in blood pressure (BP) and HR in response to catecholamine infusion in the indicated rats. The blood pressure was measured after neck surgery and common carotid artery cannulation in rats under chloral hydrate anesthesia. The arterial blood pressure and heart rate were measured through the common carotid artery with a model DTX disposable transducer (Spectramed, Oxnard, CA, USA), which was connected to a model AP‐621 G carrier amplifier for arterial blood pressure (Nihon Kohden) and a model AT‐601 G HR counter (Nihon Kohden). When the blood pressure had stabilized, epinephrine or isoproterenol (2 mL, 1:100 000) were injected into the tail vein. The SBP and HR were recorded on a model WR‐3701 recorder (Graphtec, Tokyo, Japan) at 30 seconds, 1 minute, 2 minutes, 3 minutes, 4 minutes, and 5 minutes.
Histological Analysis
Hearts were harvested, arrested in diastole with a 10% potassium chloride solution, fixed with 10% formalin, dehydrated, and embedded in paraffin. Next, frozen tissues were transversely sectioned at 5‐μm intervals. Sections at the left ventricular mid–papillary muscle level were stained with hematoxylin and eosin and picrosirius red to compare the cardiomyocyte cross‐sectional area and cardiac perivascular collagen deposition volume, respectively.
Statistical Analysis
All data are expressed as the mean±SD. Data were first analyzed for normality test (by Shapiro‐Wilk test or D'Agostino and Pearson test where indicated). Data passed the normality test were then assessed by unpaired 2‐tailed t test (2 groups) or 1‐way ANOVA with Tukey test (3 groups or more), where indicated. Non‐normally distributed data (2 groups) were analyzed by Mann‐Whitney test. P<0.05 was considered to be statistically significant. For linear regression analysis (Figure 1A and 1B), data were also first tested and passed the normality test (D'Agostino and Pearson test). Chi‐square tests and paired t‐tests were used to conduct comparisons of categorical variables and continuous variables in the human studies. No experiment‐wide multiple test correction was applied in our study. Representative images were chosen for similarity to the quantification data (close to the average expression) and the image quality. Statistical calculations in the animal experiments were performed on at least 3 independent experiments. A P<0.05 was considered to indicate statistical significance.
Figure 1. The expressions of GRK2 (G protein‐coupled receptor kinase 2) and ADRB1 (adrenergic receptor beta 1) are upregulated in patients with hypertension.
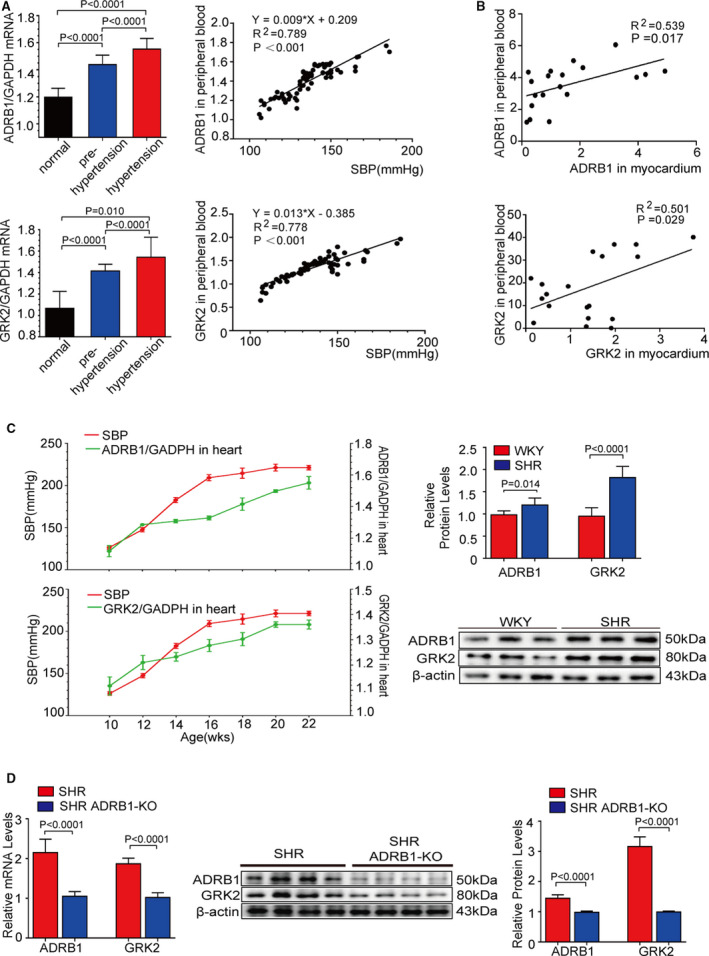
A, The relative mRNA expressions of ADRB1 and GRK2 in peripheral blood mononuclear cell from the individuals with normal blood pressure (n=24), prehypertension (n=24), and hypertension (n=24) (left); Pearson comparison analyses of the correlations between ADRB1 and GRK2 mRNA levels and systolic blood pressure. P<0.001 for all of these correlations, by Pearson correlation coefficient analysis (right). B, Pearson comparison analyses of the correlations between ADRB1 and GRK2 mRNA levels in peripheral blood mononuclear cell and those levels in the myocardium in the patients with congenital heart diseases (n=19). P<0.05 for all of these correlations, by Pearson correlation coefficient analysis. C, The relative mRNA expressions of ADRB1 and GRK2 in the myocardium (n=6) and dynamic change in systolic blood pressure (n=6) from 10‐ to 22‐week of age in spontaneously hypertensive rats ((SHRs) (left). Representative images of western blot analysis and quantitative results of ADRB1 and GRK2 protein levels in the myocardium at 22‐week of age in SHR (n=6) (right). D, The relative mRNA expressions of ADRB1 and GRK2 in the myocardium (n=6) from SHR and SHR with ADRB1 knockout at 22‐week of age (left). Representative images of western blot (middle) and quantitative results (right) of ADRB1 and GRK2 in in the myocardium (n=6) from SHR and SHR with ADRB1 knockout at 22‐week of age (n=6). Data shown are mean±SD (A, C, and D). Data were first analyzed and passed normality test (D'Agostino and Pearson test in [A and B], Shapiro‐Wilk test in [A, C, and D]). P values were shown and assessed by one‐way ANOVA with Tukey test (A) and by Mann‐Whitney test (C and D). ADRB1 indicates adrenergic receptor beta 1; GRK2, G protein‐coupled receptor kinase 2; KO, knockout mice; SHR, spontaneously hypertensive rats; and WKY, Wistar‐Kyoto rats.
Results
Expressions of GRK2 and ADRB1 are Upregulated in Patients With Hypertension
We tested the hypothesis that the expressions of GRK2 and ADRB1 are upregulated in the patients with hypertension and rodents. First, we found that the mRNA levels of GRK2 and ADRB1 were significantly upregulated in the PBMCs of individuals with pre‐hypertension and essential hypertension compared with those of normal controls. In addition, GRK2 and ADRB1 mRNA levels in PBMCs were positively correlated with SBP (r 2=0.778 and r 2=0.789, respectively) (Figure 1A). Next, we found the expressions of GRK2 and ADRB1 in PBMC are positively correlated with those expressions in heart tissue in patients with congenital heart diseases receiving heart surgery (r 2=0.501 and r 2=0.539, respectively) (Figure 1B). Consistently, the mRNA and protein expression levels of GRK2 and ADRB1 increased with the progression of hypertension in SHR hearts (n=6) (P<0.0001 and P=0.014, respectively) (Figure 1C), but kept the same level in Wistar‐Kyoto rat hearts (n=6) (Figure S2). Depletion ADRB1 gene expression significantly attenuated ADRB1 and GRK2 expression of mRNA and protein in the heart tissue (P<0.0001 and P<0.0001, respectively) (Figure 1D and Figure S1). The demographic characteristics of the recruited individuals undergoing health examinations and the patients with congenital heart diseases are listed in Tables S3 and S7. The abovementioned data suggested that the GRK2 and ADRB1 expression levels were elevated in patients with hypertension and were associated with blood pressure. In addition, GRK2 level was paralleled with the level of ADRB1 in the heart tissue in the hypertensive models.
Paroxetine is a Potent Inhibitor for GRK2‐ADRB1 Interaction
To explore the potential regulatory mechanism between GRK2 and ADRB1, we performed protein–protein interaction analysis by using the STRING protein–protein interaction database (Version 10, string‐db.org), an open‐source bioinformatics analysis tool for analyzing protein connection. A score combiner algorithm was used by STRING to determine the probability of protein connection or relation. The lines between the proteins mean the interaction evidence and confidence while the line thickness indicates the strength of data support or the type of interaction evidence. The result showed the corresponding relationship of the 7 exhibited proteins, different proteins own different colors and connection lines have different widths, representing the strength of the corresponding interaction relationship. Meanwhile, it also showed that there may be >1 connection between 2 proteins, which indicates that there are multiple interactions between the 2 proteins (both for experimental verification or data prediction results). The results indicated an interaction between GRK2 and ADRB1 protein in Homo sapiens (Figure 2A). We further screened the affinities of 22 small molecular compounds for the GRK2 protein as potential inhibitors by using surface plasmon resonance (SPR) technology. Notably, we found that paroxetine had the highest capacity to bind with GRK2 among the 22 compounds (Figure 2B). Paroxetine exhibited GRK2‐binding capability at various doses ranging from 800 to 3200 nmol/L (Figure 2C). The average association and dissociation rates (ka values and kd values, respectively), the equilibrium dissociation constants (KDs) and the absolute affinity coefficients (ABS values) for representative compounds with various affinities for GRK2 are shown in Figure 2D. The ka values and kd values can be used to determine an equilibrium dissociation constant KD. KDs were determined by SPR using a titration of concentrations of analyte and a maximum point on the sensorgram signifying saturation of the protein in order to determine a steady‐state KD. ABS value was calculated by SPR determining the absolute affinity coefficients. 49 Analysis of these indicators showed that paroxetine exhibited the highest affinity for GRK2, while aliskiren, cefoperazone, dirithromycin, and cefotetan demonstrated medium to low affinities (Figure 2D). Western blotting showed the interaction between GRK2 and ADRB1 that occurred in an immunoprecipitation assay performed with HEK293T cells overexpressing V5‐tagged GRK2 and Flag‐tagged ADRB1. Paroxetine preincubation significantly blocked the GRK2 and ADRB1 interaction in HEK293T cells (Figure 2E), and dobutamine preincubation showed that ADRB1 specific agonist further enhances the interaction (Figure S3). This evidence shows that paroxetine is capable of inhibiting GRK2 and ADRB1 interaction because of its high affinity to GRK2.
Figure 2. Paroxetine is identified as a potent inhibitor for GRK2 (G protein‐coupled receptor kinase 2) and ADRB1 (adrenergic receptor beta 1) interaction.
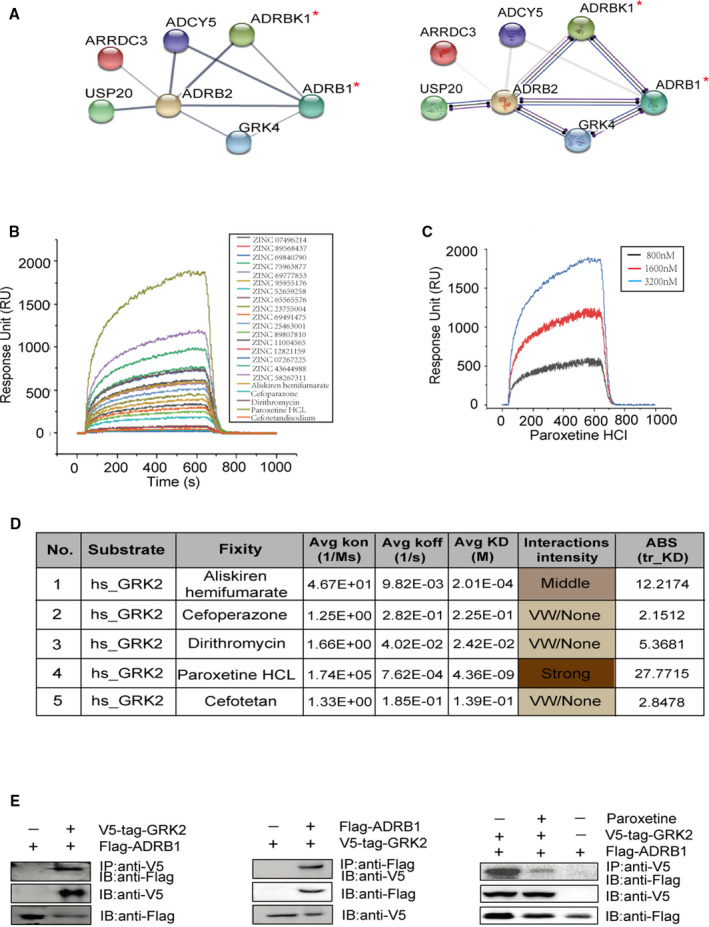
A, STRING protein–protein interaction analysis of ADRB1 interacting proteins (the protein of interest is marked by red asterisk). Line thickness indicates the strength of data support (left), line color indicates the type of interaction evidence (right). B, Surface plasmon resonance technology analysis the sensitivity of GRK2 binds to 22 selected compounds. C, Surface plasmon resonance measured the sensitivity of GRK2 binds paroxetine at doses ranged from 800 to 3200 nmol. D, Listed surface plasmon resonance sensitivity parameters between GRK2 and 5 market available drugs. E, Representative immunoblots showing interaction between V5‐tag‐GRK2 and Flag‐ADRB1 in HEK293T cells (left and middle). Representative immunoblots confirming paroxetine blocks the interaction between V5‐tag‐GRK2 and Flag‐ADRB1 (right). Co‐immunoprecipitations were performed to examine the associations between ADRB1 and GRK2. Lysates from HEK293T cells transfected with tagged GRK2 and ADRB1 expression plasmids were inputs (top panel) as described under Methods. The aliquot of input was used for immunoprecipitation (IP) with various primary antibodies including anti‐Flag or anti‐V5 as noted. Immunoblotting (IB) is to detected the protein that bound to the antibody used for IP. ADRB1 indicates adrenergic receptor beta 1; GRK2, G protein‐coupled receptor kinase 2; ADRB2, adrenergic receptor beta 2; GRK4, G protein‐coupled receptor kinase 4; ADCY5, adenylate cyclase 5; ARRDC3, Arrestin Domain Containing 3; and USP20, Ubiquitin Specific Peptidase 20.
Paroxetine Attenuates Hypertension‐Induced Cardiac Hypertrophy
Since paroxetine has proven to be effective in blocking GRK2 and ADRB1 interaction, we further explored whether paroxetine attenuated hypertension‐related cardiac hypertrophy in rodent models. Both the SHR model and the SHR+isoproterenol model were applied to test this hypothesis. The baseline cardiac phenotypes were comparable within the various rat groups. SBP and HR gradually increased in all groups from 10‐ to 14‐weeks of age. The SHR+isoproterenol group showed a greater augmentation of SBP and HR than the SHR group (Figure 3A). Intragastric administration of paroxetine significantly attenuated the heart rate increases in the SHR and SHR+isoproterenol groups but did not alter SBP in those groups (Figure 3A). However, paroxetine had no effect on SBP in Wistar‐Kyoto rats (Table S8). Cardiac hypertrophy, fibrosis and systolic function were exacerbated during the development of hypertension in SHRs, and isoproterenol infusion further potentiated these phenotypic changes (Figure 3B through 3D). We also observed ameliorated cardiac hypertrophy in the paroxetine‐treated groups compared with the non‐paroxetine‐treated SHR and SHR+isoproterenol groups, as evidenced by changes in heart weight (HW), heart weight/body weight ratios (HW/BW), heart weight/tibia length ratios (HW/TL), interventricular septal thickness in diastole, and interventricular septal thickness in systole (Figure 3B through 3D). Cardiac dysfunction (as demonstrated by ejection fraction%) (Figure 3D) and fibrosis (as demonstrated by picrosirius red staining) (Figure 3B) in the SHR and SHR+isoproterenol models were also attenuated by paroxetine treatment. Consistently, the paroxetine‐treated groups displayed lower mRNA levels of cardiac hypertrophy marker (eg, MYH7) and fibrosis markers (eg, CTGF and collagen I) than the non‐paroxetine‐treated groups (Figure 3E) at the end of the experiment. Taken together, these data suggest that paroxetine treatment ameliorates hypertension‐induced cardiac hypertrophy, dysfunction and fibrosis.
Figure 3. Treatment with GRK2 (G protein‐coupled receptor kinase 2) inhibitor protects rats from isoproterenol‐ or hypertension‐induced cardiac hypertrophy.
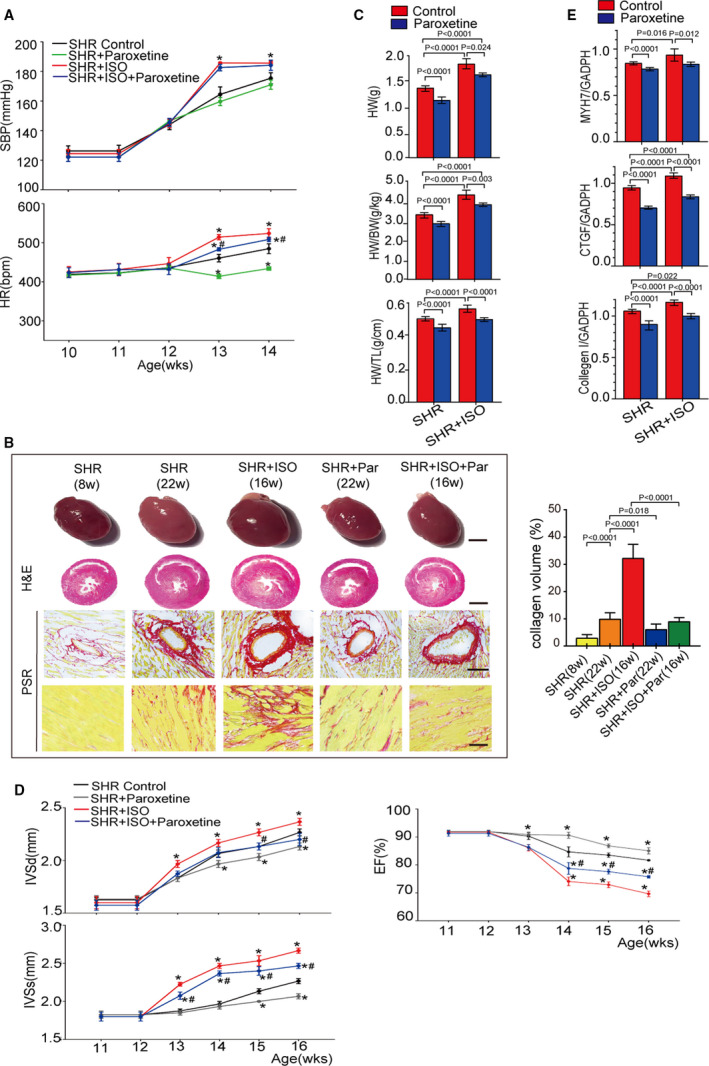
A, The dynamic change in systolic blood pressure (upper panel) and heart rate (lower panel) of spontaneously hypertensive rats (SHRs) and isoproterenol‐infused SHR (SHR+isoproterenol) or SHR and SHR+isoproterenol subjected to vehicle or paroxetine treatment (n=6 rats per group) at the age from 10 to 14 weeks. B, Left, overall comparison of representative whole‐heart tissue (first panel; scale bar, 1 cm), hematoxylin and eosin staining of whole‐heart left ventricular mid–papillary muscle plane sections (second panel; scale bar, 5 mm) and picrosirius red staining of the perivascular area (third panel; scale bar, 50 μm) and interstitial area (fourth panel; scale bar, 25 μm) in the indicated groups (n=6 rats per group). Right, quantitative results pertaining to left ventricular collagen volumes in the indicated groups (n≥20 fields per group). C, Comparison of the heart weight, heart weight to body weight ratio, and heart weight to tibia length ratio in different animal models (n=6 per group) at the age of 16 weeks (n=6 rats per group). D, Comparison of the echocardiographic parameters interventricular septal thickness at diastole, interventricular septal thickness in systole, ejection fraction and in the indicated groups at the age from 11 to 16 weeks (n=6 rats per group). E, Quantification results for real‐time polymerase chain reaction analysis of the hypertrophic marker (β‐myosin heavy chain) and fibrotic marker genes (collagen I and CTGF [connective tissue growth factor]) relative to GAPDH expression in the indicated groups (n=6 per group). Data shown are mean±SD (A through E). Data were first analyzed and passed normality test (Shapiro‐Wilk test in A through E). P values were shown and assessed by unpaired 2‐tailed t‐test (A and D) and by 1‐way ANOVA with Tukey test (B, C, and E). In (A and D), *P<0.05 (compared with the SHR group), # P<0.05 (compared with the SHR+isoproterenol group). BW indicates body weight; EF, ejection fraction; CTGF, connective tissue growth factor; H&E, hematoxylin and eosin; HR, heart rate; HW, heart weight; IVSd, interventricular septal thickness in diastole; IVSs, interventricular septal thickness in systole; MYH7, β‐myosin heavy chain; PRS, picrosirius red stain; SHR, spontaneously hypertensive rats; TL, tibia length; and WKY, Wistar‐Kyoto rats.
Paroxetine Suppresses Sympathetic Overdrive and Sensitizes Adrenergic Receptors in Hypertension
Catecholamines can increase GRK2 levels, in turn, reduce ADRB1 expression on cell surface by increasing ADRB1 phosphorylation and internalization. 14 To determine whether paroxetine therapy attenuates circulating catecholamine levels in hypertensive rat models, we measured this hormone levels in the circulation. As shown in Figure 4A through 4D, renin, norepinephrine, epinephrine, and aldosterone levels were significantly increased in SHRs at 22 weeks of age compared with Wistar‐Kyoto control rats or SHRs at 12 weeks of age. Treatment with paroxetine markedly suppressed the circulating levels of these hormones in SHRs at 22 weeks of age. Despite the pronounced blood pressure reductions in 22‐week‐old SHRs upon amlodipine administration (Figure S4), plasma epinephrine, norepinephrine, renin, and aldosterone concentrations were not altered in the amlodipine‐treated SHRs compared with the SHRs group. Thus, we hypothesized that paroxetine reduced catecholamine and sympathetic hormone levels by increasing ADRB1 sensitivity and turning down subsequent positive feedback regulation of hormone release in the context of hypertension. We further tested whether paroxetine treatment increases cardiac responses to catecholamine. SHRs treated with paroxetine for 4 weeks exhibited similar arterial blood pressures to but significantly lower heart rate than control SHR. Both epinephrine and isoproterenol venous infusion increased instantaneous blood pressure and heart rate in SHR, and paroxetine administration further potentiated these responses to catecholamines (Figure 4E). These data suggest that paroxetine suppresses circulating catecholamine and sympathetic hormone levels in the hypertension models and increases the adrenergic receptor response to catecholamines.
Figure 4. GRK2 (G protein‐coupled receptor kinase 2) inhibitor suppresses catecholamines‐releasing and increases ADRB1 (adrenergic receptor beta 1) sensitivity in spontaneously hypertensive rats (SHRs).
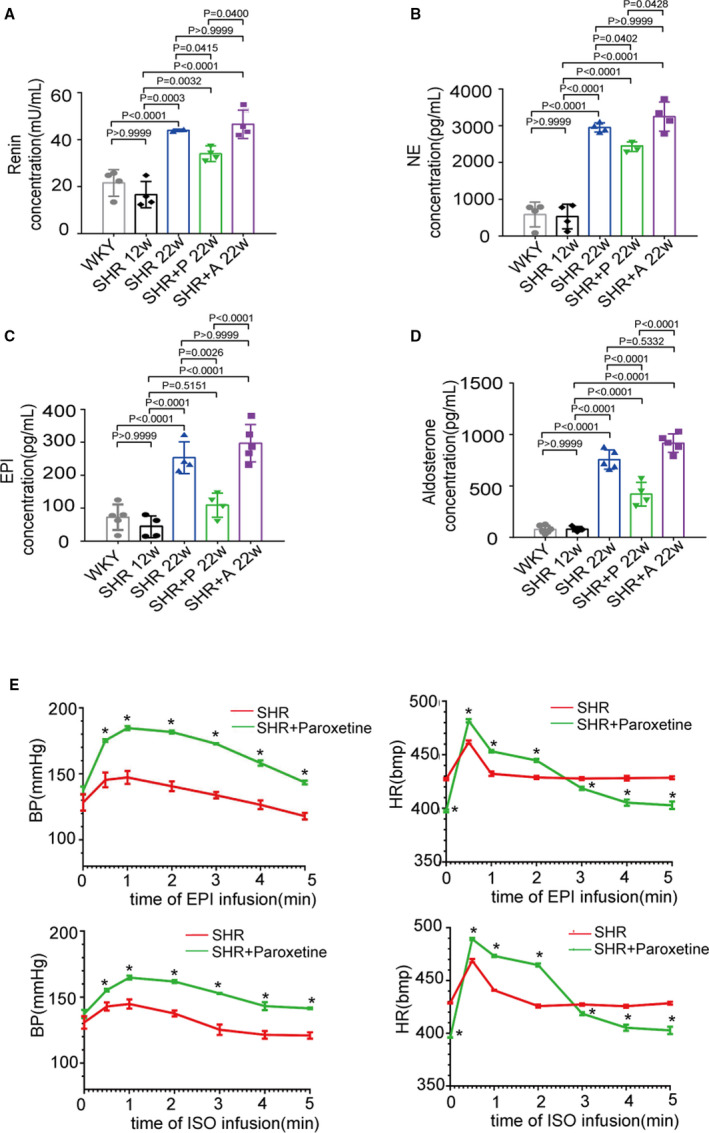
A through D, Renin, norepinephrine, epinephrine, aldosterone levels in serum was measured via ELISA kits (n=6 per group). Wistar‐Kyoto rat; SHR, SHR+paroxetine‐treated SHR (5 mg/kg per dayintragastrically), SHR+amlodipine‐treated SHR (3 mg/kg per day intragastrically). E, Dynamic changes of systolic blood pressure and heart rate after epinephrine or isoprenaline (2 mL, 1:100 000) infusion in SHR and paroxetine‐treated SHR (5 mg/kg per day intragastrically) at the age of 22 weeks. Data were recorded at 30 seconds, 1 minute, 2 minutes, 3 minutes, 4 minutes, and 5 minutes. Data shown are mean±SD (A through E). Data were first analyzed and passed normality test (Shapiro‐Wilk test in A through E). P values were shown and assessed by 1‐way ANOVA with Tukey test (A through D) and by unpaired 2‐tailed t‐test (E). In (E), *P<0.05 (compared with the SHR group). BP indicates blood pressure; HR, heart rate; SHR, spontaneously hypertensive rates; and WKY, Wistar‐Kyoto rats.
Paroxetine Attenuates Epinephrine‐Induced Cardiomyocyte Hypertrophy and ADRB1 Internalization Via GRK2 In Vitro
We further performed an in vitro experiment to test the effect of paroxetine on cardiac hypertrophic and fibrotic signaling in response to epinephrine. Exposure of cardiomyocytes to 0, 25, 50, 100, or 250 nmol/L epinephrine for 48 hours dose‐dependently increased the protein expression of GRK2, myocardial hypertrophy markers (ANP and BNP) and fibrosis markers (CTGF and collagen I) (Figure 5A). The epinephrine‐induced upregulation of the ANP, BNP, CTGF, and collagen I proteins in cardiomyocytes was markedly inhibited by paroxetine treatment (Figure 5A). Consistently, in AC16 cells, ad‐shGRK2 infection blocked the epinephrine‐induced increases in GRK2, BNP, and CTGF and collagen I protein expression (Figure 5B); in contrary, in the presence of epinephrine, lenti‐GRK2 infection augmented GRK2, BNP, CTGF, and collagen I protein expression compared with the respective control transfection (Figure 5C). Lentiviral manipulation of GRK2 expression also altered the expression of these proteins at baseline (Figure 5C). Similar results were obtained in experiments on H9C2 cells and primary rat cardiomyocytes (Figure S5). Immunofluorescence staining revealed that GRK2 transfection facilitated ADRB1 internalization under epinephrine stimulation in AC16 cells. Treatment with paroxetine inhibited the GRK2‐mediated ADRB1 internalization (Figure 5D), as evidenced by the plasmatic/membrane optical density ratio (Figure 5E). Via separating cardiomyocyte membrane from cytoplasm in SHR treated with or without paroxetine, western blots indicated paroxetine inhibited ADRB1 internalization (Figure 5F). These data suggest that paroxetine blocks epinephrine‐induced cardiomyocyte hypertrophy and fibrosis as well as ADRB1 internalization in cardiomyocytes.
Figure 5. GRK2 (G protein‐coupled receptor kinase 2) inhibitor attenuates epinephrine induced cardiomyocyte hypertrophy.
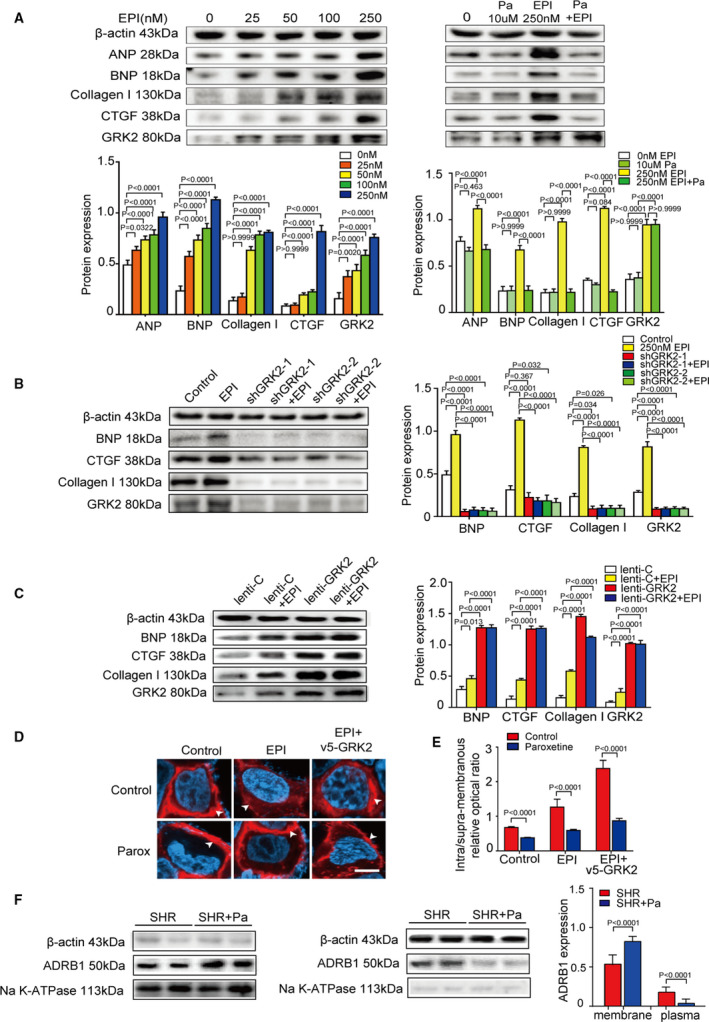
A, Representative immunoblots and quantification indicating that exposure to epinephrine dose‐dependently increase cardiomyocyte hypertrophy via the expression levels of myocardial hypertrophy markers (ANP [atrial natriuretic peptide] and BNP [B‐type natriuretic peptide]) and fibrosis markers (CTGF [connective tissue growth factor] and collagen I) in AC16 exposed to 0, 25, 50, 100, or 250 nmol/L epinephrine (left). Representative immunoblots and quantification results of the expression levels of β‐actin, ANP, BNP, CTGF, collagen I, and GRK2 in AC16 exposed to epinephrine with or without paroxetine (right). B, Immunoblots (left) and quantification (right) of expression levels of β‐actin, BNP, CTGF, collagen I, and GRK2 in cultured AC16 cells infected with the indicated recombinant adenovirus with or without stimulation by epinephrine (n=4 samples per group). C, Representative western blots and the bar graphs presenting the quantitative data for β‐actin, BNP, CTGF, collagen I, and GRK2 in the indicated groups (n=4 samples per group). D, Snapshots of immunostaining on ADRB1 (adrenergic receptor beta 1) (red) and DAPI (blue) stained AC16 cells infected with the indicated adenoviruses, epinephrine treatment or GRK2‐epinephrine treatment, with or without paroxetine. Proteins of interest appearing in red are indicated by white arrows (scale bar, 50 μm). E, Quantification of the average intra‐membranous/supra‐membranous optical density ratio of ADRB1 (n>50 cells per group). F, Representative western blots of protein expression levels of ADRB1 in cardiomyocyte membrane of spontaneously hypertensive rat (SHR) and paroxetine‐treated SHR (n=4 samples per group) (left); Representative western blots of protein expression levels of ADRB1 in cardiomyocyte plasm of SHR and paroxetine‐treated SHR (n=4 samples per group) (middle); Quantification results of the relative protein expression levels of ADRB1 (right). Data shown are mean±SD (A through C, E, and F). Data were first analyzed and passed normality test (Shapiro‐Wilk test in A through C, E, and F). P values were shown and assessed by one‐way ANOVA with Tukey test (A through C) and by Mann‐Whitney test (E and F). EPI indicates pinephrine; Pa, paroxetine; ADRB1, adrenergic receptor beta 1; ANP, atrial natriuretic peptide; BNP, B‐type natriuretic peptide; CTGF, connective tissue growth factor; and GRK2, G protein‐coupled receptor kinase 2.
Blockade of GRK2 by Paroxetine Potentiates the Effects of an ADRB1 Blocker in the Treatment of Hypertension and Cardiac Hypertrophy
Our data suggested that blockade of GRK2 by paroxetine reduced ADRB1 internalization and stabilized ADRB1 expression on the cell surface. Thus, we proposed that inhibition of GRK2 would potentiate anti‐hypertensive and cardiac protective effects of an ADRB1 blocker treatment. Intragastric administration of metoprolol significantly suppressed blood pressure and heart rate in SHR after 2 weeks of treatment. Coadministration of paroxetine further potentiated the metoprolol‐induced reductions in blood pressure and heart rate after the 4 and 2 weeks of treatment, respectively (Figure 6A). Coadministration of paroxetine and metoprolol attenuated hypertension‐induced cardiac hypertrophy (as indicated by HW, HW/BW, HW/TL, interventricular septal thickness in diastole, and interventricular septal thickness in systole) (Figure 6C and 6D), cardiac dysfunction (as indicated by ejection fraction%) (Figure 6D) and fibrosis (as indicated by picrosirius red staining) (Figure 6B) compared with metoprolol or paroxetine treatment alone in SHR at 22 weeks of age. Collectively, our data reveal a previously unappreciated role for GRK2 inhibition in the treatment of hypertension, especially in treatment regimens based on ADRB1 blockers.
Figure 6. GRK2 (G protein‐coupled receptor kinase 2) inhibitor promotes beta‐1 blockers (metoprolol) therapeutic effect in spontaneously hypertensive rat (SHR).
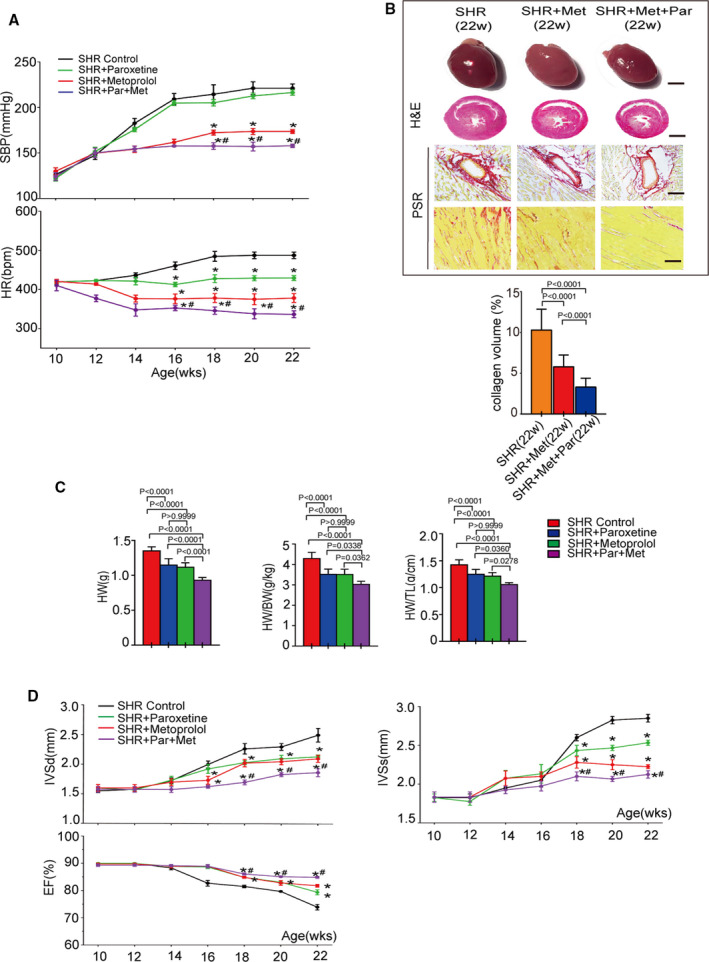
A, The dynamic change in systolic blood pressure (upper panel) and heart rate (lower panel) of SHR and SHR treated with paroxetine, metoprolol or both (n=6 rats per group) at the age from 10 to 22 weeks. B, Up, overall comparison of representative whole‐heart tissue (first panel; scale bar, 1 cm), hematoxylin and eosin staining of whole‐heart left ventricular mid–papillary muscle plane sections (second panel; scale bar, 5 mm) and picrosirius red staining of the perivascular area (third panel; scale bar, 50 μm) and interstitial area (fourth panel; scale bar, 25 μm) in the indicated groups (n=6 rats per group). Bottom, quantitative results pertaining to LV collagen volumes in the indicated groups (n≥20 fields per group). C, Comparison of the heart weight, heart weight to body weight ratio and heart weight to tibia length ratio in the indicated groups (n=6 per group) at the age of 22 weeks (n=6 rats per group). D, Comparison of the echocardiographic parameters interventricular septal thickness at diastole, interventricular septal thickness in systole, ejection fraction in the indicated groups at the age from 10 to 22 weeks (n=6 rats per group). Data shown are mean±SD (A through D). Data were first analyzed and passed normality test (Shapiro‐Wilk test in A through D). P values were shown and assessed by unpaired 2‐tailed t‐test (A and D) and by 1‐way ANOVA with Tukey test (B and C). In (A and D), *P<0.05 (compared with the SHR group), # P<0.05 (compared with the SHR+metoprolol group). BW indicates body weight; EF, ejection fraction; CTGF, connective tissue growth factor; H&E, hematoxylin and eosin; HR, heart rate; HW, heart weight; IVSd, interventricular septal thickness in diastole; IVSs, interventricular septal thickness in systole; PRS, picrosirius red stain; SHR, spontaneously hypertensive rats; and TL, tibia length.
Paroxetine Treatment May Have Cardiovascular Benefits for Hypertensive Patients Accompanies With Depression
Finally, we explored the clinical relevance of paroxetine treatment as a beneficial therapeutic strategy for patients with hypertension accompanies with depression. Thirty‐two patients with hypertension accompanied with depression were recruited from January 2017 to March 2019. Sixteen patients were treated with paroxetine for depression, and the remaining patients were treated with antidepressants other than selective serotonin reuptake inhibitors. The inclusion and exclusion criteria are shown in Data S1. All patients were in the stable stages of antidepressant and antihypertensive treatment and did not present with severe cardiovascular complications, metabolic diseases or malignant diseases. The clinical characteristics of the patients were comparable between the paroxetine group and the non‐paroxetine group (Table 1). Cardiac ultrasonography revealed that cardiac hypertrophy (interventricular septal thickness in diastole and end‐diastolic left ventricular posterior wall depth) was more pronounced in non‐paroxetine‐treated patients than in paroxetine‐treated patients (Table 2). These preliminary data indicate that paroxetine treatment may have a beneficial effect on cardiac remodeling in patients with hypertension accompanies with depression.
Table 1.
Clinical Characteristics of Patients With Depression and Hypertension
| Clinical Characteristics |
With Paroxetine (n=16) |
Without Paroxetine (n=16) |
P Value |
|---|---|---|---|
| Men, n | 8 | 8 | >0.999* |
| Women, n | 8 | 8 | >0.999* |
| Age, y | 53.63±7.96 | 53.13±5.99 | 0.837 † |
| Year of hypertension, y | 5.94±2.17 | 5.66±2.55 | 0.742 † |
| BMI, kg/m2 | 25.31±5.47 | 26.21±6.81 | 0.560 † |
| SBP, mm Hg | 128.94±12.91 | 131.88±13.56 | 0.536 † |
| DBP, mm Hg | 83.69±7.10 | 84.81±8.83 | 0.691 † |
| ALT, U/L | 19.83±5.49 | 16.51±7.73 | 0.173 † |
| AST, U/L | 18.73±3.14 | 21.45±5.52 | 0.104 † |
| ALB, g/L | 37.10±4.11 | 34.55±6.43 | 0.189 † |
| Scr, μmol/L | 77.96±15.70 | 71.42±17.28 | 0.271 † |
| BUN, mmol/L | 5.89±2.49 | 4.45±1.62 | 0.062 † |
| Triglyceride, mmol/L | 1.84±0.68 | 2.21±0.84 | 0.188 † |
| HDL‐C, mmol/L | 1.41±0.24 | 1.57±0.49 | 0.252 † |
| LDL‐C, mmol/L | 2.86±0.73 | 2.55±0.66 | 0.213 † |
| GLU, mmol/L | 4.85±0.61 | 4.60±0.84 | 0.351 † |
| CCB, n | 6 | 6 | >0.999* |
| ACEI/ARB, n | 4 | 4 | >0.999* |
| Beta‐blocker, n | 6 | 6 | >0.999* |
Data are presented as mean±SD. ACEI/ARB indicates angiotensin‐converting enzyme inhibitors/angiotensin receptor blocker; ALB, serum albumin; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; BUN, urea nitrogen; CCB, calcium channel blocker; DBP, diastolic blood pressure; GLU, blood glucose; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; SBP, systolic blood pressure; and Scr, serum creatinine.
Chi‐square test.
Paired t‐test.
Table 2.
Association Between the Using of Paroxetine and the Cardiac Hypertrophy
| Index |
With Paroxetine (n=16) |
Without Paroxetine (n=16) |
P Value |
|---|---|---|---|
| LVEDd, mm | 47.69±2.55 | 49.38±2.76 | 0.083* |
| IVSd, mm | 10.21±0.77 | 11.06±0.95 | 0.038* |
| LVPWd, mm | 10.31±0.81 | 11.11±0.83 | 0.024* |
| EF, % | 64.31±4.17 | 61.56±2.90 | 0.041* |
Data are presented as mean±SD. LVEDd indicates left ventricular end‐diastolic dimension; EF, ejection fraction; IVSd, interventricular septal thickness in diastole; and LVPWd, end‐diastolic left ventricular posterior wall depth.
Paired t‐test.
Discussion
ADRB1 is a receptor of many catecholamines that modulates cardiac output and HR, which has great implication in the pathophysiology of hypertension. In this study, we found that the expression levels of GRK2 and ADRB1 in myocardium are interrelated and also correlated with blood pressure levels in patients with hypertension. In vitro evidence showed a direct interaction in ADRB1 and GRK2 protein and genetic depletion of GRK2 expression blocks epinephrine‐induced upregulation of cardiomyocyte hypertrophic and fibrotic genes in cardiomyocytes. Further, we identified paroxetine as a potent inhibitor of GRK2 blocking its interaction with ADRB1 and cardiac hypertrophy. These beneficial effects of paroxetine were mediated primarily through inhibiting GRK2 associated ADRB1 internalization and improving its sensitivity to catecholamine hormone, thereby, circulating catecholamine and sympathetic hormone levels decreased and cardiac hypertrophy and fibrosis relieved in patients with hypertension (Figure 7).
Figure 7. Schematic illustration indicating GRK2 (G protein‐coupled receptor kinase 2) is associated with ADRB1 (adrenergic receptor beta 1) internalization and cardiac hypertrophy.
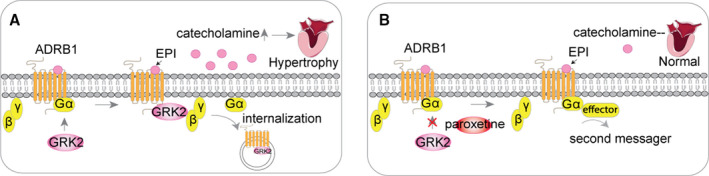
A, In hypertension, ADRB1 is activated via interacting with circulating catecholamines, eg, epinephrine. GRK2 participates in the regulation of ADRB1 by triggering ADRB1 desensitization from G proteins complex and internalization, thus contributing to signal propagation. B, Paroxetine suppresses ADRB1 internalization via functioning as the GRK2 inhibitor, thus enhances the stabilization and activity of ADRB1 on the surface of cardiomyocytes. Therefore, catecholamines in circulation decreases and reduces the stimulation of cardiomyocytes resulting in alleviation of cardiac hypertrophy. ADRB1 indicates adrenergic receptors beta 1; and GRK2, G‐protein‐coupled receptor kinase 2.
The human ADRB1, an important therapeutic target in cardiovascular diseases, and its polymorphisms impact cardiovascular disease risks and pharmacological response to ADRB1 blockers. 15 , 16 Gene regulation technologies to specifically change the expression of myocardial ADRB1 have recently gained attention, and related studies have revealed that changes in myocardial ADRB1 expression regulate blood pressure to protect against end organ damage through diverse pathways. 3 , 17 , 18 Our study found that myocardial and PBMC ADRB1 and GRK2 expressions in patients were positively correlated. Studies have shown that cardiac GRK2 is a major factor regulating inotropic and lusitropic tachyphylaxis to catecholamines, thereby contributing to its cardioprotective effects. 19 Cardiac‐specific GRK2 ablation mice had better prognosis after myocardial infarction compared with wild‐type. 20 Those findings suggest the inhibition of GRK2 in the myocardium may be beneficial in many cardiovascular diseases.
Other researchers have shown that the selective serotonin reuptake inhibitors paroxetine play a protective role in maintaining cardiac function in heart failure and acute myocardial infarction by inhibiting GRK2 activity. In the current study, we provide new evidence demonstrating that paroxetine attenuates hypertension associated cardiac hypertrophy, which has been proven to be tightly associated adverse cardiovascular events. Previous studies demonstrated that selective serotonin reuptake inhibitors have pleomorphic effects which may be beneficial for cardiac remodeling including decreased vasomotor tone and anti‐adrenergic effect. 21 , 22 , 23 , 24 In our study, we showed the positive effects of paroxetine on blocking GRK2‐ADRB1 interaction but it cannot be ruled out that paroxetine may influence cardiac remodeling by acting as selective serotonin reuptake inhibitors in animal models. However, our in vitro results showed that paroxetine attenuates epinephrine‐induced cardiomyocyte hypertrophy and ADRB1 internalization via GRK2 without 5‐hydroxytryptamine (5‐HT). It is also reported that paroxetine potentiated cardiac contraction while fluoxetine had no effects. 25 , 26 , 27 And another study also believes that paroxetine considerably improves left ventricular function and structure because of its ability to inhibit GRK2 instead of SSR. 7 Also, ADRB1 involves in the main pathological process of cardiac remodeling. 28 Our study reveals the role of paroxetine in cardiac remodeling as a GRK2 inhibitor via sensitizing ADRB1. The complex pathophysiological interactions between the catecholamine and sympathetic hormone and the importance of their modulation in the development, progression, and treatment of cardiovascular disease were well documented. Neuroendocrine modulation have become the cornerstone of medical therapy for chronic cardiovascular disease to reduce morbidity and mortality. 29 Our study discovered the effect of paroxetine in organ protection and attenuating circulating catecholamine and sympathetic hormone levels in patients with hypertension independent of the function on blood pressure control, which may provide promising new avenue for neuroendocrine modulation in cardiovascular disease.
Previous study also indicated that some stimulations stimulate the recruitment of GRK2 to ADRB2, which induces subsequently ADRB2 internalization. 30 Since previous studies have clarified the role of ADRB2 and GRK2, we did not explore the role of paroxetine targeting on ADRB2 in‐depth. 31 , 32 , 33 Different from previous studies, our finding suggested that paroxetine also sympathetic activation‐induced cardiac hypertrophy via ADRB1 internalization and sensitizing ADRB1. Also, ADRB1 blockers are widely used clinically. Therefore, based on the mechanism of paroxetine mediating ADRB1 desensitization, we speculate that there is a possibility of drug interaction between paroxetine and ADRB1 blockers, which has the guiding significance in clinical medication. Studies demonstrated that there were responders and non‐responders to beta‐blocker therapy in patients with heart failure and hypertension. 34 , 35 Human genes encoding drug response related proteins (including drug transporters, drug‐metabolizing enzymes, drug receptors, etc) have polymorphism, which may lead to changes in their functions of encoding corresponding proteins. 36 Studies about the effect of these genetic polymorphisms on the pharmacokinetics of drug response also provide explanation for the individual differences in drug response. 37 It is confirmed that polymorphism of the ADRB1 gene affects agonist‐promoted trafficking, with the Arg389 receptor form greater agonist‐mediated adenylyl cyclase activities. 38 According to our study, we postulate that GRK2 facilitates ARDB1 internalization under catecholamine stimulation in cardiomyocytes, which may lead to a low respond to ADRB1‐blocker such as metoprolol. Therefore, paroxetine functioning as GRK2 inhibitor may suppress ADRB1 internalization, thus enhancing the stabilization and activity of ADRB1 to improve the therapeutic effect of ADRB1 blocker on insensitive individuals.
Administration of isoproterenol can cause severe stress in the myocardium attributable to the activation of adrenergic system and other neurohumoral systems. 39 Earlier studies showed that repeated stimulation of beta‐adrenergic receptors by isoproterenol did not cause or expand myocardial necrosis, but myocardial resistance or adaptation against isoproterenol was developed. 40 , 41 This finding is not clearly explained yet, but according to our study, it could be related to internalization or downregulation of ADRB1. The long‐term response of ADRB1 to isoproterenol infusion needs more studies. Similarly, our results showed that the BP and HR changes to acute epinephrine or isoproterenol infusion are more obvious in SHR treated with paroxetine, which could be explained by paroxetine‐inhibited ADRB1 desensitization. Also, previous studies claimed that beta‐adrenergic receptor‐induced cardiac hypertrophy is mainly caused by the ADRB1 stimulation. 42 , 43
GRK2 is highly expressed in many tissues, including PBMCs, human visceral adipocytes, endothelial cells and liver tissue. 44 Previous studies have found that GRK2 is involved in activation of the Akt/endothelial nitric oxide synthase pathway associated with endothelial dysfunction in the aorta. 45 Chronic cardiac dysfunction and hypertrophy may involve catecholamines stimulating beta‐adrenergic receptors on cardiomyocytes or the Renin‐Angiotensin‐Aldosterone System (RAAS) activation. It has been proven that GRK2 interacts with Gαq and Gβγ at the plasma membrane. 46 With Gαq promoting cardiac hypertrophy and catecholamines being potent stimulators of Gαq via respective GPCRs, GRK2 promotes cardiac hypertrophy in response to GPCR stimulation. 47 Additionally, various additional non–GPCR‐related effects of GRK2 have been discovered, including promotion of insulin resistance, alterations in L‐type calcium channel Ca2+ handling and proapoptotic effects on mitochondria. 48 Thus, paroxetine, as a GRK2 inhibitor, may have additional systemic effect and needs to be further verified.
In conclusion, our present study provides in vitro and in vivo evidence that paroxetine promotes ADRB1 sensitivity and attenuates cardiac hypertrophy partially via blocking GRK2 mediated ADRB1 activation and internalization in the context of hypertension. This evidence implicates GRK2 as a novel therapeutic target for reducing circulating catecholamine and sympathetic hormone levels and the treatment of pathological cardiac hypertrophy.
Sources of Funding
This work was supported in part by the National Natural Science Foundation of China 81870171 (to Cai), 81770403, 81974054 (to Yuan), 81800393 (to Lu), the National Key Research and Development Projects 2019YFF0216305 (to Cai), and 2016YFC0900802 (to Yuan), the Hunan Distinguished Young Scholars 2018JJ1048 (to Cai).
Disclosures
None.
Supporting information
Data S1
Tables S1–S8
Figures S1–S5
(J Am Heart Assoc. 2021;10:e016364. DOI: 10.1161/JAHA.120.016364.)
For Sources of Funding and Disclosures, see page 18.
Contributor Information
Yao Lu, Email: luyao0719@163.com.
Jingjing Cai, Email: caijingjing83@hotmail.com.
References
- 1. Writing Group M , Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després J‐P, Fullerton HJ, et al. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. [DOI] [PubMed] [Google Scholar]
- 2. Norton GR, Woodiwiss AJ, Gaasch WH, Mela T, Chung ES, Aurigemma GP, Meyer TE. Heart failure in pressure overload hypertrophy. The relative roles of ventricular remodeling and myocardial dysfunction. J Am Coll Cardiol. 2002;39:664–671. [DOI] [PubMed] [Google Scholar]
- 3. Huang Y, Liu XL, Wen J, Huang LH, Lu Y, Miao RJ, Liu X, Li Y, Xing XW, Yuan H. Downregulation of the beta1 adrenergic receptor in the myocardium results in insensitivity to metoprolol and reduces blood pressure in spontaneously hypertensive rats. Mol Med Rep. 2017;15:703–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kl A, Oster L, Cardinal R, de Champlain J. Effects of renin‐angiotensin blockade on sympathetic reactivity and beta‐adrenergic pathway in the spontaneously hypertensive rat. Hypertension. 1997;30:278–287. [DOI] [PubMed] [Google Scholar]
- 5. Shahin MH, Conrado DJ, Gonzalez D, Gong Y, Lobmeyer MT, Beitelshees AL, Boerwinkle E, Gums JG, Chapman A, Turner ST, et al. Genome‐wide association approach identified novel genetic predictors of heart rate response to beta‐blockers. J Am Heart Assoc. 2018;7:e006463. DOI: 10.1161/JAHA.117.006463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fleg JL, Schulman S, O'Connor F, Becker LC, Gerstenblith G, Clulow JF, Renlund DG, Lakatta EG. Effects of acute beta‐adrenergic receptor blockade on age‐associated changes in cardiovascular performance during dynamic exercise. Circulation. 1994;90:2333–2341. [DOI] [PubMed] [Google Scholar]
- 7. Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, Tesmer JJ, Koch WJ. Paroxetine‐mediated GRK2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7:277ra231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tang CH, Wang CC, Chen TH, Hong CY, Sue YM. Prognostic benefits of carvedilol, bisoprolol, and metoprolol controlled release/extended release in hemodialysis patients with heart failure: a 10‐year cohort. J Am Heart Assoc. 2016;5:e002584. DOI: 10.1161/JAHA.115.002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lohse MJ, Benovic JL, Codina J, Caron MG, Lefkowitz RJ. beta‐Arrestin: a protein that regulates beta‐adrenergic receptor function. Science. 1990;248:1547–1550. [DOI] [PubMed] [Google Scholar]
- 10. Cannavo A, Komici K, Bencivenga L, D'Amico ML, Gambino G, Liccardo D, Ferrara N, Rengo G. GRK2 as a therapeutic target for heart failure. Expert Opin Ther Targets. 2018;22:75–83. [DOI] [PubMed] [Google Scholar]
- 11. Benovic JL, Strasser RH, Caron MG, Lefkowitz RJ. Beta‐adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist‐occupied form of the receptor. Proc Natl Acad Sci USA. 1986;83:2797–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang Q, Liu Y, Fu Q, Xu B, Zhang Y, Kim S, Tan R, Barbagallo F, West T, Anderson E, et al. Inhibiting insulin‐mediated beta2‐adrenergic receptor activation prevents diabetes‐associated cardiac dysfunction. Circulation. 2017;135:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yeh JL, Hsu JH, Wu PJ, Liou SF, Liu CP, Chen IJ, Wu BN, Dai ZK, Wu JR. KMUP‐1 attenuates isoprenaline‐induced cardiac hypertrophy in rats through NO/cGMP/PKG and ERK1/2/calcineurin A pathways. Br J Pharmacol. 2010;159:1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bristow MR, Ginsburg R, Minobe W, Cubicciotti RS, Sageman WS, Lurie K, Billingham ME, Harrison DC, Stinson EB. Decreased catecholamine sensitivity and beta‐adrenergic‐receptor density in failing human hearts. N Engl J Med. 1982;307:205–211. [DOI] [PubMed] [Google Scholar]
- 15. Potnuri AG, Allakonda L, Appavoo A, Saheera S, Nair RR. Association of histamine with hypertension‐induced cardiac remodeling and reduction of hypertrophy with the histamine‐2‐receptor antagonist famotidine compared with the beta‐blocker metoprolol. Hypertens Res. 2018;41:1023–1035. [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Liu ZQ, Yu BN, Xu FH, Mo W, Zhou G, Liu YZ, Li Q, Zhou HH. beta1‐Adrenergic receptor polymorphisms influence the response to metoprolol monotherapy in patients with essential hypertension. Clin Pharmacol Ther. 2006;80:23–32. [DOI] [PubMed] [Google Scholar]
- 17. Altara R, Booz GW. Deleting vascular ADAM17 sheds new light on hypertensive cardiac hypertrophy. Hypertension. 2016;68:849–850. [DOI] [PubMed] [Google Scholar]
- 18. Mialet Perez J, Rathz DA, Petrashevskaya NN, Hahn HS, Wagoner LE, Schwartz A, Dorn GW, Liggett SB. Beta 1‐adrenergic receptor polymorphisms confer differential function and predisposition to heart failure. Nat Med. 2003;9:1300–1305. [DOI] [PubMed] [Google Scholar]
- 19. Rengo G, Pagano G, Paolillo S, de Lucia C, Femminella GD, Liccardo D, Cannavo A, Formisano R, Petraglia L, Komici K, et al. Impact of diabetes mellitus on lymphocyte GRK2 protein levels in patients with heart failure. Eur J Clin Invest. 2015;45:187–195. [DOI] [PubMed] [Google Scholar]
- 20. Raake PW, Zhang X, Vinge LE, Brinks H, Gao E, Jaleel N, Li Y, Tang M, Most P, Dorn GW II, et al. Cardiac G‐protein‐coupled receptor kinase 2 ablation induces a novel Ca2+ handling phenotype resistant to adverse alterations and remodeling after myocardial infarction. Circulation. 2012;125:2108–2118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Powell JM, Ebin E, Borzak S, Lymperopoulos A, Hennekens CH. Hypothesis: paroxetine, a G protein‐coupled receptor kinase 2 (GRK2) inhibitor reduces morbidity and mortality in patients with heart failure. J Cardiovasc Pharmacol Ther. 2017;22:51–53. [DOI] [PubMed] [Google Scholar]
- 22. van Melle JP, Buikema H, van den Berg MP, van Buiten A, van Veldhuisen DJ, Boonstra PW, van Gilst WH. Sertraline causes strong coronary vasodilation: possible relevance for cardioprotection by selective serotonin reuptake inhibitors. Cardiovasc Drugs Ther. 2004;18:441–447. [DOI] [PubMed] [Google Scholar]
- 23. McFarlane A, Kamath MV, Fallen EL, Malcolm V, Cherian F, Norman G. Effect of sertraline on the recovery rate of cardiac autonomic function in depressed patients after acute myocardial infarction. Am Heart J. 2001;142:617–623. [DOI] [PubMed] [Google Scholar]
- 24. Almuwaqqat Z, Jokhadar M, Norby FL, Lutsey PL, O'Neal WT, Seyerle A, Soliman EZ, Chen LY, Bremner JD, Vaccarino V, et al. Association of antidepressant medication type with the incidence of cardiovascular disease in the ARIC Study. J Am Heart Assoc. 2019;8:e012503. DOI: 10.1161/JAHA.119.012503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, et al. Paroxetine is a direct inhibitor of G protein‐coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol. 2012;7:1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kemp AH, Fráguas R, Brunoni AR, Bittencourt MS, Nunes MA, Dantas EM, Andreão RV, Mill JG, Ribeiro ALP, Koenig J, et al. Differential associations of specific selective serotonin reuptake inhibitors with resting‐state heart rate and heart rate variability: implications for health and well‐being. Psychosom Med. 2016;78:810–818. [DOI] [PubMed] [Google Scholar]
- 27. Kemp AH, Quintana DS. The relationship between mental and physical health: insights from the study of heart rate variability. Int J Psychophysiol. 2013;89:288–296. [DOI] [PubMed] [Google Scholar]
- 28. Dorn GW II. Adrenergic pathways and left ventricular remodeling. J Card Fail. 2002;8:S370–S373. [DOI] [PubMed] [Google Scholar]
- 29. Bucciarelli V, Caterino AL, Bianco F, Caputi CG, Salerni S, Sciomer S, Maffei S, Gallina S. Depression and cardiovascular disease: the deep blue sea of women's heart. Trends Cardiovasc Med. 2019;30:170–176. [DOI] [PubMed] [Google Scholar]
- 30. Fu Q, Xu B, Parikh D, Cervantes D, Xiang YK. Insulin induces IRS2‐dependent and GRK2‐mediated beta2AR internalization to attenuate betaAR signaling in cardiomyocytes. Cell Signal. 2015;27:707–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wang Y, De Arcangelis V, Gao X, Ramani B, Jung YS, Xiang Y. Norepinephrine‐ and epinephrine‐induced distinct beta2‐adrenoceptor signaling is dictated by GRK2 phosphorylation in cardiomyocytes. J Biol Chem. 2008;283:1799–1807. [DOI] [PubMed] [Google Scholar]
- 32. Liu R, Ramani B, Soto D, De Arcangelis V, Xiang Y. Agonist dose‐dependent phosphorylation by protein kinase A and G protein‐coupled receptor kinase regulates beta2 adrenoceptor coupling to G(i) proteins in cardiomyocytes. J Biol Chem. 2009;284:32279–32287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhu W, Petrashevskaya N, Ren S, Zhao A, Chakir K, Gao E, Chuprun JK, Wang Y, Talan M, Dorn GW II, et al. Gi‐biased beta2AR signaling links GRK2 upregulation to heart failure. Circ Res. 2012;110:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Marquez MF, Hernandez‐Pacheco G, Hermosillo AG, Gomez JR, Cardenas M, Vargas‐Alarcon G. The Arg389Gly beta1‐adrenergic receptor gene polymorphism and susceptibility to faint during head‐up tilt test. Europace. 2007;9:585–588. [DOI] [PubMed] [Google Scholar]
- 35. Feely J, Crooks J, Stevenson IH. The influence of age, smoking and hyperthyroidism on plasma propranolol steady state concentration. Br J Clin Pharmacol. 1981;12:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vesell ES. Advances in pharmacogenetics and pharmacogenomics. J Clin Pharmacol. 2000;40:930–938. [DOI] [PubMed] [Google Scholar]
- 37. White RM Sr, Wong SH. Pharmacogenomics and its applications. MLO Med Lab Obs. 2005;37:20–27. [PubMed] [Google Scholar]
- 38. Mason DA, Moore JD, Green SA, Liggett SB. A gain‐of‐function polymorphism in a G‐protein coupling domain of the human beta1‐adrenergic receptor. J Biol Chem. 1999;274:12670–12674. [DOI] [PubMed] [Google Scholar]
- 39. Nichtova Z, Novotova M, Kralova E, Stankovicova T. Morphological and functional characteristics of models of experimental myocardial injury induced by isoproterenol. Gen Physiol Biophys. 2012;31:141–151. [DOI] [PubMed] [Google Scholar]
- 40. Turek Z, Kalus M, Poupa O. The effect of isoprenaline pretreatment on the size of acute myocardial necrosis induced by the same drug. Physiol Bohemoslov. 1966;15:353–356. [PubMed] [Google Scholar]
- 41. Moalic JM, Charlemagne D, Mansier P, Chevalier B, Swynghedauw B. Cardiac hypertrophy and failure—a disease of adaptation. Modifications in membrane proteins provide a molecular basis for arrhythmogenicity. Circulation. 1993;87:IV21–IV26. [PubMed] [Google Scholar]
- 42. Morisco C, Zebrowski DC, Vatner DE, Vatner SF, Sadoshima J. Beta‐adrenergic cardiac hypertrophy is mediated primarily by the beta(1)‐subtype in the rat heart. J Mol Cell Cardiol. 2001;33:561–573. [DOI] [PubMed] [Google Scholar]
- 43. Schafer M, Frischkopf K, Taimor G, Piper HM, Schluter KD. Hypertrophic effect of selective beta(1)‐adrenoceptor stimulation on ventricular cardiomyocytes from adult rat. Am J Physiol Cell Physiol. 2000;279:C495–C503. [DOI] [PubMed] [Google Scholar]
- 44. Garcia‐Guerra L, Nieto‐Vazquez I, Vila‐Bedmar R, Jurado‐Pueyo M, Zalba G, Diez J, Murga C, Fernandez‐Veledo S, Mayor F Jr, Lorenzo M. G protein‐coupled receptor kinase 2 plays a relevant role in insulin resistance and obesity. Diabetes. 2010;59:2407–2417. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45. Takayanagi T, Kawai T, Forrester SJ, Obama T, Tsuji T, Fukuda Y, Elliott KJ, Tilley DG, Davisson RL, Park J‐Y, et al. Role of epidermal growth factor receptor and endoplasmic reticulum stress in vascular remodeling induced by angiotensin II. Hypertension. 2015;65:1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tesmer VM, Kawano T, Shankaranarayanan A, Kozasa T, Tesmer JJ. Snapshot of activated G proteins at the membrane: the Galphaq‐GRK2‐Gbetagamma complex. Science. 2005;310:1686–1690. [DOI] [PubMed] [Google Scholar]
- 47. Cannavo A, Liccardo D, Eguchi A, Elliott KJ, Traynham CJ, Ibetti J, Eguchi S, Leosco D, Ferrara N, Rengo G, et al. Myocardial pathology induced by aldosterone is dependent on non‐canonical activities of G protein‐coupled receptor kinases. Nat Commun. 2016;7:10877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chen M, Sato PY, Chuprun JK, Peroutka RJ, Otis NJ, Ibetti J, Pan S, Sheu SS, Gao E, Koch WJ. Prodeath signaling of G protein‐coupled receptor kinase 2 in cardiac myocytes after ischemic stress occurs via extracellular signal‐regulated kinase‐dependent heat shock protein 90‐mediated mitochondrial targeting. Circ Res. 2013;112:1121–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Malchiodi EL, Eisenstein E, Fields BA, Ohlendorf DH, Schlievert PM, Karjalainen K, Mariuzza RA. Superantigen binding to a T cell receptor beta chain of known three‐dimensional structure.. Journal of Experimental Medicine. 1995;182:1833–1845. DOI: 10.1084/jem.182.6.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Tables S1–S8
Figures S1–S5


