Abstract
Background
The Dietary Approaches to Stop Hypertension (DASH) diet improves blood pressure in nonpregnant populations. We hypothesized that adherence to the DASH diet during pregnancy improves hemodynamic adaptations, leading to a lower risk of gestational hypertensive disorders.
Methods and Results
We examined whether the DASH diet score was associated with blood pressure, placental hemodynamics, and gestational hypertensive disorders in a population‐based cohort study among 3414 Dutch women. We assessed DASH score using food‐frequency questionnaires. We measured blood pressure in early‐, mid‐, and late pregnancy (medians, 95% range: 12.9 [9.8–17.9], 20.4 [16.6–23.2], 30.2 [28.6–32.6] weeks gestation, respectively), and placental hemodynamics in mid‐ and late pregnancy (medians, 95% range: 20.5 [18.7–23.1], 30.4 [28.5–32.8] weeks gestation, respectively). Information on gestational hypertensive disorders was obtained from medical records. Lower DASH score quartiles were associated with a higher mid pregnancy diastolic blood pressure, compared with the highest quartile (P<0.05). No associations were present for early‐ and late pregnancy diastolic blood pressure and systolic blood pressure throughout pregnancy. Compared with the highest DASH score quartile, the lower DASH score quartiles were associated with a higher mid‐ and late pregnancy umbilical artery pulsatility index (P≤0.05) but not with uterine artery resistance index. No associations with gestational hypertensive disorders were present.
Conclusions
A higher DASH diet score is associated with lower mid pregnancy diastolic blood pressure and mid‐ and late pregnancy fetoplacental vascular function but not with uteroplacental vascular function or gestational hypertensive disorders within a low‐risk population. Further studies need to assess whether the effects of the DASH diet on gestational hemodynamic adaptations are more pronounced among higher‐risk populations.
Keywords: blood pressure, Dietary Approaches to Stop Hypertension, gestational hypertension, gestational hypertensive disorders, preeclampsia
Subject Categories: Epidemiology, Hemodynamics, Pregnancy, Preeclampsia
Nonstandard Abbreviations and Acronyms
- DASH
Dietary Approaches to Stop Hypertension
- FFQ
food frequency questionnaire
- UmPI
umbilical artery pulsatility index
- UtRI
uterine artery resistance index
Clinical Perspective
What Is New?
In a low‐risk population, maternal adherence to the Dietary Approaches to Stop Hypertension diet during pregnancy is associated with a lower mid pregnancy diastolic blood pressure and tends to be associated with improved fetoplacental vascular function.
Maternal adherence to the Dietary Approaches to Stop Hypertension diet is not associated with uteroplacental vascular function or the risk of gestational hypertensive disorders in this low‐risk pregnant population.
What Are the Clinical Implications?
Our findings suggest that the Dietary Approaches to Stop Hypertension diet might have small positive effects on gestational hemodynamic adaptations in low‐risk pregnant populations.
These findings are important from an etiological perspective and on a population level.
The beneficial effects may be more pronounced in pregnant populations with high a priori risk of developing gestational hypertensive disorders.
Gestational hypertensive disorders affects up to 10% of pregnancies and are a major risk factor for maternal and neonatal morbidity and mortality. 1 In nonpregnant populations, dietary interventions have been identified as an important strategy to reduce hypertension. The Dietary Approaches to Stop Hypertension (DASH) diet is a diet high in fruits, vegetables, total grains, nuts, seeds, legumes, and non‐full‐fat dairy products and low in animal protein, sugar, and sodium. 2 Multiple observation and intervention studies have shown that adherence to the DASH diet leads to lower blood pressure levels and improves lipid profile and fasting glucose concentrations in nonpregnant adult populations. 3 , 4 , 5 , 6 , 7
Not much is known about the influence of maternal adherence to the DASH diet during pregnancy on gestational hemodynamic adaptations or the risk of gestational hypertensive disorders. Recently, a study among 511 pregnant women from Ireland showed that higher adherence to the DASH diet was associated with a lower diastolic blood pressure and mean arterial pressure in early‐ and late pregnancy. 8 An intervention study in China among 85 pregnant women diagnosed with preexistent hypertension or gestational hypertension (ie, developed <28 weeks of gestation) showed a lower incidence of preeclampsia in the group adhering to the DASH diet. 9 In contrast, 2 observational studies among 1760 American and 66 651 Danish women showed no associations of maternal adherence to the DASH diet with the risks of gestational hypertension or preeclampsia. 10 , 11
We hypothesized that maternal adherence to the DASH diet may improve maternal hemodynamic adaptations in pregnancy, leading to lower risks of gestational hypertensive disorders. 12 , 13 , 14 , 15 Therefore, we examined within a population‐based cohort study among 3414 low‐risk pregnant women, the associations of maternal DASH diet score with systolic and diastolic blood pressure and placental vascular function throughout pregnancy and the risks of gestational hypertensive disorders.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design and Study Sample
The study was embedded in the Generation R study, a population‐based prospective cohort from early pregnancy onwards in Rotterdam, The Netherlands. 16 Written informed consent was obtained of participating women. The study was approved by the Medical Ethical Committee of the Erasmus Medical Centre in Rotterdam, The Netherlands (MEC 198.782/2001/31). In total, 4096 women of Dutch ethnicity were enrolled during pregnancy. We excluded women with missing data on dietary intake (n=538), with missing data on all outcome measures (n=1), and with preexistent hypertension (n=63). Finally, we excluded loss to follow‐up (n=3), multiple gestations (n=53), and pregnancies leading to fetal death (n=16) or induced abortions (n=8), leading to a cohort for analysis of 3414 pregnant women (Figure S1).
Maternal DASH Score
Semiquantitative self‐administrated food frequency questionnaires (FFQ) of 293 food items were obtained at study enrollment at median 13.5 (95% range 10.2–23.1) weeks gestation, and assessed dietary intake in the 3 months prior. Previously, the FFQ was validated in 82 pregnant women with Dutch ethnic background. 17 , 18 As described previously, 136 of the 293 food items available from the FFQ were used to generate a DASH score. 2 This score is composed of 8 food components, based mainly on the Fung method with a scoring system based on quintile rankings. 2 , 19 For intakes of total grains, vegetables, fruits, non‐full‐fat dairy products, and nuts/seeds/legumes, participating women received a score from 1 (lowest quintile) to 5 (highest quintile). At the opposite, for intakes of red and processed meats, sugar‐sweetened beverages/sweets/added sugars and sodium, participants were scored on a reverse scale. The food component scores were summed to calculate an overall DASH score for each participant. A lower DASH score characterizes a lower dietary quality. 2 In line with previous studies, we constructed quartiles of the maternal DASH score to assess whether associations were restricted to a low DASH score only and constructed a maternal DASH SD score (SDS) to assess associations across the full range (range 10–37). 10 , 11 , 12
Blood Pressure in Pregnancy
Systolic and diastolic blood pressure measurements were performed in early pregnancy (median 12.9 weeks of gestation, 95% range 9.8–17.9), mid pregnancy (median 20.4 weeks of gestation, 95% range 16.6–23.2), and late pregnancy (median 30.2 weeks of gestation, 95% range 28.6–32.6) using a validated Omron 907 automated digital oscillometric sphygmomanometer (OMRON Healthcare Europe BV, Hoofddorp, The Netherlands). 20 Blood pressure measurements were performed with the participant in upright seated position after a minimum waiting time of 5 minutes at rest. The cuff was placed around the upper arm at the level of the heart. The mean of 2 blood pressure measurements with a 60‐second interval was used for further analysis. 21
Placental Hemodynamic Parameters
Ultrasound examinations for placental hemodynamic parameters were carried out in 2 dedicated research centers during mid‐ (median 20.5 weeks of gestation, 95% range 18.7–23.1) and late pregnancy (median 30.4 weeks of gestation, 95% range 28.5–32.8). Umbilical artery pulsatility index (UmPI), uterine artery resistance index (UtRI), and bilateral third trimester uterine artery notching were assessed as primary placental hemodynamic parameters, as these measures are most commonly used in clinical practice and strongly associated with the risks of gestational hypertensive disorders. 22 , 23 As a secondary outcome, we also assessed uterine artery pulsatility index. The umbilical artery was assessed in a free‐floating part of the umbilical cord. 23 The uterine arteries were identified at the crossover with the external iliac artery. For each Doppler measurement 3 consecutive flow velocity wave forms were recorded. The mean of 3 Doppler measurements was used. Bilateral notching resulting from increased uterine artery resistance was defined as an increase of the waveform at the start of diastole in both uterine arteries. 23 , 24
Gestational Hypertensive Disorders
Information on gestational hypertensive disorders was obtained from medical records. Women suspected of gestational hypertensive disorders based on these records were cross‐checked with the original hospital charts, as described previously. 25 , 26 Briefly, the following criteria were used to identify women with gestational hypertension: development of systolic blood pressure of at least 140 mm Hg and/or diastolic blood pressure of at least 90 mm Hg after 20 weeks of gestation in women who were previously normotensive. 25 , 26 , 27 These criteria and the presence of proteinuria (defined as 2 or more dipstick readings of 2+ or greater, 1catheter sample reading of 1+ or greater, or a 24‐hour urine collection containing at least 300 mg of protein) were used to identify women with preeclampsia. 25 , 26 , 27
Covariates
Data on maternal age, education level, parity, prepregnancy weight, folic acid supplement use, alcohol use during pregnancy, smoking during pregnancy, and total energy intake were collected by questionnaires. Height was measured at enrollment and used to calculate the prepregnancy body mass index (BMI).
Statistical Power
Power calculations within the Generation R study were performed based on 7000 subjects during the design of the study. 28 For a normally distributed continuous outcome it is possible to detect a difference of 0.08 SD with a type I error of 5% and a type II error of 20% (power 80%) if 25% of the cohort has the exposure, which corresponds to a mean difference of ≈0.90 mm Hg for systolic blood pressure and 0.70 mm Hg for diastolic blood pressure. For gestational hypertensive disorders with a prevalence of ≈7%, an odds ratio of 1.26 to 1.38 can be detected if 25% of the cohort has the relevant exposure. 28
Statistical Analysis
First, we performed a nonresponse analysis comparing characteristics of women with information on dietary intake (Figure S1) to women without information on dietary intake (Figure S2). Second, 1‐way ANOVA and chi‐square tests were used to compare population characteristics across the maternal DASH score quartiles. Third, we analyzed the associations of maternal DASH score quartiles with longitudinal systolic and diastolic blood pressure patterns in absolute values using linear mixed models, which take the correlation between repeated measurements of the same subject into account and allow for incomplete outcome. 29 We assumed a compound symmetry covariance structure and used restricted maximum likelihood estimation method. The DASH score quartiles were included in the models as intercept and as an interaction term with gestational age to examine gestational age‐independent (intercept) and gestational age‐dependent differences (interaction DASH score quartiles and gestational age). We used these models as descriptive analyses that present the absolute values for systolic and diastolic blood pressure across the DASH quartiles to reflect clinical practice. Similar methods were used to examine the associations of maternal DASH score quartiles with longitudinal UmPI and UtRI patterns from second trimester onwards. Furthermore, we examined the associations of maternal DASH score quartiles and SDS with differences in systolic and diastolic blood pressure in each pregnancy period using linear regression models to further enable assessment of small differences in blood pressure levels in each pregnancy period, which are relevant from an etiological perspective and on a population level. Fourth, we examined the associations of maternal DASH score quartiles and SDS with differences in UmPI, UtRI, and uterine artery resistance index in mid‐ and late pregnancy using linear regression models and the risk of bilateral uterine artery notching using logistic regression models. Finally, we assessed the associations of maternal DASH score quartiles and SDS with the risk of gestational hypertensive disorders using logistic regression analyses. As maternal dietary intake is known to be strongly related to other sociodemographic and lifestyle characteristics, analyses were first only adjusted for gestational age at intake in the basic model and subsequently additionally adjusted for maternal sociodemographic and lifestyle factors in the confounder model. To select potential confounders we used a directed acyclic graph and assessed whether covariates were associated with the exposure and outcome or led to a >10% change in effect estimate when added to the univariate model. 30 Using these criteria, maternal age, educational level, parity, prepregnancy BMI, folic acid supplement use, smoking habits, alcohol use, total energy intake, and gestational age at time of the measurements were included in the confounder model for the main analyses focused on the continuous outcomes systolic and diastolic blood pressure, UmPI, and UtRI. As the number of cases for the adverse binary outcomes bilateral uterine artery notching, gestational hypertensive disorders, gestational hypertension, and preeclampsia was relatively low, we selected only those confounders that led to a >10% change in effect estimate when added to the univariate model for these specific outcomes. These confounder models included parity, prepregnancy BMI, folic acid supplement use, and gestational age at the time of intake. R 2 values were obtained for the confounder models. To assess whether associations were different according to maternal prepregnancy BMI or parity, we tested statistical interaction terms but none were significant (P>0.05). 24 , 26 , 31 We performed multiple sensitivity analyses. We repeated the analyses excluding women with preexistent or gestational diabetes mellitus, hypercholesterolemia, or preexistent heart diseases, as these women represent higher‐risk populations. We repeated the analyses restricting them to women who enrolled in early pregnancy (ie, <14 weeks of gestation) as adherence to the DASH diet from preconception and early pregnancy onwards may have stronger effects on gestational hemodynamic adaptations. We repeated the analyses for binary outcomes with adjustment for a propensity score, to enable correction for a larger number of maternal sociodemographic and lifestyle‐related characteristics, considering the relatively low number of cases of adverse outcomes. We constructed a propensity score using a logistic regression model to estimate the probability of women having a dietary intake within DASH quartile 1 as compared with DASH quartile 4. The propensity score included maternal age, educational level, parity, prepregnancy BMI, folic acid supplement use, smoking habits, alcohol use, total energy intake, and gestational age at time of intake. The propensity score was then included as a covariate in the regression models. 32 , 33 Missing data of covariates were imputed using multiple imputation. The percentage of missing values was <8% for all covariates, except for prepregnancy BMI (13.7%) and folic acid supplementation (18%). Analysis were performed using IBM Statistical Package of Social Sciences version 25. The analysis for repeated measurements was performed using Statistical Analysis System version 9.4.
Results
Participant Characteristics
Population characteristics according to maternal DASH score quartiles are shown in Table 1. The mean DASH score was 24.6 (SD 4.6). Early pregnancy mean systolic and diastolic blood pressure did not differ significantly across the maternal DASH score quartiles. Mid pregnancy and late pregnancy mean systolic and diastolic blood pressure were highest in the lowest maternal DASH score quartile, decreased over the higher maternal DASH score quartiles and were lowest in the highest maternal DASH score quartile (all P values for univariate comparison across quartiles <0.05). Mid pregnancy and late pregnancy mean UtRI were highest in the lowest maternal DASH score quartile, decreased over the higher maternal DASH score quartiles and were lowest in the highest maternal DASH score quartile (all P values for univariate comparison across quartiles <0.05). Mid‐ and late pregnancy mean UmPI did not differ significantly by maternal DASH score quartiles.
Table 1.
Characteristics of the Study Population by DASH Score Quartile (n=3414)*
| Total Group |
DASH Quartile 1 Score 10–21 |
DASH Quartile 2 Score 22–24 |
DASH Quartile 3 Score 25–27 |
DASH Quartile 4 Score 28–37 |
P Value ‡ | |
|---|---|---|---|---|---|---|
| n=3414 | n=860 | n=798 | n=836 | n=920 | ||
| Maternal age at enrollment, mean (SD), y | 31.4 (4.4) | 29.7 (5.0) | 31.2 (4.2) | 32.0 (3.9) | 32.5 (3.8) | <0.001 |
| Parity, n nulliparous (%) | 2039 (59.9) | 478 (55.7) | 494 (62.1) | 481 (57.6) | 586 (63.8) | 0.001 |
| Prepregnancy BMI, mean (SD), kg/m2 | 23.1 (3.8) | 23.8 (4.4) | 23.3 (3.9) | 23.1 (3.8) | 22.4 (2.9) | <0.001 |
| Prepregnancy BMI ≥25 kg/m2, n (%) | 655 (22.2) | 217 (29.0) | 151 (22.4) | 159 (21.9) | 128 (16.1) | <0.001 |
| Gestational weight gain, mean (SD), kg | 10.8 (4.4) | 10.8 (5.1) | 10.8 (4.3) | 10.8 (4.3) | 10.8 (4.0) | 1.00 |
| Gestational age at intake, median (95% range), wk † | 14.7 (10.2–23.1) | 14.7 (9.6–23.7) | 14.6 (9.9–23.4) | 14.7 (9.9–24.0) | 14.8 (10.5–22.5) | 0.88 |
| Higher education, n (%) | 2000 (59.3) | 285 (33.7) | 456 (58.0) | 560 (67.9) | 699 (76.5) | <0.001 |
| Smoking, n continued (%) | 538 (17.0) | 259 (32.2) | 128 (17.6) | 74 (9.5) | 77 (9.0) | <0.001 |
| Alcohol consumption, n continued (%) | 1570 (50.0) | 304 (38.3) | 358 (49.4) | 425 (54.9) | 483 (56.9) | <0.001 |
| Folic acid supplement use, n (%) | 2493 (89.1) | 551 (80.8) | 575 (88.9) | 646 (92.7) | 721 (93.3) | <0.001 |
| Total energy intake, mean (SD), kcal/d | 2146.9 (511.5) | 2078.1 (548.1) | 2135.2 (535.6) | 2162.8 (491.9) | 2206.8 (462.3) | <0.001 |
| Systolic blood pressure, mean (SD), mm Hg | ||||||
| Early pregnancy | 117.3 (11.9) | 117.8 (11.9) | 117.4 (12.6) | 117.3 (12.3) | 116.6 (11.0) | 0.29 |
| Mid pregnancy | 118.5 (11.7) | 119.5 (12.0) | 118.9 (12.2) | 118.0 (11.7) | 117.5 (10.9) | 0.002 |
| Late pregnancy | 120.4 (11.4) | 121.3 (12.2) | 121.1 (11.8) | 119.7 (10.9) | 119.5 (10.8) | 0.001 |
| Diastolic blood pressure, mean (SD), mm Hg | ||||||
| Early pregnancy | 68.5 (9.2) | 68.9 (9.2) | 68.6 (10.1) | 68.4 (9.0) | 68.1 (8.5) | 0.47 |
| Mid pregnancy | 67.2 (9.3) | 68.3 (9.7) | 67.7 (9.7) | 66.9 (8.9) | 66.1 (8.5) | <0.001 |
| Late pregnancy | 69.4 (9.2) | 70.0 (9.6) | 69.6 (9.3) | 69.0 (8.7) | 68.8 (9.0) | 0.05 |
| Umbilical artery pulsatility index, mean (SD) | ||||||
| Mid pregnancy | 1.19 (0.18) | 1.20 (0.18) | 1.20 (0.18) | 1.18 (0.17) | 1.17 (0.18) | 0.01 |
| Late pregnancy | 0.98 (0.17) | 1.00 (0.18) | 0.97 (0.16) | 0.98 (0.16) | 0.96 (0.16) | <0.001 |
| Uterine artery resistance index, mean (SD) | ||||||
| Mid pregnancy | 0.535 (0.089) | 0.535 (0.091) | 0.535 (0.090) | 0.535 (0.089) | 0.535 (0.088) | 1.00 |
| Late pregnancy | 0.483 (0.078) | 0.490 (0.076) | 0.484 (0.076) | 0.480 (0.081) | 0.479 (0.08) | 0.11 |
| Third trimester bilateral uterine artery notching, n (%) | 48 (2.2) | 13 (2.5) | 11 (2.2) | 10 (1.8) | 14 (2.3) | 0.91 |
| Gestational hypertensive disorders, n (%) | ||||||
| Gestational hypertension | 173 (5.3) | 51 (6.3) | 42 (5.4) | 34 (4.2) | 46 (5.2) | 0.34 |
| Preeclampsia | 59 (1.9) | 19 (2.4) | 7 (1.0) | 20 (2.5) | 13 (1.5) | 0.07 |
BMI indicates body mass index; DASH, Dietary Approaches to Stop Hypertension.
Values are means (SD) or percentages.
Median (95% range).
P values were obtained by analysis of variance for continuous variables and by χ2 for categorical variables.
The composition of the DASH score and intake of food components according to DASH diet quartiles are shown in Table S1. Nonresponse analysis showed that no differences in blood pressure or gestational hypertensive disorders were present among women with data on dietary intake compared with women without data on dietary intake (Table S2).
Maternal DASH Score and Blood Pressure Throughout Pregnancy
Figure shows the systolic and diastolic blood pressure development during pregnancy in absolute values per maternal DASH score quartile. Women in the lowest DASH score quartile tended to have the highest overall systolic blood pressure and diastolic blood pressure throughout pregnancy, whereas women in the highest DASH score quartile tended to have the lowest overall systolic blood pressure and diastolic blood pressure throughout pregnancy. No consistent differences in the increase in blood pressure per week were present for the different maternal DASH score quartiles (P values for interaction with gestational age >0.05). The regression coefficients for gestational age‐independent (intercept) and gestational age‐dependent differences (interaction of DASH score quartile and gestational age) are given in Table S3.
Figure 1. Blood pressure patterns in different DASH categories.
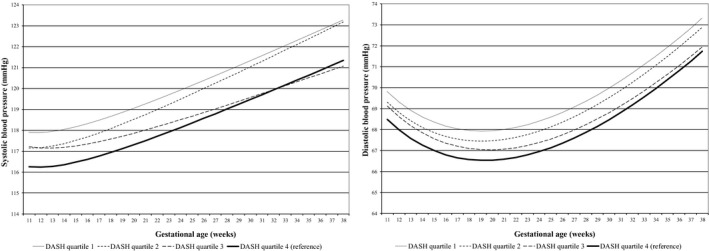
Change in systolic blood pressure (SBP) and diastolic blood pressure (DBP) in mm Hg for first quartile, second quartile, third quartile, and fourth quartile. SBP=ß0+ß1×DASH quartile+ß2×gestational age+ß3×gestational age−2+ß4×DASH quartile×gestational age. DBP=ß0+ß1×DASH quartile+ß2×gestational age+ß3×gestational age0,5+ß4×DASH quartile×gestational age. In these models, “ß0+ß1×DASH” reflects the intercept and “ß2×gestational age+ß3×gestational age−2”reflects the slope of change in blood pressure per week for SBP, and “ß2×gestational age+ß3×gestational age0,5”, reflects the slope of change in blood pressure per week for DBP. Our term of interest is ß4, which reflects the difference in change in blood pressure per week per DASH category, as compared with women in the highest DASH score quartile (healthy diet). Estimates and P values are given in Table S3. DASH indicates Dietary Approaches to Stop Hypertension.
The associations of maternal DASH score quartiles and SDS with differences in systolic and start in early‐, mid‐, and late pregnancy are given in Table 2. After adjustment for maternal sociodemographic and lifestyle factors, lower maternal DASH score quartiles, as compared with the highest maternal DASH score quartile, were associated with a higher mid pregnancy diastolic blood pressure only (P<0.05, R 2=0.16). A higher maternal DASH score across the full range was also significantly associated with a lower mid pregnancy diastolic blood pressure in the confounder model (difference −0.45 [95% CI, −0.78–−0.12] mm Hg per SDS increase in maternal DASH score, R 2=0.16) but not with diastolic blood pressure in early‐ or late pregnancy or systolic blood pressure throughout pregnancy. In the basic models, lower maternal DASH score quartiles were associated with a higher systolic and diastolic blood pressure in mid‐ and late pregnancy as compared with the highest maternal DASH score quartile (all P<0.05) (Table S4).
Table 2.
Associations of Maternal DASH Score With Systolic and Diastolic Blood Pressure in Early‐, Mid‐, and Late Pregnancy (n=3414)*
| DASH Score | Absolute Values and Differences in Systolic Blood Pressure (mm Hg) | |||
|---|---|---|---|---|
|
Early Pregnancy n=2831 |
Mid pregnancy n=3299 |
Late Pregnancy n=3321 |
||
| Quartile 1 |
Absolute mean value (SD) † Confounder model ‡ |
117.8 (11.9) −0.39 (−1.62 to 0.84) n=702 |
119.5 (12.0) 0.05 (−1.09 to 1.18) n=823 |
121.3 (12.2) −0.16 (−1.27 to 0.96) n=825 |
| Quartile 2 |
Absolute mean value (SD) † Confounder model ‡ |
117.4 (12.6) −0.35 (−1.53 to 0.82) n=664 |
118.9 (12.2) 0.08 (−0.99 to 1.15) n=773 |
121.1 (11.8) 0.45 (−0.60 to 1.49) n=782 |
| Quartile 3 |
Absolute mean value (SD) † Confounder model ‡ |
117.3 (12.3) −0.02 (−1.11 to 1.17) n=704 |
118.0 (11.7) −0.13 (−1.18 to 0.91) n=808 |
119.7 (10.9) −0.35 (−1.37 to 0.68) n=815 |
| Quartile 4 |
Absolute mean value (SD) † Confounder model ‡ |
116.6 (11.0) Reference n=761 |
117.5 (10.9) Reference n=895 |
119.5 (10.8) Reference n=899 |
| Trend§ | 0.19 (−0.26 to 0.64) | −0.01 (−0.43 to 0.40) | 0.07 (−0.33 to 0.48) | |
| DASH Score | Absolute Values and Differences in Diastolic Blood Pressure (mm Hg) | |||
|---|---|---|---|---|
|
Early Pregnancy n=2831 |
Mid pregnancy n=3298 |
Late Pregnancy n=3320 |
||
| Quartile 1 |
Absolute mean value (SD) † Confounder model ‡ |
68.9 (9.2) 0.18 (−0.77 to 1.13) n=702 |
68.3 (9.7) 1.31* (0.42 to 2.21) n=822 |
70.0 (9.6) 0.09 (−0.79 to 0.97) n=825 |
| Quartile 2 |
Absolute mean value (SD) † Confounder model ‡ |
68.6 (10.1) −0.18 (−1.09 to 0.72) n=664 |
67.7 (9.7) 0.85* (0.01 to 1.69) n=773 |
69.6 (9.3) −0.01 (−0.84 to 0.81) n=781 |
| Quartile 3 |
Absolute mean value (SD) † Confounder model ‡ |
68.4 (9.0) −0.19 (−1.07 to 0.69) n=704 |
66.9 (8.9) 0.33 (−0.49 to 1.15) n=808 |
69.0 (8.7) −0.21 (−1.02 to 0.60) n=815 |
| Quartile 4 |
Absolute mean value (SD) † Confounder model ‡ |
68.1 (8.5) Reference n=761 |
66.1 (8.5) Reference n=895 |
68.8 (9.0) Reference n=899 |
| Trend § | −0.05 (−0.39 to 0.30) | −0.45* (−0.78 to −0.12) | −0.06 (−0.38 to 0.26) | |
DASH indicates Dietary Approaches to Stop Hypertension; DBP, diastolic blood pressure; and SBP, systolic blood pressure.
P<0.05.
Values are unadjusted mean blood pressure values (SD) and reflect the absolute value in SBP and DBP per DASH quartile.
Values are regression coefficients (95% CI) and reflect the difference in mm Hg blood pressure per maternal DASH score quartile. Groups are compared with women with the highest dietary quality according to the DASH score (quartile 4) as reference. Estimates are from multiple imputed data. Models are adjusted for maternal age, educational level, parity, prepregnancy body mass index, smoking habits, alcohol use, folic acid use, total energy intake, and gestational age at time of the measurements. R 2 values: early pregnancy SBP, R 2=0.15; mid pregnancy SBP, R 2=0.15, late pregnancy SBP, R 2=0.13; early pregnancy DBP, R 2=0.14; mid pregnancy DBP, R 2=0.16; late pregnancy, R 2=0.16.
Trends were based on multiple linear regression models with DASH as SD scores. R 2 values: early pregnancy SBP, R 2=0.15; mid pregnancy SBP, R 2=0.14, late pregnancy SBP, R 2=0.12; early pregnancy DBP, R 2=0.14; mid pregnancy DBP, R 2=0.16; late pregnancy DBP, R 2=0.16.
Maternal DASH Score and Placental Vascular Function
Table S5 shows that in the basic models, compared with the highest maternal DASH score quartile, the lower maternal DASH score quartiles were associated with a higher UmPI in mid‐ and late pregnancy (P<0.05, P values for trend <0.05), but not with UtRI or bilateral notching. We observed similar results when we used repeated measurement models to examine longitudinal placental vascular development from mid pregnancy onwards (Table S6). Table 3 shows that as compared with the highest maternal DASH score quartile, the lowest maternal DASH score quartile was associated with a higher late pregnancy UmPI (P<0.05, R 2=0.04) after adjustment for maternal sociodemographic and lifestyle factors. A higher maternal DASH score across the full range was also associated with a lower late pregnancy UmPI (difference −0.008 [95% CI, −0.015–−0.002] per SDS increase in maternal DASH score, R 2=0.04). Similar tendencies were present for maternal DASH score quartiles and across the full range with mid pregnancy UmPI, but these associations were not significant. No consistent associations of maternal DASH score quartiles and SDS with mid‐ or late pregnancy UtRI or bilateral notching were present after adjustment for maternal sociodemographic and lifestyle factors. Similarly, Table S7 shows that mid‐ and late pregnancy mean uterine artery pulsatility index did not differ significantly across maternal DASH score quartiles. No associations of maternal DASH score quartiles or SDS with uterine artery pulsatility index were observed after adjustment for sociodemographic and lifestyle factors.
Table 3.
Associations of Maternal DASH Score With Placental Vascular Function (n=3414)
| DASH Score | Absolute Values and Differences in UmPI | Absolute Values and Differences in UtRI | Bilateral Notching* | |||
|---|---|---|---|---|---|---|
|
Mid pregnancy n=2527 |
Late Pregnancy n=2776 |
Mid pregnancy n=1898 |
Late Pregnancy n=2076 |
Late Pregnancy ncases=48 |
||
| Quartile 1 |
Absolute mean value (SD) † Confounder model ‡ |
1.20 (0.18) 0.012 (−0.009 to 0.033) n=598 |
1.00 (0.18) 0.026 §(0.007 to 0.044) n=672 § |
0.535 (0.091) −0.002 (−0.014 to 0.010) n=433 |
0.490 (0.076) 0.009 (−0.001 to 0.019) n=496 |
na 1.11 (0.51–2.42) ncases=13 |
| Quartile 2 |
Absolute mean value (SD) † Confounder model ‡ |
1.20 (0.18) 0.019 (0.000 to 0.039) n=600 |
0.97 (0.16) −0.002 (−0.016 to 0.019) n=644 |
0.535 (0.090) 0.000 (−0.011 to 0.011) n=448 |
0.484 (0.076) 0.005 (−0.004 to 0.014) n=477 |
na 0.94 (0.42–2.10) ncases=11 |
| Quartile 3 |
Absolute mean value (SD) † Confounder model ‡ |
1.18 (0.17) 0.009 (−0.010 to 0.028) n=630 |
0.98 (0.16) 0.015 (−0.003 to 0.031) n=693 |
0.535 (0.089) −0.001 (−0.012 to 0.010) n=468 |
0.480 (0.081) 0.000 (−0.009 to 0.009) n=516 |
na 0.82 (0.36–1.87) ncases=10 |
| Quartile 4 |
Absolute mean value (SD) † Confounder model ‡ |
1.17 (0.18) Reference n=699 |
0.96 (0.16) Reference n=767 |
0.535 (0.088) Reference n=549 |
0.479 (0.08) Reference n=587 |
na Reference ncases=14 |
| Trend ‖ | −0.007 (−0.015 to 0.001) | −0.008 § (−0.015 to −0.002) | 0.001 (−0.003 to 0.006) | −0.003 (−0.006 to 0.001) | 1.02 (0.76–1.36) | |
DASH indicates Dietary Approaches to Stop Hypertension; UmPI, umbilical artery pulsatility index; and UtRI, uterine artery resistance index.
Values are odds ratios (95% CI) that reflect difference in risks of third trimester notching per DASH quartile. Groups are compared with women with a healthy dietary pattern (quartile 4) as reference. Estimates are from multiple imputed data. R 2 values: mid pregnancy UmPI, R 2=0.07; late pregnancy UmPI, R 2=0.04; mid pregnancy UtRI, R 2=0.02; late pregnancy UtRI, R 2=0.03; bilateral notching R 2=0.01.
Values are unadjusted mean values (SD) and reflect the absolute value in UmPI and UtRI per DASH quartile.
Values are regression coefficients (95% CI) and reflect differences in UmPI and UtRI per DASH quartile. Groups are compared with women with the highest dietary quality according to the DASH score (quartile 4) as reference. Models for UmPI and UtRI are adjusted for maternal age, educational level, parity, prepregnancy body mass index, smoking habits, alcohol use, folic acid use, total energy intake, and gestational age at time of the measurements. Models for bilateral notching are adjusted for parity, prepregnancy body mass index, folic acid use, and gestational age at time of measurement.
P<0.05.
Trends were based on multiple linear regression models with DASH as SD scores for UmPI and UtRI and on multiple logistic regression models with DASH as SDS for bilateral notching. R 2 values: mid pregnancy UmPI, R 2=0.07; late pregnancy UmPI, R 2=0.04; mid pregnancy UtRI, R 2=0.02; late pregnancy UtRI, R 2=0.03; bilateral notching R 2=0.01.
Maternal DASH Score and Risks of Gestational Hypertension and Preeclampsia
Table 4 shows that maternal DASH score in quartiles and SDS were not significantly associated with the risks of any gestational hypertensive disorder, gestational hypertension, or preeclampsia in the adjusted models. Comparable findings were present in the basic models (Table S8).
Table 4.
Associations of Maternal DASH Score With the Risks of Gestational Hypertensive Disorders (n=3414)
| DASH Score | Gestational Hypertensive Disorders* | Gestational Hypertension* | Preeclampsia* |
|---|---|---|---|
|
Odds Ratio (95% CI) ncases=232 |
Odds Ratio (95% CI) ncases=173 |
Odds Ratio (95% CI) ncases=59 |
|
| Quartile 1 |
1.14 (0.78–1.67) ncases=70 |
1.04 (0.67–1.60) ncases=51 |
1.46 (0.70–3.07) ncases=19 |
| Quartile 2 |
0.84 (0.56–1.25) ncases=49 |
0.91 (0.59–1.42) ncases=42 |
0.57 (0.23–1.46) ncases=7 |
| Quartile 3 |
0.95 (0.64–1.40) ncases=54 |
0.73 (0.46–1.16) ncases=34 |
1.74 (0.85–3.55) ncases=20 |
| Quartile 4 |
Reference ncases=59 |
Reference ncases=46 |
Reference ncases=13 |
| Trend † | 0.95 (0.83–1.10) | 0.96 (0.81–1.12) | 0.94 (0.72–1.23) |
DASH indicates Dietary Approaches to Stop Hypertension; GH, gestational hypertension; GHD, gestational hypertensive disorders; and PE, preeclampsia.
Values are odds ratios (95% CI) that reflect difference in risks of gestational hypertensive disorders, gestational hypertension, and preeclampsia per DASH quartile. Groups are compared with women with the highest dietary quality according to the DASH score (quartile 4) as reference. Estimates are from multiple imputed data. Models are adjusted for parity, prepregnancy body mass index, folic acid use, and gestational age at time of intake. R 2 values: GHD, R 2=0.09; GH, R 2=0.10; PE, R 2=0.08.
Trends were based on multiple logistic regression models with DASH as SD scores. R 2 values: GHD, R 2=0.09; GH, R 2=0.10; PE, R 2=0.08.
Sensitivity Analyses
Similar results were present when we excluded women with preexistent and gestational diabetes mellitus (Tables S9 through S11) and when we excluded women with hypercholesterolemia and/or a heart condition (Tables S12 through S14). When we restricted the analysis to women who enrolled before 14 weeks of gestation, similar findings were present for systolic and diastolic blood pressure and gestational hypertensive disorders, but no associations of maternal DASH score quartiles or SDS with placental hemodynamic parameters were observed (Tables S15 through S17). When we used propensity scores to adjust for potential maternal sociodemographic and lifestyle‐related confounding factors, we observed similar results for bilateral uterine artery notching and gestational hypertensive disorders as compared with conventional covariate adjustment in the multivariable regression models (Table S18).
Discussion
Within this low‐risk population‐based cohort study, we observed that a higher maternal DASH diet score was associated with a lower mid pregnancy diastolic blood pressure but not with diastolic blood pressure in early‐ or late pregnancy or systolic blood pressure throughout pregnancy. A higher maternal DASH diet score tended to be associated with a lower mid‐ and late pregnancy UmPI but not with other placental hemodynamic parameters. No associations were present with the risks of gestational hypertensive disorders.
Interpretation of Main Findings
The DASH diet is a diet high in fruits, vegetables, total grains, nuts, seeds, legumes, and non‐full‐fat dairy products, and low in animal protein, sugar, and sodium. 2 This dietary approach has gained substantial attention for its blood pressure lowering properties in nonpregnant populations. In the original clinical trial among 459 participants with systolic blood pressure of <160 mm Hg and diastolic blood pressure of 80 to 95 mm Hg, the DASH diet led to a significant reduction of systolic and diastolic blood pressure by 5.5 and 3.0 mm Hg, with even stronger effects in hypertensive individuals. 3 These results have been reproduced in numerous other intervention and observational studies that suggest beneficial effects on cardiovascular risk factors and long‐term cardiovascular outcomes. 4 , 5 , 6 , 7 , 12 The DASH diet is accordingly recommended by the American Heart Association to manage blood pressure, improve lipid profile, and reduce the risks of heart attack and stroke. 34 We hypothesized that maternal adherence to the DASH diet during pregnancy may also reduce the risks of gestational hypertensive disorders through its potential positive effects on blood pressure and vascular function.
Not much is known about the influence of maternal adherence to the DASH diet during pregnancy on blood pressure development or placental vascular function in pregnancy. The DASH diet has some resemblance in dietary properties when compared with the Mediterranean diet. Maternal adherence to a Mediterranean dietary pattern has been associated with lower blood pressure in early‐ and mid pregnancy and lower placental vascular hemodynamic parameters in low‐risk and higher‐risk populations. 35 , 36 , 37 , 38 In line with these findings, an observational study in Ireland among 511 women with a large‐for‐gestational‐age infant in their previous pregnancy, showed that higher maternal adherence to the DASH diet in their second pregnancy was associated with a lower diastolic blood pressure and mean arterial pressure in early‐ and late pregnancy, but not in mid pregnancy. 8 Within this study dietary intake was recorded in each trimester of pregnancy using a 3‐day food diary, but no extensive adjustment for other lifestyle factors was performed. 8 A small intervention study among 34 Irani women with gestational diabetes mellitus also described a favorable influence on third trimester systolic blood pressure after adhering to the DASH diet for 4 weeks compared with a control diet. 35 , 36 , 37 , 38 , 39 Contrary, an observational study among 1760 pregnant women in the United States showed no associations of DASH diet score with third trimester blood pressure in a low‐risk multiethnic population. 10
Only partly in line with the previous studies focused on adherence to a Mediterranean diet and the DASH diet, we did not find consistent associations of a higher maternal DASH diet score with systolic and diastolic blood pressure development throughout pregnancy after adjustment for sociodemographic and lifestyle factors in a low‐risk population. A higher maternal DASH diet score was associated with a only small reduction in mid pregnancy diastolic blood pressure. A higher maternal DASH diet score also tended to be associated with lower umbilical artery vascular resistance in mid‐ and late pregnancy but not with uteroplacental vascular function. The UmPI reflects the development of the fetoplacental vascular tree. Already small increases in mid‐ and late pregnancy fetoplacental vascular resistance are associated with increased risks of gestational hypertension and preeclampsia. 22 , 23 These observed associations with mid pregnancy diastolic blood pressure and fetoplacental vascular function may be explained by improved endothelial cell function and reduction of oxidative stress through the DASH diet and potential positive effects on the renin‐angiotensin‐aldosterone system via sodium reduction. 12 , 13 , 15 Through these mechanisms, the DASH diet may positively affect physiological hemodynamic adaptations in pregnancy, which could explain the strongest effect on mid pregnancy diastolic blood pressure, when the physiological diastolic blood pressure dip in pregnancy occurs. 10 , 40 The beneficial effects on endothelial function may be more apparent on the fetoplacental vascular function than the uteroplacental vascular function, as the vasomotor tone of the fetoplacental vasculature is fully regulated by endothelial derived vasoactive mediators, whereas the uteroplacental vascular bed is also influenced by autonomic regulation. 41 , 42 , 43 Thus, this suggest that these potential beneficial effects of the DASH diet on gestational hemodynamic adaptations may be more pronounced among higher‐risk populations. 8 , 39
Three studies explored the effects of the DASH diet on the risks of gestational hypertensive disorders. A prospective cohort among 1760 pregnant women in the United States did not observe any associations of first trimester DASH diet score with gestational hypertension or preeclampsia. 10 A cohort among 66 651 women with singleton pregnancies in Denmark showed no association of maternal DASH diet score at 25 weeks gestation with the risk of gestational hypertensive disorders. 11 In line with these previous studies, we observed no significant associations of maternal DASH diet score with the risk of gestational hypertensive disorders. We observed a tendency for an association of a higher maternal DASH diet score with a lower risk of preeclampsia, but this association was not significant. This might indicate a type II error because of a relatively small number of preeclampsia cases within our low‐risk population. Contrary to our findings, a beneficial effect of the DASH diet was found in a randomized controlled trial in China among 85 high‐risk pregnant women diagnosed with preexistent hypertension or gestational hypertension. They found a lower incidence of preeclampsia when women adhered to the DASH diet compared with a control diet during a 12‐week intervention period. 9 Thus, our findings suggest that in a low‐risk pregnant population, higher maternal DASH diet score is not associated with a lower risk of gestational hypertensive disorders. Stimulating maternal adherence to the DASH diet might be more clinically relevant in pregnant populations with a high a priori risk of gestational hypertensive disorders.
Within our low‐risk Dutch population, we did not observe consistent and strong positive associations of higher maternal DASH diet score with systolic and diastolic blood pressure development throughout pregnancy, uteroplacental vascular function, or the risks of gestational hypertensive disorders after considering maternal sociodemographic and lifestyle factors. There remained only a relatively small association of higher maternal DASH diet score with a lower mid pregnancy diastolic blood pressure and a tendency to lower fetoplacental vascular resistance from mid pregnancy onwards, after adjustment for maternal sociodemographic and lifestyle factors. These observed associations were small and within the normal range of maternal blood pressure and umbilical artery vascular resistance. However, we do consider these findings important from an etiological perspective and on a population level. Overall, we observed that participating women already adhered to components of the DASH diet and subsequently the range of DASH diet score within our study population was moderate. Possibly, among pregnant populations with a larger variability in dietary intake, the influence of higher maternal adherence to the DASH diet on gestational hemodynamic adaptations is more apparent. Within our study population, blood pressure was also mainly within the normotensive range. We excluded women with preexistent hypertension. Among pregnant women with an already increased baseline blood pressure, the beneficial effects of the DASH diet on gestational hemodynamic adaptations could be more apparent as was demonstrated in earlier research in nonpregnant populations. 3 Further studies are needed to explore the effects of adherence to the DASH diet in higher‐risk multiethnic pregnant populations on gestational hemodynamic adaptations and the risks of gestational hypertensive disorders to assess whether recommending the DASH diet for these higher‐risk pregnant women may improve their pregnancy outcomes.
Strengths and Limitations
We had a prospective data collection from early pregnancy onwards and a large sample size. The response rate for participation in the Generation R cohort was 61% at baseline, which reflects the number of participating pregnant women in the study as a percentage of the total number of pregnant women who fulfilled the eligibility criteria in the study area. 16 We restricted to women of Dutch ethnicity, which may have affected the generalizability of our findings. Information on gestational hypertensive disorders was obtained from medical records, using definitions of gestational hypertensive disorders used in clinical practice at the time. 27 The definition of preeclampsia has been updated, 44 which might affect the generalizability of our findings to current clinical practice. Within our study population, we had a relatively small number of gestational hypertensive disorders and bilateral uterine artery notching cases, which might indicate a selection toward a relatively healthy low‐risk population. Additionally, it may have led to lack of statistical power for these specific analyses and the possibility of a type II error. Further studies within larger populations with more cases of placental insufficiency and gestational hypertensive disorders are needed using the most up‐to‐date classification for gestational hypertensive disorders to examine these associations in further detail with increased statistical power. Women with preexistent hypertension or other cardiovascular diseases may be at increased risk of impaired gestational hemodynamic adaptations and developing gestational hypertensive disorders. 45 Importantly, women with preexistent hypertension were excluded from our study population and we observed similar findings when we additionally excluded women with hypercholesterolemia and a heart condition from the analyses. Given the relatively young age of participating women, we consider it unlikely that a high percentage of women already had other preexistent cardiovascular diseases, but we did not have more detailed information available. Further studies with detailed assessments of maternal cardiovascular health before and during pregnancy are needed to assess whether adherence to the DASH diet has a different effect on gestational hemodynamic adaptations in low‐risk and higher‐risk populations. Although the FFQs were validated previously and are a commonly used method to assess dietary intake, reporting bias may be an issue as the FFQ was self‐administered and components of the DASH diet are food items that are generally known for their healthy or less healthy properties. We assessed maternal dietary intake by FFQ at enrollment in the study. Owing to the design of our study, the timing of the FFQ administration is relatively broad. 28 As the FFQ reflects maternal dietary intake in the 3 months prior, this approach allowed us to assess maternal dietary intake just before pregnancy and in the first half of pregnancy and reduces the risk of recall bias. Importantly, some women may have changed their diet already at an earlier stage in the preconception period in order to improve their own health and fertility or may have changed their diet when they became pregnant. Further studies from preconception onwards are needed with detailed dietary assessments in the preconception period and during pregnancy to identify critical periods for maternal dietary intake on gestational hemodynamic adaptations and the risk of gestational hypertensive disorders. Information on a large number of covariates was available within our study to adjust for potential confounding within our main analyses. We could adjust for only a relatively small set of confounders for bilateral uterine artery notching and gestational hypertensive disorders because of the relatively low number of cases. However, we observed similar results when we used a propensity score to adjust for a larger number of maternal sociodemographic and lifestyle‐related characteristics. As in any observational study residual confounding might still be an issue.
Conclusions
In a low‐risk pregnant population, higher maternal adherence to DASH diet was associated with a lower mid pregnancy diastolic blood pressure and tended to be associated with a lower mid‐ and late pregnancy umbilical artery vascular resistance but not with systolic blood pressure, uteroplacental vascular resistance, or the risk of gestational hypertensive disorders. Further studies are needed to assess whether maternal adherence to the DASH diet has more pronounced positive effects on gestational hemodynamic adaptations and the risks of gestational hypertensive disorders in higher‐risk populations.
Sources of Funding
The Generation R Study is financially supported by the Erasmus Medical Center, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development, the Netherlands Organisation for Scientific Research (NWO), the Ministry of Health, Welfare and Sport. Dr Duijts received funding from the European Union's Horizon 2020 co‐funded program ERA‐Net on Biomarkers for Nutrition and Health (ERA HDHL) (ALPHABET project [no 696295; 2017], ZonMW The Netherlands [no 529051014; 2017]). Prof Jaddoe received a grant from the European Research Council (Consolidator Grant, ERC‐2014‐CoG‐648916). Dr Romy Gaillard received funding from the Dutch Heart Foundation (grant number 2017T013), the Dutch Diabetes Foundation (grant number 2017.81.002) and the Netherlands Organization for Health Research and Development (NWO, ZonMW, grant number 543003109).
Disclosures
None.
Supporting information
Tables S1–S18
Figures S1–S2
Acknowledgments
The Generation R Study is conducted by the Erasmus Medical Center in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, Rotterdam, the Rotterdam Homecare Foundation, Rotterdam and the Stichting Trombosedienst and Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of participating mothers, general practitioners, hospitals, midwives and pharmacies in Rotterdam.
Author contributions: Wiertsema and Gaillard designed the research, wrote the article, and had primary responsibility for the statistical analysis and the final content of the paper. Mensink‐Bout constructed the Dietary Approaches to Stop Hypertension diet score within the Generation R study. All authors were responsible for critical review of the manuscript. All authors approved the final manuscript and agree to be accountable for all aspects of the work.
(J Am Heart Assoc. 2021;10:e017503. DOI: 10.1161/JAHA.120.017503.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017503
For Sources of Funding and Disclosures, see page 12.
REFERENCES
- 1. World Health Organization . WHO Recommendations for Prevention and Treatment of Pre‐Eclampsia and Eclampsia. Geneva: WHO Press; 2011. [PubMed] [Google Scholar]
- 2. Aubert AM, Forhan A, de Lauzon‐Guillain B, Chen LW, Polanska K, Hanke W, Jankowska A, Mensink‐Bout SM, Duijts L, Suderman M, et al. Deriving the Dietary Approaches to Stop Hypertension (DASH) score in women from seven pregnancy cohorts from the European ALPHABET Consortium. Nutrients. 2019;11:2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, et al. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med. 1997;336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 4. Appel LJ, Brands MW, Daniels SR, Karanja N, Elmer PJ, Sacks FM, American HA. Dietary approaches to prevent and treat hypertension: a scientific statement from the American Heart Association. Hypertension. 2006;47:296–308. [DOI] [PubMed] [Google Scholar]
- 5. Hinderliter AL, Babyak MA, Sherwood A, Blumenthal JA. The DASH diet and insulin sensitivity. Curr Hypertens Rep. 2011;13:67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Harrington JM, Fitzgerald AP, Kearney PM, McCarthy VJ, Madden J, Browne G, Dolan E, Perry IJ. DASH diet score and distribution of blood pressure in middle‐aged men and women. Am J Hypertens. 2013;26:1311–1320. [DOI] [PubMed] [Google Scholar]
- 7. Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta‐analysis. Br J Nutr. 2015;113:1–15. [DOI] [PubMed] [Google Scholar]
- 8. Courtney AU, O'Brien EC, Crowley RK, Geraghty AA, Brady MB, Kilbane MT, Twomey PJ, McKenna MJ, McAuliffe FM. DASH (Dietary Approaches to Stop Hypertension) dietary pattern and maternal blood pressure in pregnancy. J Hum Nutr Diet. 2020;33:686–697. [DOI] [PubMed] [Google Scholar]
- 9. Jiang F, Li Y, Xu P, Li J, Chen X, Yu H, Gao B, Xu B, Li X, Chen W. The efficacy of the Dietary Approaches to Stop Hypertension diet with respect to improving pregnancy outcomes in women with hypertensive disorders. J Hum Nutr Diet. 2019;32:713–718. [DOI] [PubMed] [Google Scholar]
- 10. Fulay AP, Rifas‐Shiman SL, Oken E, Perng W. Associations of the Dietary Approaches to Stop Hypertension (DASH) diet with pregnancy complications in Project Viva. Eur J Clin Nutr. 2018;72:1385–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arvizu M, Bjerregaard AA, Madsen MT, Granstrom C, Halldorsson TI, Olsen SF, Gaskins AJ, Rich‐Edwards JW, Rosner BA, Chavarro JE. Sodium intake during pregnancy, but not other diet recommendations aimed at preventing cardiovascular disease, is positively related to risk of hypertensive disorders of pregnancy. J Nutr. 2019;150:159–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH‐style diet and risk of coronary heart disease and stroke in women. Arch Intern Med. 2008;168:713–720. [DOI] [PubMed] [Google Scholar]
- 13. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, Simons‐Morton DG, et al. Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH‐Sodium Collaborative Research Group. N Engl J Med. 2001;344:3–10. [DOI] [PubMed] [Google Scholar]
- 14. Sun B, Williams JS, Svetkey LP, Kolatkar NS, Conlin PR. Beta2‐adrenergic receptor genotype affects the renin‐angiotensin‐aldosterone system response to the Dietary Approaches to Stop Hypertension (DASH) dietary pattern. Am J Clin Nutr. 2010;92:444–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maris SA, Williams JS, Sun B, Brown S, Mitchell GF, Conlin PR. Interactions of the DASH diet with the renin‐angiotensin‐aldosterone system. Curr Dev Nutr. 2019;3:nzz091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kooijman MN, Kruithof CJ, van Duijn CM, Duijts L, Franco OH,van IJzendoorn MH, de Jongste JC, Klaver CC, van der Lugt A, Mackenbach JP, et al. The Generation R Study: design and cohort update 2017. Eur J Epidemiol. 2016;31:1243–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Heppe DH, Medina‐Gomez C, Hofman A, Franco OH, Rivadeneira F, Jaddoe VW. Maternal first‐trimester diet and childhood bone mass: the Generation R Study. Am J Clin Nutr. 2013;98:224–232. [DOI] [PubMed] [Google Scholar]
- 18. Voortman T, Steegers‐Theunissen RPM, Bergen NE, Jaddoe VWV, Looman CWN, Kiefte‐de Jong JC, Schalekamp‐Timmermans S. Validation of a semi‐quantitative food‐frequency questionnaire for Dutch pregnant women from the general population using the method or triads. Nutrients. 2020;12:1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB. Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr. 2001;73:61–67. [DOI] [PubMed] [Google Scholar]
- 20. El Assaad MA, Topouchian JA, Darne BM, Asmar RG. Validation of the Omron HEM‐907 device for blood pressure measurement. Blood Press Monit. 2002;7:237–241. [DOI] [PubMed] [Google Scholar]
- 21. Gaillard R, Bakker R, Willemsen SP, Hofman A, Steegers EA, Jaddoe VW. Blood pressure tracking during pregnancy and the risk of gestational hypertensive disorders: the Generation R Study. Eur Heart J. 2011;32:3088–3097. [DOI] [PubMed] [Google Scholar]
- 22. Baschat AA, Hecher K. Fetal growth restriction due to placental disease. Semin Perinatol. 2004;28:67–80. [DOI] [PubMed] [Google Scholar]
- 23. Gaillard R, Arends LR, Steegers EA, Hofman A, Jaddoe VW. Second‐ and third‐trimester placental hemodynamics and the risks of pregnancy complications: the Generation R Study. Am J Epidemiol. 2013;177:743–754. [DOI] [PubMed] [Google Scholar]
- 24. Rurangirwa AA, Gaillard R, Steegers EA, Hofman A, Jaddoe VW. Hemodynamic adaptations in different trimesters among nulliparous and multiparous pregnant women; the Generation R Study. Am J Hypertens. 2012;25:892–899. [DOI] [PubMed] [Google Scholar]
- 25. Coolman M, de Groot CJ, Jaddoe VW, Hofman A, Raat H, Steegers EA. Medical record validation of maternally reported history of preeclampsia. J Clin Epidemiol. 2010;63:932–937. [DOI] [PubMed] [Google Scholar]
- 26. Gaillard R, Steegers EA, Hofman A, Jaddoe VW. Associations of maternal obesity with blood pressure and the risks of gestational hypertensive disorders. The Generation R Study. J Hypertens. 2011;29:937–944. [DOI] [PubMed] [Google Scholar]
- 27. Brown MA, Lindheimer MD, de Swiet M, Van Assche A, Moutquin JM. The classification and diagnosis of the hypertensive disorders of pregnancy: statement from the International Society for the Study of Hypertension in Pregnancy (ISSHP). Hypertens Pregnancy. 2001;20:IX–XIV. [DOI] [PubMed] [Google Scholar]
- 28. Jaddoe VW, Mackenbach JP, Moll HA, Steegers EA, Tiemeier H, Verhulst FC, Witteman JC, Hofman A. The Generation R Study: design and cohort profile. Eur J Epidemiol. 2006;21:475–484. [DOI] [PubMed] [Google Scholar]
- 29. Goldstein H. Multilevel statistical methods. London: Wiley; 1995. [Google Scholar]
- 30. Nash DM, Gilliland JA, Evers SE, Wilk P, Campbell MK. Determinants of diet quality in pregnancy: sociodemographic, pregnancy‐specific, and food environment influences. J Nutr Educ Behav. 2013;45:627–634. [DOI] [PubMed] [Google Scholar]
- 31. Schoenaker DA, Soedamah‐Muthu SS, Mishra GD. Quantifying the mediating effect of body mass index on the relation between a Mediterranean diet and development of maternal pregnancy complications: the Australian Longitudinal Study on Women's Health. Am J Clin Nutr. 2016;104:638–645. [DOI] [PubMed] [Google Scholar]
- 32. Rubin DB. Estimating causal effects from large data sets using propensity scores. Ann Intern Med. 1997;127:757–763. [DOI] [PubMed] [Google Scholar]
- 33. Elze MC, Gregson J, Baber U, Williamson E, Sartori S, Mehran R, Nichols M, Stone GW, Pocock SJ. Comparison of propensity score methods and covariate adjustment: evaluation in 4 cardiovascular studies. J Am Coll Cardiol. 2017;69:345–357. [DOI] [PubMed] [Google Scholar]
- 34. Carson JAS, Lichtenstein AH, Anderson CAM, Appel LJ, Kris‐Etherton PM, Meyer KA, Petersen K, Polonsky T, Van Horn L; American Heart Association Nutrition Committee of the Council on L, Cardiometabolic H, Council on Arteriosclerosis T, Vascular B, Council on C, Stroke N, Council on Clinical C, Council on Peripheral Vascular D, Stroke C . Dietary cholesterol and cardiovascular risk: a science advisory from the American Heart Association. Circulation. 2020;141:e39–e53. [DOI] [PubMed] [Google Scholar]
- 35. Timmermans S, Jaddoe VW, Silva LM, Hofman A, Raat H, Steegers‐Theunissen RP, Steegers EA. Folic acid is positively associated with uteroplacental vascular resistance: the Generation R Study. Nutr Metab Cardiovasc Dis. 2011;21:54–61. [DOI] [PubMed] [Google Scholar]
- 36. Timmermans S, Steegers‐Theunissen RP, Vujkovic M, den Breeijen H, Russcher H, Lindemans J, Mackenbach J, Hofman A, Lesaffre EE, Jaddoe VV, et al. The Mediterranean diet and fetal size parameters: the Generation R Study. Br J Nutr. 2012;108:1399–1409. [DOI] [PubMed] [Google Scholar]
- 37. Owens S, Gulati R, Fulford AJ, Sosseh F, Denison FC, Brabin BJ, Prentice AM. Periconceptional multiple‐micronutrient supplementation and placental function in rural Gambian women: a double‐blind, randomized, placebo‐controlled trial. Am J Clin Nutr. 2015;102:1450–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Reijnders IF, Mulders A, van der Windt M, Steegers EAP, Steegers‐Theunissen RPM. The impact of periconceptional maternal lifestyle on clinical features and biomarkers of placental development and function: a systematic review. Hum Reprod Update. 2019;25:72–94. [DOI] [PubMed] [Google Scholar]
- 39. Asemi Z, Tabassi Z, Samimi M, Fahiminejad T, Esmaillzadeh A. Favourable effects of the Dietary Approaches to Stop Hypertension diet on glucose tolerance and lipid profiles in gestational diabetes: a randomised clinical trial. Br J Nutr. 2013;109:2024–2030. [DOI] [PubMed] [Google Scholar]
- 40. Timmermans S, Steegers‐Theunissen RP, Vujkovic M, Bakker R, den Breeijen H, Raat H, Russcher H, Lindemans J, Hofman A, Jaddoe VW, et al. Major dietary patterns and blood pressure patterns during pregnancy: the Generation R Study. Am J Obstet Gynecol. 2011;205:337.e331–e312. [DOI] [PubMed] [Google Scholar]
- 41. Su EJ. Role of the fetoplacental endothelium in fetal growth restriction with abnormal umbilical artery doppler velocimetry. Am J Obstet Gynecol. 2015;213:S123–S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Poston L, McCarthy AL, Ritter JM. Control of vascular resistance in the maternal and feto‐placental arterial beds. Pharmacol Ther. 1995;65:215–239. [DOI] [PubMed] [Google Scholar]
- 43. Reilly FD, Russell PT. Neurohistochemical evidence supporting an absence of adrenergic and cholinergic innervation in the human placenta and umbilical cord. Anat Rec. 1977;188:277–286. [DOI] [PubMed] [Google Scholar]
- 44. Brown MA, Magee LA, Kenny LC, Karumanchi SA, McCarthy FP, Saito S, Hall DR, Warren CE, Adoyi G, Ishaku S, et al.; International Society for the Study of Hypertension in P . Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72:24–43. [DOI] [PubMed] [Google Scholar]
- 45. Bartsch E, Medcalf KE, Park AL, Ray JG; High Risk of Pre‐eclampsia Identification G . Clinical risk factors for pre‐eclampsia determined in early pregnancy: systematic review and meta‐analysis of large cohort studies. BMJ. 2016;353:i1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S18
Figures S1–S2


