Abstract
Background
Specific plaque phenotypes that predict a favorable response to statin therapy have not been systematically studied. This study aimed to identify optical coherence tomography predictors for a favorable vascular response to statin therapy.
Methods and Results
Patients who had serial optical coherence tomography imaging at baseline and at 6 months were included. Thin‐cap area (defined as an area with fibrous cap thickness <200 μm) was measured using a 3‐dimensional computer‐aided algorithm, and changes in the thin‐cap area at 6 months were calculated. A favorable vascular response was defined as the highest tertile in the degree of reduction of the thin‐cap area. Macrophage index was defined as the product of the average macrophage arc and length of the lesion with macrophage infiltration. Layered plaque was defined as a plaque with 1 or more layers of different optical density. In 84 patients, 140 nonculprit lipid plaques were identified. In multivariable analysis, baseline thin‐cap area (odds ratio [OR] 1.442; 95% CI, 1.024–2.031, P=0.036), macrophage index (OR, 1.031; 95% CI, 1.002–1.061, P=0.036), and layered plaque (OR, 2.767; 95% CI, 1.024–7.479, P=0.045) were identified as the significant predictors for a favorable vascular response. Favorable vascular response was associated with a decrease in the macrophage index.
Conclusions
Three optical coherence tomography predictors for a favorable vascular response to statin therapy have been identified: large thin‐cap area, high macrophage index, and layered plaque. Favorable vascular response to statin was correlated with signs of decreased inflammation.
Registration
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01110538.
Keywords: layered plaque, macrophage, optical coherence tomography, statin, thin‐cap area
Subject Categories: Catheter-Based Coronary and Valvular Interventions, Coronary Artery Disease, Optical Coherence Tomography (OCT)
Nonstandard Abbreviations and Acronyms
- FC
fibrous cap
- FCT
fibrous cap thickness
- TCFA
thin‐cap fibroatheroma
Clinical Perspective
What Is New?
Plaques with features of vulnerability (large thin‐cap area, high macrophage index, and layered plaque) respond more favorably to statin therapy.
Significant correlations are observed between the change in the macrophage index and the change in thin‐cap area.
The prevalence of layered (vulnerable) plaques significantly increases only in plaques with increased macrophage index.
What Are the Clinical Implications?
Statin therapy should be started immediately in patients who have optical coherence tomography predictors for a favorable response (large thin‐cap area, high macrophage index, and layered plaque).
For patients who have evidence of persistent active inflammation, additional therapies such as anti‐inflammatory agents may need to be considered.
Acute coronary syndrome (ACS) is the leading cause of morbidity and mortality in the world. 1 The most common mechanism of ACS is rupture of a lipid plaque. 2 Optical coherence tomography (OCT) studies have revealed that fibrous cap thickness (FCT) is one of the most important determinants of plaque vulnerability. 3 , 4 A previous study reported that 95% of ruptured plaques in patients had FCT <188 μm. 5 Recent studies have demonstrated that statin therapy stabilizes high‐risk plaques by increasing FCT and reducing thin‐cap area. 6 , 7 Recent studies have also shown that layered plaque is a signature of previous plaque destabilization, followed by healing, and is associated with panvascular inflammation and vulnerability. 8 , 9 Thus, the presence of a layered phenotype may be associated with a vascular response to statin therapy because statins are thought to be more effective in patients with ACS and a high baseline inflammatory status. 7 , 10 , 11 However, specific plaque phenotypes that predict a favorable response to statin therapy have not been systematically studied. In this study, we investigated OCT predictors for a favorable vascular response to statin therapy in patients who had serial OCT imaging.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Population
We identified 84 patients with 140 lipid plaques from the Massachusetts General Hospital OCT registry (ClinicalTrials.gov: NCT01110538) who had undergone serial OCT imaging at baseline and at 6 months. This study was approved by the institutional review board at each participating site. Baseline demographic and clinical data were collected, and baseline and follow‐up laboratory data and OCT images were assessed. The intensity of statin therapy was categorized as high, moderate, or low based on the published guidelines. 12 Statin naïve patients were defined as patients who were not receiving statin therapy for more than 3 months before enrollment. 7 The Massachusetts General Hospital OCT registry was approved by the institutional review board at each participating site, and all patients provided written informed consent before enrollment.
OCT Image Acquisition and Analysis
OCT imaging was performed using a frequency‐domain (C7/C8, OCT Intravascular Imaging System, St. Jude Medical, St. Paul, MN) or time‐domain (M2/M3 Cardiology Imaging Systems; Light Lab Imaging Inc) OCT system after intracoronary administration of 100 to 200 μg of nitroglycerin. All OCT images were submitted to the core laboratory at Massachusetts General Hospital for offline analysis. Analysis was performed by 2 independent investigators who were blinded to the clinical, angiographic, and laboratory data. An offline review work station (Ilumien Optis, St. Jude Medical) was used. Previously stented coronary segments and coronary segments that were going to be treated during the index procedure were excluded. Several landmarks, including stent edges, anatomical landmarks such as side branches, pericardium, plaque position and configuration, lumen shape, and/or positional or directional relationships among all these landmarks were used to identify target nonculprit plaque. 7
All OCT plaque morphologies were analyzed using previously validated criteria. 13 , 14 , 15 Nonculprit lipid plaque was identified as a plaque with more than 50% stenosis as compared with reference area, maximum lipid arc >90°, and without association with the index event or symptoms, as assessed by the treating physician. 7 A distance of at least 5 mm on the longitudinal view was required to be considered as 2 separate plaques. Lipid was identified as a low‐signal region with diffuse border. 14 The degree of lipid arc was measured. Lipid length was measured on the longitudinal view, and lipid index was obtained as the product of mean lipid arc and lipid length. 16 Minimal FCT was measured at its thinnest point 3 times and the average value was calculated. Thin‐cap fibroatheroma (TCFA) was defined as a plaque with a maximal lipid arc >90° and thinnest FCT 65 μm or less. 3 , 17 Layered plaque was defined as plaque consisting of 1 or more layers with different optical densities and a clear demarcation from underlying components in 3 or more consecutive frames. 8 , 9 , 18 Macrophages were identified as signal‐rich, distinct, or confluent punctate regions that exceed the intensity of background speckle noise. 13 Given the lack of established criteria for the quantification of macrophages, the angular extension and length of macrophages were measured to obtain a “macrophage arc” and a “macrophage length” to determine the extent of macrophage infiltration, as was done in previous studies. 19 , 20 The macrophage index was defined as the product of the average macrophage arc and macrophage length. 19 , 20 Microvessel was identified as the presence of signal‐poor structures with vesicular of tubular shapes. 13 , 16 Cholesterol crystals were identified as thin and linear regions of high signal intensity with high backscattering within a plaque. 13 , 16 Good intraobserver and interobserver agreement was noted in the OCT identification of lipid plaque (κ, 0.930 and 0.927, respectively), macrophage (κ, 0.933 and 0.867), and layered plaque (κ, 0.933 and 0.867).
Three‐Dimensional Thin‐Cap Area Measurement
The 3‐dimensional fibrous cap (FC) was volumetrically evaluated using a previously validated computer algorithm (Figure 1). 7 , 21 The FC was semiautomatically segmented by the algorithm in all frames along the entire plaque. The algorithm quantified the thickness at each point of its luminal boundary with the fully segmented FC. The FC area was calculated as the product of the frame interval and the arc length of the FC summed over all the involved frames. 22
Figure 1. Three‐dimensional thin‐cap area measurement.
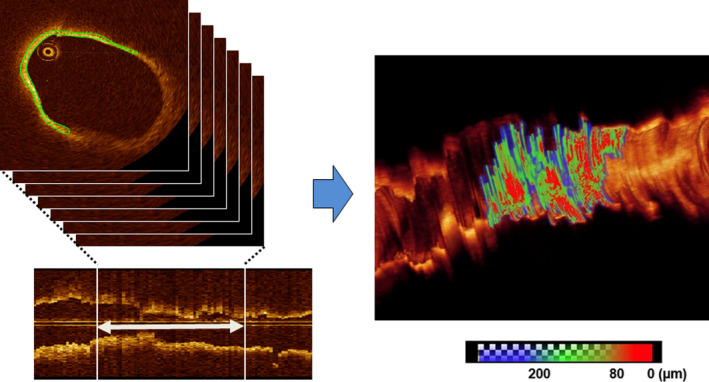
The fibrous cap (FC) was semiautomatically segmented by the algorithm in all frames along the entire plaque. The algorithm quantified the thickness at each point of its luminal boundary with the fully segmented FC. The FC area was calculated as the product of the frame interval and the arc length of the FC summed over all the involved frames. The area with FC thickness <200 µm was considered thin‐cap area (combined green and red areas in the right panel).
The thin‐cap area was defined as FC area with cap thickness <200 μm as described in previous studies. 5 , 7 The change in the thin‐cap area was subsequently calculated. Nonculprit lipid plaques were divided into 3 groups based on the tertile of absolute change in the thin‐cap area (median thin‐cap area change: −4.624 [−7.368 to −3.184] mm2 in the first tertile, −0.943 [−1.566 to −0.370] mm2 in the second tertile, 0.645 [0.121–1.481] mm2 in the third tertile). We defined the first tertile as the favorable FC response group and the remaining 2 as the less‐favorable FC response groups.
Statistical Analysis
Categorical data are presented as counts and percentages, and they were compared using the chi‐square test or Fisher exact test, as appropriate. Continuous data are presented as mean± SD or median (25th–75th percentile), as appropriate, depending on the normality of the distribution tested by the Kolmogorov‐Smirnov test. Between‐group comparisons were performed using independent‐sample t tests, either Mann‐Whitney U tests or Kruskal‐Wallis tests, as appropriate. Tests for the within‐group longitudinal changes were performed using paired‐sample t tests, Wilcoxon signed rank tests, or McNemar tests, as appropriate. Comparison of nonculprit plaque characteristics among different groups was carried out using generalized estimating equations to take into account the potential cluster effects of multiple nonculprit lipid plaques in a single patient. Multivariable logistic regression analysis was applied to identify the predictors for a favorable response to statin. Variables with a P<0.10 in the univariate test were entered into the multivariable modeling. Receiver operating characteristics with area under the curve were used to determine the best cutoff values for the baseline thin‐cap area and macrophage index. All analyses were performed using SPSS (version 25 for Windows; SPSS, Inc., Chicago, IL).
Results
Baseline Characteristics
Baseline characteristics are shown in Table 1. The median follow‐up duration was 6.3 months. Patients with a favorable response more frequently presented with ST‐segment–elevation myocardial infarction. The prevalence of dyslipidemia tended to be higher in patients with a favorable response. Statins were used in 100% of patients at discharge. The intensity of statin and the rate of statin naïve patients were not significantly different between the 2 groups.
Table 1.
Baseline Characteristics
| Patients With Favorable Response (n=39) | Patients With Less Favorable Response (n=45) | P Value | |
|---|---|---|---|
| Follow‐up duration, mo | 6.4 (6.1–12.4) | 6.3 (5.9–12.0) | 0.169 |
| Age, y | 58.2±10.9 | 59.3±8.8 | 0.320 |
| Male, n (%) | 32 (82.1) | 33 (73.3) | 0.341 |
| Clinical presentation, n (%) | 0.049 | ||
| ST‐segment–elevation myocardial infarction, n (%) | 7 (17.9) | 2 (4.4) | |
| Non‐ST‐segment–elevation acute coronary syndrome, n (%) | 21 (53.8) | 21 (46.7) | |
| Stable angina, n (%) | 11 (28.2) | 22 (48.9) | |
| Hypertension, n (%) | 26 (66.7) | 27 (60.0) | 0.528 |
| Dyslipidemia, n (%) | 35 (89.7) | 33 (73.3) | 0.056 |
| Diabetes mellitus, n (%) | 13 (33.3) | 16 (35.6) | 0.831 |
| Chronic kidney disease, n (%) | 2 (5.1) | 5 (11.1) | 0.280 |
| Smoking status, n (%) | 0.476 | ||
| Current smoker | 12 (30.8) | 14 (31.1) | |
| Former smoker | 12 (30.8) | 9 (20.0) | |
| Never smoker | 15 (38.5) | 22 (44.9) | |
| Family history of coronary artery disease, n (%) | 2 (5.1) | 2 (4.4) | 0.636 |
| Discharge medication | |||
| Dual antiplatelet therapy, n (%) | 39 (100.0) | 44 (97.8) | 0.536 |
| Statin, n (%) | 39 (100.0) | 45 (100.0) | 1.000 |
| Intensity of statin therapy | 0.771 | ||
| High‐intensity, n (%) | 1 (2.6) | 1 (2.2) | |
| Moderate‐intensity, n (%) | 34 (87.2) | 37 (82.2) | |
| Low‐intensity, n (%) | 4 (10.2) | 7 (15.6) | |
| Statin naïve, n (%) | 16 (41.0) | 17 (37.8) | 0.761 |
Values are mean±SD, n (%), or median (interquartile range). Significance was calculated by independent‐sample t tests, or by Mann‐Whitney U tests, as appropriate, depending on the normality of the distribution. Sample size: 84 patients.
Laboratory Data
Baseline and follow‐up laboratory data are shown in Table 2. Low‐density lipoprotein (LDL) cholesterol significantly decreased over time in both groups. The levels of LDL at baseline and follow‐up were not significantly different between the 2 groups. The baseline LDL level was 100.4 (74.9–130.0) mg/dL in ST‐segment–elevation myocardial infarction, 95.4 (67.9–122.3) mg/dL in non‐ST‐segment–elevation acute coronary syndrome, and 80.3 (68.7–95.0) mg/dL in stable angina; P=0.104. At follow‐up, the LDL level was 69.5 (43.8–97.9) mg/dL, 74.5 (61.6–84.5) mg/dL, 72.2 (65.0–92.0) mg/dL; P=0.821. In patients with a favorable response, triglyceride and high‐sensitivity C‐reactive protein levels significantly improved over time.
Table 2.
Laboratory Data at Baseline and Follow‐Up
| Patients With Favorable Response (n=39) | Patients With Less Favorable Response (n=45) | P Value | |
|---|---|---|---|
| LDL cholesterol (baseline), mg/dL | 78.4 (60.0 to 104.6) | 91.5 (73.1 to 116.6) | 0.063 |
| LDL cholesterol (follow‐up), mg/dL | 68.1 (53.8 to 78.6) | 76.1 (67.5 to 90.8) | 0.068 |
| LDL cholesterol change, mg/dL | −14.5 (−31.8 to 6.7) | −14.6 (−47.3 to 0.0) | 0.512 |
| P value | 0.025 | 0.001 | |
| HDL cholesterol (baseline), mg/dL | 42.1 (36.1 to 53.3) | 42.0 (38.5 to 53.2) | 0.931 |
| HDL cholesterol (follow‐up), mg/dL | 40.0 (35.9 to 46.9) | 43.1 (36.2 to 51.3) | 0.898 |
| HDL cholesterol change, mg/dL | 0.8 (−6.2 to 5.3) | −1.5 (−6.3 to 5.9) | 0.806 |
| P value | 0.538 | 0.803 | |
| Triglyceride (baseline), mg/dL | 167.0 (108.2 to 207.3) | 150.6 (98.5 to 196.9) | 0.350 |
| Triglyceride (follow‐up), mg/dL | 125.3 (95.7 to 160.6) | 128.9 (88.6 to 174.6) | 0.898 |
| Triglyceride change, mg/dL | −23.5 (−86.1 to 10.6) | 0.0 (−47.7 to 21.0) | 0.174 |
| P value | 0.007 | 0.304 | |
| hs‐CRP (baseline), mg/dL | 2.0 (1.0 to 4.0) | 1.0 (0.0 to 2.0) | 0.099 |
| hs‐CRP (follow‐up), mg/dL | 1.0 (0.5 to 1.8) | 1.0 (0.0 to 2.0) | 0.416 |
| hs‐CRP change, mg/dL | −1.0 (−2.8 to 0.0) | 0.0 (−1.0 to 0.0) | 0.221 |
| P value | 0.007 | 0.272 |
Values are median (interquartile range). Significance was calculated by Mann‐Whitney U tests and Wilcoxon signed rank tests. Sample size: 84 patients. HDL indicates high‐density lipoprotein; hs‐CRP, high‐sensitivity C‐reactive protein; and LDL, low‐density lipoprotein.
Serial OCT Findings
The results of serial OCT analysis are shown in Table 3. In all nonculprit lipid plaques, thin‐cap area was measured at baseline and at follow‐up. The favorable response group had a significantly larger thin‐cap area, thinner fibrous cap, a higher lipid index, higher macrophage index, and higher prevalence of TCFA and layered phenotype at baseline. Thin‐cap area, FCT, lipid index, macrophage index, and TCFA significantly improved over time.
Table 3.
Serial OCT Findings
| Favorable Response (n=47) | Less Favorable Response (n=93) | P Value | |
|---|---|---|---|
| 3‐dimensional OCT quantitative assessment | |||
| Thin‐cap area (baseline), mm2 | 7.137 (4.050 to 10.691) | 1.414 (0.498 to 2.983) | <0.001 |
| Thin‐cap area (follow‐up), mm2 | 1.343 (0.373 to 3.439) | 0.982 (0.175 to 2.949) | 0.295 |
| Thin‐cap area change, mm2 | −4.624 (−7.368 to −3.184) | −0.119 (−0.943 to −0.645) | <0.001 |
| P value | <0.001 | 0.281 | |
| 2‐dimensional OCT assessment | |||
| Thinnest FCT (baseline), μm | 80.0 (50.0 to 110.0) | 120.0 (90.0 to 175.0) | <0.001 |
| Thinnest FCT (follow‐up), μm | 150.0 (70.0 to 190.0) | 150.0 (95.0 to 190.0) | 0.988 |
| Thinnest FCT change, μm | 50.0 (20.0 to 90.0) | 0.0 (−30.0 to 50.0) | <0.001 |
| P value | <0.001 | 0.108 | |
| Lipid index (baseline) | 1542.5 (1151.1 to2266.4) | 1071.6 (616.0 to1460.1) | <0.001 |
| Lipid index (follow‐up) | 1221.0 (669.1 to 2033.4) | 863.4 (370.0 to 1583.8) | 0.019 |
| Lipid index change | −286.2 (−523.2 to −62.9) | −107.1 (−427.6 to 229.5) | 0.047 |
| P value | 0.001 | 0.075 | |
| Minimal lumen area (baseline), mm2 | 3.08 (2.39 to 4.02) | 2.84 (2.27 to 3.87) | 0.394 |
| Minimal lumen area (follow‐up), mm2 | 3.30 (1.73 to 4.30) | 2.83 (1.97 to 3.87) | 0.514 |
| Minimal lumen area change, mm2 | −0.20 (−1.00 to 0.74) | −0.07 (−0.53 to 0.38) | 0.445 |
| P value | 0.346 | 0.569 | |
| Macrophage index (baseline) | 275.0 (140.6 to 372.3) | 83.6 (11.4 to 169.5) | <0.001 |
| Macrophage index (follow‐up) | 100.8 (0.0 to 202.1) | 92.4 (13.3 to 192.3) | 0.674 |
| Macrophage index change | −134.7 (−227.9 to −70.0) | 0.0 (−43.0 to 47.1) | <0.001 |
| P value | <0.001 | 0.661 | |
| Microvessel (baseline), n (%) | 13 (27.6) | 29 (31.2) | 0.667 |
| Microvessel (follow‐up), n (%) | 12 (25.5) | 30 (32.3) | 0.412 |
| P value | 1.000 | 1.000 | |
| Cholesterol crystal (baseline), n (%) | 5 (10.6) | 19 (20.4) | 0.147 |
| Cholesterol crystal (follow‐up), n (%) | 3 (6.4) | 13 (14.0) | 0.182 |
| P value | 0.500 | 0.109 | |
| Calcification (baseline), n (%) | 25 (53.2) | 49 (52.7) | 0.955 |
| Calcification (follow‐up), n (%) | 23 (48.9) | 50 (53.8) | 0.589 |
| P value | 0.687 | 1.000 | |
| TCFA (baseline), n (%) | 20 (42.6) | 15 (16.1) | 0.001 |
| TCFA (follow‐up), n (%) | 7 (14.9) | 16 (17.2) | 0.728 |
| P value | 0.001 | 1.000 | |
| Layered plaque (baseline), n (%) | 26 (55.3) | 25 (26.9) | 0.001 |
| Layered plaque (follow‐up), n (%) | 28 (59.6) | 33 (35.4) | 0.007 |
| P value | 0.625 | 0.057 | |
Values are n (%), or median (interquartile range). Significance was calculated by Mann‐Whitney U tests, Wilcoxon signed rank tests, and McNemar tests. Sample size: 140 plaques. FCT indicates fibrous‐cap thickness; OCT, optical coherence tomography; and TCFA, thin‐cap fibroatheroma.
Predictors for a Favorable Response
Table 4 shows the results of the univariable and multivariable analyses. In the multivariable analysis, the baseline thin‐cap area, macrophage index, and layered plaque were found to be the significant predictors for a favorable vascular response to statin therapy. Using the receiver operating characteristics curves, the best cutoff values of each parameter to predict a favorable response were calculated as 3.144 mm2 for the baseline thin‐cap area and 173.1 for the baseline macrophage index. The relationship between individual or a combination of these parameters, and the probability of a favorable response is shown in Figure 2. When all 3 predictors (large thin‐cap area, high macrophage index, and layered plaque) are present, the probability of a favorable response increased to 78.3% (95% CI, 56.3–92.5), whereas the probability of a favorable response is 2.3% (95% CI, 0.1–12.0) when no predictors are present.
Table 4.
Univariable and Multivariable Analysis of Favorable Vascular Response to Statins
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Odds Ratio [95% CI] | P Value | Odds Ratio [95% CI] | P Value | |
| Baseline thin‐cap area, mm2 | 1.533 [1.199–1.960] | 0.001 | 1.442 [1.024–2.031] | 0.036 |
| Thin‐cap fibroatheroma (<65 μm) | 3.852 [1.765–8.404] | 0.001 | 1.217 [0.402–3.680] | 0.728 |
| Macrophage | 1.308 [0.587–2.916] | 0.511 | ||
| Macrophage index (per 10 increase) | 1.006 [1.004–1.009] | <0.001 | 1.031 [1.002–1.061] | 0.036 |
| Microvessel | 0.844 [0.415–1.716] | 0.639 | ||
| Layered plaque | 2.840 [1.197–6.736] | 0.018 | 2.767 [1.024–7.479] | 0.045 |
| Cholesterol crystal | 0.464 [0.161–1.377] | 0.155 | ||
| Spotty calcium | 1.270 [0.641–2.513] | 0.493 | ||
| Baseline lipid index (per 10 increase) | 0.999 [0.999–1.000] | <0.001 | 0.999 [0.989–1.008] | 0.777 |
| Minimum lumen area, mm2 | 1.168 [0.886–1.540] | 0.270 | ||
Multivariable logistic regression analysis was applied to identify the predictors for a favorable response to statin. Variables with a P<0.10 in the univariate test were entered into the multivariable modeling. To take into account the potential cluster effects of multiple nonculprit lipid plaques in a single patient, general estimating equations were applied. Sample size: 140 plaques.
Figure 2. The number of predictors and the probability of a favorable response.
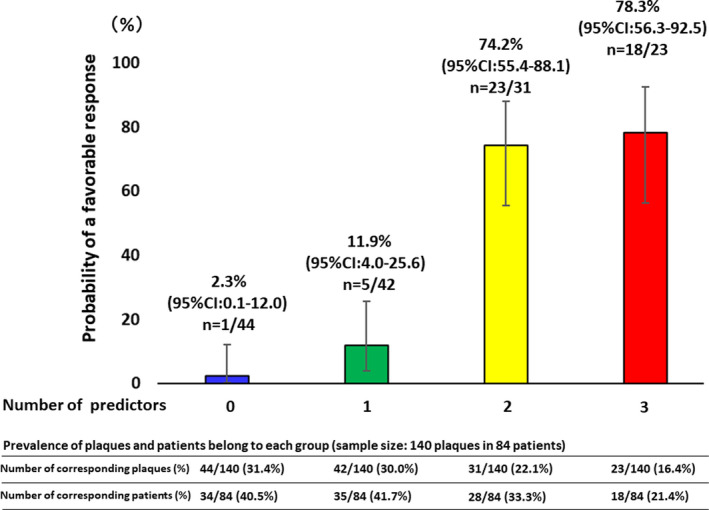
When all 3 predictors are present, the probability of a favorable response increased to 78.3%, whereas the probability of a favorable response is 2.3% when no predictors are present. Out of 140 plaques, 44 (31.4%) plaques had no, 42 (30.0%) had1, 31 (22.1%) had2, and 23 (16.4%) had 3 predictors. Out of 84 patients, 34 (40.5%) patients had plaque with no predictors, 35 (41.7%) had plaques with 1 predictor, 28 (33.3%) had plaques with 2 predictors, and 18 (21.4%) had plaques with 3 predictors. Clopper–Pearson Exact method was applied to calculate the 95% CI of the proportions. Sample size: 140 plaques in 84 patients.
In addition, we performed a sensitivity analysis which analyzes 1 randomly selected plaque from each patient. Those results are shown in Table S1.
Change of Macrophage Index and Plaque Response
The significant correlations between the change in the macrophage index, and the change in thin‐cap area and thinnest FCT are shown in Figure 3. Lower macrophage index was associated with a greater reduction in thin cap area and increase in FCT.
Figure 3. Correlation between changes in macrophage index and changes in fibrous cap.
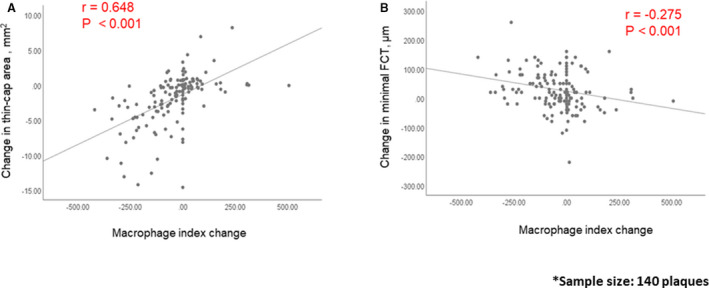
Significant correlations between the change in macrophage index and the change in thin‐cap area (A), or fibrous cap thickness (FCT) (B) were observed. Sample size: 140 plaques.
The plaques were divided into 2 groups based on changes in the macrophage index. Macrophage index increased in 46 (32.9%) plaques. Plaques in which the macrophage index did not increase had a greater improvement of the thin‐cap area (Figure 4) and a decreased prevalence of TCFA (Figure 5) at follow‐up. The prevalence of layered plaque significantly increased only in the group in which the macrophage index increased at follow‐up, indicating persistent vascular inflammation was associated with the development of new layered plaques (Figure 5).
Figure 4. Macrophage index and changes in thin‐cap area.
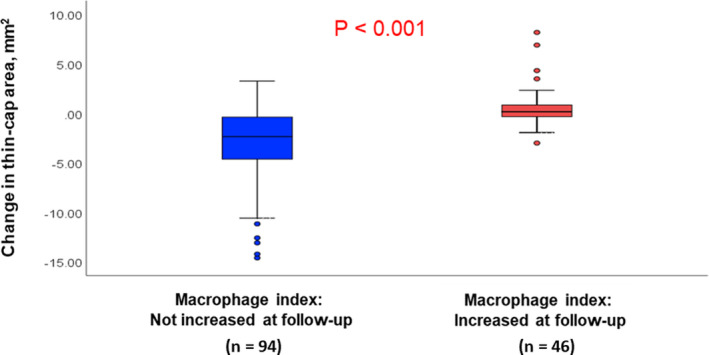
Plaques in which the macrophage index did not increase (n=94) had a greater improvement in the thin‐cap area than the plaques in which the macrophage index increased (n=46).
Figure 5. Macrophage index and changes in plaque phenotype.
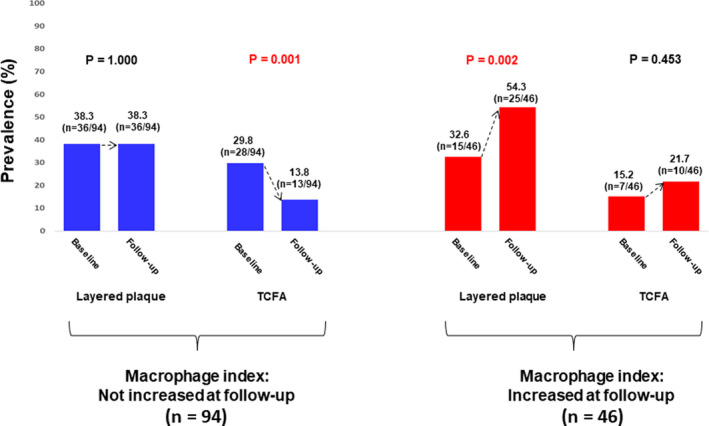
The prevalence of TCFA significantly decreased in the group in which macrophage index did not increase, whereas the prevalence of layered plaque significantly increased in the group in which macrophage index increased. These findings indicate that control of inflammation is associated with stabilization of lipid plaques. TCFA indicates thin‐cap fibroatheroma.
Predictors for a Favorable Vascular Response to Statin Therapy in Statin Naïve Patients
We evaluated if these 3 predictors (the baseline thin‐cap area, macrophage index, and layered plaque) apply to statin naïve patients (Tables S2 and S3). Fifty‐four nonculprit lipid plaques from 33 statin naïve patients were divided into 3 groups based on the absolute change in the thin‐cap area (median thin‐cap area change: −5.063 [−10.640 to −3.716] mm2 in the first tertile, −1.907 [−2.361 to −0.936] mm2 in the second tertile, 0.623 [−0.170 to 0.698] mm2 in the third tertile). Univariate analysis showed that baseline thin‐cap area and macrophage index were associated with a favorable FC response. The prevalence of layered plaque was higher in the favorable FC response group than that in the less‐favorable FC response groups, although it was not statistically significant (50.0% versus 30.6%, P=0.163).
Discussion
In this study, we demonstrated that plaques with features of vulnerability (large thin‐cap area, high macrophage index, and layered plaque) responded more favorably to statin therapy. Moreover, significant correlations were observed between the change in the macrophage index and the changes in thin‐cap area. Finally, the prevalence of layered (vulnerable) plaques significantly increased only in plaques with increased macrophage index at follow‐up, underscoring the importance of effective control of inflammation.
There have been several studies evaluating response to statin therapy. However, the focus of those studies was different: one study studied changes in plaque composition, 11 other reports were for specific subgroups such as diabetes mellitus, sex, or age. 23 , 24 , 25 Still other studies investigated only specific plaque components such as neovascularization or spotty calcium. 26 , 27 In contrast to those previously published studies, we performed comprehensive comparisons including patient demographics, laboratory parameters, and both qualitative and quantitative OCT parameters between those with and without favorable response to statin therapy. In addition, a novel 3‐dimensional fibrous cap measurement algorithm was used to minimize errors due to sampling 1 small area of a fibrous cap.
Statin Therapy and Vulnerable Plaques
Previous pathology studies of sudden cardiac death victims have reported that 60% to 70% of cases of coronary thrombosis were caused by plaque rupture. 3 , 4 Patients with ACS caused by plaque rupture had worse clinical outcomes than those with plaque erosion. 28 , 29 It is believed that plaque rupture occurs in vulnerable plaques that are characterized by a thin FC, large necrotic core, and local vascular inflammation, as evidenced by macrophage infiltration. 30 Previous studies have shown that statins stabilize vulnerable plaques not only by reducing LDL cholesterol and lipid content but also by thickening FCT and suppressing vascular inflammation. 31 , 32 , 33 Inflammation stimulates a local immune reaction and activates macrophages, mast cells, and T cells to release cytokines that inhibit collagen synthesis and proteases such as matrix metalloproteinase that digest fibrous components. 34 In particular, interferon‐γ has a powerful impact on the FC through inhibition of smooth muscle cell differentiation, procollagen‐I gene expression, and the collagen cross‐linking enzyme. 34 Statins are known to reduce these inflammatory reactions, cytokines, and degenerative enzymes. Moreover, statins promote collagen synthesis and increase smooth muscle cell content, which stabilize the FC. 33 A recent OCT study showed that statins reduced the serum high‐sensitivity C‐reactive protein and matrix metalloproteinase‐9, and that these changes were associated with an increase in FCT. 6 In our study, baseline vulnerable and inflammatory features (large thin‐cap area and high macrophage index) were significant predictors of a favorable vascular response (thickening of FC). The effect of statins on plaque stabilization appears to be greater in plaques that are more vulnerable and inflamed at baseline, although the reduction of thin‐cap area was not different by the degree of LDL cholesterol reduction (Figures S1 through S3). This is consistent with the previous studies that have shown that statins are more effective in patients with a high baseline inflammatory status compared with those with a low inflammatory status. 35 , 36 , 37 Furthermore, these results are supported by reports that have shown statins to be more effective for stabilization of plaque vulnerability in patients with ACS than in patients with stable angina. 10 , 38
Macrophage Index Change and Plaque Vulnerability
Plaques with an increased macrophage index at follow‐up showed a less favorable vascular response and a higher prevalence of layered plaque despite statin therapy (Figures 4 and 5). These results indicate that persistent active inflammation despite statin therapy may prevent plaques from having a favorable response and keep them vulnerable, as evidenced by the development of new layered plaques. More aggressive cholesterol lowering therapy or the addition of anti‐inflammatory agents may be necessary for this group of patients.
Layered Plaque and Vascular Response to Statin
Layered plaques are thought to be the consequence of previous silent plaque rupture or erosion. 3 In a histology validation report, OCT proved to have a high sensitivity and specificity for the detection of healed/layered plaques. 18 A previous OCT study revealed that patients with layered plaques at culprit lesions had elevated biomarkers of systemic inflammation, a higher prevalence of vulnerable features (plaque rupture, TCFA, and macrophage infiltration) and a higher rate of rehospitalization than those without layered phenotype. 8 Moreover, a recent study reported that layered plaques at nonculprit lesions also had more vulnerable features (larger lipid burden, higher rates of TCFA and macrophage infiltration), compared with nonlayered plaques. 9 These results indicate that a layered plaque may be a signature of panvascular inflammation and that statins may have a greater stabilization effect on their vulnerable features and high levels of inflammation than on nonlayered plaques. However, when inflammation is not under control (as evidenced by increased macrophage index at follow‐up in Figures 4 and 5), the prevalence of layered plaque increases, indicating persistent plaque vulnerability.
Internal Consistency of Plaque Responses Within Individual Patients
We analyzed the internal consistency of plaque outcomes within individual patients. Among 84 patients, 30 patients had more than 2 nonculprit plaques. Of those, 11 patients had only plaques with a less‐favorable response, 2 patients had only plaques with a favorable response, and 17 patients had both plaques with favorable and less‐favorable responses (Figure S4). Considering this result that more than half of the patients had both plaques with mixed (favorable and less‐favorable) response, each plaque may not behave consistently within each patient but may behave independently. These data indicate that both the level of vascular inflammation of each individual plaque and the level of systemic inflammation are important for predicting the response to statin therapy.
Clinical Implications
Our study suggests that the magnitude of baseline plaque abnormalities, especially inflammatory and vulnerable features, are the important indicators of favorable plaque response to statin therapy. Statins have not only a cholesterol lowering effect but also an anti‐inflammatory effect. Thus, highly inflamed vessels evidenced by high macrophage index, layered plaque, and large thin‐cap area appear to respond more favorably than those with a lower level of inflammation. Statin therapy should be started immediately in patients who have OCT predictors for a favorable response (large thin‐cap area, high macrophage index, and layered plaque). For those who do not have these predictors or who have evidence of persistent active inflammation, additional therapies such as anti‐inflammatory agents may need to be considered.
Study Limitations
This study has several limitations. First, we retrospectively selected patients who had serial OCT imaging from the registry database; therefore, selection bias cannot be excluded. Second, although we had data on the intensity of statin treatment, we did not have detailed information on the duration of statin treatment. In addition, the selection and dose of statin were at the discretion of the treating physician. All patients in the current study were from East Asia, where LDL level is low in the general population. Third, the number of patients was small. Thus, we could not analyze the predictors for a favorable response in each statin intensity group and could not perform multivariable analysis in the statin naïve cohort. Fourth, so far, there are no validated OCT criteria for macrophage quantification. Therefore, we took a semiquantitative approach using the macrophage index, as described in previous studies. 19 , 20 Fifth, although there was good agreement between histology and OCT in identifying layered plaques, the accuracy of layered plaque identification by OCT is not fully established. Sixth, we could not obtain the data for smoking duration and the details of family history of premature coronary disease, although they might affect the vascular response to statin therapy. Finally, this study was conducted with only baseline patient data and relatively short‐term follow‐up (6 months); thus the impact of FC response on clinical outcomes remains unknown.
Conclusions
Three significant predictors for a favorable vascular response to statin therapy were identified: large thin‐cap area, high macrophage index, and layered plaque. Favorable vascular response to statin was correlated with signs of decreased inflammation.
Sources of Funding
Dr Jang's research was supported by the Allan Gray Fellowship Fund in Cardiology and by Mr and Mrs Michael and Kathryn Park. They had no role in the design or conduct of this research.
Disclosures
Dr Jang has received educational grant support from Abbott Vascular and a consulting fee from Svelte Medical Systems. The remaining authors have no disclosures to report.
Supporting information
Tables S1–S3
Figures S1–S4
(J Am Heart Assoc. 2021;10:e018205. DOI: 10.1161/JAHA.120.018205.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018205
For Sources of Funding and Disclosures, see page 10.
Contributor Information
Yoshiyasu Minami, Email: nrg12391@yahoo.co.jp.
Ik‐Kyung Jang, Email: ijang@mgh.harvard.edu.
References
- 1. Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. [DOI] [PubMed] [Google Scholar]
- 2. Finn AV, Nakano M, Narula J, Kolodgie FD, Virmani R. Concept of vulnerable/unstable plaque. Arterioscler Thromb Vasc Biol. 2010;30:1282–1292. [DOI] [PubMed] [Google Scholar]
- 3. Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: a comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 2000;20:1262–1275. [DOI] [PubMed] [Google Scholar]
- 4. Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. [DOI] [PubMed] [Google Scholar]
- 5. Yonetsu T, Kakuta T, Lee T, Takahashi K, Kawaguchi N, Yamamoto G, Koura K, Hishikari K, Iesaka Y, Fujiwara H, et al. In vivo critical fibrous cap thickness for rupture‐prone coronary plaques assessed by optical coherence tomography. Eur Heart J. 2011;32:1251–1259. [DOI] [PubMed] [Google Scholar]
- 6. Komukai K, Kubo T, Kitabata H, Matsuo Y, Ozaki Y, Takarada S, Okumoto Y, Shiono Y, Orii M, Shimamura K, et al. Effect of atorvastatin therapy on fibrous cap thickness in coronary atherosclerotic plaque as assessed by optical coherence tomography: the EASY‐FIT study. J Am Coll Cardiol. 2014;64:2207–2217. [DOI] [PubMed] [Google Scholar]
- 7. Minami Y, Wang Z, Aguirre AD, Ong DS, Kim CJ, Uemura S, Soeda T, Lee H, Fujimoto J, Jang IK. Clinical predictors for lack of favorable vascular response to statin therapy in patients with coronary artery disease: a serial optical coherence tomography study. J Am Heart Assoc. 2017;6:e006241. DOI: 10.1161/JAHA.117.006241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fracassi F, Crea F, Sugiyama T, Yamamoto E, Uemura S, Vergallo R, Porto I, Lee H, Fujimoto J, Fuster V, et al. Healed culprit plaques in patients with acute coronary syndromes. J Am Coll Cardiol. 2019;73:2253–2263. [DOI] [PubMed] [Google Scholar]
- 9. Russo M, Kim HO, Kurihara O, Araki M, Shinohara H, Thondapu V, Yonetsu T, Soeda T, Minami Y, Higuma T, et al. Characteristics of non‐culprit plaques in acute coronary syndrome patients with layered culprit plaque. Eur Heart J Cardiovasc Imaging. 2020;21:1421–1430. [DOI] [PubMed] [Google Scholar]
- 10. Gin AL, Vergallo R, Minami Y, Ong DS, Hou J, Jia H, Soeda T, Hu S, Zhang S, Lee H, et al. Changes in coronary plaque morphology in patients with acute coronary syndrome versus stable angina pectoris after initiation of statin therapy. Coron Artery Dis. 2016;27:629–635. [DOI] [PubMed] [Google Scholar]
- 11. Räber L, Koskinas KC, Yamaji K, Taniwaki M, Roffi M, Holmvang L, Garcia Garcia HM, Zanchin T, Maldonado R, Moschovitis A, et al. Changes in coronary plaque composition in patients with acute myocardial infarction treated with high‐intensity statin therapy (IBIS‐4): a serial optical coherence tomography study. JACC Cardiovasc Imaging. 2019;12:1518–1528. DOI: 10.1016/j.jcmg.2018.08.024. [DOI] [PubMed] [Google Scholar]
- 12. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. Circulation. 2014;129:S1–S45. DOI: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 13. Tearney GJ, Regar E, Akasaka T, Adriaenssens T, Barlis P, Bezerra HG, Bouma B, Bruining N, Cho J‐M, Chowdhary S, et al. Consensus standards for acquisition, measurement, and reporting of intravascular optical coherence tomography studies: a report from the International Working Group for Intravascular Optical Coherence Tomography Standardization and Validation. J Am Coll Cardiol. 2012;59:1058–1072. DOI: 10.1016/j.jacc.2011.09.079. [DOI] [PubMed] [Google Scholar]
- 14. Prati F, Regar E, Mintz GS, Arbustini E, Di Mario C, Jang I‐K, Akasaka T, Costa M, Guagliumi G, Grube E, et al. Expert review document on methodology, terminology, and clinical applications of optical coherence tomography: physical principles, methodology of image acquisition, and clinical application for assessment of coronary arteries and atherosclerosis. Eur Heart J. 2010;31:401–415. DOI: 10.1093/eurheartj/ehp433. [DOI] [PubMed] [Google Scholar]
- 15. Prati F, Guagliumi G, Mintz GS, Costa M, Regar E, Akasaka T, Barlis P, Tearney GJ, Jang I‐K, Arbustini E, et al. Expert review document part 2: methodology, terminology and clinical applications of optical coherence tomography for the assessment of interventional procedures. Eur Heart J. 2012;33:2513–2520. DOI: 10.1093/eurheartj/ehs095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vergallo R, Uemura S, Soeda T, Minami Y, Cho J‐M, Ong DS, Aguirre AD, Gao L, Biasucci LM, Crea F, et al. Prevalence and predictors of multiple coronary plaque ruptures. in vivo 3‐vessel optical coherence tomography imaging study. Arterioscler Thromb Vasc Biol. 2016;36:2229–2238. DOI: 10.1161/ATVBAHA.116.307891. [DOI] [PubMed] [Google Scholar]
- 17. Kato K, Yonetsu T, Kim S‐J, Xing L, Lee H, McNulty I, Yeh RW, Sakhuja R, Zhang S, Uemura S, et al. Nonculprit plaques in patients with acute coronary syndromes have more vulnerable features compared with those with non‐acute coronary syndromes: a 3‐vessel optical coherence tomography study. Circ Cardiovasc Imaging. 2012;5:433–440. DOI: 10.1161/CIRCIMAGING.112.973701. [DOI] [PubMed] [Google Scholar]
- 18. Shimokado A, Matsuo Y, Kubo T, Nishiguchi T, Taruya A, Teraguchi I, Shiono Y, Orii M, Tanimoto T, Yamano T, et al. In vivo optical coherence tomography imaging and histopathology of healed coronary plaques. Atherosclerosis. 2018;275:35–42. [DOI] [PubMed] [Google Scholar]
- 19. Burgmaier M, Milzi A, Dettori R, Burgmaier K, Marx N, Reith S. Co‐localization of plaque macrophages with calcification is associated with a more vulnerable plaque phenotype and a greater calcification burden in coronary target segments as determined by OCT. PLoS One. 2018;13:e0205984. DOI: 10.1371/journal.pone.0205984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Reith S, Milzi A, Dettori R, Marx N, Burgmaier M. Predictors for target lesion microcalcifications in patients with stable coronary artery disease: an optical coherence tomography study. Clin Res Cardiol. 2018;107:763–771. DOI: 10.1007/s00392-018-1243-1. [DOI] [PubMed] [Google Scholar]
- 21. Galon MZ, Wang Z, Bezerra HG, Lemos PA, Schnell A, Wilson DL, Rollins AM, Costa MA, Attizzani GF. Differences determined by optical coherence tomography volumetric analysis in non‐culprit lesion morphology and inflammation in ST‐segment elevation myocardial infarction and stable angina pectoris patients. Catheter Cardiovasc Interv. 2015;85:E108–E115. DOI: 10.1002/ccd.25660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang Z, Cho Y‐S, Soeda T, Minami Y, Xing L, Jia H, Aguirre A, Vergallo R, Lee H, Fujimoto JG, et al. Three‐dimensional morphological response of lipid‐rich coronary plaques to statin therapy: a serial optical coherence tomography study. Coron Artery Dis. 2016;27:350–356. DOI: 10.1097/MCA.0000000000000370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurihara O, Thondapu V, Kim HO, Russo M, Sugiyama T, Yamamoto E, Fracassi F, Minami Y, Wang Z, Lee H, et al. Comparison of vascular response to statin therapy in patients with versus without diabetes mellitus. Am J Cardiol. 2019;123:1559–1564. DOI: 10.1016/j.amjcard.2019.02.020. [DOI] [PubMed] [Google Scholar]
- 24. Minami Y, Hou J, Xing L, Jia H, Hu S, Vergallo R, Soeda T, Lee H, Zhang S, Yu BO, et al. Serial optical coherence tomography and intravascular ultrasound analysis of gender difference in changes of plaque phenotype in response to lipid‐lowering therapy. Am J Cardiol. 2016;117:1890–1895. DOI: 10.1016/j.amjcard.2016.03.038. [DOI] [PubMed] [Google Scholar]
- 25. Nozue T, Yamamoto S, Tohyama S, Fukui K, Umezawa S, Onishi Y, Kunishima T, Sato A, Nozato T, Miyake S, et al. Impacts of age on coronary atherosclerosis and vascular response to statin therapy. Heart Vessels. 2014;29:456–463. DOI: 10.1007/s00380-013-0387-1. [DOI] [PubMed] [Google Scholar]
- 26. Liu X, Sun C, Gu X, Liu X, Wang X, Wang X, Xie Z, Tian J, Yu B. Intraplaque neovascularization attenuated statin benefit on atherosclerotic plaque in CAD patients: a follow‐up study with combined imaging modalities. Atherosclerosis. 2019;287:134–139. DOI: 10.1016/j.atherosclerosis.2019.06.912. [DOI] [PubMed] [Google Scholar]
- 27. Afolabi A, Mustafina I, Zhao L, Li L, Sun R, Hu S, Zhang S, Jia H, Guilio G, Yu B. Does spotty calcification attenuate the response of nonculprit plaque to statin therapy?: a serial optical coherence tomography study. Catheter Cardiovasc Interv. 2018;91:582–590. [DOI] [PubMed] [Google Scholar]
- 28. Yamamoto E, Yonetsu T, Kakuta T, Soeda T, Saito Y, Yan BP, Kurihara O, Takano M, Niccoli G, Higuma T, et al. Clinical and laboratory predictors for plaque erosion in patients with acute coronary syndromes. J Am Heart Assoc. 2019;8:e012322. DOI: 10.1161/JAHA.119.012322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hu S, Zhu Y, Zhang Y, Dai J, Li L, Dauerman H, Soeda T, Wang Z, Lee H, Wang C, et al. Management and outcome of patients with acute coronary syndrome caused by plaque rupture versus plaque erosion: an intravascular optical coherence tomography study. J Am Heart Assoc. 2017;6:e004730. DOI: 10.1161/JAHA.116.004730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Naghavi M, Libby P, Falk E, Casscells SW, Litovsky S, Rumberger J, Badimon JJ, Stefanadis C, Moreno P, Pasterkamp G, et al. From vulnerable plaque to vulnerable patient: a call for new definitions and risk assessment strategies: Part I. Circulation. 2003;108:1664–1672. DOI: 10.1161/01.CIR.0000087480.94275.97. [DOI] [PubMed] [Google Scholar]
- 31. Nissen SE, Tuzcu EM, Schoenhagen P, Brown BG, Ganz P, Vogel RA, Crowe T, Howard G, Cooper CJ, Brodie B, et al. Effect of intensive compared with moderate lipid‐lowering therapy on progression of coronary atherosclerosis: a randomized controlled trial. JAMA. 2004;291:1071–1080. DOI: 10.1001/jama.291.9.1071. [DOI] [PubMed] [Google Scholar]
- 32. Hiro T, Kimura T, Morimoto T, Miyauchi K, Nakagawa Y, Yamagishi M, Ozaki Y, Kimura K, Saito S, Yamaguchi T, et al. Effect of intensive statin therapy on regression of coronary atherosclerosis in patients with acute coronary syndrome: a multicenter randomized trial evaluated by volumetric intravascular ultrasound using pitavastatin versus atorvastatin (JAPAN‐ACS [Japan assessment of pitavastatin and atorvastatin in acute coronary syndrome] study). J Am Coll Cardiol. 2009;54:293–302. DOI: 10.1016/j.jacc.2009.04.033. [DOI] [PubMed] [Google Scholar]
- 33. Diamantis E, Kyriakos G, Quiles‐Sanchez LV, Farmaki P, Troupis T. The anti‐inflammatory effects of statins on coronary artery disease: an updated review of the literature. Curr Cardiol Rev. 2017;13:209–216. DOI: 10.2174/1573403X13666170426104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hansson GK, Libby P, Tabas I. Inflammation and plaque vulnerability. J Intern Med. 2015;278:483–493. DOI: 10.1111/joim.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ridker PM, Rifai N, Pfeffer MA, Sacks FM, Moye LA, Goldman S, Flaker GC, Braunwald E. Inflammation, pravastatin, and the risk of coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events (CARE) Investigators. Circulation. 1998;98:839–844. DOI: 10.1161/01.CIR.98.9.839. [DOI] [PubMed] [Google Scholar]
- 36. McMurray JJV, Kjekshus J, Gullestad L, Dunselman P, Hjalmarson Åke, Wedel H, Lindberg M, Waagstein F, Grande P, Hradec J, et al. Effects of statin therapy according to plasma high‐sensitivity C‐reactive protein concentration in the controlled rosuvastatin multinational trial in heart failure (CORONA): a retrospective analysis. Circulation. 2009;120:2188–2196. DOI: 10.1161/CIRCULATIONAHA.109.849117. [DOI] [PubMed] [Google Scholar]
- 37. Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, Gotto AM Jr. Measurement of C‐reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–1965. DOI: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 38. Tsujita K, Sugiyama S, Sumida H, Shimomura H, Yamashita T, Yamanaga K, Komura N, Sakamoto K, Oka H, Nakao K, et al. Impact of dual lipid‐lowering strategy with ezetimibe and atorvastatin on coronary plaque regression in patients with percutaneous coronary intervention: the multicenter randomized controlled PRECISE‐IVUS trial. J Am Coll Cardiol. 2015;66:495–507. DOI: 10.1016/j.jacc.2015.05.065. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S3
Figures S1–S4


