The Fourth Universal Definition of Myocardial Infarction was notable for highlighting the importance of myocardial injury. 1 It described a plethora of pathophysiologic processes associated with myocardial injury, which was defined by elevation of cardiac troponin (cTn) levels >99th percentile of the normal reference range. 1 If the cTn elevations were associated with clinical ischemia (symptoms, ECG changes, or imaging evidence), either type 1 (plaque rupture and thrombus) or type 2 (supply/demand imbalance) myocardial infarction (MI) should be diagnosed. Myocardial injury may be acute, with a rise and/or fall of cTn levels, or chronic, defined as <20% change in cTn levels beyond 48 hours previously. Chronic cTn elevation may occur in patients with stable coronary artery disease (CAD) and additionally reflect the burden of noncardiovascular disease, such as diabetes mellitus or renal disease, which may be causes of adverse outcomes and mortality long‐term.
Type 2 MI (T2MI) is common among hospital populations, accounting for at least 25% of all MI. 1 Patients with myocardial injury and T2MI have distinct cardiovascular phenotypes compared with patients with type 1 MI (T1MI), being older, more likely to be women, having lower cTn levels, and more likely to have diabetes mellitus, sepsis, pulmonary embolism, respiratory failure, hypertension, atrial fibrillation, heart failure (HF), or renal failure.
Mechanisms by which cTn can be released from cardiomyocytes include necrosis, apoptosis, normal cell turnover, release of protein degradation products, increased cell membrane permeability, and release of membranous "blebs." 2
In myocardial injury, cTn elevations may be mediated by direct toxicity from circulating cytokines, catecholamines, or vasopressors. Some of these factors are instrumental in Takotsubo syndrome, with microvascular spasm also possibly playing a part. Takotsubo syndrome is not considered an MI because of the absence of clinical ischemia, reversibility of left ventricular dysfunction, and the myocardial injury occurring beyond the distribution of a single coronary artery. 1
The amount of myocardial injury is usually larger and more localized in T1MI to the coronary artery territory supplied distal to the acute plaque event, producing higher elevations of cTn levels, than in T2MI. The occurrence of T2MI may be a more global ischemic phenomenon with regional myocardial wall motion dysfunction depending on the extent of associated CAD. The presence of underlying CAD is likely to play a major role in altering the threshold for myocardial ischemia. The incidence of associated CAD is variable, depending on the population studied, and ranges from 40% to 78%. 1 , 3 MI with nonobstructive coronary arteries may occur in either T1MI or T2MI and be associated with coronary endothelial dysfunction, epicardial coronary spasm, microvascular spasm, dissection, anemia, or bleeding.
There are 5 cardiovascular evidence‐based medicines (EBMs) for the management of T1MI (Figure), together with percutaneous coronary intervention (PCI). 4 , 5 However, for the management of myocardial injury or T2MI, there are no proven treatments. The role of invasive coronary angiography, as well as computed tomographic coronary angiography, is not well defined for patients with T2MI, and there have been few reports of the use of intravascular ultrasound and optical coherence tomography. The rates of angiography are usually low, which may be appropriate in some patients who may have renal impairment, cognitive impairment, or anemia, or be at high risk of bleeding. Consequently, management in clinical practice for both these entities varies across different hospitals and countries. Most important, patients with myocardial injury and T2MI have a worse prognosis compared with patients with T1MI. 1 , 6 , 7
Figure 1. Management of myocardial injury and type 2 myocardial infarction (MI).
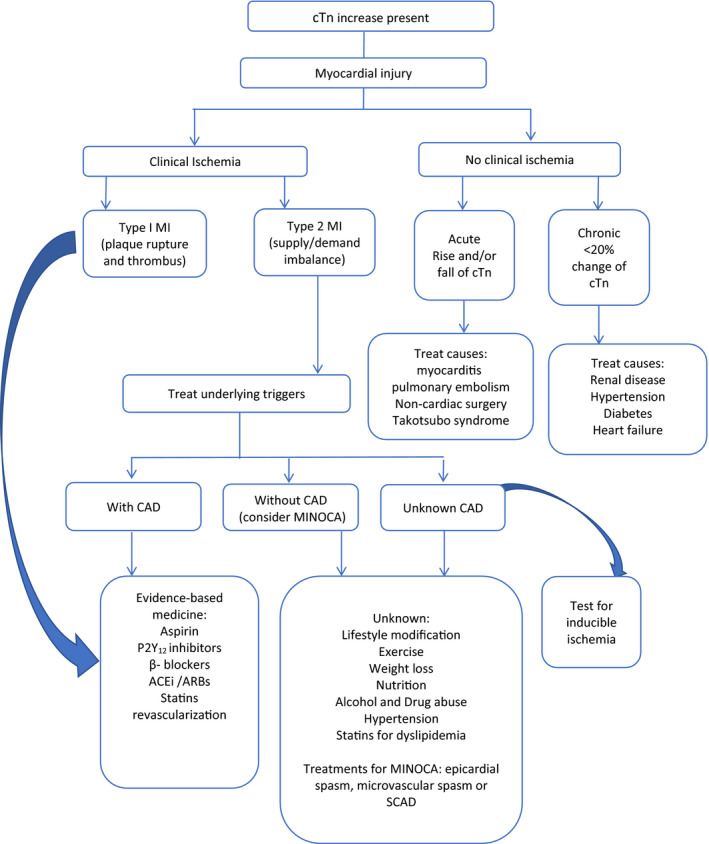
ACEi indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; CAD, coronary artery disease; cTn, cardiac troponin; MINOCA, MI with nonobstructive coronary arteries; and SCAD, spontaneous coronary artery dissection.
In this issue of the Journal of the American Heart Association (JAHA), Kadesjö et al 8 report a single‐center cohort study from Stockholm, Sweden. The aim of the study was to investigate whether EBMs were associated with a reduction in patients with acute or chronic myocardial injury and T2MI. The focus was on myocardial injury and T2MI.
Of 22 589 patients presenting to the emergency department with chest pain, 3853 with at least one level of high‐sensitivity cTn T >14 ng/L (>99th percentile upper reference limit) or <12 ng/L and a Δ‐cTn of ±≥3 ng/L were categorized following adjudication using the Fourth Universal Definition of Myocardial Infarction, into having either T1MI, acute or chronic myocardial injury or T2MI. Patients with chronic injury had stable and elevated high‐sensitivity cTn levels in the absence of an acute medical condition. No specific absolute or relative Δ criteria were applied to define stable high‐sensitivity cTn levels.
Data from all dispensed prescriptions within 180 days of the visit to emergency department were obtained for β‐blockers, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers, statins, and platelet inhibitors. The number of medications was categorized into the following 3 groups: 0 to 1, 2 to 3 or 4 medications (0–1 medication was the referent).
Less than half of all patients with acute or chronic myocardial injury and T2MI were treated with statins. Similarly, only approximately half of these patient groups were treated with aspirin or a P2Y12 inhibitor.
Overall, 29.7% had acute myocardial injury, 35% had chronic myocardial injury, and 6.5% had T2MI. Rates of coronary angiography were not reported. Within 30 days from the index date, only 1.2% of patients with acute injury, 1.3% with chronic injury, and 2.9% with T2MI underwent revascularization. During a mean follow‐up of 3.1±1.5 years, 1059 patients (27%) died.
In patients with T2MI, dispensing of 2 to 3 (50.1%) and 4 (27.9%) medications was associated with a 50% and 56% lower mortality, respectively. Corresponding reduction in death occurred in patients with acute myocardial injury of 24% (51.7% dispensed 2–3 medications) and 29% (19.5% dispensed 4 medications). In patients with chronic myocardial injury, mortality was reduced 27% (52.9% dispensed 2–3 medications) and 37% (19.2% dispensed 4 medications). For the combined outcome of death, MI, HF, and stroke, the findings were similar.
The authors concluded that patients with acute or chronic myocardial injury and T2MI are infrequently prescribed EBMs. Increasing the number of EBM treatments in patients with acute or chronic myocardial injury and T2MI was associated with a lower risk of death.
Given the lack of evidence for EBMs in patients with myocardial injury or T2MI, it is perhaps not surprising that in this study few patients with myocardial injury or T2MI were dispensed EBMs.
There are several limitations. The authors focused on the number of medication classes rather than on specific medications. In addition, patients having myocardial injury or T2MIs may have been previously prescribed β‐blockers (reported as 73%) as hypertension and atrial fibrillation requiring rate control are often associated with myocardial injury and T2MI. These patients would be expected to have worse outcome because of the underlying disease states. Also, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers may have been prescribed (reported as 61%) for patients with hypertension, diabetes mellitus, or renal disease, with similar treatment bias effects and worse outcomes.
In this study, there was no separation of aspirin and P2Y12 inhibitors and there was no reporting of the extent of CAD; thus, the role of EBMs in patients with CAD compared with those without CAD cannot be determined. It is likely that the effect of treatments in these 2 situations will be different, and a division of T2MI into patients with and patients without CAD would likely advance our understanding of these different categories of patients. Also, occassionally, coronary angiography may demonstrate ruptured plaques with thrombus, changing the diagnosis from T2MI to T1MI and mandating the use of EBMs.
The COMPLETE (Complete Versus Culprit‐Only Revascularization Strategies to Treat Multivessel Disease After Early PCI for STEMI [ST‐Segment–Elevation MI]) trial 9 showed that PCI following STEMI or non–ST‐segment–elevation MI of the nonculprit arteries at a median follow‐up of 3 years decreased cardiovascular death and MI by 26%, from 10.5% to 7.8%, compared with PCI of only the culprit artery. There was also a significant reduction in MI alone, which was mostly type 1 (70%), by 49%, from 6.3% to 3.1%, with complete revascularization of nonculprit arteries. There were 29 T2MIs with no reduction with complete revascularization. Rates of myocardial injury were not reported.
Treatment of Myocardial Injury and T2MI
The initial management should always be to reverse the triggering factor, be it hypertension, arrhythmia, hypoxemia, bleeding, or anemia (Figure). As the clinical diagnosis of T2MI means that there has not been an acute atherothrombotic event, dual antiplatelet therapy is not likely to be beneficial and could be harmful because of the risk of bleeding. Angiotensin‐converting enzyme inhibitors and β‐blockers could be beneficial by reducing left ventricular remodeling. Trials are underway to define the role of angiography and revascularization in patients with T2MI.
If there is evidence of significant CAD by angiography, patients with T2MI could be given statins, as they would be categorized in the recent European Society of Cardiology Lipid Guidelines as patients at high risk afer MI, albeit not patients with T1MI. The recommendation is to be on statins, with a goal of reducing the low‐density lipoprotein cholesterol to <54 mg/dL and to <46 mg/dL if recurrent ischemic events occur within 2 years. 10
On the basis that statin therapy is beneficial in patients with CAD, it would seem reasonable to perform invasive coronary angiography or computed tomographic coronary angiography to define the burden of CAD in many patients with T2MI, preferably beyond the time of increased risk of bleeding for the invasive approach.
The 2017 American Heart Association/American College of Cardiology clinical performance and quality measures for adults with STEMI and non–ST‐segment–elevation MI 11 reported 17 performance measures and 7 quality measures with the hope that they will improve quality of care and likely improve outcomes. The report was focused on T1MI, and there was no guidance for patients with T2MI.
Given the increasing prevalence of T2MI when updating the existing STEMI/non–ST‐segment–elevation MI measure set, consideration could be given to adding T2MI treatment of the underlying cause (eg, anemia or tachyarrhythmias). In addition, consideration could be given to defining whether underlying CAD is present (invasive angiography or computed tomographic coronary angiography), and if CAD is present, to recommending evidence‐based treatments that are recommended for T1MI as well as specific requirements for coronary artery dissection, coronary artery embolism, and MI with nonobstructive coronary arteries.
Increasing Rates of Myocardial Injury and T2MI
The increased use of high‐sensitivity troponin tests will result in an increase in the diagnosis of T2MI. Myocardial injury is also likely to be diagnosed more frequently with detection of more instances of "nonischemic" myocardial damage. It is foreseeable that myocardial injury may become more common than T2MI or T1MI. Furthermore, the introduction of an International Classification of Diseases, Tenth Revision, Clinical Modification (ICD ‐10‐CM) code for T2MI in October 2017 will likely increase the number of T2MIs. There is no coding for myocardial injury, which ideally would include the associations causing the injury.
Prevention of T2MI has been shown with the proprotein convertase subtilisin/kexin type 9 inhibitor alirocumab. 12 Other treatments that could be tested include anti‐ischemic agents, aldosterone antagonists, which have been shown in meta‐analysis to reduce cardiovascular death and HF, and hospitalization in patients with HF and reduced ejection fraction, and post‐MI, to reduce all‐cause death. 13 Sodium‐glucose cotransporter‐2 inhibitors also could be tested as they have been shown in meta‐analysis to reduce cardiovascular death/HF admissions in patients with HF and reduced ejection fraction. 14
The current coronavirus disease 2019 pandemic has seen a dramatic increase in the incidence of myocardial injury, with elevation of cTn levels being noted in 45.3% of patients positive for coronavirus disease 2019. 15 There are several possible reasons for this, including cytokine storm, bradykinin storm, myocarditis, or MI. The incidence of T2MIs is unknown.
Conclusions
There have been few studies comparing EBMs and outcomes in patients with myocardial injury and patients with T2MI. The study by Kadesjö et al 8 is important, but the results should be considered hypothesis generating and the findings should be considered associations and not causations. The study does not change recommendations for treatments. Further research is required to define strategies for prevention, diagnosis, and management of myocardial injury and T2MI.
There is much clinical ambiguity associated with determining whether an increased cTn level is attributable to myocardial injury or T2MI. If there is an added insult of ischemia, T1MI or T2MI may be diagnosed. Physicians often conflate these diagnoses with T1MI and use EBMs developed from clinical trials performed with patients with T1MI.
Both injury and T2MI are increasing in frequency, and it is incumbent on physicians to recognize this. Management is not well defined and depends on clinical judgment in the individual patient.
Disclosures
Dr White reports receiving grant support paid to the institution and fees for serving on a steering committee for the ODYSSEY OUTCOMES trial (Evaluation of Cardiovascular Outcomes After an Acute Coronary Syndrome During Treatment With Alirocumab) from Sanofi‐Aventis and Regeneron Pharmaceuticals, for the ACCELERATE study (A Study of Evacetrapib in High‐Risk Vascular Disease) from Eli Lilly, for the STRENGTH trial (Outcomes Study to Assess Statin Residual Risk Reduction With EpaNova in High CV Risk Patients With Hypertriglyceridemia) from Omthera Pharmaceuticals, for the SPIRE trial (The Evaluation of Bococizumab [PF‐04950615; RN 316] in Reducing the Occurrence of Major Cardiovascular Events in High Risk Subjects) from Pfizer USA, for the HEART‐FID study (Randomized Placebo‐Controlled Trial of FCM as Treatment for Heart Failure With Iron Deficiency) from American Regent; for the CAMELLIA‐TIMI study (A Study to Evaluate the Effect of Long‐term Treatment With BELVIQ [Lorcaserin HC] on the Incidence of Major Adverse Cardiovascular Events and Conversion to Type 2 Diabetes Mellitus in Obese and Overweight Subjects With Cardiovascular Disease or Multiple Cardiovascular Risk Factors) from Eisai Inc, for the dal‐GenE study (Effect of Dalcetrapib vs Placebo on CV Risk in a Genetically Defined Population With a Recent ACS) from DalCor Pharma UK Inc, for the AEGIS‐II study from CSL Behring, for the SCORED trial (Effect of Sotagliflozin on Cardiovascular and Renal Events in Patients With Type 2 Diabetes and Moderate Renal Impairment Who Are at Cardiovascular Risk) and the SOLOIST‐WHF trial (Effect of Sotagliflozin on Cardiovascular Events in Patients With Type2 Diabetes Post Worsening Heart Failure) from Sanofi‐Aventis Australia Pty Ltd, and for the CLEAR Outcomes Study (Evaluation of Major Cardiovascular Events in Patients With, or at High Risk for, Cardiovascular Disease Who Are Statin Intolerant Treated With Bempedoic Acid [ETC‐1002] or Placebo) from Esperion Therapeutics Inc. He was on the Advisory Board for Genentech, Inc. and received lecture fees from AstraZeneca.
(J Am Heart Assoc. 2021;10:e019796. DOI: 10.1161/JAHA.120.019796.)
The opinions expressed in this article are not necessarily those of the editors or of the American Heart Association.
For Disclosures, see page 4.
See Article by Kadesjö et al.
REFERENCES
- 1. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40:237–269. [DOI] [PubMed] [Google Scholar]
- 2. White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57:2406–2408. [DOI] [PubMed] [Google Scholar]
- 3. Neumann JT, Sörensen NA, Rübsamen N, Ojeda F, Renné T, Qaderi V, Teltrop E, Kramer S, Quantius L, Zeller T, et al. Discrimination of patients with type 2 myocardial infarction. Eur Heart J. 2017;38:3514–3520. [DOI] [PubMed] [Google Scholar]
- 4. Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, et al. 2014 AHA/ACC guideline for the management of patients with non‐ST‐elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 5. O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–e425. [DOI] [PubMed] [Google Scholar]
- 6. Sarkisian L, Saaby L, Poulsen TS, Gerke O, Jangaard N, Hosbond S, Diederichsen AC, Thygesen K, Mickley H. Clinical characteristics and outcomes of patients with myocardial infarction, myocardial injury, and nonelevated troponins. Am J Med. 2016;129:446.e5–446.e21. [DOI] [PubMed] [Google Scholar]
- 7. Cediel G, Gonzalez‐del‐Hoyo M, Carrasquer A, Sanchez R, Boqué C, Bardají A. Outcomes with type 2 myocardial infarction compared with non‐ischaemic myocardial injury. Heart. 2017;103:616–622. [DOI] [PubMed] [Google Scholar]
- 8. Kadesjö E, Roos A, Siddiqui AJ, Sartipy U, Holzmann MJ. Treatment with cardiovascular medications: prognosis in patients with myocardial injury. J Am Heart Assoc. 2020;9:e017239. DOI: 10.1161/JAHA.120.017239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, Meeks B, Di Pasquale G, López‐Sendón J, Faxon DP, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. 2019;381:1411–1421. [DOI] [PubMed] [Google Scholar]
- 10. Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, et al. 2019 ESC/EAS guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2019;41:111–188. [DOI] [PubMed] [Google Scholar]
- 11. Jneid H, Addison D, Bhatt DL, Fonarow GC, Gokak S, Grady KL, Green LA, Heidenreich PA, Ho PM, Jurgens CY, et al. 2017 AHA/ACC clinical performance and quality measures for adults with ST‐elevation and non‐ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. Circ Cardiovasc Qual Outcomes. 2017;10:e000032. DOI: 10.1161/HCQ.0000000000000032 [DOI] [PubMed] [Google Scholar]
- 12. White HD, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Erglis A, Goodman SG, Hanotin C, et al. Effects of alirocumab on types of myocardial infarction: insights from the ODYSSEY OUTCOMES trial. Eur Heart J. 2019;40:2801–2809. DOI: 10.1093/eurheartj/ehz299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frankenstein L, Seide S, Täger T, Jensen K, Fröhlich H, Clark AL, Seiz M, Katus HA, Nee P, Uhlmann L, et al. Relative Efficacy of Spironolactone, Eplerenone, and cAnRenone in patients with Chronic Heart failure (RESEARCH): a systematic review and network meta‐analysis of randomized controlled trials. Heart Fail Rev. 2020;25:161–171. DOI: 10.1007/s10741-019-09832-y [DOI] [PubMed] [Google Scholar]
- 14. Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, et al. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med. 2020;383:1413–1424. DOI: 10.1056/NEJMoa2022190 [DOI] [PubMed] [Google Scholar]
- 15. Lombardi CM, Carubelli V, Iorio A, Inciardi RM, Bellasi A, Canale C, Camporotondo R, Catagnano F, Dalla Vecchia LA, Giovinazzo S, et al. Association of troponin levels with mortality in Italian patients hospitalized with coronavirus disease 2019: results of a multicenter study. JAMA Cardiol. 2020;5:1274–1280. DOI: 10.1001/jamacardio.2020.3538 [DOI] [PMC free article] [PubMed] [Google Scholar]


