Abstract
Background
Detailed insights in temporal evolution of high‐sensitivity cardiac troponin following acute coronary syndrome (ACS) are currently missing. We aimed to describe and compare the post‐ACS kinetics of high‐sensitivity cardiac troponin I (hs‐cTnI) and high‐sensitivity cardiac troponin T (hs‐cTnT), and to determine their intra‐ and interindividual variation in clinically stable patients.
Methods and Results
We determined hs‐cTnI (Abbott) and hs‐cTnT (Roche) in 1507 repeated blood samples, derived from 191 patients with ACS (median, 8/patient) who remained free from adverse cardiac events during 1‐year follow‐up. Post‐ACS kinetics were studied by linear mixed‐effect models. Using the samples collected in the 6‐ to 12‐month post‐ACS time frame, patients were then considered to have chronic coronary syndrome. We determined (differences between) the average hs‐cTnI and average hs‐cTnT concentration, and the intra‐ and interindividual variation for both biomarkers. Compared with hs‐cTnT, hs‐cTnI peaked higher (median 3506 ng/L versus 494 ng/L; P<0.001) and was quicker below the biomarker‐specific upper reference limit (16 versus 19 days; P<0.001). In the post–6‐month samples, hs‐cTnI and hs‐cTnT showed modest correlation (r spearman=0.60), whereas the average hs‐cTnT concentration was 5 times more likely to be above the upper reference limit than hs‐cTnI. The intraindividual variations of hs‐cTnI and hs‐cTnT were 14.0% and 18.1%, while the interindividual variations were 94.1% and 75.9%.
Conclusions
Hs‐cTnI peaked higher after ACS and was quicker below the upper reference limit. In the post–6‐month samples, hs‐cTnI and hs‐cTnT were clearly not interchangeable, and average hs‐cTnT concentrations were much more often above the upper reference limit than hs‐cTnI. For both markers, the within‐patient variation fell largely below beween‐patient variation.
Registration
URL: https://www.trialregister.nl; unique identifiers: NTR1698 and NTR1106.
Keywords: biological variation, longitudinal studies, myocardial infarction, precision medicine, troponin
Subject Categories: Epidemiology, Biomarkers, Chronic Ischemic Heart Disease, Myocardial Infarction
Nonstandard Abbreviations and Acronyms
- BIOMArCS
Biomarker Study to Identify the Acute Risk of a Coronary Syndrome
- CCS
chronic coronary syndrome
- CVi
coefficient of intraindidividual or within‐subject variation
- II
index of individuality
- SWEDEHEART
System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies
- URL
upper reference limit
Clinical Perspective
What Is New?
Post–acute coronary syndrome (ACS) kinetics differ between high‐sensitivity cardiac troponin I (hs‐cTnI) and high‐sensitivity cardiac troponin T (hs‐cTnT); hs‐cTnI peaks higher and is quicker below the population upper reference limit than high‐sensitivity cardiac troponin, and in asymptomatic patients 6 months after ACS, hs‐cTnT concentrations are far more often above the population upper reference limit than measured hs‐cTnI concentrations.
Six months after ACS, both hs‐cTnI and hs‐cTnT show little within‐patient variability; in contrast, the between‐patient variation was large.
Following ACS, patients may have stable elevated high‐sensitivity cardiac troponin values without suffering from a clinical ACS, and such individuals may benefit from a patient‐specific reference value; such an individualized reference value can be derived using just 2 consecutive measurements in the majority of patients.
What Are the Clinical Implications?
Our high‐frequency blood sampling study showed that in stable asymptomatic patients following ACS, hs‐cTnI and hs‐cTnT measurements are not interchangeable.
In stable asymptomatic patients following ACS, large between‐patient variability exists for hs‐cTnI and hs‐cTnT, while the within‐patient variability is relatively small, underlining the clinical need for patient‐specific reference values for high‐sensitivity cardiac troponins.
These patient‐specific reference values for high‐sensitivity cardiac troponins could be of help to fine‐tune a personalized approach in patients following ACS, in particular in those with elevations that were found by chance (eg, in the perioperative setting) and in those presenting with unclear symptoms.
High‐sensitivity cardiac troponins (hs‐cTns) are now widely used in clinical practice and are key elements of the diagnosis of myocardial infarction (MI) in patients presenting with ischemic chest pain. 1 , 2 In the setting of suspected acute coronary syndrome (ACS), high‐sensitivity cardiac troponin I (hs‐cTnI) and high‐sensitivity cardiac troponin T (hs‐cTnT) have a comparable good performance and are practically interchangeable. 3 However, hs‐cTns are nowadays also measured for purposes other than diagnosing ACS, for example, as part of perioperative care, 4 and studies comparing hs‐cTnI concentrations and hs‐cTnT concentrations outside the setting of ACS are scarce and mostly performed in the general population. 5 , 6
In the current study, we used the BIOMArCS (Biomarker Study to Identify the Acute Risk of a Coronary Syndrome) with high‐frequency blood sampling, 7 , 8 , 9 investigating in detail the evolution of hs‐cTnI and hs‐cTnT concentrations until 1 year after ACS admission. We aimed to describe (differences in) the post‐ACS kinetics, and differences in the hs‐cTnI and hs‐cTnT concentrations after the biomarker reached stable levels. In addition, we explored the biological variation of cardiac troponins, measured with contemporary high‐sensitivity assays.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Design
The study design and main results of BIOMArCS has been published previously. 7 , 8 , 9 In short, BIOMArCS is a multicenter, prospective, observational study that was conducted in 18 participating hospitals in the Netherlands during 2008 to 2015. The study was designed to obtain detailed data on biomarker patterns until 1‐year follow‐up after ACS. Patients >40 years old presenting with ACS and at least 1 additional cardiovascular risk factor were eligible for enrollment. Exclusion criteria were ischemia precipitated by a condition other than atherosclerotic chronic coronary syndrome (CCS), a left ventricular ejection fraction <30%, end‐stage congestive heart failure (New York Heart Association [NYHA] class ≥3), severe chronic kidney disease with measured or calculated glomerular filtration rate (Cockroft‐Gault or Modification of Diet in Renal Disease‐4 formula) of <30 mL/min per 1.73 m2, or a coexistent condition with life expectancy <1 year. All patients were treated according to prevailing guidelines and at the discretion of the treating physician. The study protocol was approved by the institutional review boards of the participating hospitals, and all study subjects gave written informed consent.
Blood Sampling and Storage
Blood samples were collected at admission, at the day of hospital discharge, and subsequently every fortnight during the first 6 months after discharge. If logistic circumstances hindered inclusion during hospitalization, patients could be included on the first outpatient visit within 6 weeks after discharge. In a subset of approximately 8% of patients, additional blood samples were collected within 24, 48, 72, and 96 hours after admission and at the day of hospital discharge with the specific aim to study the early evolution and normalization of the biomarkers. Follow‐up was terminated permanently after coronary artery bypass grafting, hospital admission for heart failure, or a deterioration of renal function leading to a glomerular filtration rate <30 mL/min per 1.73 m2.
Blood samples were handled and securely stored on‐site. After preparation, aliquots were frozen at −80°C within 2 hours after withdrawal. Samples were transported under controlled conditions to the Department of Clinical Chemistry at the Erasmus Medical Center for long‐term storage.
Study Patients
For the BIOMArCS main results analysis, we applied the case‐cohort approach, including a total of 187 patients, of whom 45 reached the study end point of cardiovascular death or repeat ACS. 7 , 8 For the current analysis, we excluded these end point cases, and enriched the set with 49 patients who had daily sampling during the first 4 days after the index ACS. Hence, our analysis set consisted of 191 end point–free patients. 7 They contributed a median of 8 (25th–75th percentile; range, 5–10) repeated serum samples per patient (altogether 1507 samples), in which hs‐cTnI (Abbott Laboratories, Chicago, IL) and hs‐cTnT (Roche, Basel, Switzerland) were determined in a blinded fashion and in 1 batch. These assays have a lower limit of detection and population upper reference limit (URL; 99th percentile of the distribution in the general population) of 1.2 and 26.6 ng/L for hs‐cTnI, and 5 and 14 ng/L for hs‐cTnT, respectively. The limit of blank was equal to the lower limit of detection for hs‐cTnI and 3.0 ng/L for hs‐cTnT. Undetectable concentrations were assigned the concentration of 1.0 ng/L for hs‐cTnI and 2.9 ng/L for hs‐cTnT.
Statistical Analysis
Continuous variables are presented as mean (SD) or median (25th–75th percentile), depending on their distributions. Categorical variables are summarized as numbers and percentages. Differences between hs‐cTnI and hs‐cTnT were investigated using McNemar’s test for paired nominal data or a Wilcoxon signed‐rank test for paired continuous data.
Post‐ACS Kinetics
We used linear mixed‐effect models to describe the average cardiac troponin stabilization patterns over time. In these models, time was entered as the independent variable, and the log‐transformed (because of the nonnormal distribution) cardiac troponin value as the dependent variable. A total of 2 cubic splines were placed to model the nonlinearity of the association between time and cardiac troponin concentration. We used Akaike’s information criterion and Bayesian information criteria for the optimal placing of these splines. Random slopes as well as random intercepts were included in the models to allow for individual variation.
Using the fitted linear mixed‐effect models, we calculated the average hs‐cTnI and hs‐cTnT concentrations on a day‐to‐day basis for each patient. These concentrations were then used to estimate the peak concentration, the time until peak concentration, the median time during which cardiac troponins were elevated above the population reference value after the index ACS, and the median time until stabilization. We defined stabilization as a difference in (model‐derived) cardiac troponin concentrations of <1% between 2 consecutive days.
Measures of Biological Variation
For investigating the parameters of variability of a biomarker, it is necessary that the patients is in a (biochemically) stable status. On the basis of previous studies with repeated echocardiograms and blood measurements, we presumed that hs‐cTn concentrations would be biochemically stable at 6 months after ACS. 10 , 11 , 12 Accordingly, the analysis of biological variation was based on 446 samples (median, 4 samples per patient [range 3–9] that were collected 6 to 12 months after the index ACS and was limited to the 98 patients who had ≥3 measurements in that time window and who did not undergo a (staged) percutaneous coronary intervention; thus, iatrogenic distortion of the cardiac troponin concentrations caused by percutaneous coronary intervention was excluded. 13
We determined the coefficient of variation of hs‐cTnI and hs‐cTnT and applied the method of Fraser and Harris 14 to split the total variation into 3 components. These represent the variation attributable to the imprecision of the analytical process, the intraindividual or within‐subject variation (CVi) and the interindividual or between‐subject variation. Coefficient of analytical variation can be determined by repeatedly measuring the same sample using different assays. However, since this procedure is expensive, time consuming, and resource draining, laboratories generally use the coefficient of analytical variation that is based on a reference sample. We used the laboratory‐specific coefficient of analytical variation of 5.0% for hs‐cTnI and 3.0% for hs‐cTnT, respectively. Besides determining the different coefficients of variability, we also calculated the index of individuality (II) and the reference change value for both biomarkers. The II is the ratio of the combined within‐subject and analytical variation relative to the between‐subject variation. Previously, it was suggested that in case of an II<0.6, individual subjects should have their own reference values instead of a population‐based reference. 15 When the II>1.4, a population‐based reference is preferred. The reference change value reflects the limit of (relative) change in biomarker values in individual subjects that can be explained by the combined within‐subject and analytical variation. Finally, we investigated factors associated with the CVi using linear regression. A more detailed description of the parameters of variability and the formulas used to calculate them are included in Data S1.
Patient‐Specific Reference Value
The average time until hs‐cTnI and hs‐cTnT stabilization after the index ACS was <1 month, whereas within‐subject variability was relatively small. Therefore, we conducted a post hoc analysis of all 122 patients with >3 samples in the >1‐month time window to learn if a patient‐specific reference value could be determined this early after the index ACS, as follows: We calculated the moving average of 2 consecutive hs‐cTn measurements, which was then compared with the next measurement. If the difference was <5 ng/L, the moving average was then considered the patient‐specific reference. The 5 ng/L threshold was chosen because that value was equal to the median patient‐specific hs‐cTnT concentration times the upper limit of the reference change value.
All analyses were performed using R version 3.1.1 (R Foundation for Statistical Computing, Vienna, Austria) using packages “nlme” 16 and “splines.” 17
Results
Baseline Characteristics
Baseline characteristics are presented in Table 1. The mean age of the patients in the analysis set was 63.0 (11.1) years, and 78% were men. More than half of the population had hypertension (52.1%) and large proportions had hypercholesterolemia (47.5%) and a family history of CCS (53.5%). ST‐segment–elevation MI was the most common index event (46.2%), followed by non–ST‐segment–elevation MI (40.7%). No relevant differences in baseline characteristics were identified when comparing the full analysis set with the patients used to determine biological variation.
Table 1.
Baseline Characteristics
| Analysis Set | After 6 months | |
|---|---|---|
| (n=191) | (n=98) | |
| Age, y (SD) | 62.4 (10.6) | 62.8 (9.5) |
| Male sex, n (%) | 148 (77.5) | 77 (78.6) |
| Cardiovascular risk factors, n (%) | ||
| Diabetes mellitus | 33 (17.3) | 17 (17.3) |
| Hypertension | 101 (52.9) | 52 (53.1) |
| Hypercholesterolemia | 92 (46.5) | 54 (58.2) |
| Family history of CCS* | 87 (53.0) | 47 (59.5) |
| Current smoker | 80 (41.9) | 41 (41.8) |
| History of cardiovascular disease, n (%) | ||
| MI | 50 (26.2) | 30 (30.6) |
| CABG | 14 (7.3) | 6 (6.1) |
| PCI | 44 (23.2) | 28 (28.9) |
| Stroke | 19 (9.9) | 7 (7.1) |
| Admission diagnosis, n (%) | ||
| STEMI | 93 (49.0) | 47 (48.0) |
| NSTEMI | 74 (38.7) | 37 (37.8) |
| UAP | 24 (12.6) | 14 (14.3) |
| Physical examination | ||
| Body mass index (SD) | 27.5 (3.6) | 27.5 (3.6) |
| Killip class 1 (%) | 177 (92.7) | 94 (95.9) |
| Heart rate (IQR) | 73 (62–84) | 70 (61–81) |
| Systolic blood pressure (IQR) | 137 (117–152) | 136 (119–151) |
| eGFR, mL/min per 173 m2 (SD) | 98 (30) | 97 (28) |
| Medication, n (%) | ||
| Aspirin | 183 (96.3) | 95 (96.9) |
| Beta‐blocker | 167 (87.9) | 83 (84.7) |
| ACEI | 138 (72.6) | 68 (69.4) |
| ARB | 22 (11.6) | 11 (11.2) |
| Statin | 183 (96.3) | 96 (98.0) |
After 6 months: Analysis set minus an elective PCI >150 days after the index event and patients with <3 samples available after 6 months. ACEI indicates angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blockers; CABG, coronary artery bypass grafting; CCS, chronic coronary syndromes; eGFR, estimated glomerular filtration rate; IQR, interquartile range; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; and UAP, unstable angina pectoris.
Family history of CCS was defined as angina pectoris, MI, or sudden abrupt death without obvious cause before the age of 55 in a first‐degree blood relative.
Post‐ACS Kinetics
The average concentrations of the different biomarkers from the time of the ACS until day 50 are shown in Figure 1. Both hs‐cTnI and hs‐cTnT peaked on day 1 (median, interquartile range, 1–2) and gradually returned to concentrations beneath the population URL. The median peak concentration was 3506 ng/L (interquartile range, 2300–6596) for hs‐cTnI and 494 ng/L (397–939) for hs‐cTnT (P<0.001). Although statistically significant, there was little difference in the median time until stabilization on patient level. The median number of days was 31 (interquartile range, 30–32) days for hs‐cTnI and 30 (interquartile range, 30–31) days for hs‐cTnT (P<0.001), respectively. In contrast, hs‐cTnI was quicker below the URL than hs‐cTnT (median, 16 [13–19] days versus 19 [16–26] days; P<0.001).
Figure 1. Average stabilization patterns of high‐sensitivity cardiac troponins after ACS. The x axes depict the number of days since the acute coronary syndrome.
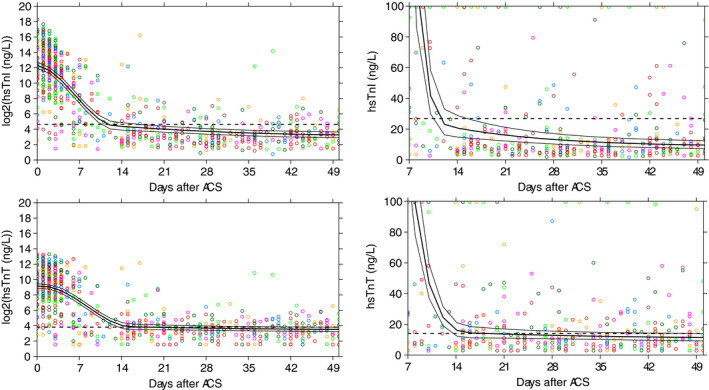
The y axes represent the cardiac troponin concentrations. The left 2 plots are on the log scale with base number 2. A 1‐point increase can thus be interpreted as a doubling of the value. The black lines depict the cohort average; the dashed lines the corresponding 95% CI. ACS indicates acute coronary syndrome; hs‐cTnI, high‐sensitivity cardiac troponin I; hs‐cTnT, and high‐sensitivity cardiac troponin T.
Biological Variation
Figure 2 depicts all pairs of hs‐cTnI and hs‐cTnT measurements taken after 6 months. All hs‐cTnI values exceeded the lower limit of detection, whereas 22.0% of hs‐cTnT values were below the lower limit of detection (9.0% below the limits of blank). In all the samples, 2.0% of hs‐cTnI and 17.2% of hs‐cTnT values exceeded the population URL (P<0.001); 3 patients had an average hs‐cTnI above the URL compared with 16 patients with an average hs‐cTnT above the URL (P=0.002). The Spearman correlation for average hs‐cTn level was r=0.60 (P<0.001).
Figure 2. Comparison of high‐sensitivity cardiac troponin I and T concentrations in the samples taken after 6 months.
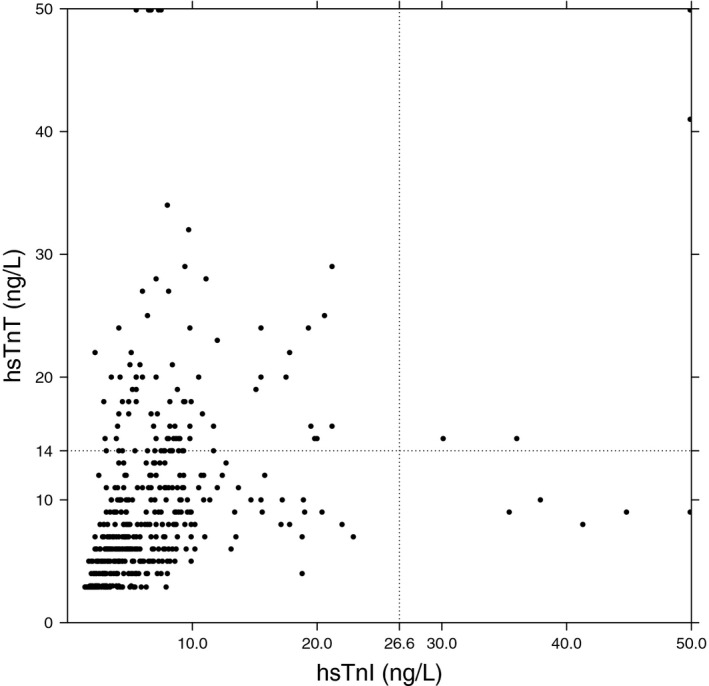
The x axis depict the hs‐cTnI concentration, while on the y axis the concentration of hs‐cTnT is given. Each dot represents a single blood sample in which thus both an hs‐cTnI and hs‐cTnT concentration has been measured. hs‐cTnI indicates high‐sensitivity cardiac troponin I; and hs‐cTnT, high‐sensitivity cardiac troponin T.
The distributions of the hs‐cTn measurements after 6 months are shown for each patient in Figure 3. CVis of hs‐cTnI and hs‐cTnT were 14.0% and 18.1%, respectively. We could not identify any baseline characteristics that were significantly associated with the observed CVis (Table S1). In contrast to the small CVis, the coefficients of interindividual or between‐subject variation were large, reflecting relatively large differences in average cardiac troponin concentrations between patients. Consequently, both biomarkers had IIs <0.6, the reference change value limits ranged between −33.6% and 50.5% for hs‐cTnI, and −39.6% and 65.5% for hs‐cTnT, respectively. Consequently, as an example, in a patient with a steady‐state hs‐cTnI concentration of 5 ng/L, a rise of 3 ng/L exceeds the combined analytical and within‐subject variation with 95% certainty and can thus be considered the consequence of pathological processes. An overview of the different parameters of biological variation is presented in Table 2.
Figure 3. Distribution of the high‐sensitivity cardiac troponins after 6 months. On the horizontal axes are the individual patients ranked based on their average cardiac troponin values.
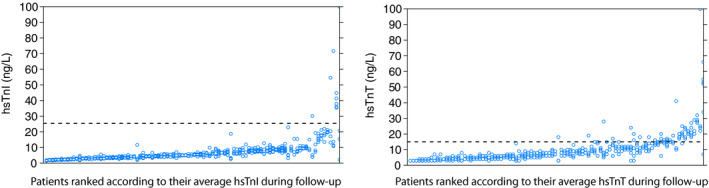
The vertical axes depict the cardiac troponin concentrations resulting from the repeated measurements. The dotted lines show the reference value of the cardiac troponin. hs‐cTnI indicates high‐sensitivity cardiac troponin I; and hs‐cTnT, high‐sensitivity cardiac troponin T.
Table 2.
Overview of Parameters of Biological Variation
| Average Patient Concentration (ng/mL) | CVa (%) | CVi (%) | CVg (%) | II | RCV (%) | Log‐Normal | ||
|---|---|---|---|---|---|---|---|---|
| RCV Low (%) | RCV Up (%) | |||||||
| hs‐cTnI | 5.3 (3.7–8.3) | 5.0 | 14.0 | 94.1 | 0.16 | 38.7 | −33.6 | 50.5 |
| hs‐cTnT | 7.8 (5.1–11.1) | 3.0 | 18.1 | 75.9 | 0.24 | 50.1 | −39.6 | 65.5 |
CVa indicates analytical coefficient of variation; CVg, interindividual coefficient of variation; CVi, intraindividual coefficient of variation; Hs‐cTnI, high‐sensitivity cardiac troponin I; hs‐cTnT, high‐sensitivity cardiac troponin T; II, index of individuality; and RCV, reference change value.
Patient‐Specific Reference Value
In the post hoc analysis of 122 patients (see the Methods section), a patient‐specific reference value could be determined in 85.2% (hs‐cTnI) and 83.6% (hs‐cTnT) using the first 2 post‐30‐day measurements. The median (25th–75th percentile) reference values were 7.1 ng/L (4.4–10.6) and 8.5 ng/L (6.5–12.9) for hs‐cTnI and hs‐cTnT, respectively. The difference between the patient‐specific baseline value and their last available measurement (on average 11 months after the index ACS) was <5 ng/L in >81.7% (hs‐cTnI) and 77.5% (hs‐cTnT) of the patients. A paired t‐test confirmed that there were no significant differences between the patient‐specific baseline value on the basis of the first 2 measurements and the last available measurement for both hs‐cTnI (mean difference, −0.37 ng/L; 95% CI, −3.26–2.53; P=0.80) and hs‐cTnT (mean difference, 0.11 ng/L; 95% CI, −1.81–2.03; P=0.91).
Discussion
In BIOMArCS, we confirmed the hs‐cTn peak, the plateau after the index ACS, and that values can remain above the population URL for a prolonged time. 18 We added that after a quick decrease, the median time to reach values below the URL was shorter for hs‐cTnI than for hs‐cTnT. In addition, post‐6‐month samples in (then) stable patients with CCS, the percentage of hs‐cTnT measurements with concentration above the population URL was far greater than that of hs‐cTnI (Figure 3) with (thus) poor interchangeability of the 2 biomarkers. The individual variation of both hs‐cTnI and hs‐cTnT were low, while differences between patients were large. This combination of characteristics led to a low II (<0.6) for both cardiac troponins, which again stresses that in patients with known stable CCS after having previously endured an ACS, patient‐specific reference values are to be preferred over the population‐based reference. 15 Finally, we were able to demonstrate that the patient‐specific reference value can already be obtained on the basis of 2 consecutive samples taken after 1 month in the vast majority of subjects following ACS.
In our study, we found some striking differences between hs‐cTnI and hs‐cTnT. After the index ACS, hs‐cTnI showed a higher peak concentration and had a quicker descent when compared with hs‐cTnT. The higher peak levels had been previously described by Laugaudin et al in 106 consecutive patients with ST‐segment–elevation MI. 19 We now add to this that hs‐cTnI is also faster below the population URL than hs‐cTnT. After 6 months, when patients were to be considered biochemically stable, there were >5 times as many patients with an average hs‐cTnT concentration above the population URL than patients with hs‐cTnI above the population URL. Moreover, despite being statistically significant, the correlation between average hs‐cTnI and hs‐cTnT concentration clearly showed that the 2 markers cannot be considered interchangeable in an asymptomatic post‐ACS population. Although obvious differences in design (single measurement versus multiple measurements) and participants (general population versus patients with ACS) are to be acknowledged, our findings are much in line with previous reports from general population cohorts comparing hs‐cTns. In a study by Kimenai et al among 1540 individuals without significant baseline disease, the correlation coefficient between hs‐cTnI and hs‐cTnT was 0.55, 6 while among 19 501 participants of the General Scotland Scottisch Family Health Study, the r was 0.46. 5 Remarkably, in the latter study, the number of patients above the population URL was much greater for hs‐cTnT than for hs‐cTnI, which is in line with our results. We add to this current body of evidence that also in patients with known CCS the correlation between hs‐cTnI and hs‐cTnT concentrations are not strong.
To date, studies on the biological variation of cardiac troponins, measured with contemporary high‐sensitivity assays, are scarce, and their sample sizes have usually been small. 20 , 21 , 22 , 23 , 24 Particularly in patients with established CCS, such as patients following ACS, little to no information is available. The parameters of variation found in our study are comparable to earlier reports in subjects sampled from the general “healthy” population. For example, Wu et al 23 reported a long‐term individual variation of 14% for hs‐cTnI based on 17 healthy subjects. The coefficient of interindividual or between‐subject variation in their report was lower than in our study, which suggests that cardiac troponins show larger variations in patients with CCS than in healthy individuals. The larger between‐subject variation in a diseased population compared with a healthy one is also confirmed by a study of Meijers et al 25 comparing biological variation in 83 patients with heart failure to 28 healthy subjects. They reported a coefficient of interindividual or between‐subject variation for hs‐cTnT of 96.6% and 51.2%, respectively. The CVis, however, were similar in both populations and comparable to our cohort.
We were able to demonstrate the feasibility of obtaining patient‐specific references values in patients with established CCS. This reference value could be retrieved in the majority of our patients following ACS based on a limited number of consecutive measurements, whereas these values showed good agreement with samples taken later during follow‐up. It is our opinion that the patient‐specific reference value can help fine‐tune the diagnostic process in specific situations. These reference values could be of help to fine‐tune a personalized approach in patients following ACS, in particular in those with asymptomatic elevations that were found by chance (eg, hs‐cTn measurements in the perioperative setting) and in those presenting with unclear symptoms. For instance, if a patient comes with atypical complaints and has slightly elevated hs‐cTn concentrations in 2 consecutive measurements. Atypical presentations are not uncommon, 26 and a rise of hs‐cTns concentrations cannot always be identified, particularly if patients come several hours after the complaints start when cardiac troponin levels might already be in the plateau phase. Comparing the hs‐cTn concentrations measured with the patient‐specific reference could help determine if this patient is more likely to have an ACS and needs to go to the catheterization laboratory or can be sent home. Also, when a patient has typical complaints but the hs‐cTn concentrations are still below population URL with a borderline rise between the 2 consecutive measurements, comparing the concentration with their individual reference value might determine the final decision. If the found concentration is (much) higher than the patient‐specific reference value (but still below population URL), then it is probably more likely to be unstable angina pectoris or an MI. Accurately diagnosing unstable angina is important, as these patients often need early percutaneous coronary intervention and have an incidence rate of future (lethal) cardiac events comparable with patients who had a non–ST‐segment–elevation MI. 27 , 28 Moreover, particularly when using hs‐cTnI, MI is known to be underdiagnosed because of the relatively high population URL. 29
Limitations
The high‐frequency blood sampling design of BIOMArCS enables an in‐depth analysis of longitudinal biomarker patterns in the population of patients with established CCS. A limitation of the current analysis is that compared with a real‐world ACS population such as the SWEDEHEART (System for Enhancement and Development of Evidence‐Based Care in Heart Disease Evaluated According to Recommended Therapies) registry, 30 the subjects included in the current study were on average 8 years younger, were more likely to have an ST‐segment–elevation MI (49% versus 35.5%), had more previous percutaneous coronary interventions performed (29.1% versus 13.8%), and had a lower prevalence of diabetes mellitus (17.3% versus 22.5%). These differences might compromise the generalizability of the results. Moreover, the generalizability of our parameters could potentially be further compromised as per study protocol; we excluded all patients with recurrent events during the 1‐year follow‐up, as we did not want to take into account possible distortion from an imminent ischemic event while calculating the parameters of variability. However, in a sensitivity analysis also comprising the patients with ischemic events, the parameters changed only marginally (data not shown). Second, information on the patient’s activities before sampling is lacking and that the timing of blood sampling during the day was not specified. Hs‐cTns are known to be influenced by (heavy) physical activity, 31 and hs‐cTnT, but not hs‐cTnI, is known to exhibit a diurnal rhythm. 32 However, we have investigated the variation of the time of sampling and found that all measurements were taken between 8 am and 4 pm. Moreover, we observed that, although not specified in the protocol, the vast majority of the patients had repeated visits for blood sampling at the same hour of the day. Hence, the within‐patient variation in biomarker concentrations found in this study cannot be explained by variations in sampling time. Third, no echocardiographic data are available, which could have been an aid in explaining chronic elevated cardiac troponin concentrations in different patients. A final limitation is that, using our data, although plausible, we cannot confirm that using a patient‐specific reference value enhances the diagnostics for future ACS. This should be the focus of future research.
Conclusions
In conclusion, hs‐cTn concentrations showed similar post‐ACS kinetics; however, after the initial peak, hs‐cTnI had a quicker median time to concentrations below population URL than hs‐cTnT. In the post‐6‐month samples, hs‐cTnI and hs‐cTnT showed modest correlation (r spearman=0.60), whereas the average hs‐cTnT concentration was 5 times more likely to be above the URL than hs‐cTnI. The within‐patient variation was small for both cardiac troponins and comparable to healthy populations. Between‐patient variation, however, is much higher in post‐ACS patients than in population controls. Consequently, our data support the use of patient‐specific reference values for hs‐cTn in patients with CCS. Patient‐specific reference values can easily be obtained in the vast majority of patients by using 2 consecutive samples during a clinically stable phase.
Appendix
BIOMArCS Investigators: Maarten de Mulder; Erasmus MC, University Medical Center Rotterdam, the Netherlands; Carl Schotborgh, HagaZiekenhuis, Den Haag, the Netherlands; Eelko Ronner; Reinier de Graaf Hospital, Delft, the Netherlands; Anho Liem; Sint Franciscus Gasthuis, Rotterdam, the Netherlands; David Haitsma; Admiraal de Ruyter Hospital, Goes, the Netherlands; Arthur Maas, Gelre Hospital, Zutphen, the Netherlands; Ben Ilmer, Havenziekenhuis, Rotterdam, the Netherlands; Rene Dijkgraaf, St. Jansdal Hospital, Harderwijk, the Netherlands; S. Hong Kie; Treant Zorggroep, Bethesda, Hoogeveen, the Netherlands; Alexander J. Wardeh, Medisch Centrum Haaglanden location Westeinde, Den Haag, the Netherlands; Walther Hermans, Elizabeth‐Tweesteden Hospital, Tilburg, the Netherlands; Etienne Cramer, Radboud University Medical Center Nijmegen, the Netherlands; Pieter A. Doevendans, University Medical Center Utrecht, the Netherlands; Maarten L. Simoons, Erasmus MC, University Medical Center Rotterdam, the Netherlands.
Sources of Funding
The work was supported and funded by the Netherlands Heart Foundation (grant number 2007B012), the Netherlands Heart Institute‐Interuniversity Cardiology Institute of the Netherlands (project number 071.01) and the Working Group on Cardiovascular Research Netherlands, all of which are noncommercial funding bodies. An unrestricted research grant was further obtained from Eli Lilly, the Netherlands.
Disclosures
None.
Supporting information
Data S1
Table S1
(J Am Heart Assoc. 2021;10:e017393. DOI: 10.1161/JAHA.120.017393.)
Supplementary material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.017393
For Sources of Funding and Disclosures, see page 8.
Contributor Information
Eric Boersma, Email: h.boersma@erasmusmc.nl.
for the BIOMArCS investigators:
Maarten Mulder, Carl Schotborgh, Eelko Ronner, Anho Liem, David Haitsma, Arthur Maas, Ben Ilmer,, Rene Dijkgraaf, S. Hong Kie, Alexander J Wardeh, Walther Hermans, Etienne Cramer, Pieter A Doevendans, and Maarten L Simoons
References
- 1. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, et al. 2015 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation: task force for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation of the European society of cardiology (ESC). Eur Heart J. 2016;37:267–315. 10.1093/eurheartj/ehv320 [DOI] [PubMed] [Google Scholar]
- 2. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 3. van der Linden N, Wildi K, Twerenbold R, Pickering JW, Than M, Cullen L, Greenslade J, Parsonage W, Nestelberger T, Boeddinghaus J. Combining high‐sensitivity cardiac troponin I and cardiac troponin T in the early diagnosis of acute myocardial infarction. Circulation. 2018;138:989–999. 10.1161/CIRCULATIONAHA.117.032003 [DOI] [PubMed] [Google Scholar]
- 4. Duceppe E, Parlow J, MacDonald P, Lyons K, McMullen M, Srinathan S, Graham M, Tandon V, Styles K, Bessissow A. Canadian cardiovascular society guidelines on perioperative cardiac risk assessment and management for patients who undergo noncardiac surgery. Can J Cardiol. 2017;33:17–32. 10.1016/j.cjca.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 5. Welsh P, Preiss D, Shah ASV, McAllister D, Briggs A, Boachie C, McConnachie A, Hayward C, Padmanabhan S, Welsh C. Comparison between high‐sensitivity cardiac troponin t and cardiac troponin i in a large general population cohort. Clin Chem. 2018;64:1607–1616. 10.1373/clinchem.2018.292086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kimenai DM, Henry RMA, van der Kallen CJH, Dagnelie PC, Schram MT, Stehouwer CDA, van Suijlen JDE, Niens M, Bekers O, Sep SJS. Direct comparison of clinical decision limits for cardiac troponin T and I. Heart. 2016;102:610–616. 10.1136/heartjnl-2015-308917 [DOI] [PubMed] [Google Scholar]
- 7. Boersma E, Vroegindewey MM, van den Berg VJ, Asselbergs FW, van der Harst P, Kietselaer B, Lenderink T, Oude Ophuis AJ, Umans V, de Winter RJ, et al. Details on high frequency blood collection, data analysis, available material and patient characteristics in biomarcs. Data Brief. 2019;27:104750. 10.1016/j.dib.2019.104750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Oemrawsingh RM, Akkerhuis KM, de Mulder M, Umans VA, Kietselaer B, Schotborgh C, Ronner E, Lenderink T, Liem A, Haitsma D, et al. High‐frequency biomarker measurements of troponin, NT‐PROBNP, and C‐reactive protein for prediction of new coronary events after acute coronary syndrome. Circulation. 2019;139:134–136. 10.1161/CIRCULATIONAHA.118.036349 [DOI] [PubMed] [Google Scholar]
- 9. Oemrawsingh RM, Akkerhuis KM, Umans VA, Kietselaer B, Schotborgh C, Ronner E, Lenderink T, Liem A, Haitsma D, van der Harst P, et al. Cohort profile of BIOMArCS: the biomarker study to identify the acute risk of a coronary syndrome‐a prospective multicentre biomarker study conducted in the Netherlands. BMJ Open. 2016;6:e012929. 10.1136/bmjopen-2016-012929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Eggers KM, Lagerqvist B, Venge P, Wallentin L, Lindahl B. Persistent cardiac troponin I elevation in stabilized patients after an episode of acute coronary syndrome predicts long‐term mortality. Circulation. 2007;116:1907–1914. 10.1161/CIRCULATIONAHA.107.708529 [DOI] [PubMed] [Google Scholar]
- 11. Funaro S, La Torre G, Madonna M, Galiuto L, Scara A, Labbadia A, Canali E, Mattatelli A, Fedele F, Alessandrini F, et al. Incidence, determinants, and prognostic value of reverse left ventricular remodelling after primary percutaneous coronary intervention: results of the acute myocardial infarction contrast imaging (AMICI) multicenter study. Eur Heart J. 2009;30:566–575. 10.1093/eurheartj/ehn529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Galiuto L, Garramone B, Scara A, Rebuzzi AG, Crea F, La Torre G, Funaro S, Madonna M, Fedele F, Agati L, et al. The extent of microvascular damage during myocardial contrast echocardiography is superior to other known indexes of post‐infarct reperfusion in predicting left ventricular remodeling: results of the multicenter amici study. J Am Coll Cardiol. 2008;51:552–559. 10.1016/j.jacc.2007.09.051 [DOI] [PubMed] [Google Scholar]
- 13. Ndrepepa G, Colleran R, Braun S, Cassese S, Hieber J, Fusaro M, Kufner S, Ott I, Byrne RA, Husser O. High‐sensitivity troponin T and mortality after elective percutaneous coronary intervention. J Am Coll Cardiol. 2016;68:2259–2268. 10.1016/j.jacc.2016.08.059 [DOI] [PubMed] [Google Scholar]
- 14. Fraser CG, Harris EK. Generation and application of data on biological variation in clinical chemistry. Crit Rev Clin Lab Sci. 1989;27:409–437. 10.3109/10408368909106595 [DOI] [PubMed] [Google Scholar]
- 15. Petersen PH, Fraser CG, Sandberg S, Goldschmidt H. The index of individuality is often a misinterpreted quantity characteristic. Clin Chem Lab Med. 1999;37:655–661. [DOI] [PubMed] [Google Scholar]
- 16. Pinheiro J, Bates D, DebRoy S, Sarkar D.R core team (2014) nlme: linear and nonlinear mixed effects models. R package version 3.1‐117. Available at http://CRAN.R‐project.org/package=nlme. 2014.
- 17. Bates MD, Venables B; Team MRC . Package “splines.” R Version. 2011;2. [Google Scholar]
- 18. Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, Storrow AB, Wu AHB, Christenson RH, Apple FS, Francis G. National academy of clinical biochemistry laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem. 2007;53:552–574. 10.1373/clinchem.2006.084194 [DOI] [PubMed] [Google Scholar]
- 19. Laugaudin G, Kuster N, Petiton A, Leclercq F, Gervasoni R, Macia J‐C, Cung T‐T, Dupuy A‐M, Solecki K, Lattuca B. Kinetics of high‐sensitivity cardiac troponin T and I differ in patients with ST‐segment elevation myocardial infarction treated by primary coronary intervention. Eur Heart J. 2016;5:354–363. [DOI] [PubMed] [Google Scholar]
- 20. Frankenstein L, Wu AH, Hallermayer K, Wians FH Jr, Giannitsis E, Katus HA. Biological variation and reference change value of high‐sensitivity troponin T in healthy individuals during short and intermediate follow‐up periods. Clin Chem. 2011;57:1068–1071. 10.1373/clinchem.2010.158964 [DOI] [PubMed] [Google Scholar]
- 21. Vasile VC, Saenger AK, Kroning JM, Jaffe AS. Biological and analytical variability of a novel high‐sensitivity cardiac troponin T assay. Clin Chem. 2010;56:1086–1090. 10.1373/clinchem.2009.140616 [DOI] [PubMed] [Google Scholar]
- 22. Vasile VC, Saenger AK, Kroning JM, Klee GG, Jaffe AS. Biologic variation of a novel cardiac troponin I assay. Clin Chem. 2011;57:1080–1081. 10.1373/clinchem.2011.162545 [DOI] [PubMed] [Google Scholar]
- 23. Wu AH, Lu QA, Todd J, Moecks J, Wians F. Short‐ and long‐term biological variation in cardiac troponin I measured with a high‐sensitivity assay: implications for clinical practice. Clin Chem. 2009;55:52–58. 10.1373/clinchem.2008.107391 [DOI] [PubMed] [Google Scholar]
- 24. Nordenskjold AM, Ahlstrom H, Eggers KM, Frobert O, Jaffe AS, Venge P, Lindahl B. Short‐ and long‐term individual variation in cardiac troponin in patients with stable coronary artery disease. Clin Chem. 2013;59:401–409. 10.1373/clinchem.2012.191700 [DOI] [PubMed] [Google Scholar]
- 25. Meijers WC, van der Velde AR, Muller Kobold AC, Dijck‐Brouwer J, Wu AH, Jaffe A, de Boer RA. Variability of biomarkers in patients with chronic heart failure and healthy controls. Eur J Heart Fail. 2017;19(3):357–365. 10.1002/ejhf.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Canto JG, Shlipak MG, Rogers WJ, Malmgren JA, Frederick PD, Lambrew CT, Ornato JP, Barron HV, Kiefe CI. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA. 2000;283:3223–3229. 10.1001/jama.283.24.3223 [DOI] [PubMed] [Google Scholar]
- 27. Eggers KM, Jernberg T, Lindahl B. Unstable angina in the era of cardiac troponin assays with improved sensitivity—a clinical dilemma. Am J Med. 2017;130:1423–1430.e5. 10.1016/j.amjmed.2017.05.037 [DOI] [PubMed] [Google Scholar]
- 28. Puelacher C, Gugala M, Adamson PD, Shah A, Chapman AR, Anand A, Sabti Z, Boeddinghaus J, Nestelberger T, Twerenbold R. Incidence and outcomes of unstable angina compared with non‐ST‐elevation myocardial infarction. Heart. 2019;105:1423–1431. 10.1136/heartjnl-2018-314305 [DOI] [PubMed] [Google Scholar]
- 29. Wildi K, Gimenez MR, Twerenbold R, Reichlin T, Jaeger C, Heinzelmann A, Arnold C, Nelles B, Druey S, Haaf P. Misdiagnosis of myocardial infarction related to limitations of the current regulatory approach to define clinical decision values for cardiac troponin. Circulation. 2015;131:2032–2040. 10.1161/CIRCULATIONAHA.114.014129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sahlen A, Varenhorst C, Lagerqvist B, Renlund H, Omerovic E, Erlinge D, Wallentin L, James SK, Jernberg T. Outcomes in patients treated with ticagrelor or clopidogrel after acute myocardial infarction: experiences from SWEDEHEART registry. Eur Heart J. 2016;37:3335–3342. 10.1093/eurheartj/ehw284 [DOI] [PubMed] [Google Scholar]
- 31. Shave R, Baggish A, George K, Wood M, Scharhag J, Whyte G, Gaze D, Thompson PD. Exercise‐induced cardiac troponin elevation: evidence, mechanisms, and implications. J Am Coll Cardiol. 2010;56:169–176. 10.1016/j.jacc.2010.03.037 [DOI] [PubMed] [Google Scholar]
- 32. Klinkenberg LJJ, Wildi K, Van Der Linden N, Kouw IWK, Niens M, Twerenbold R, Rubini Gimenez M, Puelacher C, Daniel Neuhaus J, Hillinger P. Diurnal rhythm of cardiac troponin: consequences for the diagnosis of acute myocardial infarction. Clin Chem. 2016;62:1602–1611. 10.1373/clinchem.2016.257485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1
Table S1


