Abstract
Background
In athletes with ventricular arrhythmias (VA) and otherwise unremarkable clinical findings, cardiac magnetic resonance (CMR) may reveal concealed pathological substrates. The aim of this multicenter study was to evaluate which VA characteristics predicted CMR abnormalities.
Methods and Results
We enrolled 251 consecutive competitive athletes (74% males, median age 25 [17‐39] years) who underwent CMR for evaluation of VA. We included athletes with >100 premature ventricular beats/24 h or ≥1 repetitive VA (couplets, triplets, or nonsustained ventricular tachycardia) on 12‐lead 24‐hour ambulatory ECG monitoring and negative family history, ECG, and echocardiogram. Features of VA that were evaluated included number, morphology, repetitivity, and response to exercise testing. Left‐ventricular late gadolinium‐enhancement was documented by CMR in 28 (11%) athletes, mostly (n=25) with a subepicardial/midmyocardial stria pattern. On 24‐hour ECG monitoring, premature ventricular beats with multiple morphologies or with right‐bundle‐branch‐block and intermediate/superior axis configuration were documented in 25 (89%) athletes with versus 58 (26%) without late gadolinium‐enhancement (P<0.001). More than 3300 premature ventricular beats were recorded in 4 (14%) athletes with versus 117 (53%) without positive CMR (P<0.001). At exercise testing, nonsustained ventricular tachycardia occurred at peak of exercise in 8 (29%) athletes with late gadolinium‐enhancement (polymorphic in 6/8, 75%) versus 17 athletes (8%) without late gadolinium‐enhancement (P=0.002), (P<0.0001). At multivariable analysis, all 3 parameters independently correlated with CMR abnormalities.
Conclusions
In athletes with apparently idiopathic VA, simple characteristics such as number and morphology of premature ventricular beats on 12‐lead 24‐hour ambulatory ECG monitoring and response to exercise testing predicted the presence of concealed myocardial abnormalities on CMR. These findings may help cost‐effective CMR prescription.
Keywords: cardiomyopathy, late gadolinium enhancement, preparticipation screening, sports cardiology, sudden cardiac death
Subject Categories: Arrhythmias, Sudden Cardiac Death, Magnetic Resonance Imaging (MRI)
Nonstandard Abbreviations and Acronyms
- LGE
late gadolinium enhancement
- PVB
premature ventricular beat
- VA
ventricular arrhythmia
Clinical Perspective
What Is New?
In athletes with ventricular arrhythmias and negative family history, normal ECG, and unremarkable echocardiography, simple parameters such as morphology of premature ventricular beats and response to exercise testing may help to identify the subset with the highest probability of a concealed underlying pathological substrate at cardiac magnetic resonance.
What Are the Clinical Implications?
In the diagnostic work‐up of athletes referred for apparently idiopathic ventricular arrhythmias, the study findings may help to select the subgroup for whom more in‐depth investigation by cardiac magnetic resonance imaging may be particularly cost‐effective.
The observation of premature ventricular beats (PVBs) in an apparently healthy athlete raises the concern of an underlying cardiac disease that may potentially cause risk of life‐threatening ventricular arrhythmias (VA). 1 , 2 ECG and echocardiography represent first‐line diagnostic tools, but negative results do not rule out the presence of pathological arrhythmic substrates, such as segmental left ventricular (LV) fibrosis, that can only be disclosed by cardiac magnetic resonance (CMR). 3 , 4 , 5 , 6 , 7
PVBs are not rare in competitive athletes. On 24‐hour ambulatory ECG monitoring, >100 PVBs/h or ≥1 repetitive VAs (couplet, triplets, or nonsustained VT) have been reported in 8% of young (<30‐year‐old) and in 18% of veteran (>30‐year‐old) apparently healthy athletes. 8 , 9 On exercise testing, 1 or more PVBs were recorded in 5% of athletes with negative history, physical examination, and resting ECG. 4 Investigations of all athletes with PVBs and no other abnormal clinical findings by CMR would not be cost‐effective, considering that in the majority of cases VAs are idiopathic and unrelated to underlying pathological substrates. Recently, criteria for differentiating between common (usually benign) and uncommon (potentially associated with heart disease) PVBs have been proposed, with the aim to restrict CMR prescription to high‐risk athletes. 1 However, these criteria were mostly based on small single‐center reports and have not been validated on larger samples.
The aim of this multicenter study was to evaluate which PVBs features predicted a higher probability of pathological CMR findings in a large sample of athletes with VA and negative family history, no previous cardiac arrest or sustained VT, and unremarkable electrocardiographic and echocardiographic findings.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
We enrolled a consecutive series of competitive nonprofessional athletes recruited in 3 different centers. These athletes underwent contrast‐enhanced CMR for investigation of VA detected on 24‐hour ambulatory ECG monitoring, which was prescribed either because of PVBs during preparticipation screening (that is compulsory in Italy and includes resting and exercise ECG) or because of symptoms. According to the Italian law on preparticipation screening, competitive athletes were defined as individuals engaged in competitions organized by National Sports Federations or other Sports Associations recognized by the National Olympic Committee. 10 Athletes were included in the study if they showed >100 PVBs/24 h or ≥1 repetitive VA (couplets, triplets, or nonsustained VT) on 24‐hour ambulatory ECG monitoring and no electrocardiographic or echocardiographic abnormalities (including mitral valve prolapse). Athletes with previous cardiac arrest or sustained VT, positive family history for cardiomyopathy or premature sudden death were excluded. A summary of the study methods is shown in Figure 1.
Figure 1. Summary of the study methods.
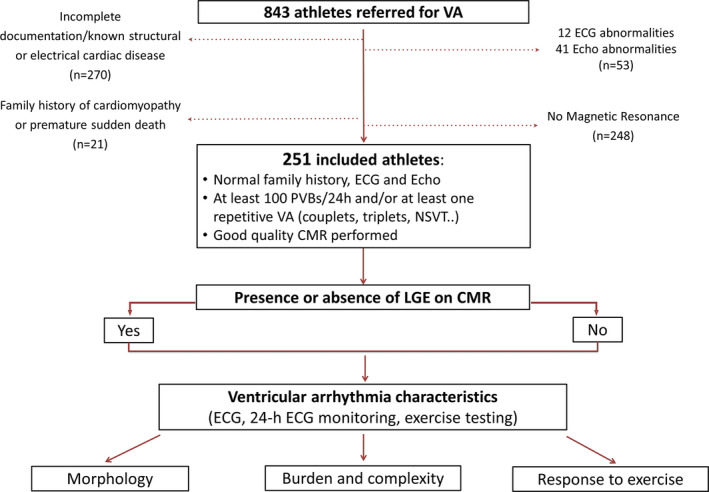
CMR indicates cardiac magnetic resonance; LGE, late gadolinium enhancement; NSVT, nonsustained ventricular tachycardia; PVB, premature ventricular beat; and VA, ventricular arrhythmia.
ECGs were interpreted as normal or abnormal according to the 2017 International criteria. 11 These criteria recognize 3 categories of abnormalities: (1) those that are typical of the athlete's heart and are considered normal (eg, sinus bradycardia, first‐degree atrioventricular block, increased QRS voltages in the precordial leads, anterior T‐wave inversion preceeded by J‐point/ST‐segment elevation in black athletes); (2) borderline findings that are considered abnormal only if 2 or more are present (atrial enlargement, QRS axis deviation and right bundle‐branch block [RBBB]); (3) abnormal changes that warrant further investigations to exclude an underlying disease (eg, ventricular pre‐excitation, left bundle‐branch block, and T‐wave inversion in lateral leads). Echocardiography was considered suggestive of physiological athlete's heart in case of eccentric left ventricular hypertrophy with harmonic dilation of all cardiac chambers that was consistent with the intensity of training. In veteran athletes (>35 years old) with risk factors for coronary artery disease, chest pain or ST‐segment depression at exercise testing, the presence of coronary artery disease was ruled out by coronary computed tomography or imaging stress testing. The study complied with the Declaration of Helsinki and was approved by the ethical committee. All participants provided written informed consent.
VA Characteristics
Twelve‐lead 24‐hour ambulatory ECG monitoring was performed in all study participants. Athletes were asked to perform a training session of at least 30 to 60 minutes during the ambulatory ECG. Recordings were reviewed by 1 investigator for each center (AZ, CC, TV): in particular, every single ectopic beat, pause, or artifact and all families of normal beats were confirmed manually. In all athletes, at least 23 hours of recording excluding artifacts were available.
The PVB morphology was classified as left‐bundle‐branch‐block‐like if the ectopic QRS complex was predominantly negative in lead V1, and as RBBB‐like if the ectopic QRS complex was predominantly positive or isodiphasic in lead V1. The QRS axis in the limb leads was labeled as inferior if the ectopic beat was negative in aVL and positive in aVF. PVBs with a QRS duration ≤130 ms resembling a typical RBBB/left or right axis deviation were considered of fascicular origin. 12 , 13 PVBs with ≥2 morphologies that accounted for ≥10% of all PVBs were classified as polymorphic. Nonsustained VT was defined as a VT of at least 3 complexes but lasting <30 s.
Exercise testing was performed with bicycle or treadmill and continued until exhaustion. The behavior of VA during exercise testing was graded as the following: (1) PVBs were present at baseline or low workload but were suppressed with increasing workload; (2) isolated only PVBs were absent at baseline and appeared with increasing workload, or were present at baseline but were not suppressed by increasing exercise; (3) recording of at least 1 exercise‐induced couplet; (4) recording of at least 1 exercise‐induced nonsustained VT. All the examinations were reviewed by 1 investigator for each center (AZ, CC, TV). Exercise testing and 24‐hour ECG monitoring were performed within 2 weeks after the initial evaluation.
Cardiac Magnetic Resonance
CMR studies were performed with 1.5‐T systems (Magnetom Avanto, Siemens Medical Solutions, Germany; Achieva, Philips North American Corporation, USA) using dedicated software, a phased‐array surface receiver coil, vectocardiogram trigger, and a uniform protocol that included cine sequences, T2 sequences for myocardial edema, T1 sequences for fatty infiltration, and postcontrast T2 sequences for late gadolinium enhancement (LGE) according to current recommendations. 14 Global ventricular volumes, systolic function, and LV myocardial mass were calculated from the short‐axis cine images, excluding papillary muscles from the myocardium, using a dedicated software (CMR42, Circle Cardiovascular Imaging Inc; InelliSpacePortal 9.0‐Philips).
Postcontrast Sequences and LGE Evaluation
Images were acquired using a steady‐state free precession sequence (true FISP) cine loops in sequential short‐axis views (slice thickness 6 mm, gap 2 mm; repetition time 2.5–3.8; echo time 1.1–1.6, average in‐plane resolution 1.5×2.4 mm, flip angle 45°–60°, temporal resolution 40–45 ms) and long‐axis views (2‐, 3‐, and 4‐chamber views). After intravenous administration of contrast agent (gadobenate dimeglumine, Multihance, Bracco, 0.2 mmol/kg of body weight) 2‐dimensional segmented fast low‐angle shot inversion recovery sequences after at least 10 minutes were acquired in the same views of cine images, covering the entire ventricles (repetition time 5.4–8.3 ms, echo time 1.3–3.9 ms, average in‐plane spatial resolution 1.4–1.5×2.2–2.4 mm, 6‐mm slice thickness, 2‐mm gap, and flip angle 20°–25°). Inversion times were adjusted to null normal myocardium using the Look‐Locker sequence and images were repeated in 2 separate phase‐encoding directions to exclude artifacts.
Myocardial LGE was assessed with an automated thresholding and considered present if signal intensity was >3 SDs above remote myocardium in 2 orthogonal views. To exclude artifacts, LGE was deemed present only if visible in 2 orthogonal views (short‐axis and long‐axis views). LGE was quantified by a semiautomatic detection and LGE mass (in grams) expressed as a percentage of total left ventricle (LV) mass. The pattern of LGE distribution and morphology was characterized as subendocardial, epicardial/midmyocardial, transmural, or patchy. If >1 pattern was present, the distribution was characterized based on the predominant pattern. Isolated junctional LGE (ie, at the insertion points of the right‐ventricular free wall to the interventricular septum) was not considered abnormal because it is a common and nonpathologic finding in athletes. 6 All CMR were reviewed by 2 experts (A.C., M.D.L.) who were blinded to clinical data; in case of disagreement a third expert was consulted (M.P.M.). CMR was performed within 2 months after the initial evaluation.
Statistical Analysis
Continuous and categorical variables were expressed as mean (±SD) or median (25th–75th percentiles), according to distribution, which was assessed with the Shapiro‐Wilk test. Categorical variables were expressed as n (%). Categorical variables were compared using the χ2 or Fisher exact test, as appropriate. Continuous data were compared using the Student t test or the Mann–Whitney U test according to distribution. The receiver operating characteristic curve analysis was used to identify the most accurate cut‐off of number of PVBs on 24‐hour ECG monitoring to discriminate between athletes with and without LGE. Univariable and multivariable binomial regression analysis was used to assess the clinical determinants of abnormal CMR. Variables that resulted in significant predictors at univariable analysis were included in the multivariable model. Goodness of fit was assessed with the Hosmer–Lemeshow test (a small P value is indicative of poor fit). A P<0.05 was considered statistically significant. Data were analyzed with SPSS version 23 (IBM).
Results
Characteristics of the Study Sample
During the study period, 843 athletes with VA were analyzed. Of those, 518 were excluded because they did not undergo CMR or had known structural/electrical heart disease; and 74 were excluded because they presented a positive family history of cardiomyopathy or premature sudden death (n=21), abnormal ECG (n=12), or abnormal echocardiography (n=41) (Figure 1). The final study sample included 251 competitive athletes [74% males, median age 25 (17–39) years] (Table 1) training a median of 7 hours per week (range 4–14). Most athletes (53%) practiced team sports (soccer 22%, volleyball 12%, basketball 8%, and other 11%); 38% were engaged in individual endurance disciplines (running 24%, cycling 10%, and other 4%) while 9% practiced other disciplines. Sixty‐eight (27%) were symptomatic, while the others were asymptomatic and were referred after recording of PVBs at preparticipation screening. Symptoms included palpitations (n=53, 21%), presyncope (n=8, 3%), chest pain (n=7, 3%), dyspnea (n=6, 2%), and syncope (n=3, 1%).
Table 1.
Characteristics of the Study Population
| Overall (n=251) | LV LGE + (n=28) | LV LGE − (N=223) | P Value | |
|---|---|---|---|---|
| General characteristics | ||||
| Age, y | 25 (17–39) | 31 (17–50) | 23 (16–39) | 0.06 |
| Male sex, n (%) | 183 (74) | 25 (89) | 158 (71) | 0.04 |
| Body mass index, kg2/m | 23±3 | 24±5 | 23±3 | 0.74 |
| Risk factors for coronary artery disease | ||||
| Hypertension, n (%) | 10 (4) | 3 (11) | 7 (3) | 0.09 |
| Dyslipidemia, n (%) | 4 (2) | 0 | 4 (2) | 1.0 |
| Smoking, n (%) | 20 (8) | 2 (7) | 18 (8) | 1.0 |
| Family history of coronary artery disease, n (%) | 46 (19) | 3 (11) | 43 (19) | 0.44 |
| Symptoms | ||||
| Syncope or presyncope, n (%) | 11 (4) | 2 (7) | 9 (4) | 0.35 |
| Palpitations, n (%) | 53 (21) | 7 (25) | 46 (21) | 0.59 |
| Chest pain, n (%) | 7 (3) | 0 | 7 (3) | 1.0 |
| Dyspnea, n (%) | 6 (2) | 0 | 6 (3) | 1.0 |
| Cardiac magnetic resonance findings | ||||
| LV EDV, mL/m2 | 84±18 | 86±20 | 83±18 | 0.74 |
| RV EDV, mL/m2 | 87±18 | 87±21 | 87±18 | 1.0 |
| LV EF, % | 63±7 | 61±9 | 63±7 | 0.53 |
| RV EF, % | 57±7 | 57±9 | 58±7 | 0.91 |
| LV myocardial edema, n (%) | 2 (1) | 2 (7) | … | … |
| LV fatty infiltration, n (%) | 2 (1) | 2 (7) | … | … |
Values are expressed as N (%) or median (interquartiles range). EDV indicates end‐diastolic volume; EF, ejection fraction; LGE, late gadolinium enhancement; LV, left ventricle; and RV, right ventricle.
At 12‐lead 24‐hour ambulatory ECG monitoring, athletes had a median of 2625 (296–10 645) PVBs. More than 3300 PVBs/d were recorded in 121 (48%), while 83 (33%) had at least 1 nonsustained VT. The majority of athletes (209, 83%) had monomorphic PVBs, with the most common pattern being left bundle‐branch block/inferior axis (n=92, 37%).
At exercise testing, the majority of athletes (189, 75%) showed disappearance of PVBs at high workload; only isolated PVBs and couplets were observed at high workload in 23 (9%) and 14 (6%) athletes, respectively, while exercise‐induced nonsustained VT were observed in the remaining 25 (10%) athletes, including 9 (4%) with polymorphic nonsustained VT.
CMR Findings
CMR showed normal LV and right ventricular global function in all athletes (mean LV and right ventricular ejection fraction 63±7% and 57±7%, respectively) and segmental LV hypokinesis involving the lateral wall in 5 (all with LGE). LV LGE was documented in 28 (11%) athletes (Figure 2). The LGE involved a median of 2 (1–3) LV segments (8% [5–14] of the total LV mass) and showed subepicardial/midmyocardial stria pattern in 25, a patchy pattern in 1, and a subendocardial/transmural (ischemic) pattern in 2.
Figure 2. Regional distribution of late gadolinium enhancement.

Myocardial edema suggestive of acute myocardial inflammation was observed in 2 patients, both also showing a subepicardial/midmyocardial stria pattern of LGE. Fatty infiltration was observed in 2 other patients and distributed in a LV territory also showing subepicardial LGE. The former pattern (edema plus LGE) suggests acute myocarditis while the latter (fatty infiltration plus LGE) might be indicative of a fibrofatty scar typical of left‐dominant arrhythmogenic cardiomyopathy. The cause of isolated LGE with a nonischemic distribution observed in the other 22 patients (21 with a stria and 1 with a patchy pattern) remained undetermined. Clinical characteristics and CMR findings of the 28 athletes with LGE are reported in Table 2.
Table 2.
Characteristics of Athletes With LGE at CMR
| No. | Sex | Age at Evaluation | Sport | Medical History | Symptoms | Exercise Test | 24 h Holter Monitoring | CMR Findings |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 18 y | Soccer | None | None | PVBs and couplets that decreased with increasing exercise intensity | 1205 PVBs; couplets | Subepicardial LGE in the mid to apical inferolateral segments; mild pericardial effusion |
| 2 | M | 47 y | Soccer | Hypertension | Palpitations | PVBs and couplets that persisted with increasing exercise intensity | 15 190 PVBs; couplets, triplets | Subepicardial/intramyocardial LGE in the LV basal inferolateral wall with fatty infiltration |
| 3 | M | 15 y | Rowing | None | None | Isolated polymorphic PVBs at peak of exercise | 6 PVBs; 1 triplet | Intramyocardial inferior LGE |
| 4 | M | 30 y | Soccer | None | Palpitations; presyncope | Polymorphic, triplets not suppressed with increasing exercise intensity | 3861 PVBs; couplets, NSVT | Subepicardial LGE in the mid to apical inferior wall |
| 5 | M | 41 y | Bicycle | None | None | Polymorphic VT that persisted with increasing exercise intensity | 4896 PVBs; couplets | Intramyocardial LGE in the mid to apical inferolateral wall |
| 6 | M | 54 y | Rowing | None | Palpitations | PVBs and couplets suppressed with increasing exercise intensity | 3267 PVBs, couplets, NSVT | Subendocardial LGE with transmural extension of the midinferolateral segment |
| 7 | M | 62 y | Bicycle | None | Palpitations | PVBs suppressed with increasing exercise intensity | 624 PVBs, couplets | Subepicardial lateral and intramyocardial inferior LGE |
| 8 | M | 57 y | Running | None | None | PVBs and NSVT suppressed with increasing exercise intensity | 1604 PVBs | Subendocardial LGE with transmural extension of the midanterolateral segment |
| 9 | M | 52 y | Running | None | None | PVBs suppressed with increasing exercise intensity | 262 PVBs, 1 couplet, NSVT | Subepicardial and intramyocardial LGE of the inferolateral wall, apical interventricular septum with fatty infiltration |
| 10 | M | 27 y | Running | None | None | PVBs suppressed with increasing exercise intensity | 119 PVBs | Subepicardial and intramyocardial LGE of the inferolateral wall of mid to basal LV |
| 11 | M | 57 y | Bicycle | Hypertension | Palpitations | PVBs at rest; polymorphic couplets and triplets with increasing exercise intensity | 2274 PVBs, couplets | Subepicardial LGE of the inferolateral wall of mid to basal LV |
| 12 | M | 48 y | Bicycle | Hypertension | None | PVBs suppressed with increasing exercise intensity | 700 PVBs; 1 couplet | Intramyocardial LGE in the midinferolateral, midinterventricular, and midanterior segments |
| 13 | M | 23 y | Bicycle | None | Palpitations | PVBs suppressed with increasing exercise intensity | 1632 PVBs | Subepicardial stria of LGE involving the LV inferolateral wall |
| 14 | M | 49 y | Running | None | None | NSVT at low effort | 5 PVBs | Subepicardial/intramyocardial LGE on the mid and basal LV lateral segments, apical inferior segment |
| 15 | M | 18 y | Basketball | None | None | PVBs exercised induced | 1300 PVBs, couplets, triplets | Intramyocardial LGE of the basal inferior wall; |
| 16 | M | 47 y | Triathlon | None | None | Polymorphic VT exercise induced | 349 PVBs, couplets | Myocardial edema on the basal inferolateral wall; subepicardial/intramyocardial LGE on the mid and basal inferolateral segments, apical inferior segment, basal interventricular septum |
| 17 | F | 14 y | Swimming | None | None | PVBs suppressed with increasing exercise intensity | 3993 PVBs, couplets | Intramyocardial LGE of the basal inferior wall, basal interventricular septum |
| 18 | M | 23 y | Athletics | Smoker; FH of cardiovascular disease | Palpitations; syncope | PVBs suppressed with increasing exercise intensity | 9 PVBs, NSVT | Subepicardial stria of LGE involving the midapical segments of the lateral wall; LGE spot in the basal anterolateral wall |
| 19 | F | 17 y | Soccer | None | None | Polymorphic VT exercise‐induced | 123 PVBs, couplets, NSVT | Intramyocardial mid inferolateral LGE |
| 20 | M | 13 y | Fencing | FH of cardiovascular disease | None | VA suppressed with increasing exercise intensity | 568 PVBs, couplets | Subepicardial LGE of the LV basal inferior wall |
| 21 | M | 49 y | Swimming | Smoker; FH of cardiovsacular disease | None | Couplets exercise‐induced | 1246 PVBs, couplets | Subepicardial/intramyocardial LGE on the LV midinferolateral segments; |
| 22 | F | 16 y | Volleyball | Smoker | None | Exercise‐induced polymorphic VT | 1226 PVBs, couplets, triplets | Subepicardial LGE of the mid LV inferior segment; patchy LGE of the midventricular septum |
| 23 | M | 12 y | Tennis | Smoker; FH of cardiovascular disease | None | PVBs, couplets suppressed with increasing exercise intensity | 13 PVBs, couplets, triplets | Myocardial edema along the lateral wall; subepicardial LGE of the LV basal lateral wall |
| 24 | M | 48 y | Soccer | None | None | PVBs exercised induced | 241 PVBs, couplets, 1 triplet | Subepicardial LGE of the LV inferolateral wall |
| 25 | M | 17 y | Runner | None | None | Couplets, triplet exercise‐induced | 49 PVBs couplets | Subepicardial/intramyocardial midbasal inferolateral LGE |
| 26 | M | 31 y | Swimming | None | None | NSVT exercise‐induced | 6 PVBs | Intramyocardial LGE on the LV midinferior segment |
| 27 | M | 17 y | Soccer | None | None | Couplets exercise‐induced | 53 PVBs, couplets, NSVT | Subepicardial LGE of the midinferior segment |
| 28 | M | 24 y | Runner | None | None | PVBs exercise induced | 12 PVBs, couplets | Intramyocardial LGE of the LV inferior wall |
CMR indicates cardiac magnetic resonance; FH, family history; LV, left ventricle; NSVT, nonsustained ventricular tachycardia; PVBs, premature ventricular beats; VA, ventricular arrhythmia; and VT, ventricular tachycardia.
Predictors of LV LGE
Athletes with LGE were not significantly older (31 [17–50] years versus 23 [16–39] years, P=0.06) and were significantly more often males (25 [89%] versus 158 [71%], P=0.04) than those without. The prevalence of athletes reporting symptoms was similar between those with and without LGE (7 [25%] versus 61 [27%], P=0.79) (Table 1).
Ventricular arrhythmia characteristics on 12‐lead 24‐hour ECG monitoring and exercise testing in patients with and without positive CMR are shown in Table 3.
Table 3.
Ventricular Arrhythmia Characteristics at 12‐Lead 24‐Hour ECG Monitoring and Exercise Testing in Patients With and Without Positive CMR
| LV LGE + (n=28) | LV LGE − (n=223) | P Value | |
|---|---|---|---|
| 12‐lead 24‐h ECG monitoring | |||
| PVBs morphology | <0.001 | ||
| LBBB/inferior axis, n (%) | 0 | 92 (41) | |
| LBBB/intermediate or superior axis, n (%) | 2 (7) | 36 (16) | |
| RBBB/narrow QRS (fascicular), n (%) | 0 | 9 (4) | |
| RBBB/inferior axis, n (%) | 1 (4) | 28 (13) | |
| RBBB/intermediate or superior axis, n (%) | 7 (25) | 34 (15) | |
| Polymorphic, n (%) | 18 (64) | 24 (11) | |
| PVBs number* | 596 (50–1625) | 3858 (372–12 200) | <0.001 |
| Couplets, n (%) | 21 (75) | 123 (55) | 0.025 |
| Nonsustained VT, n (%) | 12 (43) | 71 (32) | 0.34 |
| Exercise testing | |||
| Reduction/disappearance, n (%) | 13 (46) | 176 (79) | <0.001 |
| Persistence/appearance of isolated PVBs, n (%) | 4 (14) | 19 (9) | 0.302 |
| Persistence/appearance of couplets, n (%) | 3 (11) | 11 (5) | 0.195 |
| Exercise‐induced nonsustained VT, n (%) | 8 (29) | 17 (8) | 0.002 |
| Exercise‐induced polymorphic VT, n (%) | 6 (21) | 3 (1) | <0.001 |
CMR indicates cardiac magnetic resonance; LBBB left bundle‐branch block; LGE, late gadolinium enhancement; LV, left ventricle; PVB, premature ventricular beat; RBBB, right bundle‐branch block; and VT, ventricular tachycardia.
Data are expressed as median (interquartile range).
On 24‐hour ECG monitoring, PVBs with multiple morphologies or with RBBB and intermediate/superior axis configuration were documented in 25 (89%) athletes with versus 58 (26%) without LGE (P<0.001). It is noteworthy that none of the 92 athletes with PVBs with left‐bundle‐branch‐block and inferior axis configuration (infundibular pattern) had positive CMR (P<0.001). The receiver operating characteristic curve analysis (Figure 3) showed that the number of PVBs significantly discriminated between patients with and without LGE at CMR with an area under the curve of 0.75 (95% CI, 0.63–0.88; P=0.005). Patients with LGE had a lower number of PVBs and a number <3300/24 h was identified as the optimal cut‐off point to predict LGE, with sensitivity of 68% and specificity of 73%. More than 3300 PVBs were recorded in 4 (14%) athletes with versus 117 (52%) without positive CMR (P<0.001). Couplets were recorded in 21 (75%) athletes with versus 123 (55%) without LGE (P=0.025), while at least 1 nonsustained VT was recorded in 12 (43%) athletes with and 71 (32%) without LGE (P=0.34). At exercise testing, nonsustained VT occurred at peak of exercise in 8 (29%) athletes with versus 17 (8%) without LGE (P=0.002), while polymorphic nonsustained VT was observed in 6 (21%) athletes with and in 3 (1%) without LGE (P<0.001). Sensitivity, specificity, predictive value, and diagnostic accuracy of PVB characteristics for the presence of LGE on CMR are shown in Table 4.
Figure 3. ROC curve analysis of number of premature ventricular beats for the prediction of late gadolinium enhancement.
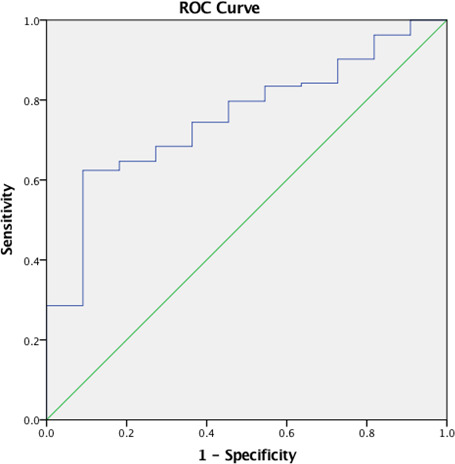
The area under the curve was 0.75 (0.63–0.88), P=0.005. The cut‐off point that best identified late gadolinium enhancement with sensitivity of 68% and specificity of 73% was 3300. ROC indicates receiver operating characteristic.
Table 4.
Sensitivity, Specificity, Predictive Value, and Diagnostic Accuracy of PVB Characteristics for the Presence of LGE on Cardiac Magnetic Resonance
| Sensitivity | Specificity | PPV | NPV | Accuracy | |
|---|---|---|---|---|---|
| Morphology: RBBB/intermediate‐superior axis or polymorphic | 89% | 98% | 30% | 98% | 76% |
| Number of PVBs: <3300/24 h | 86% | 97% | 18% | 97% | 56% |
| Exercise testing: polymorphic VT | 21% | 91% | 67% | 91% | 90% |
LGE indicates late gadolinium enhancement; NPV, negative predictive value; PPV, positive predictive value; PVB, premature ventricular beat; RBBB, right bundle‐branch block; and VT, ventricular tachycardia.
On multivariable analysis, PVBs with a RBBB and intermediate/superior axis configuration or multiple morphologies, a number of PVBs <3300/24 h and the occurrence of exercise‐induced polymorphic nonsustained VT remained independent predictors of LV LGE (Table 5).
Table 5.
Univariable and Multivariable Analysis for Predictors of Positive LGE
| Univariable Analysis | Multivariable Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Male sex | 3.4 | 1.0–11.8 | 0.053 | |||
| 24‐h ambulatory ECG monitoring | ||||||
| Morphology: RBBB/intermediate or superior axis or polymorphic | 23.7 | 6.8–81.4 | <0.001 | 19.7 | 5.3–73 | <0.001 |
| Number of PVBs: <3300/24 h | 6.6 | 2.2–19.7 | 0.001 | 5.4 | 1.6–17.8 | 0.005 |
| Complexity: ≥1 nonsustained VT | 1.6 | 0.7–3.5 | 0.246 | |||
| Exercise testing | ||||||
| Polymorphic VT exercise‐induced | 20.0 | 4.6–85.5 | <0.0001 | 7.1 | 1.4–44.9 | 0.022 |
Hosmer–Lemeshow goodness‐of‐fit test: χ2=2.7; P=0.45. LGE indicates late gadolinium enhancement; OR, odds ratio; PVBs, premature ventricular beats; RBBB, right bundle‐branch block; and VT, ventricular tachycardia.
A flow‐chart showing the prevalence of LGE according to the morphology of PVBs and the occurrence of nonsustained VT at peak of exercise testing is shown in Figure 4. Among the 15 athletes with both PVBs with multiple morphologies or with RBBB intermediate/superior axis configuration and the occurrence of exercise‐induced nonsustained VT, 8 (53%) had a positive CMR (Figure 5) including 6 (67%) with exercise‐induced polymorphic nonsustained VT. On the other hand, 3/168 (2%) athletes without PVBs with polymorphic or RBBB intermediate/superior axis configurations and without exercise‐induced nonsustained VT showed LV LGE (Figure 6). Avoidance of CMR in athletes with neither characteristic would have increased the positive predictive value from 11% (28/251) to 27% (25/93) while reducing sensitivity by 11% (3/28).
Figure 4. Summary of main study findings.
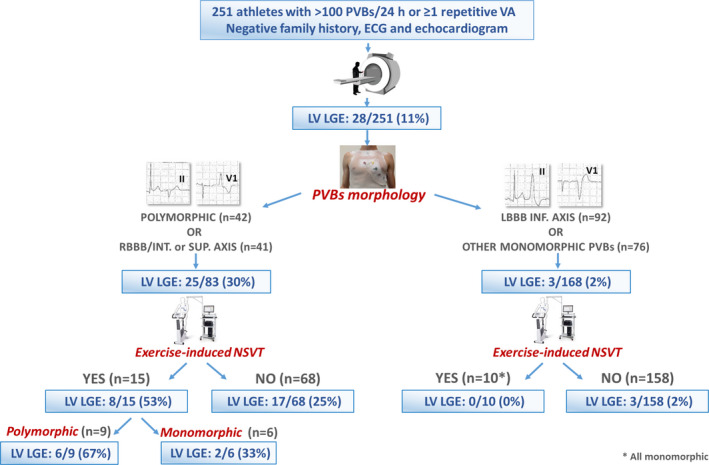
LBBB indicates left bundle‐branch block; LGE, late gadolinium enhancement; LV, left ventricle; PVBs, premature ventricular beats; RBBB, right bundle‐branch block; and VA, ventricular arrhythmias.
Figure 5. Representative example of an athlete with positive cardiac magnetic resonance.

A 41‐year‐old asymptomatic cyclist with negative resting ECG and echocardiography showed polymorphic premature ventricular beats (with both right‐bundle‐branch‐block and left‐bundle‐branch‐block configurations) and polymorphic ventricular tachycardia at exercise testing (A). Short‐axis (B) and 4‐chamber (C) postcontrast cardiac magnetic resonance views revealed a stria of late gadolinium‐enhancement suggesting myocardial fibrosis with a subepicardial/midmyocardial (nonischemic) distribution involving the basal and midapical inferolateral left ventricular segments (white arrows).
Figure 6. Representative example of an athlete with negative cardiac magnetic resonance.
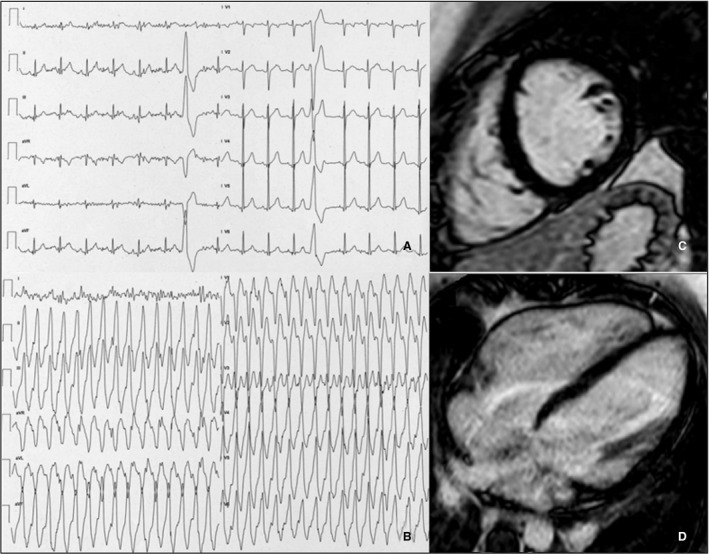
A 19‐year‐old soccer player symptomatic for palpitations showed frequent premature ventricular beats with a left‐bundle‐branch‐block configuration, precordial S/R transition in V4, and inferior axis in the limb leads, suggesting a right ventricular outflow tract origin (A). During exercise testing, symptomatic nonsustained ventricular tachycardia occurred (B). Short‐axis (C) and 4‐chamber (D) postcontrast cardiac magnetic resonance views showed no myocardial abnormalities. The athlete underwent successful catheter ablation.
Discussion
This study was designed to investigate the characteristics of VA that were associated with abnormal myocardial substrates at CMR in athletes with otherwise unremarkable clinical findings. The clinical objective was to identify criteria for appropriate CMR prescription in this particular setting. The main findings were the following: (1) 28 athletes (11%) with PVBs and a low clinical probability of an underlying heart disease (negative family history, no previous sustained VT or cardiac arrest, normal ECG and echocardiography) had evidence of LV LGE at cardiac CMR; (2) in the majority of athletes with LGE (21/28, 75%), CMR abnormalities consisted of LGE with a subepicardial/midmyocardial stria pattern in the absence of wall motion abnormalities, myocardial edema, or fatty infiltration (so‐called isolated nonischemic LV scar); (3) independent predictors of a positive CMR included the presence of PVBs with a RBBB and intermediate/superior axis configuration or multiple morphologies and the occurrence of polymorphic nonsustained VT during exercise testing; (4) none of the 92 athletes with PVBs with left‐bundle‐branch‐block and inferior axis configuration (infundibular pattern) showed positive CMR; and (5) a high PVB burden should not be considered a risk factor for an underlying cardiac disease.
LV Scar as a Substrate of VAs in the Athletes
VAs are not rare in athletes. In 2 studies on apparently healthy athletes who volunteered to undergo a 24‐hour ambulatory ECG monitoring, 8% of young (<30‐year‐old) and 18% of veteran (>30‐year‐old) athletes showed >100 PVBs/h or ≥1 repetitive VA (ie, the inclusion criteria of the present study). 8 , 9 Identification of VA in athletes raises the concern for myocardial abnormalities at risk of sudden death. In most cases, athletes with PVBs caused by a structural myocardial disease show a positive family history, ECG, and/or echocardiography. 3 , 15 However, there is emerging evidence that segmental pathological arrhythmic substrates such as LV scars may be missed by traditional clinical investigation. 3 , 4 , 5 , 6 , 7
The LV scars are classified according to the pattern of distribution into ischemic (subendocardial or transmural) and nonischemic (subepicardial or midmyocardial). 14 The etiopathological basis of nonischemic LV scars remains elusive: although they are traditionally attributed to a previous (healed) myocarditis, they may be observed in many genetically determined diseases and some hypothesize that they may also represent the hallmark of exercise‐induced myocardial damage. 6 , 7 , 16 , 17 , 18 , 19 , 20 Regardless of the origin, LV scars should not be dismissed as innocent findings because data from both the athletic and the general population demonstrated that they can be the substrate of life‐threatening VA during follow‐up. 6 , 20 Unfortunately, this potentially dangerous myocardial substrate is usually eletrocardiographically and echocardiographically silent and can only be revealed by CMR, in the form of LGE. 4 , 5 , 6 , 7
CMR in Athletes With VA
CMR is expensive and may have limited availability and, for this reason, there is a need for appropriate prescription criteria. In athletes with VA, the diagnostic role of CMR is 2‐fold. The first is to further investigate subjects with abnormal electrocardiographic or echocardiographic findings. The second is to rule out the presence of an underlying disease in athletes with high‐risk VA features but otherwise unremarkable clinical findings. In this last case, there is no consensus on when CMR should be performed.
Previous studies on small samples of athletes undergoing CMR because of VA showed that PVBs with a RBBB pattern (suggesting LV origin) and increasing arrhythmia complexity with exercise predicted an underlying LV scar. 4 , 5 , 6 , 7 , 9 A recent study on a large sample of individuals (mostly middle‐aged nonathletes) who underwent CMR because of frequent (>1000) PVBs/d and otherwise negative diagnostic work‐up confirmed that PVBs with a RBBB pattern or multiple morphologies were independent predictors of myocardial abnormalities while the PVBs burden was not. 20 However, such previous investigations also included individuals with a high disease probability because of positive family history, abnormal ECG findings, or regional wall motion abnormalities on echocardiography.
The present multicenter study was unique because it enrolled a large sample of athletes who exhibited PVBs as the only clinical abnormality (ie, negative family history, resting ECG, and echocardiography) that in the pre‐CMR era would have been labeled as idiopathic. We found a segmental LV scar in 11% of cases, mostly with a nonischemic pattern. Simple characteristics of VA such as morphology (RBBB and intermediate/superior axis or polymorphic) and response to exercise testing allowed identification of athletes at highest risk of pathological CMR. On the other hand, although the current perspective is that a higher number of PVBs is a risk factor for an underlying disease, we found that a high PVB burden was paradoxically associated with a structurally normal heart. This observation suggests that idiopathic and benign ectopic activity may generate a higher number of ventricular ectopic beats than the LV scar, hindering the predictive value of the PVB burden.
Clinical Implications
The present study confirms that PVBs may be caused by an LV scar identifiable only by CMR. The probability of a concealed myocardial substrate depends on simple VA characteristics, particularly QRS morphology (Figure 4). Athletes with VA with a RBBB pattern and intermediate/inferior axis or polymorphic PVBs that become repetitive during exercise represented the highest‐risk subgroup: the probability of an underlying LV scar was 53% and increased to 67% in the presence of exercise‐induced nonsustained polymorphic VT. Prescription of CMR in these cases appears mandatory. The probability of underlying pathological substrates in athletes with VA with multiple morphologies or RBBB/inferior or intermediate axis configuration but not nonsustained VT at exercise testing was 25%: they also probably should undergo CMR. On the other hand, the probability of LGE in athletes with monomorphic PVBs with left bundle‐branch block or RBBB/inferior axis was only 2%: avoidance of CMR in this subgroup would have reduced the sensitivity for identification of LV‐LGE by 11% (from 28 to 25 cases) but increased the diagnostic rate from 11% (28/251) to 30% (25/83).
Study Limitations
The study has some limitations. The main one was that CMR was not prescribed according to prespecified criteria. As a consequence, the prevalence of abnormal CMR findings in our study may be influenced by selection bias and may not be representative of the general population of athletes. However, it is noteworthy that in a previous study enrolling asymptomatic young athletes who volunteered to undergo 24‐hour ambulatory ECG monitoring and who underwent CMR in case of frequent (>500/d), exercise‐induced, or repetitive PVBs, the prevalence of LV scar was higher (18%). A 18% rate of LV LGE was also observed in a recent study involving patients who underwent CMR because they showed >1000/d apparently idiopathic PVBs. 20 A second limitation may arise from not including a large number of patients who were unable to undergo CMR for various reasons, but who could have been eligible for the analysis. Third, the sample is unbalanced in terms of sex (74% males). Fourth, despite its potential utility for evaluation of athletes with suspected heart disease, 21 , 22 , 23 the CMR protocol did not included the study of extracellular volume (T1‐mapping) for quantification of interstitial fibrosis, because the technique was not available at the time of enrollment. Finally, it is beyond the scope of the present study to show data on arrhythmic outcome of LV scar.
Conclusions
This multicenter study confirmed previous observations that negative ECG and echocardiography cannot definitely rule out an underlying structural myocardial abnormality in athletes with VA. Because of its ability to provide tissue characterization, CMR has a higher sensitivity for detecting concealed myocardial substrates such as the nonischemic LV scar, but it should be reserved for selected cases because of its high costs and limited availability.
According to our findings, simple VA characteristics such as morphology and response to exercise testing may help to identify the subset of athletes with the highest probability of an underlying disease, for whom CMR prescription may be particularly cost‐effective. On the other hand, contrary to current perspective, a high number of PVBs/24 h should not be considered a risk factor for an associated cardiac disease.
Sources of Funding
The study was supported by a research grant of the Italian Ministry of Health (RF‐2014‐00000394) and by the Department of Cardiac, Thoracic and Vascular Sciences and Public Health of the University of Padova, Italy (BIRD‐2016‐162733).
Disclosures
None.
(J Am Heart Assoc. 2021;10:e018206. DOI: 10.1161/JAHA.120.018206.)
For Sources of Funding and Disclosures, see page 13.
REFERENCES
- 1. Corrado D, Drezner JA, D'Ascenzi F, Zorzi A. How to evaluate premature ventricular beats in the athlete: critical review and proposal of a diagnostic algorithm. Br J Sports Med. 2020;54:1142–1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Parisi A, Tranchita E, Minganti C, Sperandii F, Guerra E, Calò L, Borrione P, Pigozzi F. Young athletes with ventricular premature beats: continuing or not intense training and competition? Scand J Med Sci Sports. 2018;28:541–548. [DOI] [PubMed] [Google Scholar]
- 3. Calo L, Martino A, Tranchita E, Sperandii F, Guerra E, Quaranta F, Parisi A, Nigro A, Sciarra L, Ruvo E, et al. Electrocardiographic and echocardiographic evaluation of a large cohort of peri‐pubertal soccer players during pre‐participation screening. Eur J Prev Cardiol. 2019;26:1444–1455. [DOI] [PubMed] [Google Scholar]
- 4. Zorzi A, Vessella T, De Lazzari M, Cipriani A, Menegon V, Sarto G, Spagnol R, Merlo L, Pegoraro C, Marra MP, et al. Screening young athletes for diseases at risk of sudden cardiac death: role of stress testing for ventricular arrhythmias. Eur J Prev Cardiol. 2020;27:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cipriani A, Zorzi A, Sarto P, Donini M, Rigato I, Bariani R, De Lazzari M, Pilichou K, Thiene G, Iliceto S, et al. Predictive value of exercise testing in athletes with ventricular ectopy evaluated by cardiac magnetic resonance. Heart Rhythm. 2019;16:239–248. [DOI] [PubMed] [Google Scholar]
- 6. Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, et al. Nonischemic left ventricular scar as a substrate of life‐threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol. 2016;9:e004229. DOI: 10.1161/CIRCEP.116.004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schnell F, Claessen G, La Gerche A, Bogaert J, Lentz PA, Claus P, Mabo P, Carré F, Heidbuchel H. Subepicardial delayed gadolinium enhancement in asymptomatic athletes: let sleeping dogs lie? Br J Sports Med. 2016;50:111–175. [DOI] [PubMed] [Google Scholar]
- 8. Zorzi A, Mastella G, Cipriani A, Berton G, Del Monte A, Gusella B, Nese A, Portolan L, Sciacca F, Tikvina S, et al. Burden of ventricular arrhythmias at 12‐lead 24‐hour ambulatory ECG monitoring in middle‐aged endurance athletes versus sedentary controls. Eur J Prev Cardiol. 2018;25:2003–2011. [DOI] [PubMed] [Google Scholar]
- 9. Zorzi A, De Lazzari M, Mastella G, Niero A, Trovato D, Cipriani A, Peruzza F, Portolan L, Berton G, Sciacca F, et al. Ventricular arrhythmias in young competitive athletes: prevalence, determinants, and underlying substrate. J Am Heart Assoc. 2018;7:e009171. DOI: 10.1161/JAHA.118.009171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Decree of the Ministry of Health 18/02/1982 "Rules for the health care of competitive sport activities".
- 11. Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, La Gerche A, Ackerman MJ, Borjesson M, Salerno JC, et al. International recommendations for electrocardiographic interpretation in athletes. Eur Heart J. 2018;39:1466–1480. DOI: 10.1093/eurheartj/ehw631. [DOI] [PubMed] [Google Scholar]
- 12. Luebbert J, Auberson D, Marchlinski F. Premature ventricular complexes in apparently normal hearts. Card Electrophysiol Clin. 2016;8:503–514. DOI: 10.1016/j.ccep.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 13. D'Ascenzi F, Zorzi A, Alvino F, Bonifazi M, Corrado D, Mondillo S. The prevalence and clinical significance of premature ventricular beats in the athlete. Scand J Med Sci Sports. 2017;27:140–151. DOI: 10.1111/sms.12679. [DOI] [PubMed] [Google Scholar]
- 14. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff‐Brenkenhoff F, Kramer CM, Pennell DJ, et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees Task Force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. DOI: 10.1186/1532-429X-15-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vessella T, Zorzi A, Merlo L, Pegoraro C, Giorgiano F, Trevisanato M, Viel M, Formentini P, Corrado D, Sarto P. The Italian preparticipation evaluation programme: diagnostic yield, rate of disqualification and cost analysis. Br J Sports Med. 2020;54:231–237. DOI: 10.1136/bjsports-2018-100293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Breuckmann F, Möhlenkamp S, Nassenstein K, Lehmann N, Ladd S, Schmermund A, Sievers B, Schlosser T, Jöckel K‐H, Heusch G, et al. Myocardial late gadolinium enhancement: prevalence, pattern, and prognostic relevance in marathon runners. Radiology. 2009;251:50–57. DOI: 10.1148/radiol.2511081118. [DOI] [PubMed] [Google Scholar]
- 17. La Gerche A. Can intense endurance exercise cause myocardial damage and fibrosis? Curr Sports Med Rep. 2013;12:63–69. [DOI] [PubMed] [Google Scholar]
- 18. Eichhorn C, Biere L, Schnell F, Schmied C, Wilhelm M, Kwong RY, Gräni C. Myocarditis in athletes is a challenge: diagnosis, risk stratification, and uncertainties. JACC Cardiovasc Imaging. 2020;13:494–507. DOI: 10.1016/j.jcmg.2019.01.039. [DOI] [PubMed] [Google Scholar]
- 19. Corrado D, van Tintelen PJ, McKenna WJ, Hauer RNW, Anastastakis A, Asimaki A, Basso C, Bauce B, Brunckhorst C, Bucciarelli‐Ducci C, et al. Arrhythmogenic right ventricular cardiomyopathy: evaluation of the current diagnostic criteria and differential diagnosis. Eur Heart J. 2020;41:1414–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muser D, Santangeli P, Castro SA, Maeda S, Casado Arroyo R, Liuba I, Benhayon D, Sadek M, Desjardins B, Garcia F, et al. Risk stratification of patients with apparently idiopathic premature ventricular contractions. A multicenter international CMR registry. JACC Clin Electrophysiol. 2020;6:722–735. [DOI] [PubMed] [Google Scholar]
- 21. Miller CA, Naish JH, Bishop P, Coutts G, Clark D, Zhao S, Ray SG, Yonan N, Williams SG, Flett AS, et al. Comprehensive validation of cardiovascular magnetic resonance techniques for the assessment of myocardial extracellular volume. Circ Cardiovasc Imaging. 2013;6:373–383. DOI: 10.1161/CIRCIMAGING.112.000192. [DOI] [PubMed] [Google Scholar]
- 22. McDiarmid AK, Swoboda PP, Erhayiem B, Lancaster RE, Lyall GK, Broadbent DA, Dobson LE, Musa TA, Ripley DP, Garg P, et al. Athletic cardiac adaptation in males is a consequence of elevated myocyte mass. Circ Cardiovasc Imaging. 2016;9:e003579. DOI: 10.1161/CIRCIMAGING.115.003579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maestrini V, Torlasco C, Hughes R, Moon JC. Cardiovascular magnetic resonance and sport cardiology: a growing role in clinical dilemmas. J Cardiovasc Transl Res. 2020;13:296–305. DOI: 10.1007/s12265-020-10022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


