Abstract
Background
Contrast‐associated acute kidney injury (CA‐AKI) is associated with substantial morbidity and may be prevented by using less contrast during percutaneous coronary intervention (PCI). However, tools for determining safe contrast volumes are limited. We developed risk models to tailor safe contrast volume limits during PCI.
Methods and Results
Using data from all PCIs performed at 18 hospitals from January 2015 to March 2018, we developed logistic regression models for predicting CA‐AKI, including simpler models (“pragmatic full,” “pragmatic minimum”) using only predictors easily derivable from electronic health records. We prospectively validated these models using PCI data from April 2018 to December 2018 and compared them to preexisting safe contrast models using the area under the receiver operating characteristic curve (AUC). The model derivation data set included 20 579 PCIs with 2102 CA‐AKI cases. When applying models to the separate validation data set (5423 PCIs, 488 CA‐AKI cases), prior safe contrast limits (5*Weight/Creatinine, 2*CreatinineClearance) were poor measures of safety with accuracies of 53.7% and 56.6% in predicting CA‐AKI, respectively. The full, pragmatic full, and pragmatic minimum models performed significantly better (accuracy, 73.1%, 69.3%, 66.6%; AUC, 0.80, 0.76, 0.72 versus 0.59 for 5 * Weight/Creatinine, 0.61 for 2*CreatinineClearance). We found that applying safe contrast limits could meaningfully reduce CA‐AKI risk in one‐quarter of patients.
Conclusions
Compared with preexisting equations, new multivariate models for safe contrast limits were substantially more accurate in predicting CA‐AKI and could help determine which patients benefit most from limiting contrast during PCI. Using readily available electronic health record data, these models could be implemented into electronic health records to provide actionable information for improving PCI safety.
Keywords: contrast‐associated acute kidney injury, contrast‐induced nephropathy, percutaneous coronary intervention
Subject Categories: Quality and Outcomes, Percutaneous Coronary Intervention
Nonstandard Abbreviations and Acronyms
- CA‐AKI
contrast‐associated acute kidney injury
- CrCl
creatinine clearance
- NCDR
National Cardiovascular Data Registry
Clinical Perspective
What Is New?
When applied to a large cohort of patients who underwent percutaneous coronary intervention (PCI), preexisting methods for calculating safe contrast volume limits during PCI (5*Weight/Creatinine, 2*CreatinineClearance) performed poorly in predicting which patients developed contrast‐associated acute kidney injury.
Contrast volume limits calculated from new multivariable models that used risk factor information easily derivable from the electronic health records were significantly more accurate in discriminating which patients developed contrast‐associated acute kidney injury.
What Are the Clinical Implications?
Multivariable models for safe contrast limits can help determine which patients benefit most from limiting contrast during PCI and how much contrast is safe.
Using the calculated safe contrast limit, approximately one‐quarter of patients undergoing PCI could have their contrast‐associated acute kidney injury risk meaningfully reduced by decreasing contrast usage.
In using information readily available from the electronic health record, these new safe contrast volume limit models could be implemented into electronic health records to provide actionable information at the point of care to improve PCI safety.
Cardiovascular disease remains the leading cause of morbidity and mortality in the United States, with coronary artery disease being the most common type of heart disease. 1 Percutaneous coronary intervention (PCI) is the treatment of choice for refractory symptomatic coronary artery disease and acute coronary syndromes, with more than 660 000 PCIs performed annually across the country. 2 Up to 14% of all PCIs are complicated by postprocedure acute kidney injury, also known as contrast‐associated acute kidney injury (CA‐AKI). 3 , 4 , 5 , 6 , 7 Patients who develop CA‐AKI suffer not only from longer and more costly hospital stays but also from increased mortality, including a 36% chance of death during hospitalization and a 12% chance of dying within 1 year of hospital discharge. 3 , 4 , 8 , 9 , 10
To address the harms of CA‐AKI and its costs to the healthcare system, risk models have been developed to predict patients at highest risk for this complication. 11 These models consistently identify contrast volume as an important risk factor, and it remains one of the few risk factors that are modifiable in the periprocedural setting. Reducing the volume of contrast used during PCI has therefore been the centerpiece of many prior efforts to prevent CA‐AKI. 12 , 13 However, despite general enthusiasm for reducing contrast usage, current methods for determining a safe contrast volume for a given patient are underdeveloped and do not account for many patient risk factors, such as hypertension or diabetes mellitus, that are known to predispose patients to CA‐AKI. 11 , 14
Developing more accurate safe contrast volume limits could substantially reduce CA‐AKI risk by giving providers a concrete value to use for procedural planning before or even during cardiac catheterization (eg, whether to stage a multivessel PCI procedure if a substantial amount of contrast has already been used or whether to use special contrast‐sparing techniques). 15 Knowing this limit has the potential to improve PCI safety and inform shared decision making. In this study, we developed a new pragmatic model for more accurately calculating safe contrast limits.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Study Cohort
We performed a multicenter retrospective analysis of all patients undergoing PCI between January 2015 and March 2018 at hospitals sharing data with Biome Analytics, a cardiovascular data analytics firm in San Francisco, California. This included a mixture of academic and nonacademic as well as public and private institutions (4 public academic, 2 private academic, 12 private nonacademic). Data fields were the same as those defined by the NCDR (National Cardiovascular Data Registry) CathPCI Registry. 16 We excluded nonindex procedures (ie, repeat PCI procedures during a hospitalization), same‐day discharge procedures (attributable to lack of postprocedure creatinine measurement), as well as those where the patient was already dialysis dependent. We further excluded any patients missing pre‐ or postprocedure creatinine values, the vast majority of which were same‐day discharge procedures (Figure 1). We prospectively validated our model with data contributed to the same data set from April 2018 to December 2018. The study protocol was approved by the institutional review board at Cedars–Sinai Medical Center and was in accordance with data‐sharing agreements signed by hospitals working with Biome Analytics.
Figure 1. Selection of PCI procedures included in retrospective and prospective datasets for creating and validating predictive models.
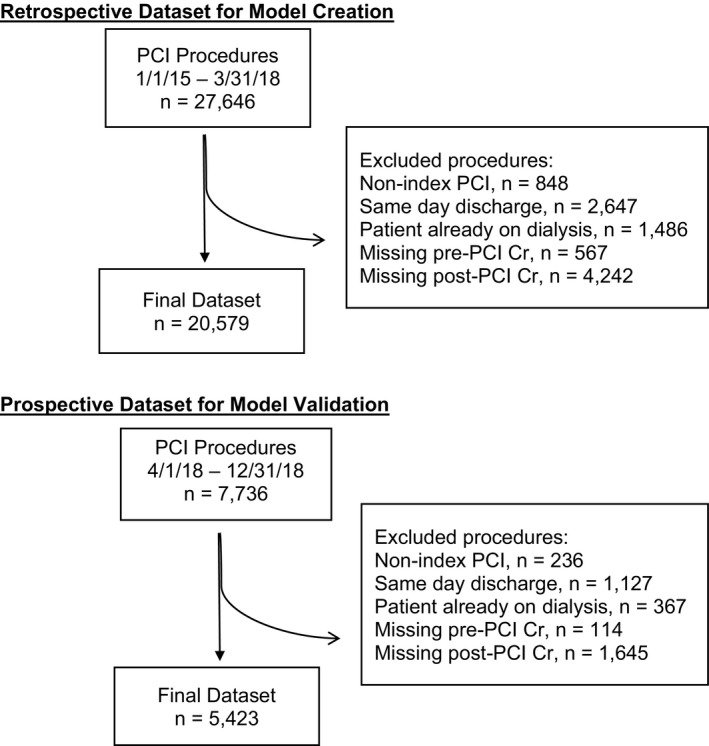
PCI indicates percutaneous coronary intervention.
For patient characteristics, continuous variables were expressed as mean±SD, and discrete variables were expressed as frequency counts and percentages. The differences in discrete variables between groups were evaluated by the chi‐square test. Differences in continuous variables were evaluated using the t test.
Prior Models for Safe Contrast Limits
We conducted a literature review to identify prior published studies on safe contrast limits during PCI (see Data S1).
Study Variables
CA‐AKI was defined according to the NCDR definition as a serum creatinine increase of ≥50% or ≥0.3 mg/dL from baseline. 17 , 18 Other predictors used in our models were also defined according to NCDR definitions. 17 As prior studies have shown that iodine content may affect CA‐AKI rates, we normalized contrast volumes to a standard iodine concentration of 300 mg/mL. 19 , 20 We analyzed the rates of CA‐AKI in patients with specific risk factors who had received a contrast volume that was <5*Weight/Creatinine or 2*CreatinineClearance (CrCl). 21 , 22 These thresholds were chosen as comparators as they were the most frequently cited existing safe contrast limits based on our literature review (Table 1).
Table 1.
Previously Published Studies of Safe Contrast Limits for Preventing CA‐AKI
| Author | Patient Cohort | CA‐AKI Definition | Contrast Limit | Performance of Limit |
|---|---|---|---|---|
| Cigarroa 1989 21 | 115 patients with renal dysfunction (creatinine >1.8) undergoing diagnostic coronary angiography | Creatinine increase >1 mg/dL by 5 d | CV <5*Weightt/Creatinine | CA‐AKI in 2% of patients below limit. CA‐AKI in 38% of patients above limit |
| Marenzi 2009 25 | 561 patients with STEMI undergoing PCI | Creatinine increase > 25% by 72 h | CV <5*Weight/Creatinine | CA‐AKI in 2.8% of patients below limit; CA‐AKI in 13% of patients above limit |
| Laskey 2007 26 | 3179 patients undergoing PCI | Creatinine increase >0.5 mg/dL from 24 to 48 h | CV <3.7*CrCl | CV/CrCl ROC curve had C‐statistic of 0.69 for predicting CA‐AKI |
| Gurm 2011 22 | 58 957 patients undergoing PCI | Creatinine increase >0.5 mg/dL by 1 wk | CV <2*CrCl | CV/CrCl ROC curve had C‐statistic of 0.67 for predicting CA‐AKI |
| Mager 2011 27 | 871 patients with STEMI undergoing PCI | Creatinine increase >0.5 mg/dL or 25% by 48 h | CV < 3.7*CrCl | CV/CrCl >3.7 had odds ratio of 3.87 for CA‐AKI |
| Ando 2014 28 | 535 patients with STEMI undergoing PCI | Creatinine increase > 0.5 mg/dL or 25% by 72 h | CV <2.5*CrCl | CV/CrCl ROC curve had C‐statistic of 0.77 for predicting CA‐AKI |
| Ogata 2014 29 | 100 patients undergoing elective PCI with CrCl <30 | Creatinine increase >0.5 mg/dL or 25% by 48 h | CV <1*CrCl | CA‐AKI in 0% below the limit compared with 11% for CV >2*CrCl but <5*Weight/Creatinine |
| Liu 2015 30 | 3273 patients undergoing coronary angiography or PCI | Creatinine increase >0.5 mg/dL from 48 to 72 h |
CV <2.44*CrCl, CV <1.87*CrCl if low hydration CV <2.93*CrCl if high hydration |
CV/CrCl ROC curve had C‐statistic of 0.78 for predicting CA‐AKI. C‐statistics of 0.74, 0.73 for low and high hydration thresholds |
| Liu 2015 30 | 1020 patients >65 years old with creatinine <1.5 mg/dL undergoing PCI | Creatinine increase >0.5 mg/dL from 48 to 72 h | CV <2.74*CrCl | CV/CrCl ROC curve had C‐statistic of 0.68 for predicting CA‐AKI |
| Nyman 2008 19 | 391 patients with STEMI undergoing PCI | Creatinine increase >44.2 μmol/L | Contrast iodine (g) < CrCl | CA‐AKI in 3% of patients below limit. CA‐AKI in 25% of patients above limit |
| Yoon 2011 20 | 226 patients undergoing elective PCI | Creatinine increase >0.5 mg/dL or 25% from 48 to 72 h | Contrast iodine (g) < 1.42*CrCl | Contrast iodine (g)/CrCl ROC curve had C‐statistic of 0.87 for predicting CA‐AKI |
CA‐AKI indicates contrast‐associated acute kidney injury, CrCl, Creatinine clearance; CV, contrast volume; PCI, percutaneous coronary intervention; ROC, receiver operating characteristic; and STEMI, ST‐segment–elevation myocardial infarction.
Model Creation
Using our retrospective cohort, we constructed 3 specific models for predicting CA‐AKI: (1) full, (2) pragmatic full, and (3) pragmatic minimum models (Table 2). The full model included all predictors used in a widely cited NCDR‐based CA‐AKI risk prediction model. 17 The pragmatic full model included only those predictors from the full model that could be theoretically extracted from an electronic health record (EHR), although some predictors may be inconsistently coded and require further interpretation (eg, history of heart failure or history of diabetes mellitus). The pragmatic minimum model included only those predictors from the full model that could be derived from an EHR's discrete data fields without further interpretation (eg, age or sex). Using these 3 sets of predictors, we created 3 CA‐AKI prediction models using logistic regression. We ensured that there were no significant predictor interactions or multicollinearity. We tested for linearity of the relationships between predictors and the logit of CA‐AKI by comparing the root mean square errors and R2 values of linear models versus polynomial, logarithmic, and general additive models. This resulted in the use of a cubic model for CrCl and a logarithmic model for preprocedure hemoglobin. While there was a slight improvement in the relationship between the logit of CA‐AKI and contrast volume using a general additive model as has been recently described, the overall effect on the multivariate model prediction was minimal given the more significant influence of other predictors. 23 A general additive model was furthermore not used since it would make solving for a contrast limit prohibitively complex for use in clinical practice.
Table 2.
Predictors Used for Each CA‐AKI Prediction Model
| Full Model | Pragmatic Full Model | Pragmatic Minimum Model |
|---|---|---|
| Contrast volume | Contrast volume | Contrast volume |
| Age | Age | Age |
| Sex | Sex | Sex |
| BMI | BMI | BMI |
| IABP before procedure | IABP before procedure | IABP before procedure |
| CrCl | CrCl | CrCl |
| Preprocedure hemoglobin | Preprocedure hemoglobin | Preprocedure hemoglobin |
| History of diabetes mellitus | History of diabetes mellitus | |
| History of hypertension | History of hypertension | |
| History of HF | History of HF | |
| Preprocedure cardiogenic shock | Preprocedure cardiogenic shock | |
| HF symptoms in past 2 weeks | ||
| History of MI | ||
| History of PCI | ||
| History of CABG | ||
| History of CVD | ||
| History of PAD | ||
| History of chronic lung disease | ||
| CAD presentation (UA, NSTEMI, STEMI) | ||
| Cardiac arrest in past 24 hours | ||
We constructed 3 specific models for predicting CA‐AKI: full, pragmatic full, and pragmatic minimum models. The full model included all predictors used in a widely cited National Cardiovascular Data Registry–based CA‐AKI risk prediction model. 17 The pragmatic full model included only those predictors from the full model that would be easily extracted from an EHR, but would potentially require further interpretation of the EHR data. The pragmatic minimum model included only those predictors from the full model that would be guaranteed to be derivable from an EHR without significant interpretation of the EHR data. Contrast volume was additionally included in all 3 models, as it was the variable of interest. BMI indicates body mass index; CA‐AKI, contrast‐associated acute kidney injury; CABG, coronary artery bypass grafting, CVD, cerebrovascular disease; CrCl, creatinine clearance; CAD, coronary artery disease; EHR, electronic health record; HF, heart failure; IABP, intra‐aortic balloon pump; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention, PAD, peripheral artery disease; STEMI, ST‐segment–elevation myocardial infarction; and UA, unstable angina.
We calculated safe contrast limit equations by starting with our logistic models for CA‐AKI risk and then solving for contrast volume as a function of tolerated CA‐AKI risk and patient characteristics (see Data S1).
Model Validation
Using the safe contrast limit equations with coefficients developed from the retrospective cohort, we predicted CA‐AKI risk in a prospective cohort that had not been used in our model creation. We compared the prediction performance of our safe contrast limit models to one another as well as to previously published models (5*Weight/Creatinine and 2*CrCl) using receiver operating characteristic curves. These receiver operating characteristic curves were constructed by continuously varying the classification cutoffs for each model. For example, for the Weight*Creatinine model, we plotted the sensitivity and 1‐specificity for 1*Weight/Creatinine, 2*Weight/Creatinine, 3*Weight/Creatinine, and so on, to create the receiver operating characteristic curve. For the CrCl model, we plotted the sensitivity and 1‐specificity for 1*CrCl, 2*CrCl, 3*CrCl, and so on. We estimated the area under the curve (AUC) using the trapezoidal rule. We assessed model calibration by using the Hosmer–Lemeshow test. We then constructed a table displaying the sensitivity, specificity, and accuracy of the specific classification cutoffs that have been previously published (ie, 5*Weight/Creatinine and 2*CrCl) compared with our new multivariate models when they were set to a classification cutoff of a tolerated acute kidney injury rate of 10%. This cutoff was chosen given the overall cohort CA‐AKI rate of 10.2%. Accuracy was defined as the average of sensitivity and specificity. The table additionally included Hosmer–Lemeshow calibration P values. All analyses werreas performed with R software (version 3.4.1; R Foundation for Statistical Computing, Vienna, Austria). We used the mgcv package for general additive models and the ResourceSelection package for the Hosmer–Lemeshow test for calibration assessment.
Defining Acute Kidney Injury Risk Groups
We defined 3 CA‐AKI risk groups based on realistic expectations for contrast usage. In our cohort, the lowest recorded contrast volumes were near 20 mL, which is consistent with volumes achieved by ultra‐low PCI methods reported in the literature. 24 At the high end of contrast volumes, we found few cases exceeding 500 mL, with 98.9% of patients in our cohort having received a contrast volume <500 mL. Therefore, we felt that it would be reasonable for operators to stay under the calculated contrast limit whenever it was calculated to be between 20 and 500 mL. We thus defined the modifiable‐risk group as anyone with a calculated contrast limit between 20 and 500 mL, the low‐risk group as any patient with a calculated safe contrast limit >500 mL, and the high‐risk group as anyone with a calculated contrast limit <20 mL. Using the prospective cohort, we plotted the risk of CA‐AKI within each risk group against the contrast volume received.
Results
Study Population
Our retrospective cohort included 27 646 PCI procedures. We excluded 7067 procedures because they were nonindex procedures (n=848), same‐day discharge (n=2647), the patient's being already dialysis dependent (n=1486), or missing pre‐ (n=567) or postprocedure creatinine values (n=4242). This resulted in a final study sample of 20 579 procedures. Our prospective cohort included 7736 PCI procedures. Using the same exclusion criteria as for the retrospective sample resulted in a final prospective sample of 5423 procedures.
Acute kidney injury occurred in 2102 (10.2%) PCI procedures. The average procedural contrast usage was 203.2 mL (SD, 95.0 mL). Significant risk factors are summarized in Table 3. Patients who developed CA‐AKI tended to be older women with worse baseline kidney function and lower hemoglobin levels. Other substantial risk factors included having a history of diabetes mellitus, heart failure, peripheral artery disease, or cerebrovascular disease. Patients were also more likely to develop CA‐AKI if they presented with an ST‐segment–elevation myocardial infarction or non–ST‐segment–elevation myocardial infarction, recent symptomatic heart failure, cardiac arrest, cardiogenic shock, or required intra‐aortic balloon pump usage.
Table 3.
Baseline Characteristics of Patients Undergoing PCI Procedures
| Patient Characteristics | Retrospective Cohort | Prospective Cohort | |||
|---|---|---|---|---|---|
|
Total (n=20 579) |
CA‐AKI (n=2102) |
No CA‐AKI (n=18 477) | P Value* | Total (n=5423) | |
| Age, y | 67.37±12.31 | 70.69±12.61 | 67.00±12.22 | <0.001 | 68.00±12.11 |
| Sex | <0.001 | ||||
| Male | 14 717 (71.5) | 1377 (65.5) | 13 340 (72.2) | 3867 (71.3) | |
| Female | 5862 (28.5) | 724 (34.5) | 5137 (27.8) | 1556 (28.7) | |
| BMI | 28.74±5.94 | 28.54±6.22 | 28.77±5.91 | 0.096 | 28.97±6.02 |
| IABP before procedure | 73 (0.4) | 40 (1.9) | 33 (0.2) | <0.001 | 14 (0.3) |
| CrCl | 78.34±29.01 | 66.42±36.10 | 79.70±27.77 | <0.001 | 75.51±25.76 |
| Mild CKD (45‐60) | 3185 (15.5) | 394 (18.7) | 2791 (15.1) | 884 (16.3) | |
| Moderate CKD (30‐45) | 1547 (7.5) | 327 (15.6) | 1220 (6.6) | 418 (7.7) | |
| Severe CKD (<30) | 648 (3.1) | 294 (14.0) | 354 (1.9) | 169 (3.1) | |
| Preprocedure hemoglobin | 13.30±2.00 | 12.21±2.30 | 13.42±1.93 | <0.001 | 13.30±2.06 |
| History of diabetes mellitus | 7800 (37.9) | 1026 (48.8) | 6774 (36.7) | <0.001 | 2086 (38.5) |
| History of HTN | 16 623 (80.8) | 1782 (84.8) | 14 841 (80.3) | <0.001 | 4371 (80.6) |
| History of HF | 3414 (16.6) | 608 (28.9) | 2806 (15.2) | <0.001 | 956 (17.6) |
| Preprocedure cardio shock | 668 (3.2) | 302 (14.4) | 366 (2.0) | <0.001 | 162 (3.0) |
| Prior 2‐week NYHA | <0.001 | ||||
| Class I | 17 306 (84.1) | 790 (62.4) | 15 994 (86.6) | 4327 (79.8) | |
| Class II | 809 (3.9) | 118 (5.6) | 691 (3.7) | 360 (6.6) | |
| Class III | 1264 (6.1) | 280 (13.3) | 984 (5.3) | 442 (8.2) | |
| Class IV | 1200 (5.8) | 392 (18.6) | 808 (4.4) | 294 (5.4) | |
| History of MI | 5429 (26.4) | 623 (29.6) | 4806 (26.0) | <0.001 | 1462 (27.0) |
| History of PCI | 7275 (35.4) | 678 (32.3) | 6597 (35.7) | 0.002 | 1933 (35.6) |
| History of CABG | 2897 (14.1) | 324 (15.4) | 2573 (13.9) | 0.068 | 802 (14.8) |
| History of CVD | 2603 (12.6) | 402 (19.1) | 2201 (11.9) | <0.001 | 793 (14.6) |
| History of PAD | 2173 (10.6) | 330 (15.7) | 1843 (10.0) | <0.001 | 538 (9.9) |
| History of chronic lung disease | 2347 (11.4) | 324 (15.4) | 2023 (10.9) | <0.001 | 730 (13.5) |
| NSTEMI or UA | 13 066 (63.5) | 1274 (60.6) | 11 792 (63.8) | 0.004 | 2807 (51.8) |
| STEMI | 4144 (20.1) | 600 (28.5) | 3544 (19.2) | <0.001 | 1073 (19.8) |
| Cardiac arrest past 24 h | 598 (2.9) | 206 (9.8) | 392 (2.1) | <0.001 | 188 (3.5) |
| Contrast (mL) | 203.21±94.97 | 209.58±107.05 | 202.48±93.47 | 0.001 | 189.25±92.83 |
| CA‐AKI | 2102 (10.2) | 2102 (100) | 18 477 (0) | 488 (9.0) | |
Proportions and mean±SD are shown. Continuous and categorical variables compared using t‐test and chi‐squared tests, respectively. BMI indicates body mass index; CA‐AKI, contrast‐associated acute kidney injury; CABG, coronary artery bypass grafting; CKD, chronic kidney disease; CrCl, creatinine clearance; CVD, cerebrovascular disease; HF, heart failure; HTN, hypertension; IABP, intra‐aortic balloon pump; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; NYHA, New York Heart Association Functional Classification; PAD, peripheral artery disease; PCI, percutaneous coronary intervention; STEMI, ST‐segment–elevation myocardial infarction; and UA, unstable angina.
P value for CA‐AKI versus No CA‐AKI groups.
Prior Models for Contrast Volume Limits
Our review of the literature showed that proposed safe contrast limit equations were either based on a factor of Weight/Creatinine or CrCl, with the most widely cited contrast limits being 5*Weight/Creatinine and 2*CrCl (Table 1). We analyzed the rates of CA‐AKI among different high‐risk patient groups who had received contrast volumes <2*CrCl or 5*Weight/Creatinine (Figure 2). There were large differences in CA‐AKI risk among these high‐risk groups. Among patients receiving a contrast volume <2*CrCl, the overall CA‐AKI rate was 7.4% and was as high as 32.8% among patients with preprocedural cardiogenic shock and 37.5% among patients using an intra‐aortic balloon pump. Those with prior heart failure had nearly double the risk of CA‐AKI, and patients with a hemoglobin <10 g/dL had nearly triple the risk of CA‐AKI compared with the overall group. Similar patterns were seen among groups for patients receiving contrast volumes <5*Weight/Creatinine.
Figure 2. Rates of contrast‐induced nephropathy for different patient risk factors.
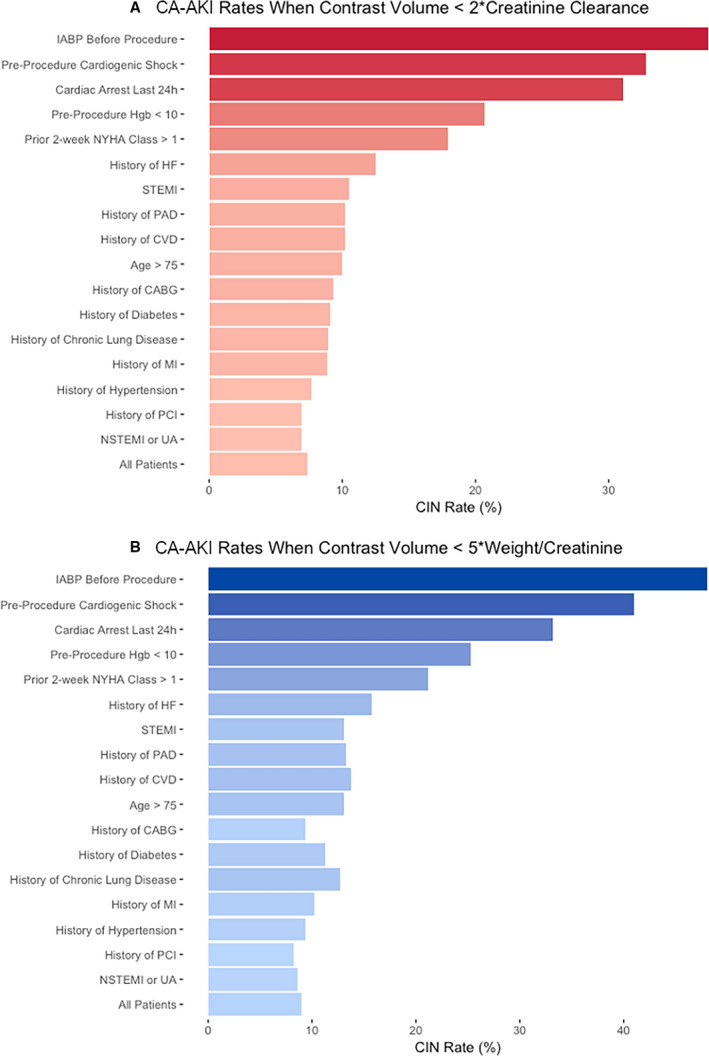
A, When contrast volume use < 2*CreatinineClearance. B, When contrast volume use < 5*Weight/Creatinine. CA‐AKI indicates contrast‐associated acute kidney injury; CABG, coronary artery bypass grafting; CVD, cerebrovascular disease; HF, heart failure; IABP, intra‐aortic balloon pump; MI, myocardial infarction; NSTEMI, non–ST‐segment–elevation myocardial infarction; PAD, peripheral artery disease; STEMI, ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; and UA, unstable angina.
New Models for Contrast Volume Limits
Using the retrospective cohort, we created multivariate logistic regression models for predicting CA‐AKI. From these models, we solved for safe contrast volume limits as a function of tolerated CA‐AKI risk and patient risk factors (Figure 3). Using the prospective validation data set, we then compared the performance of our new multivariate safe contrast limit models to prior models that used Weight/Creatinine or CrCl (Figure 4). The model using Weight/Creatinine yielded an AUC of 0.59, while the model using CrCl produced an AUC of 0.61. Our full, pragmatic full, and pragmatic minimum multivariate models had improved performance with AUCs of 0.80, 0.76, 0.72, respectively. The average calculated safe contrast limit for a patient in the validation cohort was 427.7 mL using the 5*Weight/Creatinine rule, 151.0 mL for the 2*CrCl rule, 367.6 mL for the full model, 327.0 mL for the pragmatic full model, and 299.3 for the pragmatic minimum model (Table 4). The 5*Weight/Creatinine contrast limit had high specificity for predicting CA‐AKI but low sensitivity; 2*CrCl had high sensitivity but low specificity. This translated to an overall accuracy of 53.7% for the 5*Weight/Creatinine rule and an accuracy of 56.5% for the 2*CrCl rule. In contrast, our multivariate safe contrast limits had more balanced sensitivities and specificities, with overall accuracies of 73.1% for the full model, 69.3% for the pragmatic full model, and 66.6% for the pragmatic minimum model. The multivariate safe contrast limits had appropriate calibration, whereas the 5*Weight/Creatinine and 2*CrCl models had poor calibration.
Figure 3. Equations for safe contrast limits as a function of tolerated CA‐AKI risk and patient risk factors.
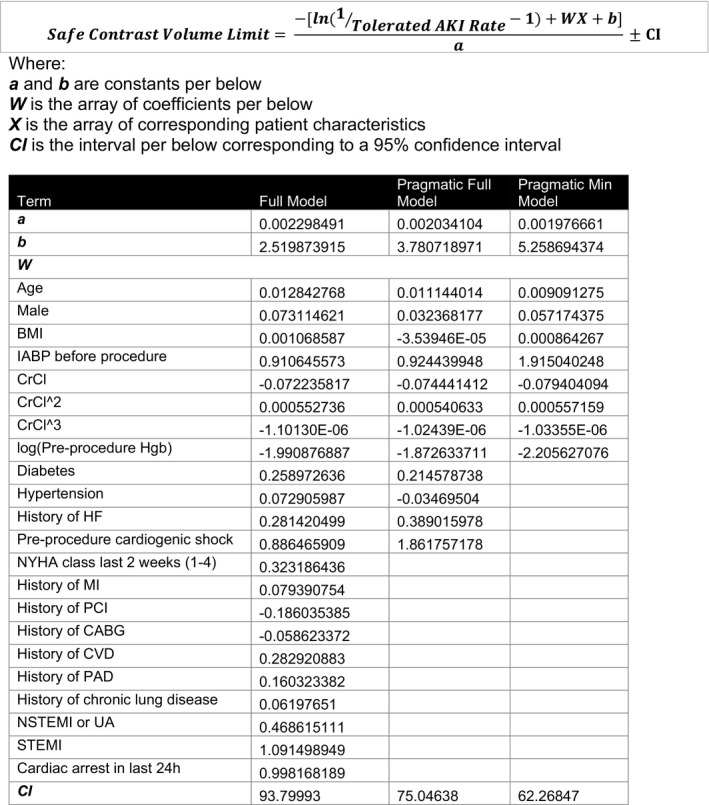
AKI indicates acute kidney injury; BMI, body mass index; CABG, coronary artery bypass grafting; CVD, cerebrovascular disease; CrCl, creatinine clearance; HF, heart failure; IABP, intra‐aortic balloon pump; MI, myocardial infarction; NYHA, New York Heart Association; NSTEMI, non–ST‐segment–elevation myocardial infarction; PCI, percutaneous coronary intervention; PAD, peripheral artery disease; STEMI, ST‐segment–elevation myocardial infarction; and UA, unstable angina.
Figure 4. Receiver operating characteristic (ROC) curves for prediction of CA‐AKI by safe contrast limit models.
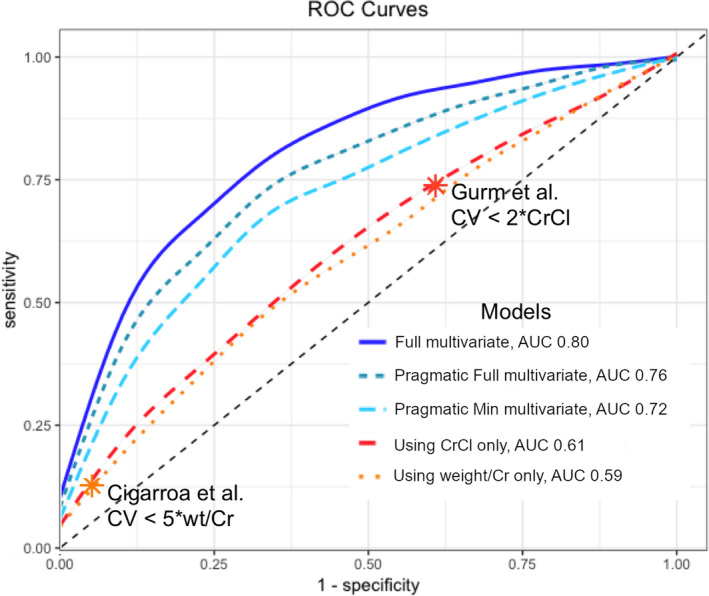
The multivariate full, pragmatic full, and pragmatic minimum models performed better than currently established models using CrCl or weight/creatinine only. *Asterisks denote specific thresholds that have been previously published: 5*Weight/Creatinine (Cigarroa et al), 2*CrCl (Gurm et al). 21 , 22 AUC indicates area under the receiver operating characteristic curves; CV, contrast volume; and CrCl, creatinine clearance.
Table 4.
Performance Characteristics of Prior Safe Contrast Limit Equations 5*Weight/Creatinine and 2*CrCl and New Multivariate Models
| Safe Contrast Limit Equation | |||||
|---|---|---|---|---|---|
|
5*Weight/Creatinine (95% CI) |
2*CrCl | Full Model | Pragmatic Full Model | Pragmatic Minimum Model | |
| Average Calculated Contrast Limit | 427.7 mL | 151.0 mL | 367.6 mL | 327.0 mL | 299.3 mL |
| Sensitivity, % |
12.7 (10.0–16.0) |
73.8 (69.6–77.6) |
70.3 (66.0–74.3) |
63.1 (58.7–67.4) |
59.6 (55.1–64.0) |
| Specificity, % |
94.8 (94.1–95.4) |
39.1 (37.8–40.5) |
75.9 (74.7–77.1) |
75.6 (74.3–76.8) |
73.7 (72.4–74.5) |
| Accuracy, % | 53.7 | 56.5 | 73.1 | 69.3 | 66.6 |
|
Calibration HL P value |
<0.05 | <0.05 | 0.14 | 0.11 | 0.10 |
Full, pragmatic full, and pragmatic minimum model safe contrast equations are presented in Figure 3. For these equations, tolerated AKI rate was set to 10%. CrCl indicates creatinine clearance; HL, Hosmer–Lemeshow test (P>0.1 is appropriately calibrated; P<0.05 is miscalibrated).
Finally, we defined 3 different groups (high‐risk, modifiable‐risk, low‐risk) based on the calculated safe contrast volume limit (Figure 5). We found that for all of our multivariate models, approximately 10% of patients had a safe contrast volume limit <20 mL and could be considered high risk. One‐quarter had a safe contrast limit between 20 and 500 mL and could therefore be considered as having a modifiable risk. Nearly two‐thirds of patients had a safe contrast limit >500 mL and were thus low risk. The overall rate of kidney injury in the high‐ versus low‐risk groups was 26.7% to 34.4% versus 2.1% to 4.7% depending on the model.
Figure 5. Kidney injury risk categories based on the calculated safe contrast volume limit.
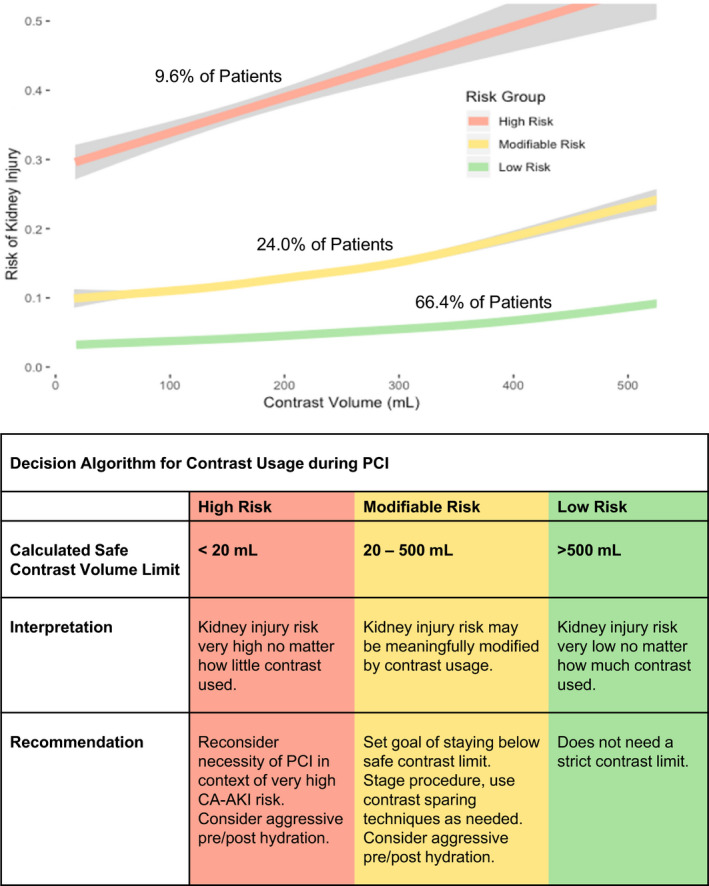
One‐tenth of patients are at high risk of kidney injury no matter how little contrast is used. One‐quarter have a modifiable risk and should receive a contrast volume less than the calculated contrast limit. About two‐thirds of patients are at low risk and can likely receive most volumes of contrast without developing kidney injury. Note: The pragmatic full model was used for defining contrast limits. Results were similar if using full or pragmatic minimum models. CA‐AKI indicates contrast‐associated acute kidney injury; and PCI, percutaneous coronary intervention.
Discussion
CA‐AKI is one of the most frequently encountered and potentially preventable complications of PCI. Using data from more than 20 000 PCI procedures, we created new pragmatic multivariate models for calculating safe contrast limits with significantly improved performance compared with prior published models. These models use information widely accessible from EHRs and could therefore be easily automated into EHR systems to be used at the point of care for guiding clinical decision making. We observed that approximately one‐quarter of patients could meaningfully reduce their CA‐AKI risk with contrast volume‐limiting efforts. In this group, an accurate, readily available, contrast volume limit provides practical information that can be used to directly improve periprocedural safety as well as facilitate more informed provider–patient discussions regarding procedural risks.
Prior studies made great strides in helping better delineate safe contrast volumes by using patient characteristics such as creatinine clearance and weight to personalize these limits. 19 , 20 , 21 , 22 , 30 Existing guidelines continue to use the formula 5*Weight/Creatinine as the standard safe contrast volume limit. 13 , 31 A more recent study proposed using 2*CrCl as a safer cutoff. 22 We found that for both of these contrast limits, CA‐AKI risk varied widely based on additional patient risk factors not accounted for in these simplified equations. Among patients who received contrast volumes <2*CrCl, factors such as a history of heart failure or a hemoglobin <10 mg/dL doubled and tripled the risk of CA‐AKI, respectively.
Our newly developed multivariate safe contrast models use only information available in the EHR, enhancing their practicality and usability. When applied to prospective data, these safe contrast limits performed substantially better than when using weight/creatinine or CrCl alone. We observed that the 5*Weight/Creatinine rule grossly overestimated true safe contrast levels with an average calculated safe contrast limit of 427.7 mL and a sensitivity of only 12.7%. This meant that 87.3% of patients who developed CA‐AKI received contrast volumes less than the safe 5*Weight/Creatinine limit. Using 5*Weight/Creatinine as the contrast limit may therefore offer false reassurance to providers regarding the safety of their contrast use and, in turn, lead to higher rates of CA‐AKI. In contrast, the 2*CrCl rule underestimated true safe contrast levels, with an average calculated safe contrast limit of 151.0 mL and a specificity of only 39.1%. This meant that 60.9% of patients who did not develop CA‐AKI received contrast volumes >2*CrCl limit. Using the 2*CrCl rule may therefore have the opposite effect of the 5*Weight/Creatinine rule and potentially overlimit providers and even discourage them from performing PCIs when such procedures could be done safely.
Our multivariate safe contrast models had substantially better accuracy for predicting CA‐AKI and struck a balance between both preventing excessive contrast use and CA‐AKI while also allowing for sufficient contrast usage to perform effective PCI. As might be expected, there was a trade‐off between the number of predictors included and model performance. Selection of a given model in clinical practice will be predicated on the availability of predictor data in an institution's EHR.
To further refine the role of the safe contrast volume limit in decision making, we used the calculated contrast limit to stratify patients into high‐, low‐, or modifiable‐risk groups. In the low‐risk group, which contained two‐thirds of all patients, the risk of kidney injury was low enough, regardless of the volume of contrast use, that providers could be assured that contrast usage would not significantly increase kidney injury risk. In the high‐risk group, the risk of kidney injury was high enough, regardless of the volume of contrast use, that limiting contrast would be unlikely to bring the kidney injury risk down to acceptable levels. These findings are consistent with the recognized hypothesis that in some patients, contrast use may be associated with, but not causative of, kidney injury following PCI. 32 For these patients, efforts to limit contrast usage may not meaningfully affect the risk of kidney injury and operators should focus more on whether PCI should be performed at all rather than how much contrast is used.
In contrast, approximately a quarter of patients fell into the modifiable‐risk category, where efforts to limit contrast volume may meaningfully reduce kidney injury risk. For this population, safe contrast limits can improve care quality across the PCI care continuum by helping with pre‐, post‐, and intraprocedural planning. Establishing a safe contrast limit before a procedure has been shown to decrease the rate of post‐PCI renal injury when incorporated in a pre‐PCI “time‐out.” 33 Such a time‐out could help operators decide ahead of time whether they should use contrast‐sparing PCI methods if the safe contrast limit is particularly low. 15 , 24 The safe contrast limit could also help guide management during procedures. An operator, for example, could decide during a PCI that the contrast use is approaching the safe limit and delay additional interventions. It may also similarly give confidence to an operator that he or she may continue a procedure and give additional contrast if the safe contrast limit has not been reached. Finally, in the postprocedure setting, there may be a role for guiding how aggressively to administer intravenous fluids or other such CA‐AKI prevention methods depending on the contrast usage relative to the safe contrast limit. We present a schematic (Figure 6) for how our new safe contrast limits might be implemented in a clinical setting. In this implementation model, the EHR automatically extracts patient risk factor data, calculates the contrast limit, and displays the contrast limit at the point of care.
Figure 6. Proposed implementation of EHR‐based safe contrast limit calculator.
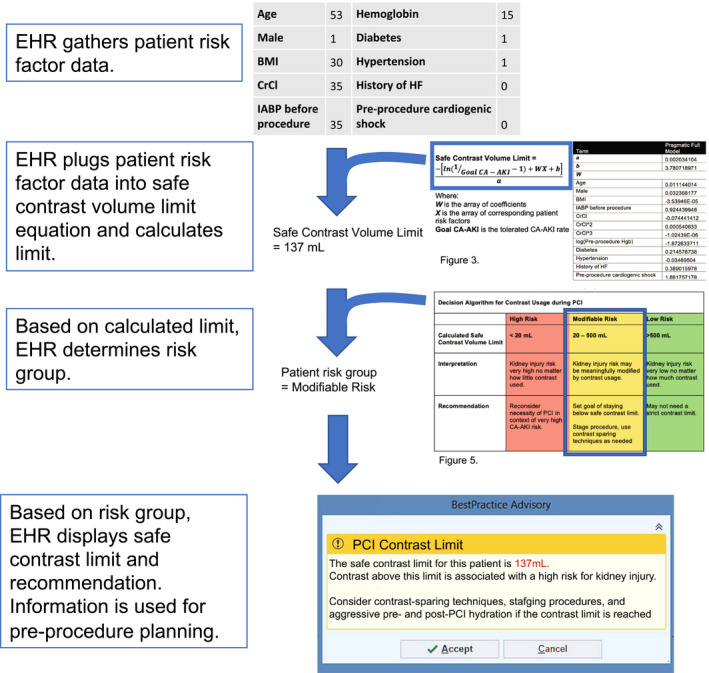
BMI indicates body mass index; CA‐AKI, contrast‐associated acute kidney injury; CrCl, creatinine clearance; EHR, electronic health records; and IABP, intra‐aortic balloon pump.
Several limitations to this study merit consideration. As our models were derived from a specific group of hospitals contributing to the Biome Analytics data set, it is possible that the patients are not representative of the overall US patient population. The data set, however, includes information from 18 hospitals with representation from academic, nonacademic, private, and public institutions. Furthermore, the sample size was large, and our derived model discovered predictors that are similar to those from other widely accepted CA‐AKI risk models and also performed similarly. 11 , 17 In determining whether patients had developed CA‐AKI, we could not include patients who did not have a postprocedure creatinine or were discharged the day of procedure, which could potentially exclude low‐risk patients from our sample. Indeed, our sample's CA‐AKI rate was somewhat higher (10.2%) than in other papers. 21 , 22 As such, our model may be more generalizable to higher‐risk patients with multiday hospital stays, who arguably may benefit most from safe contrast volume limits. 34 Finally, there are other factors that likely affect safe contrast limits that could not be included in our model, such as the use of intravenous fluids before and after PCI or the presence of concomitant medications with renal toxicities. While these were not captured in the data, future studies using EHR data may help measure the effects of these factors.
In conclusion, using data from a multicenter registry, we developed pragmatic models for safe contrast limits for preventing CA‐AKI in patients undergoing PCI. These models were substantially more accurate than prior ones and could be easily implemented in EHR systems and used at the point of care for guiding decisions. We propose a decision algorithm that uses the safe contrast limit to stratify patients into low‐, high‐, or modifiable‐risk groups. In patients in the modifiable‐risk group (about one quarter of patients), CA‐AKI risk is meaningfully affected by efforts to limit contrast usage. By helping providers decide which patients benefit from contrast reduction and how much contrast use to target, the safe contrast limit provides actionable information for pre‐, post‐, and intraprocedural planning to improve the quality of PCI care.
Sources of Funding
None.
Disclosures
K.L. works for Biome Analytics, a company contracted by Cedars‐Sinai Medical Center to conduct data analytics work using methods that are unrelated to the clinical content area of this manuscript and, thus, involving no direct or indirect financial or other interests. The remaining authors have no disclosures to report.
Supporting information
Data S1
(J Am Heart Assoc. 2021;10:e018890. DOI: 10.1161/JAHA.120.018890.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.018890
For Sources of Funding and Disclosures, see page 13.
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S., Das SR et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics‐2019 Update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. Masoudi FA, Ponirakis A, de Lemos JA, Jollis JG, Kremers M, Messenger JC, Moore JWM, Moussa I, Oetgen WJ, Varosy PD, et al. Report from 4 ACC national cardiovascular data registries. J Am Coll Cardiol. 2016;2017:1424–1426. [DOI] [PubMed] [Google Scholar]
- 3. McCullough PA, Wolyn R, Rocher LL, Levin RN, O’Neill WW. Acute renal failure after coronary intervention: incidence, risk factors, and relationship to mortality. Am J Med. 1997;103:368–375. [DOI] [PubMed] [Google Scholar]
- 4. Rihal CS, Textor SC, Grill DE, Berger PB, Ting HH, Best PJ, Singh M, Bell MR, Barsness GW, Mathew V, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. [DOI] [PubMed] [Google Scholar]
- 5. Finn WF. The clinical and renal consequences of contrast‐induced nephropathy. Nephrol Dial Transplant. 2006;21:i2–10. [DOI] [PubMed] [Google Scholar]
- 6. Giacoppo D, Madhavan MV, Baber U, Warren J, Bansilal S, Witzenbichler B, Dangas GD, Kirtane AJ, Xu KE, Kornowski R, et al. Impact of contrast‐induced acute kidney injury after percutaneous coronary intervention on short‐ and long‐term outcomes: Pooled analysis from the HORIZONS‐AMI and ACUITY trials. Circ Cardiovasc Interv. 2015;8:e002475. DOI: 10.1161/CIRCINTERVENTIONS.114.002475. [DOI] [PubMed] [Google Scholar]
- 7. Gupta R, Gurm HS, Bhatt DL, Chew DP, Ellis SG. Renal failure after percutaneous coronary intervention is associated with high mortality. Catheter Cardiovasc Interv. 2005;64:442–448. [DOI] [PubMed] [Google Scholar]
- 8. Weisbord SD, Chen H, Stone RA, Kip KE, Fine MJ, Saul MI, Palevsky PM. Associations of increases in serum creatinine with mortality and length of hospital stay after coronary angiography. J Am Soc Nephrol. 2006;17:2871–2877. [DOI] [PubMed] [Google Scholar]
- 9. Bartholomew BA, Harjai KJ, Dukkipati S, Boura JA, Yerkey MW, Glazier S, Grines CL, O’Neill WW. Impact of nephropathy after percutaneous coronary intervention and a method for risk stratification. Am J Cardiol. 2004;93:1515–1519. [DOI] [PubMed] [Google Scholar]
- 10. Subramanian S, Tumlin J, Bapat B, Zyczynski T. Economic burden of contrast‐induced nephropathy: implications for prevention strategies. J Med Econ. 2007;10:119–134. [DOI] [PubMed] [Google Scholar]
- 11. Silver SA, Shah PM, Chertow GM, Harel S, Wald R, Harel Z. Risk prediction models for contrast induced nephropathy: systematic review. BMJ. 2015;351:h4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown JR, McCullough PA, Splaine ME, Davies L, Ross CS, Dauerman HL, Robb JF, Boss R, Goldberg DJ, Fedele FA, et al. How do centers begin the process to prevent CI‐AKI: a report from a new regional collaborative. BMJ Qual Saf. 2012;21:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown JR, Solomon RJ, Sarnak MJ, McCullough PA, Splaine ME, Davies L, Ross CS, Dauerman HL, Stender JL, Conley SM, et al. Reducing contrast‐induced acute kidney injury using a regional multicenter quality improvement intervention. Circ Cardiovasc Qual Outcomes. 2014;7:693–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Keaney JJ, Hannon CM, Murray PT. Contrast‐induced acute kidney injury: how much contrast is safe? Nephrol Dial Transplant. 2013;28:1376–1383. [DOI] [PubMed] [Google Scholar]
- 15. Almendarez M, Gurm HS, Mariani J, Montorfano M, Brilakis ES, Mehran R, Azzalini L. Procedural strategies to reduce the incidence of contrast‐induced acute kidney injury during percutaneous coronary intervention. JACC Cardiovasc Interv. 2019;12:1877–1888. [DOI] [PubMed] [Google Scholar]
- 16. Moussa I, Hermann A, Messenger JC, Dehmer GJ, Weaver WD, Rumsfeld JS, Masoudi FA. The NCDR CathPCI Registry: a US national perspective on care and outcomes for percutaneous coronary intervention. Heart. 2013;99:297–303. [DOI] [PubMed] [Google Scholar]
- 17. Tsai TT, Patel UD, Chang TI, Kennedy KF, Masoudi FA, Matheny ME, Kosiborod M, Amin AP, Weintraub WS, Curtis JP, et al. Validated contemporary risk model of acute kidney injury in patients undergoing percutaneous coronary interventions: insights from the National Cardiovascular Data Registry Cath‐PCI Registry. J Am Heart Assoc. 2014;3:e001380. DOI: 10.1161/JAHA.114.001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, Levin A. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Critical Care. 2007;11:R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nyman U, Björk J, Aspelin P, Marenzi G. Contrast medium dose‐to‐GFR ratio: a measure of systemic exposure to predict contrast‐induced nephropathy after percutaneous coronary intervention. Acta Radiol. 2008;49:658–667. [DOI] [PubMed] [Google Scholar]
- 20. Yoon H‐J, Hur S‐H. Determination of safe contrast media dosage to estimated glomerular filtration rate ratios to avoid contrast‐induced nephropathy after elective percutaneous coronary intervention. Korean Circ J. 2011;41:265–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cigarroa RG, Lange RA, Williams RH, Hillis LD. Dosing of contrast material to prevent contrast nephropathy in patients with renal disease. Am J Med. 1989;86:649–652. [DOI] [PubMed] [Google Scholar]
- 22. Gurm HS, Dixon SR, Smith DE, Share D, Lalonde T, Greenbaum A, Moscucci M. BMC2 (Blue Cross Blue Shield of Michigan Cardiovascular Consortium) Registry. Renal function‐based contrast dosing to define safe limits of radiographic contrast media in patients undergoing percutaneous coronary interventions. J Am Coll Cardiol. 2011;58:907–914. [DOI] [PubMed] [Google Scholar]
- 23. Huang C, Li S‐X, Mahajan S, Testani JM, Wilson FP, Mena CI, Masoudi FA, Rumsfeld JS, Spertus JA, Mortazavi BJ, et al. Development and validation of a model for predicting the risk of acute kidney injury associated with contrast volume levels during percutaneous coronary intervention. JAMA Netw Open. 2019;2:e1916021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Azzalini L, Laricchia A, Regazzoli D, Mitomo S, Hachinohe D, Bellini B, Demir OM, Poletti E, Maccagni D, Colombo A. Ultra‐low contrast percutaneous coronary intervention to minimize the risk for contrast‐induced acute kidney injury in patients with severe chronic kidney disease. J Invasive Cardiol. 2019;31:176–182. [DOI] [PubMed] [Google Scholar]
- 25. Marenzi G, Lauri G, Assanelli E, Campodonico J, De Metrio M, Marana I, Grazi M, Veglia F, Bartorelli AL. Contrast‐induced nephropathy in patients undergoing primary angioplasty for acute myocardial infarction. J Am Coll Cardiol. 2004;44:1780–1785. [DOI] [PubMed] [Google Scholar]
- 26. Laskey WK, Jenkins C, Selzer F, Marroquin OC, Wilensky RL, Glaser R, Cohen HA, Holmes DR. NHLBI Dynamic Registry Investigators. Volume‐to‐creatinine clearance ratio: a pharmacokinetically based risk factor for prediction of early creatinine increase after percutaneous coronary intervention. J Am Coll Cardiol. 2007;50:584–590. [DOI] [PubMed] [Google Scholar]
- 27. Mager A, Vaknin Assa H, Lev EI, Bental T, Assali A, Kornowski R. The ratio of contrast volume to glomerular filtration rate predicts outcomes after percutaneous coronary intervention for ST‐segment elevation acute myocardial infarction. Catheter Cardiovasc Interv. 2011;78:198–201. [DOI] [PubMed] [Google Scholar]
- 28. Andò G, de Gregorio C, Morabito G, Trio O, Saporito F, Oreto G. Renal function‐adjusted contrast volume redefines the baseline estimation of contrast‐induced acute kidney injury risk in patients undergoing primary percutaneous coronary intervention. Circ Cardiovasc Interv. 2014;7:465–472. [DOI] [PubMed] [Google Scholar]
- 29. Ogata N, Ikari Y, Nanasato M, Okutsu M, Kametani R, Abe M, Uehara Y, Sumitsuji S. Safety margin of minimized contrast volume during percutaneous coronary intervention in patients with chronic kidney disease. Cardiovasc Interv Ther. 2014;29:209–215. [DOI] [PubMed] [Google Scholar]
- 30. Liu Y, Chen J‐Y, Tan N, Zhou Y‐L, Yu D‐Q, Chen Z‐J, He Y‐T, Liu Y‐H, Luo J‐F, Huang W‐H, et al. Safe limits of contrast vary with hydration volume for prevention of contrast‐induced nephropathy after coronary angiography among patients with a relatively low risk of contrast‐induced nephropathy. Circ Cardiovasc Interv. 2015;8:1–7. DOI: 10.1161/CIRCINTERVENTIONS.114.001859. [DOI] [PubMed] [Google Scholar]
- 31. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–184. [DOI] [PubMed] [Google Scholar]
- 32. Mehran R, Dangas GD, Weisbord SD. Contrast‐associated acute kidney injury. N Engl J Med. 2019;380:2146–2155. [DOI] [PubMed] [Google Scholar]
- 33. Jia KQ, Blais D, Porter K, Boudoulas KD, Lilly S. The effect of establishing pre‐angiography thresholds on contrast utilization. J Interv Cardiol. 2018;31:430–435. [DOI] [PubMed] [Google Scholar]
- 34. National Cardiovascular Data Registry Committee . CathPCI: Acute Kidney Injury (risk‐adjusted) Specifications and Testing Overview. Available at https://www.ncdr.com/WebNCDR/docs/default‐source/ncdr‐general‐documents/cathpci_kidney‐injury‐overview_comment‐period.pdf?sfvrsn=2. Accessed February 12, 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1


