Abstract
Background
Prevalence of cardiovascular disease risk factors and rates of atherosclerotic cardiovascular disease outcomes vary across racial/ethnic groups. This analysis examined the effects of evolocumab on LDL‐C (low‐density lipoprotein cholesterol) levels and LDL‐C goals achievement by race/ethnicity.
Methods and Results
Data from 15 phase 2 and 3 studies of treatment with evolocumab versus placebo or ezetimibe were pooled (n=7669). Results were analyzed by participant clinical characteristics and by self‐identified race/ethnicity. Key outcomes included percent change from baseline in LDL‐C, achievement of LDL‐C <70 mg/dL, and LDL‐C reduction of ≥50% at 12 weeks and at 1 to 5 years. Across 12‐week studies, mean percent change in LDL‐C from baseline in evolocumab‐treated participants was −52% to −59% for White and −46% to −67% for non‐White participants, across clinical characteristics groups. LDL‐C <70 mg/dL was achieved in 43% to 84% and 62% to 94% and LDL‐C reduction of ≥50% in 63% to 78% and 58% to 86%, respectively. In 1‐ to 5‐year studies, mean percent change in LDL‐C was −46% to −52% for White and −49% to −55% for non‐White participants. LDL‐C <70 mg/dL was achieved in 53% to 84% and 66% to 77%, and LDL‐C reduction of ≥50% in 53% to 67% and 58% to 68%, respectively. The treatment effect on mean percent change in LDL‐C differed only in participants with type 2 diabetes mellitus, with a larger reduction in Asian participants. The qualitative interaction P values were nonsignificant, indicating consistent directionality of effect.
Conclusions
Similar reduction in LDL‐C levels with evolocumab was observed across racial/ethnic groups in 12‐week and 1‐ to 5‐year studies. Among those with diabetes mellitus, Asian participants had greater LDL‐C reduction.
Keywords: ethnicity, evolocumab, lipids and lipoproteins, race
Subject Categories: Lipids and Cholesterol
Nonstandard Abbreviations and Acronyms
- PCSK9
proprotein convertase subtilisin/kexin type 9
- Q2W
every 2 weeks (dosing regimen)
- QM
once monthly (dosing regimen)
Clinical Perspective
What Is New?
Lipid‐lowering therapy with evolocumab, a PCSK9 (proprotein convertase subtilisin/kexin type 9) inhibitor, substantially reduces LDL‐C (low‐density lipoprotein cholesterol) in patients with a broad range of clinical characteristics.
In this pooled analysis, evolocumab therapy was associated with similar reductions in LDL‐C across racial/ethnic groups in both 12‐week and 1‐ to 5‐year studies (eg, in 12‐week studies, mean percent change in LDL‐C from baseline in evolocumab‐treated participants was −52% to −59% for White and −46% to −67% for non‐White participants across clinical characteristic groups, and LDL‐C <70 mg/dL was achieved in 43–84% and 62–94%, respectively).
Among those with diabetes mellitus, Asian participants had greater reduction in LDL‐C.
What Are the Clinical Implications?
These findings provide further evidence for the use of evolocumab therapy to lower LDL‐C in individuals of diverse racial/ethnic backgrounds, including those with diabetes mellitus.
Prevalence of major cardiovascular disease (CVD) risk factors and rates of CVD outcomes vary markedly by race and ethnicity. 1 , 2 Non‐Hispanic Black participants experience higher age‐adjusted rates of stroke and mortality because of CVD, and higher rates of major CVD comorbid conditions compared with non‐Hispanic White participants. 1 , 3 Rates of elevated total cholesterol and LDL‐C (low‐density lipoprotein cholesterol) levels vary by race/ethnicity (ie, rates are higher in Hispanic men compared with non‐Hispanic White men), while prevalence of elevated LDL‐C level in non‐Hispanic Black men is similar to that among non‐Hispanic White men. 1 Non‐Hispanic White women have the highest rates of elevated total cholesterol and LDL‐C levels. 1 The burden of low HDL‐C (high‐density lipoprotein cholesterol)/high triglyceride dyslipidemia appears greater in minority populations including Hispanic/Latino participants. 4 In addition, Hispanic and non‐Hispanic Black individuals are less likely to receive lipid‐lowering therapy or reach lipid treatment goals than non‐Hispanic White participants. 5 , 6 , 7
Lipid‐lowering therapy with evolocumab, a monoclonal antibody to PCSK9 (proprotein convertase subtilisin/kexin type 9), substantially and consistently reduces LDL‐C in patients with a broad range of clinical characteristics. 8 A pooled study of four 12‐week phase 3 studies reported that the LDL‐C‐lowering effect of evolocumab was comparable across racial/ethnic groups, 9 with findings similar for both approved dosing regimens of subcutaneous evolocumab (140 mg every 2 weeks [Q2W] or 420 mg once monthly [QM]); however, the majority of the pooled sample (>90%) consisted of White participants. The FOURIER (Further Cardiovascular Outcomes Research with PCSK9 Inhibition in Subjects with Elevated Risk) trial found that reductions in major cardiovascular events with evolocumab treatment were consistent in men and women, participants <65 years and ≥65 years, and White participants and non‐White participants. 10 Examination of the heterogeneity of treatment effects across racial/ethnic groups has been impeded by limited inclusion of participants from minority backgrounds in clinical trials. 11 A recent post hoc analysis of pooled data from 3 trials reported that alirocumab, another currently available PCSK9 inhibitor, significantly lowered LDL‐C regardless of racial/ethnic background. 12 The effects of evolocumab on LDL‐C levels and achievement of LDL‐C goals have not been examined by race/ethnicity. This post hoc analysis addresses this gap by conducting a meta‐analysis of individual participant data from 15 phase 2 and phase 3 clinical trials with both short‐term (12 weeks) and long‐term (up to 5 years) follow‐up to examine the effects of evolocumab on reduction in LDL‐C from baseline and attainment of LDL‐C treatment goals by race/ethnicity.
Methods
Data on participants from 15 phase 2 and phase 3 evolocumab trials were included in this analysis. 13 , 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 All evolocumab studies with a clinical study report published at the time of the analysis were included except those studies that evaluated the at‐home use of evolocumab comparing 2 different devices (THOMAS 1 and 2, Trial for Home‐use of Prefilled Auto‐Injector Pen and 3.5 ml Personal Injector in AMG 145 Administrations), studies with patients on lipoprotein apheresis (De LAVAL, Administration of Evolocumab to Reduce Lipoprotein Apheresis in Patients Who Reach LDL‐C Values Acceptably Low with Evolocumab Alone), studies involving coronary imaging (GLAGOV, Global Assessment of Plaque Regression with a PCSK9 Antibody as Measured by Intravascular Ultrasound), and those that evaluated evolocumab in patients with homozygous familial hypercholesterolemia (TESLA A and B, Trial Evaluating PCSK9 Antibody in Subjects with LDL Receptor Abnormalities). This analysis included 12 randomized, double‐blind, phase 2/3 trials of 12 weeks in duration, the DESCARTES (Durable Effect of PCSK9 Antibody Compared with Placebo Study) (NCT01516879) study, which was a randomized double‐blind trial of 52 weeks, and the open‐label randomized extension studies OSLER (Open‐Label Study of Long‐Term Evaluation Against LDL Cholesterol) (NCT01439880) and OSLER‐2 (NCT01854918), which were 5 and 3 years in duration, respectively (Table 1). A meta‐analysis of individual participant data was performed by pooling data from individual participants based on study length and clinical characteristics of the study population.
Table 1.
| Studies | n* |
n (Evolocumab); n (Comparator) |
Study Design/Phase | Trial Population | Background Lipid‐Lowering Therapy | Comparator(s) |
|---|---|---|---|---|---|---|
| Short‐term (12‐wk) studies | ||||||
| Hypercholesterolemia/mixed dyslipidemia | ||||||
|
LAPLACE‐TIMI 57 |
313 | 158; 155 | Randomized, double‐blind/phase 2 | Ages 18–80 y (51% women, 89% White) with hypercholesterolemia and fasting LDL ≥85 mg/dL while on a stable dose of statins (with or without ezetimibe) for at least 4 wk | Statin±ezetimibe | Placebo |
|
LAPLACE‐2 |
1896 | 1117; 558 (placebo); 221 (ezetimibe) | Randomized, double‐blind/phase 3 | Ages 18–80 y (45.6% women, 91% White) with screening LDL ≥150 mg/dL and no statin use, LDL ≥100 mg/dL with nonintensive statin use, or LDL ≥80 mg/dL with intensive statin use | Statin therapy | Placebo or ezetimibe (in those treated with atorvastatin only) |
|
MENDEL |
180 | 90; 90 | Randomized, double‐blind/phase 2 | Ages 18–75 y (66% women; 79% White, 16% Black, 6% other) with fasting LDL ≥100 mg/dL but <190 mg/dL, TG ≤400 mg/dL, and 10‐y Framingham risk score for CHD up to 10% | None, and no use of lipid‐lowering medication within 3 mo of enrollment | Placebo and ezetimibe |
|
MENDEL‐2 |
614 | 306; 154 (placebo); 154 (ezetimibe) | Randomized, double‐blind/phase 3 | Men and women ages 18–80 y with fasting LDL ≥100 mg/dL but <190 mg/dL, TG ≤400 mg/dL, and 10‐y Framingham risk score for CHD ≤10% | None | Placebo and ezetimibe |
|
YUKAWA |
207 | 105; 102 | Randomized, double‐blind/phase 2 | Ages 20–80 y (37.1% women, 100% Asian) classified as high risk for CVD events; with screening LDL ≥115 mg/dL and fasting TG ≤400 mg/dL and on stable statin therapy for ≥4 wk | Statin±ezetimibe | Placebo |
|
YUKAWA‐2 |
404 | 202; 202 | Randomized, double‐blind/phase 3 | Ages 20–85 y (39.6% women, 100% Asian) with LDL ≥100 mg/dL, fasting TG ≤400 mg/dL, and on stable statin therapy for ≥4 wk | Statin | Placebo |
| Heterozygous familial hypercholesterolemia | ||||||
|
RUTHERFORD |
112 | 56; 56 | Randomized, double‐blind/phase 2 | Ages 18–75 y (47% female, 89% White) with diagnosed HeFH, LDL ≥100 mg/dL and TG ≤400 mg/dL, and on stable statin and other lipid‐lowering therapy for ≥4 wk | Statin±ezetimibe | Placebo |
|
RUTHERFORD‐2 |
329 | 220; 109 | Randomized, double‐blind/phase 3 | Ages 18–80 y (42% women, 90% White) with diagnosed HeFH and LDL ≥100 mg/dL despite stable statin and other lipid‐lowering therapy for ≥4 wk | Statin±ezetimibe | Placebo |
| Statin intolerance | ||||||
|
GAUSS |
64 | 32; 32 | Randomized, double‐blind/phase 2 | Ages 18–74 y (63.7% women, 88.5% White, 5.1% Black) and statin intolerant with LDL ≥100 mg/dL with diagnosed CHD or risk equivalent per NCEP criteria, LDL ≥130 mg/dL without CHD/risk equivalent and 2+ CVD risk factors, or LDL ≥160 mg/dL without CHD/risk equivalent and 0–1 CVD risk factors | Low‐dose statin permitted | Ezetimibe |
|
GAUSS‐2 |
307 | 205; 102 | Randomized, double‐blind/phase 3 | Ages 18–80 y (45.9% women, 93.5% White), LDL >NCEP ATP III panel goal and previous intolerance to ≥2 statins | Low‐dose statin permitted | Ezetimibe |
| Type 2 diabetes mellitus | ||||||
|
BANTING |
421 | 280; 141 | Randomized, double‐blind/phase 3 | Ages ≥18 y (44% women; 77% White) with type 2 diabetes mellitus, HBA1c <10% and on stable pharmacotherapy for diabetes mellitus for ≥6 mo; and LDL ≥70 mg/dL with CVD or LDL ≥100 mg/dL without CVD and taking maximally tolerated dose of statin of at least moderate intensity | Statin | Placebo |
|
BERSON |
981 | 657; 324 | Randomized, double‐blind/phase 3 | Ages 18–80 y (50% Asian, 42.5% White), with type 2 diabetes mellitus, HbA1c <10% and on stable pharmacotherapy for diabetes mellitus for ≥6 mo; and LDL ≥130 mg/dL with no statin or LDL ≥100 mg/dL with statin | Statin | Placebo |
| Long‐term (1‐ to 5‐y) studies | ||||||
|
DESCARTES |
901 | 599; 302 | Randomized, double‐blind/phase 3 | Ages 18–75 y (52.3% women, 80.3% White) with LDL ≥75 mg/dL and TG ≤400 mg/dL (1‐y duration) | Diet±statin or Diet+statin+ezetimibe | Placebo |
|
OSLER‐2 |
3443 † | 3443; ‐‐ | Controlled, open‐label extension/phase 3 |
Ages 18–85 y (47% women, 82.7% White, 12% Asian). Open‐label extension of MENDEL‐2, LAPLACE‐2, GAUSS‐2, RUTHERFORD‐2, DESCARTES, THOMAS‐1, ‐2 studies (3‐y duration) |
Standard‐of‐care | Standard‐of‐care in the first year of the study ‡ |
|
OSLER |
1151 † | 1151; ‐‐ | Controlled, open‐label extension/phase 3 |
Ages 18–85 y (53% women, 73.5% White, 19.3% Asian, 5.9% Black). Open‐label extension of MENDEL, LAPLACE‐TIMI 57, GAUSS, RUTHERFORD, YUKAWA studies (5‐y duration) |
Standard‐of‐care | Standard‐of‐care in the first year of the study ‡ |
All studies were global; YUKAWA (Study of LDL‐Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk) and YUKAWA‐2 enrolled Japanese participants. CHD indicates coronary heart disease; CVD, cardiovascular disease; HbA1c, hemoglobin A1c; HeFH, heterozygous familial hypercholesterolemia; LDL, low‐density lipoprotein; NCEP ATP, National Cholesterol Education Program Adult Treatment Panel; TG, triglycerides; BANTING, Evolocumab Efficacy and Safety in Type 2 Diabetes Mellitus on Background Statin Therapy; BERSON, Evolocumab Efficacy for LDL‐C Reduction in Subjects with T2DM on Background Statin; DESCARTES, Durable Effect of PCSK9 Antibody Compared With Placebo Study; LAPLACE TIMI 57, LDL‐C Assessment with PCSK9 MonoclonaL Antibody Inhibition Combined With Statin Therapy‐ TIMI‐57; LAPLACE‐2, LDL‐C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined with Statin Therapy‐2; MENDEL, Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels; MENDEL‐2, Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels‐2; OSLER, Open Label Study of Long Term Evaluation Against LDL‐C Trial; OSLER‐2, Open Label Study of Long Term Evaluation Against LDL‐C Trial‐2; RUTHERFORD, Reduction of LDL‐C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study; RUTHERFORD‐2, Reduction of LDL‐C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study‐2; GAUSS, Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin Intolerant Subjects; and GAUSS‐2, Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin Intolerant Subjects ‐2.
Sample sizes include those patients who were randomized and received at least 1 dose of evolocumab (at approved doses), ezetimibe, or placebo.
The open‐label extension studies included participants who were also enrolled and counted in the parent studies. Thus, the total sample size for this analysis is lower than the sum of sample sizes across the included studies.
During year 1, patients were randomized to receive evolocumab plus standard‐of‐care or standard‐of‐care. After year 1, all patients received evolocumab plus standard‐of‐care.
All participants provided written informed consent and individual protocols were approved by each institutional review board; investigations were conducted in accordance with the Declaration of Helsinki. Data verification was performed by study monitors in all trials, and principal investigators of each trial confirmed the completeness of the data entered into the clinical trial database. Data from Amgen clinical studies may be requested by qualified researchers. Complete details are available at the following: http://www.amgen.com/datasharing.
Background statin therapy differed across the trials. The GAUSS (Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin‐Intolerant Subjects) (NCT01375764) and GAUSS‐2 (NCT01763905) trials enrolled participants who were statin‐intolerant, and MENDEL (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Subjects Currently Not Receiving Drug Therapy for Easing Lipid Levels) (NCT01375777) and MENDEL‐2 (NCT01763827) evaluated evolocumab as monotherapy. The majority of trials used placebo comparators; however, the GAUSS trials used ezetimibe comparators, the MENDEL‐2 trial and atorvastatin cohort of the LAPLACE‐2 (LDL‐C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined with Statin Therapy) (NCT01763866) study used both placebo and ezetimibe comparison groups, and the OSLER studies randomized participants to either evolocumab plus standard‐of‐care or standard‐of‐care only in the first year, after which all participants received evolocumab plus standard‐of‐care. The OSLER and OSLER‐2 studies were open‐label extension studies that enrolled participants who had completed phase 2 and phase 3 evolocumab trials, respectively. Data from 2 approved dosing regimens of evolocumab (420 mg QM and 140 mg Q2W) were pooled in this analysis because of their similar lipid‐lowering effects. 9
LDL‐C was calculated using the Friedewald equation; however, if calculated LDL‐C was <40 mg/dL or triglycerides >400 mg/dL, then LDL‐C was measured in the same blood sample by ultracentrifugation if an additional adequate sample was available. Race and ethnicity were self‐identified as White, Non‐White, Non‐Hispanic Black/African American, Asian, or Hispanic/Latino (Non‐White includes African American/Black, Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, Mixed Race, and other). The race‐ and sex‐specific pooled cohort equation was used to predict the risk of an atherosclerotic cardiovascular disease (ASCVD) event within 10 years in participants without a prior history of ASCVD. 27
Studies that focused on participants with distinct clinical characteristics at baseline and/or that used different comparator groups were pooled into groups as follows: all placebo‐comparator studies; all ezetimibe‐comparator studies; hypercholesterolemia/mixed dyslipidemia with a placebo comparator; hypercholesterolemia/mixed dyslipidemia with an ezetimibe comparator; statin intolerance; type 2 diabetes mellitus; heterozygous familial hypercholesterolemia; and studies with 1‐, 3‐, and 5‐year follow‐up duration. Only groups with adequate sample size (n>20) were examined in the results. Baseline characteristics were summarized by racial/ethnic group for participants of placebo‐comparator and ezetimibe‐comparator trials separately. Mean percent change and absolute change in LDL‐C levels from baseline, achievement of LDL‐C <70 mg/dL, and achievement of at least 50% LDL‐C reduction were summarized by racial/ethnic background. Mean percent change in LDL‐C for each racial/ethnic group was also illustrated by forest plots by clinical characteristics. Additional analyses, including mean percent change and absolute change from baseline in levels of non‐HDL‐C (non–high density lipoprotein cholesterol), apolipoprotein B, Lp(a) [lipoprotein(a)], triglycerides, and HDL‐C, were examined by racial/ethnic background.
Differences in treatment effects between White and non‐White individuals were assessed by a quantitative interaction test for treatment by race on the average LDL‐C percent change at week 12 (week 52 for the DESCARTES study) for each clinical population group. The quantitative interaction P values were obtained through an ANCOVA model, which included treatment, race, the interaction of treatment with race, baseline LDL‐C (mg/dL), study, and the interaction of treatment with study as covariates. If significant treatment‐by‐race quantitative interaction was present, a qualitative interaction was assessed using Gail‐Simon's method to determine whether treatment efficacy varied directionally among different racial groups. 28 No multiplicity adjustments were performed. All statistical analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
This analysis is based on data from 7669 participants of phase 2 and 3 trials, including 6466 individuals who received at least 1 dose of evolocumab (this includes 1499 individuals in open‐label extension studies who previously received placebo or ezetimibe in the parent studies), 2193 individuals who received at least 1 dose of placebo, and 509 who received at least 1 dose of ezetimibe. Figure 1 depicts the regional enrollment and racial/ethnic composition of participants receiving evolocumab in the double‐blind studies included in this analysis. The majority of participants from North America (n=1577) and Europe (n=1429) self‐identified as non‐Hispanic White (72% and 98%, respectively); those from Latin America (n=136) largely self‐identified as Hispanic/Latino (94%), and all participants from East Asia (n=658) self‐identified as Asian.
Figure 1. Regional enrollment of evolocumab‐treated patients in the double‐blind studies in this analysis by race and ethnicity.
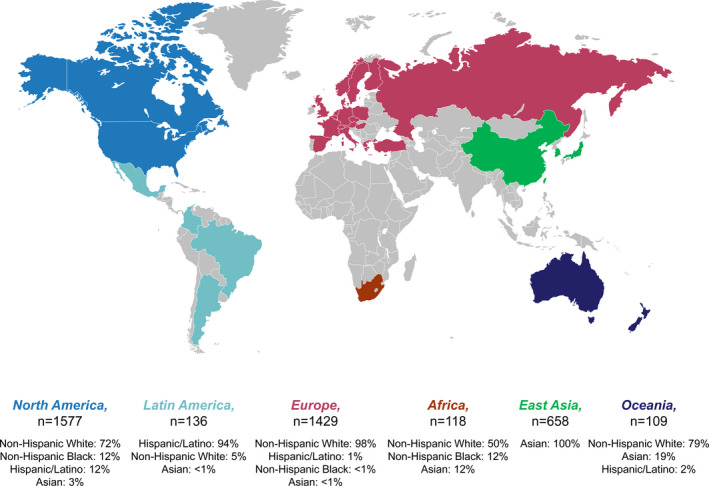
Nonpresented racial/ethnic groups include those who self‐identified as American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, and Mixed Race.
Baseline Characteristics
Among participants of placebo‐comparator studies (n=3790), the overall mean age was 58.5 years, 50.6% were men, 29.7% had ASCVD at baseline, and 35.9% had type 2 diabetes mellitus. Among participants of ezetimibe‐comparator studies (n=982), the overall mean age was 57.8 years, 48.3% were men, 21.8% had ASCVD, and 11.5% had type 2 diabetes mellitus. Table 2 shows descriptive baseline characteristics by racial/ethnic group across placebo‐comparator and ezetimibe‐comparator studies. For studies with a placebo‐comparator group, mean age and proportion of women were similar between White and non‐White groups, whereas for ezetimibe‐comparator studies, mean age was higher for White participants compared with non‐White participants, and a higher proportion of non‐White participants were women. In both types of studies, mean total cholesterol and LDL‐C levels were higher for White participants compared with non‐White participants (mean total cholesterol was 194.9 versus 185.1 mg/dL and LDL‐C was 115.7 versus 108.9 mg/dL in the placebo‐comparator versus ezetimibe‐comparator studies, respectively); however, non‐Hispanic Black participants had LDL‐C levels (115.1 mg/dL) as high as those for White participants in the placebo‐comparator studies only. Asian and Hispanic/Latino participants had among the lowest LDL‐C levels. Of note, in both types of studies, Hispanic/Latino individuals had the highest mean triglyceride levels. Lastly, a higher proportion of non‐White individuals had prevalent type 2 diabetes mellitus compared with White participants (60.0% versus 26.6% in the placebo‐comparator studies) (Table 2).
Table 2.
Baseline Demographics by Race and Ethnicity in Those Receiving Evolocumab
| Racial/Ethnic Group | |||||
|---|---|---|---|---|---|
| Placebo‐Comparator Studies* (n=3790) | |||||
|
White (n=2742) |
Non‐White † (n=1048) |
Non‐Hispanic Black (n=200) |
Asian (n=740) |
Hispanic/Latino (n=328) |
|
| Age, y, mean (SD) | 58.6 (10.9) | 58.3 (10.6) | 54.0 (10.5) | 59.7 (10.2) | 57.8 (10.5) |
| Sex, female, n (%) | 1355 (49.4) | 518 (49.4) | 124 (62.0) | 328 (44.3) | 200 (61.0) |
| BMI, kg/m2, mean (SD) | 29.7 (5.5) | 28.3 (6.1) | 34.8 (7.3) | 26.1 (3.9) | 30.4 (6.0) |
| Waist circumference, cm, mean (SD) | |||||
| Male | 103.1 (13.1) | 96.0 (11.5) | 106.4 (13.4) | 93.3 (9.3) | 102.8 (14.6) |
| Female | 96.0 (15.1) | 95.3 (14.7) | 108.6 (16.4) | 90.0 (10.1) | 96.5 (14.6) |
| Lipid profile | |||||
| LDL‐C, mg/dL, mean (SD) | 115.7 (38.9) | 108.9 (38.3) | 115.1 (33.1) | 106.7 (39.0) | 106.3 (32.8) |
| Total cholesterol, mg/dL, mean (SD) | 194.9 (43.9) | 185.1 (43.3) | 188.1 (39.5) | 184.4 (43.8) | 182.7 (39.1) |
| HDL‐C, mg/dL, mean (SD) | 52.4 (16.0) | 50.6 (13.9) | 52.1 (16.8) | 51.1 (13.2) | 47.4 (12.8) |
| Triglycerides, mg/dL, mean (SD) | 139.3 (78.0) | 132.1 (70.3) | 111.3 (52.3) | 136.9 (73.8) | 148.6 (72.3) |
| ASCVD, n (%) ‡ | 804 (29.3) | 320 (30.5) | 35 (17.5) | 256 (34.6) | 55 (16.8) |
| Type 2 diabetes mellitus, n (%) | 730 (26.6) | 629 (60.0) | 75 (37.5) | 488 (65.9) | 204 (62.2) |
| Ezetimibe‐Comparator Studies § (n=982) | |||||
|---|---|---|---|---|---|
|
White (n=890) |
Non‐White † (n=92) |
Non‐Hispanic Black (n=45) |
Asian (n=39) |
Hispanic/Latino (n=58) |
|
| Age, y, mean (SD) | 58.3 (11.4) | 53.1 (11.3) | 54.7 (10.6) | 52.3 (12.1) | 53.5 (11.7) |
| Sex, female, n (%) | 452 (50.8) | 56 (60.9) | 27 (60.0) | 25 (64.1) | 35 (60.3) |
| BMI, kg/m2, mean (SD) | 28.9 (5.4) | 29.8 (7.0) | 31.5 (6.1) | 27.6 (7.3) | 29.6 (5.4) |
| Waist circumference, cm, mean (SD) | |||||
| Male | 102.6 (13.4) | 100.5 (17.1) | 106.4 (19.2) | 92.7 (8.0) | 96.8 (11.5) |
| Female | 93.1 (13.4) | 91.5 (15.6) | 97.8 (15.8) | 86.2 (13.4) | 91.0 (11.7) |
| Lipid profile | |||||
| LDL‐C, mg/dL, mean (SD) | 141.3 (55.8) | 130.6 (38.1) | 132.6 (40.8) | 127.6 (33.2) | 130.2 (37.8) |
| Total cholesterol, mg/dL, mean (SD) | 223.7 (60.3) | 211.5 (47.1) | 213.0 (49.9) | 209.2 (44.0) | 208.3 (44.1) |
| HDL‐C, mg/dL, mean (SD) | 54.1 (16.7) | 58.0 (19.0) | 58.5 (21.8) | 58.9 (16.7) | 49.3 (13.6) |
| Triglycerides, mg/dL, mean (SD) | 141.9 (74.0) | 115.1 (59.0) | 110.5 (55.3) | 113.3 (49.0) | 144.7 (67.7) |
| ASCVD, n (%) ‡ | 203 (22.8) | 11 (12.0) | 6 (13.3) | 4 (10.3) | 9 (15.5) |
| Type 2 diabetes mellitus, n (%) | 94 (10.6) | 19 (20.7) | 9 (20.0) | 7 (17.9) | 8 (13.8) |
ASCVD indicates atherosclerotic cardiovascular disease; BMI, body mass index; HDL‐C, high‐density lipoprotein cholesterol; and LDL‐C, low‐density lipoprotein cholesterol.
BANTING (Evolocumab Efficacy and Safety in Type 2 Diabetes Mellitus on Background Statin Therapy), BERSON (Evolocumab Efficacy for LDL‐C Reduction in Subjectw with T2DM on Background Statin), RUTHERFORD (Reduction of LDL‐C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study), RUTHERFORD‐2 (Reduction of LDL‐C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study‐2), MENDEL (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels), MENDEL‐2 (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels‐2), LAPLACE (LDL‐C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined with Statin Therapy), LAPLACE‐2 (LDL‐C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined with Statin Therapy‐2), YUKAWA (Study of LDL‐Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk), YUKAWA‐2, and DESCARTES (Durable Effect of PCSK9 Antibody Compared with Placebo Study).
Non‐White includes African American/Black, Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, Mixed Race, and other.
ASCVD includes coronary artery disease, cardiovascular disease/peripheral artery disease, angina, myocardial infarction, coronary artery bypass graft, percutaneous coronary intervention, peripheral artery disease, stroke, transient ischemic attack, and carotid/ventricular assist device.
GAUSS‐1, GAUSS‐2, MENDEL‐2, and LAPLACE‐2 atorvastatin cohorts.
Change in LDL‐C From Baseline
In the short‐term (12‐week) studies, mean percent change in LDL‐C from baseline with evolocumab therapy ranged from ≈−52% to −59% among White individuals and from −46% to −67% among non‐White individuals (Table 3). In comparison, mean percent changes in LDL‐C ranged from 1% to 5% and 1% to 12% among White and non‐White participants who received placebo, respectively, and from −17% to −20% and −14% to −21% among White and non‐White individuals who received ezetimibe, respectively (Figure 2). Mean percent change in LDL‐C with evolocumab therapy was similar in White and non‐White individuals. Statistically significant quantitative interaction of treatment effect by race (White participants versus non‐White participants) was noted among those with diabetes mellitus (quantitative interaction P values for evolocumab Q2W and QM were P<0.001 and P=0.007, respectively). This difference was driven by a greater LDL‐C reduction observed in Asian participants compared with participants in other racial/ethnic groups. When qualitative interaction P values were assessed for individuals with diabetes mellitus, no significant interactions were observed for either evolocumab Q2W or evolocumab QM versus placebo (P=0.5 for both dosing regimens). As such, consistent directionality of treatment effect was demonstrated across racial groups among individuals with diabetes mellitus.
Table 3.
Effect of Evolocumab on LDL‐C by Race and Ethnicity in Various Patient Populations
| Population | Changes |
Percent (%) and Absolute (mg/dL) Changes in LDL‐C From Baseline Mean (SD) |
Treatment Effect P Value (White vs Non‐White) | |||||
|---|---|---|---|---|---|---|---|---|
| White | Non‐White* | Non‐Hispanic Black | Asian | Hispanic/Latino* | EvoMab Q2W | EvoMab QM | ||
| Pooled (all placebo‐comparator studies) † | N | 2539 | 976 | 178 | 699 | 282 | <0.001 ‡ | 0.059 |
| % | −56.9 (20.9) | −64.2 (19.9) | −50.4 (21.5) | −68.9 (17.2) | −57.3 (20.8) | |||
| mg/dL | −66.1 (32.0) | −69.6 (31.6) | −58.3 (28.9) | −73.5 (31.0) | −61.2 (30.0) | |||
| Pooled (all ezetimibe‐comparator studies) § | N | 815 | 85 | 41 | 36 | 47 | 0.48 | 0.67 |
| % | −58.4 (16.6) | −56.3 (18.2) | −51.6 (18.6) | −63.0 (15.9) | −56.6 (21.5) | |||
| mg/dL | −81.5 (35.7) | −74.0 (29.1) | −69.6 (27.9) | −80.9 (29.4) | −74.1 (32.6) | |||
| Statin‐intolerant ‖ | n | 210 | 15 | 7 | 6 | 3 | 0.14 | 0.28 |
| % | −55.4 (14.8) | −46.3 (20.9) | −46.0 (13.4) | −49.0 (29.3) | −54.6 (12.3) | |||
| mg/dL | −106.4 (37.2) | −77.4 (40.1) | −78.6 (31.1) | −76.1 (49.6) | −103.7 (39.9) | |||
| Type 2 diabetes mellitus ¶ | n | 440 | 395 | 40 | 305 | 158 | <0.001 ‡ | 0.007 ‡ |
| % | −51.5 (25.6) | −66.5 (21.2) | −50.4 (22.2) | −69.6 (20.2) | −59.8 (20.5) | |||
| mg/dL | −53.7 (30.9) | −61.3 (29.3) | −54.8 (29.1) | −63.0 (29.6) | −59.3 (26.9) | |||
| HeFH # | n | 236 | 26 | 3 | 15 | 1 | 0.55 | 0.19 |
| % | −57.3 (19.7) | −64.1 (12.0) | −73.7 (4.2) | −60.5 (12.8) | −35.7 (‐) | |||
| mg/dL | −86.8 (35.9) | −109.1 (36.2) | −88.0 (12.0) | −104.7 (38.8) | −59.5 (‐) | |||
| Hypercholesterolemia/mixed dyslipidemia (placebo comparator)** | n | 1399 | 437 | 86 | 339 | 91 | 0.42 | 0.45 |
| % | −58.8 (19.2) | −65.5 (17.0) | −51.2 (19.4) | −69.4 (13.9) | −54.6 (20.3) | |||
| mg/dL | −69.1 (31.2) | −78.7 (29.9) | −62.7 (27.3) | −82.7 (28.9) | −65.5 (29.3) | |||
| Hypercholesterolemia/mixed dyslipidemia (ezetimibe comparator) ** | n | 605 | 70 | 34 | 30 | 44 | 0.19 | 0.39 |
| % | −59.4 (17.1) | −58.4 (16.9) | −52.8 (19.4) | −65.8 (10.3) | −56.7 (22.1) | |||
| mg/dL | −72.9 (30.8) | −73.3 (26.5) | −67.7 (27.3) | −81.9 (24.7) | −72.1 (31.6) | |||
| 1‐y study ** (wk 52) | n | 436 | 106 | 47 | 34 | 28 | N/A | 0.58 |
| % | −52.1 (27.7) | −48.6 (29.2) | −50.8 (22.0) | −49.5 (29.4) | −43.0 (43.1) | |||
| mg/dL | −54.2 (31.3) | −51.1 (32.4) | −55.5 (27.2) | −49.0 (32.1) | −49.6 (52.9) | |||
| 3‐y study ** (wk 152) | n | 2564 | 465 | 91 | 321 | 99 | … | … |
| % | −45.5 (35.1) | −52.9 (31.0) | −36.4 (33.9) | −59.2 (27.5) | −28.6 (47.4) | |||
| mg/dL | −63.9 (53.5) | −61.0 (42.7) | −43.8 (43.9) | −67.2 (40.1) | −42.2 (60.0) | |||
| 5‐y study ** (wk 260) | n | 682 | 258 | 34 | 213 | 19 | … | … |
| % | −47.6 (26.4) | −54.6 (22.7) | −41.9 (33.4) | −57.7 (19.4) | −20.9 (46.0) | |||
| mg/dL | −68.8 (44.5) | −77.8 (35.2) | −57.1 (45.5) | −82.4 (31.1) | −36.6 (75.5) | |||
Treatment effect P values are not presented for the 3‐ and 5‐year studies since these studies did not use comparator groups at the final timepoint measured. EvoMab indicates evolocumab; HeFH, heterozygous familial hypercholesterolemia; LDL‐C, low‐density lipoprotein cholesterol; N/A, not applicable; Q2W, every 2 weeks; and QM, once monthly.
Twenty‐two patients self‐identified as Hispanic Black. Non‐White includes African American/Black, Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, Mixed Race, and other.
BANTING (Evolocumab Efficacy and Safety in Type 2 Diabetes Mellitus on BackGround Statin Therapy), BERSON (Evolocumab Efficacy for LDL‐C Reduction in Subjects with T2DM on Background Statin), RUTHERFORD‐1 (Reduction of LDL‐C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study), RUTHERFORD‐2 (Reduction of LDL‐C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study‐2), MENDEL‐1 (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels), MENDEL‐2 (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels‐2), LAPLACE‐TIMI‐57 (LDL‐C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined With Statin Therapy‐ TIMI‐57), LAPLACE‐2 (LDL‐C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined with Statin Therapy‐2), YUKAWA‐1 (Study of LDL‐Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk), YUKAWA‐2, and DESCARTES (Durable Effect of PCSK9 Antibody Compared wiTh Placebo Study).
Qualitative interaction P values were nonsignificant (P=0.5) via Gail‐Simon's method.
GAUSS‐1 (Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin Intolerant Subjects), GAUSS‐2 (Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin Intolerant Subjects ‐2), MENDEL‐2 (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels‐2), and LAPLACE‐2 atorvastatin cohorts.
GAUSS‐1, ‐2 (Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin Intolerant Subjects).
BANTING and BERSON.
RUTHERFORD‐1, ‐2.
MENDEL‐1, ‐2, LAPLACE‐TIMI‐57, LAPLACE‐2, and YUKAWA‐1, ‐2.
MENDEL‐2 and LAPLACE‐2 atorvastatin cohorts.
DESCARTES.
OSLER‐2 (Open Label Study of Long Term Evaluation Against LDL‐C Trial‐2).
OSLER‐1 (Open Label Study of Long Term Evaluation Against LDL‐C Trial).
The patient populations for “statin‐intolerant,” “HeFH,” “Type‐2 Diabetes,” and “Hypercholesterolemia/mixed dyslipidemia” are for short‐term [12‐week] studies.
Figure 2. Mean percent change from baseline in LDL‐C with evolocumab, ezetimibe, or placebo across racial/ethnic groups.
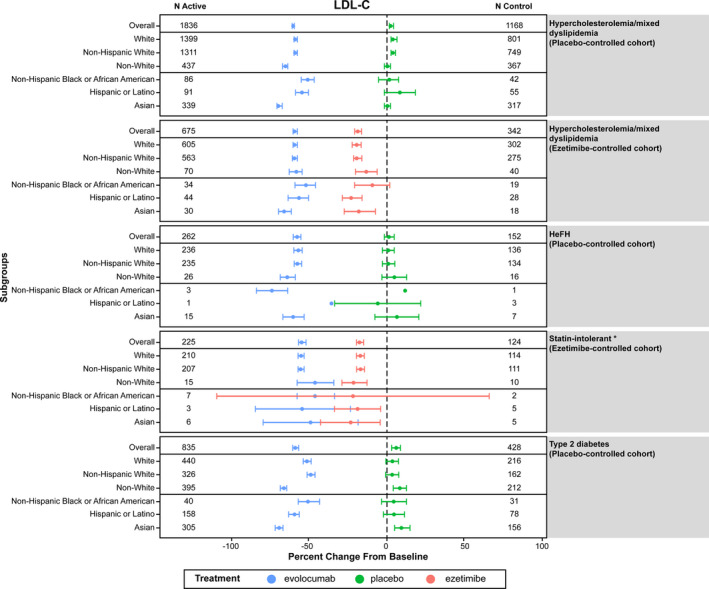
Dots represent the mean value and bars represent the 95% CIs. *Mean percent change in LDL‐C from baseline (95% CI) for the evolocumab and ezetimibe treatment arms were: −46.0% (−58.3, −33.6) and −22.1% (−109.3, 65.1), respectively, for Non‐Hispanic Black or African American persons; −54.6% (−85.3, −24.0) and −19.0% (−33.9, −4.2), respectively, for Hispanic or Latino persons; and −49.0% (−79.7, −18.2) and −23.5% (−42.5, −4.6), respectively, for Asian persons. HeFH indicates heterozygous familial hypercholesterolemia; and LDL‐C, low‐density lipoprotein cholesterol.
In the long‐term studies (ie, with 1, 3, and 5 years of follow‐up), mean percent change in LDL‐C with evolocumab therapy ranged from −46% to −52% among White participants and from −49% to −55% among non‐White participants (Table 3). In pooled analyses of all studies with a placebo comparator, evolocumab treatment was associated with a mean percent change in LDL‐C of −56.9% and −64.2% among White and non‐White participants, respectively. A significant quantitative difference in treatment effect was detected for White participants versus non‐White participants (quantitative interaction P values for evolocumab 140 mg Q2W and evolocumab 420 mg QM were P<0.001 and P=0.059, respectively). However, no significant qualitative interaction was seen (P=0.5). The mean percent change in LDL‐C was −50.4% among non‐Hispanic Black participants, −68.9% among Asian participants, and −57.3% among Hispanic/Latino participants. In pooled analyses of all studies with an ezetimibe comparator, mean percent change in LDL‐C was −58.4% among White participants, −56.3% among non‐White participants, −51.6% among non‐Hispanic Black participants, −63.0% among Asian participants, and −56.6% among Hispanic/Latino participants. There was no significant difference in treatment effect between White and non‐White individuals (Table 3). Of note, treatment with evolocumab was also associated with consistent and stable reductions in a panel of lipids including non‐HDL‐C, apolipoprotein B, Lp(a), and triglycerides across all participants, and with increase in HDL‐C in almost all populations studied (Tables S1 through S5).
Achievement of LDL‐C Treatment Goals
In the short‐term studies, LDL‐C <70 mg/dL was achieved at 12 weeks in 43% to 83% of White participants and 27% to 94% of non‐White participants receiving evolocumab. The lower end of the ranges for White and non‐White participants was set by the statin‐intolerance studies in which participants did not receive background statin therapy. Similarly, a ≥50% reduction in LDL‐C was achieved by 63% to 79% of White participants and 60% to 86% of non‐White participants (Table 4).
Table 4.
Achievement of LDL‐C Treatment Goals by Race and Ethnicity With Evolocumab
| Achievement of LDL‐C <70 mg/dL, %/Achievement of at Least 50% LDL‐C Reduction, % | |||||
|---|---|---|---|---|---|
| White | Non‐White* | Non‐Hispanic Black | Asian | Hispanic/Latino* | |
| Pooled (all placebo‐comparator studies) † |
82.6/73.8 n=2539 |
89.2/81.7 n=976 |
77.5/60.1 n=178 |
94.1/90.0 n=699 |
85.8/71.6 n=282 |
| Pooled (all ezetimibe‐comparator studies) ‡ |
70.6/76.0 n=815 |
75.3/72.9 n=85 |
70.7/63.4 n=41 |
88.9/91.7 n=36 |
72.3/68.1 n=47 |
| Statin‐intolerant |
42.9/68.6 n=210 |
26.7/60.0 n=15 |
14.3/42.9 n=7 |
50.0/83.3 n=6 |
0/66.7 n=3 |
| Type 2 diabetes mellitus |
82.3/63.0 n=440 |
93.9/82.3 n=395 |
90.0/62.5 n=40 |
95.1/86. 9 n=305 |
89.2/75.3 n=158 |
| HeFH |
66.1/73.7 n=236 |
61.5/84.6 n=26 |
100/100 n=3 |
53.3/80.0 n=15 |
0/0 n=1 |
| Hypercholesterolemia/mixed dyslipidemia (placebo comparator) |
83.3/77.6 n=1399 |
89.7/86.3 n=437 |
74.4/60.5 n=86 |
94.7/93.8 n=339 |
79.1/69.2 n=91 |
| Hypercholesterolemia/mixed dyslipidemia (ezetimibe comparator) |
80.2/78.5 n=605 |
85.7/75.7 n=70 |
82.4/67.6 n=34 |
96.7/93.3 n=30 |
77.3/68.2 n=44 |
| 1‐y study |
84.4/67.4 n=436 |
73.6/57.5 n=106 |
74.5/59.6 n=47 |
76.5/58.8 n=34 |
82.1/60.7 n=28 |
| 3‐y study |
63.4/58.0 n=2564 |
77.0/68.0 n=465 |
57.1/42.9 n=91 |
84.4/76.6 n=321 |
49.5/41.4 n=99 |
| 5‐y study |
52.8/53.4 n=682 |
66.3/66.7 n=258 |
55.9/44.1 n=34 |
69.5/72.8 n=213 |
26.3/26.3 n=19 |
HeFH indicates heterozygous familial hypercholesterolemia; and LDL‐C, low‐density lipoprotein cholesterol.
A total of 22 patients receiving evolocumab self‐identified as Hispanic Black. Non‐White includes African American/Black, Asian, American Indian or Alaska Native, Native Hawaiian or Other Pacific Islander, Mixed Race and other.
BANTING (Evolocumab Efficacy and Safety in Type 2 Diabetes Mellitus on Background Statin Therapy), BERSON (Evolocumab Efficacy for LDL‐C Reduction in Subjects with T2DM On Background Statin), RUTHERFORD‐1 (Reduction of LDL‐C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study), RUTHERFORD‐2 (Reduction of LDL‐C With PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder Study‐2), MENDEL‐1 (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels), MENDEL‐2 (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels‐2), LAPLACE‐TIMI‐57 (LDL‐C Assessment with PCSK9 MonoclonaL Antibody Inhibition Combined With Statin Therapy‐ TIMI‐57), LAPLACE‐2 (LDL‐C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined with Statin Therapy‐2), YUKAWA‐1 (Study of LDL‐Cholesterol Reduction Using a Monoclonal PCSK9 Antibody in Japanese Patients With Advanced Cardiovascular Risk), YUKAWA‐2, and DESCARTES (Durable Effect of PCSK9 Antibody Compared with Placebo Study).
GAUSS‐1 (Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin Intolerant Subjects), GAUSS‐2 (Goal Achievement After Utilizing an Anti‐PCSK9 Antibody in Statin Intolerant Subjects ‐2), MENDEL‐2 (Monoclonal Antibody Against PCSK9 to Reduce Elevated LDL‐C in Adults Currently Not Receiving Drug Therapy for Easing Lipid Levels‐2), and LAPLACE‐2 (LDL‐C Assessment with PCSK9 Monoclonal Antibody Inhibition Combined with Statin Therapy‐2) atorvastatin cohorts.
The patient populations for “statin‐intolerant,” “HeFH,” “Type‐2 Diabetes Mellitus,” and “Hypercholesterolemia/mixed dyslipidemia” are for short‐term [12‐week] studies.
In the long‐term studies (1, 3, and 5 years of follow‐up) with evolocumab treatment, 53% to 84% and 66% to 77% of White and non‐White participants, respectively, achieved LDL‐C <70 mg/dL, and 53% to 67% and 58% to 68% of White and non‐White participants, respectively, achieved ≥50% reduction in LDL‐C. In pooled analyses on all placebo‐comparator studies, LDL‐C <70 mg/dL was achieved by 82.6% of White and 89.2% of non‐White participants; a ≥50% reduction in LDL‐C level was achieved by 73.8% of White and 81.7% of non‐White participants. Similar findings were observed in analyses pooling all ezetimibe‐comparator studies, with achievement of LDL‐C <70 mg/dL in 70.6% of White and 75.3% of non‐White participants and achievement of at least 50% reduction in LDL‐C in 76.0% of White and 72.9% of non‐White participants (Table 4).
Discussion
This pooled analysis of data from 15 phase 2 and phase 3 clinical trials demonstrated that both dosing regimens of evolocumab led to consistent and marked reductions in LDL‐C and greater achievement of an LDL‐C goal of <70 mg/dL and of reduction in LDL‐C by at least 50% across self‐identified racial/ethnic backgrounds than the comparators. Among participants with diabetes mellitus, mean percent change in LDL‐C with evolocumab treatment was greater in non‐White participants compared with White participants (ie, significant quantitative P interaction was seen), with Asian participants with diabetes mellitus experiencing the largest reduction in LDL‐C among all racial/ethnic groups, followed by Hispanic/Latino participants. Of note, this finding was driven by the results of the BERSON (Evolocumab Efficacy for LDL‐C Reduction in Subjects with T2DM on Background Statin) study (981 patients, of which 451 were Chinese) in which Asian participants experienced a greater reduction in LDL‐C compared with the overall study population 24 , 29 (ie, LDL‐C reduction by 85% after a 12‐week therapy with 140 mg of evolocumab in BERSON participants from China 29 versus a 72% reduction in the overall study sample 24 ). Among individuals with diabetes mellitus, no significant qualitative interactions were observed in those receiving evolocumab versus placebo (ie, the directionality of treatment effect was consistent across racial groups). Evolocumab treatment was also associated with reductions in non‐HDL‐C, apolipoprotein B, Lp(a), and triglycerides, and increases in HDL‐C across all racial/ethnic groups.
Evolocumab therapy has previously demonstrated stable and substantial lowering of LDL‐C across various patient populations. Stroes et al conducted a pooled analysis of four 12‐week phase 3 trials of 3146 participants (mean age 57.8 [SD ±11.2 years]; 49.4% women; 91.5% White) with primary hypercholesterolemia, statin intolerance, and familial hypercholesterolemia (the MENDEL‐2, LAPLACE‐2, RUTHERFORD‐2, and GAUSS‐2 trials). 9 They reported significant and consistent reductions in LDL‐C with evolocumab regardless of race and ethnicity (mean percent changes [95% CI] in LDL‐C from baseline were −52.9 [−62.2, −43.7] in Asian participants, −47.3 [−61.0, −33.6] in Black participants, and −40.6 in White participants [−43.4, −37.7]; quantitative P interaction=0.034; qualitative P interaction=0.875), 9 which is consistent with our findings. Reductions in LDL‐C did not differ between Hispanic and non‐Hispanic individuals (mean percent changes [95% CI] in LDL‐C from baseline: −38.1 [−52.1, −24.2] and −41.6 [−44.2, −38.9], respectively; P interaction=0.63). 9 The current post hoc analysis extended these previous findings by evaluating the effects of evolocumab in a significantly larger pooled sample of participants from 15 phase 2 and 3 trials for Asian, Hispanic/Latino, and Black populations and across additional subpopulations, including individuals with diabetes mellitus, statin intolerance, and heterozygous familial hypercholesterolemia, and over an extended duration of up to 5 years. It has also been recently reported that alirocumab significantly lowered LDL‐C, Lp(a), and apolipoprotein B levels regardless of racial/ethnic background. 12
One limitation of the current pooled analysis was the heterogeneity of the individual study populations in terms of inclusion criteria, background statin therapy, and clinical characteristics including history of ASCVD and type 2 diabetes mellitus. While analyses stratified by clinical characteristics were conducted in order to control for potential confounding because of these differences, these stratified analyses had limited ability to detect differences across specific racial/ethnic groups with certain clinical characteristics because of inadequate representation of non‐White participants, particularly the statin‐intolerant and heterozygous familial hypercholesterolemia cohorts for which there were fewer contributing studies or studies involving small numbers of participants. Moreover, considerable heterogeneity exists within the broadly defined racial/ethnic groups used in this study. For instance, the Hispanic/Latino population encompasses individuals with diverse backgrounds, cultures, and exposures, and substantial variation in CVD risk factors and CVD outcomes have been reported across Hispanic/Latino ethnic backgrounds. 30 , 31 , 32 However, this heterogeneity could not be taken into consideration because of the limited sample sizes for the non‐White racial/ethnic groups. The ASCVD risk calculator used in this analysis was derived in Americans and may not apply internationally. Nonetheless, the current analyses provide new information on the short‐term and long‐term effect of evolocumab in lowering LDL‐C among non‐Hispanic Black, Hispanic/Latino, and Asian individuals. The focus of the current article was to analyze the effects of evolocumab on LDL‐C lowering across racial/ethnic groups, which was the primary end point of the clinical trial program; the assessment of other lipids and lipoproteins is outside the scope of the current analysis. Since the current analyses include data from randomized trials, the majority of which were blinded, and given the focus on LDL‐C levels rather than clinical end points, the findings presented here are unlikely to be affected by selection, performance, or outcome ascertainment biases. Moreover, in the analysis assessing treatment difference between White and non‐White participants, individual study and interaction of treatment with study were included as covariates in the analytic model to account for possible biases from individual studies.
The influence of race and ethnicity is notably multifactorial and affected by socioeconomic status, environmental exposures, cultural factors, and access to health care. In addition to elevated total cholesterol and LDL‐C levels, specific patterns of dyslipidemia may differentially impact certain racial/ethnic groups, in particular, Hispanics/Latino participants and Asian participants. 31 In the Hispanic Community Health Study/Study of Latinos, prevalence of mixed dyslipidemia (ie, both elevated triglycerides and low HDL‐C) was 10.7% overall. 32 This pattern that is associated with higher CVD risk irrespective of LDL‐C level may be more prevalent among Hispanic participants compared with non‐Hispanic White participants. 33 Moreover, Hispanic participants of the Multi‐Ethnic Study of Atherosclerosis had higher age–sex adjusted odds of combined hyperlipidemia (ie, age–sex‐specific LDL‐C and triglyceride levels ≥75th percentile based on a mostly White reference population) compared with White participants. 34 Despite the high burden of CVD risk in racial/ethnic minority populations and the variations in risk across groups, enrollment of non‐White individuals in clinical trials has been historically limited for most therapeutic agents. The small number of individuals in some racial/ethnic groups in the current study, despite the fact that data from 15 clinical trials were pooled, underscores the need for intensive, culturally sensitive efforts to promote inclusion of diverse populations in future studies, and for research on the efficacy of lipid‐lowering therapies in individuals across a range of dyslipidemia patterns to ensure that research findings are generalizable to vulnerable minority populations.
In conclusion, this pooled analysis of data from 15 clinical trials confirms that treatment with evolocumab markedly reduced LDL‐C with similar beneficial effects across race and ethnicity in participants with hypercholesterolemia/mixed dyslipidemia and type 2 diabetes mellitus. These findings provide additional evidence for the use of evolocumab therapy 35 in individuals of diverse racial/ethnic backgrounds, including those with diabetes mellitus.
Sources of Funding
This work was supported by Amgen Inc.
Disclosures
Rodriguez has received grant funding from Amgen Inc. López, Monsalvo, and Wu are employees and shareholders of Amgen Inc. Ferdinand is a consultant for Sanofi, Amgen Inc., Boehringer Ingelheim, Eli Lilly, and Janssen. Daviglus has no disclosures to report.
Supporting information
Tables S1–S5
Acknowledgments
The authors thank Maya Shehayeb, of Amgen Inc., for medical writing and editorial support, and Huei Wang, of Amgen Inc., for statistical support.
(J Am Heart Assoc. 2021;10:e016839. DOI: 10.1161/JAHA.120.016839.)
Supplementary Material for this article is available at https://www.ahajournals.org/doi/suppl/10.1161/JAHA.120.016839
For Sources of Funding and Disclosures, see page 12.
See Editorial by Kalra
References
- 1. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella‐Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carnethon MR, Pu J, Howard G, Albert MA, Anderson CAM, Bertoni AG, Mujahid MS, Palaniappan L, Taylor HA Jr, Willis M, et al.; American Heart Association Council on Epidemiology and Prevention; Council on Cardiovascular Disease in the Young; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Functional Genomics and Translational Biology; and Stroke Council . Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136:e393–e423. [DOI] [PubMed] [Google Scholar]
- 4. Rodriguez C, Pablos‐Méndez A, Palmas W, Lantigua R, Mayeux R, Berglund L. Comparison of modifiable determinants of lipids and lipoprotein levels among African‐Americans, Hispanics, and non‐Hispanic Caucasians > or =65 years of age living in New York City. Am J Cardiol. 2002;89:178–183. [DOI] [PubMed] [Google Scholar]
- 5. Frank ATH, Zhao B, Jose PO, Azar KMJ, Fortmann SP, Palaniappan LP. Racial/ethnic differences in dyslipidemia patterns. Circulation. 2014;129:570–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Goff DC Jr, Bertoni AG, Kramer H, Bonds D, Blumenthal RS, Tsai MY, Psaty BM. Dyslipidemia prevalence, treatment, and control in the Multi‐Ethnic Study of Atherosclerosis (MESA): gender, ethnicity, and coronary artery calcium. Circulation. 2006;113:647–656. [DOI] [PubMed] [Google Scholar]
- 7. Leigh JA, Alvarez M, Rodriguez CJ. Ethnic minorities and coronary heart disease: an update and future directions. Curr Atheroscler Rep. 2016;18:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giugliano RP, Sabatine MS. Are PCSK9 inhibitors the next breakthrough in the cardiovascular field? J Am Coll Cardiol. 2015;65:2638–2651. [DOI] [PubMed] [Google Scholar]
- 9. Stroes E, Robinson JG, Raal FJ, Dufour R, Sullivan D, Kassahun H, Ma Y, Wasserman SM, Koren MJ. Consistent LDL‐C response with evolocumab among patient subgroups in PROFICIO: a pooled analysis of 3146 patients from phase 3 studies. Clin Cardiol. 2018;41:1328–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, et al. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 11. Sardar MR, Badri M, Prince CT, Seltzer J, Kowey PR. Underrepresentation of women, elderly patients, and racial minorities in the randomized trials used for cardiovascular guidelines. JAMA Intern Med. 2014;174:1868–1870. [DOI] [PubMed] [Google Scholar]
- 12. Ferdinand KC, Jacobson TA, Koren A, Elassal J, Thompson D, Deedwania P. Alirocumab efficacy and safety by race and ethnicity: analysis from 3 ODYSSEY phase 3 trials. J Clin Lipidol. 2019;13:586–593.e5. [DOI] [PubMed] [Google Scholar]
- 13. Giugliano RP, Desai NR, Kohli P, Rogers WJ, Somaratne R, Huang F, Liu T, Mohanavelu S, Hoffman EB, McDonald ST, et al. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 in combination with a statin in patients with hypercholesterolaemia (LAPLACE‐TIMI 57): a randomised, placebo‐controlled, dose‐ranging, phase 2 study. Lancet. 2012;380:2007–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Robinson JG, Nedergaard BS, Rogers WJ, Fialkow J, Neutel JM, Ramstad D, Somaratne R, Legg JC, Nelson P, Scott R, et al. Effect of evolocumab or ezetimibe added to moderate‐ or high‐intensity statin therapy on LDL‐C lowering in patients with hypercholesterolemia: the LAPLACE‐2 randomized clinical trial. JAMA. 2014;311:1870–1882. [DOI] [PubMed] [Google Scholar]
- 15. Koren MJ, Scott R, Kim JB, Knusel B, Liu T, Lei L, Bolognese M, Wasserman SM. Efficacy, safety, and tolerability of a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 as monotherapy in patients with hypercholesterolaemia (MENDEL): a randomised, double‐blind, placebo‐controlled, phase 2 study. Lancet. 2012;380:1995–2006. [DOI] [PubMed] [Google Scholar]
- 16. Koren MJ, Lundqvist P, Bolognese M, Neutel JM, Monsalvo ML, Yang J, Kim JB, Scott R, Wasserman SM, Bays H, et al. Anti‐PCSK9 monotherapy for hypercholesterolemia: the MENDEL‐2 randomized, controlled phase III clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2531–2540. [DOI] [PubMed] [Google Scholar]
- 17. Hirayama A, Honarpour N, Yoshida M, Yamashita S, Huang F, Wasserman SM, Teramoto T. Effects of evolocumab (AMG 145), a monoclonal antibody to PCSK9, in hypercholesterolemic, statin‐treated Japanese patients at high cardiovascular risk–primary results from the phase 2 YUKAWA study. Circ J. 2014;78:1073–1082. [DOI] [PubMed] [Google Scholar]
- 18. Kiyosue A, Honarpour N, Kurtz C, Xue A, Wasserman SM, Hirayama A. A phase 3 study of evolocumab (AMG 145) in statin‐treated Japanese patients at high cardiovascular risk. Am J Cardiol. 2016;117:40–47. [DOI] [PubMed] [Google Scholar]
- 19. Raal F, Scott R, Somaratne R, Bridges I, Li G, Wasserman SM, Stein EA. Low‐density lipoprotein cholesterol‐lowering effects of AMG 145, a monoclonal antibody to proprotein convertase subtilisin/kexin type 9 serine protease in patients with heterozygous familial hypercholesterolemia: the Reduction of LDL‐C with PCSK9 Inhibition in Heterozygous Familial Hypercholesterolemia Disorder (RUTHERFORD) randomized trial. Circulation. 2012;126:2408–2417. [DOI] [PubMed] [Google Scholar]
- 20. Raal FJ, Stein EA, Dufour R, Turner T, Civeira F, Burgess L, Langslet G, Scott R, Olsson AG, Sullivan D, et al. PCSK9 inhibition with evolocumab (AMG 145) in heterozygous familial hypercholesterolaemia (RUTHERFORD‐2): a randomised, double‐blind, placebo‐controlled trial. Lancet. 2015;385:331–340. [DOI] [PubMed] [Google Scholar]
- 21. Stroes E, Colquhoun D, Sullivan D, Civeira F, Rosenson RS, Watts GF, Bruckert E, Cho L, Dent R, Knusel B, et al. Anti‐PCSK9 antibody effectively lowers cholesterol in patients with statin intolerance: the GAUSS‐2 randomized, placebo‐controlled phase 3 clinical trial of evolocumab. J Am Coll Cardiol. 2014;63:2541–2548. [DOI] [PubMed] [Google Scholar]
- 22. Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, Wasserman SM, Stein EA. Effect of a monoclonal antibody to PCSK9 on low‐density lipoprotein cholesterol levels in statin‐intolerant patients: the GAUSS randomized trial. JAMA. 2012;308:2497–2506. [DOI] [PubMed] [Google Scholar]
- 23. Rosenson RS, Daviglus ML, Handelsman Y, Pozzilli P, Bays H, Monsalvo ML, Elliott‐Davey M, Somaratne R, Reaven P. Efficacy and safety of evolocumab in individuals with type 2 diabetes mellitus: primary results of the randomised controlled BANTING study. Diabetologia. 2019;62:948–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lorenzatti AJ, Eliaschewitz FG, Chen Y, Lu J, Baass A, Monsalvo ML, Wang N, Hamer AW, Ge J. Randomised study of evolocumab in patients with type 2 diabetes and dyslipidaemia on background statin: primary results of the BERSON clinical trial. Diabetes Obes Metab. 2019;21:1455–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blom DJ, Hala T, Bolognese M, Lillestol MJ, Toth PD, Burgess L, Ceska R, Roth E, Koren MJ, Ballantyne CM, et al. A 52‐week placebo‐controlled trial of evolocumab in hyperlipidemia. N Engl J Med. 2014;370:1809–1819. [DOI] [PubMed] [Google Scholar]
- 26. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, et al. Efficacy and safety of evolocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1500–1509. [DOI] [PubMed] [Google Scholar]
- 27. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, Greenland P, Lackland DT, Levy D, O'Donnell CJ, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gail M, Simon R. Testing for qualitative interactions between treatment effects and patient subsets. Biometrics. 1985;41:361–372. [PubMed] [Google Scholar]
- 29. Chen Y, Yuan Z, Lu J, Eliaschewitz FG, Lorenzatti AJ, Monsalvo ML, Wang N, Hamer AW, Ge J. Randomized study of evolocumab in patients with type 2 diabetes and dyslipidaemia on background statin: pre‐specified analysis of the Chinese population from the BERSON clinical trial. Diabetes Obes Metab. 2019;21:1464–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodriguez CJ, Allison M, Daviglus ML, Isasi CR, Keller C, Leira EC, Palaniappan L, Piña IL, Ramirez SM, Rodriguez B, et al. Status of cardiovascular disease and stroke in Hispanics/Latinos in the United States: a science advisory from the American Heart Association. Circulation. 2014;130:593–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jacobson TA, Maki KC, Orringer CE, Jones PH, Kris‐Etherton P, Sikand G, La Forge R, Daniels SR, Wilson DP, Morris PB, et al. National Lipid Association recommendations for patient‐centered management of dyslipidemia: part 2. J Clin Lipidol. 2015;9(6 suppl):S1–122.e1. [DOI] [PubMed] [Google Scholar]
- 32. Rodriguez CJ, Daviglus ML, Swett K, González HM, Gallo LC, Wassertheil‐Smoller S, Giachello AL, Teng Y, Schneiderman N, Talavera GA, et al. Dyslipidemia patterns among Hispanics/Latinos of diverse background in the United States. Am J Med. 2014;127:1186–1194.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Haffner Steven M, D’Agostino R, Goff D, Howard B, Festa A, Saad Mohammed F, Mykkänen L. LDL size in African Americans, Hispanics, and non‐Hispanic Whites: the insulin resistance atherosclerosis study. Arterioscler Thromb Vasc Biol. 1999;19:2234–2240. [DOI] [PubMed] [Google Scholar]
- 34. Paramsothy P, Knopp R, Bertoni AG, Tsai MY, Rue T, Heckbert SR. Combined hyperlipidemia in relation to race/ethnicity, obesity, and insulin resistance in the Multi‐Ethnic Study of Atherosclerosis. Metabolism. 2009;58:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Orringer CE, Jacobson TA, Saseen JJ, Brown AS, Gotto AM, Ross JL, Underberg JA. Update on the use of PCSK9 inhibitors in adults: recommendations from an Expert Panel of the National Lipid Association. J Clin Lipidol. 2017;11:880–890. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S5


