Abstract
Background
The inflammation and apoptosis of podocytes contribute to the pathological progression of diabetic nephropathy. Gasdermin D (GSDMD) plays an executive role in pyroptosis, but its effect on high-glucose (HG)-induced inflammation and apoptosis remains unclear. The aim of this study was to investigate the effect of GSDMD on high-glucose-induced inflammation and apoptosis in podocytes.
Material/Methods
Mouse podocytes were cultivated by high- or normal-glucose medium. We used western blot analysis, reverse transcription-quantitative polymerase chain reaction (RT-qPCR), and immunofluorescence to detect the expression and localization of GSDMD in high-glucose-induced podocytes, and the expression of apoptosis-related proteins Bax and Bcl-2, inflammatory factors IL-1β, IL-6, and TNF-α, and JNK pathways in high-glucose-induced podocytes. Western blot and immunofluorescence were used to detect the expression and localization of synaptopodin under GSDMD knockdown and JNK-specific blocker SP600125. MitoSOX Red was used to detect the production of ROS in mitochondria under siGSDMD. The intracellular ROS generation was detected using a reactive oxygen species assay kit.
Results
We found that GSDMD knockdown and JNK inhibition reduced the expression of Bax, Bcl-2, cleaved caspase-3, IL-1β, IL-6, and TNF-α. Our results showed that GSDMD knockdown can inhibit HG-induced mitochondrial ROS production and JNK phosphorylation.
Conclusions
This study indicates that GSDMD knockdown can attenuate HG-induced inflammation and apoptosis by inhibiting the phosphorylation of JNK via mitochondrial ROS.
Keywords: Blood Glucose; Diabetes Mellitus, Type 2; JNK Mitogen-Activated Protein Kinases; Mitochondrial Diseases
Background
Diabetes is a group of metabolic diseases characterized by hyperglycemia, and it is a major health problem worldwide. According to the latest global diabetes map (Diabetes Atlas 9th Edition) released by the International Diabetes Federation (IDF) in 2019 [1], there are approximately 463 million adults age 20–79 years with diabetes. Moreover, it is estimated that by 2030, the number of diabetic patients will reach 578.4 million, and 700.2 million by 2045. Diabetic nephropathy (DN) is the most serious long-term microvascular complication of diabetes and is also one of the main causes of end-stage renal failure. Podocyte injury is an important factor of glomerulosclerosis in DN. Moreover, studies have shown that the production of ROS increases in a high-glucose (HG) environment [2] and that the mitochondria are the main source of ROS production [3].
C-Jun N-terminal kinase (JNK) is an important member of the mitogen-activated protein kinase (MAPK) family. Activation of the JNK regulatory pathways can trigger several pathophysiological processes such as cell proliferation and cell death [4]. Previous studies have shown that in rats with adenine-induced nephropathy, activation of the TGF-β1/JNK signaling pathway plays a major role in the apoptosis of renal tubular epithelial cells and renal damage [5]. The JNK pathway is usually activated in diabetic nephropathy and the long non-coding RNA (lncRNA) CASC2 can be used to accurately predict DN. Moreover, in mouse podocytes treated with high glucose, the lncRNA CASC2 expression level was decreased, while the JNK phosphorylation level was increased. lncRNA CASC2 overexpression has also been shown to inhibit the apoptosis of podocytes and reduce JNK phosphorylation levels [6]. Therefore, inhibition of the JNK pathway is currently considered a target for the treatment of DN.
Gasdermin D (GSDMD) belongs to the conserved gasdermin domain protein family [7], which includes GSDMA, GSDMB, GSDMC, GSDMD, DFNA5, and DFNB59 in humans, while mice harbor 3 GSDMAs (GSDMA1–3) and 4 GSDMCs (GSDMC1–4) [8,9]. GSDMD is the final executor of pyroptotic cell death and serves as the direct substrate of activated caspase-1 in the canonical inflammation signaling pathway and as that of caspase-11/4/5 in the noncanonical pathway [10,11]. Inflammatory caspases cleave GSDMD into a 32-kDa N-terminal domain (GSDMD-N) and a 22-kDa C-terminal domain (GSDMD-C). The GSDMD-N acts as the direct executor of pyroptosis by inducing formation of pores in the plasma membrane [12]. GSDMD also plays a significant role in chronic kidney diseases. Previous research has suggested that pyroptosis can participate in the development of DN, as high-glucose levels elevate GSDMD, GSDMD-N, and cleaved caspase-1 protein levels in renal glomerular endothelial cells [13]. However, while the molecular mechanism underlying GSDMD-mediated pyroptosis in DN is well understood, it remains unclear whether GSDMD also plays a role in other inflammatory and apoptotic processes.
In the present study, we assessed the regulatory role of mitochondrial ROS (mtROS) in GSDMD cleavage. Moreover, we further explored the role of GSDMD in inflammation and apoptosis in a high-glucose environment.
Material and Methods
Chemicals and Regents
D-glucose and the JNK inhibitor SP600125 were obtained from Sigma (St. Louis, USA). The RPMI 1640 medium was purchased from Thermo Fisher (Carlsbad, USA) and DMEM-F12 medium was obtained from Corning (Steuben County, NY, USA). Fetal bovine serum (FBS) was purchased from GIBCO Invitrogen (Carlsbad, CA, USA). IFN-γ was obtained from MedChem Express (New Jersey, USA). The antibodies against GSDMD, GSDMD-N, JNK, and p-JNK were purchased from Abcam (Cambridge, UK), while the antibody against cleaved caspase-3 was obtained from Cell Signaling Technology (Beverly, USA). The antibodies against synaptopodin, Bax, Bcl-2, SOD2, TNF-α, IL-1β, IL-6, and β-actin were purchased from Proteintech (Chicago, USA).
Cell Culture
Conditionally immortalized mouse podocytes were cultured as previously reported [14]. To induce differentiation, podocytes cells were cultured at 37°C in a 95% air/5% CO2 atmosphere without IFN-γ for 2 weeks and then were used for subsequent experiments. The podocytes were cultured in DMEM-F12 (5: 1) supplemented with normal-glucose (NG, 5.5 mM) or high-glucose (HG, 30 mM) concentrations for 6 h, 12 h, 24 h, or 48 h after being pre-treated with the JNK inhibitor SP600125 (15 μM) for 4 h.
Western Blotting
Western blotting was performed according to the standard methods. Briefly, the podocytes were lysed in RIPA lysis buffer containing 0.2% protease inhibitors (BestBio, Shanghai, China) and 1% phosphatase inhibitors (Roche, Bale, Switzerland). The BCA protein assay kit (Solarbio, Beijing, China) was used to measure the total protein concentration. The primary antibodies used were: anti-GSDMD, anti-GSDMD-N, anti-JNK, anti-phospho-JNK, anti-cleaved caspase-3, anti-Bax, anti-synaptopodin, anti-Bcl-2, anti-SOD2, and anti-β-actin. The protein bands were visualized using an ECL detection kit (Biosharp, Shenzhen, China), and detected using the Odyssey Fc System (LI-COR, USA).
Quantitative Real-time PCR (qRT-PCR)
The qRT-PCR experiments were performed according to the methods described by Zhao et al [14]. Total RNA was extracted from the samples using TRIzol reagent (Thermo Fisher, Carlsbad, USA). The cDNA was synthesized using the Reverse Transcription Kit (Thermo Fisher, Carlsbad, USA). PCR amplification was conducted on the Agilent Mx3000PQPCR System (Biosystems, USA) using SYBR Green Master Mix (Vazyme, Nanjing, China). The primers were designed and synthesized by Sangon Biotech (Shanghai, China). Primer sequences for the genes analyzed were:
-
GSDMD: 5′-CTAGCTAAGGCTCTGGAGACAA-3′ and
5′-GATTCTTTTCATCCCAGCAGTC-3′.
-
Actin: 5′-GGC TGTATTCCCCTCCAT CG-3′ and
5′-CCAGTTGGTAACAATGCCATGT-3′.
The relative expression levels were analyzed using the 2−ΔΔCT method.
Immunofluorescence Staining
Conditionally immortalized mouse podocytes were grown and stimulated for 24 h, then incubated with the primary antibodies (anti-GSDMD anti-synaptopodin). After being washed, the cells were treated with FITC-conjugated secondary antibodies for 2 h at 37°C, then the nuclei were stained with DAPI for 10 min at room temperature. The localization intensity was assessed using a fluorescence microscope (Olympus BX63, Japan).
Mitochondrial ROS Generation
The experiments were performed according to the methods described by Zhao et al [14]. mtROS production was measured using MitoSOX Red (Sigma, St. Louis, USA). After the treatment, podocytes were incubated with 5 μM MitoSOX Red at 37°C in the dark for 30 min. The fluorescent intensity was then detected using a confocal microscope (Leica, Germany).
siRNA Transfection
The day before transfection, 3–8×105 mouse podocytes were seeded on 6-well plates and were grown to 70–90% confluence. The cells were transfected with 20 μM of GSDMD siRNA or control siRNA using Lipofectamine 3000 reagents (Invitrogen, CA, USA) according to the manufacturer’s protocol. After 4–6 h of incubation in serum-free DMEM-F12 (5: 1) medium, the medium was replaced with the original medium. After siRNA transfection, the cells were treated and kept for subsequent experiments. Western blotting was used to verify whether the transfection was successful.
Statistical Analysis
All experiments were done in triplicate. Dates are expressed as the mean±standard deviation (SD). Date were analyzed using SPSS software version 23.0 (SPSS, Inc, Chicago, IL, USA). The differences among groups were analyzed for statistical significance using one-way ANOVA. Differences with P<0.05 were considered to be statistically significant.
Results
High-glucose Induces GSDMD Expression in Mouse Podocytes
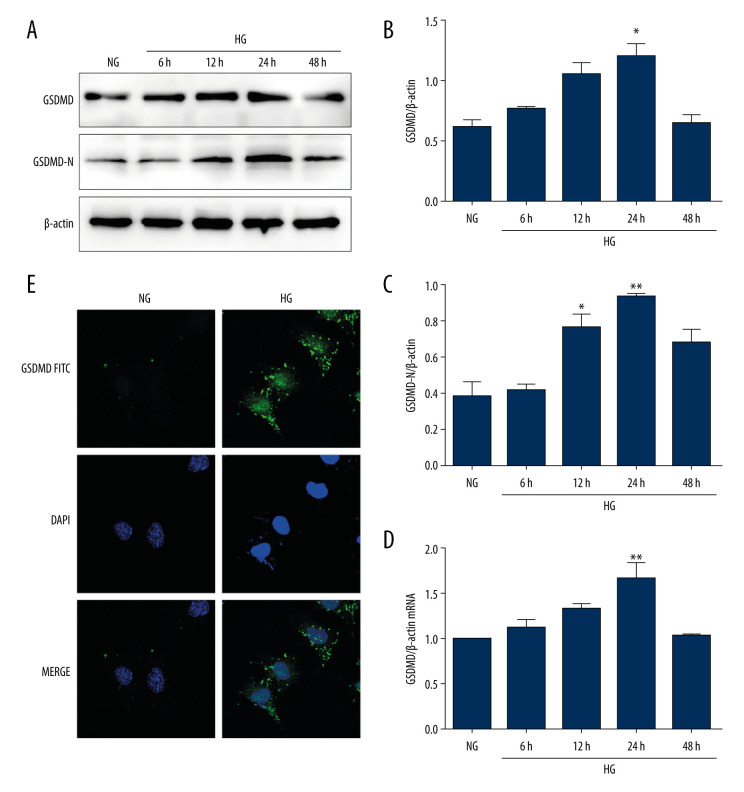
To explore the role of HG in the activation of GSDMD in vitro, mouse podocytes were cultured with HG for different time periods. The protein and mRNA levels of GSDMD and the protein level of GSDMD-N were then detected. As shown in Figure 1A–1D, GSDMD and GSDMD-N protein levels were significantly increased by HG in a time-dependent manner, with the highest levels being detected in the podocytes that were incubated with HG for 24 h. The immunofluorescence assay demonstrated that GSDMD was mainly distributed in the cytoplasm of mouse podocytes. Compared with the NG group, the GSDMD immunofluorescence intensity was found to be markedly increased in the HG group at 24 h (Figure 1E), which is consistent with the results obtained in the western blot and qRT-PCR analyses.
Figure 1.
Activation of GSDMD in HG-cultured mouse podocytes at various time points. (A–C) Western blot showing GSDMD and GSDMD-N protein expression levels in NG and HG-cultured mouse podocytes. (D) The expression of GSDMD-N mRNA was determined by RT-qPCR. (E) The expression of GSDMD in mouse podocytes was detected by immunofluorescence. NG – normal-glucose; HG – high-glucose; Data represent the average of 3 measurements. Mean±SD of 3 experiments is shown. * P<0.05, ** P<0.01 vs NG.
GSDMD Knockdown Protects Mouse Podocytes Against HG-induced Inflammation and Apoptosis
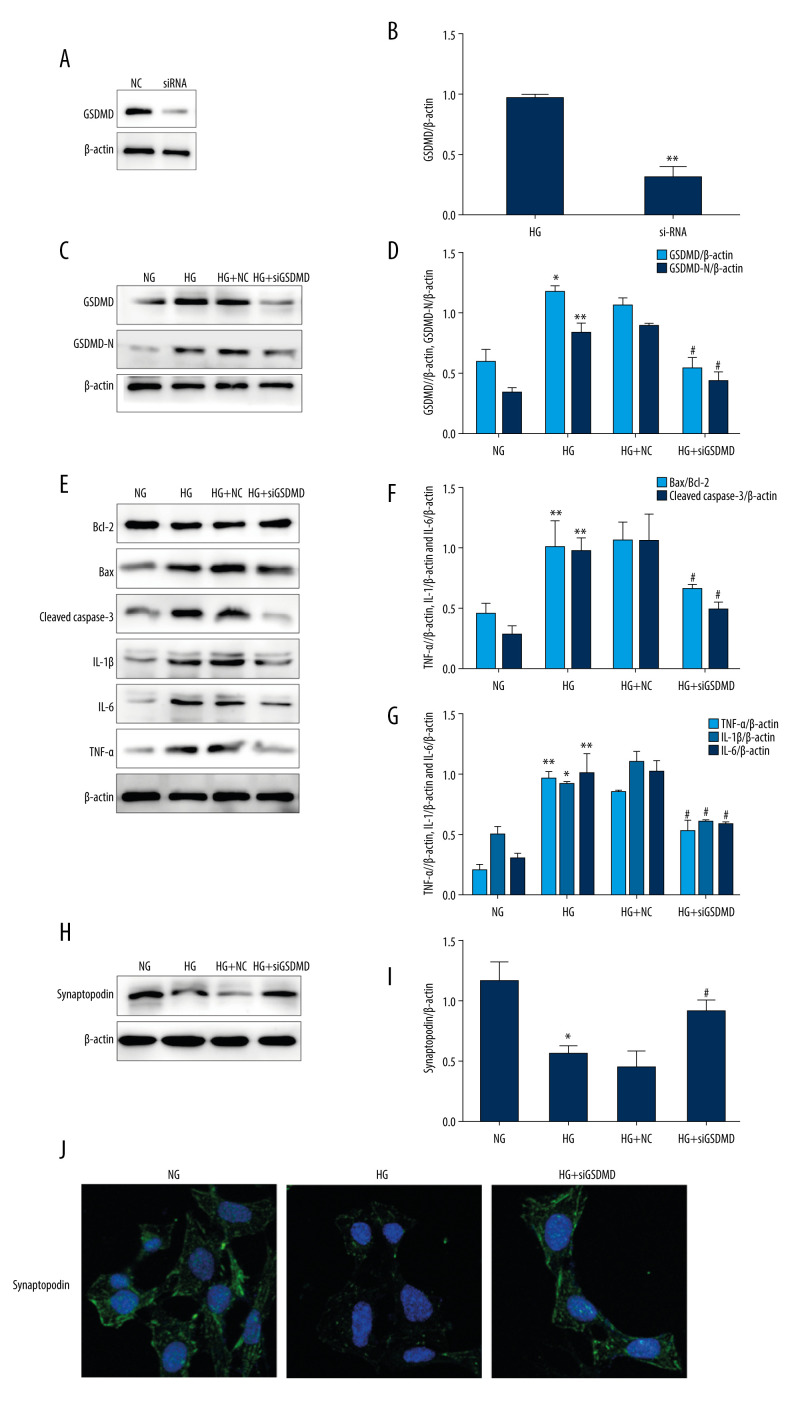
To identification the effect of GSDMD knockdown on HG-induced inflammation and apoptosis, GSDMD siRNA and NC siRNA were transfected into podocytes. Then, western blot was used to detect the protein expression. Our results showed that, compared with the NC group, siGSDMD protein expression was significantly decreased, indicating GSDMD was successfully knocked down (Figure 2A, 2B). As shown in Figure 2C and 2D, GSDMD and GSDMD-N protein levels were significantly decreased by siGSDMD in HG-cultured mouse podocytes. Moreover, the Bax/Bcl-2 ratio and the expressed level of cleaved caspase-3 were markedly increased after HG incubation compared to the NG group (Figure 2E–2G). The expression of IL-1β, IL-6, and TNF-α was also found to be higher in the HG group. Thus, our results show that the expression of these proteins was significantly decreased by transfection with siGSDMD, which indicates that siGSDMD can attenuate HG-induced inflammation and apoptosis. Synaptopodin is one of the cytoskeleton proteins found in differentiated mature podocytes, which helps maintain the functional structure of podocytes and stabilize glomerular filtration [15]. Under stimulation with HG, synaptopodin protein expression levels were found to be significantly decreased compared to the NG group. However, after siGSDMD treatment, synaptopodin expression levels were found to be significantly increased. In addition, immunofluorescence detection of synaptopodin confirmed the results of the western blot analysis (Figure 2H–2J). These findings suggest that GSDMD knockdown can protect podocytes from HG-induced injury.
Figure 2.
Effects of GSDMD gene knockout on inflammation and apoptosis in mouse podocytes induced by HG. (A–D) The expression of siGSDMD protein was detected by western blot. (E–G) The activity of Bax, Bcl-2, cleaved caspase-3, IL-1β, IL-6, and TNF-α was evaluated by western blotting. (H–J) The activities of synaptopodin in mouse podocytes was evaluated by western blotting and immunofluorescence. NC – siRNA negative control; siRNA – GSDMD siRNA (20 μM); NG – normal-glucose; HG – high-glucose; HG+NC – high-glucose+siRNA negative control; HG+siGSDMD – high-glucose (30mM)+GSDMD siRNA (20 μM). Date represent the average of 3 measurements. Mean±SD of 3 experiments is shown. * P<0.05, ** P<0.01 vs NG, # P<0.05 vs HG.
Inhibition of the JNK Pathway Alleviates HG-induced Inflammation and Apoptosis
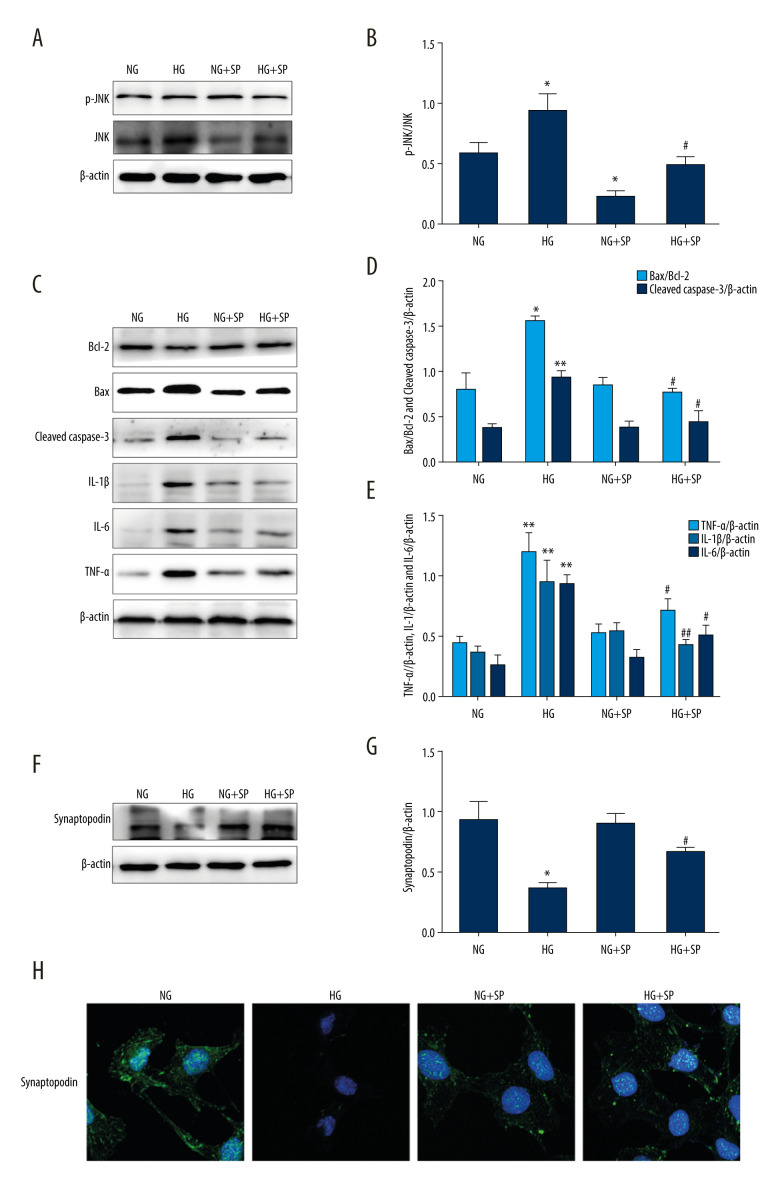
The JNK-specific inhibitor SP600125 (15 μM) was used to assess the effect of the JNK pathway on HG-induced inflammation and apoptosis. Our results showed that JNK and p-JNK protein levels were reduced in both the NG and HG groups after pretreatment with SP600125 (Figure 3A, 3B). However, the HG+SP600125 group showed a lower Bax/Bcl-2 ratio and lower cleaved caspase-3, IL-1β, IL-6, and TNF-α protein expression levels compared to the HG group, as determined by western blot analysis (Figure 3C–3E). Moreover, as shown in Figure 3F–3H, the expression of synaptopodin protein in the HG+SP600125 group was significantly higher than that in the HG group. The detection of synaptopodin using immunofluorescence and the results of the western blot analysis were consistent. Therefore, our results demonstrate that pretreatment with SP600125 significantly suppresses HG-induced inflammation and apoptosis.
Figure 3.
Effects of JNK inhibitor on inflammation and apoptosis in mouse podocytes induced by HG. (A, B) The activities of JNK and p-JNK were evaluated via western blotting. (C–E) The activities of Bax, Bcl-2, cleaved caspase-3, IL-1β, IL-6, and TNF-α were evaluated via western blotting. (F–H) The intensity of synaptopodin in mouse podocytes was detected via western blotting and immunofluorescence. NG – normal-glucose; HG – high-glucose; NG+SP – normal-glucose+SP600125; HG+SP – high-glucose+SP600125. Data represent the average of 3 measurements. Mean±SD of 3 experiments is shown. * P<0.05, ** P<0.01 vs NG, # P<0.05, ## P<0.01 vs HG.
GSDMD Knockdown Suppresses HG-induced Activation of JNK via ROS
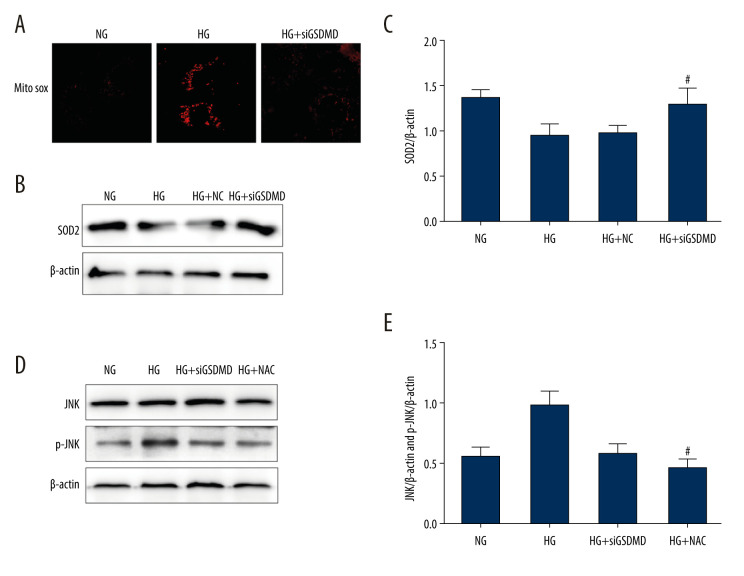
In the mitochondria, which are the major source of ROS, superoxide dismutase (SOD2) plays a critical role in maintaining the redox balance to avoid or repair oxidative damage, which can lead to dysfunction and cell death. To further explore the effect of GSDMD on the mitochondria, we assessed mtROS levels using the MitoSOX Red fluorescence probe and we measured SOD2 protein levels using western blot. Our results showed that the enhanced mtROS production and reduced expression of SOD2 induced by HG were ameliorated by siGSDMD treatment (Figure 4A–4C). N-acetylcysteine (NAC) is an antioxidant that can effectively inhibit the production of endogenous ROS. As shown in Figure 4D, 4E, we found that the expression levels of p-JNK protein in the HG+siGSDMD and HG+NAC groups were significantly lower than those in HG group. Moreover, there was no significant difference in the levels of p-JNK between the HG+siGSDMD group and HG+NAC group. On the whole, our research demonstrates that GSDMD is a key regulator of mtROS production and that GSDMD knockdown suppresses HG-induced activation of JNK via ROS.
Figure 4.
Effect of GSDMD knockdown on HG-induced mitochondrial ROS production and p-JNK expression levels in mouse podocytes. (A) Mitochondrial ROS production was measured using a confocal microscope. (B, C) SOD2 activity was evaluated by western blotting. (D, E) The protein activity of JNK and p-JNK was detected by western blotting. NG – normal-glucose; HG – high-glucose; HG+siGSDMD – high-glucose+siGSDMD; HG+SP – high-glucose+SP600125. Data represent the average of 3 measurements. Mean±SD of 3 experiments is shown. (C) * P<0.05 vs NG, # P<0.05 vs HG; (E) * P<0.05 vs HG, # P<0.05 vs HG.
Discussion
Proteinuria, including microalbuminuria, occurs during the early stages of DN, and its pathological manifestations are mainly characterized by podocyte injury [16,17]. Podocytes are terminal differentiated cells with no regeneration ability, which together with the glomerular basement membrane and endothelial cells constitute the glomerular filtration barrier. Damage to the glomerular filtration barrier is the main pathological mechanism of proteinuria. Accumulating evidence has demonstrated that overproduction of ROS is the common denominator which links the altered metabolic pathways with disrupted renal hemodynamics in DN. These pathways ultimately lead to inflammation, fibrosis, and endothelial dysfunction [18].
Our study demonstrated that GSDMD plays a critical role in the development of DN. In our study, we observed that GSDMD protein and mRNA expression levels significantly increased when mouse podocytes were treated with HG for 24 h. Moreover, the immunofluorescence staining experiments showed that activated GSDMD mainly localized to the cell membrane. Several recent studies have shown that there is crosstalk between pyroptosis and apoptosis. GSDMD is a newly defined pyroptotic executor. In addition, Gao et al found that GSDMD knockdown facilitates the intrinsic mitochondrial apoptotic pathway and attenuates tumor proliferation in non-small cell lung cancer [19]. Inflammatory responses are known to be a major component and play an important role in the pathophysiology of DN [20]. However, it is not known whether GSDMD knockdown affects the apoptosis and inflammation induced by HG in mouse podocytes. Accordingly, we transfected mouse podocytes with a GSDMD siRNA after treatment with HG and found that transfection with the GSDMD siRNA reversed HG-induced inflammation and apoptosis in mouse podocytes. Synaptopodin is an important protein marker of podocytes and can be used to determine the structural integrity of podocytes. After being treated with siGSDMD, synaptopodin protein expression levels were shown to increase significantly in an HG environment. Moreover, the HG-induced podocyte damage was found to be alleviated by siGSDMD treatment. Thus, we speculated that a mechanism of endogenous activation may be involved in this process.
JNK is a stress-activated protein kinase that induces transactivation by phosphorylating the N-terminal Ser-63 and Ser-73 residues factors [3]. The JNK pathway is considered to be an important regulatory pathway for cell apoptosis. as it regulates members of the Bcl-2 family [21,22]. JNK can inactivate anti-apoptotic Bcl-2 proteins and activate the mitochondrial translocation of Bax [23]. SP600125, a JNK inhibitor, downregulates the expression of p-JNK, Bax, and cleaved caspase-3, thereby blocking HG-induced apoptosis. The JNK pathway plays a crucial role in the molecular mechanism underlying cardiac pathological processes initiated by inflammation and oxidative stress [24,25]. In lipopolysaccharide (LPS)-stimulated mouse neurons, the suppression of JNK activation can improve inflammatory responses, while the release of inflammatory markers such as IL-1β, TNF-α, and IL-6 has been found to be significantly reduced [26]. Our results suggest that the HG-induced inflammatory response was alleviated by blocking JNK activation, while the protein expression of IL-1β, TNF-α, and IL-6 was decreased. Several studies have revealed that ROS, which play a key role in the pathogenesis of DN, are mainly derived from mitochondrial dysfunction [27,28]. Previous studies have also found that the insertion of GSDMD-N into the mitochondrial membrane drives mitoROS production [29]. Our findings confirmed that mitoROS production is triggered by GSDMD and further showed that GSDMD knockdown enhances the antioxidant activity. Incubation with NAC was also shown to inhibit HG-induced ROS production and reduced p-JNK the protein expression levels. Thus, treatment with siGSDMD protects against HG-induced inflammation and apoptosis by blocking the JNK signaling pathway.
Conclusions
Collectively, the results of our study revealed a novel mechanism through which GSDMD drives mitoROS production upstream of the JNK pathway. Our results provide new understanding of GSDMD in regulating HG-induced inflammation and apoptosis. Moreover, we demonstrated that the siGSDMD treatment markedly repressed inflammation and apoptosis, an effect which was largely dependent on blockage of the JNK pathway. The present study enhances our knowledge regarding the molecular mechanisms underlying inflammation and apoptosis and illustrates the important role of GSDMD in DN, and it might be a strong candidate for the development of novel DN treatments.
Acknowledgments
The authors are thankful for assistance from the Department of Pathology of Hebei Medical University (Shijiazhuang, China).
Footnotes
Source of support: This research was supported by the National Natural Science Foundation of China (grant no. 81770717)
References
- 1.Yuen L, Saeedi P, Riaz M, et al. Projections of the prevalence of hyperglycaemia in pregnancy in 2019 and beyond: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res Clin Pract. 2019;157:107841. doi: 10.1016/j.diabres.2019.107841. [DOI] [PubMed] [Google Scholar]
- 2.Forbes JM, Coughlan MT, Cooper ME. Oxidative stress as a major culprit in kidney disease in diabetes. Diabetes. 2008;57(6):1446–54. doi: 10.2337/db08-0057. [DOI] [PubMed] [Google Scholar]
- 3.Addabbo F, Montagnani M. Mitochondria and reactive oxygen species. Hypertension. 2009;53(6):885–93. doi: 10.1161/HYPERTENSIONAHA.109.130054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dhanasekaran DN, Reddy EP. JNK-signaling. A multiplexing hub in programmed cell death. Genes Cancer. 2017;8(9–10):682–94. doi: 10.18632/genesandcancer.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tu Y, Sun W, Wan YG, et al. Dahuang Fuzi Decoction ameliorates tubular epithelial apoptosis and renal damage via inhibiting TGF-β1-JNK signaling pathway activation in vivo. J Ethnopharmacol. 2014;156:115–24. doi: 10.1016/j.jep.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 6.Yang H, Kan QE, Su Y, Man H. Long non-coding RNA CASC2 improves diabetic nephropathy by inhibiting JNK pathway. Exp Clin Endocrinol Diabetes. 2019;127(8):533–37. doi: 10.1055/a-0629-9958. [DOI] [PubMed] [Google Scholar]
- 7.Kayagaki N, Warming S, Lamkanfi M, et al. Non-canonical inflammasome activation targets caspase-11. Nature. 2011;479(7371):117–21. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 8.Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526(7575):660–65. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 9.Shi J, Gao W, Shao F. Pyroptosis: Gasdermin-mediated programmed necrotic cell death. Trends Biochem Sci. 2017;42(4):245–54. doi: 10.1016/j.tibs.2016.10.004. [DOI] [PubMed] [Google Scholar]
- 10.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526(7575):666–71. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 11.Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535(7610):153–58. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen X, He WT, Hu L, et al. Pyroptosis is driven by non-selective gasdermin-D pore and its morphology is different from MLKL channel-mediated necroptosis. Cell Res. 2016;26(9):1007–20. doi: 10.1038/cr.2016.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu J, Huang W, Zhang W, et al. Sodium butyrate alleviates high-glucose-induced renal glomerular endothelial cells damage via inhibiting pyroptosis. Int Immunopharmaco. 2019;75:105832. doi: 10.1016/j.intimp.2019.105832. [DOI] [PubMed] [Google Scholar]
- 14.Zhao K, Li Y, Wang Z, et al. Carnosine protects mouse podocytes from high glucose induced apoptosis through PI3K/AKT and Nrf2 pathways. Biomed Res Int. 2019;2019 doi: 10.1155/2019/4348973. 4348973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yu H, Kistler A, Faridi MH, et al. Synaptopodin Limits TRPC6 podocyte surface expression and attenuates proteinuria. J Am Soc Nephrol. 2016;27(11):3308–19. doi: 10.1681/ASN.2015080896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Izumi Y, Kawahara K, Nonoguchi H. Combined angiotensin inhibition in diabetic nephropathy. N Engl J Med. 2014;370(8):777–78. doi: 10.1056/NEJMc1315504. [DOI] [PubMed] [Google Scholar]
- 17.Si X, Li P, Zhang Y, et al. Renoprotective effects of olmesartan medoxomil on diabetic nephropathy in streptozotocin-induced diabetes in rats. Biomed Rep. 2014;2(1):24–28. doi: 10.3892/br.2013.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jha JC, Banal C, Chow BS, et al. Diabetes and kidney disease: Role of oxidative stress. Antioxid Redox Signal. 2016;25(12):657–84. doi: 10.1089/ars.2016.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao J, Qiu X, Xi G, et al. Downregulation of GSDMD attenuates tumor proliferation via the intrinsic mitochondrial apoptotic pathway and inhibition of EGFR/Akt signaling and predicts a good prognosis in non small cell lung cancer. Oncol Rep. 2018;40(4):1971–84. doi: 10.3892/or.2018.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yaribeygi H, Atkin SL, Sahebkar A. Interleukin-18 and diabetic nephropathy: A review. J Cell Physiol. 2019;234(5):5674–82. doi: 10.1002/jcp.27427. [DOI] [PubMed] [Google Scholar]
- 21.Liu GY, An ZM. [Protective effect of rosiglitazone sodium on islet beta-cell of STZ induced diabetic rats through JNK pathway]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2009;40(3):430–34. [in Chinese] [PubMed] [Google Scholar]
- 22.Lee HJ, Wang CJ, Kuo HC, et al. Induction apoptosis of luteolin in human hepatoma HepG2 cells involving mitochondria translocation of Bax/Bak and activation of JNK. Toxicol Appl Pharmacol. 2005;203(2):124–31. doi: 10.1016/j.taap.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Li Q, Cheng X, et al. Oligomannuronate prevents mitochondrial dysfunction induced by IAPP in RINm5F islet cells by inhibition of JNK activation and cell apoptosis. Chin Med. 2020;15:27. doi: 10.1186/s13020-020-00310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Drosatos K, Drosatos-Tampakaki Z, Khan R, et al. Inhibition of c-Jun-N-terminal kinase increases cardiac peroxisome proliferator-activated receptor alpha expression and fatty acid oxidation and prevents lipopolysaccharide-induced heart dysfunction. J Biol Chem. 2011;286(42):36331–39. doi: 10.1074/jbc.M111.272146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feng L, Ning R, Liu J, et al. Silica nanoparticles induce JNK-mediated inflammation and myocardial contractile dysfunction. J Hazard Mater. 2020;391:122206. doi: 10.1016/j.jhazmat.2020.122206. [DOI] [PubMed] [Google Scholar]
- 26.Wang JL, Ren CH, Feng J, et al. Oleanolic acid inhibits mouse spinal cord injury through suppressing inflammation and apoptosis via the blockage of p38 and JNK MAPKs. Biomed Pharmacother. 2020;123:109752. doi: 10.1016/j.biopha.2019.109752. [DOI] [PubMed] [Google Scholar]
- 27.Yang S, Han Y, Liu J, et al. Mitochondria: A novel therapeutic target in diabetic nephropathy. Curr Med Chem. 2017;24(29):3185–202. doi: 10.2174/0929867324666170509121003. [DOI] [PubMed] [Google Scholar]
- 28.Han Y, Xu X, Tang C, et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis [published correction appears in Redox Biol, 2019;24:101216] Redox Biol. 2018;16:32–46. doi: 10.1016/j.redox.2018.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platnich JM, Chung H, Lau A, et al. Shiga toxin/lipopolysaccharide activates caspase-4 and gasdermin D to trigger mitochondrial reactive oxygen species upstream of the NLRP3 inflammasome. Cell Rep. 2018;25(6):1525–36. doi: 10.1016/j.celrep.2018.09.071. [DOI] [PubMed] [Google Scholar]