Abstract
Since the commercialization of the first liposomes used for drug delivery, Doxil/Caelyx® and Myocet®, tremendous progress has been made in understanding interactions between nanomedicines and biological systems. Fundamental work at the interface of engineering and medicine has allowed nanomedicines to deliver therapeutic small molecules and nucleic acids more efficiently. While nanomedicines are used in oncology for immunotherapy or to deliver combinations of cytotoxics, the clinical successes of gene silencing approaches like patisiran lipid complexes (Onpattro®) have paved the way for a variety of therapies beyond cancer. In parallel, the global severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic has highlighted the potential of mRNA vaccines to develop immunization strategies at unprecedented speed. To rationally design therapeutic and vaccines, chemists, materials scientists, and drug delivery experts need to better understand how nanotechnologies interact with the immune system. This review presents a comprehensive overview of the innate and adaptative immune systems and emphasizes the intricate mechanisms through which nanomedicines interact with these biological functions.
KEY WORDS: Cancer immunotherapy, mRNA vaccine, Complement activation, Macrophage, In vivo clearance, Anti-PEG antibody, Nanoparticle, mRNA-1273, BNT162b2, Immunology
Graphical abstract
Upon administration, nanomedicines interact with the innate and adaptive immune systems in intricate ways, these interactions will impact their pharmacology and tolerability.
1. Introduction
Nanomedicines share physicochemical characteristics with pathogens: dimensions which are a fraction of the cell diameter, significant liquid–solid interfaces, and patterned surfaces1. Some mechanisms involved in the protection against microbes are therefore also implicated in the recognition of nanomedicines. However, all biological processes protecting against microbial colonization might not equally affect the fate of nanomedicines. While bacteria and viruses have the biological machinery necessary for proliferation, therapeutics are administered at a finite dose; some processes inhibiting the replication of microbes might not affect the clearance of nanomedicines.
The implications of individual biological responses might also be different: while immunological memory against pathogens help protect against infections, immune reactions toward therapeutic nanomedicines can impede their therapeutic effect or trigger adverse reactions. In many countries struggling with the pandemic of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), mRNA vaccines have drawn of a lot of attention. The shape, size, and chemical compositions of these vaccines strongly resemble delivery systems destined to treat cancer or genetic diseases. In light of the physicochemical similarities between vaccines and therapeutic nanomedicines, it seems timely to revise current understanding on the interactions between nano-sized materials and the immune system.
This work offers a perspective of the components of the immune systems and recapitulates how they interact with nanomedicines. The following sections will address innate immunity, the links between the innate and adaptive responses, and the adaptive immune system. Highlighting the intricate interactions between nanomedicines and each of these components will help scientists design more efficient and better tolerated nanotechnologies to treat and prevent human diseases.
2. The immune system
The innate and adaptive immune systems work in orchestrated ways to achieve two distinct purposes: clearing senescent cells and protecting against invading pathogens2. The former involves efficient removal of the millions of cells which undergo apoptosis every day. This physiological process must therefore be carefully regulated to avoid disproportionate inflammatory responses3. The second function aims at maintaining homeostasis against a variety of microorganisms which have evolved to infect mammals: viruses, bacteria, fungi, and parasites. To overcome very quick replication and possible resistance mechanisms, the control of pathogens involves amplification of biological cues, crosstalk between cells and redundant defense functions. Untamed, this response can sustain inflammation and have deleterious effects on the host.
The innate immune response is a series of biological processes involving proteins and phagocytic cells that occur without much specificity, when a naïve organism is exposed to a pathogen for the first time. Components of the innate immune systems have been conserved throughout evolution or are the result of early interactions of mammals with commensal flora4. The innate immune system can rapidly recognize certain molecular patterns shared by pathogens and eliminate them through sequestration in phagocytes. The production of cytokines, i.e., biological mediators that can trigger the death of infected cells and prime for a more sustained response, can further prevent the installation of infection. Examples of cytokines produced by the innate immune system are interferons, interleukins, and some fragments of the complement cascade5.
The adaptive immune system allows a second wave of defense and prepares for future contacts with the pathogens. This delayed reaction results in both the cellular and the humoral responses, that is the development of mature immune cells and antibodies able to specifically recognize certain antigens on the pathogens6. In time, these specialized cells and proteins will allow a quicker and more specific reaction. Importantly, a fraction of the clones of lymphocytes produced during the adaptive response may remain after an infection, creating an immunological memory. This memory, directed against proteins, glycoproteins, and polysaccharides on the surface of the infecting pathogen, can protect the host against other microorganisms sharing structural similarities.
2.1. Cell components of the immune system
The cells of the immune system are called leukocytes or white blood cells. These cells can be separated based on their origin, their histological appearance, and their surface markers (Fig. 1). Like all blood cells, leukocytes originate from hematopoietic stem cells in the bone marrow and are derived from two progenitor lines: myeloid and lymphoid cells. Myeloid cells mature mostly into innate immune cells: monocytes, dendritic cells, mast cells, and granulocytes. The latter include neutrophils, eosinophils, and basophils. In contrast, lymphoid cells are the progenitors of natural killer cells (part of the innate immune system) and the B and T lymphocytes, which constitute the adaptive immune system. After a first stage of maturation in the bone marrow, leukocytes migrate to the blood, the lymphatic system, and tissues, where they further differentiate6.
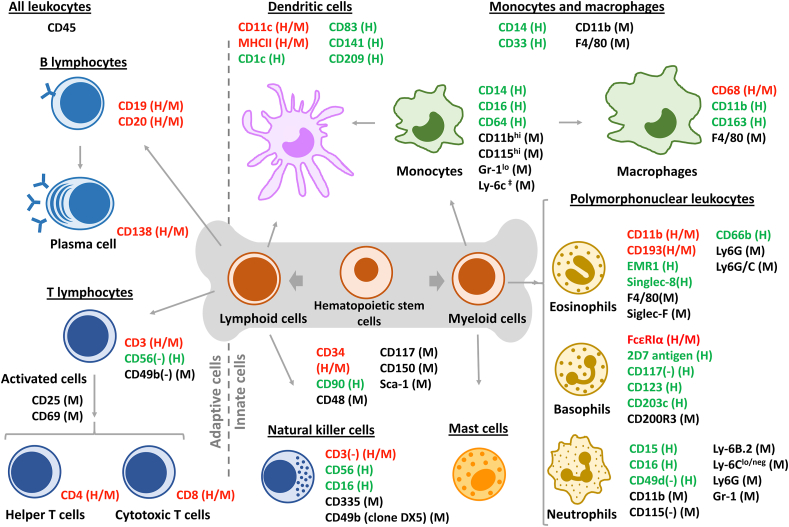
Figure 1.
Leukocytes originate from hematopoietic stem cells in the bone marrow. Stem cells give rise to lymphoid and myeloid cells, which are common progenitors of leukocytes and other blood cells. Monocytes (the precursor of macrophages), mast cells and granulocytes (basophils, neutrophils, and eosinophils) originate from myeloid cells. Lymphoid cells produce B- and T-lymphocytes and natural killer cells. Dendritic cells can originate from monocytes or lymphoid precursors. All leukocytes exhibit the CD45 protein on their surface. Common receptors for each cell type in mice (M) and humans (H) are presented in red (both M/H), green (only H) or black (only M). According to their main function in the immune system, leukocytes can be subdivided in adaptive cells (left) or innate cells (right).
Histologically, the different families of blood cells can also be separated based on the structure of their nuclei. Mature red blood cells and platelets are anuclear. Monocytes, macrophages, mast cells, and lymphocytes have spherical nuclei (i.e., mononuclear) and are distinct from polymorphonuclear cells, also known as granulocytes (i.e., neutrophils, eosinophils, and basophils). The lower density of mononuclear cells allows isolation from the blood by centrifugation, and peripheral blood mononucleated cells (PBMCs) are easily accessible to study the interactions of nanomedicines with immune cells7.
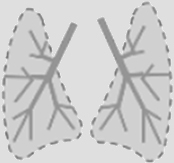
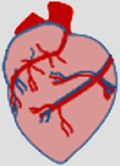
Leukocytes all express a common surface marker, the CD45 surface protein. CD45 is a large protein (180–220 kDa) which plays a key role in regulating immune functions via its phosphatase activity, notably the activation of the T- and B-cell receptors. Natural ligands of CD45 include placental protein 14, lectins (CD22, galectin-1 and -3) and pUL11, a protein found on the cytomegalovirus (CMV)8. Leukocytes are distributed differently among organs and tissues, which contributes to their particular immune functions (Table 1).
Table 1.
Approximative distribution of leukocytes in mouse organs and human blood.
| Cell type |
 |
 |
 |
 |
 |
 |
 |
 |
 |
|
|---|---|---|---|---|---|---|---|---|---|---|
| Lung | Heart | Liver | Spleen | Small intestine | Kidney | Eye | Skin | Human blood | ||
 |
T cells | 31% | 9% | 17% | 38%–42% | 34% | 20% | 8% | 40% | 15%–34% |
 |
B cells | 23% | 10% | 11% | 38%–42% | 30% | 5% | 4% | – | 2%–10% |
 |
NK cells | 6% | 2% | 2% | – | – | 1% | 2% | – | 2% |
 |
Neutrophils | 12% | 7% | 10% | 15% | 1% | 3% | 5% | 0.6% | 45%–75% |
 |
Eosinophils | 2% | 0.7% | 0.7% | 0.7% | 2% | 0.7% | – | 4% | 1%–7% |
 |
Basophils | – | – | – | – | – | – | – | – | 1% |
 |
Mast cells | – | – | – | – | – | – | – | 13% | – |
 |
Dendritic cells | 5% | 1.5% | 3% | 2% | 3% | 4% | 5% | 20%a | – |
 |
Monocytes | 2% | 2% | 2% | – | 1% | 3% | 4% | 0.6% | 4%–10% |
 |
Macrophages | 19% | 62% | 54%b | 1% | 28% | 63% | 71%c | 22% | – |
 |
Blast cells | – | – | – | 4% | – | – | – | – | – |
Includes Langerhans cells.
also known as Kupffer cells.
Includes Microglia.
3. The innate immunity
3.1. Cellular functions in the innate immunity
To clear microbes, cells of the innate immune system share general functions which can be exerted alone or in collaboration with opsonins, i.e., soluble proteins acting as biological ‘flags’ driving cellular responses (see below).
3.1.1. Phagocytosis
Phagocytosis is responsible for the sequestration of pathogens as well as the removal of senescent cells from tissues. It consists of the actin-dependent engulfment of microbes or debris inside a phagocyte, usually without the involvement of clathrin9. Neutrophils, macrophages, and dendritic cells are called “professional phagocytes”, but other cell types, like fibroblasts and endothelial cells, can also participate in the clearance of apoptotic bodies10.
Phagocytosis involves 1) recognition of the microbe/particle, 2) internalization, and 3) maturation of the phagosome. Multiple successive events initiate phagocytosis: the engagement of extracellular receptors triggers their clustering on the cell membrane and intracellular phosphorylation events which induce actin polymerization and the remodeling of the cell cytoskeleton9,10. These events culminate in the wrapping of the phagocyte membrane around the target and its internalization in an intracellular vesicle. The maturation of the phagosome which follows serves two distinct functions: the degradation of the internalized pathogen and the sensing of its composition to drive additional responses, if needed.
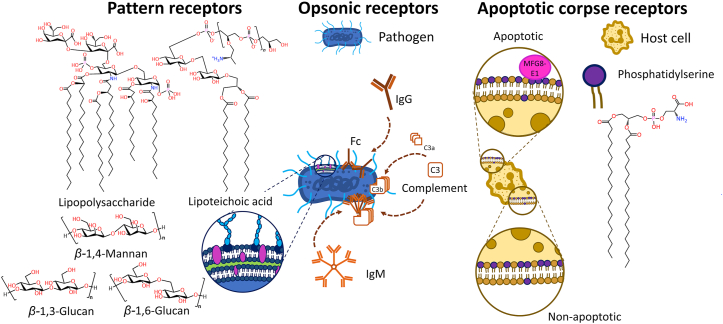
Phagocytes have a variety of cellular receptors that allow the detection and engagement of particles in their environment10. In mammals, these receptors can be separated in three different classes: pattern-recognition receptors, opsonic receptors, and apoptotic corpse receptors (Fig. 2). Pattern-recognition receptors are a group of receptors able to recognize common chemical characteristics conserved by microbes. Examples of ligands include various components of the wall of bacteria and fungi like lipopolysaccharides, lipoteichoic acid, and various β-glucans11. In contrast, opsonic receptors engage pathogens following conformational changes in endogenous fluid-phase proteins. For example, Fc receptors (FcR) recognize the Fc fragments of immunoglobulins when they are patterned on immune complexes. The FcRs expressed on phagocytes have distinct affinity for the different isotypes of immunoglobulins. Other important ligands of opsonic receptors are proteins of the complement system which will be described in more details below. Finally, apoptotic corpse receptors detect conformational changes in the phospholipid membrane of apoptotic cells. During apoptosis, phosphatidylserine relocates from the cytoplasmic leaflet to face the extracellular environment, increasing the concentration of this phospholipid by ∼300-fold in the outer monolayer of the cell membrane12. Macrophages can bind phosphatidylserine directly or via the involvement of soluble proteins (e.g., MFG8-E1, Gas6, or protein S)10.
Figure 2.
Phagocytes have different types of receptors enabling phagocytosis. Pattern receptors directly recognize molecules on the surface of pathogens. Opsonic receptors recognize changes in conformation of soluble proteins when the latter bind to a pathogen. Apoptotic corpse receptors recognize extracellular exposure of phosphatidylserine on the surface of dying cells.
Recruitment of V-ATPases from the cytosol to the phagosome membrane drives gradual acidification of the lumen via the pumping of H+ and Cl− ions10. Acidification to a pH of 4.5–5.0 restricts bacterial growth, facilitates hydrolysis, and regulates the functions of proteolytic proteins. The NOX2 enzyme consumes protons from the lumen to form reactive oxygen species (ROS) and superoxide anions able to further degrade pathogens. Myeloperoxidase also uses hydrogen peroxide (H2O2) and chloride ions to form the strong oxidizer hypochlorous acid (HOCl)13. Finally, cytosolic vesicles fuse with the phagosome to deliver antimicrobial peptides and proteins. These molecules interfere with functions of the pathogen by restricting access to essential metal cofactors: for example, lactoferrin binds ferric ions (Fe3+) and the natural resistance-associated macrophage protein 1 (NRAMP-1) binds Zn2+ and Mn2+. The maturation of the phagosome into the phagolysosome also implicates proteins with direct hydrolase activities: lysozyme can degrade β1–4 glycosidic bonds, while different pH-dependent cathepsins cleave peptide bonds, at various stages of phagosome maturation10.
Strictly speaking, phagocytosis describes the internalization of particles with diameters above 0.5 μm9,10. In rat alveolar macrophages, cultured in vitro in the presence of polystyrene beads (diameters between 1 and 9 μm), phagocytosis was found to be maximal for particles with a diameter of 2–3 μm, irrespective of opsonization14. Another in vitro study suggests that murine bone marrow macrophages can eventually ingest IgG-opsonized particles with diameters ca. 20 μm, over a period of 60 min15. Beyond this size, or if particles present an elongated aspect-ratio and inadequate orientation16, the spreading of the membrane on the particle can occur and drive frustrated phagocytosis15,16.
3.1.2. Molecular sensing
Pattern-recognition receptors on the cell surface, like C-type lectin receptors (CLRs) and Toll-like receptors (TLRs), participate in the internalization of pathogens, but other pattern-recognition receptors, like RIG1-like receptors and NOD-like receptors, are also distributed intracellularly2. Together these receptors bind molecular patterns associated with pathogens or tissue damage, respectively named pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs)2. Activation of the receptors induces as series of signaling events and the production of cytokines that affect the phagocyte and surrounding cells.
In innate immune cells, TLRs are particularly important because they participate both in extracellular and intracellular sensing of pathogens. TLRs are a family of transmembrane proteins highly conserved between species. Until now, 10 human and 12 murine TLRs have been identified17. TLR1, 2, 4, 5, 6, and 11 are found on the plasma membrane. In collaboration with coreceptors, they bind lipopolysaccharides (LPS), lipopeptides, peptidoglycans, and bacterial flagella by hydrophobic and electrostatic interactions. The binding of LPS to TLR4 and its co-receptor MD2 induces internalization17. Intracellular TLRs (3, 7, 8, and 9) are expressed in endosomes, lysosomes, and the endoplasmic reticulum, and can be recruited to the phagosome to sense its content. Examples of molecules that can be sensed by intracellular TLRs are single and double-stranded RNA, as well as CpG DNA motifs. These molecules are ligands for TLR7/8 (sRNA), TLR3 (dsRNA), and TLR9 (DNA). Activation of TLRs by their ligand induces cascades of signaling events which can translate into a type 1 interferon response or the production of inflammatory cytokines [interleukin-1β (IL-1β), IL-6, IL-12, IL-18, and tumor necrosis factor-alpha (TNFα)]17. These cytokines will impact the polarization of surrounding cells (see below).
3.1.3. Extracellular degranulation
A third function shared by some innate immune cells is the ability to secrete, in the extracellular fluid, cytotoxic and antibacterial molecules similar to those found in the phagosome18. This additional protection mechanism can prevent the replication of pathogens by damaging them in situ and interfering with some of their metabolic functions. Due to the presence of intracellular granules, mast cells, and granulocytes (neutrophils, eosinophils, and basophils) are well equipped for these secretory functions10. Frustrated phagocytosis or direct activation of cellular receptors by ligands coming from the pathogen can trigger extracellular degranulation. Upon release, the content of these granules, which also include cytokines, acts as biological cues for surrounding cells. For example, the granules of eosinophils contain both pro- and anti-inflammatory cytokines, as well as IL-5, chemokines, and growth factors which will influence chemotaxis and immune responses19,20.
Degranulation can originate from the total lysis of the cell (i.e., cytolytic degranulation) or from “piecemeal” degranulation, a process that maintains viability and ulterior functions20. One type of cytolytic degranulation is the production of extracellular traps by neutrophils21 and eosinophils19. These structures consist of entanglements of DNA and bactericidal proteins which are expelled from the cell by the disruption of the plasma membrane. These structures physically entangle pathogens and act as strong DAMPs that can be sensed by surrounding cells, they appear to have protective functions in sepsis22, but might also be implicated in disease23.
3.1.4. Cytotoxic activity
Finally, a subset of innate cells from lymphoid origin, natural killer lymphocytes (NK cells), also help to maintain homeostasis24. NK lymphocytes can trigger apoptosis by directly discharging bactericidal molecules to the cytoplasm of cells expressing distress signals. This process shares similarities with the activity of cytotoxic CD8+ lymphocytes discussed below, notably the need for a cell–cell synapse and the involvement of adapter proteins. An important distinction between NK cells and cytotoxic lymphocytes is that the formers do not require maturation to engage pathogens. This allows rapid control of proliferation in infected cells without the need for clonal selection and expansion. NK cells also have a role in the immunosurveillance against the spontaneous development of cancer25.
Receptors on the surface of NK cells stem from germline encoded genes, in contrast to receptors from B and T cells which originate from somatic recombination (see below). Another distinction is that, although clones of B and T lymphocytes each express one single antigen-specific receptor, NK cells possess random amounts of multiple receptors26. This ensures phenotypic diversity despite a limited repertoire24.
The effector functions of NK cells are closely regulated by adaptor proteins and activating or inhibitory receptors. An important activating receptor is FcRγRIIIA (CD16) which binds to the Fc portion of IgG immune complexes to trigger antibody-dependent cellular cytotoxicity (ADCC)25. This receptor, which bridges innate immunity and the presence of antigen-specific antibodies, is implicated in the efficacy of some therapeutic monoclonal antibodies27. NK cells can also be activated in the absence of immunoglobulins, via natural cytotoxicity receptors28. These receptors bind diverse ligands with activating or suppressing functions, including components of the extracellular matrix, proteins upregulated in cancer and viral infections, proteins of the complement, lectins (e.g., galectin-3) and growth factors28.
Finally, like other immune cells, NK cells are impacted by surrounding cytokines. The presence of IL-15, IL-12, and IL-18, secreted by proximal macrophages or dendritic cells, appears necessary to fully prime NK cell functions25. Accordingly, NK cells also affect their environment via the production of pro-inflammatory interferon-γ (IFN-γ) and TNFα, as well as anti-inflammatory IL-1029. Importantly, the lysis of target cells by NK cells promotes the release of free antigens which can be captured and presented by dendritic cells, eventually playing a role in the adaptive immune response25.
3.2. Interactions of nanomedicines with innate immune cells
Even if the diameter of nanomaterials is below the range where phagocytosis is the most efficient, interactions of nanomedicines with monocytes and macrophages remains an important factor governing their biological fate29. Seminal studies in the 1990s showed that steric protection on the surface of liposomes30,31 and polymer nanoparticles32 decreased unwanted distribution to the liver and the spleen, and prompted enhanced blood circulation times in rodents. This spurred interest in studying the in vitro interactions of nanoparticles with monocytes33,34 and macrophages35, and eventually their distribution in the organs of the mononuclear phagocyte system (MPS) in vivo.
Walkey et al.35 evaluated the uptake of gold nanoparticles with different poly(ethylene glycol) (PEG) densities in cultures of reticulum cell sarcoma (J774A.1 cells, a cancerous cell line resembling macrophages). After 4 h of incubation in vitro, they reached three conclusions: 1) the cellular uptake of nanoparticles with no/low PEGylation (<32 PEG chains per 100 nm2) was higher than that of nanoparticles with denser steric protection, 2) the uptake of larger particles (60 and 90 nm in diameter) was more efficient than that of smaller colloids (15 and 30 nm), and 3) cellular uptake became independent of the presence of surface proteins as the density of PEG on nanoparticles increased. Looking at human neutrophils isolated from peripheral blood, Bisso and colleagues36 similarly confirmed that in vitro internalization increased with larger diameters until ca. 200 nm, and that protein adsorption could increase or decrease uptake based on the nanoparticle characteristics. Interestingly, in other in vitro settings, incubation of neutrophils with gold nanorods37, cationic silica nanostructures38, inorganic calcium phosphate nanoparticles39, or cationic lipid nanoparticles40 triggered the rapid degranulation of extracellular traps. The relevance of extracellular traps on the in vivo fate of nanoparticles remains unclear.
In vivo, the involvement of the MPS on the clearance of nanomedicines was evidenced notably by studying the impact of the dose on circulation times41,42. For nanoparticles with no steric protection (i.e., high intrinsic clearance), independent studies showed that augmenting the injected dose resulted in non-linear increase in blood exposure41, 42, 43, 44. This phenomenon was attributed to the limited quantity of opsonins available in the bloodstream41 or to the saturation of liver Kupffer cells and other phagocytes42, 43, 44. Interestingly, for nanoparticles which have lower affinity for the MPS due to their steric protection (i.e., PEGylated), increasing the dose within a 100-fold range did not prolong circulation times42,43. Recently, our group confirmed that a threshold of approximately 20 PEG chains per 100 nm2 might be necessary to prevent early clearance of polymer nanoparticles by the MPS, but that higher PEGylation densities did not necessarily translate into higher blood exposure45.
Various groups have highlighted the importance of circumventing distribution to the MPS to increase the efficacy of therapeutic nanomedicines. For example, the injection of large liposomes containing clodronate can efficiently deplete Kupffer cells in the liver46. This model was used to increase the circulation times and tumor distribution of PEGylated doxorubicin liposomes and other types of nanoparticles47,48. Although this strategy can result in increased therapeutic efficacy47, its clinical relevance remains questionable as it might also make animals (and potentially humans) more susceptible to bacterial infections47. Interestingly, in both aforementioned studies, the depletion of Kupffer cells resulted in increased splenic distribution47,48. This supports the role of the spleen to sieve colloids that are not efficiently retained by the liver49.
Decoy colloids can also be used to partially bypass the MPS. For example, the tumor distribution and efficacy of small PEGylated nanoparticles was significantly increased when mice were pre-dosed with high quantities of non-PEGylated liposomes (375 mg/kg), 1.5 h before treatment50. Likewise, in a thorough and elegant study, Nikitin and colleagues51 proposed that pre-dosing rodents with allogeneic anti-erythrocytes IgG2a 12 h before the injection of nanomedicines could prolong the circulation times of various colloidal systems, including PEGylated liposomal doxorubicin. The antibodies induced the forced clearance of red blood cells and resulted in increased circulation times compared to those observed in animals primed with vehicle. Although particles with very high clearance benefited the most from the phenomenon, forced clearance of erythrocytes appeared to induce a 1.6-fold increase in the blood exposure of PEGylated liposomal doxorubicin51. Importantly, in a murine model of melanoma, PEGylated liposomes showed improved antitumor efficacy when the MPS was saturated.
Finally, in a provocative but well-designed study, Chan and his group52 suggested recently that the capacity of the MPS might be regulated in terms of numbers of particles instead of mass. Using a variety of techniques, including intravital imaging, they showed that the clearance rates of gold nanoparticles significantly increased when a minimum threshold of 1012 injected particles per mouse was reached. Doses above this number resulted in decreased accumulation in macrophages and increased tumor deposition, for gold and silica nanoparticles, as well as PEGylated liposomes52. To further showcase the relevance of this threshold, one single administration of a fixed quantity of PEGylated doxorubicin liposomes (ca. 5 × 1012 particles, 2 mg/kg of doxorubicin) had significantly increased antitumor efficacy when delivered with a large quantity of empty particles (ca. 5 × 1013 empty particles). Time will tell if this threshold can translate to humans by allometric conversions, and whether the phenomenon, obtained with single dose administrations, holds true for the multiple dose regimens used in the clinics.
In patients receiving PEGylated liposomes encapsulating irinotecan53 or a camptothecin analog54, Zamboni and his group53,54 observed that individuals with higher plasma clearance also had a more significant decrease in blood monocytes. In these single intravenous administration studies, blood exposure to the drug was measured by the plasma concentration vs. time curve (AUC), and monocyte counts were measured at nadir, (i.e., 9 or 11 days after the dosing of the camptothecin analog and irinotecan, respectively). For both drugs, younger patients (<60 years old) experienced a larger decrease in monocytes than patients >60 years of age53,54. The authors propose that uptake in monocytes partly explains the clearance of the liposomes and that the delayed monocytopenia is a consequence of the encapsulated cytotoxic payload53,54. Interestingly, both liposomal drugs appeared to be 1.5- to 2.5-fold more toxic for monocytes than for neutrophils. In comparison, the non-encapsulated camptothecin analog was more toxic and affected monocytes and neutrophils equally54. It remains unclear if these interactions between the liposomes and the monocytes occur in the bloodstream or in the bone marrow, and whether differences in initial counts of monocytes existed between young and older patients before the administration of liposomes.
Multiple-dose pharmacokinetic studies in patients also support the role of the MPS in the clearance of nanomedicines. In an open label study in 15 patients, Gabizon et al.55 have observed that repeated administration of PEGylated liposomal doxorubicin resulted in gradually increasing plasma exposure to the drug. Over three cycles of intravenous treatments given every 4 weeks, the AUC of the drug increased by >40%. The authors ascribed this increase in blood exposure to damage caused by the drug to the mononuclear phagocyte system55. A follow-up analysis suggested that patients who had important decreases in monocytes also experienced higher gradual increase in blood exposure, over the 3 cycles56.
In the clinics, both PEGylated and non-PEGylated nanomedicines are used (Table 2). Liposomal doxorubicin (Myocet®), liposomal vincristine (Marqibo®), and liposomes containing a synergistic combination of daunorubicin and cytarabine (Vyxeos®) are examples of nanomedicines formulated without PEG coating. In contrast, PEGylated liposomal doxorubicin (Doxil®/Caelyx®), PEGylated liposomal irinotecan (Onivyde®), and patisiran lipid complexes (Onpattro®) have steric protection. At least for liposomes, the absence of PEG does not compromise clinical efficacy; encapsulation of doxorubicin in PEGylated and non-PEGylated liposomes significantly increases the blood exposure compared to the free drug, 260-fold with PEGylated liposomes and 20-fold with their non-PEGylated counterpart57.
Table 2.
Name and indications of various nanomedicines used clinically.
| Disease | Formulation | Tradename | Active ingredient | Lipid-based | PEG | Approved indication |
|---|---|---|---|---|---|---|
| Oncology | PEGylated liposomal doxorubicin | Doxil/Caelyx® | Doxorubicin | Yes | Yes | Kaposi's sarcoma, multiple myeloma (USA), metastatic breast cancer (Canada), ovarian cancer (2nd line) |
| PEGylated liposomal irinotecan | Onivyde® | Irinotecan | Yes | Yes | Pancreatic cancer (2nd line, with fluorouracil and folinic acid) | |
| Liposomal doxorubicin | Myocet liposomal® | Doxorubicin | Yes | No | Metastatic breast cancer (Europe, Canada) | |
| Liposomal daunorubicin/cytarabine | Vyxeos® | Daunorubicin/cytarabine (1:5 molar ratio) | Yes | No | Newly-diagnosed therapy-related acute myeloid leukemia (in adults), AML with myelodysplasia-related changes (in adults) | |
| Liposomal vincristine | Marqibo® | Vincristine | Yes | No | Philadelphia chromosome negative acute lymphoblastic leukemia (2nd line, in adults) | |
| Hafnium oxide nanoparticles | Hensify® | Hafnium oxide nanoparticles (radioenhancer) | No | No | Locally advanced tissue sarcoma (Europe) | |
| Albumin-bound paclitaxel | Abraxane® | Paclitaxel | No | No | Pancreatic cancer, non-small cell lung cancer (with carboplatin), metastatic breast cancer (2nd line) | |
| Infectious disease | Liposomal amphotericin B | Ambisome® | Amphotericin B (antifungal) | Yes | No | Cryptococcal meningitis, leishmaniasis, and fungal infections (immunocompromised and neutropenic patients or renal sensitivity) |
| Liposomal amikacin (suspension for oral inhalation) | Arikayce® | Amikacin (antibiotic) | Yes | No | Refractive Mycobacterium avium complex infections | |
| Orphan disease | Patisiran lipid complex | Onpattro® | Patisiran (siRNA) | Yes | Yes | Hereditary transthyretin-mediated amyloidosis |
| Vaccines | Tozinameran | Comirnaty® | mRNA encoding SARS-CoV-2 spike protein | Yes | Yes | Vaccination against COVID-19 (USA: emergency use; Europe: conditional marketing approval) |
| mRNA-1273 | Not available | mRNA encoding SARS-CoV-2 spike protein | Yes | Yes | Vaccination against COVID-19 (USA: emergency use; Europe: conditional marketing approval) | |
| Others | Liposomal verteporfin | Visudyne® | Verteporfin (photodynamic therapy) | Yes | No | Phototherapy in subfoveal choroidal neovascularization in age-related macular degeneration |
| Ferumoxytol | Feraheme® | Iron oxide nanoparticles | No | No | Iron-deficiency in adults with chronic kidney disease | |
| Loteprednol etabonate (ophthalmic suspension) | Eysuvis®/Inveltys® | Loteprednol nanocrystals | No | Yes | Short-term treatment of dry eye disease (<2 weeks), post-operative inflammation and pain following ocular surgery |
3.3. Proteins of the innate immunity
3.3.1. The complement system
The complement cascade includes approximately 50 proteins58, which circulate in the fluid-phase (plasma, lymph, and interstitial fluids)59 or are attached to the extracellular membrane of leukocytes, platelets, erythrocytes, epithelial cells, endothelial cells, skin keratinocytes, and kidney podocytes60. Upon contact with the surface of pathogens, these proteins are cleaved in sequence and their enzymatic functions are activated, triggering a self-amplifying cascade. The deposition of complement proteins on pathogens decorates them with signals that facilitate phagocytosis and send messages to cells of the adaptive immune system. These proteins are called opsonins. Fragments of the complement cascade which do not bind to the pathogen can also act as chemokines and trigger effects more systemically.
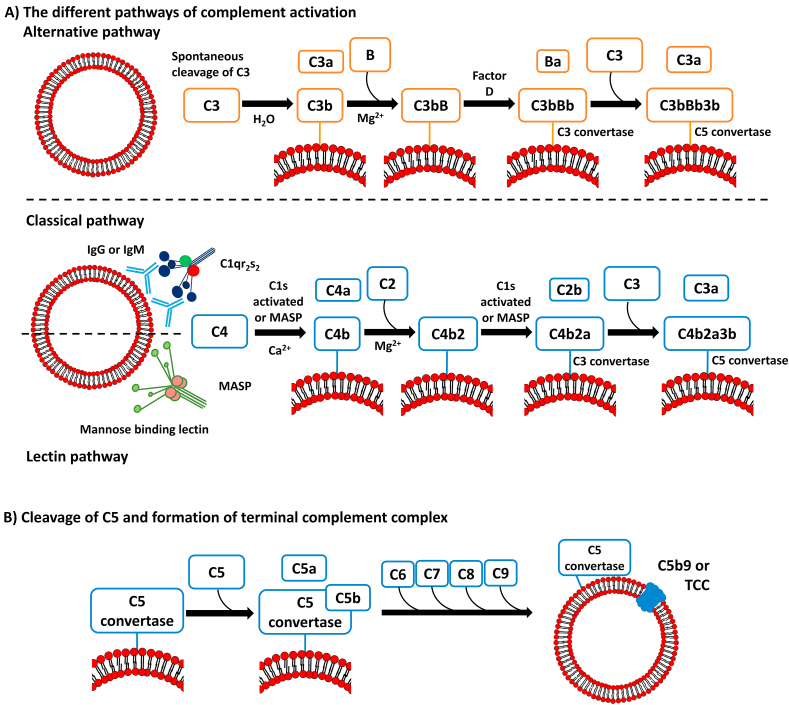
The two key components of the complement cascade are the C3 and C5 proteins which are responsible for most of the biological effects of the cascade. These proteins are cleaved into smaller fragments (C3a/C3b and C5a/C5b) by three well-known activation mechanisms: the alternative, classical, and lectin-mediated pathways. Each activation route leads to the formation of distinct C3 and C5 convertases, molecular complexes responsible for the conversion of C3 and C5 into their activated fragments (Fig. 3A).
Figure 3.
The complement cascade can be initiated by the alternative, classical, or lectin pathways (A). Independently of the pathway, the binding of C6, C7, C8, and C9 proteins to the C5b protein leads to formation of C5b9, also known as terminal complement complex (B).
The alternative pathway is initiated by the spontaneous hydrolysis of C3 protein into C3a and C3b. The C3b fragment can covalently bind to carbohydrates and amines on the surface of the pathogen, via a thioester moiety which is exposed upon activation. The C3a protein does not directly interact with the pathogen but is involved in recruiting neutrophils, monocytes, and macrophages to the site of infection. The classical pathway involves the binding of C1q protein to the Fc portion of immunoglobulins found on the surface of pathogens (IgM or IgG). C1q also bind to lipid A, β-sheet amyloid fibrils, pentraxins, and apoptotic cells60. The lectin pathway differs from the classical pathway only in the initial stimuli responsible for the conversion of C4 into its bioactive fragments. Instead of relying on the presence of surface antibodies, the lectin pathway is initiated by lectin binding to mannose residues of glycoproteins and glycolipids, or by ficolins binding to N-acetylated surfaces. Each mannose binding lectin (MBL) or ficolin associates with serine proteases, forming the MBL-associated serine proteases (MASPs) complexes.
All activation pathways result in the production of different C3 convertases protein complexes (Fig. 3). These complexes amplify the cascade by cleaving additional C3 protein and releasing more C3a and C3b. At this point, additional C3b molecules can join the C3 convertase complex and form the C5 convertase. Cleavage of the protein C5 by the C5 convertase leads to the release of the anaphylatoxin C5a, while the larger fragment, C5b protein, remains bound to the C5 convertase. The enzymatic cascade ends on the formation of C5b-9, terminal complement complex (TCC), or membrane attack complex, resulting from the association of C5b with the proteins C6, C7, C8, and C9. The TCC creates a pore in the microorganism's membrane, leading to its lysis (Fig. 3B).
In general, the complement cascade produces cytokines, opsonins, and terminal lytic complex. Cytokines C3a, C4a, and C5a recruit macrophages and mast cells, which eliminate the antigens marked by C1q, C4b, C3b, or other fragments of C3 (iC3b and C3c). Besides these immediate actions, complement proteins can also participate in the adaptative humoral response58.
3.3.2. Nanomedicines and the complement cascade
For almost 30 years, evaluating how nanomedicines activate the complement cascade ex vivo has been a common and practical assay to study interactions with biological systems61, 62, 63, 64, 65. These interactions are regulated by dynamic interfacial forces and physicochemical properties of the material, such as charge, size, shape, hydrophobicity, hydrophilicity, chemical composition, and coverage by functional groups61,62,64,65. As such, they offer a convenient way to discriminate between materials, and the challenges and opportunity associated with studying complement–nanomedicine interactions have been detailed elsewhere recently66.
However, the impact of complement activation on the fate of nanomedicines in vivo remains poorly understood. Despite the necessary role of complement proteins to fight some pathogen infections67,68, bona fide demonstrations of the impact of complement on the blood clearance of nanoparticles remain scarce. As early as the 1990s, some reports showed that in vitro complement activation by liposomes was not always predictive of circulation times in vivo69. Szoka's group70 showed that inhibiting the complement system in mice did not significantly alter the pattern of gene expression, in the lungs, liver, and spleen, observed after intravenous injection of cationic transfecting liposomes.
More recently, our group compared the circulation profiles of PEGylated nanoparticles in C57bl/6 wildtype controls and transgenic animals unable to activate the cascade of the complement, that is C3 knockout (KO) mice45. Nanoparticles with long and short circulation times were tested (i.e., 7-fold variation in AUC0–6 h between the fastest and slowest clearance). For all nanoparticles, intravenous injection resulted in similar pharmacokinetics in C3−/− animals and control animals. Similar results were reproduced by depleting the complement activity in BALB/c mice, via the injection of intraperitoneal cobra venom factor (CVF)71. This toxin acts as a soluble C3 convertase, and its injection to animals temporarily depletes circulating levels of C3 and their ability to activate the complement system. In this follow-up study, the circulation profiles of a first dose of PEGylated nanoparticles were superimposable, irrespective of the animal's ability to activate complement71. We also looked at the circulation profiles of PEGylated and non-PEGylated liposomes in BALB/c mice and Sprague Dawley rats treated with vehicle or CVF72. Again, comparable circulation profiles were observed in animals with or without the ability to activate the complement, when 20 mg/kg of PEGylated and non-PEGylated liposomes were injected. However, when non-PEGylated liposomes were administered at a lower dose of 2 mg/kg (which resulted in a very fast clearance), a 1.3- and 1.5-fold increase in AUC0–24 h was observed when complement was depleted in mice and rats, respectively. This increase in blood exposure was mostly attributable to the blood concentrations measured between 6 and 24 h after the injection, when residual circulating levels were low72.
Other groups have also observed that complement minimally impacted the clearance rates of PEGylated emulsions73, iron oxide nanoparticles51, but also alphaviruses74 and adenoviruses75. Some evidence exist that complement proteins pre-adsorbed on the surface of nanomaterials could be dynamically exchanged in vivo76. To reach this conclusion, the groups of Moghimi and Simberg76 coated paramagnetic iron-oxide nanoworms with complement proteins and injected them to C3 KO animals. Five minutes after injection, they recovered the nanomaterial from the blood and observed no trace of the initial complement proteins. If dynamic exchanges also occur with other materials, the phenomenon could explain the difficulty of observing the impact of complement in vivo.
Finally, when investigating the tissue distribution of fluorescently-labeled, non-PEGylated liposomes in the presence and absence of complement, we observed that depletion of the cascade resulted in decreased distribution of liposomes to splenic B cells72. Twenty-four hours after injection, animals injected with CVF had a 4-fold decrease in the proportion of splenic B cells containing liposomes, compared to mice treated with vehicle. Others have also observed that paramagnetic iron-oxide nanoworms had different distribution in circulating leukocytes, in wildtype and C3 KO mice77. It is therefore possible that complement could qualitatively affect the distribution of nanomedicines to organs of the MPS, while not always significantly affecting the levels found in circulation.
3.3.3. The importance of other proteins on the clearance of nanomedicines
Recently, Schöttler et al.78 studied the uptake of 100-nm polystyrene nanoparticles with relatively sparse steric protection (8–10 chains per 100 nm2) in cultures of leukemic macrophages (RAW264.7 cells). Like in other reports36, they observed that cellular uptake could be prevented by pre-incubation of the nanoparticles with plasma proteins. Interestingly, they showed that the adsorption of the protein clusterin (apolipoprotein J) on the surface of nanoparticles could inhibit the in vitro uptake in macrophages by >70%78. In our own hands, pre-incubation of PEGylated nanoparticles with clusterin before intravenous injection to healthy mice resulted in increased blood circulation times only for particles with very low PEG densities (<20 PEG chains per 100 nm2)45. In the same work, we also showed that nanoparticles with low steric protection were cleared much faster in transgenic mice which did not express apolipoprotein E (ApoE), a protein responsible for lipid trafficking in the blood. It is therefore possible that clusterin and other apolipoproteins, which interact physiologically with hydrophobic biological constituents, bind to the surface of nanoparticles with low PEG densities to somehow stabilize them. This effect would be less perceptible with systems which have inherently higher steric protection45.
Further evidences also support a possible role for apolipoproteins on the in vivo fate of nanomedicines. The 60-nm hepatitis B virus is known to highjack apolipoproteins to evade immune mechanisms and enter hepatocytes79. Likewise, lipid nanoparticles encapsulating siRNA lose their gene silencing properties in vitro in the absence of ApoE, and in vivo in Apoe−/− mice80. Finally, we showed that, irrespective of PEG coverage, the circulation times of polymer nanoparticles in animals that did not express the receptor for low density lipoproteins (Ldlr−/−) were longer than those observed in wildtype animals45. Further investigation might therefore consolidate a more definitive role for apolipoproteins in the biological fate of nanomedicines.
Finally, two recent reports highlighted the possible importance of “natural” antibodies on the fate of nanomedicines81,82. These antibodies are non-specific immunoglobulins which circulate in the blood in the absence of infection. In vitro incubation of PEGylated liposomal doxorubicin (LipoDox®), PEGylated liposomal irinotecan (Onivyde®), and carbohydrate coated iron oxide nanoparticles (FeraHeme®), resulted in decreased complement activation, when the plasma of healthy donors and breast cancer patients was depleted of antibodies by exposure to protein A81. Complement activation could be restored with the reintroduction of polyclonal IgGs, but not monoclonal (non-specific) trastuzumab. In a parallel study, the quantity of natural IgM adsorbed on the surface of peptide-targeted PEGylated liposomes were correlated with decreases in circulation times in mice and rats82.
4. The links between innate and adaptative immunity
4.1. Type 1 and type 2 immune responses
Tissue-resident macrophages and infiltrating neutrophils, as first responders to tissue aggression, recruit additional monocytes, and macrophages during the initiation phase of inflammation. The cytokines released in the tissue will influence how recruited cells will respond. For example, most of the neutrophils that accumulate in tissues do not return to the circulation. Neutrophils that phagocytose particles enter phagocytosis-induced cell death which prompts their clearance by macrophages3. The sensing of pathogens or infected cells, notably via TLR signaling, promotes the production of interferon and inflammatory cytokines. This induces a pro-inflammatory phenotype in newly arrived and tissue-resident cells, that is the type 1 response83. Type 1 response translates into increased phagocytic and cytotoxic activities. In opposition, when immune cells sense anti-inflammatory cytokines, for example the minimal danger cues associated with phagocytosis of apoptotic corpses, they will adopt a type 2 response. This phenotype initiates the resolution phase. The balance between type 1 and 2 responses is closely regulated and threads a fine line between fighting a pathogen and maintaining tissue functions84.
Type 1/2 polarization was first described for T helper lymphocytes (CD4+, TH cells)83, but the observation that macrophages responded to similar biological cues prompted a model where pro- and anti-inflammatory phenotypes were coined as M1 and M2, respectively. Although the dichotomous M1/M2 paradigm is practical, it does not fully appreciate the plethora of macrophage with mixed M1/M2 phenotypes that exist in health and disease85. It is increasingly clear that the phenotype of macrophages in tissues initiates the polarization of TH cells, and not the other way around84. Likewise, while macrophages can maintain their phenotype without the involvement of lymphocytes, implication of polarized TH cells potentiates their effect. Type 1 and 2 responses therefore represent cascades of biological responses implicating a variety of cells.
At the onset, Type 1 response is prompted by IL-12, but the cascade is sustained mostly by the production of IFN-γ, IL-2, IL-6, and TNFα83. Pro-inflammatory macrophages are effector cells and produce bactericidal nitric oxide (NO) and ROS, but also high quantities of IL-1β, TNFα, and IL-6 which act as potent positive amplification signals84. The pro-inflammatory immune response inhibits and kills pathogens; it is necessary to fight leishmania, bacterial, mycobacterial, and fungal infections83. However, untamed type 1 response can cause tissue damage, predispose toward neoplastic transformation or promote insulin resistance84.
In contrast, type 2 response is driven by IL-4, but also IL-10 and IL-13. Physiologically, most tissue-resident macrophages are polarized toward an anti-inflammatory phenotype which drives growth and healing84. In this state, macrophages produce IL-10 and tissue growth factor-β (TGF-β) which sustain the type 2 phenotype. Anti-inflammatory macrophages have high levels of scavenger, mannose, and galactose-type receptors. Disproportionate type 2 response is associated with tissue fibrosis and allergy83,84.
4.2. Nanomedicines and immune polarization
Due to imbalances in the production of IL-4 and IL-12, the responses of certain inbred strains of mice are skewed toward type 1 or type 2 reactions83. While C57BL/6 mice exhibit a general susceptibility to type 1 polarization, the response of BALB/c mice is biased toward type 2. In a very interesting study, the teams of Bear and DeSimone86 have compared the clearance of nanoparticles in type 1- and type 2-biased mouse strains. During the 2 h that followed the intravenous injection of cylindrical, negatively charged, 300-nm PEG-hydrogels, type 1-biased mice (C57BL/6 and B10D2) had at least a 4-fold higher blood exposure than their type 2 counterparts (BALB/c and DBA2)86. These differences were also noticeable for 30-nm quantum dots, but not for 6-μm microparticles. The authors ascribe these distinctions to a higher expression of mannan receptors on the surface of phagocytes from BALB/c mice, compared to C57BL/6 animals. It is unclear how this phenomenon translates to other materials. In this experiment, the clearances of the studied materials were relatively fast, as all had <25% of the initial signal remaining in the blood 2 h after injection86. Independently, we also compared the pharmacokinetics of 90-nm polymer nanoparticles with different PEG densities in BALB/c and C57BL/6 mice45. Over 6 h after intravenous injection, the blood exposure observed with nanoparticles with low steric protection (i.e., 15 PEG chains per 100 nm2) was approximately 1.8-fold higher in C57BL/6, compared to BALB/c mice. Interestingly, these differences disappeared for nanoparticles with slower clearances45. Altogether, this suggests that differences between type 1/2-biased strains might be more important for nanoparticles which are quickly removed from the circulation than for longer-circulating systems.
In cancer, systemically injected nanoparticles can preferentially distribute to solid tumors87 where they can target cancer cells and macrophages alike88. The ability of nanomedicines to polarize tumor-associated macrophages toward an anticancer phenotype has therefore raised significant interest. The general concept relies on using the sensing machinery in the macrophage (i.e., TLRs and other pattern receptors) to locally prompt the phagocyte towards a type 1 immune response, for example by encapsulating TLR agonists89, IL-12, or CpG motifs90.
In a recent report, Chen's group91 has designed hybrid nanovesicles decorated with signal regulatory protein alpha (SIRPα) and combining the tumor homing properties of platelet-derived exosomes and the pro-inflammatory macrophage-derived extracellular microvesicles. Three intravenous injections of these sophisticated nanovesicles to C57BL/6 mice bearing subcutaneous B16F10 melanoma, resulted in increased tumor levels of IFN-γ, TNFα, and IL-12, and reduced amounts of IL-10, strongly supporting a shift toward a type 1 response91. In this metastatic-prone cancer model, treatment with nanovesicles was able to prevent cancer recurrences after surgical tumor ablation and significantly prolong survival. Interestingly, the nanovesicles were much less potent in a triple-negative breast cancer model (4T1 cells) implanted in BALB/c mice. To achieve comparable efficacy in these type 2-biased animals, encapsulation in nanovesicles of cyclic GMP-AMP, a strong ligand of the intracytoplasmic pattern receptor STING, was necessary91.
4.3. Antigen presentation and the major histocompatibility complex
Dendritic cells, macrophages and B lymphocytes are professional antigen presenting cells (APCs) which contribute to the adaptative immune response via their ability to present antigens to T lymphocytes92. T cells can only recognize peptides with a length of 10–30 amino acids93. Therefore, APCs specialize in intracellularly processing danger signals and presenting representative segments on their surface via dedicated transmembrane proteins, the major histocompatibility complex (MHC) molecules.
In mammals, MHC molecules are highly polymorphic (i.e., large differences exist between individuals) and represent a unique and necessary footprint used by lymphocytes to discriminate between self and non-self. Two categories of MHC molecules exist: MHC class I molecules present what is inside the cytoplasm, and MHC class II what has been phagocytosed. In other words, class I provides surveillance against cellular distresses that are replicating in the cytoplasm (including pathogens), while class II molecules are used by phagocytes to display what they have internalized94. Because all nucleated cells can be infected, MHC class I molecules are ubiquitous. In contrast, mostly professional APCs present MHC class II molecules. For reasons inherent to their effector functions detailed below, CD8+ cytotoxic T cells recognize antigenic peptides presented on MHC class I molecules, while CD4+ helper lymphocytes bind only peptides on MCH class II complexes (i.e., interact with APCs).
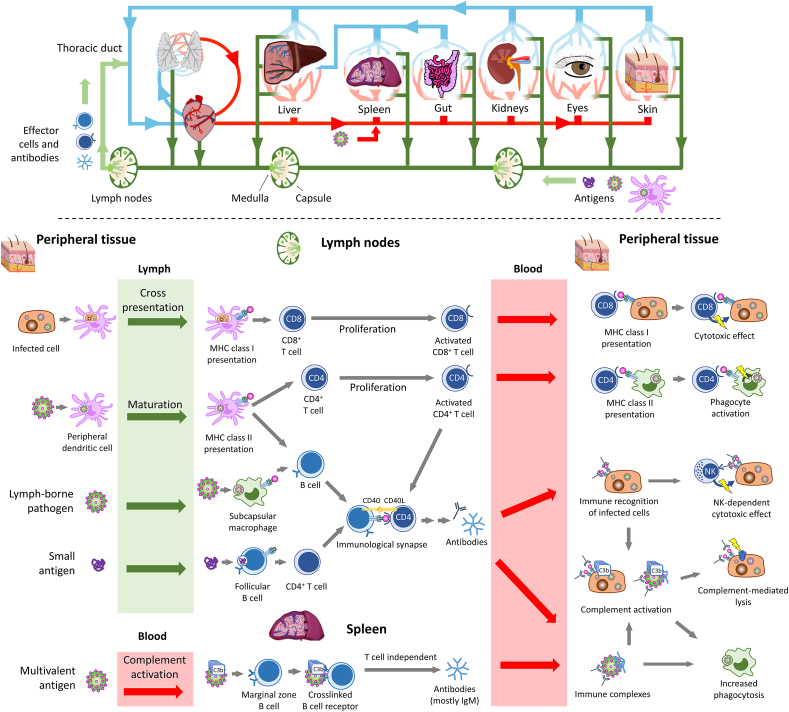
Antigen presentation is implicated in three important immune functions: 1) the differentiation of naïve T cells toward an antigen-specific response (for this function, APCs are mostly dendritic cells), 2) the T-dependent production of antibodies (APCs are B cells, in lymphoid organs), and 3) the response of effector T lymphocytes (APCs are mostly peripheral macrophages)84. The first two functions exploit the mobility of antigens and immune cells through the lymphatic circulation to facilitate contacts between dendritic cells and lymphocytes (Fig. 4). Peripheral dendritic cells that have internalized a pathogen and sensed inflammatory signals, notably the presence of IFN-γ, can migrate toward lymph nodes to present antigens to naïve lymphocytes. The presence of chemokines, notably ligands of the receptor CCR7 expressed on dendritic cells and lymphocytes, coordinates the movement of both populations towards the lymph nodes6. During their journey, if dendritic cells sense sufficient danger signals from the internalized pathogen (e.g., activation of TLR by DAMPs), it will upregulate the costimulatory protein B7 involved in the activation of T cells. Free antigens from the extracellular fluid can also passively drain to lymphoid organs where they can be internalized and presented by residing dendritic cells or B cells95. Some evidence also exists that monocytes can enter tissues without differentiation, up-regulate expression of MHC class II molecules, take up antigens and deliver them to lymph nodes96. Finally, the last function of APCs, which relates to their interactions with effector cells, relies on the patrolling of differentiated T cells in the bloodstream and their chemotaxis to infected peripheral tissues.
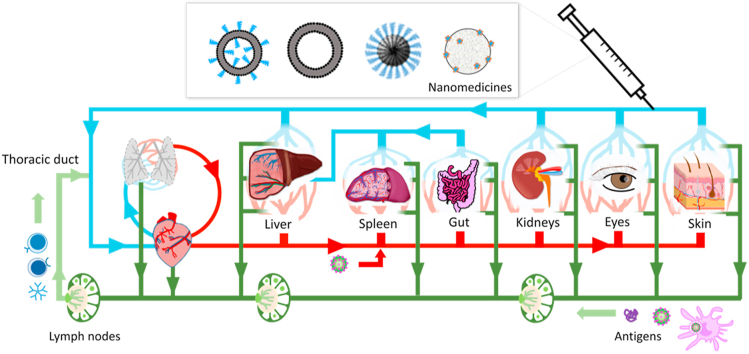
Figure 4.
Leukocytes and antigens travel through the blood and the lymph. The lymph is a mix of extracellular fluids and leukocytes drained from tissues of the entire body. The lymph flows from tissues to lymph nodes, reentering the venous circulation through the thoracic duct. Adaptative immune response results from the encounters between antigens and leukocytes.
4.4. Nanomedicines in the lymphatic circulation
In healthy organs, the interstitial fluid is passively drained to lymph nodes via afferent lymphatic vessels (Fig. 4)6. Lymphatic vessels have a diameter between 10 μm and 2 mm and contain the lymph, a mixture of extracellular fluid, leukocytes and free antigens97,98. In the absence of hydrodynamic pressure, the progression of the lymph is slow (superficial velocities range from 3 to 10 μm/s), and is sustained by the contraction of smooth muscles and the presence of unidirectional valves98. The thoracic duct returns the fluid back to the venous circulation via the brachiocephalic vein (Fig. 4).
Lymph nodes are capsular tissues consisting of multiple side-by side lobules surrounded by sinuses97. Their basic structure is made of a fibrovascular tissue filled with lymphocytes, macrophages, dendritic cells, and erythrocytes97. Afferent lymphatic vessels empty in the subcapsular space, while the efferent vessels, the vein, and the artery stem from the medulla. Antibody-producing B cells also reside in the medulla. Each lobule can be subdivided in the superficial cortex, which contains follicles of naïve B cells, and the paracortex which contains T cells97.
The size of a free antigen can affect how fast it leaves the extracellular fluid to reach the lymph, but also its diffusion through the lymph nodes. Protein antigens with small sizes (molecular weight <70 kDa) can distribute to the follicular region of afferent lymph nodes within minutes of intradermal injection95,99. In contrast, lymph-borne vesicular stomatitis viruses (70 nm × 180 nm cylinders) are captured by macrophages in the subcapsular sinus100. These distinctions might be of importance to the biological fate of nanomedicines and exploited to design functional materials.
The diffusion of fluorescent colloids through the dermal tissue can offer an elegant way to monitor lymphatic drainage in health and disease101. Fluorescent PEG conjugates and liposomes were allowed to evidence how metastases in sentinel lymph nodes could impact lymphatic flow101. In a parallel study, intradermal injection of non-PEGylated polymer nanoparticles with diameters of 25 and 100 nm showed very different patterns of diffusion to the lymphatics102. Twenty-four hours after injection, significant quantities of ultra-small nanoparticles were detected in the subcapsular sinus of the afferent lymph nodes, internalized by CD11c+ dendritic cells and macrophages. In comparison, the uptake in dendritic cells was 8-fold lower for nanoparticles with a diameter of 100 nm. Beyond size, this work also evidenced that activation of the complement by the nanoparticles prompted maturation of the dendritic cells and more potent immune response102.
5. The adaptive immunity
Adaptive immunity regroups T and B lymphocytes which have very distinct functions: T cells are mostly effector cells which can adapt to fight pathogens, while B cells are responsible for the production of antibodies. Both T and B lymphocytes are produced in the bone marrow in the form of individual clones with exceptional ability to recognize antigens.
Through a gene rearrangement process unique to B and T cells called V(D)J recombination, each clone expresses a single and randomly generated surface receptor. Although the biological mechanisms responsible for clonal diversity are beyond the scope of this review, the outcome is that each B- or T-cell receptor on immature lymphocytes recognizes a unique peptide. At this stage, the receptors that bind antigens are generated randomly and without consideration to self or non-self. Cells which are not useful or possibly harmful are eradicated from the repertoire by the selection of clones. Cells that bind self MHC class molecules too weakly or too strongly and those that recognize peptides belonging to the host (i.e., possibly self-reactive) will be eliminated by apoptosis. By the end of this process, each mature lymphocyte can recognize one out of 106 different possible foreign peptides.
From the thymus and the spleen where T and B cells respectively mature, they return to the lymph and blood to wander between secondary lymphoid organs (e.g., lymph nodes and mucosae). Clonal expansion occurs when a T cell with the correct specificity binds an APC with the right antigen–MHC complex24. The APCs responsible for priming T cells are mostly dendritic cells. While phagocytosed antigens can be presented on MHC class II molecules (to activate CD4+ cells), the MHC class I presentation (necessary for CD8+ activation) is usually restrained to antigens which have invaded the cytoplasm. Dendritic cells possess a unique cross presentation capacity, that is the ability to phagocytose infected or diseased cells in the periphery, release phagocytosed antigens in their cytoplasm and present them on MHC class I molecules103.
Clonal expansion requires a triad of signals: 1) antigen-specific activation of the T cell receptor complex, 2) costimulatory signals between the lymphocyte and the APC, and 3) the presence of cytokines24. Costimulatory signals originate from the binding of lymphocyte receptors to proteins on primed APCs, notably the involvement of the receptors in the CD28 superfamily which act as stimulatory (CD28, ICOS) and inhibitory (PD-1, CTLA-4) immune checkpoints. Cytokines can originate from the lymphocyte itself (e.g., in an IL-2-dependent autocrine loop) or from surrounding cells (e.g., CD4+ cells help the expansion of CD8+ cells). Under these conditions, antigen-specific CD4+ clones expand 1000 to 10,000-fold, and CD8+ clones up to 50,000 times, significantly increasing the numbers of lymphocytes that can recognize the antigen. Differentiated CD4+ cells also start expressing CD40L which is key to their effector functions.
After clonal expansion, differentiated T cells return to peripheral tissues where they can exert their function, but some CD4+ cells also migrate within the lymphoid organ to the B cell-rich follicle, to help drive the humoral response.
5.1. Cellular immune responses
Via MHC class I molecules, differentiated CD8+ cytotoxic lymphocytes recognize cells that have been infected by pathogens or are expressing distress signals (e.g., cancer cells). Like NK cells, CD8+ lymphocytes can trigger apoptosis by discharging cytotoxic proteins (perforin and granzymes) to the cytoplasm of infected cells. The immunological synapse involves the binding of the specific T cell receptor to its antigen, the binding of the coreceptor CD8 to the MHC class I molecules, as well as the involvement of activating costimulatory signals. Costimulatory signals involve immune checkpoints like those discussed for clonal expansion, but also LFA-1 on the T cell and integrin ICAM-1 on the distressed cell. CD8+ lymphocytes can also induce apoptosis by directly binding the FAS death receptor on the distressed cells by the expression of the protein FAS ligand.
In parallel, CD4+ helper lymphocytes recognize antigens presented on MHC class II molecules through interactions between 1) the presented antigen and T cell receptor, 2) CD4 and the MHC class II molecules, and 3) CD40L and CD40, a protein constitutively expressed on APCs. The immunological synapse sustains a positive amplification loop skewed toward a type 1 or type 2 response, based on the cell polarization and surrounding milieu. TH1 cells produce cytokines that recruit and activate additional phagocytes and directly stimulate the production of bactericidal molecules by the macrophage involved in the synapse. This enhances the phagocyte's ability to kill its intracellular content by increasing the production of digestive enzymes, and in turn augments the number of digested antigens presented on its surface. Immunological synapses involving TH2 cells suppress the inflammatory phenotype by secretion of IL-4 and IL-10 and contribute to the activation of eosinophils by secretion of IL-5. When TH2 cells form immunological synapses with B cells, the humoral response can be skewed toward the production of IgE.
5.2. The humoral immune response
The humoral response corresponds to the production of neutralizing antibodies that bind pathogens and enhance their clearance. Through recombination and maturation, naïve B lymphocytes exhibit a diversity of random receptors comparable to that of T cells. However, while receptors of T cells bind only peptides, those of B cells can recognize peptides, proteins, polysaccharides, lipids, and small chemicals. Furthermore, B cells are distinct from T cells in that they act both as APCs and effector cells104. This allows B cells to bind and internalize free antigens in the form of digested peptides, full proteins, or complex structures with repeated surface patterns.
The first step of the humoral response involves the binding of an antigen to a B cell receptor exhibiting the correct specificity104. This happens when free antigens diffuse to the follicle of the lymph nodes or when blood-borne pathogens reach the spleen. The mechanisms of B cell activation differ based on whether the antigen is a globular protein or a larger polyvalent pattern.
After binding their specific B cell receptor, antigenic proteins are internalized by naïve B cells and traditionally presented on MHC class II molecules. While these events are not enough to stimulate the production of antibodies, they induce phenotypic changes in the B cell's chemokine receptors and initiate its migration toward the T cell zone of the lymphoid tissue104. At this location, the activated B cell presents its surface antigen to a differentiated CD4+ T follicular lymphocytes sharing specificity for the same antigen. Prompted by the secreted cytokines and the activation of its own CD40 by the T cell's CD40L, the antigen-specific B cell proliferates, forming a germinal center in the follicle. There, under the influence of T helper lymphocytes and follicular dendritic cells, B cells undergo a series of hypermutation and selection events which translate into the development of antibodies with very high affinity toward the antigen. Under continuous stimuli from CD40 and cytokines (notably IL-2, IL-4, and IL-6), B cells eventually adopt a plasma cell phenotype able to secrete antibodies.
Contrary to antigenic proteins, large polyvalent patterns like polysaccharides and nucleic acids can trigger antibody production in the absence of T cell involvement. This is due to their large repeating structures which can crosslink multiple B cell receptors simultaneously. In that context, complement proteins deposited on the antigen also bind to coreceptors CD21 (complement receptor 2), fully engaging the response105. B cells stimulated in the absence of T helper lymphocytes adopt a short-lived plasma cell phenotype, and rarely induce memory responses.
5.3. Nanomedicines and adaptive immune functions
PEG was initially chosen for its inert character, but it is now appreciated that its patterning on the surface of a nanomedicine can increase interactions with the immune system71. Dams and colleagues106 showed that the clearance of PEGylated liposomes was much increased when rats and Rhesus monkeys had received a first ‘sensitizing’ dose 5–7 days prior. They showed that the phenotype could be transferred from sensitized to naïve animals by plasma transfusions.
Significant efforts subsequently devoted to study the phenomenon confirmed that increased clearance was due to anti-PEG IgM107, which were not observed in splenectomized animals108. The T-independent nature of the immune response was established by the observation that mice without T cells (nude BALB/c) still developed this phenotype, but not animals without T and B cells (SCID)109. Our group and others evidenced that, in animals with anti-PEG IgMs, activation of the complement cascade was in part responsible for the accelerated clearance71,73.
Beyond liposomes, the phenomenon was observed with various types of PEGylated colloids, including polymer nanoparticles71, lipid complexes of nucleic acids110, proteins and viruses111. The physicochemical characteristics of the nanomedicines appear to play a role in the production of anti-PEG IgM112, but also the injected dose113 and the encapsulation of anticancer payloads114. In the latter condition, it is believed that cytotoxic nanomedicines would kill the B cells upon internalization and prevent their proliferation. This is consistent with the absence of accelerated clearance seen in patients who received multiple doses of anticancer nanomedicines. However, multiple reports have evidenced the presence of anti-PEG antibodies in the sera of healthy donors, without known prior exposure to PEGylated therapeutics115, 116, 117. In these studies, conducted in patients from the U.S., Austria, and China, the prevalence of anti-PEG IgM and IgG ranged between 20% and 70% of samples analyzed115, 116, 117. The impact of these antibodies on the performance of nanomedicines in patients remains unclear.
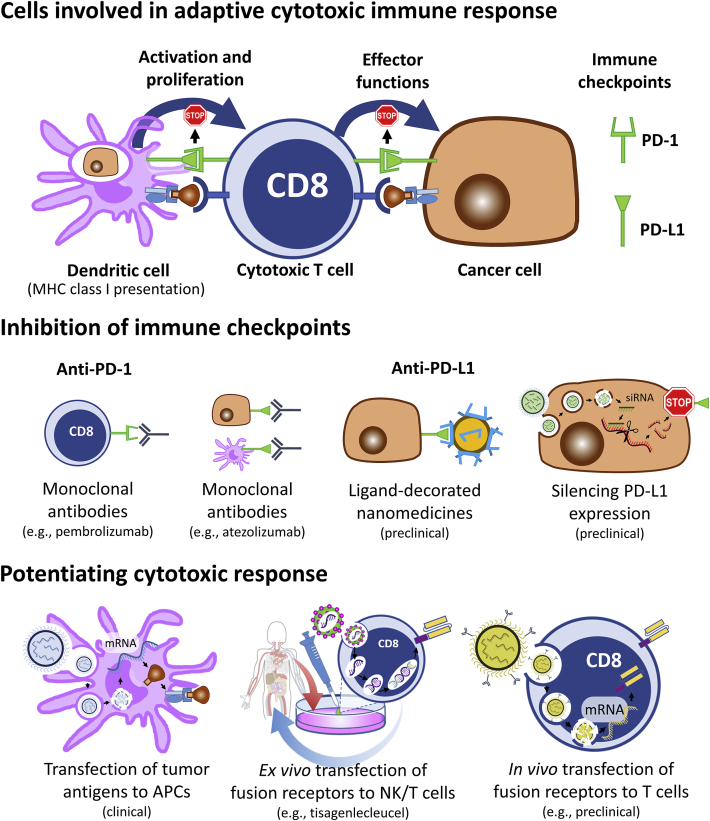
The interactions of nanomedicines with the adaptive immune system can also be exploited therapeutically. For example, the physiological PD-1/PD-L1 immunoregulatory pathway is an inhibitory checkpoint which prevents CD8+ from eliciting their cytotoxic activity118. In healthy tissues, the binding of PD-1 to PD-L1 protects against self-reactivity, but these cell–cell interactions can also be exploited by cancer cells to bypass immune surveillance. Anti-PD-1 and anti-PD-L1 antibodies, which disrupt the inhibitory effect, achieve very high success rates in patients suffering from melanoma, non-small cell lung cancer, and other types of cancers, but some tumors remain refractive118.
To increase immune responsiveness, Lebel et al.119 used 80-nm protein nanoparticles derived from the papaya mosaic virus to trigger a polarizing IFN-α response in tumor-bearing animals. Injected intratumorally, these nanoparticles act as TLR7 agonists and induce the production of interferon gamma inducible proteins-10 (IP-10), monocyte chemoattractant protein-1 (MCP-1), and other inflammatory cytokines. The ensuing tumor infiltration of CD8+ cytotoxic lymphocytes translates into prolonged survival in a model of B16F10 melanoma119. Importantly, in the same model, the treatment with the nanoparticles synergized strongly with anti-PD1 antibodies. The authors ascribe this synergy to the increased capacity of their nanoparticles to prime CD8+ T cells, which can freely exert their cytotoxic effect in the presence of checkpoint inhibitors.
More recently, other systems have been proposed to potentiate the therapeutic inhibition of immune checkpoints and further mitigate the ability of cancer cells to stall cytotoxic lymphocytes. For example, nanomedicines exhibiting polyvalent PD-L1 surface ligands were shown to be have higher affinity and selectivity than free antibodies toward cancer cells, in vitro and in vivo120. In parallel, targeted nanoparticles were also used to silence PD-L1 expression and significantly decrease tumor volume in a murine model of non-small cell lung cancer121.
Beyond implications of the PD-1/PD-L1 pathway, Oberli et al.122 described lipid nanoparticles encapsulating mRNA that were able to efficiently transfect phagocytes to express protein antigens. Their rationale was that proteins expressed in the cytoplasm of APCs could be presented to CD8+ cells and induce clonal expansion. In vivo screening of a small library of nanoparticles (composed of phospholipids, cholesterol, surfactants, and ionizable and PEGylated lipids) identified formulations which resulted in the prevalence of antigen-specific CD8+ lymphocytes122. Using a mock reporter luciferase mRNA, they showed that subcutaneously injected lipid nanoparticles transfected ∼5% of the dendritic cells in the draining lymph node, ∼3% of the neutrophils and ∼1% of the macrophages. mRNA encoding the tumor antigen TRP2, transfected using a formulation which contained the TLR–agonist LPS, resulted in prolonged survival in a B16F10 melanoma model122. Similar strategies, combining mRNA to other TLR agonists, like the small molecule R848, have also been proposed123.
In clinical oncology, T cells can be transfected ex vivo with chimeric antigen receptor (CAR) to confer them specificity toward tumor cells, irrespective of their original receptor configuration124. Reinjection of autologous transfected CAR T cells to patient results in large numbers of tumor-specific lymphocytes that can fight the cancer, without the need for antigen presentation and clonal expansion. Tisagenlecleucel (Kymriah®, Novartis) and Axicabtagene ciloleucel (Yescarta®, Kite/Gilead) are examples of CAR-T cell technologies approved by the U.S. Food and Drug Administration (FDA) which cost upward of 300,000 $ per patient. To palliate to the complex and costly process of transfecting autologous cells ex vivo, CD3-targeted polymer-based nanomedicines to transfect the CAR to T cells in vivo have been proposed125. Intravenous injection of these antibody-coated nanomedicines containing mRNA resulted in efficient transcription in approximately 8% of the splenic T cells, 3% of macrophages and 2% dendritic cells. Expression was transient and lasted for approximately 7–10 days. Using the cancer specific 1928z CAR, the authors showed that weekly intravenous injections could significantly prolong survival of immunocompetent mice models of leukemia and solid tumor125. Importantly, the authors also showed the potential of the technology to induce cellular response against hepato-cellular carcinoma caused by hepatitis B virus. Altogether, these approaches confirm the potential of mRNA nanomedicine to vaccinate against cancer. Should these promises be confirmed in patients, they could significantly alter the practices in cancer immunotherapy, including making CAR technology more accessible for a variety of prevalent cancers. Examples of cancer immunotherapy approaches that can be enabled by nanomedicines are presented in Fig. 5.
Figure 5.
Nanomedicines can impact the field of cancer immunotherapy and prompt the immune system to fight tumors more efficiently.
Due to the global SARS-CoV-2 pandemic, mRNA vaccines to protect against pathogens have also attracted tremendous attention. The topic has been discussed recently in many excellent reviews126. Briefly, the American company Moderna (candidate mRNA-1273)127, the European collaboration between BioNTech and Pfizer (tozinameran, BNT162b2)128 and other groups129 have developed comparable technologies where molecules of messenger RNA are encapsulated in PEGylated lipid nanoparticles. These systems all contain proprietary ionizable lipid allowing endosomal escape, which enables the cytoplasmic translation of mRNA coded protein. The mRNA used by Moderna and Pfizer/BioNTech encodes a stabilized version of the full transmembrane SARS-CoV-2 spike protein, while the mRNAs of the Chinese company Suzhou Abogen Biosciences (candidate ARCoV) encode solely the receptor-binding domain of the spike protein. In an unprecedented accomplishment in the field of vaccine development, these technologies entered clinical evaluation only a few months after the SARS-CoV-2 genome was made available.
A report on the preclinical development of ARCoV offers some insight on the mechanisms of mRNA vaccines against COVID-19129. They showed that intramuscular injection translated into rapid transfection of the injected muscle and the liver, mainly in monocytes, macrophages, and dendritic cells, but also in hepatocytes129. Intravenous injection of their candidate also resulted in significant plasma concentration of the encoded protein, but this administration method is not currently used in clinical vaccination regimens. A two-dose vaccination treatment (14 days apart) elicited neutralizing antibodies, a TH1-biased CD4+ response and specific CD8+ cells in mice and non-human primates.
High neutralizing IgG titers, TH1 biased CD4+ polarization and CD8+ response against subunit S1 of the spike were also obtained in mice with the mRNA-1273 candidate from Moderna130. A follow up study in primates showed that two intramuscular injections of 100 μg of mRNA-1273, at a 4-week interval, could raise a neutralizing humoral response in rhesus macaques127. In that study, the presence of spike-specific CD4+ follicular lymphocytes and TH1 cells was confirmed, and no TH2 polarization nor specific CD8+ T cells were detected. Four weeks after the second vaccination, antibodies could protect animals against intratracheal challenges with 7.6 × 105 plaque-forming units of the virus, as evidenced by genome counts in bronchoalveolar fluid and nasal swabs127. The interim results of the phase I clinical trial in 45 healthy patients (18–55 years of age) showed that two injections of mRNA-1273, 28 days apart, induced dose-dependent antigen-binding titers131. Combining in vitro pseudovirus and wild-type virus neutralizing assays, antibody protection appeared comparable to that measured in sera from convalescent patients, especially after the second vaccination. Similar to data obtained in primates, a CD4+ TH1-biased response was confirmed by the expression of TNFα, IL-2, and IFN-γ, and low levels of spike-specific CD8+ response was observed131. A randomized, placebo-controlled, phase III study was conducted in +30,000 patients to evaluate two 100-μg intramuscular doses of the vaccine, administered 28 days apart132. Over 120 days following randomization, 11 infections were diagnosed in patients that had received mRNA-1273, compared to 185 in the placebo group, hence an estimated efficacy of 94%132. Importantly, the vaccine appeared to efficiently protect against the severe form of the disease which occurred in 30 participants form the placebo group, but none from the treatment arm.
In essence, preclinical133 and clinical128,134 evaluation of Pfizer/BioNTech's technology afforded very similar results. Intramuscular injection of the vaccine induced the production of neutralizing antibodies in mice and rhesus macaques133, presumably by transfecting the antigen in APCs from the draining lymph nodes and the spleen. Non-human primates (2–4 years of age) were also protected against infection, upon challenge with 1.05 × 106 plaque forming units of the virus133. In contrast with data obtained with mRNA-1273, vaccination with BNT162b2 showed evidence of spike-specific CD8+ cellular response in macaques133 and humans135. In the randomized, placebo-controlled phase III trial, the regimen consisted of two 30-μg doses administered intramuscularly at a 21-day interval. Over 120 days, vaccination resulted in a 95% efficacy (i.e., 8 cases of COVID-19 with the vaccines vs. 165 in the placebo group, +43,000 patients randomized). In the whole study, only 10 cases of severe disease were reported, with one in the vaccine group occurring >60 days after the second dose.
These very positive results led to recent regulatory milestones for both Moderna's and Pfizer/BioNTech's vaccines. In December 2020, the FDA authorized emergency use for both vaccines, while the European Medical Agency recommended conditional marketing authorizations. Broad vaccination campaigns have since then been initiated.
6. Conclusions and perspectives
Mammals have developed superb capabilities to protect themselves against microbes. When designing novel therapeutics, understanding of the complex relationships between biological functions might be valuable to predict possible inefficacies and adverse reactions. Whether the intended purpose of the technology is to deliver therapeutic payloads more efficiently or to vaccinate against pathogens, being able to foresee how the host will react to single and multiple doses is critical.
Until now, numerous efforts in the field of nanomedicine have been directed toward fighting cancer. The preclinical development of such technologies often necessitates the use of immunocompromised models which do not fully embody what occurs in immunocompetent hosts. For this reason, interactions of nanomedicines with the immune systems are sometimes overlooked. Likewise, while the clinical successes (and failures) of nanomedicines might provide some understanding on how to develop drugs that will help patients the most, many of the commercialized products are highly cytotoxic. With such agents, deconvoluting the effects of the drug from those of the carrier remains difficult.
The recent commercialization of systemic RNA-based therapies, notably patisiran lipid complexes (Onpattro®), has paved the way for the use of nanomedicines beyond cancer. Opportunities will continue to arise to encapsulate, protect and efficiently deliver drugs and other molecules for a variety of human diseases. Similarly, although it is too soon to predict whether nanosized vaccines will be our ticket out of the global COVID-19 pandemic, the rapid development of Moderna's mRNA-1273 and BioNTech's BNT162b1 have clearly consolidated interest toward mRNA vaccines. Altogether, this justifies concerted efforts toward a better understanding of the intricacies governing how nanomedicines interact with biological systems.
Author contributions
Iara Maíra de Oliveira Viana and Nicolas Bertrand wrote the article, Iara Maíra de Oliveira Viana, Sabrina Roussel, and Nicolas Bertrand prepared the figures, all authors revised and commented on the article.
Conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
We are grateful for the financial support of the Canadian agencies Natural Sciences and Engineering Research Council of Canada, the Canada Foundation for Innovation, and the Fondation du CHU de Quebec. NB is a Junior 1 Research Scholar from the Fonds de Recherche du Québec – Santé.
Footnotes
Peer review under responsibility of Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences.
References
- 1.Nel A.E., Mädler L., Velegol D., Xia T., Hoek E.M.V., Somasundaran P. Understanding biophysicochemical interactions at the nano-biointerface. Nat Mater. 2009;8:543–557. doi: 10.1038/nmat2442. [DOI] [PubMed] [Google Scholar]
- 2.Netea M.G., Domínguez-Andrés J., Barreiro L.B., Chavakis T., Divangahi M., Fuchs E. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20:375–388. doi: 10.1038/s41577-020-0285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Poon I.K.H., Lucas C.D., Rossi A.G., Ravichandran K.S. Apoptotic cell clearance: basic biology and therapeutic potential. Nat Rev Immunol. 2014;14 doi: 10.1038/nri3607. l66–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belkaid Y., Hand T. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121–141. doi: 10.1016/j.cell.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lacy P., Stow J.L. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118:9–18. doi: 10.1182/blood-2010-08-265892. [DOI] [PubMed] [Google Scholar]
- 6.Murphy K., Travers P., Walport M., Janeway C. 8th ed. Garland Science; New York: 2012. Janeway's immunobiology. [Google Scholar]
- 7.Ab Kadir R., Ariffin S.H.Z., Wahab R.M.A., Senafi S. Molecular characterisation of human peripheral blood stem cells. South Afr J Sci. 2012;108 [Google Scholar]
- 8.Rheinländer A., Schraven B., Bommhardt U. CD45 in human physiology and clinical medicine. Immunol Lett. 2018;196:22–32. doi: 10.1016/j.imlet.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Aderem A., Underhill D.M. Mechanisms of phagocytosis in macrophages. Annu Rev Immunol. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 10.Flannagan R.S., Jaumouillé V., Grinstein S. The cell biology of phagocytosis. Annu Rev Pathol. 2012;7:61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki A., Medzhitov R. Control of adaptive immunity by the innate immune system. Nat Immunol. 2015;4:343–353. doi: 10.1038/ni.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borisenko G.G., Matsura T., Liu S.X., Tyurin V.A., Jianfei J., Serinkan F.B. Macrophage recognition of externalized phosphatidylserine and phagocytosis of apoptotic Jurkat cells—existence of a threshold. Arch Biochem Biophys. 2003;413:41–52. doi: 10.1016/s0003-9861(03)00083-3. [DOI] [PubMed] [Google Scholar]
- 13.Harrison J.E., Schultz J. Studies on the chlorinating activity of myeloperoxidase. J Biol Chem. 1976;251:1371–1374. [PubMed] [Google Scholar]
- 14.Champion J.A., Walker A., Mitragotri S. Role of particle size in phagocytosis of polymeric microspheres. Pharm Res. 2008;25:1815–1821. doi: 10.1007/s11095-008-9562-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cannon G.J., Swanson J.A. The macrophage capacity for phagocytosis. J Cell Sci. 1992;101:907–913. doi: 10.1242/jcs.101.4.907. [DOI] [PubMed] [Google Scholar]
- 16.Champion J.A., Mitragotri S. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A. 2006;103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kawai T., Akira S. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol. 2010;11:373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 18.Mayadas T.N., Cullere X., Lowell C.A. The multifaceted functions of neutrophils. Annu Rev Pathol. 2014;9:181–218. doi: 10.1146/annurev-pathol-020712-164023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aoki A., Hirahara K., Kiuchi M., Nakayama T. Eosinophils: cells known for over 140 years with broad and new functions. Allergol Int. 2020;70:3–8. doi: 10.1016/j.alit.2020.09.002. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg H.F., Dyer K.D., Foster P.S. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22. doi: 10.1038/nri3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D.S. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 22.Clark S.R., Ma A.C., Tavener S.A., McDonald B., Goodarzi Z., Kelly M.M. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs T.A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D.D. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A. 2010;107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adams N.M., Grassmann S., Sun J.C. Clonal expansion of innate and adaptive lymphocytes. Nat Rev Immunol. 2020;11:694–707. doi: 10.1038/s41577-020-0307-4. [DOI] [PubMed] [Google Scholar]
- 25.Vivier E., Raulet D.H., Moretta A., Caligiuri M.A., Zitvogel L., Lanier L.L. Innate or adaptive immunity?. The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Horowitz A., Strauss-Albee D.M., Leipold M., Kubo J., Nemat-Gorgani N., Dogan O.C. Genetic and environmental determinants of human NK cell diversity revealed by mass cytometry. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan A.C., Carter P.J. Therapeutic antibodies for autoimmunity and inflammation. Nat Rev Immunol. 2010;10:301–316. doi: 10.1038/nri2761. [DOI] [PubMed] [Google Scholar]
- 28.Barrow A.D., Martin C.J., Colonna M. The natural cytotoxicity receptors in health and disease. Front Immunol. 2019;10:909. doi: 10.3389/fimmu.2019.00909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moghimi S.M., Hunter A.C., Murray J.C. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacol Rev. 2001;53:283–318. [PubMed] [Google Scholar]
- 30.Allen T.M., Hansen C., Martin F., Redemann C., Yau-Young A. Liposomes containing synthetic lipid derivatives of poly(ethylene glycol) show prolonged circulation half-lives in vivo. Biochim Biophys Acta. 1991;1066:29–36. doi: 10.1016/0005-2736(91)90246-5. [DOI] [PubMed] [Google Scholar]
- 31.Gabizon A., Papahadjopoulos D. Liposome formulations with prolonged circulation time in blood and enhanced uptake by tumors. Proc Natl Acad Sci U S A. 1988;85:6949–6953. doi: 10.1073/pnas.85.18.6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gref R., Minamitake Y., Peracchia M.T., Trubetskoy V., Torchilin V.P., Langer R. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263:1600–1602. doi: 10.1126/science.8128245. [DOI] [PubMed] [Google Scholar]
- 33.Leroux J.C., De Jaeghere F., Anner B., Doekler E., Gurny R. An investigation on the role of plasma and serum opsonins on the internalization of biodegradable poly(d,l-lactic acid) nanoparticles by human monocytes. Life Sci. 1995;57:695–703. doi: 10.1016/0024-3205(95)00321-v. [DOI] [PubMed] [Google Scholar]
- 34.Bazile D., Prud'homme C., Bassoullet M.T., Marlard M., Spenlehauer G., Veillard M. Stealth Me.PEG-PLA nanoparticles avoid uptake by the mononuclear phagocytes system. J Pharmaceut Sci. 1995;84:493–498. doi: 10.1002/jps.2600840420. [DOI] [PubMed] [Google Scholar]
- 35.Walkey C.D., Olsen J.B., Guo H., Emili A., Chan W.C.W. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J Am Chem Soc. 2012;134:2139–2147. doi: 10.1021/ja2084338. [DOI] [PubMed] [Google Scholar]
- 36.Bisso P.W., Gaglione S., Guimarães P.P.G., Mitchell M.J., Langer R. Nanomaterial interactions with human neutrophils. ACS Biomater Sci Eng. 2018;4:4255–4265. doi: 10.1021/acsbiomaterials.8b01062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bartneck M., Keul H.A., Zwadlo-Klarwasser G., Groll J. Phagocytosis independent extracellular nanoparticles clearance by human immune cells. Nano Lett. 2010;10:59–63. doi: 10.1021/nl902830x. [DOI] [PubMed] [Google Scholar]
- 38.Rizzi M., Carniato F., Tonello S., Migliario M., Invernizzi M., Rocchetti V. Charged molecular silica trigger in vitro NETosis in human granulocytes via both oxidative and autophagic pathways. Eur Rev Med Pharmacol Sci. 2018;22:7058–7068. doi: 10.26355/eurrev_201810_16178. [DOI] [PubMed] [Google Scholar]
- 39.Peng H.H., Liu Y.J., Ojcius D.M., Lee C.M., Chen R.H., Huang P.R. Mineral particles stimulate innate immunity through neutrophil extracellular traps containing HMGB1. Sci Rep. 2017;7:16628. doi: 10.1038/s41598-017-16778-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang T.L., Aljuffali I.A., Hung C.F., Chen C.H., Fang J.Y. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs) Chem Biol Interact. 2015;235:106–114. doi: 10.1016/j.cbi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 41.Oja C.D., Semple S.C., Chonn A., Cullis P.R. Influence of dose on liposome clearance: critical role of blood proteins. Biochim Biophys Acta. 1996;1281:31–37. doi: 10.1016/0005-2736(96)00003-x. [DOI] [PubMed] [Google Scholar]
- 42.Allen T.M., Hansen C. Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta. 1991;1068:133–141. doi: 10.1016/0005-2736(91)90201-i. [DOI] [PubMed] [Google Scholar]
- 43.Panagi Z., Beletsi A., Evangelatos G., Livianou E., Ithakissios D., Avgoustakis K. Effect of dose on the biodistribution and pharmacokinetics of PLGA and PLGA-mPEG nanoparticles. Int J Pharm. 2001;221:143–152. doi: 10.1016/s0378-5173(01)00676-7. [DOI] [PubMed] [Google Scholar]
- 44.Chow D.D., Essien H.E., Padki M.M., Hwang K.L. Targeting small unilamellar liposomes to hepatic parenchymal cells by dose effect. J Pharmacol Exp Therapeut. 1989;248:506–513. [PubMed] [Google Scholar]
- 45.Bertrand N., Grenier P., Mahmoudi M., Lima E.M., Appel E.A., Dormont F. Mechanistic understanding of in vivo protein corona formation on polymeric nanoparticles and impact on pharmacokinetics. Nat Commun. 2017;8:777. doi: 10.1038/s41467-017-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Van Rooijen N., Kors N., Van de Ende M., Dijkstra C. Depletion and repopulation of macrophages in spleen and liver of rat after intravenous treatment with liposome-encapsulated dichloromethylene diphosphonate. Cell Tissue Res. 1990;260:215–222. doi: 10.1007/BF00318625. [DOI] [PubMed] [Google Scholar]
- 47.Ohara Y., Oda T., Yamada K., Hashimoto S., Akashi Y., Miyamoto R. Effective delivery of chemotherapeutic nanoparticles by depleting host Kupffer cells. Int J Cancer. 2012;131:2402–2410. doi: 10.1002/ijc.27502. [DOI] [PubMed] [Google Scholar]
- 48.Tavares A.J., Poon W., Zhang Y.N., Dai Q., Besla R., Ding D. Effect of removing Kupffer cells on nanoparticle tumor delivery. Proc Natl Acad Sci U S A. 2017;114:E10871–E10880. doi: 10.1073/pnas.1713390114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bertrand N., Leroux J.C. The journey of a drug carrier in the body: an anatomo-physiological perspective. J Control Release. 2012;161:152–163. doi: 10.1016/j.jconrel.2011.09.098. [DOI] [PubMed] [Google Scholar]
- 50.Liu T., Choi H., Zhou R., Chen I.W. RES blockade: a strategy for boosting efficiency of nanoparticle drug. Nano Today. 2015;10:11–21. [Google Scholar]
- 51.Nikitin M.P., Zelepukin I.V., Shipunova V.O., Sokolov I.L., Deyev S.M., Nikitin P.I. Enhancement of the blood-circulation time and performance of nanomedicines via the forced clearance of erythrocytes. Nat Biomed Eng. 2020;4:717–731. doi: 10.1038/s41551-020-0581-2. [DOI] [PubMed] [Google Scholar]
- 52.Ouyang B., Poon W., Zhang Y.N., Lin Z.P., Kingston B.R., Tavares A.J. The dose threshold for nanoparticle tumour delivery. Nat Mater. 2020;19:1362–1371. doi: 10.1038/s41563-020-0755-z. [DOI] [PubMed] [Google Scholar]
- 53.Wu H., Infante J., Keedy V., Jones S., Chan E., Bendell J. Factors affecting the pharmacokinetics and pharmacodynamics of PEGylated liposomal irinotecan (IHL-305) in patients with advanced solid tumors. Int J Nanomed. 2015;10:1201–1209. doi: 10.2147/IJN.S62911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zamboni W.C., Maruca L.J., Strychor S., Zamboni B.A., Ramalingam S., Edwards R.P. Bidirectional pharmacodynamic interaction between pegylated liposomal CKD-602 (S-CKD602) and monocytes in patients with refractory solid tumors. J Liposome Res. 2011;21:158–165. doi: 10.3109/08982104.2010.496085. [DOI] [PubMed] [Google Scholar]
- 55.Gabizon A., Isacson R., Rosengarten O., Tzemach D., Shmeeda H., Sapir R. An open-label study to evaluate dose and cycle dependence of the pharmacokinetics of pegylated liposomal doxorubicin. Cancer Chemother Pharmacol. 2008;61:695–702. doi: 10.1007/s00280-007-0525-5. [DOI] [PubMed] [Google Scholar]
- 56.La-Beck N.M., Zamboni B.A., Gabizon A., Schmeeda H., Amantea M., Gehrig P.A. Factors affecting the pharmacokinetics of pegylated liposomal doxorubicin in patients. Cancer Chemother Pharmacol. 2012;69:43–50. doi: 10.1007/s00280-011-1664-2. [DOI] [PubMed] [Google Scholar]
- 57.Mross K., Niemann B., Massing U., Drevs J., Unger C., Bhamra R. Pharmacokinetics of liposomal doxorubicin (TLC-D99; Myocet) in patients with solid tumors: an open-label, single-dose study. Cancer Chemother Pharmacol. 2004;54:514–524. doi: 10.1007/s00280-004-0825-y. [DOI] [PubMed] [Google Scholar]
- 58.Hajishengallis G., Reis E.S., Mastellos D.C., Ricklin D., Lambris J.D. Novel mechanisms and functions of complement. Nat Immunol. 2017;18:1288–1298. doi: 10.1038/ni.3858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kolev M., Le Friec G., Kemper C. Complement — tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 60.Reis E.S., Mastellos D.C., Hajishengallis G., Lambris J.D. New insights into the immune functions of complement. Nat Rev Immunol. 2019;19:503–516. doi: 10.1038/s41577-019-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chonn A., Cullis P.R., Devine D.V. The role of surface charge in the activation of the classical and alternative pathways of complement by liposomes. J Immunol. 1991;146:4234–4241. [PubMed] [Google Scholar]
- 62.Devine D.V., Wong K., Serrano K., Chonn A., Cullis P.R. Liposome-complement interactions in rat serum: implications for liposome survival studies. Biochim Biophys Acta. 1994;1191:43–51. doi: 10.1016/0005-2736(94)90231-3. [DOI] [PubMed] [Google Scholar]
- 63.Alléman É., Gravel P., Leroux J.C., Balant L., Gurny R. Kinetics of blood component adsorption on poly(d,l-lactic acid) nanoparticles: evidence of complement C3 component involvement. J Biomed Mater Res. 1997;37:229–234. doi: 10.1002/(sici)1097-4636(199711)37:2<229::aid-jbm12>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 64.Hamad I., Al-Hanbali O., Hunter A.C., Rutt K.J., Andresen T.L., Moghimi S.M. Distinct polymer architecture mediates switching of complement activation pathways at the nanosphere-serum interface: implications for stealth nanoparticle engineering. ACS Nano. 2010;4:6629–6638. doi: 10.1021/nn101990a. [DOI] [PubMed] [Google Scholar]
- 65.Perry J.L., Reuter K.G., Kai M.P., Herlihy K.P., Jones S.W., Luft J.C. Nanoparticles: the impact of peg density on protein binding, macrophage association, biodistribution, and pharmacokinetics. Nano Lett. 2012;12:5304–5310. doi: 10.1021/nl302638g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Moghimi S.M., Simberg D., Papini E., Farhangrazi Z.S. Complement activation by drug carriers and particulate pharmaceuticals: principles, challenges and opportunities. Adv Drug Deliv Rev. 2020;157:83–95. doi: 10.1016/j.addr.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bottermann M., Foss S., Caddy S.L., Clift D., van Tienen L.M., Vaysburd M. Complement C4 prevents viral infection through capsid inactivation. Cell Host Microbe. 2019;25:617–629. doi: 10.1016/j.chom.2019.02.016. e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li E., Tako E.A., Singer S.M. Complement activation by Giardia duodenalis parasites through the lectin pathway contributes to mast cell responses and parasite control. Infect Immun. 2016;84:1092–1099. doi: 10.1128/IAI.00074-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ahl P.L., Bhatia S.K., Meers P., Roberts P., Stevens R., Dause R. Enhancement of the in vivo circulation lifetime of l-α-distearoylphosphatidylcholine liposomes: importance of liposomal aggregation versus complement opsonization. Biochim Biophys Acta. 1997;1329:370–382. doi: 10.1016/s0005-2736(97)00129-6. [DOI] [PubMed] [Google Scholar]
- 70.Barron L.G., Meyer K.B., Szoka F.C.J. Effects of complement depletion on the pharmacokinetics and gene delivery mediated by cationic lipid–DNA complexes. Hum Gene Ther. 1998;9:315–323. doi: 10.1089/hum.1998.9.3-315. [DOI] [PubMed] [Google Scholar]
- 71.Grenier P., Viana I.M.O., Lima E.M., Bertrand N. Anti-polyethylene glycol antibodies alter the protein corona deposited on nanoparticles and the physiological pathways regulating their fate in vivo. J Control Release. 2018;287:121–131. doi: 10.1016/j.jconrel.2018.08.022. [DOI] [PubMed] [Google Scholar]
- 72.Viana I.M.O., Grenier P., Defrêne J., Barabé F., Lima E.M., Bertrand N. Role of the complement cascade on the biological fate of liposomes in rodents. Nanoscale. 2020;12:18875–18884. doi: 10.1039/d0nr04100a. [DOI] [PubMed] [Google Scholar]
- 73.Wang L., Su Y., Wang X., Liang K., Liu M., Tang W. Effects of complement inhibition on the ABC phenomenon in rats. Asian J Pharm Sci. 2016;3:250–258. doi: 10.1016/j.ajps.2016.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Carpentier K.S., Davenport B.J., Haist K.C., McCarthy M.K., May N.A., Robison A. Discrete viral E2 lysine residues and scavenger receptor MARCO are required for clearance of circulating alphaviruses. ELife. 2019;8:49163. doi: 10.7554/eLife.49163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu Z., Tian J., Smith J.S., Byrnes A.P. Clearance of adenovirus by Kupffer cells is mediated by scavenger receptors, natural antibodies, and complement. J Virol. 2008;82:11705–11713. doi: 10.1128/JVI.01320-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen F., Wang G., Griffin J.I., Brenneman B., Banda N.K., Holers V.M. Complement proteins bind to nanoparticle protein corona and undergo dynamic exchange in vivo. Nat Nanotechnol. 2016;12:387–393. doi: 10.1038/nnano.2016.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Inturi S., Wang G., Chen F., Banda N.K., Holers V.M., Wu L. Modulatory role of surface coating of superparamagnetic iron oxide nanoworms in complement opsonization and leukocyte uptake. ACS Nano. 2015;9:10758–10768. doi: 10.1021/acsnano.5b05061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schöttler S., Becker G., Winzen S., Steinbach T., Mohr K., Landfester K. Protein adsorption is required for stealth effect of poly(ethylene glycol)- and poly(phosphoester)-coated nanocarriers. Nat Nanotechnol. 2016;11:372–377. doi: 10.1038/nnano.2015.330. [DOI] [PubMed] [Google Scholar]
- 79.Bankwitz D., Doepke M., Hueging K., Weller R., Bruening J., Behrendt P. Maturation of secreted HCV particles by incorporation of secreted ApoE protects from antibodies by enhancing infectivity. J Hepatol. 2017;67:480–489. doi: 10.1016/j.jhep.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 80.Dong Y., Love K.T., Dorkin J.R., Sirirungruang S., Zhang Y., Chen D. Lipopeptide nanoparticles for potent and selective siRNA delivery in rodents and nonhuman primates. Proc Natl Acad Sci U S A. 2014;111:3955–3960. doi: 10.1073/pnas.1322937111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Vu V.P., Gifford G.B., Chen F., Benasutti H., Wang G., Groman E.V. Immunoglobulin deposition on biomolecule corona determines complement opsonization efficiency of preclinical and clinical nanoparticles. Nat Nanotechnol. 2019;14:260–268. doi: 10.1038/s41565-018-0344-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ding T., Guan J., Wang M., Long Q., Liu X., Qian J. Natural IgM dominates in vivo performance of liposomes. J Control Release. 2020;319:371–381. doi: 10.1016/j.jconrel.2020.01.018. [DOI] [PubMed] [Google Scholar]
- 83.Spellberg B., Edwards J.E. Type 1/type 2 immunity in infectious diseases. Clin Infect Dis. 2001;32:76–102. doi: 10.1086/317537. [DOI] [PubMed] [Google Scholar]
- 84.Italiani P., Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514. doi: 10.3389/fimmu.2014.00514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liddiard K., Taylor P.R. Understanding local macrophage phenotypes in disease: shape-shifting macrophages. Nat Med. 2015;21:119–120. doi: 10.1038/nm.3798. [DOI] [PubMed] [Google Scholar]
- 86.Jones S.W., Roberts R.A., Robbins G.R., Perry J.L., Kai M.P., Chen K. Nanoparticle clearance is governed by Th1/Th2 immunity and strain background. J Clin Invest. 2013;123:3061–3073. doi: 10.1172/JCI66895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bertrand N., Wu J., Xu X., Kamaly N., Farokhzad O.C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv Drug Deliv Rev. 2014;66:2–25. doi: 10.1016/j.addr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miller M.A., Zheng Y.R., Gadde S., Pfirschke C., Zope H., Engblom C. Tumour-associated macrophages act as a slow-release reservoir of nano-therapeutic Pt(IV) pro-drug. Nat Commun. 2015;6:8692. doi: 10.1038/ncomms9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodell C.B., Arlauckas S.P., Cuccarese M.F., Garris C.S., Li R., Ahmed M.S. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour-associated macrophages to enhance cancer immunotherapy. Nat Biomed Eng. 2018;2:578–588. doi: 10.1038/s41551-018-0236-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shan H., Dou W., Zhang Y., Qi M. Targeted ferritin nanoparticle encapsulating CpG oligodeoxynucleotides induces tumor-associated macrophage M2 phenotype polarization into M1 phenotype and inhibits tumor growth. Nanoscale. 2020;12:22268–22280. doi: 10.1039/d0nr04520a. [DOI] [PubMed] [Google Scholar]
- 91.Rao L., Wu L., Liu Z., Tian R., Yu G., Zhou Z. Hybrid cellular membrane nanovesicles amplify macrophage immune responses against cancer recurrence and metastasis. Nat Commun. 2020;11:4909. doi: 10.1038/s41467-020-18626-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Steinman R.M., Witmer M.D. Lymphoid dendritic cells are potent stimulators of the primary mixed leukocyte reaction in mice. Proc Natl Acad Sci U S A. 1978;75:5132–5136. doi: 10.1073/pnas.75.10.5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ekeruche-Makinde J., Miles J.J., van den Berg H.A., Skowera A., Cole D.K., Dolton G. Peptide length determines the outcome of TCR/peptide-MHCI engagement. Blood. 2013;121:1112–1123. doi: 10.1182/blood-2012-06-437202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sant A.J. Endogenous antigen presentation by MHC class II molecules. Immunol Res. 1994;13:253–267. doi: 10.1007/BF02935617. [DOI] [PubMed] [Google Scholar]
- 95.Pape K.A., Catron D.M., Itano A.A., Jenkins M.K. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity. 2007;26:491–502. doi: 10.1016/j.immuni.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 96.Jakubzick C., Gautier E.L., Gibbings S.L., Sojka D.K., Schlitzer A., Johnson T.E. Minimal differentiation of classical monocytes as they survey steady-state tissues and transport antigen to lymph nodes. Immunity. 2013;39:599–610. doi: 10.1016/j.immuni.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Willard-Mack C.L. Normal structure, function, and histology of lymph nodes. Toxicol Pathol. 2006;34:409–424. doi: 10.1080/01926230600867727. [DOI] [PubMed] [Google Scholar]
- 98.Swartz M.A. The physiology of the lymphatic system. Adv Drug Deliv Rev. 2001;50:3–20. doi: 10.1016/s0169-409x(01)00150-8. [DOI] [PubMed] [Google Scholar]
- 99.Roozendaal R., Mempel T.R., Pitcher L.A., Gonzalez S.F., Verschoor A., Mebius R.E. Conduits mediate transport of low-molecular-weight antigen to lymph node follicles. Immunity. 2009;30:264–276. doi: 10.1016/j.immuni.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Junt T., Moseman E.A., Iannacone M., Massberg S., Lang P.A., Boes M. Subcapsular sinus macrophages in lymph nodes clear lymph-borne viruses and present them to antiviral B cells. Nature. 2007;450:110–114. doi: 10.1038/nature06287. [DOI] [PubMed] [Google Scholar]
- 101.Proulx S.T., Luciani P., Christiansen A., Karaman S., Blum K.S., Rinderknecht M. Use of a PEG-conjugated bright near-infrared dye for functional imaging of rerouting of tumor lymphatic drainage after sentinel lymph node metastasis. Biomaterials. 2013;34:5128–5137. doi: 10.1016/j.biomaterials.2013.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Reddy S.T., van der Vlies A.J., Simeoni E., Angeli V., Randolph G.J., O'Neil C.P. Exploiting lymphatic transport and complement activation in nanoparticles vaccines. Nat Biotechnol. 2007;25:1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 103.Palucka K., Banchereau J. Cancer immunotherapy via dendritic cells. Nat Rev Cancer. 2012;12:265–277. doi: 10.1038/nrc3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Akkaya M., Kwak K., Pierce S.K. B cell memory: building two walls of protection against pathogens. Nat Rev Immunol. 2020;20:229–238. doi: 10.1038/s41577-019-0244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ochsenbein A.F., Zinkernagel R.M. Natural antibodies and complement link innate and acquired immunity. Immunol Today. 2000;21:624–630. doi: 10.1016/s0167-5699(00)01754-0. [DOI] [PubMed] [Google Scholar]
- 106.Dams E.T.M., Laverman P., Oyen W.J.G., Storm G., Scherphof G.L., Van der Meer J.W.M. Accelerated blood clearance and altered biodistribution of repeated injections of sterically stabilized liposomes. J Pharmacol Exp Therapeut. 2000;292:1071–1079. [PubMed] [Google Scholar]
- 107.Wang X.Y., Ishida T., Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119:236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 108.Ishida T., Ichihara M., Wang X.Y., Kiwada H. Spleen plays an important role in the induction of accelerated blood clearance of PEGylated liposomes. J Control Release. 2006;115:243–250. doi: 10.1016/j.jconrel.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 109.Ishida T., Wang X.Y., Shimizu T., Nawata K., Kiwada H. PEGylated liposomes elicit an anti-PEG IgM response in a T cell-independent manner. J Control Release. 2007;122:349–355. doi: 10.1016/j.jconrel.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 110.Tagami T., Nakamura K., Shimizu T., Ishida T., Kiwada H. Effect of siRNA in PEG-coated siRNA-lipoplex on anti-PEG IgM production. J Control Release. 2009;137:234–240. doi: 10.1016/j.jconrel.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 111.Shimizu T., Ichihara M., Yoshioka Y., Ishida T., Nakagawa S., Kiwada H. Intravenous administration of polyethylene glycol-coated (PEGylated) proteins and PEGylated adenovirus elicits an anti-PEG immunoglobulin M response. Biol Pharm Bull. 2012;35:1336–1342. doi: 10.1248/bpb.b12-00276. [DOI] [PubMed] [Google Scholar]
- 112.Wang X.Y., Ishida T., Ichihara M., Kiwada H. Influence of the physicochemical properties of liposomes on the accelerated blood clearance phenomenon in rats. J Control Release. 2005;104:91–102. doi: 10.1016/j.jconrel.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 113.Ishida T., Harada M., Wang X.Y., Ichihara M., Irimura K., Kiwada H. Accelerated blood clearance of PEGylated liposomes following preceding liposome injection: effects of lipid dose and PEG surface-density and chain length of the first-dose liposomes. J Control Release. 2005;105:305–317. doi: 10.1016/j.jconrel.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 114.Ishida T., Atobe K., Wang X.Y., Kiwada H. Accelerated blood clearance of PEGylated liposomes upon repeated injections: effect of doxorubicin-encapsulation and high dose first injection. J Control Release. 2006;115:251–258. doi: 10.1016/j.jconrel.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 115.Yang Q., Jacobs T.M., McCallen J.D., Moore D.T., Huckaby J.T., Edelstein J.N. Analysis of pre-existing IgG and IgM antibodies against polyethylene glycol (PEG) in the general population. Anal Chem. 2016;88:11804–11812. doi: 10.1021/acs.analchem.6b03437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lubich C., Allacher P., de la Rosa M., Bauer A., Prenninger T., Horling F.M. The mystery of antibodies against polyethylene glycol (PEG)—what do we know? Pharm Res. 2016;33:2239–2249. doi: 10.1007/s11095-016-1961-x. [DOI] [PubMed] [Google Scholar]
- 117.Chen B.M., Su Y.C., Chang C.J., Burnouf P.A., Chuang K.H., Chen C.H. Measurement of pre-existing IgG and IgM antibodies against polyethylene glycol in healthy individuals. Anal Chem. 2016;88:10661–10666. doi: 10.1021/acs.analchem.6b03109. [DOI] [PubMed] [Google Scholar]
- 118.Rizvi N.A., Hellmann M.D., Snyder A., Kvistborg P., Makarov V., Havel J.J. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lebel M.È., Chartrand K., Tarrab E., Savard P., Leclerc D., Lamarre A. Potentiating cancer immunotherapy using papaya mosaic virus-derived nanoparticles. Nano Lett. 2016;16:1826–1832. doi: 10.1021/acs.nanolett.5b04877. [DOI] [PubMed] [Google Scholar]
- 120.Bu J., Nair A., Iida M., Jeong W.J., Poellmann M.J., Mudd K. An avidity-based PD-L1 antagonist using nanoparticle–antibody conjugates for enhanced immunotherapy. Nano Lett. 2020;20:4901–4909. doi: 10.1021/acs.nanolett.0c00953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Han X., Wang L., Li T., Zhang J., Zhang D., Li J. Beyond blocking: engineering RNAi-mediated targeted immune checkpoint nanoblocker enables T-cell-independent cancer treatment. ACS Nano. 2020;14:17524–17534. doi: 10.1021/acsnano.0c08022. [DOI] [PubMed] [Google Scholar]
- 122.Oberli M.A., Reichmuth A.M., Dorkin J.R., Mitchell M.J., Fenton O.S., Jaklenec A. Lipid nanoparticle assisted mRNA delivery for potent cancer immunotherapy. Nano Lett. 2017;17:1326–1335. doi: 10.1021/acs.nanolett.6b03329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Islam M.A., Rice J., Reesor E., Zope H., Tao W., Lim M. J. Adjuvant-pulsed mRNA vaccine nanoparticle for immunoprophylactic and therapeutic tumor suppression in mice. Biomaterials. 2021;266:120431. doi: 10.1016/j.biomaterials.2020.120431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rosenberg S.A., Restifo N.P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science. 2015;348:62–68. doi: 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Parayath N.N., Stephan S.B., Koehne A.L., Nelson P.S., Stephan M.T. In vitro-transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat Commun. 2020;11:6080. doi: 10.1038/s41467-020-19486-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim J., Eygeris Y., Gupta M., Sahay G. Self-assembled mRNA vaccines. Adv Drug Deliv Rev. 2021;170:83–112. doi: 10.1016/j.addr.2020.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N Engl J Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 129.Zhang N.N., Li X.F., Deng Y.Q., Zhao H., Huang Y.J., Yang G. A thermostable mRNA vaccine against COVID-19. Cell. 2020;182:1271–1283. doi: 10.1016/j.cell.2020.07.024. e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M. Immunogenic BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021;592:283–289. doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 134.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020 https://ncrc.jhsph.edu/research/bnt162b2-induces-sars-cov-2-neutralising-antibodies-and-t-cells-in-humans/ Available from: [Google Scholar]