Abstract
Background
Short-term exposure to ozone and nitrogen dioxide is a risk factor for acute exacerbation (AE) of idiopathic pulmonary fibrosis (AE-IPF). The comprehensive roles of exposure to fine particulate matter in AE-IPF remain unclear. We aim to investigate the association of short-term exposure to fine particulate matter with the incidence of AE-IPF and to determine the exposure-risk time window during 3 months before the diagnosis of AE-IPF.
Methods
IPF patients were retrospectively identified from the nationwide registry in Japan. We conducted a case–control study to assess the correlation between AE-IPF incidence and short-term exposure to eight air pollutants, including particulate matter < 2.5 µm (PM2.5). In the time-series data, we compared monthly mean exposure concentrations between months with AE (case months) and those without AE (control months). We used multilevel mixed-effects logistic regression models to consider individual and institutional-level variables, and also adjusted these models for several covariates, including temperature and humidity. An additional analysis with different monthly lag periods was conducted to determine the risk-exposure time window for 3 months before the diagnosis of AE-IPF.
Results
Overall, 152 patients with surgically diagnosed IPF were analyzed. AE-IPF was significantly associated with an increased mean exposure level of nitric oxide (NO) and PM2.5 30 days prior to AE diagnosis. Adjusted odds ratio (OR) with a 10 unit increase in NO was 1.46 [95% confidence interval (CI) 1.11–1.93], and PM2.5 was 2.56 (95% CI 1.27–5.15). Additional analysis revealed that AE-IPF was associated with exposure to NO during the lag periods lag 1, lag 2, lag 1–2, and lag 1–3, and PM2.5 during the lag periods lag 1 and lag 1–2.
Conclusions
Our results show that PM2.5 is a risk factor for AE-IPF, and the risk-exposure time window related to AE-IPF may lie within 1–2 months before the AE diagnosis. Further investigation is needed on the novel findings regarding the exposure to NO and AE-IPF.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-021-01671-6.
Keywords: Acute exacerbation, Air pollution exposure, Idiopathic pulmonary fibrosis, Particulate matter, Risk factors
Background
Idiopathic pulmonary fibrosis (IPF) is a fibrotic lung disease characterized by the progressive impairment of lung function and poor prognosis [1]. The natural history of IPF is heterogeneous and its median survival is 2–5 years [1–3]. The associations of increased exposure levels of particulate matter < 2.5 µm (PM2.5) and particulate matter < 10 µm (PM10) and the mortality of patients with IPF are reported, but not correlated with the incidence of acute exacerbation (AE) of IPF (AE-IPF) [4]. The two antifibrotic drugs pirfenidone and nintedanib have recently been identified for the treatment of IPF [5, 6]. Several registries have shown the survival benefits of these two antifibrotic drugs on patients with IPF [7, 8]; however, IPF still remains a life-threatening disease.
Some patients with IPF experience severe deteriorations that are associated with rapid disease progression and high mortality, which is termed AE-IPF [9, 10]. AE-IPF is defined as the acute worsening of respiratory symptoms combined with new radiographic lung opacities on high-resolution computed tomography (HRCT) without any identifiable causes [9, 10]. The most common cause of death in Japanese patients with IPF is AE [3]. In addition, the percentage of AE-related deaths (40%) in Japan is reportedly higher than that observed in Western countries (18%) [3]. Viral infection [11], microaspiration [12] and SLB [13] are established triggers for AE-IPF. Regarding exposure to ambient air pollution as potential risk factors of AE-IPF, a significant association between the incidence of AE-IPF and increased mean exposure levels of ozone (O3) and nitrogen dioxide (NO2) during 0–42 days prior to AE was shown in South Korea [14]. Similarly, a French study showed that short-term exposure to increased level of O3 was positively related to AE-IPF [4].
Among ambient air pollutants, fine particulate matter is considered as particularly dangerous. Indeed, an association has been shown between the elevated levels of airborne fine particulate matter and the risk of hospital admissions for patients with asthma, chronic obstructive pulmonary disease (COPD) and cardiovascular disease [15–18]. Dales et al. demonstrated that exposure to fine particulate matter is a risk factor for hospitalization of patients with IPF [19]. The two previous studies (South Korea and France) suggested that fine particulate matter may be a risk factor for AE-IPF; however, their results were not statistically significant [4, 14]. Moreover, these studies applied the exposure time window as “the 0–42 days” prior to the diagnosis of AE according to the definition of AE-IPF (i.e. onset should be within 1 month prior to diagnosing AE-IPF) [4, 9, 10, 14]. However, the specific exposure time window during which patients with IPF are at risk of developing AEs remains undefined.
We hypothesized that the short-term exposure to fine particulate matter could increase the incidence of AE-IPF. Therefore, we investigated whether the incidence of AE-IPF associated with increased mean exposure level of fine particulate matter in the month with AE. Furthermore, we performed an additional analysis with different monthly lag periods to determine the exposure-risk time window during 3 months before the diagnosis of AE. We also investigated the correlations between the AE of idiopathic interstitial pneumonias (AE-IIPs) and the air pollutant exposure levels. The exposure levels of eight air pollutants, namely sulfur dioxide (SO2), nitric oxide (NO), NO2, nitrogen oxides (NOX), carbon monoxide (CO), O3, PM2.5, and PM10, were evaluated using the nationwide surgically diagnosed IPF and IIPs registry in Japan [20].
Methods
Study design
We performed a case–control study to investigate the correlation between short-term exposure to air pollutants and the incidence of AE. A time-series data was used to compare monthly mean exposure concentrations between 41 months with AE (case months) and 5742 months without AE (control months) (Additional file 1: Fig. S1). Using a multilevel approach, we adjusted the effects for individual confounders because this case–control study included the individual time-series data such as air pollutant levels. Finally, we adjusted the effects for individual and institution confounders using the multilevel mixed-effects logistic regression models with a two-level structure of patients nested within the 33 hospitals [21, 22]. Although case–crossover is the most common design for analyzing the health-related effects of air pollution [23], this statistical methodology (the case–control study partially including case–crossover design) allowed us to use all the single months without AE as controls (Additional file 1: Fig. S1).
Source database and study subjects
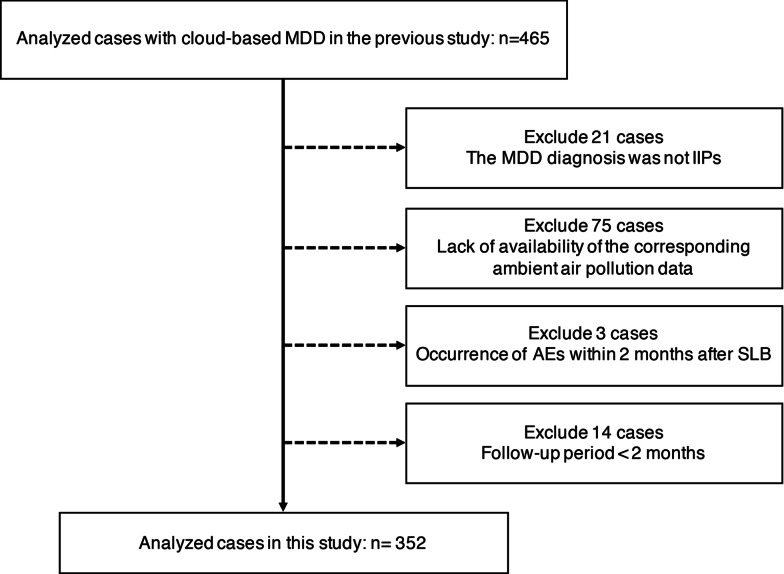
Japanese patients were identified from the nationwide cloud-based integrated database for IIPs in Japan [20]. In the online database, 465 patients with an institutional diagnosis of IIPs who had undergone chest HRCT and SLB from April 2009 to March 2014 were retrospectively collected from 39 institutions. Subsequently, a cloud-based MDD involving respiratory physicians, radiologists and pathologists with expertise in interstitial lung disease (ILD) was conducted via video-conferencing according to the International IPF statements and IIPs classification (see Additional file 1: Methods) [1, 24, 25]. From the database, we excluded patients who met the following criteria: (a) MDD diagnosis was not an IIPs; (b) the corresponding ambient air pollution data were unavailable (patients registered before 2008 were excluded due to unavailable air pollution data from the nationwide database); (c) AE occurred within 2 months after the SLB procedure and (d) follow-up period was < 2 months.
AE-IPF was diagnosed based on the following criteria established by the American Thoracic Society/European Respiratory Society [9]: (1) within 1 month of the clinical course of IPF disease progression, the following two conditions should have been satisfied: (a) worsening of dyspnea and (b) presence of new ground-glass opacities on chest HRCT and (2) exclusion of other identifiable causes [9]. Patients with AE-IIPs were diagnosed based on the criteria for AE-IPF [9]. Among patients who experienced ≥ 2 AEs, only the first event was included in the analysis. This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review boards of the University of Occupational and Environmental Health, Kitakyushu, Japan (18-013) and the Hamamatsu University School of Medicine, Hamamatsu, Japan (19-003) approved the protocol.
Measurement
We extracted the following information at the time of SLB from the database as baseline characteristics: age, sex, smoking status (pack-years), percentage of predicted value of forced vital capacity (FVC), percentage of predicted value of diffusing capacity of the lung for carbon monoxide (DLCO), HRCT pattern [1], and data pertaining to therapy with antifibrotic drugs (e.g., pirfenidone and nintedanib). The date of the SLB procedure and the diagnosis of AE from April 1, 2009, to March 31, 2017, were obtained from the database.
Daily and monthly mean concentrations of SO2, NO, NO2, NOX, CO, O3, PM2.5 and PM10 were obtained from the nationwide database of the Atmospheric Environment Regional Observation System using the website of the National Institute for Environmental Studies, Japan (http://www.nies.go.jp/igreen/index.html). O3 measurements were obtained during the 15-h period of daylight (5:00–20:00). For the PM10 measurements, the measurements obtained as suspended particulate matter (SPM) from the website were used because SPM is defined as airborne particulate matter with a diameter smaller than or equal to 10 in Japan. Daily and monthly mean temperature and humidity values were obtained from the database of the Japan Meteorological Agency (https://www.data.jma.go.jp/gmd/risk/obsdl/index.php). We selected the air monitoring stations located nearest to the registered hospitals from 1907 air monitoring stations in Japan and obtained the levels of air pollutants, temperature, and humidity. The patients who developed AE were admitted to these registered hospitals. Demographic data for each prefecture and neighborhood-level factors in Japan were obtained from the 2015 national census (https://www.e-stat.go.jp/).
Statistical analysis
Multilevel mixed-effects logistic regression models were used to evaluate the association between the incidence of AE-IPF and monthly mean exposure for each air pollutant by matching data on the case month with that on the control month. For each AE diagnosis, the case month was defined as 30 days before AE diagnosis (Additional file 1: Fig. S1). The control months were defined as all the single months during the date of SLB procedure to the date of death or censoring in patients without AE. In patients with AE, we served all the single months other than the case month as control months (see Additional file 1: Methods and Fig. S1). Patients who were alive without the incidence of AE on March 31, 2017 were censored. Patients lost to follow-up were censored at the date of last contact/follow-up. We adjusted the effects for individual and institution confounders using the multilevel regression models with a two-level structure of patients nested within the 33 hospitals [21, 22].
In addition, we estimated the single month lag exposure (lag 1 to lag 3) and cumulative exposure (lag 1–2 to lag 1–3). For example, lag 1 exposure refers to the exposure during 30 days prior to AE diagnosis, while lag 1–2 exposure refers to the exposure during 60 days before AE diagnosis. The definition of control and case periods in the additional analysis is shown in Additional file 1: Fig. S2.
These models were adjusted for temperature, humidity, age, sex, smoking status (pack-years), the percentage of the predicted value of FVC and DLCO at the time of SLB (risk factors identified in previous studies [26]), and neighborhood-level factors, such as regional characteristics of population density and per capita income [27]. Results are presented as odds ratios (ORs) with 95% confidence intervals (CIs) associated with a 10-unit increase in SO2 (ppb), NO (ppb), NO2 (ppb), NOX (ppb), CO (ppb), O3 (ppb), PM2.5 (µg/m3) and PM10 (µg/m3). We also assessed the association between the incidence of AE-IIPs and monthly mean exposure for each air pollutant using the same methodology used for AE-IPF. All analyses were conducted at a significant α level of 0.05. All statistical analyses were performed using the STATA 16.1 software (StataCorp, College Station, TX, USA). Complete details of the methods are available in the Additional file 1: Material.
Results
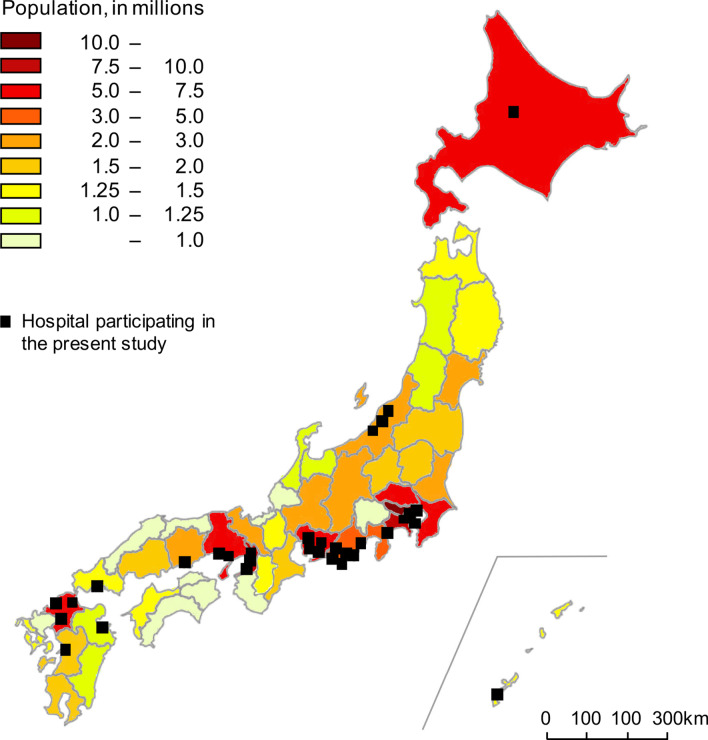
Among the 465 patients included in the previous study [20], 113 were excluded for the following reasons: MDD diagnosis was not an IIPs (n = 21); lack of the corresponding ambient air pollution data (n = 75); AE occurred within 2 months after SLB (n = 3); follow-up period was < 2 months (n = 14) (Fig. 1). Finally, 352 patients with IIPs (152 IPF, 35 idiopathic nonspecific interstitial pneumonia, four cryptogenic organizing pneumonia, seven desquamative interstitial pneumonia/respiratory bronchiolitis-ILD and 15 idiopathic pleuroparenchymal fibroelastosis and 139 unclassifiable IIPs) were enrolled in the present study (Table 1). Baseline (at SLB) clinical characteristics of the participants with or without AE-IPF and AE-IIPs are shown in Table 2 and Additional file 1: Table S1, respectively. The proportion of the UIP pattern in HRCT was 15% of the IPF patients (Table 2). Figure 2 shows the demographic data and the locations of the 33 hospitals participating in the present study; all monitoring stations were located within 10-km radius of the hospitals (Additional file 1: Figs. S3 and S4–6). The distribution of meteorological and air pollutant-exposure levels in patients with IPF and IIPs are shown in Table 3 and Additional file 1: Table S2, respectively.
Fig. 1.
Patient flow diagram. MDD multidisciplinary discussion, IIPs idiopathic interstitial pneumonias, AE acute exacerbation, SLB surgical lung biopsy
Table 1.
Number of patients diagnosed through MDD in the present study
| MDD diagnosis | All | Acute exacerbation | No acute exacerbation |
|---|---|---|---|
| IIPs | 352 | 74 | 278 |
| IPF | 152 | 41 | 111 |
| iNSIP | 35 | 6 | 29 |
| COP | 4 | 0 | 4 |
| DIP/RB-ILD | 7 | 1 | 6 |
| iPPFE | 15 | 5 | 10 |
| Unclassifiable IIPs | 139 | 21 | 118 |
Data presented as frequencies. MDD multidisciplinary discussion, IIPs idiopathic interstitial pneumonias, IPF idiopathic pulmonary fibrosis, iNSIP idiopathic nonspecific interstitial pneumonia, COP cryptogenic organizing pneumonia, DIP/RB-ILD desquamative interstitial pneumonia/respiratory bronchiolitis-interstitial lung disease, iPPFE idiopathic pleuroparenchymal fibroelastosis
Table 2.
Characteristics of patients with IPF at the time of SLB
| All | Acute exacerbation | No acute exacerbation | |
|---|---|---|---|
| Subjects | 152 | 41 | 111 |
| Case or control months | 5,783 | 41 | 5,742 |
| Age (years) | 65 [61–70] | 68 [63–71] | 65 [60–70] |
| Male | 106 (70%) | 29 (71%) | 77 (69%) |
| Pack-years | 30 [0–45] | 30 [0–53] | 26 [0–45] |
| Pulmonary function tests | |||
| FVC (% predicted) | 83.9 [75.1–96.4] | 83.6 [75–92.2] | 85.2 [75.2–97.2] |
| DLCO (% predicted) | 71.6 [55.5–85.6] | 61.0 [48.5–75.9] | 74.8 [57.2–88.4] |
| HRCT pattern | |||
| UIP | 23 (15%) | 9 (22%) | 14 (13%) |
| Possible UIP | 122 (80%) | 30 (73%) | 92 (83%) |
| Inconsistent with UIP | 7 (5%) | 2 (5%) | 5 (4%) |
| Antifibrotic therapy | |||
| Pirfenidone | 55 (36%) | 18 (44%) | 37 (33%) |
| Nintedanib | 19 (13%) | 3 (7%) | 16 (14%) |
Data presented as median [interquartile rage] or frequencies (%)
IPF idiopathic pulmonary fibrosis, SLB surgical lung biopsy, FVC forced vital capacity, DLCO diffusing capacity of the lung for carbon monoxide, HRCT high-resolution computed tomography
Fig. 2.
Demographic data and geographic location of the 33 hospitals. The demographic data in 2015 in Japan is shown in different colors for each prefecture. The numbers on the top left represent the population and correspond to each color. The black boxes indicate the geographic location of 33 Japanese hospitals participating in the present study
Table 3.
Distribution of meteorological and air pollutant exposure levels in patients with IPF
| Air pollutants | Acute exacerbation | No acute exacerbation | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean | Min | Median | Max | Mean | Min | Median | Max | |
| Temperature (°C) | 14.1 | − 3.3 | 13.4 | 28.0 | 16.3 | − 9.4 | 16.4 | 30.0 |
| Humidity (%) | 67.4 | 42.7 | 68.6 | 87.0 | 66.8 | 36.0 | 67.0 | 87.0 |
| SO2 (ppb) | 2.6 | 0.0 | 2.6 | 12.5 | 2.8 | 0.0 | 3.0 | 8.0 |
| NO (ppb) | 10.8 | 1.1 | 3.4 | 98.0 | 5.6 | 1.0 | 4.0 | 69.0 |
| NO2 (ppb) | 16.7 | 3.1 | 14.7 | 37.0 | 14.9 | 2.0 | 14.0 | 46.0 |
| NOX (ppb) | 26.4 | 2.5 | 16.5 | 133.0 | 20.5 | 4.0 | 17.0 | 106.0 |
| CO (ppb) | 460.4 | 81.6 | 412.1 | 2101.0 | 411.4 | 100.0 | 400.0 | 1700.0 |
| O3 (ppb) | 28.0 | 6.2 | 28.2 | 47.6 | 29.2 | 5.0 | 28.0 | 57.0 |
| PM2.5 (µg/m3) | 17.0 | 6.0 | 16.8 | 42.6 | 15.3 | 1.6 | 14.8 | 43.6 |
| PM10 (µg/m3) | 20.1 | 7.7 | 19.3 | 30.3 | 20.7 | 6.0 | 20.0 | 51.0 |
SO2 sulfur dioxide, NO nitric oxide, NO2 nitrogen dioxide, NOX nitrogen oxides, CO carbon monoxide, O3 ozone, PM2.5 particulate matter < 2.5 µm, PM10 particulate matter < 10 µm
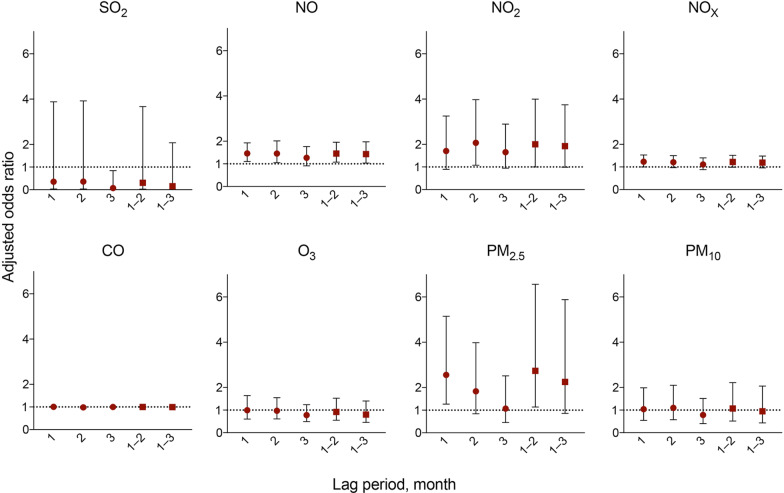
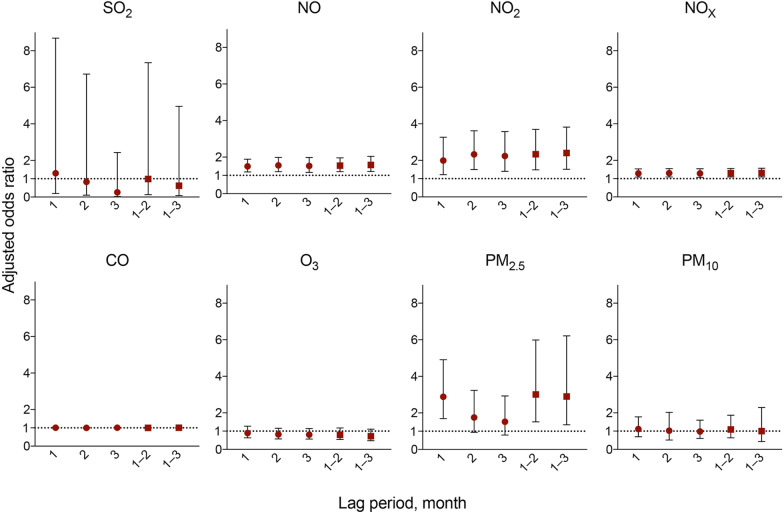
The adjusted OR for AE-IPF associated with a 10 unit increase in exposure to NO was 1.46 (95% CI 1.11–1.93; p = 0.008) and PM2.5 was 2.56 (95% CI 1.27–5.15; p = 0.009) (Table 4). Figure 3 shows the results with different monthly lag periods for AE-IPF. Significant positive associations were observed between the monthly mean exposure to NO (lag 1, lag 2, lag 1–2, and lag 1–3), NO2 (lag 2 and lag 1–2), PM2.5 (lag 1 and lag 1–2), and AE-IPF (Fig. 3). The adjusted OR for AE-IIPs incidence associated with a 10 unit increase in exposure to NO was 1.50 (95% CI 1.19–1.88; p = 0.001), NO2 was 1.99 (95% CI 1.22–3.27; p = 0.006), NOX was 1.29 (95% CI 1.08–1.53; p = 0.004), and PM2.5 was 2.88 (95% CI 1.69–4.91; p ≤ 0.001) (Table 5). Figure 4 shows the results with different monthly lag periods for AE-IIPs. Significant positive associations were observed between the monthly mean exposure to NO (lag 1, lag 2, lag 3, lag 1–2, and lag 1–3), NO2 (lag 1, lag 2, lag 3, lag 1–2, and lag 1–3), NOX (lag 1, lag 2, lag 3, lag 1–2, and lag 1–3), PM2.5 (lag 1, lag 1–2 and lag 1–3), and AE-IIPs. (Fig. 4). Complete details of the results are available in the Additional file 1: Result.
Table 4.
Association between exposure to air pollutants and the incidence of AE-IPF
| Air pollutants | Increase | Adjusted OR | 95% CI | p-value |
|---|---|---|---|---|
| SO2 | 10 ppb | 0.35 | 0.03–3.88 | 0.39 |
| NO | 10 ppb | 1.46 | 1.11–1.93 | 0.008 |
| NO2 | 10 ppb | 1.71 | 0.89–3.25 | 0.105 |
| NOX | 10 ppb | 1.24 | 0.99–1.53 | 0.052 |
| CO | 10 ppb | 1.01 | 0.99–1.02 | 0.52 |
| O3 | 10 ppb | 0.99 | 0.60–1.64 | 0.983 |
| PM2.5 | 10 µg/m3 | 2.56 | 1.27–5.15 | 0.009 |
| PM10 | 10 µg/m3 | 1.04 | 0.55–1.99 | 0.90 |
Results are presented as adjusted ORs and 95% CIs; the model was adjusted for temperature, humidity, age, sex, smoking status (pack-years), percentage of predicted value of FVC and DLCO and neighbourhood-level factors. The adjusted ORs are presented per 10-unit increase in levels of SO2 (ppb), NO (ppb), NO2 (ppb), NOX (ppb), CO (ppb), O3 (ppb), PM2.5 (µg/m3) and PM10 (µg/m3). p-values statistically significant are presented in bold
AE-IPF acute exacerbation of idiopathic pulmonary fibrosis, OR odds ratio, CI confidence interval, FVC forced vital capacity, DLCO diffusing capacity of the lung for carbon monoxide, SO2 sulfur dioxide, NO nitric oxide, NO2 nitrogen dioxide, NOX nitrogen oxides, CO carbon monoxide, O3 ozone, PM2.5 particulate matter < 2.5 µm, PM10 particulate matter < 10 µm
Fig. 3.
Adjusted ORs and 95% CIs for the incidence of AE-IPF with different monthly lag periods. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) showing the increased risk of acute exacerbation of idiopathic pulmonary fibrosis (AE-IPF) associated with a 10-unit increase in the levels of sulfur dioxide (SO2), nitric oxide (NO), nitrogen dioxide (NO2), nitrogen oxides (NOX), carbon monoxide (CO), ozone (O3), particulate matter < 2.5 µm (PM2.5) and particulate matter < 10 µm (PM10) with different monthly lag periods. The adjusted ORs were estimated using multilevel mixed-effects logistic regression models, adjusting for temperature, humidity, age, sex, smoking status (pack-years) and percentage of the predicted value of forced vital capacity and diffusing capacity of the lung for carbon monoxide, and neighbourhood-level factors
Table 5.
Association between exposure to air pollutants and the incidence of AE-IIPs
| Air pollutant | Increase | Adjusted OR | 95% CI | p-value |
|---|---|---|---|---|
| SO2 | 10 ppb | 1.30 | 0.19–8.69 | 0.79 |
| NO | 10 ppb | 1.50 | 1.19–1.88 | 0.001 |
| NO2 | 10 ppb | 1.99 | 1.22–3.27 | 0.006 |
| NOX | 10 ppb | 1.29 | 1.08–1.53 | 0.004 |
| CO | 10 ppb | 1.01 | 0.99–1.03 | 0.16 |
| O3 | 10 ppb | 0.90 | 0.64–1.27 | 0.55 |
| PM2.5 | 10 µg/m3 | 2.88 | 1.69–4.91 | < 0.001 |
| PM10 | 10 µg/m3 | 1.11 | 0.70–1.78 | 0.65 |
Results are presented as adjusted ORs and 95% CIs; the model was adjusted for temperature, humidity, age, sex, smoking status (pack-years), percentage of predicted value of FVC and DLCO and neighbourhood-level factors. The adjusted ORs are presented per 10-unit increase in levels of SO2 (ppb), NO (ppb), NO2 (ppb), NOX (ppb), CO (ppb), O3 (ppb), PM2.5 (µg/m3), and PM10 (µg/m3). p-values statistically significant are presented in bold
AE-IPF acute exacerbation of idiopathic pulmonary fibrosis, OR odds ratio, CI confidence interval, FVC forced vital capacity, DLCO diffusing capacity of the lung for carbon monoxide, SO2 sulfur dioxide, NO nitric oxide, NO2 nitrogen dioxide, NOX nitrogen oxides, CO carbon monoxide, O3 ozone, PM2.5 particulate matter < 2.5 µm, PM10 particulate matter < 10 µm
Fig. 4.
Adjusted ORs and 95% CIs for the incidence of AE-IIPs with different monthly lag periods. Adjusted odds ratios (ORs) and 95% confidence intervals (CIs) showing the increased risk of acute exacerbation of idiopathic interstitial pneumonias (AE-IIPs) associated with a 10-unit increase in the levels of sulfur dioxide (SO2), nitric oxide (NO), nitrogen dioxide (NO2), nitrogen oxides (NOX), carbon monoxide (CO), ozone (O3), particulate matter < 2.5 µm (PM2.5) and particulate matter < 10 µm (PM10) with different monthly lag periods. The adjusted ORs were estimated using multilevel mixed-effects logistic regression models, adjusting for temperature, humidity, age, sex, smoking status (pack-years) and percentage of the predicted value of forced vital capacity and diffusing capacity of the lung for carbon monoxide, and neighbourhood-level factors
Discussion
Based on the nationwide surgically diagnosed IPF registry, the present study demonstrated that there was a positive relationship between short-term monthly exposure to PM2.5 and the incidence of AE-IPF. An increase in 10 µg/m3 of PM2.5 amplified the risk of AE-IPF by approximately 2.5-fold.
In the previous two studies (South Korea and France), a significant positive association was observed between the increased levels of O3 and NO2 during 0–42 days prior to the diagnosis of AE-IPF [4, 14]. Moreover, these studies suggested potential (but not statistically significant) risks of elevated levels of PM2.5 (France) or PM10 (South Korea) for AE-IPF [4, 14]. Our study demonstrated that the increased mean level of PM2.5 30 days before AE diagnosis has significant positive association with the increased risk of AE-IPF after adjusting for temperature, humidity, age, sex, smoking status, and percentage of the predicted value of FVC, DLCO, and neighborhood-level factors. On the other hand, no significant relationship was observed between AE-IPF incidence and exposure to NO2 and O3. The reason of the difference between our study and the previous studies on NO2 and O3 is unclear; however, the difference in the definition of IPF diagnosis, ethnicity, environment of studied areas and statistical methodology might explain that there was no relationship between these irritant pollutants and the incidence of AE-IPF in our study. In this study, the proportion of UIP pattern in HRCT was 15% of the IPF patients, which was less than that of the previous study [8]. Future studies are required to resolve these different findings.
Several in-vivo and in-vitro findings support the biological plausibility of the correlation between the elevated PM2.5 level and the incidence of AE-IPF [28]. PM2.5 tends to be deposited in the lower airways [28] inducing subsequent inflammation that could exacerbate asthma and COPD [29]. Furthermore, particulate matter causes mitochondrial damage in macrophages and produces reactive oxygen species, which can damage cellular proteins, lipids, membranes, and DNA [30–32]. Therefore, it was speculated that exposure to airborne PM2.5 triggers an inflammatory reaction and induces tissue damage in the lungs; thus, contributing significantly to AE-IPF.
Previous studies applied the exposure time window as “the 0–42 days” prior to the diagnosis of AE [4, 14] because the clinical course of AE-IPF should be within 1 month prior to AE diagnosis in the definition [9, 10]; however, the specific risk-exposure time window prior to the diagnosis of AE-IPF remains unclear. Significant positive associations between the exposure to increased PM2.5 levels and the AE-IPF diagnosis were observed during lag 1 and lag 1–2 months in our additional analysis. An increased mean level of PM2.5 during 15–21 days (OR 3.65; 95% CI 1.95–6.83; p ≤ 0.001) showed the strongest impact on AE-IPF (Additional file 1: Fig. S7) in our weekly analysis. These results suggest that “the 0–42 days” prior to AE-IPF diagnosis is useful in assessing the risk for AE-IPF regarding exposure to ambient PM2.5.
The health benefits of pollution reduction strategies have been documented in the Asia–Pacific Region, including Japan [33]. The Japanese government has passed a legislation to limit emissions in 2001, with a subsequent decrease in the mean levels of PM2.5 from 38 to 26 µg/m3 (2009) [33]. In our study, the mean ambient PM2.5 level in patients with IPF was 15.4 (Table 4). Our findings showed that elevated levels of PM2.5, albeit lower than the PM2.5 level of 26 µg/m3—the mean levels of PM2.5 established by the Japanese government in 2009—may be a risk factor for AE-IPF. Additionally, the mean exposure level of patients with IPF to PM2.5 was higher than the annual level recommended by the World Health Organisation (not exceeding an annual level of 10 µg/m3 for PM2.5) [34]. Accordingly, our findings suggest that the current ambient PM2.5 level remains a possible risk factor of AE-IPF in Japan.
We demonstrated a novel correlation between short-term monthly exposure to NO and AE-IPF. There was no report that exposure to ambient NO is a risk factor for respiratory diseases, including AE-IPF, presumably because few countries measure ambient NO in the atmosphere. In addition, significant negative association between AE-IPF and exposure to SO2 during lag 3 was observed. The negative correlation between exposure to SO2 and AE-IPF has been unclear. Further research is needed to determine the clinical significance of these results.
This study establishes the significant positive correlations between the AE-IIPs and short-term monthly exposure levels of NO, NO2, NOX, and PM2.5. This is the first study to show the correlation between AE-IIPs and short-term exposure to ambient air pollutants. Further research is necessary to clarify the relationship between exposure to air pollutants, including fine particulate matter and AE-IIPs.
The strengths of the present study are as follows. First, accurate IPF diagnoses were obtained in our study. All patients with IIPs participated in this study were diagnosed using SLB samples and MDD using a new video-conferencing system were performed [20]. Although SLB is not necessary in the diagnosis of IPF for all patients [1, 35], the most reliable method of diagnosing IPF is obtained from information on SLB and following MDD with ILD experts [36, 37]; this is because of an unignorable rate of commingling fibrosing lung diseases, such as fibrotic hypersensitivity pneumonitis [38]. Second, we adjusted the models for age, sex, and risk factors reported in previous studies (e.g., smoking status and percentage of the predicted value of FVC) [26] and time-varying variables (e.g., temperature and humidity).
Several limitations of this study should be acknowledged. First, this study was a retrospective study. Second, the sample size was relatively small and consisted only of Japanese patients; however, it comprised only patients with surgically diagnosed IPF, the most robust method for IPF diagnosis. Third, socioeconomic information that should be used for adjustment were not available in the database. Fourth, the addresses of patients were not available in the database; however, the hospital addresses were used as an alternative, because the addresses of patients are approximately 10 min away by ambulance from the nearby hospitals in Japan (Additional file 1: Table S7). In addition, all distances between the registered hospitals and monitoring stations were within 10 km (Additional file 1: Figs. S3 and S4–6). Fifth, we were unable to distinguish between the levels of air pollutant at home and in the workplace.
Conclusions
The results of the case–control study suggest that short-term monthly exposure to PM2.5 may be a contributing risk factor to AE-IPF. We identified that the exposure periods of ambient PM2.5 recorded between 1 to 2-month prior to AE diagnosis were positively associated with AE-IPF in the additional analysis. Consistent with previous reports, we confirmed that “the 0–42 days” period preceding diagnosis may be useful in evaluating the relationship between the mean level of airborne PM2.5 and incidence of AE-IPF. Although the recent reduction in the levels of air pollutants has conferred health benefits, further efforts are required to decrease exposure to PM2.5 and reduce the risk of AE-IPF in Japan. We also identified the positive association between short-term exposure to NO and AE-IPF. Prospective cohort studies are expected to validate the relationship between exposure to ambient PM2.5, NO, and AE-IPF.
Supplementary Information
Additional file 1. Supplementary Material.
Acknowledgements
We wish to thank all members participating in the nationwide IIPs database, and our research is based on the underlying research conducted by their hard work and efforts. The underlying research reported by Fujisawa and colleagues was funded by the Practical Research Project for Rare Intractable Disease from the Japan Agency for Medical Research Group under the aegis of the Ministry of Health, Ministry of Health, Labour and Welfare, Japan. We also wish to thank Mrs. Kumiko Matsuyama for her efforts to edit the Fig. 2.
Abbreviations
- AE
Acute exacerbation
- AE-IPF
Acute exacerbation of idiopathic pulmonary fibrosis
- CI
Confidence interval
- CO
Carbon monoxide
- COPD
Chronic obstructive pulmonary disease
- DLCO
Diffusing capacity of the lung for carbon monoxide
- FVC
Forced vital capacity
- HRCT
High-resolution computed tomography
- IIPs
Idiopathic interstitial pneumonias
- ILD
Interstitial lung disease
- IPF
Idiopathic pulmonary fibrosis
- KL-6
Krebs von den Lungen-6
- MDD
Multidisciplinary discussion
- NO
Nitric oxide
- NOX
Nitrogen oxides
- NO2
Nitrogen dioxide
- OR
Odds ratio
- O3
Ozone
- SLB
Surgical lung biopsy
- SO2
Sulfur dioxide
- SP-D
Surfactant protein-D
- SPM
Suspended particulate matter
- PM10
Particulate matter < 10 µm
- PM2.5
Particulate matter < 2.5 µm
Authors’ contributions
MT designed the study, had full access to all the data in this study, performed statistical analysis and wrote the initial draft. YF gave advice on the study design and performed statistical analysis. KY, KO, TK, NS and KY substantially contributed to the conception and design of the study. KK, RE, MH, YS, TF and TS prepared the nationwide IIPs database. MT, KY, KO, NS, TK, YS, HM and KY participated in drafting and critically revising the article for important intellectual content. KY was attributable for the final responsibility for the decision to submit the article for publication. All authors have read and approved the final manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used for the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
This study was conducted in accordance with the amended Declaration of Helsinki. The institutional review boards of the University of Occupational and Environmental Health, Kitakyushu, Japan (18-013) and the Hamamatsu University School of Medicine, Hamamatsu, Japan (19-003) approved the protocol.
Consent for publication
Not applicable.
Competing interests
MT received grants from GlaxoSmithKline and speakers’ fees from Boehringer Ingelheim and MSD, all outside the submitted work. YF received grants from Hitachi Systems, Ltd. and NIPPON STEEL CORPORATION and personal fees from Asahi Kasei, The Asahi Shimbun Company, AstraZeneca, CHUGAI, NTT DATA MSE CORPORATION, Pfizer, Sompo Health Support Inc., and THE LOFT CO., LTD., all outside the submitted work. KY received speakers’ fees from Novartis outside the submitted work. TK received speakers’ fees from Asahi Kasei, AstraZeneca, Boehringer Ingelheim, and TEIJIN outside the submitted work. NS received consultancy or speakers’ fees from Boehringer Ingelheim, CHUGAI, and Daiichi Sankyo outside the submitted work. TK received speakers’ fees from KYORIN outside the submitted work. KK received speakers’ fees from Boehringer Ingelheim outside the submitted work. RE received grants from the Japan Society for the Promotion of Science and speakers’ fees from AstraZeneca, Boehringer Ingelheim, Daiichi Sankyo, EISAI, KYORIN, and SHIONOGI, all outside the submitted work. YS received grants from the Japan Society for the Promotion of Science and the Ichiro Kanehara Foundation for the Promotion of Medical Sciences and Medical Care, as well as received industry-academic funding from MSD and Novartis, all outside the submitted work. HM received industry-academic funding from Asahi Kasei, Astellas, Boehringer Ingelheim, CHUGAI, Daiichi Sankyo, EISAI, Eli Lilly, Fujifilm, GlaxoSmithKline, KYORIN, Meiji Seika, MSD, Novartis, ONO PHARMACEUTICAL CO., LTD., Oxford Immunotec, Inc., Pfizer, SHIONOGI, Sumitomo Dainippon, TAIHO, TAISHO, Takeda, TEIJIN, TORAY, TORII, Toyama Chemical, and Yakult, as well as consultancy or speakers’ fees from Abbott, Asahi Kasei, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers, CHUGAI, Daiichi Sankyo, Denka Seiken, Eli Lilly, Fujifilm, GlaxoSmithKline, KYORIN, Meiji Seika, MSD, Mylan, Nihon Pharmaceutical, Nikkei Radio, Novartis, ONO, Pfizer, SHIONOGI, Sumitomo Dainippon, TAIHO, TAISHO, TEIJIN, TORAY, and Toyama Chemical, all outside the submitted work. TS received industry-academic funding from Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers, CHUGAI, Eli Lilly, Daiichi Sankyo, KYORIN, MITSUBISHI, MSD, Novartis, ONO, Pfizer, Sanofi, SHIONOGI, TAIHO, TAISHO, Taisho Toyama, Takeda, TANABE, and TEIJIN, as well as consultancy or speakers’ fees from Astellas, AstraZeneca, Boehringer Ingelheim, CHUGAI, Eli Lilly, KYORIN, Daiichi Sankyo, SHIONOGI, MSD, Novartis, ONO, Pfizer, TAISHO, Taisho Toyama, and Tanabe Mitsubishi, all outside the submitted work. KY received industry-academic funding from Boehringer Ingelheim, GlaxoSmithKline, KYORIN, MSD, Novartis, ONO, Pfizer, TAIHO, TAISHO, and TEIJIN, as well as consultancy or speakers’ fees from Asahi Kasei, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers, CHUGAI, Daiichi Sankyo, Eli Lilly, GlaxoSmithKline, KYORIN, MSD, Novartis, ONO, Pfizer, SHIONOGI, TAIHO, TAISHO, TEIJIN, and TOA EIYO LTD., all outside the submitted work. The other authors declare they have no actual or potential competing financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King TE, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet. 2011;378:1949–1961. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 3.Natsuizaka M, Chiba H, Kuronuma K, Otsuka M, Kudo K, Mori M, Bando M, Sugiyama Y, Takahashi H. Epidemiologic survey of Japanese patients with idiopathic pulmonary fibrosis and investigation of ethnic differences. Am J Respir Crit Care Med. 2014;190:773–779. doi: 10.1164/rccm.201403-0566OC. [DOI] [PubMed] [Google Scholar]
- 4.Sese L, Nunes H, Cottin V, Sanyal S, Didier M, Carton Z, Israel-Biet D, Crestani B, Cadranel J, Wallaert B, et al. Role of atmospheric pollution on the natural history of idiopathic pulmonary fibrosis. Thorax. 2018;73:145–150. doi: 10.1136/thoraxjnl-2017-209967. [DOI] [PubMed] [Google Scholar]
- 5.King TE, Jr, Bradford WZ, Castro-Bernardini S, Fagan EA, Glaspole I, Glassberg MK, Gorina E, Hopkins PM, Kardatzke D, Lancaster L, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083–2092. doi: 10.1056/NEJMoa1402582. [DOI] [PubMed] [Google Scholar]
- 6.Richeldi L, du Bois RM, Raghu G, Azuma A, Brown KK, Costabel U, Cottin V, Flaherty KR, Hansell DM, Inoue Y, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071–2082. doi: 10.1056/NEJMoa1402584. [DOI] [PubMed] [Google Scholar]
- 7.Jo HE, Glaspole I, Grainge C, Goh N, Hopkins PM, Moodley Y, Reynolds PN, Chapman S, Walters EH, Zappala C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J. 2017 doi: 10.1183/13993003.01592-2016. [DOI] [PubMed] [Google Scholar]
- 8.Guenther A, Krauss E, Tello S, Wagner J, Paul B, Kuhn S, Maurer O, Heinemann S, Costabel U, Barbero MAN, et al. The European IPF registry (eurIPFreg): baseline characteristics and survival of patients with idiopathic pulmonary fibrosis. Respir Res. 2018;19:141. doi: 10.1186/s12931-018-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collard HR, Moore BB, Flaherty KR, Brown KK, Kaner RJ, King TE, Jr, Lasky JA, Loyd JE, Noth I, Olman MA, et al. Acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2007;176:636–643. doi: 10.1164/rccm.200703-463PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collard HR, Ryerson CJ, Corte TJ, Jenkins G, Kondoh Y, Lederer DJ, Lee JS, Maher TM, Wells AU, Antoniou KM, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An International Working Group Report. Am J Respir Crit Care Med. 2016;194:265–275. doi: 10.1164/rccm.201604-0801CI. [DOI] [PubMed] [Google Scholar]
- 11.Wootton SC, Kim DS, Kondoh Y, Chen E, Lee JS, Song JW, Huh JW, Taniguchi H, Chiu C, Boushey H, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:1698–1702. doi: 10.1164/rccm.201010-1752OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee JS, Song JW, Wolters PJ, Elicker BM, King TE, Jr, Kim DS, Collard HR. Bronchoalveolar lavage pepsin in acute exacerbation of idiopathic pulmonary fibrosis. Eur Respir J. 2012;39:352–358. doi: 10.1183/09031936.00050911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kondoh Y, Taniguchi H, Kitaichi M, Yokoi T, Johkoh T, Oishi T, Kimura T, Nishiyama O, Kato K, du Bois RM. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med. 2006;100:1753–1759. doi: 10.1016/j.rmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Johannson KA, Vittinghoff E, Lee K, Balmes JR, Ji W, Kaplan GG, Kim DS, Collard HR. Acute exacerbation of idiopathic pulmonary fibrosis associated with air pollution exposure. Eur Respir J. 2014;43:1124–2113. doi: 10.1183/09031936.00122213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 16.Dominici F, Peng RD, Bell ML, Pham L, McDermott A, Zeger SL, Samet JM. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA. 2006;295:1127–1134. doi: 10.1001/jama.295.10.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014;69:660–665. doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li MH, Fan LC, Mao B, Yang JW, Choi AMK, Cao WJ, Xu JF. Short-term exposure to ambient fine particulate matter increases hospitalizations and mortality in COPD: a systematic review and meta-analysis. Chest. 2016;149:447–458. doi: 10.1378/chest.15-0513. [DOI] [PubMed] [Google Scholar]
- 19.Dales R, Blanco-Vidal C, Cakmak S. The association between air pollution and hospitalization of patients with idiopathic pulmonary fibrosis in chile: a daily time series analysis. Chest. 2020;158:630–636. doi: 10.1016/j.chest.2020.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Fujisawa T, Mori K, Mikamo M, Ohno T, Kataoka K, Sugimoto C, Kitamura H, Enomoto N, Egashira R, Sumikawa H, et al. Nationwide cloud-based integrated database of idiopathic interstitial pneumonias for multidisciplinary discussion. Eur Respir J. 2019;53:1802243. doi: 10.1183/13993003.02243-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diez-Roux AV. Multilevel analysis in public health research. Annu Rev Public Health. 2000;21:171–192. doi: 10.1146/annurev.publhealth.21.1.171. [DOI] [PubMed] [Google Scholar]
- 22.Navidi W, Thomas D, Stram D, Peters J. Design and analysis of multilevel analytic studies with applications to a study of air pollution. Environ Health Perspect. 1994;102(Suppl 8):25–32. doi: 10.1289/ehp.94102s825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carracedo-Martinez E, Taracido M, Tobias A, Saez M, Figueiras A. Case-crossover analysis of air pollution health effects: a systematic review of methodology and application. Environ Health Perspect. 2010;118:1173–1182. doi: 10.1289/ehp.0901485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.American Thoracic S, European Respiratory S: American Thoracic Society/European Respiratory Society International Multidisciplinary Consensus Classification of the Idiopathic Interstitial Pneumonias. This joint statement of the American Thoracic Society (ATS), and the European Respiratory Society (ERS) was adopted by the ATS board of directors, June 2001 and by the ERS Executive Committee, June 2001. Am J Respir Crit Care Med 2002;165:277–304. [DOI] [PubMed]
- 25.Travis WD, Costabel U, Hansell DM, King TE, Jr., Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU, et al: An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. 2013;188:733–748 [DOI] [PMC free article] [PubMed]
- 26.Song JW, Hong SB, Lim CM, Koh Y, Kim DS. Acute exacerbation of idiopathic pulmonary fibrosis: incidence, risk factors and outcome. Eur Respir J. 2011;37:356–363. doi: 10.1183/09031936.00159709. [DOI] [PubMed] [Google Scholar]
- 27.Bowe B, Xie Y, Yan Y, Al-Aly Z. Burden of cause-specific mortality associated with PM2.5 air pollution in the United States. JAMA Netw Open. 2019;2:e1915834. doi: 10.1001/jamanetworkopen.2019.15834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Falcon-Rodriguez CI, Osornio-Vargas AR, Sada-Ovalle I, Segura-Medina P. Aeroparticles, composition, and lung diseases. Front Immunol. 2016;7:3. doi: 10.3389/fimmu.2016.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. J Allergy Clin Immunol. 1998;102:539–554. doi: 10.1016/S0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- 30.Nel A. Atmosphere. Air pollution-related illness: effects of particles. Science. 2005;308:804–806. doi: 10.1126/science.1108752. [DOI] [PubMed] [Google Scholar]
- 31.Gilliland FD, Li YF, Saxon A, Diaz-Sanchez D. Effect of glutathione-S-transferase M1 and P1 genotypes on xenobiotic enhancement of allergic responses: randomised, placebo-controlled crossover study. Lancet. 2004;363:119–125. doi: 10.1016/S0140-6736(03)15262-2. [DOI] [PubMed] [Google Scholar]
- 32.Mukae H, Vincent R, Quinlan K, English D, Hards J, Hogg JC, van Eeden SF. The effect of repeated exposure to particulate air pollution (PM10) on the bone marrow. Am J Respir Crit Care Med. 2001;163:201–209. doi: 10.1164/ajrccm.163.1.2002039. [DOI] [PubMed] [Google Scholar]
- 33.North CM, Rice MB, Ferkol T, Gozal D, Hui C, Jung SH, Kuribayashi K, McCormack MC, Mishima M, Morimoto Y, et al. Air pollution in the Asia-Pacific Region: A Joint Asian Pacific Society of Respirology/American Thoracic Society perspective (Republication) Respirology. 2019;24:484–491. doi: 10.1111/resp.13531. [DOI] [PubMed] [Google Scholar]
- 34.Europe, W. H. O. R. O. for & Organization, W. H. Air Quality Guidelines: Global update 2005: Particulate matter, ozone, nitrogen dioxide and sulfur dioxide. World Health Organization, 2006 [PubMed]
- 35.Raghu G, Remy-Jardin M, Myers JL, Richeldi L, Ryerson CJ, Lederer DJ, Behr J, Cottin V, Danoff SK, Morell F, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med. 2018;198:44–68. doi: 10.1164/rccm.201807-1255ST. [DOI] [PubMed] [Google Scholar]
- 36.Raghu G, Mageto YN, Lockhart D, Schmidt RA, Wood DE, Godwin JD. The accuracy of the clinical diagnosis of new-onset idiopathic pulmonary fibrosis and other interstitial lung disease: a prospective study. Chest. 1999;116:1168–1174. doi: 10.1378/chest.116.5.1168. [DOI] [PubMed] [Google Scholar]
- 37.Hunninghake GW, Zimmerman MB, Schwartz DA, King TE, Jr, Lynch J, Hegele R, Waldron J, Colby T, Muller N, Lynch D, et al. Utility of a lung biopsy for the diagnosis of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2001;164:193–196. doi: 10.1164/ajrccm.164.2.2101090. [DOI] [PubMed] [Google Scholar]
- 38.Lederer DJ, Martinez FJ. Idiopathic pulmonary fibrosis. N Engl J Med. 2018;378:1811–1823. doi: 10.1056/NEJMra1705751. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplementary Material.
Data Availability Statement
The datasets used for the current study are available from the corresponding author on reasonable request.