Abstract
Although they comprise a small number of primary tumors of the heart, papillary fibroelastomas (PFEs) are the second most common type of benign cardiac tumor. PFEs of the right heart are uncommon, and those arising from the right-ventricular (RV) wall are extremely rare, with only a handful of reported cases in the literature. Removal of these tumors has been described, primarily through a median sternotomy approach, with only one report of using a right-sided mini-thoracotomy technique. The advantages of endoscopic robotic-assisted cardiac surgery have been demonstrated and described extensively. We report on a case of an incidentally found PFE in the RV that was successfully removed with a totally endoscopic robotic-assisted approach. The focus of our report is on the uniqueness of both the right-sided nonvalvular PFE and the treatment with a robotic totally endoscopic surgical approach.
Keywords: Cardiac tumor, Papillary fibroelastoma, Robotics, Minimally invasive surgery, Cardiac surgery, Right ventricle mass
Introduction
Benign primary cardiac tumors are a rare phenomenon overall. Of these, papillary fibroelastomas (PFEs) comprise only 10–14%. [1, 2] They are overwhelmingly associated with the heart valves, making up over three-fourths of the 24% of cardiac tumors found on valves [1]. PFEs are least commonly associated with the right-side of the heart [2]. We found fifteen reports in the literature of nonvalvular right-ventricle (RV) PFEs [2, 3]. Each of these, with only one exception, was reported to have been removed via a median sternotomy. We report on a case of incidentally found nonvalvular PFE on the RV wall, successfully removed with a robotic totally-endoscopic approach. To our knowledge, this is the first reported case of endoscopic robotic-assisted excision of a right-ventricular PFE.
Case report
The patient is an active 56-year-old woman with no significant past medical or surgical history who was sent for a stress test as part of routine check-up. An echocardiogram was requested by the patient’s family as there was a family history of cardiac disease. This showed a 1.3 × 1.2 cm multi-lobulated echodense mass in the RV (adherent to the ventricular septum), normal left ventricular (LV) function, and normal valvular function. Specifically, the mass appeared adjacent to and possibly involved with the tricuspid valve (TV) subvalvular apparatus. The patient was asymptomatic and denied chest pain, shortness of breath, palpitations, orthopnea, lower extremity edema, and paroxysmal nocturnal dyspnea.
Surgical technique
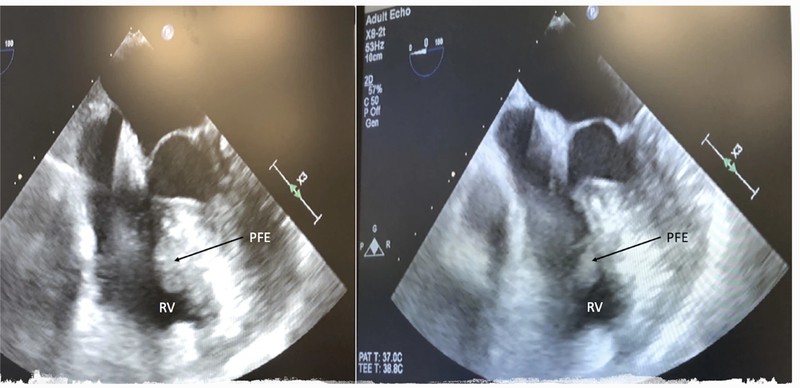
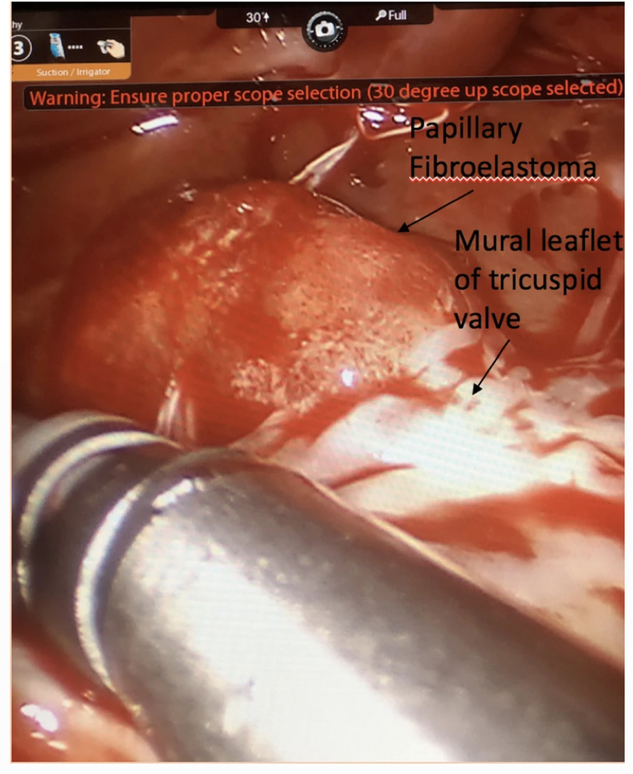
Surgical resection of the mass was performed using a robotic-assisted totally endoscopic approach on cardiopulmonary bypass (CPB) through a right atriotomy. Intraoperative transesophageal echocardiography (TEE) confirmed presence of the mass, which appeared to be attached to the TV subvalvular apparatus (Fig. 1). General anesthesia was administered using a single-lumen endotracheal tube. Right femoral–femoral CPB was instituted and the endo-balloon (Intraclude™ Edwards, Irvine CA) was used to arrest the heart. Bicaval cannulation was achieved through the right femoral vein (via cut-down) and the right internal jugular vein (percutaneously). Standard right-sided mitral valve (MV) robotic ports were placed in the second, fourth and sixth intercostal spaces (ICS) in the anterior axillary line. A 15-mm working port was made in the fourth ICS. CPB was initiated, the heart was decompressed and the Da Vinci™ robot was docked. The pericardium was opened anterior to the right phrenic nerve. Snares were placed around the superior and inferior vena cavae. Once right atrial isolation was achieved, a transverse right atriotomy was performed and the dynamic atrial retractor was applied. The mass was visualized on the ventricular side of the TV. It appeared to be gelatinous, similar to a sea anemone. It was attached to the ventricular septum and entangled in the cords of the TV mural leaflet, (Fig. 2). An attempt was made to visualize the mass using a beating-heart technique, however, this proved difficult and the heart was arrested with cold Del Nido™ cardioplegia solution, which provided optimal visualization. The attachment site on the ventricular septum was delineated and the mass was fully extirpated from its base. Low electrocautery was used to treat the base of the mass to prevent further recurrence. In excising the mass, a secondary cord of the TV was sacrificed, however, this did not affect valve function as evidenced by intraoperative TEE. The patient was rewarmed, the endo-balloon deflated, and right atriotomy closed.
Fig. 1.
Transesophageal echo showing RV PFE
Fig. 2.
Robotic view of RV PFE under the mural leaflet of the tricuspid valve
The patient tolerated the procedure well and came off-pump without inotropes. The cardiac arrest time was 39 min. The post-procedure TEE showed absence of the mass with a competent, well-functioning TV. Postoperative histological analysis revealed the mass to be a PFE sized at 1.5 × 0.7 × 0.3 cm. The patient was extubated within 1 h of surgery, and had an unremarkable postoperative course. She was discharged within 36 h of the procedure. At 30-day follow-up the patient reported feeling well. Within 10 days of surgery she had returned to full activity, including her baseline of walking 1 h everyday. She was off of all pain medication within 5 days of surgery, and denied any symptoms including chest pain, shortness of breath, palpitations or discomfort at the incision sites. She was planning to return to a substitute teaching job shortly after her postoperative visit.
Discussion
Primary cardiac tumors are rare. Gowda and colleagues report a frequency of primary cardiac tumors of 0.02% found at autopsy, or two tumors in 10,000 autopsies. Of primary cardiac tumors, myxomas are the most common, comprising 70–80%. These are followed by papillary fibroelastomas (PFEs), with a frequency between 10 and 14% [1, 2]. PFEs are overwhelmingly associated with the endocardium, and are reported to account for over 75% of valvular cardiac tumors [1, 4, 5]. In particular, the aortic valve (AV) has been reported as the most common site for PFEs to occur, followed by the MV [2, 4, 5]. The LV is the most common nonvalvular site for PFEs to occur, with the least common sites being the right-sided free walls including right atrium (RA), RV or right atrial appendage [4]. Given the risk for systemic/pulmonary thromboembolism, stroke or valvular dysfunction, PFEs are predominantly indicated for surgical removal [3].
We report on a case of incidental finding of PFE attached to the right-ventricular wall. There are only a few cases of nonvalvular right-ventricular wall PFEs in the literature, particularly relative to the number of reported valve-associated PFEs. Overall, under 5% of PFEs are found in the right heart. In a comprehensive literature analysis of 725 cases of PFEs, nine were found in the RV. [4] Niino and colleagues reported a rare case of PFE attached to the RV free-wall which was removed through the RV and main pulmonary artery. Interestingly, this patient underwent workup due to palpitations. Our patient’s PFE was an incidental finding on a routine echo. It has been noted that the majority of PFEs are discovered incidentally. They are less commonly discovered in the context of physical findings/symptoms, and rarely from complications such as stroke or pulmonary embolism [4]. Given the rarity of symptoms seen in PFEs, it may be possible that the prevalence of these tumors is higher than currently thought. However, given the risk for serious complications, when discovered it is recommended that PFEs be removed [4, 5].
In our review of the literature we found 15 cases of nonvalvular RV-PFEs described in 4 articles. In one report of a single-center’s experience with the removal of 88 PFEs over 17-years (Ngaage and colleagues), there were 3 cases of nonvalvular RV-PFEs [4]. All were removed via median sternotomy. We found only one case of a minimally-invasive approach to removal of a RV-PFE in the literature, described as a right-sided mini-thoracotomy. This report by Bossert and colleagues on their series of 77 cardiac tumor removals included 11 PFEs, two being on the free-RV wall. Of the 77 primary cardiac tumors removed, 24 were operated on through a mini-thoracotomy. These were the only cases in the literature that reported removal of PFEs in any location with a minimally-invasive approach. We found no cases of PFE excision using robotics.
Our case is unique in that it was performed using a totally-endoscopic robotic-assisted technique for the removal of a PFE in a rare location in the heart. Over the last 10-years we have developed an extensive robotic-assisted surgical experience in a myriad of different procedures, including both epi and intracardiac cases [6–8]. Our experience with robotic-assisted excision of primary cardiac tumors includes 13 intracardiac masses over 5 years. Of these, five were PFEs: three on the AV, one from the RA, and one from the RV as described in this case report. All 13 cases were completed using a totally-endoscopic robotic-assisted approach, and four were removed with an arrested heart. The advantages of robotic and minimally invasive cardiac surgery have been extensively described, most notably with regards to improved patient outcomes including shorter hospital stay, less blood transfusions, and earlier return to activities/work [9–11].
The benefits of the robotic approach were realized in this patient with a rare cardiac tumor as it allowed for excellent visualization under the TV. She had no intra or perioperative complications and was discharged 36-h after surgery. From our experience in many various types of robotic cardiac procedures, we believe this approach is advantageous in terms of improved outcomes, swift recovery, and accelerated return to work/activities for patients who are candidates for this type of procedure. Extensive experience with intracardiac robotic procedures and peripheral perfusion is required for successful outcomes in these cases. Our decision to arrest the heart was taken after opening the RA on the beating heart and finding that visualization of the RV tumor was less than adequate for effective removal. It should be mentioned that many right heart tumors/masses can be removed without arresting the heart, as long as adequate caval isolation is achieved.
In conclusion, we describe the first case of using a totally-endoscopic robotic-assisted surgical approach for successful excision of a rare, nonvalvular right-ventricular papillary fibroelastoma.
Footnotes
Compliance with ethical standards
Conflict of interest Author Husam Balkhy, MD is a proctor for Intuitive Surgical, manufacturer of the Da Vinci robot. Author Sarah Nisivaco declares that she has no conflict of interest. Author Michael Henry, MD declares that he has no conflict of interest. Author Parker Ward, MD declares that he has no conflict of interest.
Ethical approval This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) with the Helsinki Declaration of 1975, as revised in 2000. Informed consent was obtained from all patients for being included in the study. Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- 1.Hoffmeier A, Sindermann JR, Scheld HH, Martens S (2014) Cardiac tumors—diagnosis and surgical treatment. Dtsch Arztebl Int 111:205–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bossert T, Gummert JF, Battellini R, Richter M, Barten M, Walther T et al. (2005) Surgical experience with 77 primary cardiac tumors. Interact Cardiovasc Thorac Surg 4:311–315 [DOI] [PubMed] [Google Scholar]
- 3.Niino T, Satoshi U (2014) Papillary fibroelastoma of the right ventricular free wall. Case Rep Surg 2014:654641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gowda RM, Khan IA, Nair CK, Mehta NJ, Vasavada BC, Sacchi TJ (2003) Cardiac papillary fibroelastoma: a comprehensive analysis of 725 cases. Am Heart J 146:404–410 [DOI] [PubMed] [Google Scholar]
- 5.Ngaage DL, Mullany CJ, Daly RC, Dearani JA, Edwards WD, Tazelaar HD et al. (2005) Surgical treatment of cardiac papillary fibroelastoma: a single center experience with eighty-eight patients. Ann Thorac Surg 80:1712–1718 [DOI] [PubMed] [Google Scholar]
- 6.Balkhy HH, Wann LS, Krienbring DJ, Arnsdorf SE (2011) Integrating coronary anastomotic connectors and robotics toward a totally endoscopic beating heart approach: review of 120 cases. Ann Thorac Surg 92:821–827 [DOI] [PubMed] [Google Scholar]
- 7.Maciolek K, Asfaw ZE, Krienbring DJ, Arnsdorf SE, Balkhy HH (2016) Robotic endoscopic off-pump total pericardiectomy in constrictive pericarditis. Innovations (Phila) 11:134–137 [DOI] [PubMed] [Google Scholar]
- 8.Kitahara H, Patel B, McCrorey M, Nisivaco S, Balkhy HH (2017) Morbid obesity does not increase morbidity or mortality in robotic cardiac surgery. Innovations (Phila) 12:434–439 [DOI] [PubMed] [Google Scholar]
- 9.Poston RS, Tran R, Collins M, Reynolds M, Connerney I, Reicher B et al. (2008) Comparison of economic and patient outcomes with minimally invasive versus traditional off-pump coronary artery bypass grafting techniques. Ann Surg 248:638–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chitwood WR Jr (2016) Robotic mitral valve surgery: overview, methodology, results and perspective. Ann Surg 5:544–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giambruno V, Chu MW, Fox S, Swinamer SA, Rayman R, Markova Z et al. (2018) Robotic-assisted coronary artery bypass surgery: an 18-year single-centre experience. Int J Med Robot 14:e1891. [DOI] [PubMed] [Google Scholar]