Abstract
Objectives
To assess the influence of corticosteroid pulses on 60-day mortality in hospitalized patients with severe COVID-19.
Methods
We designed a multicenter retrospective cohort study in three teaching hospitals of Castilla y León, Spain (865,096 people). We selected patients with confirmed COVID-19 and lung involvement with a pO2/FiO2<300, excluding those exposed to immunosuppressors before or during hospitalization, patients terminally ill at admission, or those who died in the first 24 hours. We performed a propensity score matching (PSM) adjusting covariates that modify the probability of being treated. Then, we used a Cox regression model in the PSM group to consider factors affecting mortality.
Results
From 2933 patients, 257 fulfilled the inclusion and exclusion criteria. 124 patients were on corticosteroid pulses (250 mg of methylprednisolone for three days), and 133 were not. 30.3% (37/122) of patients died in the corticosteroid pulse group and 42.9% (57/133) in the nonexposed cohort. These differences (12.6%, 95% CI [8·54-16.65]) were statically significant (log-rank 4.72, p = 0, 03). We performed PSM using the exact method. Mortality differences remained in the PSM group (log-rank 5.31, p = 0.021) and were still significant after a Cox regression model (HR for corticosteroid pulses 0.561; p = 0.039).
Conclusions
This study provides evidence about treatment with corticosteroid pulses in severe COVID-19 that might significantly reduce mortality. Strict inclusion and exclusion criteria with that selection process set a reliable frame to compare mortality in both the exposed and nonexposed groups.
1. Introduction
In December 2019, a new betacoronavirus called SARS-CoV-2 induced severe bilateral pneumonia similar to severe acute respiratory syndrome (SARS), described in 2003. This coronavirus disease (COVID-19) had lower mortality than SARS-CoV-1 infection but higher infective capacity. The epidemic began in Wuhan, mainland China, but in a few months became pandemic.
Spain was one of the world's most affected countries, especially in Madrid, Catalonia, and Castilla y León regions [1].
After 32 885 641 cases were confirmed, mortality rates are between 3 and 4% [2], mostly due to acute respiratory distress syndrome (ARDS) and micropulmonary embolism. These symptoms are related to a hyperinflammatory state and a cytokine storm syndrome in some patients [3]. Thus, several authors have postulated that immunosuppressor agents (like corticosteroids, anakinra [4, 5], or tocilizumab [6, 7]) might be useful for these patients.
Several studies have tried corticosteroids for the treatment of viral pneumonia (including Flu and SARS-CoV-1) and ARDS, with different results [8–26]. Only a few studies demonstrate the benefits of corticosteroids on mortality [15, 16, 27, 28]. The Recovery trial's preliminary results obtained mortality benefits with dexamethasone treatment in COVID-19 patients that required oxygen supplementation [29].
Corticosteroids inhibit the migration of leukocytes to inflamed tissues, enhancing their migration from bone marrow to blood [30] and decreasing leukocyte apoptosis [31]. They also inhibit leukocyte reactive oxygen species, increase IL-10 [32, 33], and alter the maturation and differentiation of dendritic cells [34–36]. Corticosteroids modify NK cytolytic activity and monocyte activation [36]. They also downregulate IL-1, IL-2, IL-6, IL-8, IFN-γ, or TNF-α by transrepression [37].
The dose and the timing of corticosteroids are essential to determine their effect. There are three moments in which the use of corticosteroids might be especially useful. These are the onset of acute lung injury, the initial phase of ARDS, and ARDS refractory to treatment [38].
At thirty to one hundred mg of prednisone equivalent daily dose, corticosteroids act over cytosolic glucocorticoid receptors (cGCR), following the so-called genomic pathway [38, 39]. The genomic pathway effect is highest at 100 mg daily dose. The complex formed by glucocorticoid and its cytosolic GCR has two actions: promotion of anti-inflammatory transcription factors (transactivation) like IL-10 and annexin 1 and inhibition of inflammatory transcription factors (transrepression) like IL-1, IL-2, IL-6, interferon-γ (IFN-γ), prostaglandins or tumor necrosis factor α (TNF-α), and IL-8. All these changes carry out from hours to days.
If we use an equivalent dose of prednisone higher than 100 mg daily (so-called pulse corticosteroids), we obtain the maximum effect of the genomic pathway and additional responses from the faster “nongenomic pathway” [37]. These nongenomic mechanisms include membrane dysfunction in all immune cells (including lymphocytes), with a delayed flow across the membrane in the calcium and sodium channels with subsequent decreased ATP production. Other nongenomic effects are binding to membrane GCR in T cells [37] or the release of Src protein from the complex cGCR multiprotein (anti-inflammatory effects). This quick (in hours) and effective action [40] justifies their use in life-threatening situations in autoimmune diseases.
2. Methods
We analyzed patients with COVID-19 admitted between March 12th and May 20th to three tertiary teaching hospitals in Castilla y León, Spain: Hospital Clínico Universitario de Valladolid (HCUV), Hospital Universitario de Salamanca (HUSA), and Hospital Universitario de Burgos (HUBU). The three hospitals cover all hospital admissions in a geographical area corresponding to 865 096 people.
The treating team decided on the prescription of all drugs without any intervention from investigators. We obtained the local ethics committee (CEIC) permission to perform the study. Informed consent was obtained. We designed a retrospective cohort study and compared a cohort of patients exposed to corticosteroid pulses and an unexposed one.
2.1. Data Source
We analyzed paper and electronic records in all hospitals. We recorded variables related to clinical outcomes and corticosteroids exposure (supplementary material section A (available here)).
2.2. Inclusion and Exclusion Criteria
We included patients older than 18 years, testing positive on SARS-CoV-2 PCR (nasopharyngeal or oropharyngeal swab specimens). Patients with positive ELISA serology and consistent clinical symptoms were also considered confirmed cases.
All included patients had a significant lung involvement, defined as a pO2/FiO2<300, maintained for 24 hours or repeated for three days. We measured pO2/FiO2 in arterial gasometry or estimated it from pulse oximetry data (nonlinear estimate model) [41, 42].
We excluded patients receiving classic immunosuppressors or cytokine blockers (as cyclosporine, tocilizumab, or anakinra). Concomitant drugs allowed were hydroxychloroquine, azithromycin, remdesivir, lopinavir/ritonavir, and colchicine. We excluded patients who died in the first 24 hours of admission. Patients on corticosteroid treatment in a different regimen than the one described in this study were excluded. We also excluded pregnant women, terminally ill patients, and patients under a limitation of therapeutic efforts during the first 24 hours of admission.
2.3. Corticosteroid Pulse Definition
We considered exposure to corticosteroid pulses if administered at a daily dose of 125 to 500 mg of intravenous methylprednisolone for two to five days. We did not include patients with repeated corticosteroid pulses nor treatments longer than five days. About timing, we considered corticosteroid pulses in the ±3 days, respecting the inclusion criterion date.
2.4. Endpoints
The primary endpoint was 60-day mortality in exposed versus nonexposed patients. Secondary endpoints were 30-day mortality, intensive care unit (ICU) admission, in-hospital stay, viral shedding until negative PCR, and serious adverse events, including infections.
2.5. Statistical Analysis
We expressed continuous variables with the median and interquartile range (mean and standard deviation if they had normal distribution). We used chi-square to compare qualitative variables and the t-test (if normal distribution) or the Mann Withney test to compare two quantitative variables. We performed the Kolmogorov-Smirnov test to examine normal distribution.
We performed a propensity score matching to balance the difference of covariates related to exposure to corticosteroid pulses.
To select suitable covariates to control, we set biologically plausible variables related to the probability of being treated with corticosteroids pulses (propensity score). First, we analyzed variables associated with the propensity score in univariate analysis. Variables found significant were dichotomized, and then, we performed a binary logistic regression to evaluate independent variables associated with the propensity score. We chose three matching methods (propensity score matching) to preprocess the sample: the nearest neighbor, the nearest neighbor with a caliper (at a distance of 0.05, 0.1, 0.2, and 0.3), and exact matching. We performed the propensity score matching using the R software, with the MatchIt and Cobalt libraries.
We checked the balance of the propensity score matching through the “difference of means” to ensure that the distribution of covariates was similar in the treated and control group, and we picked the best-matched model.
Once we completed the propensity score matching, we performed a Cox proportional hazard regression analysis to evaluate mortality and intensive care admission.
3. Results
3.1. Patients
From 2933 patients in our cohort, 257 fulfilled the inclusion and exclusion criteria. 767 fulfilled the inclusion criterion, and 546 had any exclusion criteria (see Figure 1). We diagnosed with COVID-19 in 243 patients based on SARS-CoV-2 PCR in the nasopharyngeal or oropharyngeal swabbing and 14 patients based on positive serology with compatible symptoms. 124 patients were on corticosteroid pulses, and 133 were not. The most used corticosteroid pulses dose was 250 mg daily for three days (92%,114/124).
Figure 1.
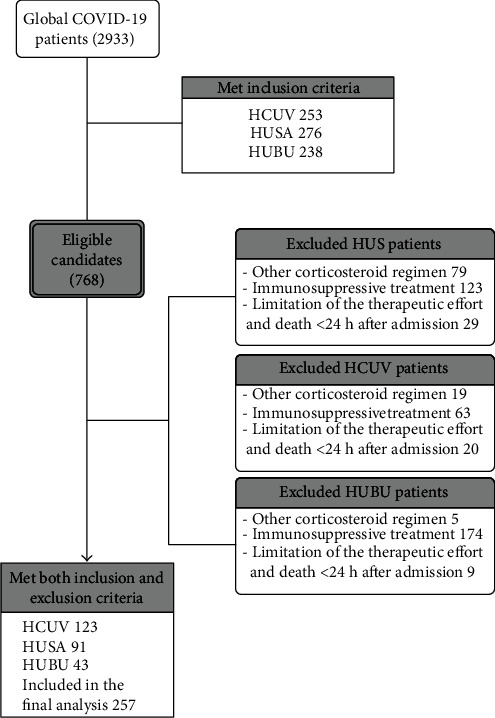
Flow diagram of COVID-19 included patients in this study.
3.2. Propensity Score Matching
We calculated the propensity score (probability of being treated with corticosteroid pulses) in each participant from a binary logistic regression. Variables statically significant in the binary logistic regression were as follows: epidemiological week, presence of bilateral infiltrates or not, Center in Castilla y León, Ferritin, and COVID-gram score (see supplementary material section B (available here)).
After performing the propensity score, we tried several preprocessing methods for matching (see Methods), and we selected the exact matching method as it got the minimum difference between groups with the minimum sample loss (28 controls and 2 treated patients).
The difference of means was zero in the treated versus the controls because we used the exact matching method (Figure 2 and supplementary material section B (available here)).
Figure 2.
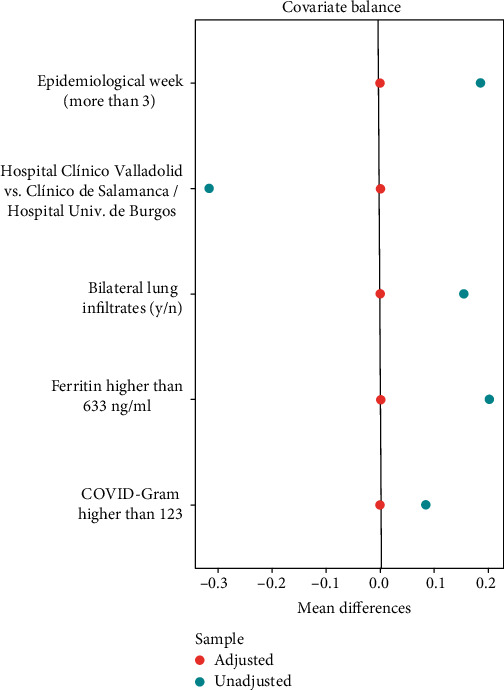
Love plot of the propensity score matching using the exact method.
After the propensity score matching, the sample consisted of 207 patients (119 treated and 88 controls).
3.3. Baseline Features
The median age in all participants (257) was 75 [63.5-83] years. One hundred and eleven participants (43.2%) were women. Their median classic Charlson score was 1 [0-3].
Comparing patients exposed to corticosteroid pulses and the nonexposed ones, we found that age and comorbidities were similar in both groups, without significant differences. The COVID-gram score [43] was 155.8 in the bolus group and 152.3 in the control group, even decreasing these differences after matching. The classic Charlson score was significantly higher (0.7 points) in the control group (p = 0.012). These differences disappeared after dichotomizing the variable in the matched group (≤2 or >2 more than 2 points, p = 0.171) (see Table 1).
Table 1.
Baseline features of patients from all centers combined.
| Treatment | Overall population | Matched population | ||
|---|---|---|---|---|
| Methylprednisolone (n = 124) | Usual care (n = 133) | Methylprednisolone (n = 117) | Usual care (n = 88) | |
| Baseline characteristics | ||||
| Gender (male) no- (%) | 77 (62%) | 69 (51.8%) | 74 (63.3%) | 45 (51.1%) |
| Age (mean-years) | 74 [59-83] | 76 [65-84] | 75[60-83] | 76 [66-83] |
| > 80 years no- (%) | 37 (29.8%) | 47 (35.3%) | 36 (30.8%) | 36 (30.8%) |
| Autoimmune disease | 4 (3.2%) | 3 (2.3%) | 3 (2.5%) | 3 (3.4%) |
| Cancer (%) | 17 (13.7%) | 17 (12.8%) | 17 (14.5%) | 11 (12.5%) |
| Chronic kidney disease (%) | 6 (4.8%) | 14 (10.5%) | 6 (5.1%) | 9 (10.2%) |
| Dementia (%) ∗ | 7 (5.6%) | 21 (18.7%) | 6 (5.1%) | 12 (13.6%) |
| Diabetes | 40 (32.3%) | 52 (39.1%) | 38 (32.5%) | 36 (40.9%) |
| Dyslipidemia | 44 (35.8%) | 40 (30.1%) | 42 (35.9%) | 29 (33%) |
| Hypertension | 63 (50.8%) | 67 (50.4%) | 42 (35.9%) | 50 (56.8%) |
| Obesity | 22 (17.8%) | 18 (13.5%) | 20 (17.1%) | 13 (14.7%) |
| Smoker | 32 (25.8%) | 29(21.8%) | 30(25.6%) | 15 (17%) |
| Prior AIT/stroke (%) | 7 (5.6%) | 9 (6.8%) | 7 (6%) | 5 (5.7%) |
| Prior IHD (%) | 12 (9.7%) | 12 (9%) | 12 (10.2%) | 10 (11.4%) |
| Prior lung disease | 20 (16.1%) | 23 (17.3%) | 20 (17.1%) | 17 (19.3%) |
| Charlson comorbidity index | 1 [0-2] | 1 [0-4] | 1 [0-2] | 1 [0-2] |
|
| ||||
| Previous treatment | ||||
| Corticosteroids | 4 (3.2%) | 2 (1.5%) | 4 (3.4%) | 1 (1.2%) |
| Anticoagulants | 14 (11.2%) | 10 (7.5%) | 14 (11.9%) | 6 (6.8%) |
|
| ||||
| Main findings at admission | ||||
| Respiratory insufficiency | 104 (83.9%) | 105 (80.8%) | 99 (84.6%) | 73 (83.9%) |
| Bilateral infiltrates chest X-ray | 103 (83.1%) | 91 (68.4%) | 105 (89.7%) | 67 (76.1%) |
| pO2/FiO2 | 239.5 [142.2-276.1] | 238.1 [192.8-285.7] | 250 [153.2-276.2] | 238 [180.5-276.1] |
| COVID GRAM | 155.8 [83.3-230] | 152.3 [69-235.6] | 157.5 [83.6-231.6] | 157.7 [82-233.2] |
|
| ||||
| Laboratory findings at admission | ||||
| Glucose (mg/dl) | 114.5 [94.2-145] | 118.5 [100.1-168.2] | 114.6 [94.5-147] | 114.3 [99.2-180] |
| C-reactive protein (mg/L) | 126.2 [84-200.2] | 85.2 [36.1-180] | 124.5 [66.7-194.5] | 111.5 [60.9-213.5] |
| Creatinine (mg/dL) | 1.06 [0.81-1.47] | 0.99 [0.78-1.53] | 1.06 [0.8-1.45] | 1.01 [0.79-1.62] |
| D-dimer (ng/mL)∗ | 842 [448-1.450] | 1,105 [540.2-2,532] | 848 [4.127-1,425] | 1,250 [678-2.532] |
| Ferritin (ng/mL) | 1,533.5 [764-2,351.2] | 921 [359.5-1,466] | 1,475 [742-2,405] | 1,104 [556-1,650] |
| Interleukin-6 (pg/mL) | 35.5 [10.15-118.7] | 51[17.4-119] | 35.5 [10.1-118.7] | 59 [17.6-123] |
| Lactate dehydrogenase (UI/L) | 348.5 [283.7-460.7] | 312[248-394] | 341 [283-455] | 326 [259-396.5] |
| Lymphocytes (cells/mm3) | 1,000 [672-1,357] | 1,000 [715-1,415] | 1,000 [670-1,320] | 1,010 [730-1,467] |
| Neutrophils (cells/mm3 × 103) | 5,050 [3,575-7,212] | 5,670 [3,895-8,805] | 5,997 [3,580-7,085] | 5,155 [133-179] |
| Procalcitonin (ng/mL) | 0.18 [0.1-0.37] | 0.15 [0.07-0.5] | 0.16 [0.1-0.3] | 0.19 [0.1-1.5] |
|
| ||||
| Specific COVID-19 treatment | ||||
| Azithromycin∗ | 115 (92.7%) | 94 (71.2%) | 108 (92.3%) | 64 (73.3%) |
| Interferon beta-1b∗ | 48 (38.7%) | 23 (17.4%) | 44 (37.6%) | 20 (23%) |
| Hydroxychloroquine∗ | 120 (96.8%) | 121 (91.7%) | 113 (96.6%) | 80 (92%) |
| Lopinavir/ritonavir∗ | 106 (85.5%) | 92 (69.7%) | 102 (87.2%) | 65 (74.7%) |
| Colchicine∗ | 16 (12.9%) | 2 (1.5%) | 15 (12.8%) | 2 (2.3%) |
|
| ||||
| Nonspecific COVID-19 treatment | ||||
| Prophylactic anticoagulation∗ | 69 (56.1%) | 90 (68.7%) | 66 (56.4%) | 57 (66.3%) |
| Intermediate anticoagulation∗ | 12 (9.8%) | 4 (3.1%) | 10 (8.5%) | 4 (4.7%) |
| Full anticoagulation∗ | 26 (21.1%) | 16 (12.2%) | 26 (22.2%) | 13 (15.1%) |
Data are shown as the median (IQR) or n (%). ∗Significant (p < 0, 05) difference between methylprednisolone and usual care population in both groups, the overall population, and propensity score match. It was calculated using the χ2 test or Kruskal-Wallis test as appropriate. PaO2/FiO2 = ratio of arterial oxygen partial pressure to fractional inspired oxygen.
LDH and ferritin at admission were higher in the pulse group, but these differences disappeared after matching. Peak ferritin and peak LDH during hospitalization were significantly higher in the pulse group, even after matching (see Table 1).
Concomitant treatments with colchicine, interferon beta-1b, lopinavir/ritonavir, and azithromycin were more common in the corticosteroid pulse group than in the controls, both before and after matching (see Table 1).
We did not find differences in pO2/FiO2 between the pulses and control group (p = 0.183 in all participants and p = 0.69 in the matched group), but bilateral lung infiltrates were more frequent in the corticosteroid pulse group (p = 0.006). That difference disappeared after matching (p = 0.299).
3.4. Outcomes
3.4.1. Primary Endpoint
Ninety-four patients died during the 60 days after admission, representing 36.9% (94/255) of the sample. 30.3% (37/122) of patients died in the corticosteroid pulse group and 42.9% (57/133) in the nonexposed cohort. These differences (12.6%) were statically significant in the Kaplan Meier curve (log-rank 4.72, p = 0.03) (see Figure 3).
Figure 3.
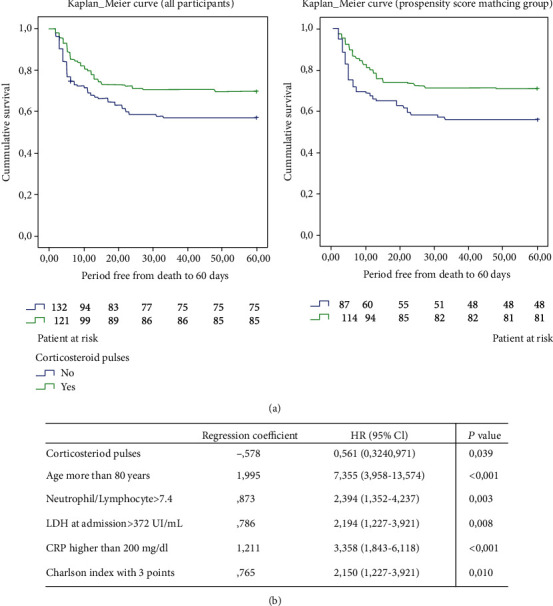
(a) Kaplan-Meier estimates of 60-day mortality in patients with and without corticosteroid treatment. (b) Cox regression for evaluating the 60-day mortality in the matched population.
We carried out a propensity score matching and calculated the mortality in the matched group. The corticosteroid pulse group had a 60 days mortality of 29.6% (34/115), while mortality in the control group was 44.3% (39/88). Again, these differences were statically significant (log-rank 5.31, p = 0.021) (see Figure 3 and Table 2).
Table 2.
Primary and secondary outcomes of the global and matched population.
| Outcome | Overall population | Matched population | ||||
|---|---|---|---|---|---|---|
| Methylprednisolone (n = 124) | Usual care (n = 133) | p value | Methylprednisolone (n = 117) | Usual care (n = 88) | p value | |
| Primary outcome | ||||||
| 60-day mortality | 37/122 (30.3%) | 57 (42.8%) | 0.026 | 34/115 (29.6%) | 39/88 (44.3%) | 0.022 |
|
| ||||||
| Secondary outcomes | ||||||
| 30-day mortality | 37/122 (30.3%) | 56/133 (42.1%) | 0.034 | 34 (29%) | 38 (43.2%) | 0.031 |
| Hyperglycemia | 10 (8.0%) | 5 (3.7%) | n.s | 10 (8.5%) | 5 (5.6%) | n.s |
| ICU admission | 23 (18.5%) | 26 (19.5%) | n.s | 19 (16.2%) | 21 (23.8%) | n.s |
| In-hospital mortality | 36 (29%) | 56 (42.1%) | 0,02 | 33 (28.2%) | 38 (43.2%) | 0.019 |
| LOS (days) | 8 (10) | 12 (13) | 8 (11) | 12 (12) | n.s | |
| Mechanical ventilation | 19 (15.3%) | 25 (18.7%) | n.s | 16 (13.6%) | 21 (23.8%) | n.s |
| Nosocomial infection | 29 (23.4%) | 32 (24%) | n.s | 26 (22.2%) | 25 (28.4%) | n.s |
Data are shown as the median (IQR) or n (%).ICU: intensive care unit; LOS: length of stay; n.s: not significant.
We performed a multivariate analysis using a Cox regression model in the propensity score matching group, after dichotomizing variables, and those independently related to 60-day mortality were as follows: corticosteroid pulses (HR 0.561, p = 0.039), age older than 80 years (HR 7.3, p < 0.001), CPR at admission higher than 200 mg/dL (HR 3.35, p < 0.001), neutrophil/lymphocyte index > 7.4 (HR 2.15, p = 0.010), Charlson index higher than 2 points (HR 2.15, p = 0.018), and LDH at admission > 372 UI/mL (HR 2.29, p = 0.008) (see Figure 4). In the equation, we did not include other variables (like pO2/FiO2, lung infiltrates, or D-dimer) that were not significant in the multivariate model, although important in the univariate analysis.
Figure 4.
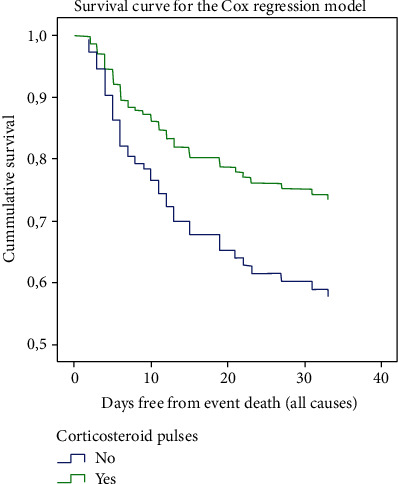
Survival for the Cox regression model in corticosteroids exposed and nonexposed patients (in the PSM group).
We studied the possible association of colchicine, azithromycin, and lopinavir/ritonavir on mortality, as those treatments were more frequently used in the corticosteroid pulse group. The three treatments were associated with lower mortality in the Kaplan-Meier curves (p = 0.008 for colchicine, p = 0.032 for lopinavir/ritonavir, and p < 0.001 for azithromycin). These differences disappear after adjusting for age higher than 80 years in both three drugs (p = 0.1 for colchicine, p = 0.794 for lopinavir/ritonavir, and p = 0.378 for azithromycin).
Patients on corticosteroid pulses had a higher, but not significant, anticoagulation rate (86.9% vs. 83.9%, p = 0.49). Patients on pulses also used significantly lower prophylactic dose heparin and higher intermediate and anticoagulant dose. Still, we did not find in our study an association between the absence of anticoagulation and mortality (log-rank 0.9, p = 0.338) (see supplementary material Section D).
3.4.2. Secondary Endpoints
The thirty-day mortality was 30.3% (37/122) in the corticosteroid pulse group and 42.1% (56/133) in the nonexposed cohort. The difference was statically significant (log-rank = 4.3, p = 0.038) in the Kaplan-Meier curve. That difference remained in the propensity score matching group (p = 0.03) (See supplementary material (available here)). After using the same Cox regression model used in the 60-days mortality analysis, corticosteroid pulses remain a protective factor for 30-days mortality (p = 0.049) (see supplementary material section C1 (available here) and Figure 3).
Forty-nine patients were admitted to ICU during this period. ICU admission was 18.5% (23/124) in the corticosteroid pulses cohort and 19.5% (26/133) in the nonexposed group (p = 0.838). We found similar results in the propensity score matching group (ICU admission 16.2% in the corticosteroid group and 23.9% in the control group, p = 0.173). The time from hospital admission to ICU admission was zero to four days, and 83.6% of patients were admitted in the first 24 hours. Mortality among the ICU admitted patients was 17.4% (4/23) in the exposed group and 61.5% (16/26) in the nonexposed group. This difference was statistically significant for 60-day mortality (log-rank = 9.7, p = 0.002). This difference remains in the propensity score-matched group (15.8% vs. 61.9%, p = 0.003). After adjusting for several variables (peak LDH, peak CRP, number of comorbidities, D-dimer at admission, SaO2/FiO2, and age) in a Cox regression, those differences in mortality were not significant (see supplementary material section C2 (available here)). The ICU average stay was 19.2 and 21.67 days for both the exposed and nonexposed cohorts, respectively (p = 0.750).
The in-hospital median stay was 12 [7.25-19.75] days in the treated cohort and 8 [5–15] days in the nonexposed group (p < 0.001). These differences remain in the matched group (p = 0.001). However, differences were not statistically significant if we analyzed them in the survivor's group (difference of means 1.2 days, p = 0.619).
Viral shedding until negative PCR was shorter (but not significant, p = 0.279) in the corticosteroid cohort (25.02 days in the corticosteroid group and 30.65 days in the nonexposed).
We reported serious adverse events in 17 patients in the exposed cohort and 15 patients in the control group (p = 0.133). Hemorrhage happened in 9 patients, without difference between groups (p = 0.053). In-hospital infections were not higher in the corticosteroid bolus group (29/124) than in the nonexposed cohort (32/129) (p = 0.792).
We found 27 patients with pulmonary embolism by CT scan (8 in the exposed group and 19 in nonexposed, p = 0.066). We also reported two ischemic strokes and one acute myocardial infarction.
4. Discussion
Corticosteroid pulses have been widely used in Spain, especially in the Castilla y León region, but not in other countries to treat COVID-19. The rationale of its use is to stop the systemic inflammation process [3] that develop in some patients with severe COVID-19. Some studies [44–46] have described the positive effects of corticosteroid pulses on mortality in patients with severe COVID-19.
We found a significant improvement in survival in patients treated with corticosteroid pulses. We designed a retrospective cohort study to confirm this statement, which is the main limitation of our work. As there is no randomization, unknown confounders might be unattended.
We carried out a multicenter study with three teaching hospitals in the Castilla y León region in Spain. This fact is one of the main strengths of the study. On the one hand, we summarized various treatment protocols in each center, showing a wider specter of treatment options for severe COVID-19. This protocol variety determines different probabilities of being treated with corticosteroids in each center and enables us to adjust them in the latter propensity score matching. It also considers different hospital admission criteria and different extrahospital resources that may change the baseline features of hospitalized patients.
On the other hand, it represents all hospital admissions in a geographic area in Castilla y León with more than 865 000 people that have similar epidemiological features. They also had the same timing of lockdown and the same mobility restrictions over time.
We used strict inclusion and exclusion criteria to avoid mixing the effects of other immunosuppressive treatments in mortality. They were also useful to find an adequate patient profile who, a priori, should benefit on an anti-inflammatory treatment as corticosteroid pulses. We selected patients, at the inclusion time, with the onset of an acute respiratory distress syndrome.
To limit potential biases, we performed a propensity score matching (PSM) using the exact method. Thus, we obtained a more homogeneous sample with baseline features that were similar in both groups. Both the exposed and nonexposed cohorts in the propensity score matching group had comparable age, a similar pretest probability of dying (through the COVID-gram score), probability of being treated with corticosteroids, and uniform comorbidities. We again found, in this PSM group, the same mortality decrease in the corticosteroid pulse arm. Then, to adjust other possible mortality causes, we performed a Cox regression multivariate model on the PSM group, once more finding a significant protective role of corticosteroid pulses in mortality.
Altogether, joining these strict inclusion and exclusion criteria with all this selection process sets a reliable frame to compare mortality in both the exposed and nonexposed groups. Thus, corticosteroid pulses might be a good option for the treatment of severe COVID-19, as they have been shown to be effective in reducing mortality in our cohort and they are inexpensive and highly available worldwide. Our results can only extrapolate to patients with severe COVID-19, with a pO2/FiO2 lower than 300, not exposed to any kind of immunosuppression, and in the absence of a terminally ill situation at admission.
Some recent studies have confirmed that oral or intravenous low-dose corticosteroids positively affect mortality [29]. There was a decrease in mortality between 3.1 and 12.1% (in the ICU admitted group), lower than the 12.6% of global mortality reduction (even higher in the ICU subset) that we found. We hypothesized that the effect of corticosteroid pulses might be higher than the low-dose corticosteroids because they act in different pathways (genomic vs. nongenomic) and behave, in fact, as different drugs [37]. Our study is not powered to compare both low-dose and pulse corticosteroid treatments, so we cannot assure this statement. Future studies must investigate this topic.
Pulse corticosteroids did not reduce the ICU admission rate in our study. Most patients were moved to the ICU in the first 24 hours of hospitalization (83.6%), so we understand that those patients were critically ill at admission time, requiring ICU in any case.
The in-hospital stay was significantly longer in the pulses corticosteroid arm, but those differences disappear in the survivor's group. Thus, this difference in the hospital stay was due to higher survival in the corticosteroid pulse group.
The rate of adverse events and serious adverse events declared was similar in both groups. In the same way, in-hospital infection and viral shedding time were similar in both groups, but some of these adverse events and infections might be underreported. The study was not powered to detect these adverse events because they were not always reported in the medical record in all patients.
In conclusion, this study provides evidence about the treatment with corticosteroid pulses in severe COVID-19 that might significantly reduce mortality. This data must be confirmed in prospective randomized studies.
Acknowledgments
Roche granted the present study through the IECSCYL foundation. Ainhoa Cusacovich contributed to the paper translation.
Abbreviations
- ARDS:
Acute respiratory distress syndrome
- COVID-19:
Coronavirus disease 2019
- ICU:
Intensive care unit
- PSM:
Propensity score matching
- SARS-CoV-2:
Severe acute respiratory syndrome coronavirus 2
- PCR:
Polymerase chain reaction
- IFN-γ:
Interferon-γ
- TNF-α:
Tumor necrosis factor α
- LDH:
Lactate dehydrogenase
- CRP:
C-reactive protein.
Data Availability
We recorded all data in a database. These data could be requested if there is an important reason.
Additional Points
Bullet Points. (i) Corticosteroid pulses can improve mortality in severe COVID-19 patients. (ii) The Kaplan Meier curve with the propensity score matching and multivariate analysis in this group is reliable. (iii) There are no substantial changes in ICU admission and hospital stay.
Disclosure
We sent a preprint to MedRxiv, with the following doi:10.1101/2020.09.30.20204719.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
All authors contributed to data collection. Ivan Cusacovich, Álvaro Aparisi, Miguel Marcos, David Andaluz-Ojeda, Roberto Gonzalez-Fuentes, and Carlos Dueñas contributed to the study design. Ivan Cusacovich and David Andaluz-Ojeda performed the statistical analysis. Ivan Cusacovich, Álvaro Aparisi, David Andaluz-Ojeda, Roberto Gonzalez-Fuentes, Miguel Marcos, and Carlos Dueñas contributed to writing the draft. David Andaluz-Ojeda and Roberto Gonzalez-Fuentes contributed equally to this work.
Supplementary Materials
Section A: Variable Description and Coding in the Study. Section B: Construction of the Propensity Score and Matching Process. Section C: Secondary Endpoints. Section D: Anticoagulation and Survival Analysis.
References
- 1.Centro de coordinación y alertas sanitarias. Gobierno de España. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_109_COVID-19.pdf.
- 2.Du R. H., Liang L. R., Yang C. Q., et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Europen Respiratory Journal. 2020;55(5, article 200024) doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehta P., McAuley D. F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cavalli G., de Luca G., Campochiaro C., et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. The Lancet Rheumatology. 2020;2(6):e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P., Cron R. Q., Hartwell J., Manson J. J., Tattersall R. S. Silencing the cytokine storm: the use of intravenous anakinra in haemophagocytic lymphohistiocytosis or macrophage activation syndrome. The Lancet Rheumatology. 2020;2(6):e358–e367. doi: 10.1016/S2665-9913(20)30096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan K. W., Wong V. T., Tang S. C. W. COVID-19: an update on the epidemiological, clinical, preventive and therapeutic evidence and guidelines of integrative Chinese-Western medicine for the management of 2019 novel coronavirus disease. The American Journal of Chinese Medicine. 2020;48(3):737–762. doi: 10.1142/S0192415X20500378. [DOI] [PubMed] [Google Scholar]
- 7.Sorbello M., el-Boghdadly K., di Giacinto I., et al. The Italian coronavirus disease 2019 outbreak: recommendations from clinical practice. Anaesthesia. 2020;75(6):724–732. doi: 10.1111/anae.15049. [DOI] [PubMed] [Google Scholar]
- 8.Luce J. M., Montgomery A. B., Marks J. D., Turner J., Metz C. A., Murray J. F. Ineffectiveness of high-dose methylprednisolone in preventing parenchymal lung injury and improving mortality in patients with septic shock. The American Review of Respiratory Disease. 1987;138(1):62–68. doi: 10.1164/ajrccm/138.1.62. [DOI] [PubMed] [Google Scholar]
- 9.Russell C. D., Millar J. E., Baillie J. K. Clinical evidence does not support corticosteroid treatment for 2019-nCoV lung injury. Lancet. 2020;395(10223):473–475. doi: 10.1016/S0140-6736(20)30317-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindstrom S. Efficacy and safety of corticosteroids for persistent acute respiratory distress syndrome. The New England Journal of Medicine. 2006;360:2605–2615. doi: 10.1056/NEJMoa051693. [DOI] [PubMed] [Google Scholar]
- 11.on behalf of the GETGAG Study Group, Moreno G., Rodríguez A., et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Medicine. 2018;44(9, article 5332):1470–1482. doi: 10.1007/s00134-018-5332-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao B., Gao H., Zhou B., et al. Adjuvant corticosteroid treatment in adults with influenza a (H7N9) viral pneumonia. Critical Care Medicine. 2016;44(6):e318–e328. doi: 10.1097/CCM.0000000000001616. [DOI] [PubMed] [Google Scholar]
- 13.Ni Y. N., Chen G., Sun J., Liang B. M., Liang Z. A. The effect of corticosteroids on mortality of patients with influenza pneumonia: a systematic review and meta-analysis. Critical Care. 2019;23(1):99–99. doi: 10.1186/s13054-019-2395-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peter J. V., John P., Graham P. L., Moran J. L., George I. A., Bersten A. Corticosteroids in the prevention and treatment of acute respiratory distress syndrome (ARDS) in adults: meta-analysis. BMJ. 2008;336(7651):1006–1009. doi: 10.1136/bmj.39537.939039.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meduri G. U., Headley A. S., Golden E., et al. Effect of prolonged methylprednisolone therapy in unresolving acute respiratory distress syndrome: a randomized controlled trial. Journal of the American Medical Association. 1998;280(2):159–165. doi: 10.1001/jama.280.2.159. [DOI] [PubMed] [Google Scholar]
- 16.Keel J. B. P., Hauser M., Stocker R., Baumann P. C. S. R., Speich R. Established acute respiratory distress syndrome: benefit of corticosteroid rescue therapy. Respiration. 1998;65(4):258–264. doi: 10.1159/000029273. [DOI] [PubMed] [Google Scholar]
- 17.Bone R. C., Fisher C. J., Clemmer T. P., Slotman G. J., Metz C. A. Early methylprednisolone treatment for septic syndrome and the adult respiratory distress syndrome. Chest. 1987;92(6):1032–1036. doi: 10.1378/chest.92.6.1032. [DOI] [PubMed] [Google Scholar]
- 18.Bernard G. R., Luce J. M., Sprung C. L., et al. High-Dose corticosteroids in patients with the adult respiratory distress syndrome. The New England Journal of Medicine. 1987;317(25):1565–1570. doi: 10.1056/NEJM198712173172504. [DOI] [PubMed] [Google Scholar]
- 19.Ríos F. G., Estenssoro E., Villarejo F., et al. Lung function and organ dysfunctions in 178 patients requiring mechanical ventilation during the 2009 influenza a (H1N1) pandemic. Critical Care. 2011;15(4):R201–R212. doi: 10.1186/cc10369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H., Yang S. G., Gu L., et al. Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A(H1N1)pdm09 viral pneumonia. Influenza and Other Respiratory Viruses. 2017;11(4):345–354. doi: 10.1111/irv.12456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brun-Buisson C., Richard J. C. M., Mercat A., Thiébaut A. C. M., Brochard L. Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. American Journal of Respiratory and Critical Care Medicine. 2011;183(9):1200–1206. doi: 10.1164/rccm.201101-0135OC. [DOI] [PubMed] [Google Scholar]
- 22.Diaz E., Martin-Loeches I., Canadell L., et al. Corticosteroid therapy in patients with primary viral pneumonia due to pandemic (H1N1) 2009 influenza. The Journal of Infection. 2012;64(3):311–318. doi: 10.1016/j.jinf.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Viasus D., Ramón Paño-Pardo J., Cordero E., et al. Effect of immunomodulatory therapies in patients with pandemic influenza A (H1N1) 2009 complicated by pneumonia. The Journal of Infection. 2011;62(3):193–199. doi: 10.1016/j.jinf.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 24.Meduri G. U., Golden E., Freire A. X., et al. Methylprednisolone infusion in early severe ards: results of a randomized controlled trial. Chest. 2007;131(4):954–963. doi: 10.1378/chest.06-2100. [DOI] [PubMed] [Google Scholar]
- 25.Weigelt J. A., Norcross J. F., Borman K. R., Snyder WH 3rd Early steroid therapy for respiratory failure. Archives of Surgery. 1985;120(5):536–540. doi: 10.1001/archsurg.1985.01390290018003. [DOI] [PubMed] [Google Scholar]
- 26.Villar J., Ferrando C., Martínez D., et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. The Lancet Respiratory Medicine. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 27.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA internal medicine. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group, Sterne J. A. C., Murthy S., et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19. JAMA. 2020;324(13):1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The RECOVERY Collaborative Group, Emberson J. R. Dexamethasone in hospitalized patients with Covid-19 — preliminary report. New England Journal of Medicine. 2020 doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavalcanti D. M. H., Lotufo C. M. C., Borelli P., Ferreira Z. S., Markus R. P., Farsky S. H. P. Endogenous glucocorticoids control neutrophil mobilization from bone marrow to blood and tissues in non-inflammatory conditions. British Journal of Pharmacology. 2007;152(8):1291–1300. doi: 10.1038/sj.bjp.0707512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liles W. C., Dale D. C., Klebanoff S. J. Glucocorticoids inhibit apoptosis of human neutrophils. Blood. 1995;86(8):3181–3188. doi: 10.1182/blood.V86.8.3181.3181. [DOI] [PubMed] [Google Scholar]
- 32.Dandona P., Mohanty P., Hamouda W., Aljada A., Kumbkarni Y., Garg R. Effect of dexamethasone on reactive oxygen species generation by leukocytes and plasma interleukin-10 concentrations: a pharmacodynamic study. Clinical Pharmacology and Therapeutics. 1999;66(1):58–65. doi: 10.1016/S0009-9236(99)70054-8. [DOI] [PubMed] [Google Scholar]
- 33.Llewellyn-Jones C. G., Hill S. L., Stockley R. A. Effect of fluticasone propionate on neutrophil chemotaxis, superoxide generation, and extracellular proteolytic activity in vitro. Thorax. 1994;49(3):207–212. doi: 10.1136/thx.49.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rozkova D., Horvath R., Bartunkova J., Spisek R. Glucocorticoids severely impair differentiation and antigen presenting function of dendritic cells despite upregulation of toll-like receptors. Clinical Immunology. 2006;120(3):260–271. doi: 10.1016/j.clim.2006.04.567. [DOI] [PubMed] [Google Scholar]
- 35.Piemonti L., Monti P., Allavena P., et al. Glucocorticoids affect human dendritic cell differentiation and maturation. Journal of Immunology. 1999;162(11):6473–6481. [PubMed] [Google Scholar]
- 36.Zaza G., Leventhal J., Signorini L., Gambaro G., Cravedi P. Effects of antirejection drugs on innate immune cells after kidney transplantation. Frontiers in Immunology. 2019;10:1–10. doi: 10.3389/fimmu.2019.02978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stahn C., Buttgereit F. Genomic and nongenomic effects of glucocorticoids. Nature Clinical Practice. Rheumatology. 2008;4(10):525–533. doi: 10.1038/ncprheum0898. [DOI] [PubMed] [Google Scholar]
- 38.Meduri G. U., Chrousos G. P. Effectiveness of prolonged glucocorticoid treatment in acute respiratory distress syndrome: the right drug, the right way? Critical Care Medicine. 2006;34(1):236–238. doi: 10.1097/01.CCM.0000196088.75067.4C. [DOI] [PubMed] [Google Scholar]
- 39.Ruiz-Irastorza G., Danza A. Khamashta M. Glucocorticoid use and abuse in SLE. Rheumatol (United Kingdom) 2012;51:1145–1153. doi: 10.1093/rheumatology/ker410. [DOI] [PubMed] [Google Scholar]
- 40.Hughes R. Pulse glucocorticoid therapy. Journal of Chemical Information and Modeling. 2012;53:p. 287. [Google Scholar]
- 41.Brown S. M., Grissom C. K., Moss M., et al. Nonlinear imputation of PaO2/FIO2 from SpO2/FIO2 among patients with acute respiratory distress syndrome. Chest. 2016;150(2):307–313. doi: 10.1016/j.chest.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown S. M., Duggal A., Hou P. C., et al. Nonlinear imputation of PaO2/FIO2 from SpO2/FIO2 among mechanically ventilated patients in the ICU. Critical Care Medicine. 2017;45(8):1317–1324. doi: 10.1097/CCM.0000000000002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liang W., Liang H., Ou L., et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Internal Medicine. 2020;180(8):1081–1089. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz-Irastorza G., Pijoan J.-I., Bereciartua E., et al. Second week methyl-prednisolone pulses improve prognosis in patients with severe coronavirus disease 2019 pneumonia: an observational comparative study using routine care data. PLoS One. 2020;15(9, article e0239401):1–17. doi: 10.1371/journal.pone.0239401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edalatifard M., Akhtari M., Salehi M., et al. intravenous methylprednisolone pulse as a treatment for hospitalised severe COVID-19 patients: results from a randomised controlled clinical trial. The European Respiratory Journal. 2020;56(6, article 2002808) doi: 10.1183/13993003.02808-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Callejas Rubio J. L., Luna del Castillo J. d. D., de la Hera Fernández J., Guirao Arrabal E., Colmenero Ruiz M., Ortego Centeno N. Effectiveness of corticoid pulses in patients with cytokine storm syndrome induced by SARS-CoV-2 infection. Medicina Clínica (Barcelona) 2020;155(4):159–161. doi: 10.1016/j.medcli.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Section A: Variable Description and Coding in the Study. Section B: Construction of the Propensity Score and Matching Process. Section C: Secondary Endpoints. Section D: Anticoagulation and Survival Analysis.
Data Availability Statement
We recorded all data in a database. These data could be requested if there is an important reason.


