Abstract
Adipose tissue is composed of a variety of cells distributed in different depots and playing various metabolic roles. In a recent issue of Nature, Sun et al. (2020) use snRNA-seq and functional studies to identify a population of adipocytes that can suppress the thermogenic activity of neighboring adipocytes by secretion of acetate.
Adipocytes are a major hub for energy balance and fuel metabolism in the body. Inter- and intra-depot heterogeneity of adipocytes offers a wide range of functional diversity enabling short- and long-term regulation of energy storage and dissipation. White adipocytes store energy in the form of triglycerides in lipid droplets and are found in discrete white adipose tissue (WAT) depots, as well as dispersed in other tissues throughout the body. Excess WAT is associated with insulin resistance and metabolic syndrome. Brown and beige adipocytes, on the other hand, are specialized to burn energy and generate heat. Classical brown adipocytes are present in a distinct brown adipose tissue (BAT) depot, while beige/brite adipocytes appear intermixed in WAT depots, where they are induced or activated in response to certain environmental cues, such as chronic cold exposure, exercise training, or pharmacologic treatment with the β-adrenergic receptor activators. In general, increased brown/beige fat is associated with improved metabolic health.
Previous studies using lineage tracing and clonal isolation of adipocyte progenitors have revealed developmental and functional diversity of adipocytes within the brown or white adipose depots (Lee et al., 2019; Min et al., 2019). In their recent paper, Sun et al. have uncovered a new adipocyte subtype that is present in both WAT and BAT that can negatively regulate the thermogenic activity of neighboring adipocytes (Sun et al., 2020). To identify these cells, Sun et al. employed a novel variation of single-nucleus RNA sequencing (snRNA-seq) using murine adipocytes expressing a fluorescent nuclear label. This identified a population of CYP2E1+/ALDH1A1+ adipocytes that represent about 10% of adipocytes in interscapular BAT and 20% of adipocytes in subcutaneous WAT in mice. snRNA-seq of human BAT and WAT demonstrated the presence of similar adipocytes in human adipose tissue. Importantly, they found that the frequency of CYP2E1+/ALDH1A1+ adipocytes in mice decreases upon cold exposure and increases in thermoneutrality, suggesting they may play a negative role in thermogenesis and metabolism.
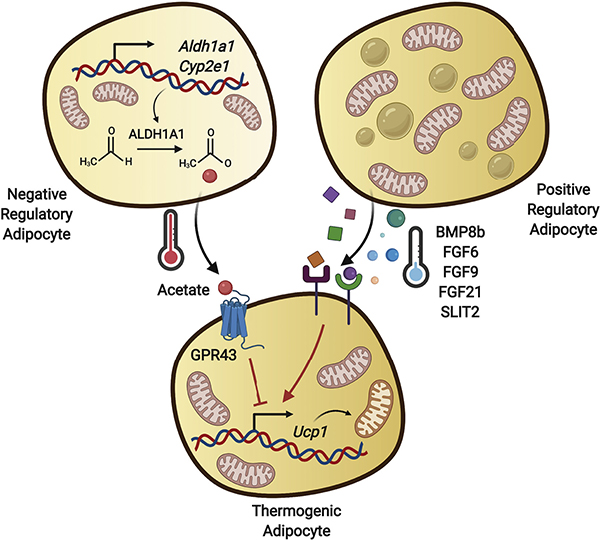
Previous studies have reported a role for ALDH1A1 (aldehyde dehydrogenase 1 family member A1) in the regulation of adipose thermogenesis and metabolism (Kiefer et al., 2012; Ziouzenkova et al., 2007). ALDH1A1 is a cytosolic enzyme that catalyzes the conversion of retinaldehyde to retinoic acid. Mice lacking Aldh1a1 display enhanced energy expenditure and are protected against diet-induced obesity and insulin resistance (Ziouzenkova et al., 2007). Aldh1a1 deficiency in WAT leads to accumulation of retinaldehyde, which activates the retinoic acid receptor and recruits the coactivator PGC-1α to the UCP1 regulatory region (Kiefer et al., 2012). To address the functional role of CYP2E1+/ALDH1A1+ adipocytes in BAT thermogenesis, Sun et al. used a combination of in vivo and in vitro gain- and loss-of-function studies. Consistent with the above studies, knockdown of Aldh1a1 in adipocytes resulted in an elevated level of UCP1, the key thermogenic protein, leading to increased BAT glucose uptake and enhanced oxygen consumption upon cold exposure. Conversely, overexpression of Aldh1a1 in BAT reduced UCP1 expression and blunted cold-induced energy expenditure. Importantly, co-culture of wild-type adipocytes with adipocytes overexpressing Aldh1a1 led to decreased UCP1 expression and cellular respiration in the wild-type adipocytes, indicating that the ALDH1A1-expressing cells modulate the thermogenic capacity of neighboring adipocytes through a paracrine factor. A likely candidate for this factor was acetate, since ALDH1A1 mediates the conversion of acetaldehyde to acetate. Indeed, reduction of ALDH1A1 in adipocytes lowered the concentration of acetate in media, whereas treatment of adipocytes with acetate decreased the number of UCP1-expressing cells. Together this suggested that acetate produced by the ALDH1A1-expressing adipocytes was acting as the paracrine factor repressing thermogenic activity in neighboring adipocytes (Figure 1).
Figure 1. Regulation of Thermogenesis by Inter-Adipocyte Communications.
Multiple subtypes of adipocytes are present in a single white or brown adipose depot. Signaling peptides, including BMP8b, FGF6, FGF9, FGF21, and SLIT2, released by brown or beige adipocytes function in an autocrine/paracrine manner to promote thermogenesis. In contrast, acetate secreted by the CYP2E1+/ALDH1A1+ adipocytes negatively regulates UCP1 expression in the neighboring thermogenic adipocytes.
Emerging evidence indicates the key role of the adipose microenvironment in regulating adipocyte differentiation and function. The coordinated response of adipose to environmental stimuli requires extensive cellular crosstalk between the adipocytes themselves, as well as with the various cell types in the adipose niche (Knights et al., 2020). Previous studies have identified several of these adipocyte paracrine/endocrine factors, including BMP8b, FGF6, FGF9, FGF21, and SLIT2. In contrast to the effect of acetate, which is to suppress thermogenesis, all of these adipocyte factors enhance the thermogenic activity of surrounding adipocytes (Figure 1). Although it is currently unknown whether these factors are produced by specific adipocyte subtypes, considering the heterogeneity of adipocytes and the other cells in the adipose niche, this seems likely. Indeed, data suggest that there is even a population of cells in adipose tissue that exerts an anti-adipogenic effect on other cells in the same depot, although the exact mediator remains unknown (Lee et al., 2019; Schwalie et al., 2018).
Understanding the pathways that regulate the development and function of distinct adipocyte subtypes, as well as the potential interconversion of these adipocytes, is fundamental to harnessing their potential to improve metabolic health. Moving forward, it is also crucial to determine whether the different adipocyte subtypes are derived from distinct progenitor populations or a common source. Additionally, it will be important to determine if an individual adipocyte can transition to these different cellular states in response to different environmental cues. Lineage tracing experiments could help resolve these important issues.
While metabolites can serve as mediators of cellular communication, the role of acetate appears more complex than this simple model suggests. In general, the gut microbiome is a major source of short-chain fatty acids, including butyrate and acetate, both of which have been shown to have beneficial effects on metabolism. A previous study showed that peripheral administration of acetate induces browning of subcutaneous WAT and enhances whole-body thermogenesis in mice (Sahuri-Arisoylu et al., 2016), rather than suppressing it, as suggested for locally secreted acetate. Likewise, evidence from several studies suggests a beneficial role for acetate in increasing fat oxidation and energy expenditure in humans (Canfora et al., 2017; van der Beek et al., 2016). While these differences may reflect differences in the systemic versus local action of acetate, it is also possible that one or more other mediators are involved in the paracrine or systemic effects of this molecule. Thus, delineating the role of acetate in the adipose microenvironment will require further investigation. In summary, this study and others highlight the important role of functional heterogeneity of adipocytes within a single depot beyond the known brown/white paradigm and introduce metabolites as potential mediators of cellular crosstalk in regulation of adipose biology and energy balance.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants R01DK077097, R01DK102898, R01DK122808 (to Y.-H.T.), R01DK121967, and R01DK082659 (to C.R.K.) and by grant P30DK036836 (to Joslin Diabetes Center’s Diabetes Research Center) from the National Institute of Diabetes and Digestive and Kidney Diseases. F.S. was supported by NIH grant K01DK125608. We apologize to the many scientists whose work we could not cite due to space limitations.
Footnotes
DECLARATION OF INTERESTS
Y.-H.T. and C.R.K. are consultants for Cellarity, a company that employs single-cell and single-nucleus gene expression data to understand tissue metabolism and growth.
REFERENCES
- Canfora EE, van der Beek CM, Jocken JWE, Goossens GH, Holst JJ, Olde Damink SWM, Lenaerts K, Dejong CHC, and Blaak EE (2017). Colonic infusions of short-chain fatty acid mixtures promote energy metabolism in overweight/obese men: a randomized crossover trial. Sci. Rep. 7, 2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiefer FW, Vernochet C, O’Brien P, Spoerl S, Brown JD, Nallamshetty S, Zeyda M, Stulnig TM, Cohen DE, Kahn CR, and Plutzky J (2012). Retinaldehyde dehydrogenase 1 regulates a thermogenic program in white adipose tissue. Nat. Med. 18, 918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knights AJ, Wu J, and Tseng YH (2020). The heating microenvironment: intercellular cross talk within thermogenic adipose tissue. Diabetes 69, 1599–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Luong Q, Sharma R, Dreyfuss JM, Ussar S, and Kahn CR (2019). Developmental and functional heterogeneity of white adipocytes within a single fat depot. EMBO J. 38, e99291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min SY, Desai A, Yang Z, Sharma A, DeSouza T, Genga RMJ, Kucukural A, Lifshitz LM, Nielsen S, Scheele C, et al. (2019). Diverse repertoire of human adipocyte subtypes develops from transcriptionally distinct mesenchymal progenitor cells. Proc. Natl. Acad. Sci. USA 116, 17970–17979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahuri-Arisoylu M, Brody LP, Parkinson JR, Parkes H, Navaratnam N, Miller AD, Thomas EL, Frost G, and Bell JD (2016). Reprogramming of hepatic fat accumulation and ‘browning’ of adipose tissue by the short-chain fatty acid acetate. Int. J. Obes. 40, 955–963. [DOI] [PubMed] [Google Scholar]
- Schwalie PC, Dong H, Zachara M, Russeil J, Alpern D, Akchiche N, Caprara C, Sun W, Schlaudraff KU, Soldati G, et al. (2018). A stromal cell population that inhibits adipogenesis in mammalian fat depots. Nature 559, 103–108. [DOI] [PubMed] [Google Scholar]
- Sun W, Dong H, Balaz M, Slyper M, Drokhlyansky E, Colleluori G, Giordano A, Kovanicova Z, Stefanicka P, Balazova L, et al. (2020). snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature 587, 98–102. [DOI] [PubMed] [Google Scholar]
- van der Beek CM, Canfora EE, Lenaerts K, Troost FJ, Damink SWMO, Holst JJ, Masclee AAM, Dejong CHC, and Blaak EE (2016). Distal, not proximal, colonic acetate infusions promote fat oxidation and improve metabolic markers in overweight/obese men. Clin. Sci. (Lond.) 130, 2073–2082. [DOI] [PubMed] [Google Scholar]
- Ziouzenkova O, Orasanu G, Sharlach M, Akiyama TE, Berger JP, Viereck J, Hamilton JA, Tang G, Dolnikowski GG, Vogel S, et al. (2007). Retinaldehyde represses adipogenesis and diet-induced obesity. Nat. Med. 13, 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]