STRUCTURED ABSTRACT
Background:
Natriuretic peptides are substrates of neprilysin; hence B-type natriuretic peptide (BNP) concentrations rise with neprilysin inhibition. Thus, the clinical validity of measuring BNP in sacubitril/valsartan-treated patients has been questioned, and use of N-terminal pro-BNP (NT-proBNP) has been preferred and recommended.
Objectives:
To determine the prognostic performance of BNP measurements before and during treatment with sacubitril/valsartan.
Methods:
BNP and NT-proBNP were measured before and after 4–6 weeks, 8–10 weeks, and 9 months of treatment with sacubitril/valsartan in the PARADIGM-HF trial. We assessed the association of levels of these natriuretic peptides with the subsequent risk of cardiovascular death or hospitalization for HF.
Results:
Median BNP concentration (before treatment: 202 [Q1–Q3 126–335] ng/L) increased to 235 (Q1–Q3 128–422) ng/L after 8–10 weeks of treatment. A total of 141 (18%) patients experiencing a doubling and 49 (6%) experienced a tripling of BNP during the first 8–10 weeks of sacubitril/valsartan. In contrast, such striking increases in NT-proBNP following the use of the neprilysin inhibitor were extremely rare. Treatment with sacubitril/valsartan caused a rightward shift in the distribution of BNP when compared with NT-pro-BNP, but both peptides retained their prognostic accuracy (C-statistics of 63–67% for BNP and C-statistics of 64–70% for NT-proBNP) with no difference between the 2 biomarkers. Increases in both BNP and NT-proBNP during 8–10 weeks of sacubitril/valsartan were associated with worse outcomes (P=0.003 and P=0.005, respectively).
Conclusions:
Circulating levels of BNP may increase meaningfully early after initiation of sacubitril/valsartan. In comparison, NT-proBNP is less directly influenced by neprilysin inhibition, and thus may lead to less clinical confusion when measured within 8–10 weeks of drug initiation. However, during treatment, either biomarker predicts the risk of major adverse outcomes in patients treated with angiotensin receptor-neprilysin inhibitors.
Clinical Trial Registration:
Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure [PARADIGM-HF]; ClinicalTrials.gov Unique identifier: NCT01035255.
Keywords: biomarker, heart failure, natriuretic peptides, prognostication, risk stratification, treatment
CONDENSED ABSTRACT
The interpretability of B-type natriuretic peptide (BNP) in sacubitril/valsartan-treated patients is uncertain. We assessed the association between BNP at several time-points (prior to and after 4–6 weeks, 8–10 weeks, and 9 months of treatment) and composite cardiovascular death and heart failure hospitalization in patients treated with sacubitril/valsartan enrolled in the PARADIGM-HF trial. Sacubitril/valsartan led to doubling in BNP after 8–10 weeks in 18% of patients. Sacubitril/valsartan caused a rightward shift in the distribution of BNP when compared with N-terminal proBNP, but absolute concentrations of both natriuretic peptides during treatment with sacubitril/valsartan were associated with the primary outcome.
Natriuretic peptides (NP) are widely employed in routine clinical practice (1,2) for prognostication and risk stratification. C-terminal B-type NP (BNP) has been demonstrated to have similar clinical utility as N-terminal pro-BNP (NT-proBNP) (1,3), and is the only assay available in many hospital systems (4). The angiotensin receptor-neprilysin inhibitor (ARNI) sacubitril/valsartan has been shown to improve cardiovascular outcomes in patients with heart failure with reduced ejection fraction (HFrEF) (5) and is recommended as a replacement for angiotensin-converting enzyme inhibitors (ACEi) or angiotensin II receptor blockers (ARB) (1,2). Neprilysin is a widely expressed enzyme involved in the degradation of several beneficial vasoactive peptides, including NPs. However, while A-type NP (ANP) and C-type NP (CNP) are effectively cleaved by neprilysin, BNP is a relatively poor substrate for neprilysin (6,7). Nevertheless, treatment with sacubitril/valsartan has been associated with an overall increase in BNP, and thus, the clinical utility and interpretability of BNP in sacubitril/valsartan-treated patients has been called into question by clinical practice guidelines, expert consensus statements, and decision pathways (1,8–10). We assessed the relative prognostic value of BNP and NT-proBNP before and during treatment with sacubitril/valsartan in the Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial.
METHODS
Study design and patient population
PARADIGM-HF was a randomized, double-blind, parallel group, active-controlled trial comparing the long-term efficacy and safety of sacubitril/valsartan compared with enalapril in patients with HFrEF, as previously described (5,11). Patients enrolled had an ejection fraction ≤40% (changed during the trial to ≤35% by amendment), New York Heart Association (NYHA) class II–IV symptoms, and elevated NPs (if no recent hospitalization for HF: BNP ≥150 ng/L or N-terminal pro-BNP ≥600 ng/L; if hospitalization for HF within 12 months: BNP ≥100 ng/L and NT-proBNP ≥400 ng/L). Patients were excluded with an estimated glomerular filtration rate (eGFR) <30 ml/min/1.73m2, hyperkalemia (potassium concentration >5.2 mmol/L at screening or >5.4 mmol/L at randomization), hypotension (symptomatic, or a systolic blood pressure <100 mmHg at screening or <95 mmHg at randomization), history of angioedema or intolerance to ACEi or ARB. Patients were also required to tolerate ACEi or ARB equivalent to enalapril 10 mg daily for ≥4 weeks and be maintained on stable doses of a β-blocker and mineralocorticoid receptor antagonist (if indicated). Eligible patients entered 4–6 weeks of single-blind enalapril run-in, followed by an additional 4–6 weeks single-blind sacubitril/valsartan run-in. Patients were then randomized in a 1:1 ratio to enalapril 10 mg twice daily or sacubitril/valsartan 200 mg twice daily, if both drugs were tolerated at target dose during the run-in periods. Patients were followed for a mean 2.4 years from randomization and the primary outcome was a composite of death from cardiovascular cause or first hospitalization for HF.
BNP and NT-proBNP measurements
NP measurements were analyzed in a central laboratory from frozen venous blood samples drawn before run-in (n=1,656), after run-in with both enalapril and sacubitril/valsartan (time of randomization, n=2,075 in both study arms), 1 month after randomization (n=994 in the sacubitril/valsartan arm and n=1,007 in the enalapril arm), and 8 months after randomization (n=908 in the sacubitril/valsartan arm and n=901 in the enalapril arm). Plasma BNP was measured by the Advia Centaur chemiluminescent immunoassay (Siemens Healthcare Diagnostics, Tarrytown, NY) with a reporting range of 2.7 to 4,590 ng/L. NT-proBNP was measured by the Roche Elecsys proBNP assay (Roche Diagnostics GmbH, Penzberg, Germany) with a coefficient of variation <2.5% at all levels tested between 47 ng/L and 34,160 ng/L (12).
Statistical analysis
Analyses were performed at 4 time points; before run-in (baseline), at randomization (after 4–6 weeks of treatment), 1-month post- randomization (after 8–10 weeks of treatment) and 8-months post-randomization (after 9 months of treatment). Patient characteristics are presented by quartiles of BNP concentrations after 8–10 weeks of treatment with sacubitril/valsartan (time point with highest median BNP concentration). Categorical and continuous variables were compared by trend across quartiles using standard parametric or non-parametric methods, as appropriate. BNP and NT-proBNP are presented as median (quartile 1 to quartile 3 [Q1–Q3]). The ratio between NT-proBNP and BNP was calculated by dividing the concentration of NT-proBNP by the concentration in BNP, using the same units. This NP ratio measured early after drug initiation has been hypothesized to identify patients with greater target response to neprilysin inhibition (13).Spearman’s rank correlation coefficients (rs) were calculated to characterize the correlation between BNP and NT-proBNP at each time-point in sacubitril/valsartan-treated patients. The % changes in BNP and NT-proBNP during treatment with sacubitril/valsartan were also calculated between pre-run-in (before treatment) and 1 month after randomization (after 8–10 weeks of treatment). Baseline clinical profiles were compared based on BNP trajectory during 8–10 weeks of treatment: increased (greater than +10%), stable (±10%), or decreased (greater than −10%).
The prognostic significance of log-transformed NP concentrations at each time-point, changes in NPs, and the ratio between NT-proBNP and BNP at each time-point, was evaluated with respect to the primary outcome. All Cox proportional hazards models included covariates determined a priori based on clinical factors known to influence NP levels and/or clinical outcomes (age, sex, race, body mass index [BMI], history of diabetes mellitus, hypertension, coronary artery disease, follow-up systolic blood pressure, and follow-up eGFR (measured at the contemporaneous time-points as NP concentrations). Model discrimination using BNP and NT-proBNP was estimated and compared using Harrell’s C-statistics. For the landmark analysis, we included all patients with available NP measurements who had not experienced the primary outcome prior to this time-point. Only events that occurred after NP sampling were included in the analysis for each time point. For the delta-analysis from before to 8–10 weeks after treatment, only events that occurred after 8–10 weeks were included, and these analyses were also adjusted for baseline NP concentrations before treatment. Kaplan-Meier survival curves were constructed to display time-to-first primary outcomes by quartiles of BNP and NT-proBNP measured at 4–6 weeks and 8–10 weeks of treatment with sacubitril/valsartan. All patients provided written informed consent, and the study was approved by institutional review boards or ethics committees at each participating institution. Statistical analyses were performed using STATA 14.1 (College Station, TX, USA).
RESULTS
Changes in BNP and NT-proBNP during treatment with sacubitril/valsartan
Median BNP before both run-in phases and any treatment exposure was 202 (Q1–Q3 126–335) ng/L. Median BNP increased to a peak median concentration of 235 (Q1–Q3 128–422) ng/L after 8–10 weeks of sacubitril/valsartan, and decreased to a median 181 (Q1–3 109–310) ng/L after 8–10 weeks of enalapril therapy (Figure 1). These changes were consistent when assessing only patients in the sacubitril/valsartan arm with available blood samples at all time points (n=622), as a sensitivity analysis (Online Figure 1).
Figure 1. Natriuretic Peptide Trajectories in the PARADIGM-HF Trial.
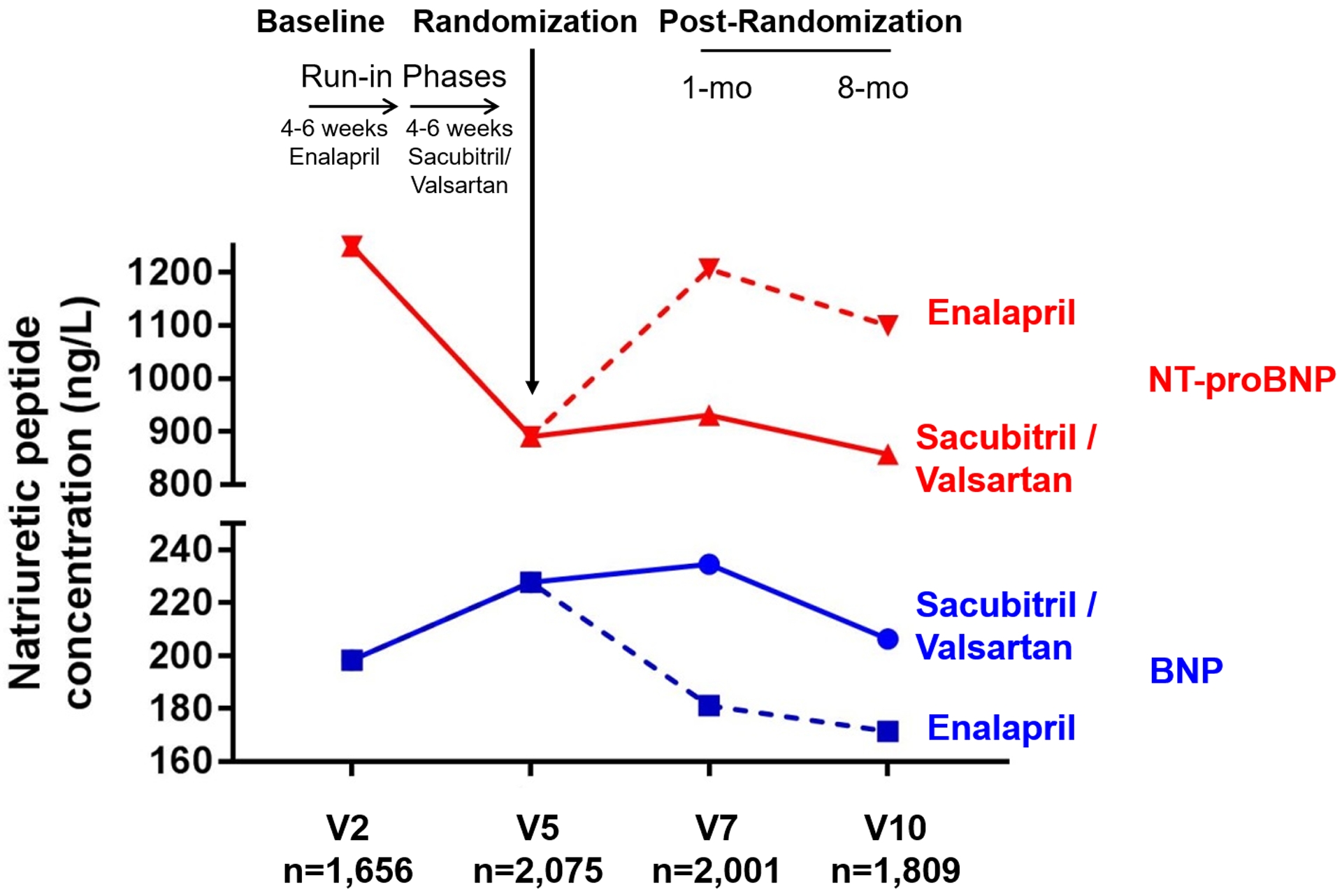
Median concentration of B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) before (V2) and during (V5, V7, V10) treatment with sacubitril/valsartan and enalapril.
BNP concentrations at 8–10 weeks of treatment were available in 2,001 (24%) of all patients in PARADIGM-HF (Table 1). Compared with patients with missing follow-up BNP data, patients with available measurements were older, more likely to be men and white, carried more comorbidities, and had higher blood pressure and lower eGFR (Online Table 1). During 8–10 weeks of treatment with sacubitril/valsartan (95% on target dose), BNP increased by a median of 19% (Q1 22% reduction, Q3 75% increase), with 141 (18%) of patients experiencing doubling and 49 (6%) tripling of BNP during 8–10 weeks of sacubitril/valsartan, while 105 (14%) had stable concentrations (±10% change) (Figure 2). In the same patients, during 8–10 weeks of treatment with sacubitril/valsartan, there was a median reduction in NT-proBNP of 28% (Q1 52% reduction, Q3 1% reduction), with only 18 (2%) experiencing doubling and 4 (0.5%) experiencing tripling of NT-proBNP during 8–10 weeks of sacubitril/valsartan, while 93 (12%) had stable concentrations (±10% change) (Figure 2). In comparison, 8–10 weeks of treatment with enalapril led to a median decrease of 6% in BNP with 75 (10%) and 31 (4%) experiencing doubling and tripling of BNP levels, respectively. Similarly, a median decrease of 5% in NT-proBNP was observed during enalapril treatment with 53 (7%) and 18 (2%) experiencing double and tripling of NT-proBNP levels, respectively. Overall, treatment with sacubitril/valsartan shifted the distribution of BNP concentrations rightward compared with that of NT-proBNP. Patients increasing (more than +10%) in BNP during 8–10 weeks of treatment with sacubitril/valsartan were older, more likely to be male, had higher prevalence of previous myocardial infarction, and ischemic cardiomyopathy, and had lower eGFR and BNP concentrations before treatment compared with patients decreasing (more than −10%) in BNP (Online Table 2). Patients experiencing a doubling or more in BNP during 8–10 weeks of treatment with sacubitril/valsartan were older (69±9 years vs. 67±11 years, p=0.009) with lower baseline, pre-treatment BNP (164 [Q1–Q3 106–345] ng/L vs. 212 [Q1–Q3 135–333] ng/L, p=0.02) compared with patients that decreased or increased <100% in BNP.
Table 1.
Patient characteristics according to quartiles of BNP levels at 8–10 weeks of treatment with sacubitril/valsartan (sacubitril/valsartan arm only)
| BNP Q1 | BNP Q2 | BNP Q3 | BNP Q4 | ||
|---|---|---|---|---|---|
| BNP range (ng/L) | <128 | 128–235 | 235–422 | >422 | |
| n=249 | n=249 | n=249 | n=247 | P | |
| 62.9 ± 11.6 | 67.1 ± 9.6 | 69.1 ± 9.2 | 69.7 ± 9.3 | <0.001 | |
| Women | 46 (18.5%) | 49 (19.7%) | 47 (18.9%) | 31 (12.6%) | 0.09 |
| White race | 229 (92.0%) | 240 (96.4%) | 237 (95.2%) | 239 (96.8%) | 0.032 |
| History of: | |||||
| Diabetes mellitus | 91 (36.5%) | 106 (42.6%) | 91 (36.5%) | 100 (40.5%) | 0.68 |
| Stroke | 18 (7.2 %) | 21 (8.4 %) | 20 (8.0 %) | 27 (10.9%) | 0.18 |
| Hypertension | 193 (77.5%) | 190 (76.3%) | 189 (75.9%) | 195 (78.9%) | 0.74 |
| Myocardial infarction | 95 (38.2%) | 121 (48.6%) | 131 (52.6%) | 144 (58.3%) | <0.001 |
| Ischemic cardiomyopathy | 126 (50.6%) | 147 (59.0%) | 181 (72.7%) | 182 (73.7%) | <0.001 |
| New York Heart Association class | 0.26 | ||||
| I | 5 (2.0 %) | 3 (1.2 %) | 9 (3.6 %) | 8 (3.2 %) | |
| II | 195 (78.3%) | 185 (74.3%) | 172 (69.1%) | 177 (71.7%) | |
| III | 49 (19.7%) | 58(23.3%) | 68 (27.3%) | 60 (24.3%) | |
| IV | 0 (0.0 %) | 3 (1.2 %) | 0 (0.0 %) | 2 (0.8 %) | |
| Left ventricular ejection fraction (%) | 30.9 ± 5.7 | 31.3 ± 5.9 | 30.7 ± 5.9 | 28.6 ± 6.7 | <0.001 |
| Background therapies | |||||
| Angiotensin-converting enzyme inhibitor | 209 (83.9%) | 204 (81.9%) | 206 (82.7%) | 197 (79.8%) | 0.28 |
| Angiotensin II receptor blocker | 42 (16.9%) | 46 (18.5%) | 44 (17.7%) | 52 (21.1%) | 0.29 |
| Mineralocorticoid receptor antagonist | 118 (47.4%) | 94 (37.8%) | 104 (41.8%) | 103 (41.7%) | 0.35 |
| Diuretic | 198 (79.5%) | 197 (79.1%) | 196 (78.7%) | 215 (87.0%) | 0.05 |
| Beta-blocker | 240 (96.4%) | 237 (95.2%) | 233 (93.6%) | 240 (97.2%) | 0.90 |
| Cardiac resynchronization therapy | 20 (8.0 %) | 24 (9.6 %) | 32 (12.9%) | 28 (11.3%) | 0.13 |
| Implantable cardioverter-defibrillator | 68 (27.3%) | 60 (24.1%) | 72 (28.9%) | 79 (32.0%) | 0.14 |
| Measurements at Visit 7 | |||||
| Systolic blood pressure (mmHg) | 123.1 ± 15.6 | 123.5 ± 17.5 | 123.0 ± 17.7 | 123.7 ± 18.2 | 0.75 |
| Estimated glomerular filtration rate (mL/min/m2) | 66.4 ± 19.0 | 65.5 ± 17.3 | 63.3 ± 15.6 | 58.9 ± 17.3 | <0.001 |
| Potassium (mmol/L) | 4.6 ± 0.5 | 4.5 ± 0.4 | 4.5 ± 0.4 | 4.4 ± 0.5 | 0.006 |
| NT-proBNP (ng/L) | 357 [221, 580] | 682 [481, 969] | 1162 [808, 1510] | 2346 [1551, 4011] | <0.001 |
Abbreviations: BNP = B-type natriuretic peptide; NT-proBNP = N-terminal pro-B-type natriuretic peptide.
Figure 2. Distribution of Natriuretic Peptide Responses after 8–10 Week Treatment with Sacubitril/Valsartan.
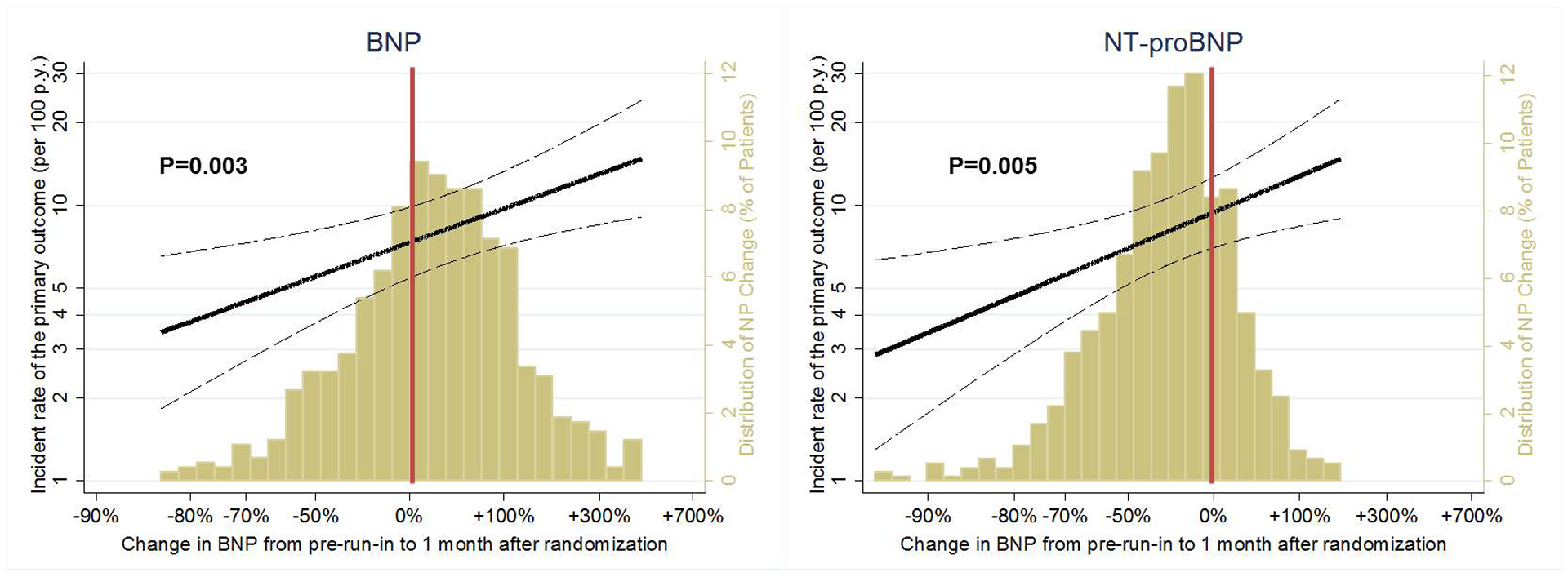
The gold histogram represents changes in B-type natriuretic peptide (BNP) and N-terminal pro-BNP (NT-proBNP) during 8–10 weeks of treatment with sacubitril/valsartan, presented as % change from pre-run-in to 1 month after randomization. The histogram corresponds to the secondary (right-sided) Y-axis.
The solid black line represents an estimation by Poisson regression of the association between change in natriuretic peptides and the primary outcome after adjusting for age, sex, race, body mass index, diabetes mellitus, hypertension, coronary artery disease, systolic blood pressure, estimated glomerular filtration rate, and pre-run-in natriuretic peptide concentrations. Dotted lines represent the 95% confidence intervals. Incidence rates are displayed on the primary (left-sided) Y-axis.
Prognostic value of BNP and NT-proBNP before and during treatment with sacubitril/valsartan
BNP and NT-proBNP had comparable prognostic performance in the total cohort at screening (n=8,348; C-statistics 64.0% and 63.6%, respectively, p=0.37). Concentrations of BNP and NT-proBNP correlated strongly before treatment with sacubitril/valsartan (rs=0.77), and at each of the time-points after treatment initiation (rs =0.80 at 4–6 weeks of treatment, rs=0.75 at 8–10 weeks of treatment, and rs=0.78 at 9 months of treatment). Log-transformed concentrations of BNP and NT-proBNP were strongly associated with the primary endpoint at each time-point, independent of key demographic and clinical factors (P<0.001 for all time-points; Table 2). Survival curves by quartiles of BNP and NT-proBNP after 4–6 weeks of treatment are displayed in Online Figure 2 and after 8–10 weeks of treatment in Online Figure 3. C-statistics for the primary endpoint ranged from 63% to 67% for BNP and from 64% to 70% for NT-proBNP, and there were no significant differences between the 2 biomarkers in predicting outcome at any of the time-points before and during treatment with sacubitril/valsartan (P>0.05 for all time-points; Table 2). The association between quartiles of BNP and NT-proBNP and subsequent primary outcomes is presented in Figure 3 (Central Illustration).
Table 2.
Association between log-transformed concentrations of natriuretic peptides and the primary outcome before and during treatment with sacubitril/valsartan
| Events, incidence rates (95% CI) per 100 p.y. | BNP (log-transformed) | NT-proBNP (log-transformed) | P for difference in C-statistics | ||||
|---|---|---|---|---|---|---|---|
| Adjusted HR (95% CI)* |
Adjusted HR (95% CI)* |
||||||
|
V2 (n=1,656) Before treatment with sacubitril/valsartan |
n=370, 9.41 (8.50–10.42) |
1.75 (1.49–2.05) |
1.89 (1.62–2.21) |
0.42 | |||
|
V5 (n=2,075) After 4–6 weeks with sacubitril/valsartan |
n=464, 10.02 (9.15–10.98) |
1.60 (1.45–1.77) |
1.80 (1.62–2.01) |
0.58 | |||
|
V7 (n=994) After 8–10 weeks with sacubitril/valsartan |
n=194, 8.94 (7.76–10.29) |
1.71 (1.45–2.01) |
2.01 (1.69–2.39) |
0.29 | |||
|
V10 (n=908) After 9 months with sacubitril/valsartan |
n=124, 7.80 (6.54–9.30) |
1.73 (1.41–2.12) |
2.12 (1.71–2.62) |
0.06 | |||
Adjusted for age, sex, race, body mass index, diabetes mellitus, hypertension, coronary artery disease, and systolic blood pressure at the same visit, and estimated glomerular filtration rate at the same visit, Abbreviations: CI = confidence interval; HR = hazard ratio
Figure 3 (Central Illustration). Association Between Natriuretic Peptide Concentrations and Subsequent Cardiovascular Events Before and During Treatment with Sacubitril/Valsartan.

Association between log-transformed B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) and the primary outcome before (V2) and during (V5, V7 and V10) treatment with sacubitril/valsartan after adjusting for age, sex, race, body mass index, diabetes mellitus, hypertension, coronary artery disease, and systolic blood pressure at the same visit, estimated glomerular filtration rate at the same visit. Spearman’s rank correlation coefficients (rs) were calculated to characterize the correlation between BNP and NT-proBNP at each time-point. Abbreviations: CI = confidence interval; HR = hazard ratio.
Relative changes in concentrations of both BNP (p=0.003) and NT-proBNP (p=0.005) during 8–10 weeks of treatment with sacubitril/valsartan were associated with the primary outcome, even after accounting for demographics, comorbidities, blood pressure, eGFR, and baseline NP levels prior to treatment (Figure 2).
Ratio between NT-proBNP and BNP
The median ratio between NT-proBNP and BNP was 6.3 (Q1–Q3 4.5–8.8) before treatment and was 3.8 (2.8–5.5) at 4–6 weeks, 3.8 (2.7–5.3) at 8–10 weeks, and 3.9 (2.7–5.7) at 9 months of treatment with sacubitril valsartan. In contrast, the median ratio of NT-proBNP and BNP did not change with 8–10 weeks (6.5 [4.7–9.2]) and 9 months (6.4 [4.7–9.2]) of treatment with enalapril. The NT-proBNP:BNP ratio at each time-point was not independently associated with the primary outcome in sacubitril/valsartan-treated patients (P>0.10 for each of the time-points; Online Table 3).
DISCUSSION
Sacubitril/valsartan led to meaningful increases in BNP concentrations in many patients with peak levels detected at 8–10 weeks during treatment. Sacubitril/valsartan shifts the distribution of BNP concentrations rightward early after treatment initiation. In contrast, NT-proBNP is less directly influenced by sacubitril/valsartan, and its measurement may be preferred within 8–10 weeks of drug initiation. However, despite its initial rise, on-treatment BNP concentrations remained robustly and independently associated with adverse cardiovascular outcomes, with comparable discrimination of risk to that of on-treatment NT-proBNP concentrations. Greater relative increases in BNP and NT-proBNP during treatment with sacubitril/valsartan were independently associated with higher risk of the primary outcome.
Natriuretic peptide biology
NPs are key regulators of volume and blood pressure homeostasis, and are upregulated as a compensatory mechanism to cardiomyocyte stretch (14). The beneficial effects of NPs occur through a complex signaling system that involves upregulation of intracellular cyclic guanosine monophosphate (cGMP), which induces vasodilation and excretion of water and sodium in the kidneys. Neprilysin is the key enzyme responsible for the breakdown of these peptides. Neprilysin cleaves ANP and CNP efficiently, while BNP is a poorer substrate and the inactive fragment NT-proBNP is not affected by neprilysin (6,7,15). In PARADIGM-HF, combined neprilysin and renin-angiotensin-aldosterone-system inhibition with sacubitril/valsartan was associated with lower cardiovascular mortality and hospitalization for HF compared with renin-angiotensin-aldosterone-system inhibition alone (5). The marked increase in urinary cGMP observed in patients treated with sacubitril/valsartan suggests that enhanced intracellular effects of NPs may be an important pharmacodynamic mechanism related to the drug (16).
Measurement of NPs in sacubitril/valsartan-treated patients in clinical practice
On average, treatment with sacubitril/valsartan has been recognized to be associated with an initial increase in BNP and decrease in NT-proBNP (5). As such, current clinical guidelines (1) and expert consensus statements (8) call into question the utility of on-treatment BNP testing. For instance, the 2017 American College of Cardiology Expert Consensus Decision Pathway (8) states that “BNP concentrations will increase (while NT-proBNP will most often fall) with ARNI therapy, and thus it may be more prudent to check only NT-proBNP in patients on ARNI.”
In the present study, we provide additional data demonstrating that increases in BNP are modest (on average, ~20% increases) in most patients, but more substantial in some (~20% of patients experience doubling of baseline levels after ARNI initiation). BNP increases occur early after treatment initiation (8–10 weeks peak effect). As such, until BNP reaches a “steady state” during the maintenance phase of ARNI treatment, measurement of NT-proBNP, which is much less subject to direct drug effects, may introduce less clinical confusion and is preferred.
However, our data suggest that both biomarkers convey similar prognostic information given the relatively uniform rightward shift in BNP concentrations by sacubitril/valsartan. BNP carried a consistent association with cardiovascular events before and during treatment with sacubitril/valsartan. Baseline (pre-treatment) levels of NPs, potentially reflecting background risk, are important determinants of subsequent measurements, even in the presence of a neprilysin inhibitor and when either BNP or NT-proBNP assays are used. As such, the prognostic performance of on-treatment BNP levels was comparable to NT-proBNP at every time-point and there was a consistent and strong correlation between NT-proBNP and BNP both before and during treatment with sacubitril/valsartan.
Taken together, although BNP concentrations are increased with initiation of sacubitril/valsartan, on-treatment measurement remains reliable in predicting risk. Natriuretic peptides may be strongly influenced by comorbid diseases and adiposity, genetic determinants and race/ethnicity, physiological states (such as pregnancy), and severity of HF. We tested the hypothesis of whether pharmacological modification of the distribution of BNP concentrations would influence its prognostic value. Similar to certain populations (such as atrial fibrillation) where the entire biomarker distribution is shifted rightward, but remain interpretable and meaningful (17), absolute and relative changes in NPs remain prognostically important in ARNI-treated patients, regardless of assay.
Increases in NPs (either BNP or NT-proBNP) during 8–10 weeks of sacubitril/valsartan are consistently associated with adverse cardiovascular risk. Given the modest increases BNP concentrations after treatment initiation (especially relative to baseline values) in most patients and known long-term cardiovascular benefits of sacubitril/valsartan, the prognostic relevance of more substantial BNP increases likely reflects disease progression rather than blunted NP degradation by sacubitril/valsartan alone. Early improvement or worsening in HF status influences BNP changes and may overshadow the relatively modest direct ARNI-related effects on NP clearance. Consistently, in the PIONEER-HF (comParIson Of sacubitril/valsartaN versus Enalapril on Effect on nt-pRo-bnp in patients stabilized from an acute Heart Failure episode) trial (18), both NT-proBNP and BNP fell over 4–8 weeks after in-hospital initiation of either sacubitril/valsartan or enalapril, likely driven by net post-discharge improvement in congestion status and compensation of HF. Given the competing contributions of disease activity and blunted NP degradation, we were unable to identify an expected or acceptable rise in BNP after sacubitril/valsartan initiation. However, given the potential effects of disease activity on serial BNP concentrations in ARNI-treated patients, all BNP increases should not be dismissed as being related to drug effects alone.
Although BNP predicts adverse cardiovascular risk in sacubitril/valsartan-treated patients, it should not be used in clinical decision-making regarding treatment continuation. Specifically, early modest rises in BNP which may be expected with starting the drug should not be a reason that sacubitril/valsartan is dose-reduced, interrupted, or discontinued in clinical practice. In most sacubitril/valsartan-treated patients, BNP concentrations are expected to reach steady-state or decline after several months of maintenance dosing. Although NT-proBNP-to-BNP ratio after initiation of sacubitril/valsartan has previously been suggested as clinically meaningful (13), we did not observe an association between this NP ratio and clinical outcomes at any time-point during treatment with sacubitril/valsartan.
Study limitations
The study is subject to several limitations. First, the analyses were limited to the subset of patients with available NP concentrations that in general carried more cardiovascular risk factors compared with the original PARADIGM-HF trial. Second, we relied on pre-treatment measurements of NPs from prior to run-in of both enalapril and sacubitril/valsartan (n=1,656), because the number of available blood samples between run-in with enalapril and sacubitril/valsartan was limited (n=903). However, the run-in with enalapril did not change BNP concentrations (before: median BNP 198 [Q1–Q3 123–340] ng/L vs. after: 198 [123–340]. Third, we only used one assay to analyze BNP and NT-proBNP. Given the substantial assay-specific, glycosylation-dependent (19,20), cross-reactivity of the precursor pro-BNP with commercial BNP and NT-proBNP assays, different assays could potentially detect different epitopes on the peptide (15,21). Although certain NP assays have been shown to be stable when stored under freezing conditions (22), freeze-thaw cycles required for processing and measurement by the central laboratory may have influenced concentrations. Mechanistic studies, such as PROVE-HF (Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure; ClinicalTrials.gov identifier: NCT02887183), are underway and are anticipated to provide comprehensive understanding of the effects of neprilysin inhibition on a broad range of biomarkers (23). Finally, early BNP trajectories may differ in patients by baseline neprilysin levels, which were not measured in this study.
CONCLUSIONS
Sacubitril/valsartan increases BNP early after treatment initiation to a modest extent (~20%) in most, but more substantially in some treated patients. As NT-proBNP is the least vulnerable to degradation by neprilysin and thus relatively less subject to direct ARNI effects, its measurement (if available) is preferred to limit clinical confusion during initial drug initiation, consistent with current recommendations (8). However, in hospital systems where BNP is the only NP-assay available, its measurement during treatment with sacubitril/valsartan reliably reflects clinical prognosis with comparable performance to NT-proBNP. Importantly, early increases after drug initiation in either NP level, especially to a greater magnitude, should not be ascribed to drug effects alone, and identify patients at heightened risk for clinical events. As such, BNP or NT-proBNP can continue to be used based on local laboratory availability in monitoring risk by clinicians (as indicated) in sacubitril/valsartan-treated patients. Given short-term variability in BNP responses to treatment with sacubitril/valsartan, this marker should not however be used to determine treatment adherence or degree of treatment response, or lack thereof, in individual patients. Although BNP is right-shifted during treatment with sacubitril/valsartan which may cause confusion in the initial phases, it remains an important and clinically valid biomarker that carries independent prognostic value in patients treated with sacubitril/valsartan that is similar to that of NT-proBNP.
Supplementary Material
CLINICAL PERSPECTIVES.
Competency in Medical Knowledge:
Sacubitril/valsartan causes an early, but modest rise in BNP levels in most treated patients.
Competency in Patient Care and Procedural Skills:
BNP remains a reliable marker of prognosis and can continue to be used in monitoring risk (as indicated) in sacubitril/valsartan-treated patients.
Translational Outlook:
Future mechanistic studies, such as PROVE-HF (Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure), will define the temporal profile of effects of neprilysin inhibition on the broad spectrum of NPs and assays used to detect them.
FUNDING
The PARADIGM-HF trial was funded by Novartis AG.
DISCLOSURES
Dr. Myhre has received speaker fees from Novartis and is supported by a postdoctoral research grant from the South-Eastern Norway Regional Health Authority (Dr. Røsjø), Akershus University Hospital, Norway (Dr. Omland), the Norwegian Medical Association and the Unger Vetlesen Medical Fund. Dr. Vaduganathan is supported by the KL2/Catalyst Medical Research Investigator Training award from Harvard Catalyst | The Harvard Clinical and Translational Science Center (NIH/NCATS Award UL 1TR002541), and serves on advisory boards for AstraZeneca, Bayer AG, and Baxter Healthcare. Dr. Packer has consulted for Amgen, Actavis, AstraZeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Pfizer, Relypsa, Sanofi, Cytokinetics, and Cardiorentis. Prof McMurray’s employer, University of Glasgow, was paid by Novartis for Prof McMurray’s time spent as co-chairman of the PARADIGM-HF trial. Dr. Desai has received research grant support from Novartis and consulting fees from Novartis, AstraZeneca, Abbott, Relypsa, and DalCor Pharma. Dr. Swedberg has received honoraria from Novartis for sponsored lectures. Dr. Zile is a consultant for and has received grants from Novartis. Dr. Solomon has received research grants from Alnylam, Amgen, AstraZeneca, Bellerophon, Celladon, Gilead, GlaxoSmithKline, Ionis Pharmaceutics, Lone Star Heart, Mesoblast, MyoKardia, NIH/NHLBI, Novartis, Sanofi Pasteur, Theracos, and has consulted for Alnylam, Amgen, AstraZeneca, Bayer, Bristol-Myers Squibb, Corvia, Gilead, GlaxoSmithKline, Ironwood, Merck, Novartis, Pfizer, Takeda, and Theracos. All other authors report no other relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- ARNI
angiotensin receptor-neprilysin inhibitor
- BNP
brain natriuretic peptide
- CI
confidence interval
- HR
hazard ratio
- HF
heart failure
- HFrEF
heart failure with reduced ejection fraction
- NP
natriuretic peptide
- NT-proBNP
N-terminal pro-B-type natriuretic peptide
- PARADIGM-HF
Prospective Comparison of ARNI with ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure
REFERENCES
- 1.Yancy CW, Jessup M, Bozkurt B et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017. [DOI] [PubMed] [Google Scholar]
- 2.Ponikowski P, Voors AA, Anker SD et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016;37:2129–200. [DOI] [PubMed] [Google Scholar]
- 3.Clerico A, Fontana M, Zyw L, Passino C, Emdin M. Comparison of the diagnostic accuracy of brain natriuretic peptide (BNP) and the N-terminal part of the propeptide of BNP immunoassays in chronic and acute heart failure: a systematic review. Clin Chem 2007;53:813–22. [DOI] [PubMed] [Google Scholar]
- 4.Hammerer-Lercher A, Collinson P, van Dieijen-Visser Marja P et al. Do laboratories follow heart failure recommendations and guidelines and did we improve? The CARdiac MArker Guideline Uptake in Europe (CARMAGUE). Clin Chem Lab Med 2013:1301. [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Packer M, Desai AS et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014;371:993–1004. [DOI] [PubMed] [Google Scholar]
- 6.Pankow K, Schwiebs A, Becker M, Siems WE, Krause G, Walther T. Structural substrate conditions required for neutral endopeptidase-mediated natriuretic Peptide degradation. J Mol Biol 2009;393:496–503. [DOI] [PubMed] [Google Scholar]
- 7.Walther T, Stepan H, Pankow K, Becker M, Schultheiss HP, Siems WE. Biochemical analysis of neutral endopeptidase activity reveals independent catabolism of atrial and brain natriuretic peptide. Biol Chem 2004;385:179–84. [DOI] [PubMed] [Google Scholar]
- 8.Yancy CW, Januzzi JL Jr., Allen LA et al. 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure With Reduced Ejection Fraction: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J Am Coll Cardiol 2018;71:201–230. [DOI] [PubMed] [Google Scholar]
- 9.Lippi G, Sanchis-Gomar F. Monitoring B-type natriuretic peptide in patients undergoing therapy with neprilysin inhibitors. An emerging challenge? Int J Cardiol 2016;219:111–4. [DOI] [PubMed] [Google Scholar]
- 10.Ibrahim NE, Januzzi J. Monitoring Biomarkers in Patients Receiving Neprilysin Inhibitors. Curr Emerg Hosp Med Rep 2018;6:8–16. [Google Scholar]
- 11.McMurray JJ, Packer M, Desai AS et al. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013;15:1062–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zile MR, Claggett BL, Prescott MF et al. Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients With Heart Failure. J Am Coll Cardiol 2016;68:2425–2436. [DOI] [PubMed] [Google Scholar]
- 13.Mair J, Lindahl B, Giannitsis E et al. Will sacubitril-valsartan diminish the clinical utility of B-type natriuretic peptide testing in acute cardiac care? Eur Heart J Acute Cardiovasc Care 2017;6:321–328. [DOI] [PubMed] [Google Scholar]
- 14.Volpe M, Rubattu S, Burnett JJ. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur Heart J 2014;35:419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semenov AG, Katrukha AG. Different Susceptibility of B-Type Natriuretic Peptide (BNP) and BNP Precursor (proBNP) to Cleavage by Neprilysin: The N-Terminal Part Does Matter. Clin Chem 2016;62:617–622. [DOI] [PubMed] [Google Scholar]
- 16.Packer M, McMurray JJ, Desai AS et al. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015;131:54–61. [DOI] [PubMed] [Google Scholar]
- 17.Myhre PL, Vaduganathan M, Claggett BL et al. Association of Natriuretic Peptides With Cardiovascular Prognosis in Heart Failure With Preserved Ejection Fraction: Secondary Analysis of the TOPCAT Randomized Clinical Trial. JAMA Cardiol 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Velazquez EJ, Morrow DA, DeVore AD et al. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N Engl J Med 2018. (in press). [DOI] [PubMed] [Google Scholar]
- 19.Seferian KR, Tamm NN, Semenov AG et al. Immunodetection of glycosylated NT-proBNP circulating in human blood. Clin Chem 2008;54:866–73. [DOI] [PubMed] [Google Scholar]
- 20.Mair J Clinical significance of pro-B-type natriuretic peptide glycosylation and processing. Clin Chem 2009;55:394–7. [DOI] [PubMed] [Google Scholar]
- 21.Luckenbill KN, Christenson RH, Jaffe AS et al. Cross-reactivity of BNP, NT-proBNP, and proBNP in commercial BNP and NT-proBNP assays: preliminary observations from the IFCC Committee for Standardization of Markers of Cardiac Damage. Clin Chem 2008;54:619–21. [DOI] [PubMed] [Google Scholar]
- 22.Nowatzke WL, Cole TG. Stability of N-terminal pro-brain natriuretic peptide after storage frozen for one year and after multiple freeze-thaw cycles. Clin Chem 2003;49:1560–2. [DOI] [PubMed] [Google Scholar]
- 23.Januzzi JL, Butler J, Fombu E et al. Rationale and methods of the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF). Am Heart J 2018;199:130–136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


