Abstract
Purpose
Most randomized controlled trials (RCTs) in patients with acute respiratory distress syndrome (ARDS) revealed indeterminate or conflicting study results. We aimed to systematically evaluate between-trial heterogeneity in reporting standards and trial outcome.
Methods
A systematic review of RCTs published between 2000 and 2019 was performed including adult ARDS patients receiving lung-protective ventilation. A random-effects meta-regression model was applied to quantify heterogeneity (non-random variability) and to evaluate trial and patient characteristics as sources of heterogeneity.
Results
In total, 67 RCTs were included. The 28-day control-group mortality rate ranged from 10 to 67% with large non-random heterogeneity (I2 = 88%, p < 0.0001). Reported baseline patient characteristics explained some of the outcome heterogeneity, but only six trials (9%) reported all four independently predictive variables (mean age, mean lung injury score, mean plateau pressure and mean arterial pH). The 28-day control group mortality adjusted for patient characteristics (i.e. the residual heterogeneity) ranged from 18 to 45%. Trials with significant benefit in the primary outcome reported a higher control group mortality than trials with an indeterminate outcome or harm (mean 28-day control group mortality: 44% vs. 28%; p = 0.001).
Conclusion
Among ARDS RCTs in the lung-protective ventilation era, there was large variability in the description of baseline characteristics and significant unexplainable heterogeneity in 28-day control group mortality. These findings signify problems with the generalizability of ARDS research and underline the urgent need for standardized reporting of trial and baseline characteristics.
Supplementary Information
The online version of this article (10.1007/s00134-021-06370-w) contains supplementary material, which is available to authorized users.
Keywords: ARDS, Critical Care Research, Heterogeneity
Introduction
The Acute Respiratory Distress Syndrome (ARDS) is a clinically and biologically heterogeneous syndrome that contributes significantly to morbidity and mortality in critically ill patients [1–3]. ARDS has long been a focal point of critical care research, but hundreds of randomized controlled trials (RCTs) have led to merely two guideline recommendations supported by high-level evidence: low tidal volume ventilation and prone positioning in patients with severe ARDS [4, 5].
The paucity of high-level evidence is due to indeterminate and conflicting trial results. Many RCTs in the ARDS population report an indeterminate outcome—detecting neither significant benefit nor harm of investigated therapeutic strategies [6]. Several other large RCTs demonstrated contradictory results, with seemingly beneficial therapies being found ineffective in subsequent trials [7, 8].
It has become clear that treatment effects of interventions in ARDS are highly dependent on the details of the intervention, as small variations of the same treatment have led to disparate results. For example, different definitions of ‘low’ and ‘high’ tidal volumes [9–13] or differences in neuromuscular blockade and sedation [7, 8, 14] have led to different trial outcomes. On the other hand, it is much less clear how differences between methodological trial characteristics and patient characteristics affect study outcomes. Between-trial heterogeneity refers to the non-random variation in treatment effect of an intervention due to methodological or clinical differences between patient populations. Unmeasured or unexplainable heterogeneity—both among patients in a single trial and between trial populations—may adversely affect the validity and generalizability of study results (see: ‘Panel: A practical example of the problem with unexplainable between-trial heterogeneity’) [15, 16].
In this study, we set out to quantify the consistency of reporting baseline characteristics and to measure between-trial heterogeneity in 28-day control group mortality among all ARDS RCTs in the lung-protective ventilation era. Our aim was to determine to which extent between-trial differences in control group mortality could be explained by differences in trial and patient characteristics. We hypothesized that between-trial heterogeneity would be large and trial populations often poorly characterized, leading to a discrepancy between inclusion criteria and patient characteristics on the one hand, and control group outcomes on the other hand.
Panel: A practical example of the problem with unexplainable between-trial heterogeneity
We note two high-profile trials published in the same journal issue [17, 18]. Both trials investigated high-frequency oscillatory ventilation in the same target population of moderate to severe ARDS patients, but they reported a different effect on mortality. Judging by the control group patient characteristics, there were clinically meaningful differences between the trial populations: there was a 32% relative difference in baseline Acute Physiology and Chronic Health Evaluation (APACHE II) scores (22 vs. 29 points) and there was a 41% relative difference in control group mortality (41% vs 29% at 30 days). But, paradoxically, the trial with the lowest baseline mean APACHE II score had the highest control group mortality. This makes the interpretation of the conflicting trial results exceedingly difficult. One trial demonstrated significant harm from the intervention while the other trial found no effect. Was the difference in treatment effect due to subtle unreported differences in the intervention, due to unreported differences in the patient populations, or due to differences in the standard of care? Were the patients more severely ill at baseline in the trial with the highest APACHE II score or in the trial with the highest control group mortality rate? It is clear that unexplainable outcome heterogeneity reduces the generalizability of intervention effects to the global ARDS population [19].
Methods
This systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). The study protocol and statistical analysis plan were registered online at the International Prospective Register of Systematic Reviews (PROSPERO, registration number: CRD42020161809).
Systematic search
A comprehensive search was conducted in MEDLINE, Embase and Scopus for randomized clinical trials including adult ARDS patients published from January 1st, 2000 until January 31st, 2020. Eligible studies included a) adult ARDS patients diagnosed according to the AECC guidelines from 1994 [20] or the Berlin definition from 2012 [21], subjected to b) invasive lung-protective mechanical ventilation according to the ARDSnet protocol [11], or reporting a tidal volume of ≤ 8 ml/kg. Included studies were c) randomized clinical trials reporting on d) 28-day, hospital, intensive care unit (ICU) or 60-day mortality. There were no restrictions with regards to the intervention or phase of the study. More details about the review process are provided in the supplementary appendix, Sect. 1.1 and 1.2.
Outcome measures
For each study, we recorded trial characteristics, intervention, inclusion and exclusion criteria, mean patient baseline characteristics and mortality outcomes.
Primary outcome was the between-trial heterogeneity based on the 28-day control group mortality rate (I2). The 28-day control group mortality rate reflects the baseline risk of death of a patient population of an individual trial. Secondary outcomes included associations between 28-day control group mortality and characteristics of trial design and outcome, inclusion- and exclusion criteria, as well as baseline characteristics.
Estimation of 28-day control group and intervention group mortality
All analyses investigating heterogeneity were conducted using the 28-day control group mortality rate. For trials reporting solely on the hospital, ICU or 60-day mortality, 28-day control group mortality was estimated with linear regression using data from trials reporting on both, 28-day mortality and any of the other mortality outcomes [22]. 28-day intervention group mortality was estimated in the same manner for analyses investigating differences between control and intervention group mortality. A sensitivity analysis was conducted, using only the trials reporting 28-day control group mortality.
Estimation and quantification of between-trial heterogeneity
The 28-day control group mortality rates across studies were analyzed using a random-effects meta-regression model with the log odds of mortality as the dependent variable. Each individual trial was weighted by the inverse of the sampling variance of the mortality rates. A maximum likelihood estimator was applied to estimate the mean mortality (random-effects pooled estimate), the between-study standard deviation due to heterogeneity (τ), and heterogeneity (the percentage of variation in control group mortality due to heterogeneity rather than chance, I2). To make heterogeneity interpretable in a clinically meaningful manner, we calculated the 95% prediction interval. The prediction interval represents the distribution of estimated underlying mortality after correction for random chance and predictive covariates [for further details see method section reference [22]]. This model and its corresponding outcomes were used to present the distribution of 28-day control group mortality between all trials, and to investigate differences between individual trial characteristics.
Associations between patient characteristics and mortality rates
The associations between 28-day control group mortality and reported patient characteristics were estimated by adding each individual covariate separately to the random-effect model as moderators in univariate analysis. The goodness-of-fit of the log-linear, quadratic and power models were compared, and the model with the lowest Akaike information criterion (AIC) was selected [23]. For each model, the regression coefficient (b) and unadjusted R2 were reported. R2 represents the proportion of between-trial heterogeneity in 28-day control group mortality explained by the individual baseline characteristic—for the n trials reporting the covariate.
Prediction of control group mortality based on significant patient characteristics
To predict between-trial differences in mortality based on patient characteristics, a comprehensive multivariate logistic regression model was constructed. Missing observations were imputed using multiple imputation generating 20 datasets with predictive mean matching. For a detailed description of the process, we refer to the supplementary appendix, Sect. 2.5. Significant baseline characteristics reported in at least 25% of all trials with a univariate regression R2 ≥ 0.10 were eligible for the model. The threshold R2 of 0.10 was a compromise between the number of variables and the limited number of observations, as described before [22]. A stepwise backward selection procedure was applied removing regressors if p ≥ 0.05 for the final model. To facilitate comparisons between the individual covariates the standardized regression coefficient (β) and the standardized standard error (SSE) were reported in the supplementary appendix, Sect. 2.5.
Control group mortality differences between trials demonstrating benefit vs. no benefit
A trial demonstrating significant benefit was defined as a reported p value of < 0.05 for the primary endpoint (as defined by authors) in favor for the intervention group. Comparisons between trials demonstrating significant benefit and trials with an indeterminate outcome or harm were performed using the Mann–Whitney U test. Linear mixed-effects and regression models were applied to estimate the probability of a significant trial outcome based on the observed control group mortality and intervention group mortality, respectively.
Statistical analyses
A p-value of < 0.05 was considered statistically significant.
Statistical analyses were performed with R Studio interface (Version 1.1.447. R core team. R: A Language and Environment for Statistical Computing. 2013. http://www.r–project.org/) using the packages ‘tidyverse, ‘dplyr’, ‘metafor’, ‘mice’, ‘Hmisc’, ‘wCorr’, ‘data.table’, ‘MASS’ and ‘ggplot2’.
Results
Systematic search
The literature search yielded 3479 results. A total of 67 RCTs met all inclusion and exclusion criteria and were included in the analyses (eFigure 1) [7, 8, 17, 18, 24–86]. Table 1 provides an overview of the included trial characteristics.
Table 1.
Characteristics of included randomized clinical trials
| Number (%) or median (IQR) | |
|---|---|
| Number of included trials | 67 |
| Control group sample size | 55 (26 – 169) |
| Multicenter trials | 41 (61) |
| Trial site*: | |
| Europe | 30 (45) |
| North America | 23 (34) |
| Middle- and South America | 2 (3) |
| Asia | 22 (33) |
| Australia / New Zealand | 6 (9) |
| Language: | |
| English | 63 (94) |
| Chinese | 4 (6) |
| Intervention: | |
| Ventilation strategy | 23 (34) |
| Drug | 21 (31) |
| Prone positioning | 4 (9) |
| Neuromuscular blockage | 5 (7) |
| Nutrition | 5 (7) |
| ECMO | 1 (1) |
| Others | 8 (11) |
| Explicitly stated primary endpoint: | 59 (88) |
| Mortality | 23 (34) |
| Ventilator-free days at day 28 | 10 (15) |
| PaO2/FiO2 ratio | 9 (13) |
| Others | 18 (26) |
| Power analysis performed | 50 (75) |
| Early termination of trial | 19 (28) |
| Trials demonstrating significant benefit** | 11 (16) |
| Trials with an indeterminate outcome*** | 40 (54) |
| Trials demonstrating significant harm**** | 4 (6) |
| Jadad score | 2 (1–4) |
| Composites of the Jadad score: | |
| Randomization | 67 (100) |
| Randomization adequate | 60 (93) |
| Blinding | 28 (42) |
| Blinding adequate | 23 (34) |
| Description of withdrawals and lost to follow-up | 36 (54) |
Data are presented as numbers and percentages (%) or median and interquartile range (IQR) according to the type of variable. Legend: *: more than one option possible. **: p < 0.05 for the primary outcome (as defined and reported by authors) for the intervention group. ***: p > 0.05 for the primary outcome (as defined and reported by authors). ****: p < 0.05 for the primary outcome (as defined and reported by authors) for the control group. ECMO extracorporeal membrane oxygenation
Estimation of 28-day control group and intervention group mortality
The 28-day control group mortality was reported in 45 trials. For trials reporting another mortality timeframe, 28-day mortality could be reliably estimated (adjusted R2 ≥ 0.98 for all estimation models). Linear equations and corresponding regression plots are shown in eTable 1 and eFigure 2. For the sensitivity analysis for trials reporting solely on 28-day mortality we refer to the supplementary appendix, Sect. 2.3.
Estimation and quantification of between-trial heterogeneity
The 28-day control group mortality ranged from 9.7 to 66.7% with a weighted mean mortality rate of 30.9%. Between-trial heterogeneity was large with 87.5% of the differences between control group mortality rates not explained by chance (I2 = 87.5%, τ = 0.509, p < 0.0001). The 95% prediction interval (the estimated range of mortality rates, corrected for small trials and random error) was 14 to 55%. The mean mortality and the magnitude of heterogeneity were similar for all subgroups of trial characteristics (Fig. 1). The exclusion criteria were too manifold for valid analyses. However, the number of reported exclusion criteria was associated with a lower 28-day control group mortality (p = 0.04, eFigure 3).
Fig. 1.
28-day control group mortality for individual trial characteristics. The diamond represents the mean mortality rate (peak) with the corresponding 95% confidence interval (length of diamond). The black line denotes the 95% prediction interval, which is the estimated between-trial variability in mortality rates after adjusting for random chance and sample size, i.e. the between-trial heterogeneity. I2 represents the proportion of between-trial variability that cannot be explained by chance.
Associations between patient characteristics and mortality rates
Table 2 presents the most-reported baseline characteristics and their associations with 28-day control group mortality. For the goodness-of-fit statistics of the individual associations, we refer to eTable 2 in the supplementary appendix. Figure 2 provides an overview of between-trial differences in control group mortality and patient characteristics. We observed an association between 28-day control group mortality and the following patient characteristics in univariate analyses: mean age; mean body mass index; mean APACHE II score; the proportion of patients treated with vasopressors; the proportion of patients presenting with shock at baseline; mean lung injury score; mean oxygenation index; mean plateau pressure; mean PaO2/FiO2 ratio and mean arterial pH. Individual regression plots are shown in eFigure 3.
Table 2.
Univariate associations between 28-day control group mortality and commonly reported mean baseline patient characteristics
| Covariate | Number of trials reporting on variable (%) | Mean (SD) | Regression coefficient | R2 | p-value |
|---|---|---|---|---|---|
| Publication (year) | 67 (100) | 2012 (4.6) | − 0.01 | 0.00 | 0.429 |
| Age (years) | 65 (97) | 54.9 (4.3) | 0.00 | 0.16 | 0.011* |
| Male gender (%) | 60 (90) | 59.7 (7.5) | 0.76 | 0.04 | 0.320 |
| BMI | 13 (19) | 28.5 (2.3) | − 0.00 | 0.51 | 0.004** |
| Illness severity scores: | |||||
| APACHE II | 37 (55) | 22.6 (3.6) | 0.05 | 0.036* | |
| APACHE III | 10 (15) | 95 (8.9) | 0.01 | 0.398 | |
| APACHE IV | 1 (2) | – | – | 0.13 | – |
| SAPS II | 16 (24) | 48.5 (3.3) | 0.01 | 0.14 | 0.894 |
| SAPS III | 1 (2) | – | – | 0.00 | – |
| SOFA | 24 (36) | 9.5 (1.2) | 0.07 | 0.08 | 0.351 |
| Use of vasopressors (%) | 11 (16) | 51.1 (17) | 0.02 | 0.058 | 0.003** |
| Shock at baseline (%) | 4 (6) | 56.0 (9.8) | 0.00 | 0.74 | 0.036* |
| Risk factors for ARDS (%): | |||||
| Pneumonia | 44 (66) | 51.6 (11.7) | 0.01 | 0.00 | 0.990 |
| Aspiration | 34 (51) | 13.1 (5.5) | -0.74 | 0.00 | 0.634 |
| Sepsis | 31 (46) | 31.4 (16.7) | 0.13 | 0.00 | 0.827 |
| Trauma | 24 (37) | 6.9 (7.9) | -0.41 | 0.00 | 0.746 |
| Transfusion | 15 (22) | 3.4 (2.9) | 6.02 | 0.08 | 0.154 |
| Pancreatitis | 11 (16) | 5.3 (3.2) | 0.68 | 0.00 | 0.680 |
| Pulmonary severity scores: | |||||
| Lung injury score (LIS) | 22 (33) | 2.7 (0.3) | 1.23 | 0.31 | 0.023* |
| Oxygenation index | 14 (21) | 13.8 (3.2) | 0.00 | 0.35 | 0.044* |
| Mechanical ventilation: | |||||
| Tidal volume (ml/kg) | 50 (75) | 7.1 (0.9) | − 0.11 | 0.05 | 0.250 |
| Plateau pressure (cmH2O) | 45 (67) | 25.8 (2.4) | 0.09 | 0.27 | 0.001** |
| Minute ventilation (L/min) | 21 (31) | 10.5 (1.1) | − 0.11 | 0.10 | 0.355 |
| Driving pressure (cmH2O) | 9 (13) | 13.6 (1.9) | 0.08 | 0.16 | 0.240 |
| PEEP (cm H2O) | 51 (76) | 10.8 (1.8) | 0.04 | 0.04 | 0.364 |
| FiO2 (%) | 21 (31) | 0.72 (0.1) | 1.95 | 0.15 | 0.109 |
| Respiratory rate (/min) | 27 (40) | 24.6 (2.6) | 0.05 | 0.11 | 0.120 |
| PaO2/FiO2 ratio | 60 (90) | 134.2 (29.7) | 0.00 | 0.21 | 0.011* |
| Arterial pH | 26 (39) | 7.33 (0.04) | − 5.67 | 0.50 | 0.003** |
| PaCO2 (mmHg) | 26 (39) | 44.9 (4.3) | − 0.00 | 0.08 | 0.097 |
Associations were estimated using a weighted random-effects model with mortality on the log-odds scale. Some baseline characteristics were reported by a minority of trials, which resulted in low power to detect significant associations. R2 can be interpreted as the proportion of heterogeneity that is explained by the population characteristic for the n trials reporting that characteristic
APACHE Acute Physiology and Chronic Health Evaluation, BMI body mass index, FiO2 fraction of inspired oxygen, PaCO2 partial pressure of carbon dioxide in arterial blood, PaO2 partial pressure of oxygen in arterial blood, SAPS Simplified Acute Physiology Score, SD standard deviation, SOFA Sequential Organ Failure Assessment Score, PEEP positive end-expiratory pressure
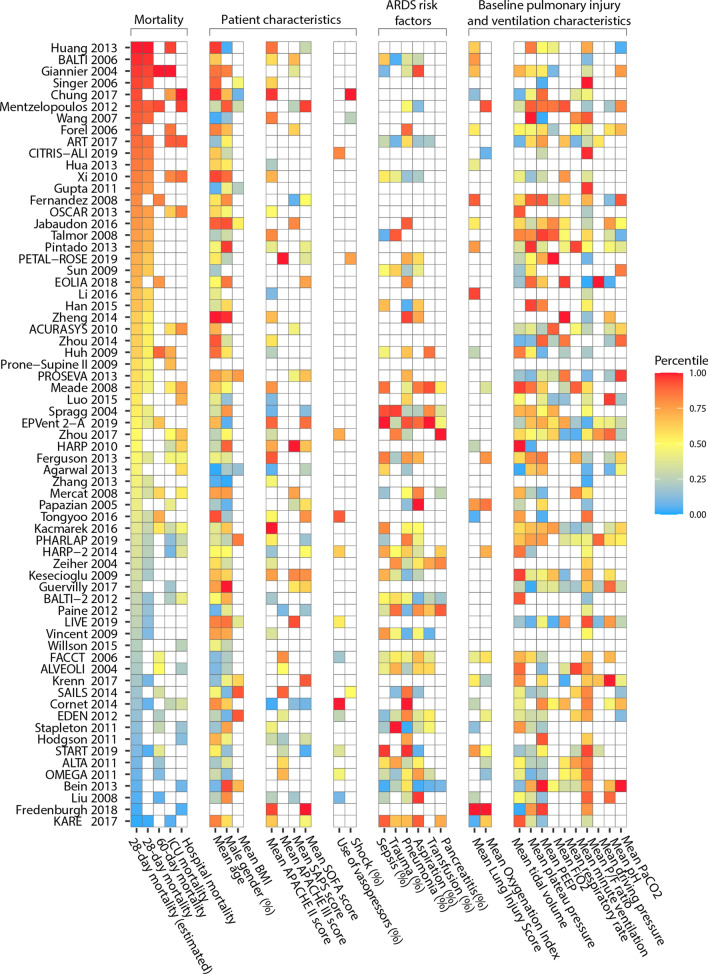
Fig. 2.
Heatmap of control group outcomes and baseline characteristics. On the y-axis, all included trials are ordered from highest to lowest (estimated) 28-day mortality rate. The color of a tile represents whether, for a specific trial, a reported variable was lowest (blue) or highest (red) among all trials that reported the variable. A white tile represents a variable not reported by a specific trial. The X-axis depicts the most reported baseline characteristics. Some show a concordant pattern (e.g. age) with 28-day mortality while others do not (e.g. SAPS II score, SOFA score). Most importantly, the distribution of white tiles demonstrates the large variability in the reporting of baseline characteristics.
Prediction of control group mortality based on significant patient characteristics
A detailed description of variable selection and construction of the multivariate logistic regression model is available in the supplementary appendix, Sect. 2.5. Significant variables for the final logistic regression model were: mean age (p < 0.0001), mean LIS (p = 0.0099), mean plateau pressure (p = 0.0078) and mean arterial pH (p = 0.0119). The residual 95% prediction interval adjusted for the significant predictors was 18 to 45%. Six trials reported all four variables in the final model.
Control group mortality differences between trials demonstrating benefit vs. no benefit
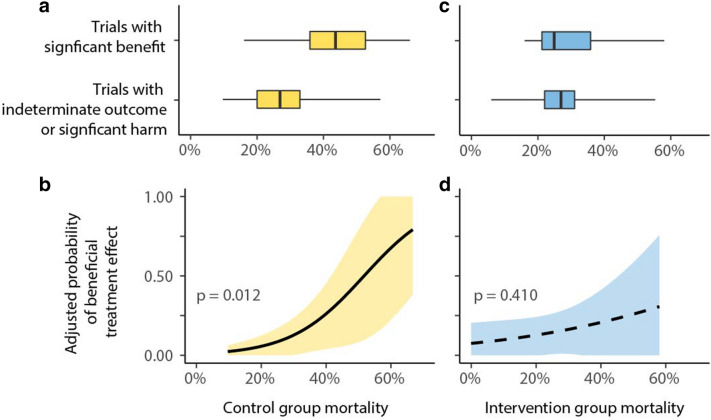
As shown in Fig. 3a, trials demonstrating significant benefit reported a higher 28-day control group mortality compared to trials with an indeterminate outcome or harm (mean 28-day control group mortality rate: 0.275 vs. 0.439; p = 0.001). Figure 3b demonstrates that trials with higher control group mortality were more likely to demonstrate significant benefit. Conversely, Fig. 3c shows that intervention group mortality did not differ between trials demonstrating significant benefit and compared to trials with an indeterminate outcome or harm (mean 28-day intervention group mortality rate: 0.271 vs. 0.301; p = 0.697). Figure 3d demonstrates that trials with higher or lower intervention group mortality rates were not more or less likely to demonstrate significant benefit.
Fig. 3.
Differences in 28-day control group and intervention group mortality between significant and indeterminate trials, and the corresponding probability of a significant treatment effect. a Mean 28-day control group mortality was 43.9% in trials with a beneficial outcome versus 27.5% in trials with an indeterminate outcome or significant harm (p = 0.001). b The higher control group mortality, the higher the probability to obtain a beneficial trial outcome for the intervention group (p = 0.012). c Mean 28-day intervention group mortality does not differ between trials with significant benefit and trials with the indeterminate outcome or significant harm. (27.1% vs. 30.1%; p = 0.697). d The probability to obtain a beneficial trial outcome was not affected by intervention group mortality (p = 0.410)
Discussion
This systematic analysis of 67 ARDS RCTs in the lung-protective ventilation era revealed a statistically significant and clinically relevant amount of between-trial heterogeneity in reporting and outcome.
The description of patient characteristics was variable and often incomplete. Basic ventilation characteristics such as mean respiratory rate, FiO2, pH and PaCO2 were reported in a minority of trials.
The estimated range of 28-day control group mortality corrected for small trials and random error was 14 to 55%. This between-trial heterogeneity in control group outcomes could not be explained by differences in trial characteristics. Reported baseline patient characteristics explained some of the outcome heterogeneity, but the residual (unexplainable) range in control group mortality was still 18 to 45% after adjusting for the most predictive baseline characteristics. There was no secular trend in mortality outcomes over the period 2000–2019.
Notably, trials with higher control group mortality were more likely to report a significant benefit, also after adjustment for baseline mean severity of illness.
Relevance for clinicians
To assess the applicability of a trial’s result to individual patients, clinicians need a clear description of the study population and concomitant treatment from trial reports. In the present study, we identified important problems in this respect.
The variation in reporting of baseline variables was considerable, with ≥ 90% of all studies reporting on age and gender, but only 75% describing observed tidal volumes and PEEP, down to only a third of all trials reporting on lung injury scores or results from blood gas analyses.
After adjustment for significant baseline characteristics (mean age, mean LIS, mean plateau pressure, mean arterial pH), the residual (unexplainable) range in control group mortality was 18 to 45%. In other words, among trials with comparable inclusion criteria and comparable baseline patient characteristics, there were inexplicable 2.5-fold mortality differences (45%/18%). This indicates that there are very important yet unreported differences in ARDS populations, and possibly also differences in co-interventions and standard care.
This silent heterogeneity between trials makes it nearly impossible to evaluate whether RCT results are valid outside of the immediate trial context (i.e., the exact population in the actual participating centers). At its most extreme, this can be thought of as a generalizability crisis in ARDS research: we cannot know which trial results are transportable to which patients outside the trial [87, 88]. The generalizability crisis comes into clear focus when different RCTs show conflicting and statistically mutually exclusive results (benefit vs. no benefit or harm) of the same intervention. Conflicting study results are often ascribed to subtle differences in the intervention, while, in fact, it may be important yet unreported differences in the population or standard of care that are driving conflicting outcomes.
The finding that the number of exclusion criteria is inversely associated with the control group mortality rate only exacerbates the generalizability problem. It means that trial populations, especially those with a large number of exclusion criteria, likely differ from the intended (broader) target population.
Clinicians trying to gauge the applicability of a trial’s result should carefully review not only inclusion criteria and baseline characteristics, but also whether the control group mortality fits the apparent patient characteristics.
Relevance for ARDS researchers
What could account for the large unexplainable heterogeneity? Possible factors may be found in biology, standards of care and co-interventions, or measurement variability.
Statistical cluster analyses of various biological and clinical characteristics led to the identification of distinct ARDS subphenotypes, each associated with a different mortality risk, a different biochemical inflammatory profile and importantly, differential responses to treatments such as PEEP, fluids, low-dose macrolide therapy or simvastatin [89–94]. We are only just beginning to appreciate the extraordinary biological heterogeneity of ARDS [95], which is undoubtedly one of the causes of between-trial variability in outcomes. It is currently not clear whether larger more pragmatic trials or smaller high-adherence trials in more selected populations will provide more useful clinical information in the future.
Variability in standards of care and co-interventions may be another likely cause of between-trial heterogeneity. The LUNG-SAFE study revealed that many ARDS patients are undertreated or not treated according to the current best practices [1]. We cannot know the implications for interpreting RCTs, because standard care and co-interventions are almost never described in trial papers. Historically, this was due to restrictions in space and words allotted by scientific journals. However, there is an urgent need for future studies to report these details, nowadays enabled by the use of supplementary materials and public data repositories.
A final likely cause of between-trial heterogeneity may be measurement variability of baseline characteristics and clinical outcomes. Severity of illness scores, for example, are notoriously dependent on small variations in measurement definitions [96, 97]. The call for standardization of baseline and outcome measures in ARDS trials is not new, but the current study underlines its urgency and importance [98, 99].
Consequently, comprehensive reporting in a standardized manner on patient characteristics, standard care and concomitant treatments may be one step towards solving the generalizability crisis in ARDS research. Clearly, heterogeneity is not the only cause of a large number of indeterminate and conflicting trial results. Statistical shortcomings, such as underpowered studies [100] or overestimations of effect size [101], equally contribute to indeterminate trial outcomes. Moreover, qualifying studies as ‘indeterminate’ is the consequence of the frequentist statistical paradigm, while Bayesian analyses offer another perspective providing often useful information about trial results [102].
Because we were limited to trial-level data in this study, we should be careful to avoid the ‘ecological fallacy’: individual-level relationships cannot be inferred from group-level data. This important limitation of the present study is also an important message: we will continue to fail to understand outcome heterogeneity between ARDS trials as long as we must rely on aggregated study-level data. Sharing (anonymized) individual patient data is likely to provide a path forward and provides an immense opportunity to stratify and subphenotype patients, to detect treatment benefit and harm for specific patient groups, and to find valuable therapeutic strategies in an inherently heterogeneous syndrome.
Complete standardization of reporting is unwarranted and can even be detrimental. Reported characteristics and outcomes should be tailored to the research question at hand. But the results in this study indicate that different trials lack sufficient common ground to validly compare trial populations. Creating this common ground for between-trial comparisons requires the reporting of a ‘core baseline set’: a commonly agreed-upon minimum set of descriptors to characterize the patient population of a trial (including standards of care and co-interventions). Developing this core population characteristics set requires meta-epidemiological data (which this study provides) and clinical domain expertise from a diverse sample of ARDS researchers.
Conclusion
Randomized controlled ARDS trials in the lung-protective ventilation era present a statistically significant and clinically relevant amount of heterogeneity in reporting and mortality outcomes. Differences in baseline characteristics partly explained the variability in outcome, but large unexplainable heterogeneity remained after extensive statistical adjustments. This study underlines the urgent need for standardized and comprehensive reporting of trial and baseline characteristics to diminish between-trial heterogeneity and to support the transportability of study results across populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Electronic supplementary material 1 (DOCX 17142 kb)
Authors’ contributions
JJ, PRT, NPJ, MJS, SAL, ARJG and HJdG developed the study design of the present study. JJ, TG and HJdG conducted the search and retrieved trial information according the PRISMA guidelines. JJ en HJdG performed the statistical analyses. JJ, PRT, TG, NPJ, MJS, SAL, ARJG and HJdG interpreted the data. JJ, PRT, NPJ, MJS, ARJ and HJdG drafted the manuscript. All authors reviewed the manuscript and approved the final submitted version.
Declarations
Competing interests
The authors declare that there is no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 Countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Caldwell E, Peabody E, Weaver J, Martin DP, Neff M, et al. Incidence and outcomes of acute lung injury. N Engl J Med. 2005;353(16):1685–1693. doi: 10.1056/NEJMoa050333. [DOI] [PubMed] [Google Scholar]
- 3.Villar J, Blanco J, Añón JM, Santos-Bouza A, Blanch L, Ambrós A, et al. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med. 2011;37(12):1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 4.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society Of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 2017;195(9):1253–1263. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 5.Papazian L, Aubron C, Brochard L, Chiche JD, Combes A, Dreyfuss D, et al. Formal guidelines: management of acute respiratory distress syndrome. Ann Intensive Care. 2019;9(1):69. doi: 10.1186/s13613-019-0540-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tonelli AR, Zein J, Adams J, Ioannidis JP. Effects of interventions on survival in acute respiratory distress syndrome: an umbrella review of 159 published randomized trials and 29 meta-analyses. Intensive Care Med. 2014;40(6):769–787. doi: 10.1007/s00134-014-3272-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Heart L, Blood Institute PCTN, Moss M, Huang DT, Brower RG, Ferguson ND, et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N Engl J Med. 2019;380(21):1997–2008. doi: 10.1056/NEJMoa1901686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Papazian L, Forel JM, Gacouin A, Penot-Ragon C, Perrin G, Loundou A, et al. Neuromuscular blockers in early acute respiratory distress syndrome. N Engl J Med. 2010;363(12):1107–1116. doi: 10.1056/NEJMoa1005372. [DOI] [PubMed] [Google Scholar]
- 9.Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, et al. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338(6):347–354. doi: 10.1056/NEJM199802053380602. [DOI] [PubMed] [Google Scholar]
- 10.Brochard L, Roudot-Thoraval F, Roupie E, Delclaux C, Chastre J, Fernandez-Mondejar E, et al. Tidal volume reduction for prevention of ventilator-induced lung injury in acute respiratory distress syndrome. The Multicenter Trail Group on Tidal Volume reduction in ARDS. Am J Respir Crit Care Med. 1998;158(6):1831–1838. doi: 10.1164/ajrccm.158.6.9801044. [DOI] [PubMed] [Google Scholar]
- 11.Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342(18):1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 12.Brower RG, Shanholtz CB, Fessler HE, Shade DM, White P, Jr, Wiener CM, et al. Prospective, randomized, controlled clinical trial comparing traditional versus reduced tidal volume ventilation in acute respiratory distress syndrome patients. Crit Care Med. 1999;27(8):1492–1498. doi: 10.1097/00003246-199908000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Stewart TE, Meade MO, Cook DJ, Granton JT, Hodder RV, Lapinsky SE, et al. Evaluation of a ventilation strategy to prevent barotrauma in patients at high risk for acute respiratory distress syndrome Pressure- and Volume-Limited Ventilation Strategy Group. N Engl J Med. 1998;338(6):355–361. doi: 10.1056/NEJM199802053380603. [DOI] [PubMed] [Google Scholar]
- 14.Tarazan N, Alshehri M, Sharif S, Al Duhailib Z, Moller MH, Belley-Cote E, et al. Neuromuscular blocking agents in acute respiratory distress syndrome: updated systematic review and meta-analysis of randomized trials. Intensive Care Med Exp. 2020;8(1):61. doi: 10.1186/s40635-020-00348-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwashyna TJ, Burke JF, Sussman JB, Prescott HC, Hayward RA, Angus DC. Implications of heterogeneity of treatment effect for reporting and analysis of randomized trials in critical care. Am J Respir Crit Care Med. 2015;192(9):1045–1051. doi: 10.1164/rccm.201411-2125CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santhakumaran S, Gordon A, Prevost AT, O'Kane C, McAuley DF, Shankar-Hari M. Heterogeneity of treatment effect by baseline risk of mortality in critically ill patients: re-analysis of three recent sepsis and ARDS randomised controlled trials. Crit Care. 2019;23(1):156. doi: 10.1186/s13054-019-2446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferguson ND, Cook DJ, Guyatt GH, Mehta S, Hand L, Austin P, et al. High-frequency oscillation in early acute respiratory distress syndrome. N Engl J Med. 2013;368(9):795–805. doi: 10.1056/NEJMoa1215554. [DOI] [PubMed] [Google Scholar]
- 18.Young D, Lamb SE, Shah S, MacKenzie I, Tunnicliffe W, Lall R, et al. High-frequency oscillation for acute respiratory distress syndrome. N Engl J Med. 2013;368(9):806–813. doi: 10.1056/NEJMoa1215716. [DOI] [PubMed] [Google Scholar]
- 19.Lesko CR, Buchanan AL, Westreich D, Edwards JK, Hudgens MG, Cole SR. Generalizing study results: a potential outcomes perspective. Epidemiology. 2017;28(4):553–561. doi: 10.1097/EDE.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, Hudson L, et al. The American-European Consensus Conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149(3 Pt 1):818–824. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 21.Force ADT, Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 22.de Grooth HJ, Postema J, Loer SA, Parienti JJ, Oudemans-van Straaten HM, Girbes AR. Unexplained mortality differences between septic shock trials: a systematic analysis of population characteristics and control-group mortality rates. Intensive Care Med. 2018;44(3):311–322. doi: 10.1007/s00134-018-5134-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moses T, Holland PW. A comparison of statistical selection strategies for univariate and bivariate log-linear models. Br J Math Stat Psychol. 2010;63(Pt 3):557–574. doi: 10.1348/000711009X478580. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R, Srinivasan A, Aggarwal AN, Gupta D. Adaptive support ventilation for complete ventilatory support in acute respiratory distress syndrome: a pilot, randomized controlled trial. Respirology. 2013;18(7):1108–1115. doi: 10.1111/resp.12126. [DOI] [PubMed] [Google Scholar]
- 25.Bein T, Weber-Carstens S, Goldmann A, Muller T, Staudinger T, Brederlau J, et al. Lower tidal volume strategy ( approximately 3 ml/kg) combined with extracorporeal CO2 removal versus 'conventional' protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med. 2013;39(5):847–856. doi: 10.1007/s00134-012-2787-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beitler JR, Sarge T, Banner-Goodspeed VM, Gong MN, Cook D, Novack V, et al. Effect of titrating positive end-expiratory pressure (PEEP) with an esophageal pressure-guided strategy vs an empirical high PEEP-Fio2 strategy on death and days free from mechanical ventilation among patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2019;321(9):846–857. doi: 10.1001/jama.2019.0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, et al. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351(4):327–336. doi: 10.1056/NEJMoa032193. [DOI] [PubMed] [Google Scholar]
- 28.Cavalcanti AB, Suzumura EA, Laranjeira LN, Paisani DM, Damiani LP, Guimaraes HP, et al. Effect of lung recruitment and titrated positive end-expiratory pressure (PEEP) vs low PEEP on mortality in patients with acute respiratory distress syndrome: a randomized clinical trial. JAMA. 2017;318(14):1335–1345. doi: 10.1001/jama.2017.14171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chung FT, Lee CS, Lin SM, Kuo CH, Wang TY, Fang YF, et al. Alveolar recruitment maneuver attenuates extravascular lung water in acute respiratory distress syndrome. Medicine (Baltimore) 2017;96(30):e7627. doi: 10.1097/MD.0000000000007627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Combes A, Hajage D, Capellier G, Demoule A, Lavoue S, Guervilly C, et al. Extracorporeal membrane oxygenation for severe acute respiratory distress syndrome. N Engl J Med. 2018;378(21):1965–1975. doi: 10.1056/NEJMoa1800385. [DOI] [PubMed] [Google Scholar]
- 31.Constantin JM, Jabaudon M, Lefrant JY, Jaber S, Quenot JP, Langeron O, et al. Personalised mechanical ventilation tailored to lung morphology versus low positive end-expiratory pressure for patients with acute respiratory distress syndrome in France (the LIVE study): a multicentre, single-blind, randomised controlled trial. The Lancet Respiratory medicine. 2019;7(10):870–880. doi: 10.1016/S2213-2600(19)30138-9. [DOI] [PubMed] [Google Scholar]
- 32.Cornet AD, Groeneveld AB, Hofstra JJ, Vlaar AP, Tuinman PR, van Lingen A, et al. Recombinant human activated protein C in the treatment of acute respiratory distress syndrome: a randomized clinical trial. PLoS ONE. 2014;9(3):e90983. doi: 10.1371/journal.pone.0090983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O'Kane CM, Elborn JS, et al. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study) Am J Respir Crit Care Med. 2011;183(5):620–626. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez R, Trenchs X, Klamburg J, Castedo J, Serrano JM, Besso G, et al. Prone positioning in acute respiratory distress syndrome: a multicenter randomized clinical trial. Intensive Care Med. 2008;34(8):1487–1491. doi: 10.1007/s00134-008-1119-3. [DOI] [PubMed] [Google Scholar]
- 35.Forel JM, Roch A, Marin V, Michelet P, Demory D, Blache JL, et al. Neuromuscular blocking agents decrease inflammatory response in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2006;34(11):2749–2757. doi: 10.1097/01.CCM.0000239435.87433.0D. [DOI] [PubMed] [Google Scholar]
- 36.Fowler AA, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, et al. Effect of Vitamin C Infusion on Organ Failure and Biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fredenburgh LE, Perrella MA, Barragan-Bradford D, Hess DR, Peters E, Welty-Wolf KE, et al. A phase I trial of low-dose inhaled carbon monoxide in sepsis-induced ARDS. JCI Insight. 2018;3(23):124039. doi: 10.1172/jci.insight.124039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gainnier M, Roch A, Forel JM, Thirion X, Arnal JM, Donati S, et al. Effect of neuromuscular blocking agents on gas exchange in patients presenting with acute respiratory distress syndrome. Crit Care Med. 2004;32(1):113–119. doi: 10.1097/01.CCM.0000104114.72614.BC. [DOI] [PubMed] [Google Scholar]
- 39.Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, et al. Effect of intravenous beta-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379(9812):229–235. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guerin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, et al. Prone positioning in severe acute respiratory distress syndrome. N Engl J Med. 2013;368(23):2159–2168. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 41.Guervilly C, Bisbal M, Forel JM, Mechati M, Lehingue S, Bourenne J, et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med. 2017;43(3):408–418. doi: 10.1007/s00134-016-4653-4. [DOI] [PubMed] [Google Scholar]
- 42.Gupta A, Govil D, Bhatnagar S, Gupta S, Goyal J, Patel S, et al. Efficacy and safety of parenteral omega 3 fatty acids in ventilated patients with acute lung injury. Indian J Crit Care Med. 2011;15(2):108–113. doi: 10.4103/0972-5229.83019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han F, Sun R, Ni Y, Hu X, Chen X, Jiang L, et al. Early initiation of continuous renal replacement therapy improves clinical outcomes in patients with acute respiratory distress syndrome. Am J Med Sci. 2015;349(3):199–205. doi: 10.1097/MAJ.0000000000000379. [DOI] [PubMed] [Google Scholar]
- 44.Hodgson CL, Cooper DJ, Arabi Y, King V, Bersten A, Bihari S, et al. Maximal recruitment open lung ventilation in acute respiratory distress syndrome (PHARLAP). A phase II, multicenter randomized controlled clinical trial. Am J Respir Crit Care Med. 2019;200(11):1363–1372. doi: 10.1164/rccm.201901-0109OC. [DOI] [PubMed] [Google Scholar]
- 45.Hodgson CL, Tuxen DV, Davies AR, Bailey MJ, Higgins AM, Holland AE, et al. A randomised controlled trial of an open lung strategy with staircase recruitment, titrated PEEP and targeted low airway pressures in patients with acute respiratory distress syndrome. Crit Care. 2011;15(3):R133. doi: 10.1186/cc10249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hua F, Wang X, Zhu L. Terlipressin decreases vascular endothelial growth factor expression and improves oxygenation in patients with acute respiratory distress syndrome and shock. J Emerg Med. 2013;44(2):434–439. doi: 10.1016/j.jemermed.2012.02.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Z, Wang SR, Yang ZL, Liu JY. Effect on extrapulmonary sepsis-induced acute lung injury by hemoperfusion with neutral microporous resin column. Ther Apheresis Dial. 2013;17(4):454–461. doi: 10.1111/j.1744-9987.2012.01083.x. [DOI] [PubMed] [Google Scholar]
- 48.Huh JW, Jung H, Choi HS, Hong SB, Lim CM, Koh Y. Efficacy of positive end-expiratory pressure titration after the alveolar recruitment manoeuvre in patients with acute respiratory distress syndrome. Crit Care. 2009;13(1):R22. doi: 10.1186/cc7725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jabaudon M, Boucher P, Imhoff E, Chabanne R, Faure JS, Roszyk L, et al. Sevoflurane for sedation in acute respiratory distress syndrome. A randomized controlled pilot study. Am J Respir Crit Care Med. 2017;195(6):792–800. doi: 10.1164/rccm.201604-0686OC. [DOI] [PubMed] [Google Scholar]
- 50.Kacmarek RM, Villar J, Sulemanji D, Montiel R, Ferrando C, Blanco J, et al. Open lung approach for the acute respiratory distress syndrome: a pilot, Randomized Controlled Trial. Crit Care Med. 2016;44(1):32–42. doi: 10.1097/CCM.0000000000001383. [DOI] [PubMed] [Google Scholar]
- 51.Kesecioglu J, Beale R, Stewart TE, Findlay GP, Rouby JJ, Holzapfel L, et al. Exogenous natural surfactant for treatment of acute lung injury and the acute respiratory distress syndrome. Am J Respir Crit Care Med. 2009;180(10):989–994. doi: 10.1164/rccm.200812-1955OC. [DOI] [PubMed] [Google Scholar]
- 52.Krenn K, Lucas R, Croize A, Boehme S, Klein KU, Hermann R, et al. Inhaled AP301 for treatment of pulmonary edema in mechanically ventilated patients with acute respiratory distress syndrome: a phase IIa randomized placebo-controlled trial. Crit Care. 2017;21(1):194. doi: 10.1186/s13054-017-1795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li JQ, Li N, Han GJ, Pan CG, Zhang YH, Shi XZ, et al. Clinical research about airway pressure release ventilation for moderate to severe acute respiratory distress syndrome. Eur Rev Med Pharmacol Sci. 2016;20(12):2634–2641. [PubMed] [Google Scholar]
- 54.Liu KD, Levitt J, Zhuo H, Kallet RH, Brady S, Steingrub J, et al. Randomized clinical trial of activated protein C for the treatment of acute lung injury. Am J Respir Crit Care Med. 2008;178(6):618–623. doi: 10.1164/rccm.200803-419OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo J, Wang MY, Liang BM, Yu H, Jiang FM, Wang T, et al. Initial synchronized intermittent mandatory ventilation versus assist/control ventilation in treatment of moderate acute respiratory distress syndrome: a prospective randomized controlled trial. J Thorac Dis. 2015;7(12):2262–2273. doi: 10.3978/j.issn.2072-1439.2015.12.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, et al. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184(5):561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, et al. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. The Lancet Respiratory medicine. 2019;7(2):154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McAuley DF, Cross LM, Hamid U, Gardner E, Elborn JS, Cullen KM, et al. Keratinocyte growth factor for the treatment of the acute respiratory distress syndrome (KARE): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Respir Med. 2017;5(6):484–491. doi: 10.1016/S2213-2600(17)30171-6. [DOI] [PubMed] [Google Scholar]
- 59.McAuley DF, Laffey JG, O'Kane CM, Perkins GD, Mullan B, Trinder TJ, et al. Simvastatin in the acute respiratory distress syndrome. N Engl J Med. 2014;371(18):1695–1703. doi: 10.1056/NEJMoa1403285. [DOI] [PubMed] [Google Scholar]
- 60.Meade MO, Cook DJ, Guyatt GH, Slutsky AS, Arabi YM, Cooper DJ, et al. Ventilation strategy using low tidal volumes, recruitment maneuvers, and high positive end-expiratory pressure for acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):637–645. doi: 10.1001/jama.299.6.637. [DOI] [PubMed] [Google Scholar]
- 61.Mentzelopoulos SD, Malachias S, Zintzaras E, Kokkoris S, Zakynthinos E, Makris D, et al. Intermittent recruitment with high-frequency oscillation/tracheal gas insufflation in acute respiratory distress syndrome. Eur Respir J. 2012;39(3):635–647. doi: 10.1183/09031936.00158810. [DOI] [PubMed] [Google Scholar]
- 62.Mercat A, Richard JC, Vielle B, Jaber S, Osman D, Diehl JL, et al. Positive end-expiratory pressure setting in adults with acute lung injury and acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2008;299(6):646–655. doi: 10.1001/jama.299.6.646. [DOI] [PubMed] [Google Scholar]
- 63.Paine R, 3rd, Standiford TJ, Dechert RE, Moss M, Martin GS, Rosenberg AL, et al. A randomized trial of recombinant human granulocyte-macrophage colony stimulating factor for patients with acute lung injury. Crit Care Med. 2012;40(1):90–97. doi: 10.1097/CCM.0b013e31822d7bf0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Papazian L, Gainnier M, Marin V, Donati S, Arnal JM, Demory D, et al. Comparison of prone positioning and high-frequency oscillatory ventilation in patients with acute respiratory distress syndrome. Crit Care Med. 2005;33(10):2162–2171. doi: 10.1097/01.ccm.0000181298.05474.2b. [DOI] [PubMed] [Google Scholar]
- 65.Perkins GD, McAuley DF, Thickett DR, Gao F. The beta-agonist lung injury trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173(3):281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 66.Pintado MC, de Pablo R, Trascasa M, Milicua JM, Rogero S, Daguerre M, et al. Individualized PEEP setting in subjects with ARDS: a randomized controlled pilot study. Respir Care. 2013;58(9):1416–1423. doi: 10.4187/respcare.02068. [DOI] [PubMed] [Google Scholar]
- 67.Rice TW, Wheeler AP, Thompson BT, deBoisblanc BP, Steingrub J, Rock P. Enteral omega-3 fatty acid, gamma-linolenic acid, and antioxidant supplementation in acute lung injury. JAMA. 2011;306(14):1574–1581. doi: 10.1001/jama.2011.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rice TW, Wheeler AP, Thompson BT, Steingrub J, Hite RD, Moss M, et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA. 2012;307(8):795–803. doi: 10.1001/jama.2012.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singer P, Theilla M, Fisher H, Gibstein L, Grozovski E, Cohen J. Benefit of an enteral diet enriched with eicosapentaenoic acid and gamma-linolenic acid in ventilated patients with acute lung injury. Crit Care Med. 2006;34(4):1033–1038. doi: 10.1097/01.CCM.0000206111.23629.0A. [DOI] [PubMed] [Google Scholar]
- 70.Spragg RG, Lewis JF, Walmrath HD, Johannigman J, Bellingan G, Laterre PF, et al. Effect of recombinant surfactant protein C-based surfactant on the acute respiratory distress syndrome. N Engl J Med. 2004;351(9):884–892. doi: 10.1056/NEJMoa033181. [DOI] [PubMed] [Google Scholar]
- 71.Stapleton RD, Martin TR, Weiss NS, Crowley JJ, Gundel SJ, Nathens AB, et al. A phase II randomized placebo-controlled trial of omega-3 fatty acids for the treatment of acute lung injury. Crit Care Med. 2011;39(7):1655–1662. doi: 10.1097/CCM.0b013e318218669d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun JJ, Yang MW, Wang CH, Li CH, Qin WH, Li YL, et al. Clinical effects of low-stretch ventilation on acute respiratory distress syndrome. Chin Crit Care Med. 2009;21(10):609–612. [PubMed] [Google Scholar]
- 73.Taccone P, Pesenti A, Latini R, Polli F, Vagginelli F, Mietto C, et al. Prone positioning in patients with moderate and severe acute respiratory distress syndrome: a randomized controlled trial. JAMA. 2009;302(18):1977–1984. doi: 10.1001/jama.2009.1614. [DOI] [PubMed] [Google Scholar]
- 74.Talmor D, Sarge T, Malhotra A, O'Donnell CR, Ritz R, Lisbon A, et al. Mechanical ventilation guided by esophageal pressure in acute lung injury. N Engl J Med. 2008;359(20):2095–2104. doi: 10.1056/NEJMoa0708638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tongyoo S, Permpikul C, Mongkolpun W, Vattanavanit V, Udompanturak S, Kocak M, et al. Hydrocortisone treatment in early sepsis-associated acute respiratory distress syndrome: results of a randomized controlled trial. Crit Care. 2016;20(1):329. doi: 10.1186/s13054-016-1511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Truwit JD, Bernard GR, Steingrub J, Matthay MA, Liu KD, Albertson TE, et al. Rosuvastatin for sepsis-associated acute respiratory distress syndrome. N Engl J Med. 2014;370(23):2191–2200. doi: 10.1056/NEJMoa1401520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Vincent JL, Artigas A, Petersen LC, Meyer C. A multicenter, randomized, double-blind, placebo-controlled, dose-escalation trial assessing safety and efficacy of active site inactivated recombinant factor VIIa in subjects with acute lung injury or acute respiratory distress syndrome. Crit Care Med. 2009;37(6):1874–1880. doi: 10.1097/CCM.0b013e31819fff2c. [DOI] [PubMed] [Google Scholar]
- 78.Wang XZ, Lu CJ, Gao FQ, Li XH, Hao D, Ning FY. Comparison of the effects of BiPAP ventilation combined with lung recruitment maneuvers and low tidal volume A/C ventilation in patients with acute respiratory distress syndrome. Chin J Tuberculosis Respir Dis. 2007;30(1):44–47. [PubMed] [Google Scholar]
- 79.Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354(24):2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 80.Willson DF, Truwit JD, Conaway MR, Traul CS, Egan EE. The Adult Calfactant in Acute Respiratory Distress Syndrome Trial. Chest. 2015;148(2):356–364. doi: 10.1378/chest.14-1139. [DOI] [PubMed] [Google Scholar]
- 81.Xi XM, Jiang L, Zhu B. Clinical efficacy and safety of recruitment maneuver in patients with acute respiratory distress syndrome using low tidal volume ventilation: a multicenter randomized controlled clinical trial. Chin Med J. 2010;123(21):3100–3105. [PubMed] [Google Scholar]
- 82.Zeiher BG, Artigas A, Vincent JL, Dmitrienko A, Jackson K, Thompson BT, et al. Neutrophil elastase inhibition in acute lung injury: results of the STRIVE study. Crit Care Med. 2004;32(8):1695–1702. doi: 10.1097/01.ccm.0000133332.48386.85. [DOI] [PubMed] [Google Scholar]
- 83.Zhang JC, Chu YF, Zeng J, Ren HS, Meng M, Jiang JJ, et al. Effect of continuous high-volume hemofiltration in patients with severe acute respiratory distress syndrome. Zhonghua wei zhong bing ji jiu yi xue. 2013;25(3):145–148. doi: 10.3760/cma.j.issn.2095-4352.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 84.Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, et al. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:39. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou X, Liu D, Long Y, Zhang Q, Cui N, He H, et al. The effects of prone position ventilation combined with recruitment maneuvers on outcomes in patients with severe acute respiratory distress syndrome. Zhonghua Nei Ke Za Zhi. 2014;53(6):437–441. [PubMed] [Google Scholar]
- 86.Zhou Y, Jin X, Lv Y, Wang P, Yang Y, Liang G, et al. Early application of airway pressure release ventilation may reduce the duration of mechanical ventilation in acute respiratory distress syndrome. Intensive Care Med. 2017;43(11):1648–1659. doi: 10.1007/s00134-017-4912-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hernán MA, VanderWeele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011;22(3):368–377. doi: 10.1097/EDE.0b013e3182109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Westreich D, Edwards JK, Lesko CR, Stuart E, Cole SR. Transportability of Trial Results Using Inverse Odds of Sampling Weights. Am J Epidemiol. 2017;186(8):1010–1014. doi: 10.1093/aje/kwx164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bos LD, Schouten LR, van Vught LA, Wiewel MA, Ong DSY, Cremer O, et al. Identification and validation of distinct biological phenotypes in patients with acute respiratory distress syndrome by cluster analysis. Thorax. 2017;72(10):876–883. doi: 10.1136/thoraxjnl-2016-209719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, et al. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2(8):611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Calfee CS, Delucchi KL, Sinha P, Matthay MA, Hackett J, Shankar-Hari M, et al. Acute respiratory distress syndrome subphenotypes and differential response to simvastatin: secondary analysis of a randomised controlled trial. Lancet Respir Med. 2018;6(9):691–698. doi: 10.1016/S2213-2600(18)30177-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, et al. Acute respiratory distress syndrome subphenotypes respond differently to randomized fluid management strategy. Am J Respir Crit Care Med. 2017;195(3):331–338. doi: 10.1164/rccm.201603-0645OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simonis FD, de Iudicibus G, Cremer OL, Ong DSY, van der Poll T, Bos LD, et al. Macrolide therapy is associated with reduced mortality in acute respiratory distress syndrome (ARDS) patients. Ann Transl Med. 2018;6(2):24. doi: 10.21037/atm.2017.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sinha P, Delucchi KL, Thompson BT, McAuley DF, Matthay MA, Calfee CS, et al. Latent class analysis of ARDS subphenotypes: a secondary analysis of the statins for acutely injured lungs from sepsis (SAILS) study. Intensive Care Med. 2018;44(11):1859–1869. doi: 10.1007/s00134-018-5378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bos LDJ, Scicluna BP, Ong DSY, Cremer O, van der Poll T, Schultz MJ. Understanding heterogeneity in biologic phenotypes of acute respiratory distress syndrome by leukocyte expression profiles. Am J Respir Crit Care Med. 2019;200(1):42–50. doi: 10.1164/rccm.201809-1808OC. [DOI] [PubMed] [Google Scholar]
- 96.Féry-Lemonnier E, Landais P, Loirat P, Kleinknecht D, Brivet F. Evaluation of severity scoring systems in ICUs–translation, conversion and definition ambiguities as a source of inter-observer variability in Apache II. SAPS and OSF Intens Care Med. 1995;21(4):356–360. doi: 10.1007/BF01705416. [DOI] [PubMed] [Google Scholar]
- 97.Klein Klouwenberg PM, Ong DS, Bonten MJ, Cremer OL. Classification of sepsis, severe sepsis and septic shock: the impact of minor variations in data capture and definition of SIRS criteria. Intensive Care Med. 2012;38(5):811–819. doi: 10.1007/s00134-012-2549-5. [DOI] [PubMed] [Google Scholar]
- 98.Needham DM, Sepulveda KA, Dinglas VD, Chessare CM, Friedman LA, Bingham CO, et al. Core outcome measures for clinical research in acute respiratory failure survivors an international modified delphi consensus study. Am J Respir Crit Care Med. 2017;196(9):1122–1130. doi: 10.1164/rccm.201702-0372OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Williamson PR, Altman DG, Bagley H, Barnes KL, Blazeby JM, Brookes ST, et al. The COMET Handbook: version 1.0. Trials. 2017;18(Suppl 3):280. doi: 10.1186/s13063-017-1978-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubenfeld GD. Confronting the frustrations of negative clinical trials in acute respiratory distress syndrome. Ann Am Thoracic Soc. 2015;12(Suppl 1):S58–63. doi: 10.1513/AnnalsATS.201409-414MG. [DOI] [PubMed] [Google Scholar]
- 101.Aberegg SK, Richards DR, O'Brien JM. Delta inflation: a bias in the design of randomized controlled trials in critical care medicine. Crit Care. 2010;14(2):R77. doi: 10.1186/cc8990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yarnell CJ, Abrams D, Baldwin MR, Brodie D, Fan E, Ferguson ND, et al. Clinical trials in critical care: can a Bayesian approach enhance clinical and scientific decision making? Lancet Respir Med. 2021;9(2):207–216. doi: 10.1016/S2213-2600(20)30471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Electronic supplementary material 1 (DOCX 17142 kb)