Abstract
Background
Natural orifice specimen extraction surgery (NOSES) has been successfully applied to the treatment of gastric, colorectal cancer (CRC). However, the development of NOSES is still in the exploratory stage, and there is still no strong evidence-based medical evidence.
Patients and Methods
From January 2013 to June 2017, consecutive patients with colorectal cancer who underwent transluminal resection, anastomosis, and specimen extraction and those who underwent conventional laparoscopic resection were enrolled. Propensity score matching was used to align clinicopathological features between the two groups.
Results
A total of 372 patients were eventually included in this study, 186 in each group. According to perioperative information and postoperative follow-up in both groups, the NOSES group had less blood loss (P = 0.011), shorter time to recovery of gastrointestinal function (P < 0.001), shorter postoperative hospital stay (P = 0.037). The NOSES group had fewer postoperative analgesics (P < 0.001), lower postoperative pain scores (P < 0.001), and lower incidence of postoperative complications (P = 0.017). Compared with the LA (laparoscopic) group, the NOSES group had better physical function (P<0.05), role function (P<0.001), emotional function (P<0.001) and global health status than LA group, while symptoms such as pain (P<0.001), insomnia (P<0.001), constipation (P<0.001) and diarrhea (P<0.05) were less severe in the NOSES group. In addition, the NOSES group had higher body image scores. Overall survival (OS) and disease-free survival (DFS) were not significantly different between the two groups.
Conclusion
For surgical treatment of colorectal cancer, NOSES has advantages in reducing postoperative pain, recovery of gastrointestinal function, postoperative quality of life, and improving patients’ satisfaction with abdominal wall aesthetics. There was no difference in long-term survival between NOSES and conventional laparoscopic surgery.
Keywords: colorectal cancer, laparoscopic surgery, perioperative efficacy, natural orifice specimen extraction, survival
Introduction
With the increasing incidence, colorectal cancer (CRC) has become one of the major causes of cancer-related death worldwide.1,2 Radically resection is usually the preferred treatment for CRC patients. However, abdominal incision cannot be avoided by either open or laparoscopic-assisted resection. Incisions increase the risk of postoperative complications, pain and potential psychological stress.3–5 Therefore, minimizing trauma to patients is one of the therapeutic goals of surgeons.
NOSES refers to the use of traditional laparoscopic instruments, transanal endoscopic microsurgery (TEM) or soft endoscopy to obtain specimens through natural orifice (oral cavity, vagina or anus) without the aid of abdominal auxiliary incision after abdominal operation.6 NOSES has been successfully used in the surgical treatment of gastric cancer, colorectal cancer and other abdominal/pelvic organ diseases.7,8
However, NOSES is still in the exploratory stage and there is no high-grade evidence of evidence-based medicine for its feasibility and safety. This study retrospectively analyzed the short-term and long-term effects of NOSES on CRC patients, in order to prove the safety and feasibility of NOSES, and to provide more objective and reliable evidence-based medicine evidence for NOSES promotion and development.
Patients and Methods
This retrospective study included all patients who were histologically diagnosed with colorectal cancer and underwent radical resection at the Department of Colorectal Surgery, the Second Affiliated Hospital of Harbin Medical University from January 2013 to June 2017. These cases are consecutive. The patients who underwent natural orifice specimen extraction surgery were in the NOSES group, and the patients who underwent conventional laparoscopic surgery were in the LA group. All operations were performed by two specific surgeons in the same operating center. All surgeons are qualified for noses and laparoscopic radical resection of colorectal cancer. They have skillfully performed at least 100 cases of laparoscopic radical resection and laparoscopic radical resection of colorectal cancer, and have completed the learning curve of noses. The patient was fully aware of the procedure and made the final decision based on the MDT’s recommendations. All patients were informed on admission that all their treatment-related information would be retained and potentially available for scientific research. However, the information we collected will be kept strictly confidential and will not have any impact on the best treatment that patients received. Ethical approval for this study was granted by the ethics committee of the Second Affiliated Hospital of Harbin Medical University. All procedures performed in this study were in accordance with the ethical standards of our hospital and in compliance with the Declaration of Helsinki.
The inclusion criteria were as follow: 1) Age: 18–80 years old; 2) Complete preoperative colonoscopy and colonoscopy biopsy, and pathologically confirmed malignant tumor; 3) All patients underwent CT or MRI before surgery, and preoperative imaging examination (CT, MRI) suggested tumor diameter ≤5.0 cm; 4) Body mass index of the patient <30 kg/m2; 5) Obtain the informed consent of the patient.
The exclusion criteria were as follow: 1) contraindications for laparoscopic surgery; 2) cases of emergency surgery due to acute intestinal obstruction, perforation or bleeding; 3) combined with primary malignant tumors of other organs; 4) patients with multiple primary colorectal cancer; 5) patients received preoperative chemoradiotherapy; 6) patients with a history of cancer; 7) incomplete data or missing follow-up data; 8) Patients with prophylactic ostomy or those with ostomy for other reasons.
The inclusion criteria and exclusion criteria of both groups were referenced to the above criteria.
Surgical Procedure
Patients in both groups underwent prescribed preoperative preparation. After anesthesia, the patients were placed in the modified lithotomy position to establish pneumoperitoneum. A 10-mm trocar was placed at the umbilicus for laparoscopy, and two 5-mm working trocars were, respectively, inserted in the lower left quadrant and the upper right quadrant. A 5 mm or 12 mm trocar was inserted in the upper left quadrant and a 12 mm trocar was inserted in the lower right quadrant. The specific location and size of trocar were determined by the surgical procedure. First of all, the liver, gallbladder, stomach, spleen, tomentum, colon, small intestine, rectum and pelvis were checked. Then the location of the tumor was explored and the corresponding mesangial dissection and vascularization were performed. Finally, different methods were used for specimen removal and digestive tract reconstruction in the NOSES and LA groups.
The LA group took samples through an auxiliary incision above the navel. Then, under the direct visual observation of the surgeon, the specimen was detached to complete the anastomosis of the two ends. Finally, the abdominal cavity was rinsed with sterile distilled water and normal saline, and the incision and cannula pinhole were closed.
In the NOSES group, the NOSES performed in low rectal cancer was taken as an example. Following the gentle anus dilation, proximal bowel division was performed through the right lower quadrant cannula using the 60 mm straight linear stapler (Figure 1A). Povidone gauze was used to disinfect both proximal and distal stumps. A large clamp was reintroduced into the bowel lumen through the anal canal to grab the rectal stump and to gently withdraw extracorporeally (Figure 1B). The surgeon made an incision on the rectal wall extra abdominally (Figure 1C). Then the anvil was introduced into pelvic cavity from the incision on the rectal wall (Figure 1D). The reverse rectal specimen was flushed extra abdominally with cytotoxic solution (eg, 1% povidone-iodine, 500 mL). The distal rectal resection was performed extra abdominally using the curved cutter stapler with 1–2 cm lower tumor margin preserved (Figure 1E), and the specimen was then removed. The rectal stump was delivered back to pelvic cavity. The surgeon made a small incision on the stump of sigmoid colon; povidone gauze was used to disinfect the incision (Figure 1F). The anvil was then introduced into the bowel lumen of sigmoid colon (Figure 1G). The 60 mm straight linear stapler was used to close the incision (Figure 1H). The center rod of the anvil head was extracted from the proximal bowel lumen (Figure 1I). An end-to-end anastomosis was then performed very carefully, to certify the surrounding tissues not being caught in the anastomotic site (Figure 1J). The integrity of the proximal and the distal rings were verified. An air test was performed to check for anastomotic leak. We recommended routine use of two drainage tubes near the anastomotic area in the pelvic cavity (Figure 1K). For rectal cancer patients who underwent the ultra-low anastomosis surgery, the anastomosis should be firmly sutured extra abdominally. Finally, the abdominal cavity was rinsed with sterile distilled water and normal saline, and the incision and cannula pinhole were closed. There was no incision in the patient’s abdominal wall at the end of the procedure (Figure 1L). For patients undergoing left hemicolectomy, the scope of resection and lymph node dissection was the same as that of radical surgery for left hemicolectomy, and end-to-end anastomosis of rectum-transverse colon was performed. For patients undergoing right hemicolectomy, the scope of resection and lymph node dissection was the same as that of radical surgery for right hemicolectomy, and a side-to-side ileo-transverse colon anastomosis was performed. The surgical specimen was removed transanally through a sterile protective sleeve; or removed transvaginally through a sterile protective sleeve after opening the posterior vaginal fornix.
Figure 1.
Specimen removal and gastrointestinal reconstruction of low rectal cancer in NOSES group. (A) Cut and closed sigmoid colon. (B) The specimen was pulled out of the body through the anus. (C) Cut through the rectal wall. (D) Sent the nail base into the pelvic cavity through the anus. (E) The specimen was resected by means of kaito. (F) Cut the wall of sigmoid colon and sterilize it. (G) The nail base was placed into the proximal sigmoid colon. (H) Closed sigmoid wall. (I) Removed the connecting rod of the nail seat. (J) An end-to-end anastomosis was performed. (K) Bilateral drainage tube was inserted. (L) Postoperative abdominal wall of the patient.
Data Collection and Follow-Up Data
Body Image Questionnaire (BIQ) is composed of 8 items, which are used to evaluate the body image and cosmesis after surgery.9 Body image scale was used to explore patients’ perceptions, attitudes and satisfaction with their body appearance (items 1–5). Cosmetic scale was used to assess patient satisfaction with scar appearance (items 6–8). For the body image questionnaire, see Supplementary Figure S1. According to the guidelines as described, we linearly transformed all scores of BIQ so that all scores ranged from 0 to 100. After standardization of BIQ scores, higher scores indicated better body image and higher satisfaction with scars. In this study, we used this score to assess satisfaction with their bodily appearance of patients one month after surgery.
The continence score (Wexner Incontinence Score) is determined by adding points from the above table, which takes into account the type and frequency of incontinence and the extent to which it alters the patient’s life.10 In this study, we used this score to evaluate anal function in patients six months after surgery (Supplementary Table 1).
The PFDI-20 (Pelvic Floor Distress Inventory-20) was originally used to measure the extent to which lower urinary tract, lower gastrointestinal tract, and pelvic organ prolapse symptoms affect the quality of life of women who suffer from disorders of the pelvic floor.11 Here, we applied PFDI-20 to evaluate patients’ symptoms of lower urinary tract, lower gastrointestinal tract and pelvic organ prolapse 3 months after surgery. EORTC QLQ-C30 is a core scale in the European organization for research and treatment (EORTC)’s quality of life scale system for determining the quality of life of all cancer patients. It measures commonality, and adds specific items (modules) of different cancers on the basis of commonality to form specific scales of different cancers.12,13 According to EORTC guidelines, we linearly transformed all scores of QLQ-C30 so that all scores ranged from 0 to 100. In the Functional Scale, higher scores indicate better functional status, while in the Symptom Scale, higher scores indicate more severe symptoms or problems. In both scales, a score change of ≥10 points was considered the threshold for a clinically meaningful change.13 In this study, we used this score to assess patients' quality of life at 3 months after surgery.
Follow-up data: patients who underwent surgery in our department were followed up every 3 months for 2 years after surgery, and every 6 months for 3 years after surgery, until death or termination of the study. If the patient did not return for observation, the information was obtained by letter or telephone. All patients were followed until death or the end of the study in September 2019. Therefore, all patients were followed for at least 36 months or until death.
Statistical Analysis
Propensity score matching (PSM) was used to balance the baseline data between the two groups because it reduced selection bias. Propensity scores were matched 1:1 based on baseline information, including gender, age, BMI, American Society of Anesthesiologists score (ASA score), preoperative CEA level, stage T, stage N, tumor size and tumor location. The logistic regression model was used to analyze the variables assigned to the baseline data of 186 patients, and the caliper value was 0.01. The quantitative data were expressed as mean ± standard deviation and compared by independent samples t-test (in case of normal distribution) or Mann–Whitney-U test (in case of abnormal distribution). Classification variables are expressed by chi-square test or FISH exact probability method. Disease-free survival and overall survival were estimated using the Kaplan–Meier method and compared by the Log rank test. P<0.05 was considered statistically significant. All statistical analyses were performed using the IBM SPSS Statistics ver. 25.0 (IBM Co., Armonk, NY, USA).
Results
Patients and Characteristics
A total of 598 patients from our center were included in this study, including 239 in the LA group and 259 in the NOSES group. Before propensity matching, LA group and NOSES group were unevenly distributed in baseline data such as gender (P<0.001), age (60.7±10.9 vs 57.8±11.1 years, P=0.004), BMI (23.1±3.2 vs 22.4±2.9 kg/m2, P=0.014) and T stages (P<0.001). After PSM, 186 pairs of cases have reached a statistical matching. Baseline information such as gender, age, BMI, ASA score, tumor location, preoperative CEA, T stage, N stage, and preoperative PFDI-20 scores was balanced and comparable in all 186 pairs (Table 1).
Table 1.
Baseline Information for Two Groups of Patients
| Characteristics | Before PSM | After PSM | ||||||
|---|---|---|---|---|---|---|---|---|
| LA (N=239) | NOSES (N=259) | t/χ2 | P value | LA (N=186) | NOSES (N=186) | t/χ2 | P value | |
| Gender | 16.351 | <0.001 | 0.043 | 0.836 | ||||
| Male | 143 (59.8%) | 108 (41.7%) | 92 (49.5%) | 94 (50.5%) | ||||
| Female | 96 (40.2%) | 151 (58.3%) | 94 (50.5%) | 92 (49.5%) | ||||
| Age (years) | 60.7±10.9 | 57.8±11.1 | 2.876 | 0.004 | 59.9±11.3 | 59.0±10.4 | 0.881 | 0.379 |
| BMI (kg/m2) | 23.1±3.2 | 22.4±2.9 | 2.473 | 0.014 | 22.8±3.2 | 22.9±2.8 | −0.463 | 0.643 |
| ASA grade | 0.024 | 0.876 | 0.000 | 1.000 | ||||
| I/II | 199 (83.3%) | 217 (83.8%) | 163 (87.6%) | 163 (87.6%) | ||||
| III | 40 (16.7%) | 42 (16.2%) | 23 (12.4%) | 23 (12.4%) | ||||
| Location of tumor | 5.524 | 0.238 | 1.037 | 0.904 | ||||
| Ascending colon | 18 (7.5%) | 13 (5.0%) | 10 (5.4%) | 9 (4.8%) | ||||
| Transverse colon | 14 (5.9%) | 8 (3.1%) | 7 (3.8%) | 6 (3.2%) | ||||
| Descending colon | 14 (5.9%) | 10 (3.9%) | 9 (4.8%) | 7 (3.8%) | ||||
| Sigmoid colon | 30 (12.6%) | 40 (15.4%) | 25 (13.4%) | 31 (16.7%) | ||||
| Rectum | 163 (68.2%) | 188 (72.6%) | 135 (72.6%) | 133 (71.5%) | ||||
| Preoperative CEA | 0.410 | 0.522 | 0.318 | 0.573 | ||||
| Positive | 42 (17.6%) | 40 (15.4%) | 32 (17.2%) | 28 (15.1%) | ||||
| Negative | 197 (82.4%) | 219 (84.6%) | 154 (82.8%) | 158 (84.9%) | ||||
| Preoperative CA199 | 1.301 | 0.254 | 0.040 | 0.842 | ||||
| Positive | 19 (7.9%) | 14 (5.4%) | 13 (7.0%) | 14 (7.5%) | ||||
| Negative | 220 (92.1%) | 245 (94.6%) | 173 (93.0%) | 172 (92.5%) | ||||
| T Stage | 27.460 | <0.001 | 3.723 | 0.293 | ||||
| Tis/T1 | 45 (18.8%) | 67 (25.9%) | 40 (21.5%) | 40 (21.5%) | ||||
| T2 | 30 (12.6%) | 58 (22.4%) | 28 (15.1%) | 36 (19.4%) | ||||
| T3 | 142 (59.4%) | 94 (36.3%) | 106 (57.0%) | 91 (48.9%) | ||||
| T4 | 22 (9.2%) | 40 (15.4%) | 12 (6.5%) | 19 (10.2%) | ||||
| N Stage | 0.440 | 0.507 | 0.209 | 0.648 | ||||
| N0 | 168 (70.3%) | 189 (73.0%) | 134 (72.0%) | 130 (69.9%) | ||||
| N1/N2 | 71 (29.7%) | 70 (27.0%) | 52 (28.0%) | 56 (30.1%) | ||||
| Preoperative PFDI-20 scores | 7.17±2.16 | 7.06±1.69 | 0.657 | 0.512 | 7.03±2.15 | 6.99±1.67 | 0.189 | 0.850 |
Note: Results in the table are presented as mean ± standard deviation or number (%).
Comparison of Perioperative Indexes Between Two Groups
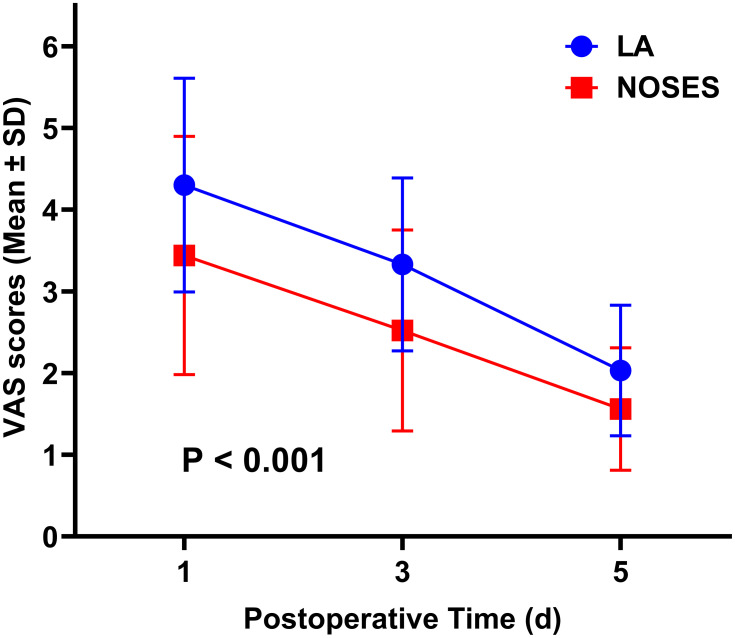
By comparing intraoperative data between the two groups, we found that the blood loss was significantly less in the NOSES group than in the LA group (P=0.011, Table 2). Obviously, the length of abdominal incision in the NOSES group was significantly shorter than that in the LA group (P<0.001, Table 2). The NOSES group was superior to the LA group in terms of time to recovery of gastrointestinal function (P<0.001) and use of other analgesics (P<0.001) after surgery (Table 2), and the NOSES group had lower postoperative pain scores (Figure 2). Postoperative incision-related complications in NOSES group were significantly less than those in LA group (P<0.001, Table 2). There was no significant difference in PDFI-20 score between NOSES group and LA group (Supplementary Table 2).
Table 2.
Comparison of Postoperative Conditions Between the Two Groups
| Outcome | After PSM | |||
|---|---|---|---|---|
| LA (N=186) | NOSES (N=186) | t/χ2 | P value | |
| Operative time (min) | 181.1±53.1 | 188.1±54.8 | −1.245 | 0.214 |
| Blood loss (mL) | 79.0±92.9 | 56.7±76.0 | 2.541 | 0.011 |
| Notch length (cm) | 6.4±0.8 | 1.3±0.4 | 74.552 | <0.001 |
| Harvested Lymph node (pieces) | 14.1±5.3 | 14.4±4.4 | −0.419 | 0.675 |
| Positive Lymph node (pieces) | 1.0±2.1 | 0.8±1.8 | 0.985 | 0.325 |
| Positive margin | 0 (0) | 0 (0) | NA | NA |
| Intraoperative complications | 0 (0) | 0 (0) | NA | NA |
| Grade | 2.674 | 0.263 | ||
| Well-differentiated | 17 (9.1%) | 18 (9.7%) | ||
| Moderately-differentiated | 156 (83.9%) | 146 (78.5%) | ||
| Poor-differentiated | 13 (7.0%) | 22 (11.8%) | ||
| Histology | 0.304 | 0.859 | ||
| Adenocarcinoma | 179 (96.2%) | 177 (95.2%) | ||
| Tubular adenocarcinoma | 2 (1.1%) | 3 (1.6%) | ||
| Mucinous | 5 (2.7%) | 6 (3.2%) | ||
| Usage of additional analgesics | 95 (51.1%) | 57 (30.6%) | 16.064 | <0.001 |
| VAS scores | / | <0.001* | ||
| Day 1 postoperatively | 4.30±1.31 | 3.44±1.46 | ||
| Day 3 postoperatively | 3.33±1.06 | 2.52±1.23 | ||
| Day 5 postoperatively | 2.03±0.80 | 1.56±0.75 | ||
| Time to recovery of gastrointestinal function (hour) | 62.3±27.6 | 49.4±26.5 | 4.584 | <0.001 |
| Length of postoperative hospital stays (day) | 12.9±5.9 | 11.6±6.0 | 2.089 | 0.037 |
| Postoperative complication | 26 (14.0%) | 12 (6.5%) | 5.745 | 0.017 |
| Anastomotic leakage | 2 (1.1%) | 8 (4.3%) | ||
| Intra-abdominal infection | 2 (1.1%) | 0 (0%) | ||
| Ileus | 1 (0.5%) | 0 (0%) | ||
| Pneumonia | 4 (2.2%) | 1 (0.5%) | ||
| Incision-related complications | 17 (9.1%) | 3 (1.6%) | ||
| Pulmonary embolism | 0 (0%) | 0 (0%) | ||
| Postoperative PFDI-20 scores | 6.95±1.50 | 7.01±1.37 | 0.344 | 0.731 |
| Reoperation | 2 (1.1%) | 1 (0.5%) | 0.000 | 1.000 |
| Medical expenses (RMB) | 68256±18254 | 69640±17656 | 0.743 | 0.458 |
Notes: Results in the table are presented as mean ± standard deviation or number (%); *the P-value was calculated by repeated measures statistical analysis.
Figure 2.
Comparison of ASA scores between two groups of patients after operation. The P-value was calculated by repeated measures statistical analysis.
Abbreviations: LA, laparoscopy group; NOSES, natural orifice specimen extraction surgery group; ASA, American Society of Anesthesiologists.
Comparison of Short-Term Quality of Life Between Two Groups
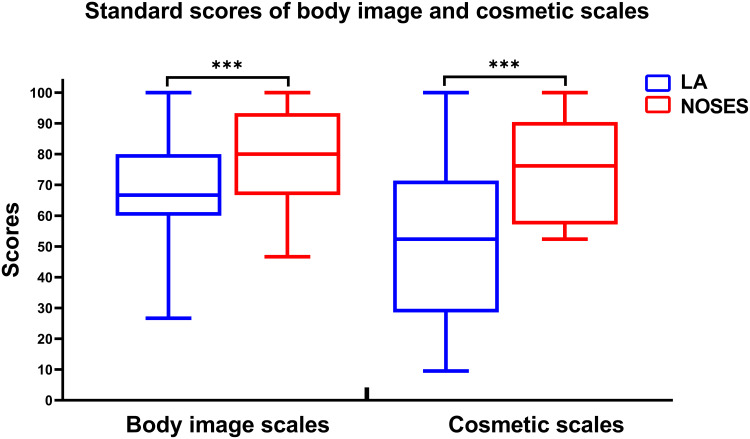
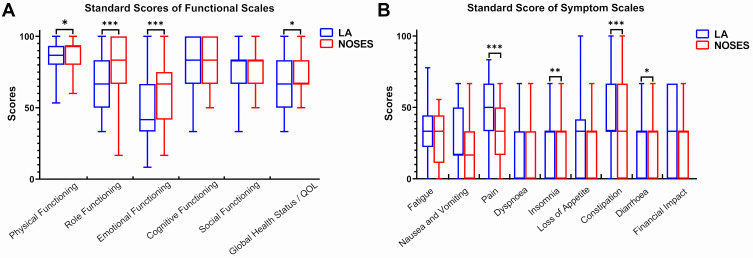
The standardized BIQ score in the NOSES group was significantly higher than that in the LA group at 1 month after surgery (Figure 3, Supplementary Table 3). At 3 months after surgery, the NOSES group had significantly better physical function (P<0.05), role function (P<0.001), emotional function (P<0.001) and global health status than LA group (Figure 4A). In addition, symptoms such as pain (P<0.001), insomnia (P<0.001), constipation (P<0.001) and diarrhea (P<0.05) were less severe in the NOSES group at 3 months after surgery (Figure 4B). However, the anal functions of the NOSES and LA groups remained at the same level in terms of anal function assessment at 6 months after surgery (Table 3, Figure 5).
Figure 3.
Comparison of standard scores of body image scales and cosmetic scales between two groups. ***P<0.001.
Abbreviations: LA, laparoscopy group; NOSES, natural orifice specimen extraction surgery group.
Figure 4.
Comparison of EORCT Quality of Life Questionnaire-Core 30 results between the two groups. The results of the questionnaire were presented by a functional scale (A) and a symptom scale (B). *p<0.05, **p<0.01, ***p<0.001.
Abbreviations: LA, laparoscopy group; NOSES, natural orifice specimen extraction surgery group.
Table 3.
Postoperative Wexner Scores in Both Groups
| Type of Incontinence | After PSM | |||
|---|---|---|---|---|
| LA (N=186) | NOSES (N=186) | t/χ2 | P value | |
| Solid | 2.52 | 2.54 | −0.171 | 0.872 |
| Liquid | 1.82 | 2.09 | −1.904 | 0.058 |
| Gas | 3.76 | 3.81 | −0.749 | 0.459 |
| Wears pad | 0.06 | 0.05 | 0.301 | 0.758 |
| Lifestyle alteration | 1.91 | 1.96 | 1.531 | 0.132 |
Note: Results in the table are presented as mean.
Figure 5.
Comparison of postoperative Wexner scores between the two groups.
Abbreviations: LA, laparoscopy group; NOSES, natural orifice specimen extraction surgery group; ns, no significant difference.
Long-Term Survival Outcomes Between the Two Groups
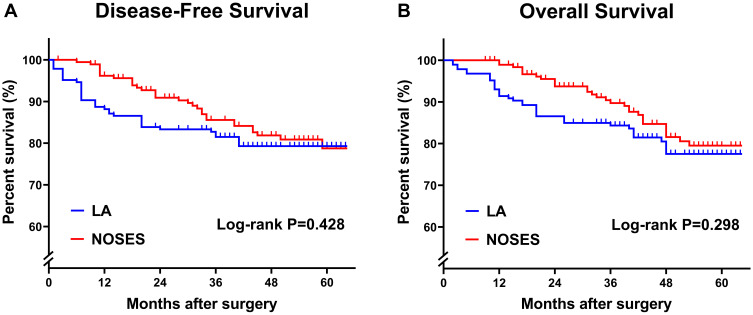
Cumulative survival analysis showed that there was no significant difference in disease-free survival and overall survival between LA group and NOSES group (Figure 6).
Figure 6.
Kaplan–Meier curves comparing the differences between the two groups in terms of disease-free survival (A) and overall survival (B). P-value is calculated by Log rank test.
Abbreviations: LA, laparoscopy group; NOSES, natural orifice specimen extraction surgery group.
Discussion
Minimally invasive is always the pursuit of surgeons. A large number of researches have shown that laparoscopic-assisted radical resection is as effective as laparotomy in the treatment of colorectal cancer. Compared with traditional open surgery, laparoscopic-assisted radical resection not only reduces trauma, but also improves patients’ quality of life.14,15 To avoid trauma-related complications and improve the quality of life, a variety of minimally invasive techniques, such as single-incision laparoscopy, have been developed and gradually applied to patients.16–18 However, single-incision laparoscopy is difficult to perform and does not significantly prevent incision-related complications as the surgeons might expect.18,19 Therefore, NOSES as a new minimally invasive technology arises at the historic moment.
However, NOSES is still in the exploratory stage, and there is no strong evidence-based medical evidence to prove its feasibility and safety. As the first NOSES surgical medical center in China, our department has great advantages in cases and follow-up data. We have collected the clinical data in recent years for retrospective analysis, and combined them with years of clinical practice, to demonstrate that NOSES outperforms traditional laparoscopic surgery in terms of both short-term outcomes and long-term survival. We balanced baseline information between the two groups by strict inclusion and exclusion criteria and using PSM. We assessed the short-term efficacy of NOSES by comparing operative time, blood loss, complications, and recovery on the premise that baseline information matched between the two groups.20 We can find that the intraoperative blood loss in the NOSES group was significantly less than that in the LA group. The reason is not only that the NOSES does not require an auxiliary incision of about 7 cm at the time of specimen removal, but also that the NOSES procedure was performed more meticulously and gently under full laparoscopic surveillance. In terms of postoperative recovery, the recovery time of gastrointestinal function and postoperative hospital stay in the NOSES group were shorter than those in the LA group. However, in terms of postoperative complications, there was no significant difference between NOSES group and LA group in the occurrence of anastomotic leakage, abdominal infection and intestinal obstruction. Meanwhile, the incidence of incision-related complications was much higher in the LA group than in the NOSES group. In addition, postoperative pain scores and use of analgesics were higher in patients in the LA group. However, there was no significant difference in hospitalization costs between the two groups. In terms of long-term efficacy, we found no significant difference in disease-free survival and overall survival between the two groups. As specimens are taken from natural foramen, some scholars will question that NOSES may impair anal sphincter function in patients.21 This study chose Wexner Incontinence Score to assess patients’ anal function at six months after surgery. We can see that there was no significant difference in postoperative anal function between the two groups of patients. In the indications of NOSES, we mentioned the choice of patients, and we have strict control over the size of the tumor. Therefore, there will be no damage to the patient’s anal sphincter during the process of taking the specimen.
Many retrospective and prospective studies have not paid much attention to the psychological health of patients after surgery. But in fact, for the patients, psychological health is no less important than physical health. It is critical to the quality of life of the patient after surgery. In this study, we used the EORTC QLQ-C30 quality of life score scale. We can see that patients in the NOSES group have better postoperative quality of life, role function, physiological function, and emotional function than the LA group. In terms of symptom function scale, patients in the NOSES group had less pain, insomnia, constipation, and diarrhea than those in the LA group. In summary, we can find that the quality of life of patients in the NOSES group is better than that of the LA group.
In the following, we will explain the necessity of NOSES from the feasibility and safety of NOSES.
Feasibility of NOSES
For the feasibility of a new technology or technique, it is very important to master its indications and contraindications. According to China’s “Concept for Colorectal Cancer NOSES Expert Consensus”, the suitable population for colorectal tumor NOSES surgery mainly includes: the tumor invasion depth is preferably T2-T3, and the rectal NOSES specimen’s circumference diameter is <3 cm. The vaginal NOSES technique is recommended to have a diameter of 3–5 cm. Relative contraindications include late onset of local tumors, large lesions, and obese patients (BMI ≥ 30 kg/m2). Therefore, the audience of NOSES is very wide and has good feasibility.
Safety of NOSES
For a new technology to be widely developed and promoted, its security must be the primary prerequisite. Under the premise of ensuring the safety of patients, the new technology which benefits patients is the real weapon in the hands of surgeons. First of all, from the perspective of aseptic technique, many people suspect the potential infection risk of NOSES, because the intestine is cut and closed in the abdominal, and the specimen is taken from the natural orifice. But in this study, we can see that there is no significant difference in the incidence of postoperative infection between the two groups of patients. These data suggest that the NOSES group is not inferior to the LA group. In addition, during surgery, before we open the stump, we can use a mixture of sterile saline and iodophor to perform intestinal lavage, which can reduce the risk of infection. These procedures contribute to achieve the principle of sterility and tumor free. Interestingly, Laitinen et al found that opening the intestine with sufficient disinfection did not increase the chance of infection, nor did it increase the likelihood of tumor cells entering the abdominal cavity.22 This also confirms our point. In summary, we can say that NOSES is in accordance with the principle of sterility. The safety of new surgery includes not only the principle of sterility, but also the principle of tumor free. We found no significant differences in lymph node detection and positive margins between the two groups of patients, which can reflect the principle of tumor-free. In addition, the postoperative recurrence rate is often the simplest and most powerful way to measure this principle, and the survival outcome of patients is also a reliable method to evaluate this principle. Comparing the data of the two groups, we found that NOSES follows the principle of tumor free. Therefore, we can say that NOSES may be a safe new technology, in line with the principles of sterility and tumor free.
The authors acknowledge the limitations of this study. First of all, we admit that the technical errors caused by the different surgeons are unavoidable. However, these primary surgeons have performed at least 100 laparoscopic radical colorectal cancer resections and completed the learning curve for NOSES before data collection. Thus, each surgeon has extensive surgical experience and a high level of professionalism. Secondly, this study is a retrospective study and there must be a selection bias among the selected patients. Both the two groups of patients were selected according to the same inclusion and exclusion criteria, between whom deviations were minimized by matching the baseline information based on PSM, and then the short-and long-term effects were analyzed. We can see that for colorectal cancer patients, NOSES seem to have a better short-term effect, and the long-term effect is not inferior to conventional laparoscopic patients, but also adhere to the principle of aseptic, tumor free. Our results are consistent with those of other studies.23,24 However, the postoperative follow-up and survival analysis were more complete and the sample size was larger. Quality of life for colorectal cancer patients after surgery has always been the goal of surgeons. We look forward to more prospective studies comparing the strengths and weaknesses of NOSES with those of traditional laparoscopy. Also, we hope to use the NOSES procedure for colorectal cancer as a starting point to widely apply the NOSES concept to total abdominal visceral resection and even abdominopelvic visceral resection, which in fact our colleagues are already exploring.7,8,25
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Das V, Kalita J, Pal M. Predictive and prognostic biomarkers in colorectal cancer: a systematic review of recent advances and challenges. Biomed Pharmacother. 2017;87:8–19. doi: 10.1016/j.biopha.2016.12.064 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi: 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 3.DeSouza A, Domajnko B, Park J, Marecik S, Prasad L, Abcarian H. Incisional hernia, midline versus low transverse incision: what is the ideal incision for specimen extraction and hand-assisted laparoscopy? Surg Endosc. 2011;25(4):1031–1036. doi: 10.1007/s00464-010-1309-2 [DOI] [PubMed] [Google Scholar]
- 4.Singh R, Omiccioli A, Hegge S, McKinley C. Does the extraction-site location in laparoscopic colorectal surgery have an impact on incisional hernia rates? Surg Endosc. 2008;22(12):2596–2600. doi: 10.1007/s00464-008-9845-8 [DOI] [PubMed] [Google Scholar]
- 5.Winslow ER, Fleshman JW, Birnbaum EH, Brunt LM. Wound complications of laparoscopic vs open colectomy. Surg Endosc. 2002;16(10):1420–1425. doi: 10.1007/s00464-002-8837-3 [DOI] [PubMed] [Google Scholar]
- 6.Xishan W. Present situation and prospect of colorectal cancers-notes surgery. Chin Colorectal Cancer Electron J Dis. 2015;4(1):11–16. doi: 10.3877/cma.j.issn.2095-3224.2015.04.04 [DOI] [Google Scholar]
- 7.Zhang L, Sun D, Zhang Y, Gao F, Guo Y. Natural orifice specimen extraction surgery in laparoscopic pancreaticoduodenectomy: a single-center case series. Int J Surg. 2020;82:95–99. doi: 10.1016/j.ijsu.2020.07.043 [DOI] [PubMed] [Google Scholar]
- 8.Sun P, Wang XS, Liu Q, Luan YS, Tian YT. Natural orifice specimen extraction with laparoscopic radical gastrectomy for distal gastric cancer: a case report. World J Clin Cases. 2019;7(24):4314–4320. doi: 10.12998/wjcc.v7.i24.4314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunker MS, Stiggelbout AM, van Hogezand RA, Ringers J, Griffioen G, Bemelman WA. Cosmesis and body image after laparoscopic-assisted and open ileocolic resection for Crohn’s disease. Surg Endosc. 1998;12(11):1334–1340. doi: 10.1007/s004649900851 [DOI] [PubMed] [Google Scholar]
- 10.Jorge JM, Wexner SD. Etiology and management of fecal incontinence. Dis Colon Rectum. 1993;36(1):77–97. doi: 10.1007/BF02050307 [DOI] [PubMed] [Google Scholar]
- 11.Barber MD, Walters MD, Bump RC. Short forms of two condition-specific quality-of-life questionnaires for women with pelvic floor disorders (PFDI-20 and PFIQ-7). Am J Obstet Gynecol. 2005;193(1):103–113. doi: 10.1016/j.ajog.2004.12.025 [DOI] [PubMed] [Google Scholar]
- 12.Whistance RN, Conroy T, Chie W, et al. Clinical and psychometric validation of the EORTC QLQ-CR29 questionnaire module to assess health-related quality of life in patients with colorectal cancer. Eur J Cancer. 2009;45(17):3017–3026. doi: 10.1016/j.ejca.2009.08.014 [DOI] [PubMed] [Google Scholar]
- 13.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 14.Christoforidis D, Clerc D, Demartines N. Transrectal specimen extraction after laparoscopic left colectomy: a case-matched study. Colorectal Dis. 2013;15(3):347–353. doi: 10.1111/codi.12006 [DOI] [PubMed] [Google Scholar]
- 15.Demarquay JF, Perrin H, Hastier P, et al. Large iatrogenic colonic perforation treated by endoscopic suturing. Gastroenterol Clin Biol. 2010;34(2):150–153. doi: 10.1016/j.gcb.2009.09.006 [DOI] [PubMed] [Google Scholar]
- 16.Senft JD, Warschkow R, Diener MK, et al. The transvaginal hybrid NOTES versus conventionally assisted laparoscopic sigmoid resection for diverticular disease (TRANSVERSAL) trial: study protocol for a randomized controlled trial. Trials. 2014;15:454. doi: 10.1186/1745-6215-15-454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leung AL, Cheung HY, Fok BK, Chung CC, Li MK, Tang CN. Prospective randomized trial of hybrid NOTES colectomy versus conventional laparoscopic colectomy for left-sided colonic tumors. World J Surg. 2013;37(11):2678–2682. doi: 10.1007/s00268-013-2163-x [DOI] [PubMed] [Google Scholar]
- 18.Rieder E, Spaun GO, Khajanchee YS, et al. A natural orifice transrectal approach for oncologic resection of the rectosigmoid: an experimental study and comparison with conventional laparoscopy. Surg Endosc. 2011;25(10):3357–3363. doi: 10.1007/s00464-011-1726-x [DOI] [PubMed] [Google Scholar]
- 19.Waespe W, Wichmann W. Oculomotor disturbances during visual-vestibular interaction in Wallenberg’s lateral medullary syndrome. Brain. 1990;113(Pt 3):821–846. doi: 10.1093/brain/113.3.821 [DOI] [PubMed] [Google Scholar]
- 20.Hida K, Yamaguchi T, Hata H, et al. Risk factors for complications after laparoscopic surgery in colorectal cancer patients: experience of 401 cases at a single institution. World J Surg. 2009;33(8):1733–1740. doi: 10.1007/s00268-009-0055-x [DOI] [PubMed] [Google Scholar]
- 21.Matran R. Nervous control of bronchial circulation in pigs: application to the airway stimulation. Pathol Biol (Paris). 1991;39(3):223–229. [PubMed] [Google Scholar]
- 22.Rautio M, Sipponen A, Lohi J, Lounatmaa K, Koukila-Kähkölä P, Laitinen K. In vitro fungistatic effects of natural coniferous resin from Norway spruce (Picea abies). Eur J Clin Microbiol Infect Dis. 2012;31(8):1783–1789. doi: 10.1007/s10096-011-1502-9 [DOI] [PubMed] [Google Scholar]
- 23.Zhu Z, Wang KJ, Orangio GR, et al. Clinical efficacy and quality of life after transrectal natural orifice specimen extraction for the treatment of middle and upper rectal cancer. J Gastrointest Oncol. 2020;11(2):260–268. doi: 10.21037/jgo.2020.03.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li XW, Wang CY, Zhang JJ, Ge Z, Lin XH, Hu JH. Short-term efficacy of transvaginal specimen extraction for right colon cancer based on propensity score matching: a retrospective cohort study. Int J Surg. 2019;72:102–108. doi: 10.1016/j.ijsu.2019.07.025 [DOI] [PubMed] [Google Scholar]
- 25.Guan X, Liu Z, Parvaiz A, et al. International consensus on natural orifice specimen extraction surgery (NOSES) for gastric cancer (2019). Gastroenterol Rep (Oxf). 2020;8(1):5–10. doi: 10.1093/gastro/goz067 [DOI] [PMC free article] [PubMed] [Google Scholar]