Abstract
Background: The COVID-19 pandemic challenges multiple sclerosis services to be innovative in delivering infusible therapies. To reduce time in clinical settings, and potential staff or space losses, we implemented rapid infusion protocols for selected patients.
Objective: To analyse the rate of infusion related reactions and patient experience of rapid infusions of natalizumab and ocrelizumab. To document time reduction patients spent in clinical settings during the COVID-19 pandemic.
Methods: Patients with prior exposure to at least three natalizumab or two 300mg ocrelizumab infusions were approved for rapid protocols. A retrospective audit and survey were completed.
Results: We analysed 269 rapid natalizumab infusions and 100 rapid ocrelizumab infusions. Infusion related reactions during the natalizumab or ocrelizumab infusions occurred in two patients (1.52%) and eight patients (8%), respectively. All infusion related reactions were mild to moderate and did not require infusion discontinuation. No infusion reactions occurred during the post-infusion observation. Patient experience was positive.
Conclusion: Frequency or severity of infusion related reactions in rapid infusions were no different compared to published data. In the setting of COVID-19, pandemic rapid infusion protocols could potentially save hospital resources and limit patient exposure to a high-risk clinical setting while still maintaining ongoing treatment of multiple sclerosis.
Keywords: Multiple Sclerosis, COVID-19 pandemic, resource utilisation, infusion related reactions, rapid infusions, natalizumab, ocrelizumab, post observational time
1. Introduction
Highly effective disease modifying therapies (DMTs), including intravenously delivered monoclonal antibodies natalizumab (NTZ) and ocrelizumab (OCR), modify the course of relapsing multiple sclerosis (MS) with marked reduction in relapse rate and disability progression (Brandstadter and Katz Sand, 2017, McCormack, 2013, Mulero et al., 2018).
MS Brain Health consensus guidelines recommend that, if an infusible DMT is selected as the most appropriate therapy for a person with MS (pwMS), it should be offered within 4 weeks, with an ideal goal of initiating treatment within 7 days (Hobart et al., 2019). Persistence and adherence to these therapies are crucial for optimal benefit.
Infusible DMT therapies are administered in dedicated infusion centres with infusion protocols based on individual product information. NTZ 300mg doses are administered every 28 days over one hour with a post infusion observational period of one hour (5). A total infusion centre time of 2.5 hours/150 minutes (min) is required. Maintenance doses of OCR 600mg are administered every six months over 3.5 hours with a post infusion observational period of one hour (Therapeutic Goods Administration 2019). Standard premedications of oral paracetamol 1000mg, oral cetirizine 10mg and intravenous 100mg methylprednisolone are administered to all patients prior to OCR infusion. The total scheduled infusion centre time is 5 hours 50 minutes/350 min.
The COVID-19 pandemic in Australia emerged several weeks later than in other countries (Therapeutic Goods Administration 2019). This window allowed rapid strategic service planning to consider ongoing delivery of infusible DMTs. We anticipated COVID19-associated reduced access to infusion locations and qualified staff for chronic diseases (Nesbitt et al., 2020, Emanuel and Persad, 2020). We developed a strategy of rapid infusion protocols based on current safety data of DMTs and COVID-19 (Giovannoni et al., 2020, Brownlee et al., 2020), because of the high risk of rebound disease activity if therapies, especially NTZ, was delayed or ceased (Sorensen et al., 2014). At the same time, we wanted to reduce exposure duration of immunocompromised pwMS to COVID-19 in the clinical setting. We considered these aims in light of the available evidence (Vollmer et al., 2019, Bermel and Waubant, 2019, Lee et al., 2019) on the utility and safety of shorter infusions (Sacco et al., 2020, (Loonstra et al., 2020)). Our consensus decision was to develop and implement rapid infusion protocols in two tertiary centres in Melbourne, Australia. We achieved protocol development, approval and implementation within 2 weeks in a coordinated effort by neurologists, nursing, pharmacy staff and hospital executives.
We developed an audit tool to monitor safety and acceptance of the protocols.
Here, we report the safety, and patient experience in pwMS who received rapid infusions of NTZ or OCR during the COVID-19 pandemic. We report actual reduction in time spent in the infusion centre.
2. Methods
This was a prospectively planned audit of pwMS attending two academic tertiary hospital infusion services in Victoria, Australia from April to July 2020. Rapid infusions of NTZ were performed at Site A (Alfred Health) and Site B (Melbourne Health), however OCR rapid infusions, and patient experience survey was only performed at Site A. The survey and audit were approved by the relevant ethics committees.
3. Study population and infusion protocols
We included all pwMS who previously received a minimum of three standard, 4-weekly infusions of NTZ 300mg and with no previous documented severe infusion related reactions (IRR). NTZ infusion time was reduced from 60 to 30 minutes with a reduction in post infusion observational time from 60 to 30 minutes. PwMS who had previously received two 300mg initiation doses of OCR without any severe IRR were eligible for rapid administration of the OCR 600mg maintenance dose. Infusion time was reduced from 3.5 hours to 2 hours with no reduction in the one-hour post infusion observation time. Protocols were offered to all eligible pwMS with the option to accept or decline the rapid infusion.
4. Data collection and assessments
We collected age, sex, number of previous infusions, Expanded Disability Status Scale Score (EDSS) (Kurtzke, 1983) and disease duration. We collected IRR incidence, severity, and onset as well as incidence of infusion rate adjustment and administration of symptomatic medication. All participants were invited to self-report any delayed reactions by contacting dedicated nursing staff. Participants at Site A completed an anonymous rapid infusion patient experience survey.
5. Management of IRRs
The site-specific NTZ infusion protocols required NTZ to be interrupted for any IRR. Symptomatic medication was given if indicated, and a medical review completed prior to recommencement or discontinuation of the infusion (Supplementary 1). In accordance with the local OCR infusion protocols, mild and moderate infusion reactions were managed by slowing the infusion rate to 30mL/hr and administration of symptomatic medications. Infusion rate was restored once the IRR was resolved. Infusion was interrupted or discontinued if any emergency haemodynamic criteria were met (Supplementary 2).
6. Data Capture
Data capture at Site A was by review of electronic medical records (EMR) which enabled accurate recording of IRRs and the dose (mg /ml) of infusion delivered at the time of event. Local clinical practice for infusion management requires baseline observations (blood pressure, heart rate, temperature, and respiratory rate), repeated every 30 minutes until discharge. These observations populated directly into the EMR, and their alteration was indicative of IRRs. At Site B, data was extracted from a combination of paper-based records (baseline and subsequent observations) and electronic medical records. A baseline nursing assessment prior to NTZ or OCR screened for any new medical conditions or new neurological symptoms. Data on IRRs were collected by the MS nurse consultants. A senior neurology consultant and an experienced infusion centre pharmacist reviewed
IRR documentation and verified these as DMT-related.
7. Patient experience survey development
The patient experience survey (Supplementary 3) was adapted from the Australian hospital patient experience set (© Australian Commission on Safety and Quality in Health Care 2018). Our aim was to obtain feedback on communication strategies, and pwMS’ experience of the process and sense of safety. The survey was piloted with two pwMS attending the infusion service who were receiving NTZ or OCR infusions. The pilot did not identify any obvious issues with health literacy and pwMS acceptance of the survey.
8. Study endpoints
We assessed frequency, type, severity and onset of IRRs in rapid infusions of NTZ and OCR using recognised terminology from common terminology criteria for adverse events (CTCAE) (US Department of Health and Human Services 2017) (Table 1 ) and identified any associated demographic or clinical factors.
Table 1.
Infusion Related Reactions (Severity and Grading).
| Numerical Grade | Severity | Description | Infusion adjustment | Symptomatic medication |
|---|---|---|---|---|
| Grade 1 | Mild | asymptomatic or transient mild symptoms | No | Yes |
| Grade 2 | Moderate | respond promptly to infusion interruption and administration of symptomatic treatment (e.g. antihistamines and IV steroids | Yes Infusion slowing/interruption and recommencement |
Yes |
| Grade 3 | Severe | recurrence and/or prolongation of reactions despite interventions. Hospitalization might be indicative | Yes Infusion interruption and cessation of therapy |
Yes |
| Grade 4 | Life threatening | reactions have life-threatening consequences and require urgent medical interventions. | Yes Infusion cessation |
Yes |
| Grade 5 | Death | reactions are any adverse event that results in death. | Yes Infusion cessation |
Yes |
9. Statistical Analysis
Baseline demographic and disease-specific data were tabulated using Microsoft Excel (Microsoft standard 2016). We compared the NTZ covariates between the two sites using a two-tailed Mann-Whitney U test for numeric data and Fishers exact test for categorical data. Univariate logistic regression analysis was used to examine the relationship between the covariates “Disease duration”, “EDSS”, “Age” and “Infusion number” and dependent variable “IRR” for both NTZ and OCR. All covariates were included in the initial multivariable logistic regression model, with backwards stepwise selection using Akaike information criterion (AIC) to select the best multivariate model.
10. Results
During the observation period, at Site A, 82 and 121 pwMS were scheduled to receive NTZ and OCR, respectively. A total of 91% of pwMS scheduled for NTZ and 82% scheduled for OCR qualified for rapid infusion protocols. No eligible pwMS declined to receive either rapid infusion protocol.
At Site B, 132 pwMS were scheduled to receive NTZ of which n= 56 (42%) qualified for the rapid infusion protocol audit. 76 of patients were excluded due to receiving NTZ on a six weekly extended dosing interval (n=27), longer infusion time of 45 minutes (n=4) or incomplete data (n=45). The main reason for incomplete data was paper data collection and unavailable medical histories making disease duration, EDSS and current dose difficult to obtain.
11. Rapid NTZ
We analysed 269 rapid NTZ infusions in 131 pwMS, 78% of these infusions were from site A and 22% from site B. Patient Demographics are summarized in Table 2 . The median EDSS was 1.5 (ISQ 2.0), mean 2.0 (SD 1.83) and mean disease duration was 7.9 (SD 6.9) years. Mean number of doses received was 42, (range = 4-158). Site B is operationally older than Site A, and the number of previous NTZ infusions per patient was significantly higher at Site B than Site A (Supplementary 4).
Table 2.
Demographics: pwMS receiving rapid protocol infusions of NTZ and OCR
| pwMS receiving NTZ | Totaln = 131 | With IRRsn = 6 | Without IRRsn = 125* |
|---|---|---|---|
|
Age (years) Mean (SD) |
39.42 (9.70) |
41 (13.16) |
39.34 (9.57) |
|
Gender, n (%) Female |
103 (79%) |
4 (3%) |
99 (76%) |
|
Dose Mean (SD) |
51.66 (41.64) |
51.74 (54.93) |
51.66 (41.18) |
|
EDSS Mean (SD) |
2.02 (1.08) |
2.92 (2.42) |
1.97 (1.97) |
|
Disease Duration Mean (SD) |
7.9 (6.9) |
12.9 (14.6) |
7.6 (6.3) |
| pwMS receiving OCR | Totaln = 100 | With IRRsn = 8 | Without IRRsn = 92 |
|---|---|---|---|
|
Age (years) Mean (SD) |
46.05 (12.5) |
46.25 (13.72) |
46.03 (12.47) |
|
Gender, n (%) Female |
75 (75%) |
6 (6%) |
69 (69%) |
|
Dose Mean (SD) |
4.57 (1.44) |
3.80 (1.44) |
4.64 (1.43) |
|
EDSS Mean (SD) |
3.42 (2.26) |
3.69 (1.98) |
3.39 (2.30) |
|
Disease duration Mean (SD) |
10.91 (8.35) |
9.79 (7.07) |
11.01 (8.48) |
1 missing EDSS.
Six pwMS (4.58%) experienced 20 IRRs (7.43% of total NTZ IRRs) including presyncope, fatigue and headache (see Table 3 ). Of the six pwMS that experienced IRRs, one was at dose four of NTZ, one at dose five and the others were at dose 9, 59,104 and 129. Two pwMS experienced IRR during infusions administration. The first pwMS experienced three episodes of subjective presyncope, rated as a moderate reaction, at doses five, six and seven; with no haemodynamic changes. Infusions were not ceased during these moderate reactions, but the infusion rate was slowed in two cases and interrupted in one case. No symptomatic medication was required. The second pwMS had headache and fatigue, rated as mild at both doses 129 and 130, with no infusion rate adjustment or symptomatic medication given, details summarized in supplementary five. Both of these pwMS had no IRRs with the prior standard infusion rate.
Table 3.
Number of pwMS with IRRs during and after rapid protocol infusions of NTZ and OCR
| pwMS receiving NTZ rapid | Totaln= 131n (%) | During the infusionn (%) | During the 30mins post obsn (%) | Self-reported post dischargen (%) |
|---|---|---|---|---|
| pwMS with IRRs | 6(4.58%) | 2(1.52%) | 0 | 4(3.06%) |
| Mild (G1) | 5(3.82%) | 1(0.76%) | 0 | 4(3.06%) |
| Moderate (G2) | 1(0.76%) | 1(0.76%) | 0 | 0 |
| Severe (G3) | 0 | 0 | 0 | 0 |
| Life threatening (G4) | 0 | 0 | 0 | 0 |
| Mono-symptomatic | 1(0.76%) | 1(0.76%) | 0 | 0 |
| Multiple symptoms | 5(3.82%) | 0 | 0 | 5(3.82%) |
| pwMS receiving rapid OCR | Totaln = 100n (%) | During the infusionn (%) | During the 60mins post obsn (%) | Self-reported post dischargen (%) |
|---|---|---|---|---|
| pwMS with IRRs | 8(8.2%) | 8(8.2%) | 0 | 0 |
| Mild (G1) | 0(0%) | 0 | 0 | 0 |
| Moderate (G2) | 8(8.2%) | 8(8.2%) | 0 | 0 |
| Severe (G3) | 0 | 0 | 0 | 0 |
| Life Threatening (G4) |
0 | 0 | 0 | 0 |
| Mono-symptomatic | 7(7.2%) | 7(7.2%) | 0 | 0 |
| Multiple symptoms | 1(1%) | 1(1%) | 0 | 0 |
No pwMS experienced IRRs in the post-infusion observational period. Four pwMS self-reported IRRs post discharge – all mild reactions, with onset approximately 30 minutes after leaving the infusion centre. One pwMS experienced a single symptom and five pwMS (3.82%) experienced multiple symptoms (a combination of headache, fatigue, presyncope and nausea), see Table 4
Table 4.
Number and summary of IRRs during and after rapid infusion protocol of NTZ or OCR
| NTZ rapid infusions | Total infusionn = 269n (%) | During the infusionn (%) | During the 30mins obsn (%) | Self-reported post dischargen (%) |
|---|---|---|---|---|
| IRR | 20(7.43%) | 3(1.42%) | 0(0%) | 17(5.69%) |
| Symptoms | ||||
| Presyncope | 8 | 3 | 0 | 5 |
| Fatigue | 7 | 2 | 0 | 5 |
| Headache Nausea |
4 1 |
2 0 |
0 0 |
2 1 |
| OCR rapid infusions | Total infusionn=100n (%) | During the infusionn (%) | During the 60mins obsn (%) | Self-reported post dischargen (%) |
|---|---|---|---|---|
| IRR | 9(9.27%) | 9(9.27%) | 0(0%) | 0(0%) |
| Symptoms | ||||
| Throat irritation | 6 | 6 | 0 | 0 |
| Akathisia | 1 | 1 | 0 | 0 |
| Headache | 1 | 1 | 0 | 0 |
| Paresthesia | 1 | 1 | 0 | 0 |
NTZ IRRs increases pwMS time in clinical settings. In our cohort of 125 pwMS on NTZ without any IRRs, the mean infusion time was 30.5 minutes (SD 2.82). For six pwMS with IRRs during NTZ infusion, the mean infusion time was 36.3 minutes (SD 13.28), an increase of 5.8 minutes. One patient did not have infusion time recorded and was excluded.
12. Rapid OCR
We analysed 100 rapid OCR infusions in 100 pwMS, all administered at Site A. The mean EDSS was 3 (SD 2.26), median 3 (IQR 4.0) and mean disease duration was 10.91 years (SD 8.35) and mean current dose 4 (range 2-6). (Table 2).
Eight pwMS (8.2%) experienced IRRs. All IRRs occurred during the infusions (between a rate of 200mls (cumulative infused volume-44mls =52.8mg) and 300ml (cumulative infused volume- 424ml=508.8mg) and were classified moderate. No pwMS experienced any IRRs during the standard 60-minute post-infusion observational time (see Table 4). No pwMS had their infusion ceased, but the infusion rate was slowed in four cases and symptomatic medications were given. After infusion rate adjustment and symptomatic treatment, 7 of 9 pwMS resumed the faster infusion rate summarised in supplementary 6. IRRs included throat irritation, akathisia, headache and paraesthesia. We did not observe any severe or life-threatening IRRs in the OCR cohort.
OCR IRRs, however increased pwMS time in clinical settings. In our cohort of 82 pwMS who did not have any IRRs mean infusion time was 130 minutes (SD 13). However, pwMS with IRRs had a mean infusion time of 189 minutes (SD 35.11), an increase of 59 minutes.
13. Patient experience Results
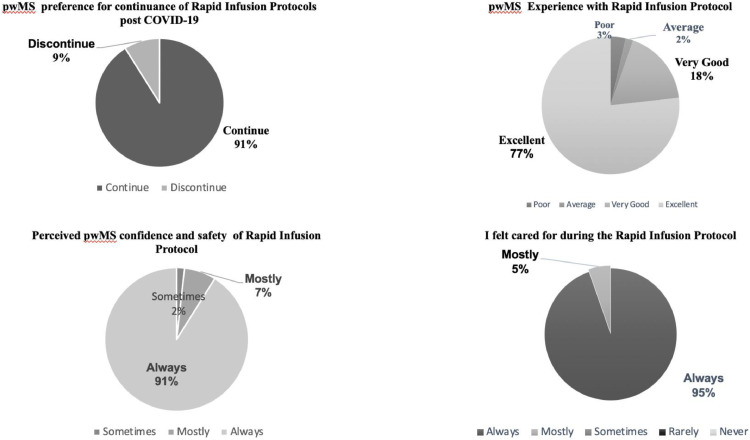
Fifty-six pwMS (32%) from site A (n=175) completed the anonymous patient experience survey. (NTZ 59%, OCR 37.5% and 3.5% with no documented infusion therapy. The experience of the rapid infusion protocol is summarized in Figure 1 . We explored views on continuance of protocols, perceived safety, sense of care and satisfaction with HCP communication.
Figure 1.
pwMS experience of Rapid Infusion Protocols
Free text responses were documented in 61% surveys (n=34). Of the 34 comments, 76% were positive.
14. Resource Utilisation
At Site A, we retrospectively collected data in the EMR to compare the overall admission time to the infusion centre to receive NTZ or OCR. Admission time was defined by time from admission to discharge in minutes.
Prior to COVID-19, the mean admission time of a pwMS for a standard duration NTZ infusion was 125 minutes (SD 41.75) (Range 74-304 mins). Notably, 71.6% of pwMS requested to “sign out” against medical advice before the one-hour observation time had been completed. After the commencement of the rapid infusion protocols, the mean admission time was reduced to 74 minutes (SD 22.9) (Range 44-191 mins) with 62.6% of pwMS still requesting to “sign out” before the 30 min observation time was completed.
The mean admission time to receive an OCR 600mg standard rate infusion was 379 min (SD 48.96) (range 285-561mins) with 26% of pwMS requesting to “sign out” against medical advice before the one-hour observation time was completed. With the implementation of the faster OCR protocol, the mean admission time was 259 min (SD 46.53) (range 186-437), equating to a mean reduction in admission time of 120 min. 50% of pwMS receiving the rapid OCR 600mg “signed out” before the one-hour observation was completed.
15. Discussion
NTZ and OCR are highly effective monoclonal antibody treatments for people with relapsing remitting MS. Although the pivotal studies and clinical experience demonstrate that these treatments are well tolerated, rare life-threatening IRRs have been reported for both treatments (Polman et al., 2006, Mayer et al., 2019). We showed that most pwMS are suitable for rapid infusion of NTZ and OCR. IRRs are rare and we rated all as mild to moderate. All pwMS received the full dose of their infusion. In addition, our results demonstrate that rapid infusion protocols lead to shorter admission times thus reducing exposure of pwMS to potential high-risk COVID-19 clinical settings and increasing resource utilisation.
For 269 NTZ infusions, we reduced admission time by 51 minutes. IRRs occurred in 4.6% overall and were all mild and moderate in classification. The IRR all occurred during the infusion or were reported after discharge. Resource utilisation analysis shows that most pwMS decline to stay for the full observational time, with “sign outs” above 60% for both the standard and rapid infusion protocols.
Our results compare favourably with the IRRs reported in the AFFIRM study (Polman et al., 2006), where these occurred in 24% of NTZ-naïve pwMS. Headache was the most common reaction in 5% of patients. Hypersensitivity reactions occurred in 4% pwMS but only 0.8% were serious in classification, and the majority of these reactions occurred at dose two. Whilst dose number risk was well documented, the timing of these events was not precise and only classified as within 2 hours post infusion start. The need for a fixed post infusion observational time is controversial. Our results add to other post marketing studies challenging the requirement for post-infusion observation time (Lee et al., 2019,Sacco et al., 2020,Loonstra et al., 2020). These studies all demonstrate that pwMS without IRRs during the infusion are extremely unlikely to develop severe or life-threatening reactions in the specified post-infusion observation time period.
OCR pivotal trial pooled data shows IRRs occur in up to 40% of infusions. Of these, 27.5% occurred during the first infusion of 300mg OCR, were mild to moderate and recurrence rate decreased with each further dose (Mayer et al., 2019). Predominantly, reactions were classified by CTCAE as pruritis and rash (10%) and throat irritation (5.3-8.1%). Safety and experience of shorter OCR infusions has been explored in several post-marketing studies (Bermel and Waubant, 2019). Our data demonstrate a similar frequency, severity, and symptom-type of IRRs to those published in other post marketing studies of rapid OCR 600mg administration (Vollmer et al., 2019).
In particular, the ENSEMBLE-PLUS (Hartung, 2020) () substudy reported 8% moderate IRRs occurring in 23 patients (n=289) who, at first randomised dose, may be at initial 600mg infusion or at dose 3,4,5 or 6. IRR onset was only defined as “during infusion” or at “24 hours” followed up by phone call, IRR during OCR infusions in this 2-hour infusion protocol occurred in 56.3% of 40 patients (n=71) and replicated exactly at 24 hour post-infusion time period. All IRRs except one was classified mild or moderate.
As in the pivotal OCR trials (Mayer et al., 2019), throat irritation was the predominate IRR but occurred at a higher frequency (31%) of participants. A smaller study by Bermel and colleagues, the CHORDS substudy (Bermel and Waubant, 2019), reported a similar IRR rate (12.4%) and severity to the data reported here with no severe or life-threatening reactions or infusion discontinuations.
Previous studies have however not addressed the requirement for the one-hour observation time post OCR infusion to increase patient safety. The pivotal pooled and post marketing studies and ENSEMBLE-PLUS only separated IRRs timing as “during infusion” or up to “post 24 hours. Our study with exact timing demonstrated no IRRs occurring in the post observation time. Those experienced post-discharge are generally mild and, as documented in the patient experience outcomes, do not sufficiently concern pwMS to prompt reporting.
Our data on self-discharges or “sign outs” with both the standard and rapid infusion protocols illustrated that the vast majority of pwMS do not feel this post-infusion observation time adds to their safety. This is supported by the absence of any infusion reactions recorded during the post-infusion observation periods for either NTZ or OCR in this study. The requirement for a mandated post-infusion observation time in pwMS who have considerable previous infusion exposure should be studied further in larger studies as it may not be warranted for many pwMS.
PwMS rated their satisfaction with the faster infusion protocols as high and they valued reduced time spent at an infusion centre with less interruption to employment, family and personal time. In addition, shorter infusions and reduction in observational time also allows other care models of infusion delivery to be explored, in particular home-based infusions.
Our study has several limitations, in particular generalisability, as it took place in only two infusion centres associated with large tertiary referral clinics and all staff were highly experienced in the infusion protocols. A multicentre randomised cohort study over a longer observation duration is required and the ENSEMBLE-PLUS (Hartung et al., 2020) study is continuing. Capturing comorbidities in future studies may assist with predictors of IRRs in pwMS. Self-reporting IRRs post discharge potentially did not capture all mild IRRs and future studies may consider systematic HCP phone follow up to capture these mild IRRs.
16. Conclusion
We demonstrate that rapid infusions of NTZ and OCR are safe and well tolerated in pwMS who have had at least three standard infusions of NTZ 300mg or two infusions of 300mg OCR. IRR incidence and severity did not increase during faster infusions. NTZ and OCR post-infusion observation time did not appear to improve safety. In particular, rapid infusions allowed pwMS to have reduced contact time in infusion services during the COVID-19 pandemic, improved resource utilisation, and enhanced pwMS satisfaction.
Conflict of Interest Statement
The authors declare that there is no conflict of interest.
Dr van der Walt served on advisory boards and receives unrestricted research grants from Novartis, Biogen, Merck and Roche She has received speaker's honoraria and travel support from Novartis, Roche, and Merck. She receives grant support from the National Health and Medical Research Council of Australia and MS Research Australia.
Ms Rath served on advisory boards from Biogen, Merck and Roche She has received speaker's honoraria and travel support from Biogen, Novartis, Roche, and Merck
Dr Butzkueven has received compensation for consulting, talks, advisory/steering board activities from Biogen, Merck, Novartis, Genzyme, Alfred Health; research support from Novartis, Biogen, Roche, Merck, NHMRC, Pennycook Foundation, MSRA; received compensation for same activities from Oxford Health Policy Forum, Merck, Biogen, Novartis.
Dr Nguyen received research grants from Novartis, Biogen, Merck Serono and MS Research Australia; speaker honoraria and consulting fees from Biogen, Teva and Merck Serono; conference travel support from Genzyme-Sanofi, Biogen and Roche
Dr Kalincik served on scientific advisory boards for Roche, Celgene, Genzyme-Sanofi, Novartis, Merck and Biogen, steering committee for Brain Atrophy Initiative by Genzyme, received conference travel support and/or speaker honoraria from WebMD Global, Novartis, Biogen, Sanofi-Genzyme, Teva, BioCSL and Merck and received research or educational event support from Biogen, Novartis, Genzyme, Roche, Celgene and Merck.
Dr Skibina received travel support, speaker honoraria for Biogen, Merck, Genzyme and Novartis and served on scientific advisory board of Merck and Biogen.
Ms Taylor has received travel support from Biogen, Novartis, BioCSL and Roche.
Ms Baker has received travel support from Merck, Roche, Biogen and Novartis; nurse advisory consultant support from Merck and Roche Biogen, Genzyme-Sanofi, Teva, BioCSL and Merck and received research support from Biogen.
Dr Wesselingh has received travel support from Merck, Roche and Honoraria from Biogen
Dr Zhong has received conference support from Roche
CRediT authorship contribution statement
Louise Rath: Project administration, Writing - original draft, Writing - review & editing. Minh Viet Bui: Investigation. Julian Ellis: Supervision, Writing - review & editing. John Carey: Resources, Investigation. Josephine Baker: Resources. Lisa Taylor: Resources. Hasini Fernando: Resources. Nicola Taylor: Resources. Poppy Savage: Resources. Janene Richards: Resources. Michael Zhong: Resources. Tomas Kalincik: Writing - review & editing. Olga Skibina: . Robb Wesselingh: Methodology, Formal analysis. Ai-Lan Nguyen: Writing - review & editing. Mastura Monif: Writing - review & editing. Helmut Butzkueven: Supervision, Writing - review & editing. Anneke van der Walt: Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgment
Thank you to the pwMS who attend Alfred Health and Melbourne Health who shared their experiences of rapid infusible therapies during this unprecedented year
Declaration of funding statement
This research did not receive any specific funding and was supported by internal departmental resources.
References
- Brandstadter R, Katz Sand I. The use of natalizumab for multiple sclerosis. Neuropsychiatr Dis Treat. 2017;13:1691–1702. doi: 10.2147/NDT.S114636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack P. Natalizumab: a review of its use in the management of relapsing-remitting multiple sclerosis. Drugs. 2013;73(13):1463–1481. doi: 10.1007/s40265-013-0102-7. Sep. [DOI] [PubMed] [Google Scholar]
- Mulero P, Midaglia L, Montalban X. Ocrelizumab: a new milestone in multiple sclerosis therapy. Ther Adv Neurol Discord. 2018;11 doi: 10.1177/1756286418773025. May 10eCollection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung HP, ENSEMBLE Steering Committee members and study investigators Ocrelizumab shorter infusion: Primary results from the ENSEMBLE PLUS substudy in patients with MS. Neurol Neuroimmunol Neuroinflamm. 2020 doi: 10.1212/NXI.0000000000000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartung HP, Berger T, Brochet B. Shorter infusion time of ocrelizumab: Results from the randomized, double-blind ENSEMBLE PLUS substudy in patients with relapsing-remitting multiple sclerosis. Mult Scler Relat Disord. 2020;24 doi: 10.1016/j.msard.2020.102492. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobart J., Bowen A., Pepper G. International consensus on quality standards for brain health-focused care in multiple sclerosis. Multiple Sclerosis Journal. 2019;25(13):1809–1818. doi: 10.1177/1352458518809326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maintaining essential health services: Operational guidance for COVID-19 context. WHO REFERENCE NUMBER: WHO/2019-nCoV/essential_health_services/2020.2 accessed.
- Therapeutic Goods Administration Product and consumer information. Ocrelizumab . 2019 https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=&q=Ocrelizumab information. Last accessed 6th Dec. [Google Scholar]
- Therapeutic Goods Administration. Product and consumer information. Natalizumab. information https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=&q=natalizumab. Last accessed 6th Dec 2019.
- Nesbitt C, Rath L, Yeh W. MSCOVID19: Using social media to achieve rapid dissemination of health information. Multiple sclerosis and related disorders. 2020;45 doi: 10.1016/j.msard.2020.102338. [DOI] [PubMed] [Google Scholar]
- Emanuel E, Persad G Upshure R. Fair allocation of scare medical resources in time of COVID-19. N Engl J Med. 2020;382:2049. doi: 10.1056/NEJMsb2005114. 2055. [DOI] [PubMed] [Google Scholar]
- Giovannoni G., Hawkes C., Lechner-Scott The COVID-19 pandemic and the use of MS disease-modifying therapies. Multiple sclerosis and related disorders. 2020;39 doi: 10.1016/j.msard.2020.102073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownlee W, Bourdette D, Broadlley S. Treating multiple sclerosis and neuromyelitis optica spectrum disorder during the COVID-19 pandemic. Neurology. 2020;94(22):949–952. doi: 10.1212/WNL.000000000000950. [DOI] [PubMed] [Google Scholar]
- Sorensen P.S., Koch-Henriksen N., Petersen T. Recurrence or rebound of clinical relapses after discontinuation of natalizumab therapy in highly active MS patients. J Neurol. 2014;261:1170–1177. doi: 10.1007/s00415-014-7325-8. [DOI] [PubMed] [Google Scholar]
- Vollmer T, Alvarez E, Nair KV et al. Safety results of administering Ocrelizumab per a shorter infusion protocol in patients with primary progressive and relapsing multiple sclerosis. Poster 1406 ECTRIMS Sweden 2019. [DOI] [PubMed]
- Bermel R, Waubant E, Pardo G et al Evaluation of shorter infusion times in Ocrelizumab treatment in an extension substudy of the Phase 111b CHORDS Trial. Poster 1408 Sweden 2019.
- Lee L, Kasliwal R, Patel Y et al. Timing and Incidence of Infusion-Related Reactions Associated with Natalizumab Treatment in Patients with Multiple Sclerosis. Poster (DXT 60) at Consortium of Multiple Sclerosis Centres I May 28 – June 1, 2019 I Seattle, WA.
- Sacco R, Disanto G, Maraffi I. Infusion related reactions during Natalizumab treatment: Do we still need a post-infusion observationa period? Multiple sclerosis and related disorders. 2020;38 doi: 10.1016/j.msard.2019.101523. Volume. [DOI] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurological impairment in multiple sclerosis. An expanded disability status scale (EDDS). Neurology. 1983;33(11):1444. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- © Australian Commission on Safety and Quality in Health Care 2018. This publication is licensed for use and distribution under a Creative Commons Attribution-Noncommercial-Share Alike (BY-NC-SA) v4.0 licence. https://creativecommons.org/licenses/by-nc-sa/4.0/.
- US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. national institutes of health, national cancer institute; 2017.
- Polman CH, O'Connor PW, Havrdova E. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. [DOI] [PubMed] [Google Scholar]
- Loonstra F, van Rossum J, van Kempen Z. Infusion-related events during natalizumab: No need for post-infusion monitoring. Mult Scler. 2020 doi: 10.1177/1352458519860415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer L., Kappos L., Rackeet Ocrelizumab infusion experience in patients with relapsing and primary progressive multiple sclerosis: Results from the phase 3 randomized OPERA I, OPERA II, and ORATORIO studies. Mult. Scler. Relat. Disord. 2019;30:236–243. doi: 10.1016/j.msard.2019.01.044. [DOI] [PubMed] [Google Scholar]