Abstract
Patients with Beckwith-Wiedemann spectrum (BWSp) undergo quarterly alpha-fetoprotein measurement for hepatoblastoma (HB) screening up to 4 years of age, paralleling the epidemiology of nonsyndromic HB. However, specific data on the timing of HB development in BWSp are lacking. Here we compare the timing of presentation of HBs in BWSp with a control cohort of consecutive HB cases, demonstrating that halving screening duration of screening procedures in BWSp likely will not impact its effectiveness.
Keywords: alpha-fetoprotein, Beckwith-Wiedemann, cancer screening, hepatoblastoma
1 |. INTRODUCTION
Beckwith-Wiedemann spectrum (BWSp) is the most common overgrowth disorder1 and presents with multiple features of variable severity including macroglossia, abdominal wall defects, macrosomia, hyperinsulinism, ear anomalies, and cancer predisposition.2 Hepatoblastoma (HB) represents the second most common cancer to develop in patients affected by BWSp.3–5 Patients with BWSp experience an over 2200 times greater HB risk than the general population and up to 1.7% will develop HB.3,6 Among BWSp molecular subgroups, the highest HB risk is observed in patients with paternal chromosome 11 uniparental disomy, occurring in 3.5–4.7% of patients3,4 and risk appears to be even greater in patients with genome-wide paternal uniparental disomy.5,7 In patients with BWSp and KCNQ1OT1:TSS-DMR loss of methylation, HB occurs in 0.7% of cases, representing the most common cancer.4
The tumor marker alpha-fetoprotein (αFP) is secreted in more than 95% of HBs starting at very early stages8,9 and has been used for cancer screening in this context, using quarterly measurement of its concentration in conjunction with abdominal ultrasound, with the aim of early diagnosis.3,5,10,11 As nonsyndromic HB occurs before 5 years of age,8 screening in BWSp is adopted during the first 4 years of life.12 However, specific data on the timing of HB development in the BWSp population are lacking. Here we provide data on HB epidemiology in BWSp, showing differences in the age of presentation compared to sequential cases of children with HB, supporting a reduction in the duration of αFP screening in these patients.
2 |. METHODS
A literature search was performed in PubMed from 1980 through 2017 to identify reports of patients with BWSp with HB and data concerning age at the diagnosis. Given the retrospective nature of this investigation, patients were selected notwithstanding molecular diagnosis and the clinical criteria used for the BWS diagnosis, as varying clinical criteria and molecular diagnostic methods were available at the time of these reports. Additional unpublished patients with BWSp and HB were identified through a search of registries at the authors’ institutions. Institutional review board approval and patient consent were obtained for personal cases included.
The age at HB tumor development was collected from the patients with BWSp for comparison with 254 sequential patients with HB reported by Litten et al.8 Data were analyzed using GraphPad Prism 7.0 (La Jolla, CA, USA).
3 |. RESULTS
A total of 76 patients with BWSp and HB were identified (64 from the literature4,13–22 and 12 unpublished patients from the authors’ cohorts; 10 Kalish/Duffy, 2 Musso/Ferrero).
Mean age at tumor diagnosis in patients with BWSp was 7.1 ± 6.0 months (median 5.0 months). The distribution of age at diagnosis (Figure 1) depicts a left-skewed log-normal distribution (R2 0.558). Mean age at diagnosis in nonsyndromic HB patients was significantly higher (19.5 ± 14.7 months, median 16.0 months, P < 0.001).
FIGURE 1.
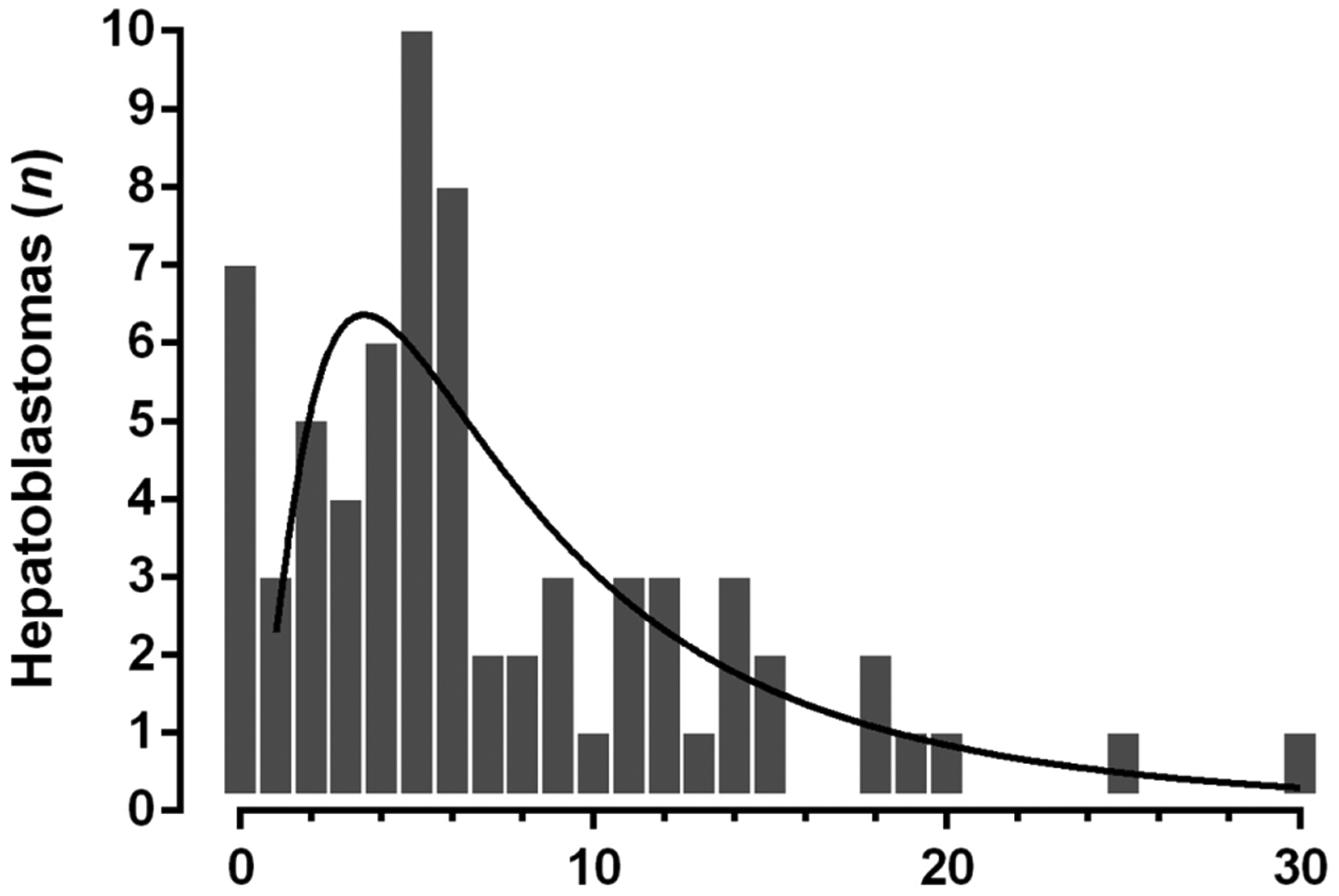
Hepatoblastoma (HB) diagnosed by month of age in patients with Beckwith-Wiedemann spectrum (BWSp) Note: The distribution of age at diagnosis depicts a left-skewed log-normal distribution (R2 0.558).
The cumulative incidence by age at HB diagnosis in patients with BWSp and nonsyndromic patients is shown in Figure 2. In patients with BWSp, 80% of HB was diagnosed before 12 months of age, 95% before 18 months, 97% before 24 months, and 100% before 30 months, while HB incidence in the control cohort was 39%, 53%, 68% and 79%, respectively. A significant difference in Kaplan-Meier curves of HB incidence of the BWSp cohort and HB cohort was found from the age of 5 months (log-rank Mantel-Cox test P < 0.001).
FIGURE 2.
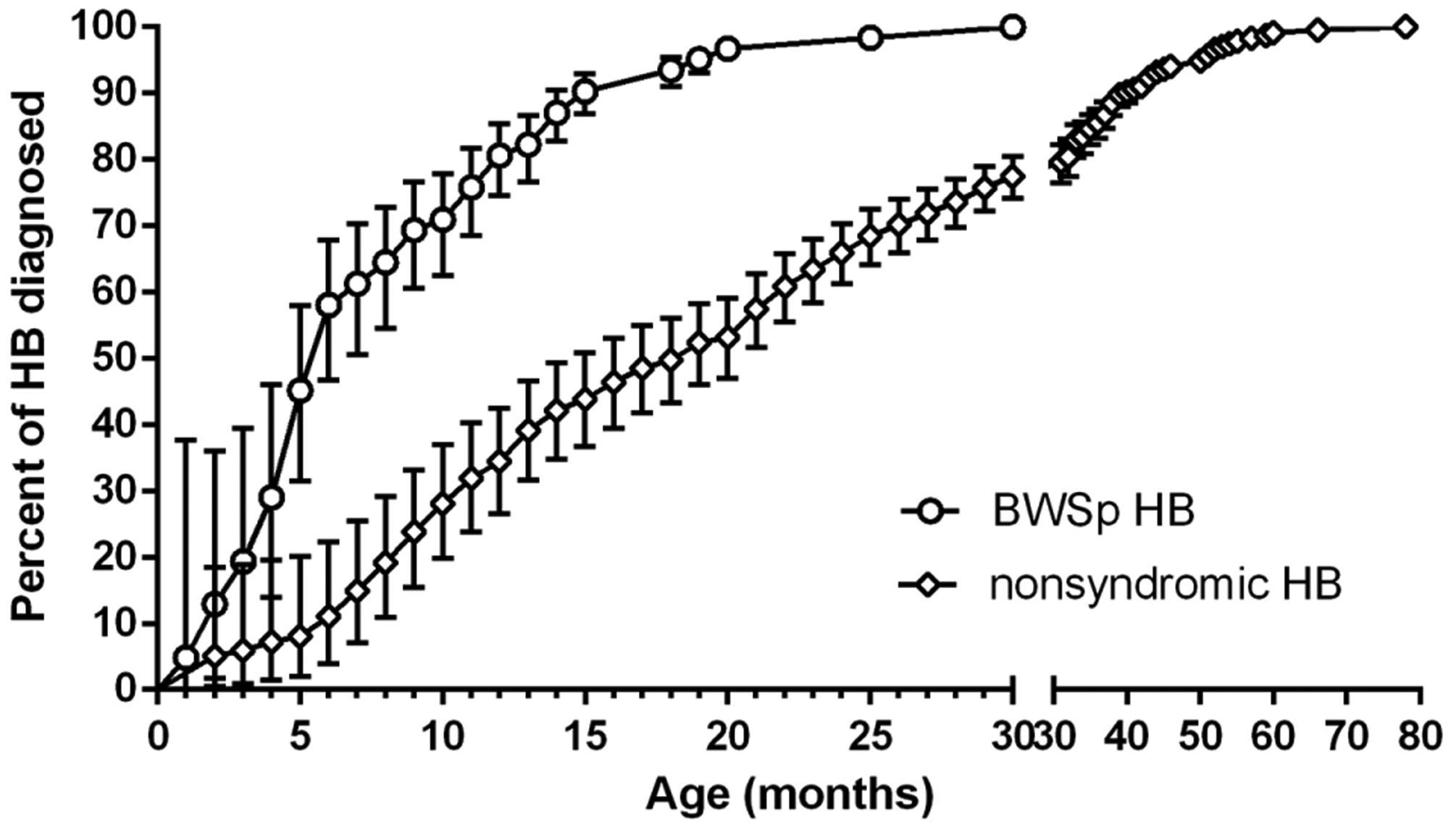
Cumulative incidence of hepatoblastomas (HBs) diagnosed by month of age in patients with Beckwith-Wiedemann spectrum (BWSp; empty circles) and in the comparator historical cohort of HB patients published by Litten et al8 (empty diamonds) with respective 95% confidence interval (vertical bars) Note: Kaplan-Meier curves were significantly different from the age of 5 months (log-rank Mantel-Cox test P < 0.001).
4 |. DISCUSSION
HB screening in BWSp is a debated issue and it has been questioned if HB deserves specific screening with αFP measurements.23–26 Current recommendations on αFP use differ between health care systems based on the threshold for acceptable risk: a 5% risk threshold has been utilized in Europe compared to a 1% risk threshold adopted in the United States. As a result, αFP screening was not recommended in a recent European Consensus on BWSp due to the challenges in interpreting αFP results,5 while αFP screening from birth or BWSp diagnosis until 4 years of age has been recommended by American Association for Cancer Research (AACR) Workshop on Childhood Cancer Predisposition in the United States,12 keeping with the recommendations employed worldwide for more than a decade.11,27
Although no specific studies have evaluated the yield of this screening strategy and its impact on survival, several data indicate that quarterly αFP measurement can result in a downgrade shift of tumor stage at diagnosis,10,21 potentially reducing mortality and treatment complications. This practice seems also to allow an earlier recognition with respect to liver ultrasound, as αFP rise usually anticipates imaging diagnosis.10,22,27 The importance of an early detection lies in prognosis implications. The overall 5-year survival rate of HB based on current treatment regimens is 70%,28 ranging from 92% to 31% based on stage at diagnosis and age, with children diagnosed <1 year of age having a better prognosis.29
Despite this evidence, drawbacks to this screening strategy include difficulties in interpreting αFP values in the first 2 years of life and absence of standardized protocols for managing αFP increases. Repeated blood draws in early infancy may create a burden for patients and their families, representing an indirect cost and factor affecting compliance. However, a recent survey among parents of patients with BWSp found that parents are often comforted by screening30 and less invasive methods have been proposed, such as αFP measurement on capillary blood.31
The duration of αFP screening up to the fourth year of age in BWSp has been chosen to parallel the epidemiology of nonsyndromic HB, where most tumors develop before the fifth year of age.8 However, it has been suggested that HB in BWSp has different characteristics compared to the nonsyndromic HB.21 In this study, we provide the first data on HB epidemiology in BWSp, demonstrating that age at tumor diagnosis in patients with BWSp is lower than that observed in a cohort of HB cases. Cumulative HB incidence diagnosed by age in patients with BWSp reaches a plateau well before that observed in the control HB patients cohort, with all HB diagnosed before the age of 30 months in BWSp, suggesting that screening up to 24–30 months may be sufficient to detect nearly all tumors in this population. Although the value of this observation is somewhat limited due to the retrospective nature of this study, the differences in the HB diagnosis trend across early infancy are clear. Actually, it should be noted that the 254 cases by Litten et al used as comparator cohort are consecutive and were not examined with respect to potential syndromic diagnoses: therefore, it is possible that some of them had BWSp or other tumor predisposing diagnoses. Moreover, this review did not evaluate the molecular diagnosis or clinical features present in patients and further investigation including these data may be beneficial to identify patients with BWSp most at risk for developing HB.
These data imply that it is approximately possible to halve the duration and number of blood draws in patients with BWSp: 10 blood draws may be sufficient to allow the detection of most HBs. The present data also highlight the importance of an early diagnosis of BWSp and immediate initiation of screening protocol in patients with a suspected diagnosis, as more than 80% of the HB cases occurred in the first year of life.
In conclusion, these retrospective data suggest that a reduction of duration of HB screening from 4 years to 30 months in BWSp can be considered, allowing reduction of direct and indirect costs and patients’ burden with likely no impact on effectiveness of screening with respect to current practice.
Funding information
National Institutes of Health, Grant/Award Number: K08CA193915; Alex’s Lemonade Stand Foundation; and St. Baldrick’s Foundation Scholar Award
Abbreviations:
- αFP
alpha-fetoprotein
- BWSp
Beckwith-Wiedemann spectrum
- HB
hepatoblastoma
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest relevant to this article to disclose.
REFERENCES
- 1.Mussa A, Russo S, De Crescenzo A, et al. Prevalence of Beckwith-Wiedemann syndrome in North West of Italy. Am J Med Genet A. 2013;161A:2481–2486. [DOI] [PubMed] [Google Scholar]
- 2.Mussa A, Russo S, Larizza L, Riccio A, Ferrero GB. (Epi)genotype-phenotype correlations in Beckwith-Wiedemann syndrome: a paradigm for genomic medicine. Clin Genet. 2016;89:403–415. [DOI] [PubMed] [Google Scholar]
- 3.Mussa A, Molinatto C, Baldassarre G, et al. Cancer risk in Beckwith-Wiedemann syndrome: a systematic review and meta-analysis outlining a novel (epi)genotype specific histotype targeted screening protocol. J Pediatr. 2016;176:142–149. [DOI] [PubMed] [Google Scholar]
- 4.Maas SM, Vansenne F, Kadouch DJ, et al. Phenotype, cancer risk, and surveillance in Beckwith-Wiedemann syndrome depending on molecular genetic subgroups. Am J Med Genet A. 2016;170:2248–2260. [DOI] [PubMed] [Google Scholar]
- 5.Brioude F, Kalish JM, Mussa A, et al. Expert consensus document: clinical and molecular diagnosis, screening and management of Beckwith-Wiedemann syndrome: an international consensus statement. Nat Rev Endocrinol. 2018;14:229–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeBaun MR, Tucker MA. Risk of cancer during the first four years of life in children from the Beckwith-Wiedemann Syndrome Registry. J Pediatr. 1998;132:398–400. [DOI] [PubMed] [Google Scholar]
- 7.Kalish JM, Conlin LK, Bhatti TR, et al. Clinical features of three girls with mosaic genome-wide paternal uniparental isodisomy. Am J Med Genet A. 2013;161:1929–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Litten JB, Tomlinson GE. Liver tumors in children. Oncologist. 2008;13:812–820. [DOI] [PubMed] [Google Scholar]
- 9.Spector LG, Birch J. The epidemiology of hepatoblastoma. Pediatr Blood Cancer. 2012;59:776–779. [DOI] [PubMed] [Google Scholar]
- 10.Clericuzio CL, Chen E, McNeil DE, et al. Serum alpha-fetoprotein screening for hepatoblastoma in children with Beckwith-Wiedemann syndrome or isolated hemihyperplasia. J Pediatr. 2003;143:270–272. [DOI] [PubMed] [Google Scholar]
- 11.Mussa A, Di Candia S, Russo S, et al. Recommendations of the Scientific Committee of the Italian Beckwith-Wiedemann Syndrome Association on the diagnosis, management and follow-up of the syndrome. Eur J Med Genet. 2016;59:52–64. [DOI] [PubMed] [Google Scholar]
- 12.Kalish JM, Doros L, Helman LJ, et al. Surveillance recommendations for children with overgrowth syndromes and predisposition to Wilms tumors and hepatoblastoma. Clin Cancer Res. 2017;23:115–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mussa A, Russo S, De Crescenzo A, et al. (Epi)genotype-phenotype correlations in Beckwith-Wiedemann syndrome. Eur J Hum Genet. 2016;24:183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brioude F, Lacoste A, Netchine I, et al. Beckwith-Wiedemann syndrome: growth pattern and tumor risk according to molecular mechanism, and guidelines for tumor surveillance. Horm Res Paediatr. 2013;80:457–465. [DOI] [PubMed] [Google Scholar]
- 15.Ibrahim A, Kirby G, Hardy C, et al. Methylation analysis and diagnostics of Beckwith-Wiedemann syndrome in 1,000 subjects. Clin Epigenet. 2014;6:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luk HM. Clinical and molecular characterization of Beckwith-Wiedemann syndrome in a Chinese population. J Pediatr Endocrinol Metab. 2017;30:89–95. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Jung SH, Kim MS, et al. Genomic profiles of a hepatoblastoma from a patient with Beckwith-Wiedemann syndrome with uniparental disomy on chromosome 11p15 and germline mutation of APC and PALB2. Oncotarget. 2017;8:91950–91957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bachmann N, Crazzolara R, Bohne F, et al. Novel deletion in 11p15.5 imprinting center region 1 in a patient with Beckwith-Wiedemann syndrome provides insight into distal enhancer regulation and tumorigenesis. Pediatr Blood Cancer. 2017. 10.1002/pbc.26241. [DOI] [PubMed] [Google Scholar]
- 19.Kawasaki Y, Makimoto M, Samejima A, et al. Hepatoblastoma in an extremely low birth-weight infant with Beckwith-Wiedemann syndrome. Pediatr Neonatol. 2017. 10.1016/j.pedneo.2017.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Calton EA, Temple IK, Mackay DJ, et al. Hepatoblastoma in a child with a paternally-inherited ABCC8 mutation and mosaic paternal uniparental disomy 11p causing focal congenital hyperinsulinism. Eur J Med Genet. 2013;56:114–117. [DOI] [PubMed] [Google Scholar]
- 21.Trobaugh-Lotrario AD, Venkatramani R, Feusner JH. Hepatoblastoma in children with Beckwith-Wiedemann syndrome: does it warrant different treatment. J Pediatr Hematol Oncol. 2014;36:369–373. [DOI] [PubMed] [Google Scholar]
- 22.Mussa A, Ferrero GB, Ceoloni B, et al. Neonatal hepatoblastoma in a newborn with severe phenotype of Beckwith-Wiedemann syndrome. Eur J Pediatr. 2011;170:1407–1411. [DOI] [PubMed] [Google Scholar]
- 23.Mussa A, Ferrero GB. Screening hepatoblastoma in Beckwith-Wiedemann syndrome: a complex issue. J Pediatr Hematol Oncol. 2015;37:627. [DOI] [PubMed] [Google Scholar]
- 24.Mussa A, Ferrero GB. Serum alpha-fetoprotein screening for hepatoblastoma in Beckwith-Wiedemann syndrome. Am J Med Genet A. 2017;173:585–587. [DOI] [PubMed] [Google Scholar]
- 25.Kalish JM, Deardorff MA. Tumor screening in Beckwith-Wiedemann syndrome-To screen or not to screen. Am J Med Genet A. 2016;170:2261–2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Duffy KA, Deardorff MA, Kalish JM. The utility of alpha-fetoprotein screening in Beckwith-Wiedemann syndrome. Am J Med Genet A. 2017;173:581–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarate YA, Mena R, Martin LJ, et al. Experience with hemihyperplasia and Beckwith-Wiedemann syndrome surveillance protocol. Am J Med Genet A. 2009;149A:1691–1697. [DOI] [PubMed] [Google Scholar]
- 28.Kremer N, Walther AE, Tiao GM. Management of hepatoblastoma: an update. Curr Opin Pediat. 2014;26:362–369. [DOI] [PubMed] [Google Scholar]
- 29.Czauderna P, Lopez-Terrada D, Hiyama E, et al. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr. 2014;26:19–28. [DOI] [PubMed] [Google Scholar]
- 30.Duffy KA, Grand KL, Zelley K, Kalish JM. Tumor screening in Beckwith-Wiedemann syndrome: parental perspectives. J Genet Couns. 2017;27:844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mussa A, Pagliardini S, Pagliardini V, et al. α-Fetoprotein assay on dried blood spot for hepatoblastoma screening in children with overgrowth-cancer predisposition syndromes. Pediatr Res. 2014; 76:544–548. [DOI] [PubMed] [Google Scholar]


