Abstract
Background and aims
COVID-19 is a pandemic that has affected beyond 100 million and caused nearly 3 million deaths globally. Vitamin D is a known risk factor for COVID-19. Therefore, we aimed to investigate the association of prevalence of vitamin D deficiency and mean vitamin D level with COVID-19 infection and mortality in Asia, predicting with other confounding factors such as median age, obesity, and diabetes.
Methods
COVID-19 infections and mortalities among the Asian countries were retrieved from the Worldometer website. Information on prevalence of vitamin D deficiency and mean vitamin D values in each Asian country was retrieved through literature searching on PubMed® and Google scholar. The associations between COVID-19 infections and mortalities with prevalence of vitamin D deficiency and mean vitamin D level were explored with correlation coefficients. As a predictive analysis, multiple linear regression was carried out with all confounders.
Results
Positive correlations were observed for prevalence of vitamin D deficiency with COVID-19 infections (r = 0.55; p = 0.01; R2 = 0.31) and mortalities (r = 0.50; p = 0.01; R2 = 0.25). Moreover, the associations for the COVID-19 infections and mortalities improved to r = 0.76 (p = 0.002; R2 = 0.58) and r = 0.65 (p = 0.03; R2 = 0.42), respectively, after predicting with confounding factors. Similarly, mean vitamin D level had a significant negative correlation with COVID-19 infections (r = −0.77; p = 0.04; R2 = 0.59) and mortalities (r = −0.80; p = 0.03; R2 = 0.63) when combining with confounders.
Conclusion
Prevalence of vitamin D deficiency is significantly positively associated whereas the mean vitamin D level is significantly negatively associated with both infection and mortality rate of COVID-19 among Asian countries upon predicting with all confounders.
Keywords: Vitamin D, COVID-19, Infection, Mortality, Asia
Abbreviations: 1 M, One million; 25(OH)D, 25-hydroxyvitamin D3; BMI, Body mass index; CIA, Central Intelligence Agency; COVID-19, Coronavirus disease; ISO, International Organization for Standardization; NCD, Non-communicable disease; NMH, Non-communicable Diseases and Mental Health Cluster; PCR, Polymerase Chain Reaction; VD, Vitamin D; VDD, Vitamin D deficiency; WHO, World Health Organization
1. Introduction
The coronavirus disease (COVID-19) outbreak has rapidly extended globally reaching through to almost every country. The current COVID-19 infections and mortalities are at a staggering number of more than 100 million and nearly 3 million, respectively, across the world [1]. COVID-19 pandemic has emerged as a public health crisis globally testing the resilience of the health systems and it has been established that severity of the cases as well as the mortality is linked with underlying health conditions [2]. Determining the associated comorbidities with the virus is twofold; firstly, on an individual level which allows health workers to tailor the appropriate treatment and secondly in a national level allowing the country’s government to improve public health recommendations [3]. Public health infrastructure, prevention of infection and control measures are an integral part of the COVID-19 management [4].
A systematic review conducted by Yang et al. on the prevalence of comorbidities in patients infected with the virus showed that both age and comorbidities are risk factors for critical patients [5]. Among other comorbid illnesses such as hypertension and cardiovascular diseases, diabetic individuals with dysregulated immune cell populations and activity may result in aggravating the severity of the disease [6]. Studying diabetes mellitus as a risk factor, an investigation showed that the biomarkers related to inflammation were significantly higher (p < 0.01) in diabetic patients comparatively to the non-diabetic [6]. Additionally, an underlying chronic condition obesity as a potential risk factor was studied and findings showed; total population affected (r = 0.46; p < 0.001) and mortalities per million population (r = 0.34; p < 0.05) by COVID-19 were both significantly positively correlated with obesity prevalence [7]. Asia being the largest continent includes an estimated equivalent to 60% of the world’s population closing in on 4.7 billion currently [8] and expected to see an increased incidence of COVID-19 [9]. The first report and subsequent outbreak was traced in China [10] and spreading to other neighbouring Asian countries [11]. The rate of COVID infections is uneven across countries and having contributed tremendously to the growth of the global economy the socio-economic evolution among the countries in Asia may cause to be a determinant besides the preparedness and management [9].
Differences in economic status with ethnicity and underlying health conditions may cumulatively provide to the unequal impact of COVID-19 [12]. Therefore, understanding the association between ethnicity and COVID-19 is necessary in order to reduce the disproportionate burden of disease in various ethnic groups; a meta-analysis showed individuals from Asian and Black ethnic groups are more likely to be infected by the virus than compared to those of White ethnicity [13]. The COVID-19 trend in Asia may have been contributed by multiple biological factors, environmental conditions, and the public health response [14]. Projections at the beginning had placed South Asia at a higher risk due to certain factors as its greater population density, increased burden of comorbidities and vast socioeconomic vulnerabilities [15]. These determinants are particularly salient in the Asian context, with its diversity among countries in terms of sociocultural heritage, healthcare setup and availability of resources [16]. Hence, in a situation as the COVID-19 pandemic, the study of the associated factors in the Asian countries plays an important role.
The key feature of COVID-19 is immune dysregulation, as a result restoring the immune balance and preventing the hyper-inflammatory cytokine storm could be a strategy to combat the virus [17]. Adequate amounts of certain micronutrients are essential to ensure proper function of the immune cells, among vitamins; A and D showed beneficial effect particularly in deficient populations [18]. Vitamin D (VD), is a steroid hormone endogenously produced via ultraviolet radiation effects on the skin or available exogenously from food sources or dietary supplements [19]. This vitamin is showed to play a critical role in acute respiratory tract infections [20]. One hypothesis for observed associations between ethnicity, obesity, and worse COVID-19 out-comes is vitamin D deficiency (VDD) among COVID-19 patients [21]. A review studying serum VD concentration with incidence/severity of COVID-19 showed that they are inversely correlated [22]. Therefore, VDD showed an increase in thrombotic episodes which were observed frequently in COVID-19 patients, mostly with obese and diabetic individuals [23].
The association between the severity and mortality of COVID-19 with VDD is distinct. On the whole, VD could act as an important risk factor for COVID-19 severity. Therefore, to further explore the association of VDD and COVID-19 in countries that belong to the Asia. This includes the multiple variants present across all countries; we aimed to investigate the country specific prevalence of VDD as well as mean VD with COVID-19 infection and mortality rates in Asia, predicting with other confounders such as median age, prevalence of obesity and diabetes.
2. Methods
2.1. Data sources of COVID-19 statistics
Real time numerical data on COVID-19 infections and mortalities among the Asian countries, as of December 31st, 2020, were retrieved from the Worldometer website [24], which includes records derived directly from official government reports of individual countries and/or indirectly through reliable local media resources. The countries which have not conducted minimum 10,000 PCR tests per one million (1 M) of the country population as of December 31st, 2020, were excluded from the analysis.
2.2. Data sources of VDD
A comprehensive electronic search was performed in both PubMed® to retrieve the information on the prevalence of VDD among the selected countries. The articles were searched by using the key words, such as “Vitamin D″ or “25-hydroxyvitamin D3”, combined with “deficiency”, “prevalence” or “status” and the name of each Asian country. Additional search was performed in the Google Scholar® for the missing countries using the similar key words. The search was limited to articles published in the last ten years and contained data for the adult population (≥18 years). The resulted articles were screened based on the following inclusion criteria: a) Population-based studies; b) studies reporting non-institutional adults; c) studies defining VDD as the serum concentration of 25(OH)D < 20 ng/ml or <50 nmol/l; d) studies reporting VDD as the prevalence of the sample population. In addition, conference proceedings, editorials, commentaries, book chapters/book reviews and studies confined to selective sample, such as pregnant women, elderly people, and patients with diagnosed illnesses were excluded. Finally, out of the screened articles for each country, the most recently published study, with the most representative sample of each country was selected for extracting data on VDD and mean VD values in the respective populations.
2.3. Data sources of other confounders
The data on the prevalence of obesity and Diabetes Mellitus (DM) were extracted from Non-communicable disease (NCD) country profiles released recently by the Non-communicable Diseases and Mental Health Cluster (NMH) of World Health Organization, where BMI levels ≥ 30 kgm−2 and fasting plasma glucose concentrations ≥7.0 mmol/l had been considered as obesity and DM, respectively [25]. In addition, the median age of each country population was retrieved from the world fact book of Central Intelligence Agency (CIA), which constitutes basic information on socio demographic factors of 266 world entities [26].
2.4. Data extraction
As of December 31st, 2020, the numbers of both COVID-19 infections and mortalities per 1 M of the total country population, and as well as the number of PCR tests done per 1 M population were retrieved from the Worldometer website for each Asian country [24]. From the selected studies reporting VDD among these Asian countries, name of the first author, published year, sample size, age range of the study population, prevalence of VDD and mean VD level were extracted. Moreover the prevalence of both obesity and DM of the adult population and as well as the published year of the individual reports were obtained from the NCD country profiles [25]. The median age of the total country population as estimated for the year 2020, was retrieved from CIA website [26]. All data were extracted by one reviewer (DTJ) using a standardized form and were checked for accuracy by a second reviewer (TVF). Discrepancies in the extracted data were resolved by consensus, with involvement of a third reviewer when necessary (RJ).
2.5. Data analysis
Statistical analysis was performed using SPSS (version 20.0; IBM, Inc.). Data normality was analysed using the Kolmogorov-Smirnov test. The relationship between the independent variables - prevalence of VDD and mean VD level and dependent variables, such as total number of COVID-19 infections and mortalities per 1 M population were explored with Pearson correlation coefficients. As a predictive analysis, multiple linear regression was carried out to determine the association between the rate of COVID-19 infections and as well as mortalities with regards to prevalence of VDD and mean VD level, combined with other confounding factors - median age of the population, prevalence of obesity and prevalence of DM. All the outcomes were visually represented as scatter diagrams and evaluated by drawing a regression line in Microsoft Excel (version 2013; Microsoft Corp.). The countries in the scatter diagrams were denoted by a 3-letter country code based on the ISO (International Organization for Standardization) 3166, according to the Terminology Bulletin Country Names and the Country and Region Codes for Statistical Use maintained by the United Nations Statistics Divisions [27].
3. Results
A total of 24 countries, satisfying the inclusion/exclusion criteria were selected for the analysis (Table 1 ) [[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51]]. Study population size varied extensively, from 107 (Japan) to 142,131 (Lebanon). All studies have both men and women participants from the age group of over 18 years with some limited to adult group (<65 years) but most studies included both adults and older adults. VDD was reported in all countries and mean VD level was only reported in 15 countries. Among three quarter of the countries, more than 50% of the adult population were VD deficient. The lowest VDD was reported in Vietnam with only 2.0% and the highest was reported in Oman at 87.5%. As expected, an inverse relationship was observed between the prevalence of VDD and mean VD levels and the countries, Oman and Vietnam had the lowest (32.5 nmol/l) and the highest (83.8 nmol/l) mean VD levels, respectively.
Table 1.
The prevalence of vitamin D deficiency and mean vitamin D levels among 24 Asian countries.
| Country | Author, Year | Sample size (M/F) | Age (years) | Prevalence of VDD (%) | Mean VD level (nmol/l) ± SD |
|---|---|---|---|---|---|
| Bahrain [6] | Almesri et al., 2020 | 314 (164/150) | >30 | 79.9 | NR |
| Bangladesh [7] | Acherjya et al., 2019 | 160 (69/91) | ≤70 | 63.7 | 46.5 ± 16.5 |
| Brunei [8] | Leong et al., 2016 | 408 (77/331) | ≥18 | 60.5 | 50.4 ± 22.3 |
| China [9] | Jiang et al., 2019 | 14302 (3002/11299) | 18–65 | 50.3 | 55.8 ± 50.8 |
| India [10] | Mechenro et al., 2018 | 424 (179/245) | ≥18 | 55.2 | 51.3 ± 23.3 |
| Iran [11] | Esmaeili et al., 2018 | 6089 (2837/3252) | 19–65 | 70.3 | NR |
| Iraq [12] | Al-Hilali, 2016 | 300 (120/180) | 25–70 | 72.9 | 38.8 ± NR |
| Japan [13] | Asakura et al., 2020 | 107 (53/54) | 20–69 | 28.2 | 44.8 ± NR |
| Jordan [14] | Khasawneh et al., 2018 | 3007 (710/2297) | 15–83 | 67.9 | NR |
| Kazakhstan [15] | Gromova et al., 2019 | 1347 (528/819) | ≥18 | 70.0 | 37.5 ± (NR) |
| Kuwait [16] | Zhang et al., 2016 | 960 (436/524) | ≥20 | 83.0 | 34.5a |
| Lebanon [17] | Saad et al., 2020 | 142131 (46099/96032) | >18 | 35.5 | 69.0 ± 45.0 |
| Malaysia [18] | Shafinaz and Moy, 2016 | 858 (77/781) | ≥18 | 67.4 | 45.0 ± 18.3 |
| Mongolia [19] | Bromage et al., 2016 | 320 (160/160) | 20–58 | 35.5 | 37.8 ± 13.8 |
| Nepal [20] | Sherchand et al., 2018 | 300 (109/191) | ≥18 | 51.3 | 47.5a |
| Oman [21] | Abiaka et al., 2012 | 206 (101/105) | 18–55 | 87.5 | 32.8 ± 15.4 |
| Pakistan [22] | Kandhro et al., 2019 | 1244(519/725) | ≤84 | 51.5 | NR |
| Qatar [23] | Zainel et al., 2019 | 102339 (34946/67393) | 18–65 | 71.4 | NR |
| Saudi Arabia [24] | Altowijri et al., 2018 | 350 (150/200) | ≥20 | 74.6 | NR |
| Singapore [25] | Bi et al., 2016 | 114 (59/55) | ≥21 | 42.0 | 54.0 ± 17.3 |
| Thailand [26] | Rajatanavin et al., 2018 | 120 (56/64) | 25–60 | 19.2 | 66.3 ± 19.2 |
| Turkey [27] | Gotkas et al., 2020 | 11734 (2592/9142) | ≥18 | 65.3 | 41.5 ± 28.8 |
| United Arab Emirates [28] | Al Zarooni et al., 2019 | 12346 (4561/7785) | ≥18 | 72.0 | NR |
| Vietnam [29] | Ho-Pham et al., 2011 | 637 (205/432) | 18–87 | 2.0 | 83.8 ± 20.1 |
Median values; F-Female; M-Male; NR: Not reported; VD: Vitamin D; VDD: Vitamin D deficiency; SD: Standard deviation.
As of December 31st, 2020, with regards to the number of COVID-19 infections per 1 M of the total population, Vietnam reported the lowest with 15/1 M while Bahrain had the highest with 53,679/1 M infections (Table 2 ). In the case of COVID-19 mortalities per 1 M population, Vietnam documented the lowest number of 0.4 deaths/1 M whereas the highest number of 655 deaths/1 M occurred in Iran. Particularly, a distinctive pattern related to COVID-19 data was observed among the countries included in the analysis. All the Western Asian countries (e.g Qatar, Lebanon and Kuwait) reported higher values for both COVID-19 infections and mortalities compared to South-Eastern Asian countries (e.g Vietnam, Singapore and Brunei). Median age of the population lies mostly in the thirties across more than half of the countries, with Japan as the outlier with a median age of 48.6 years. The prevalence of DM across the 24 countries were of close proximity, and a predominant of the countries reported a prevalence close to 10%. In contrast, obesity had a wider prevalence range with the highest value of 35% in Bahrain and the lowest of 2% in Vietnam.
Table 2.
COVID-19 infection and mortality rates and other confounding variables among 24 Asian countries.
| Country | COVID-19 infections/1 M populationa | COVID-19 mortalities/1 M populationa | Median age of population | Prevalence of DM (%) | Prevalence of obesity (%) |
|---|---|---|---|---|---|
| Bahrain | 53679 | 203 | 32.9 | 9 | 29 |
| Bangladesh | 3108 | 46 | 27.9 | 8 | 3 |
| Brunei | 357 | 7 | 31.1 | 9 | 15 |
| China | 60 | 3 | 38.4 | 9 | 7 |
| India | 7429 | 108 | 28.7 | 8 | 4 |
| Iran | 14567 | 655 | 31.7 | 10 | 26 |
| Iraq | 14658 | 315 | 21.2 | 13 | 27 |
| Japan | 1856 | 27 | 48.6 | 10 | 4 |
| Jordan | 28843 | 376 | 23.5 | 13 | 33 |
| Kazakhstan | 8230 | 120 | 31.6 | 12 | 21 |
| Kuwait | 35066 | 218 | 29.7 | 15 | 37 |
| Lebanon | 27003 | 217 | 33.7 | 13 | 31 |
| Malaysia | 3533 | 15 | 29.2 | 10 | 15 |
| Mongolia | 369 | 0.3 | 29.8 | 10 | 20 |
| Nepal | 8878 | 63 | 25.3 | 9 | 4 |
| Oman | 24919 | 290 | 26.2 | 8 | 23 |
| Pakistan | 2162 | 46 | 22.0 | 10 | 8 |
| Qatar | 51301 | 87 | 33.7 | 13 | 34 |
| Saudi Arabia | 10342 | 178 | 30.8 | 14 | 35 |
| Singapore | 9982 | 5 | 35.6 | 9 | 7 |
| Thailand | 102 | 0.9 | 39.0 | 10 | 11 |
| Turkey | 26,190 | 249 | 32.2 | 13 | 32 |
| UAE | 21,072 | 67 | 38.4 | 8 | 30 |
| Vietnam | 15 | 0.4 | 31.9 | 5 | 2 |
As of December 31st, 2020; DM: Diabetes mellitus; M: Million.
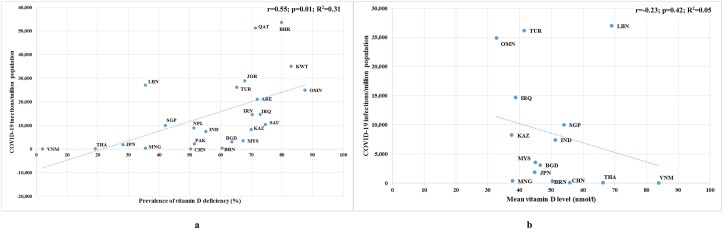
The number of COVID-19 infections per 1 M population displayed a significant positive correlation (r = 0.55; p = 0.01) with prevalence of VDD and an insignificant negative correlation (r = −0.23; p = 0.42) with mean VD levels (Fig. 1 ). Moreover, our comparison suggested that the variation in the total infections can be attributed to the prevalence of VDD (R2 = 0.31) and the mean VD level (R2 = 0.05) of the total country population at the proportions of 33% and 5%, respectively. With regards to prevalence of VDD, the regression line generated from the correlation analysis showed an upward trend, and as expected, most countries were scattered around the line, with a few countries as outliers (Lebanon, Qatar and Bahrain). Moreover, the correlation effect improved to r = 0.66, after removing the outliers (Lebanon, Qatar and Bahrain) from the analysis (p = 0.001; R2 = 0.44). As expected, the regression line generated from the mean VD level analysis against COVID-19 infections, exhibited a downward trend, with only a few countries distributed across the line. However, the association increased to r = −0.44, upon the elimination of outliers such as Oman, Turkey and Lebanon from the analysis (p = 0.17; R2 = 0.20).
Fig. 1.
Scatter diagrams of a) prevalence of vitamin D deficiency b) mean vitamin D levels, against COVID-19 infections as of December 31st, 2020.
ARE: United Arab Emirates; BGD: Bangladesh; BHR: Bahrain; BRN: Brunei; CHN: China; IND: India; IRN: Iran; IRQ: Iraq; JOR: Jordan; JPN: Japan; KAZ: Kazakazhtan; KWT: Kuwait; LBN: Lebanon; MNG: Mongolia; MYS: Malaysia; NPL: Nepal; OMN: Oman; QAT: Qatar; PAK: Pakistan; SAU: Saudi Arabia; SGP: Singapore; THA: Thailand; TUR: Turkey; VNM: Vietnam.
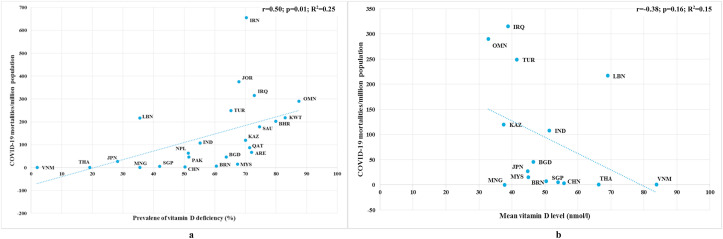
Similarly, COVID-19 mortalities per 1 M population also showed a significant positive correlation (r = 0.50; p = 0.01) with the prevalence of VDD and an insignificant negative correlation (r = −0.38; p = 0.16) with mean VD level, as depicted in Fig. 2 . Moreover, the regression lines resulted from the scatter diagrams revealed that prevalence of VDD and mean VD level were attributed to 25% (R2 = 0.25) and 15% (R2 = 0.15) variability among COVID-19 mortalities, respectively. With respect to the prevalence of VDD, almost all of the countries were found to be spread along the ascending regression line whereas Iran was the single outlier. Furthermore, the association reached the value of r = 0.58, when Iran was removed from the analysis (p = 0.004; R2 = 0.33). In the case of mean VD levels, only some of the countries were seemed to lie across the descending regression line. Countries such as Lebanon, Oman, Iraq and Turkey were positioned far away from the regression line and the correlation changed to r = −0.41, when they were omitted in the analysis (p = 0.22; R2 = 0.17).
Fig. 2.
Scatter diagrams of a) prevalence of vitamin D deficiency b) mean vitamin D levels against COVID-19 mortalities as of December 31st, 2020.
ARE: United Arab Emirates; BGD: Bangladesh; BHR: Bahrain; BRN: Brunei; CHN: China; IND: India; IRN: Iran; IRQ: Iraq; JOR: Jordan; JPN: Japan; KAZ: Kazakazhtan; KWT: Kuwait; LBN: Lebanon; MNG: Mongolia; MYS: Malaysia; NPL: Nepal; OMN: Oman; QAT: Qatar; PAK: Pakistan; SAU: Saudi Arabia; SGP: Singapore; THA: Thailand; TUR: Turkey; VNM: Vietnam.
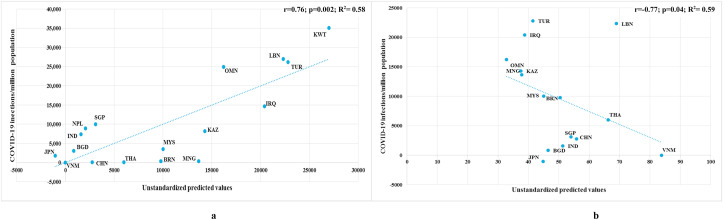
As shown in Table 3 , when the other confounders -median age of the population, prevalence of obesity and prevalence of DM were included along with the prevalence of VDD and mean VD levels in the multiple regression analysis, the respective associations improved gradually. With regards to COVID-19 infection rate, when the model was predicted with VDD prevalence and median age of the population, the correlation increased from r = 0.55 (p = 0.01; R2 = 0.31) to r = 0.58 (p = 0.01; R2 = 0.33) and jumped to r = 0.75 (p = 0.001; R2 = 0.56) with the addition of obesity prevalence. Finally the correlation reached to 0.76 (p = 0.002; R2 = 0.58) upon the inclusion of DM prevalence to the predicted model (Fig. 3 a). On the other hand, when the model was projected with mean VD level and median age of the population, the association with COVID-19 infections per 1 M population increased from r = −0.23 (p = 0.42; R2 = 0.05) to r = -0.34 (p = 0.48; R2 = 0.12) and then improved to r = -0.76 (p = 0.02; R2 = 0.58) when obesity prevalence was added together. Lastly, when the model was constructed based on all confounders by including the prevalence of DM, a strong correlation of r = -0.77 (p = 0.04; R2 = 0.59) was observed (Fig. 3b).
Table 3.
The summary of multiple regression analysis based on all independent variables.
| Model summary | COVID-19 infection ratea |
COVID-19 mortality ratea |
||||
|---|---|---|---|---|---|---|
| Correlation (r) | P value | Variance (R2) | Correlation (r) | P value | Variance (R2) | |
| VDD | 0.55 | 0.01 | 0.31 | 0.50 | 0.01 | 0.25 |
| VDD + MA | 0.58 | 0.01 | 0.33 | 0.52 | 0.04 | 0.27 |
| VDD + MA + OB | 0.75 | 0.001 | 0.56 | 0.64 | 0.01 | 0.40 |
| VDD + MA + OB + DM | 0.76 | 0.002 | 0.58 | 0.65 | 0.03 | 0.42 |
| MVD | −0.23 | 0.42 | 0.05 | −0.38 | 0.16 | 0.15 |
| MVD + MA | −0.34 | 0.48 | 0.12 | −0.56 | 0.10 | 0.31 |
| MVD + MA + OB | −0.76 | 0.02 | 0.58 | −0.79 | 0.01 | 0.62 |
| MVD + MA + OB + DM | −0.77 | 0.04 | 0.59 | −0.80 | 0.03 | 0.63 |
As of December 31st, 2020; VDD: Vitamin D deficiency; MA: Median age of the population; MVD: Mean vitamin D level; OB: Prevalence of obesity; DM: Prevalence of diabetes mellitus.
Fig. 3.
Scatter diagrams based on all confounders with a) prevalence of vitamin D deficiency b) mean vitamin D levels, against COVID-19 infections as of December 31st, 2020.
ARE: United Arab Emirates; BGD: Bangladesh; BHR: Bahrain; BRN: Brunei; CHN: China; IND: India; IRN: Iran; IRQ: Iraq; JOR: Jordan; JPN: Japan; KAZ: Kazakazhtan; KWT: Kuwait; LBN: Lebanon; MNG: Mongolia; MYS: Malaysia; NPL: Nepal; OMN: Oman; QAT: Qatar; PAK: Pakistan; SAU: Saudi Arabia; SGP: Singapore; THA: Thailand; TUR: Turkey; VNM: Vietnam.
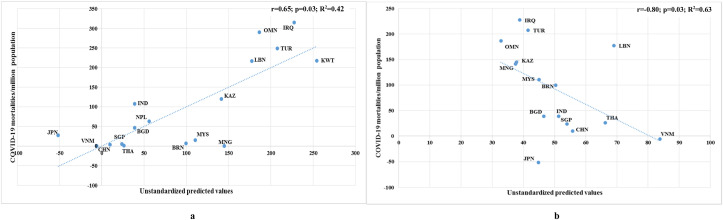
With respect to COVID-19 mortality rate, as depicted in Table 3, upon the addition of median age of the population to the predictive model, the correlation improved from r = 0.50 (p = 0.01; R2 = 0.25) to r = 0.52 (p = 0.04; R2 = 0.27) for the VDD prevalence and from r = −0.38 (p = 0.16; R2 = 0.15) to r = −0.56 (p = 0.10; R2 = 0.31) for the mean VD level. Later, when the obesity prevalence was included in the above model, the association reached to r = 0.64 (p = 0.01; R2 = 0.40) and r = −0.79 (p = 0.01; R2 = 0.62) for VDD prevalence and mean VD level respectively. Finally, the correlation of VDD prevalence reached the level of r = 0.65 (p = 0.03; R2 = 0.42) when the model was predicted with all the confounders including DM prevalence (Fig. 4 a) and with respect to mean VD level and all the other cofounders, a correlation of r = −0.80 (p = 0.03; R2 = 0.63) was achieved (Fig. 4b).
Fig. 4.
Scatter diagrams based on all confounders with a) prevalence of vitamin D deficiency b) mean vitamin D levels, against COVID-19 mortalities as of December 31st, 2020.
ARE: United Arab Emirates; BGD: Bangladesh; BHR: Bahrain; BRN: Brunei; CHN: China; IND: India; IRN: Iran; IRQ: Iraq; JOR: Jordan; JPN: Japan; KAZ: Kazakazhtan; KWT: Kuwait; LBN: Lebanon; MNG: Mongolia; MYS: Malaysia; NPL: Nepal; OMN: Oman; QAT: Qatar; PAK: Pakistan; SAU: Saudi Arabia; SGP: Singapore; THA: Thailand; TUR: Turkey; VNM: Vietnam.
4. Discussion
To the best of our knowledge this is the first analysis of VDD prevalence and mean VD level with COVID-19 infections and mortalities in Asian countries. The strength of this study is that other main confounders also have been included in a predictive analysis and as expected, it improved the correlation substantially.
Historically, discovery of vitamins as leading to major deficiency began in the 19th century with vitamin A as one of the earliest causing xerophthalmia [52]. Although VDD was recognized as rickets/osteomalacia in the early 1600s, it was only a century ago that VD as a nutritional factor was discovered [53]. Human body’s best indicator of VD status is the concentration of serum 25(OH)D and is known to be obtained by sun exposure or dietary sources [54]. Many countries either have a low supply of foods rich in VD and or inadequate exposure to sunlight but supplement use, age, geographic latitude, cultural and lifestyle factors increase or reduce risk of being VD deficient [55].
Similar to previous studies, VDD is distinctively associated with COVID-19 infections and mortalities. Gulf region countries receives endless supply of sunlight except, surprisingly continues with alarmingly high prevalence of VDD as the cultural and social habits limit exposure to sunlight [56]. Vietnam had a VDD prevalence of 2.0% which was comparatively lower, nevertheless showed slight variability between men and women as attitude differences towards clothing coverage of skin and exposure to sunlight explain the variations [51]. According to our correlation analysis of COVID-19 infections and mortalities with VDD prevalence, a significant positive correlation was seen. Individual Asian countries have reported association between VDD and COVID-19 infection and mortality, a study carried out in Chinese people revealed that 25(OH)D concentration on admission were significantly lower in COVID-19 patients than in controls (3.32 ± 0.04 vs. 3.46 ± 0.02 nmol/l) [57].
Once the confounding factors such as median age of the population, prevalence of obesity and prevalence of DM were included in the analysis, the total COVID-19 infections and mortalities increased gradually. Therefore, the addition of confounding factors as mentioned are additional risk factors with VDD for COVID-19 outcome. Obesity as a potential risk factor was investigated, obesity prevalence with the total number of COVID-19 infections and mortalities among selected countries observed a significant positive correlation (r = 0.46; p < 0.001) [7]. A review on DM as a comorbidity reported a 14.3% prevalence with confirmed COVID-19 patients and showed a greater risk of severe illness and in hospital mortality among pre-existing diabetic patients [58]. As immunity often decreases with aging, the older adults would be considered as high risk patients for developing COVID-19. Disruption of the immune system, both innate and adaptive arms is a recognized feature of aging which makes them more susceptible to adverse outcomes of the virus infection [59]. Therefore, median age of a country is a key decisive factor to predict both infection and mortality rate.
The occurrences of COVID-19 varied uneven across Asian countries as it may also be determined by predictors such as government action for COVID-19 containment and the degree of preparedness. A country level analysis showed government policy of lockdowns strongly associated with recovery rates and similarly the number of days to any border closure was associated with the number of cases per million [60]. Furthermore, COVID-19 data on infections and mortalities were extracted only up to December 31st, 2020, as the vaccination gradually reduced the rate of infections and mortalities in vaccinated individuals [61].
Most of the studies included were not of national level surveys although a representative sample of each country population recently studying VD were carefully selected for the analysis for both VDD prevalence and mean VD level. Expectedly, an inverse relationship was observed with VDD prevalence and mean VD levels across the Asian countries. A study outlining mean VD levels in different states of the Indian population, interestingly resulted in an inverse correlation between the mean VD and infection rate (r = −0.43; p = 0.02) and mortality rate (r = −0.42; p = 0.02) [62]. Additionally, another study analysed the correlation of mean VD and the number of infections and deaths per 1 M in the Asian Pacific region and showed an inverse correlation (r = −0.39; p = 0.016) [63].
Mean VD levels alone with COVID-19 infections and mortalities showed no significant correlation as this was due to the less number of studies that reported mean VD levels. When other confounders were added to mean VD levels, significant correlations were observed with the COVID-19 infections and mortalities. Growing body of evidence connects obesity with COVID-19 as mean VD levels alone are not important but with adiposity the circulatory mean VD levels may change as low mean VD is one of the metabolic disturbances that is associated with excess adiposity in particular the visceral adiposity [64].
Results of this study showcased that VDD and mean VD levels of the Asian countries have an association towards the incidence and mortality of the novel coronavirus disease. People who are deficient in VD may receive daily or weekly supplementation as it is studied to protect against acute respiratory tract infections [20]. Thus, the population at higher risk of VDD during this global pandemic should consider taking VD supplements to maintain the circulating 25(OH)D in optimal levels (75–125 nmol/l) [19]. On that account it shows that VD is a possible protective effect in reducing the infectivity and mortality of COVID-19.
4.1. Limitations
VDD and mean VD values included from the studies that report one particular value, which may affect the accuracy of the populations’ VD status as most countries have seasonal changes that may differ the mean VD levels measured. Age group ranged from young adults to older adults covering a wider pool but high risk of COVID-19 is seen in older adults. Consequently, studying only older adults would have been better. Obesity and DM prevalence data were extracted from WHO databases which is accurate but may be out-dated. Ideally prevalence data for each country would be more precise if searched for each country individually. Obesity cut off values may differ from this database (BMI<30 kgm2) when focused on Asia as the cut off value would be lower due to the different body composition [65].
5. Conclusion
Strong significant positive correlation was observed for prevalence of VDD with COVID-19 infections (r = 0.55; p = 0.01; R2 = 0.31) and mortalities (r = 0.50; p = 0.01; R2 = 0.25). Moreover, once confounding factors such as median age of population, prevalence of obesity and prevalence of DM were included in the multiple regression analysis with VDD prevalence, the associations for the number of COVID-19 infections and mortalities improved to r = 0.76 (p = 0.002; R2 = 0.58) and r = 0.65 (p = 0.03; R2 = 0.42) respectively. On the other hand, mean VD level alone with COVID-19 infections and mortalities did not result with significant correlation but once predicted with the other confounding factors, high correlations were achieved with the number of COVID-19 infections (r = −0.77; p = 0.04; R2 = 0.59) and mortalities (r = −0.80; p = 0.03; R2 = 0.63).
Authors’ contributions
RJ conceived and designed the study. DTJ, TVF and RJ collected data. DTJ analysed the data. DTJ, TVF, and RJ drafted the manuscript. AM revised the paper. All authors read and approved the final manuscript.
Funding
Not relevant for this study.
Consent for publication
Not relevant for this study.
Ethics approval and consent to participate
Not relevant for this study.
Declaration of competing interest
The authors declare that there are no conflicts of interest regarding the publication of this paper.
Acknowledgements
There are no further acknowledgements.
References
- 1.Worldometer COVID-19 Coronovirus Pandemic. https://www.worldometers.info/coronavirus/ Available online:
- 2.Weston S., Frieman M.B. COVID-19: knowns, unknowns, and questions. mSphere. 2020;5(2):e00203–e00220. doi: 10.1128/mSphere.00203-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu L., Wang B., Yuan T., Chen X., Ao Y., Fitzpatrick T. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: a systematic review and meta-analysis. J Infect. 2020;80:656–665. doi: 10.1016/j.jinf.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaswa R., Govender I. Novel coronavirus pandemic: a clinical overview. S Afr Fam Pract. 2020;62(1):e1–5. doi: 10.4102/safp.v62i1.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang J., Zheng Y., Gou X., Pu K., Chen Z., Guo Q. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erener S. Diabetes, infection risk and COVID-19. Mol Metab. 2020;39:101044. doi: 10.1016/j.molmet.2020.101044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jayawardena R., Jeyakumar D.T., Misra A., Hills A.P., Ranasinghe P. Obesity: a potential risk factor for infection and mortality in the current COVID-19 epidemic. Diabetes & Metabolic Syndrome: Clin Res Rev. 2020;14:2199–2203. doi: 10.1016/j.dsx.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Worldometer World population. https://www.worldometers.info/world-population/ accessed on, Available online:
- 9.Varkey R.S., Joy J., Sarmah G., Panda P.K. Socioeconomic determinants of COVID-19 in Asian countries: an empirical analysis. J Publ Aff. 2020 doi: 10.1002/pa.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Y.-C., Kuo R.-L., Shih S.-R. COVID-19: the first documented coronavirus pandemic in history. Biomed J. 2020;43:328–333. doi: 10.1016/j.bj.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin Microbiol Infect. 2020;26:729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Townsend M.J., Kyle T.K., Stanford F.C. Outcomes of COVID-19: disparities in obesity and by ethnicity/race. Int J Obes. 2020;44:1807–1809. doi: 10.1038/s41366-020-0635-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sze S., Pan D., Nevill C.R., Gray L.J., Martin C.A., Nazareth J. Ethnicity and clinical outcomes in COVID-19: a systematic Review and Meta-analysis. EClinicalMedicine. 2020;29:100630. doi: 10.1016/j.eclinm.2020.100630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yoon K.H., Lee J.H., Kim J.W., Cho J.H., Choi Y.H., Ko S.H. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 15.Babu G.R., Khetrapal S., John D.A., Deepa R., Narayan K.V. Pandemic preparedness and response to COVID-19 in South Asian countries. Int J Infect Dis. 2020;104:169–174. doi: 10.1016/j.ijid.2020.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lim W.S., Liang C.K., Assantachai P., Auyeung T.W., Kang L., Lee W.J. COVID-19 and older people in Asia: Asian working group for sarcopenia calls to action. Geriatr Gerontol Int. 2020;20:547–558. doi: 10.1111/ggi.13939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma C., Zhou L., Xu W., Ma S., Wang Y. Associations of physical activity and screen time with suboptimal health status and sleep quality among Chinese college freshmen: a cross-sectional study. PloS One. 2020;15 doi: 10.1371/journal.pone.0239429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jayawardena R., Sooriyaarachchi P., Chourdakis M., Jeewandara C., Ranasinghe P. Enhancing immunity in viral infections, with special emphasis on COVID-19: a review. Diabetes & Metabolic Syndrome: Clin Res Rev. 2020;14:367–382. doi: 10.1016/j.dsx.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13(10):1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12:988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercola J., Grant W.B., Wagner C.L. Evidence regarding vitamin D and risk of COVID-19 and its severity. Nutrients. 2020;12:3361. doi: 10.3390/nu12113361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weir E.K., Thenappan T., Bhargava M., Chen Y. Does vitamin D deficiency increase the severity of COVID-19? Clin Med. 2020;20:e107. doi: 10.7861/clinmed.2020-0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worldometer COVID-19 Coronovirus Pandemic. https://www.worldometers.info/coronavirus/ Available online:
- 25.Noncommunicable diseases country profiles, World Health Organization. https://www.who.int/nmh/countries/en/ Available online:
- 26.The World Factbook, Central Intelligence Agency (CIA). Available online: https://www.cia.gov/the-world-factbook/countries/(accessed on 08 Jan 2021).
- 27.ISO 3166 Country Codes, The International Standard for country codes and codes for their subdivisions, International Organization for Standardization. Available online: https://www.iso.org/obp/ui/#search (accessed on 08 Jan 2021).
- 28.Almesri N., Das N.S., Ali M.E., Gumaa K., Giha H.A. Gender-dependent association of vitamin D deficiency with obesity and hypercholesterolemia (LDLC) in adults. Endocr Metab Immune Disord - Drug Targets. 2020;20:425–436. doi: 10.2174/1871530319666191009154528. [DOI] [PubMed] [Google Scholar]
- 29.Acherjya G., Ali M., Tarafder K., Akhter N., Chowdhury M., Islam D. Study of vitamin D deficiency among the apparently healthy population in Jashore, Bangladesh. Mymensingh Med J. 2019;28:214–221. [PubMed] [Google Scholar]
- 30.Leong J.F., Yakob M., Fung E.C., Pande K.C. High prevalence of vitamin D insufficiency and deficiency in a mixed sample of patients in Brunei Darussalam. Brunei Int Med J. 2016;12(4):134–139. [Google Scholar]
- 31.Jiang W., Wu D.B., Xiao G.B., Ding B., Chen E.Q. An epidemiology survey of vitamin D deficiency and its influencing factors. Med Clin. 2020;154:7–12. doi: 10.1016/j.medcli.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 32.Mechenro J., Venugopal G., Kumar M.B., Balakrishnan D., Ramakrishna B.S. Vitamin D status in Kancheepuram District, Tamil Nadu, India. BMC Publ Health. 2018;18:1345. doi: 10.1186/s12889-018-6244-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esmaeili S.A., Mohammadian S., Radbakhsh S., Momtazi-Borojeni A.A., Kheirmand Parizi P., Atabati H. Evaluation of vitamin D3 deficiency: a population-based study in northeastern Iran. J Cell Biochem. 2019;120:10337–10341. doi: 10.1002/jcb.28317. [DOI] [PubMed] [Google Scholar]
- 34.Al-Hilali K. Prevalence of hypovitaminosis D in adult Iraqi people including postmenopausal women. Sci Res J. 2016;4:53–62. [Google Scholar]
- 35.Asakura K., Etoh N., Imamura H., Michikawa T., Nakamura T., Takeda Y., Mori S., Nishiwaki Y. Vitamin D status in Japanese adults: relationship of serum 25-hydroxyvitamin D with simultaneously measured dietary vitamin D intake and ultraviolet ray exposure. Nutrients. 2020;12:743. doi: 10.3390/nu12030743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khasawneh R., Hiari M., Khalaileh M., Khasawneh H., Alzghoul B., Almomani A. Frequency of vitamin D deficiency and insufficiency in a Jordanian cohort: a hospital based study. Journal of the Royal Medical Services. 2018;102:1–4. [Google Scholar]
- 37.Gromova O., Doschanova A., Lokshin V., Tuletova A., Grebennikova G., Daniyarova L. Vitamin D deficiency in Kazakhstan: cross-sectional study. J Steroid Biochem Mol Biol. 2020;199:105565. doi: 10.1016/j.jsbmb.2019.105565. [DOI] [PubMed] [Google Scholar]
- 38.Zhang F.F., Al Hooti S., Al Zenki S., Alomirah H., Jamil K.M., Rao A. Vitamin D deficiency is associated with high prevalence of diabetes in Kuwaiti adults: results from a national survey. BMC Publ Health. 2016;16:100. doi: 10.1186/s12889-016-2758-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saad R.K., Akiki V.C., Rahme M., Ajjour S., Assaad M., Fuleihan G.A.E.H. Time trends and predictors of hypovitaminosis D across the life course: 2009–2016. Metabolism. 2020;105:154138. doi: 10.1016/j.metabol.2020.154138. [DOI] [PubMed] [Google Scholar]
- 40.Shafinaz I., Moy F. Vitamin D level and its association with adiposity among multi-ethnic adults in Kuala Lumpur, Malaysia: a cross sectional study. BMC Publ Health. 2016;16:232. doi: 10.1186/s12889-016-2924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bromage S., Rich-Edwards J.W., Tselmen D., Baylin A., Houghton L.A., Baasanjav N., Ganmaa D. Seasonal epidemiology of serum 25-hydroxyvitamin D concentrations among healthy adults living in rural and urban areas in Mongolia. Nutrients. 2016;8:592. doi: 10.3390/nu8100592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sherchand O., Sapkota N., Chaudhari R.K., Khan S.A., Baranwal J.K., Pokhrel T. Association between vitamin D deficiency and depression in Nepalese population. Psychiatr Res. 2018;267:266–271. doi: 10.1016/j.psychres.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 43.Abiaka C., Delghandi M., Kaur M., Al-Saleh M. Vitamin D status and anthropometric indices of an Omani study population. Sultan Qaboos Univ Med J. 2013;13:224. doi: 10.12816/0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kandhro F., Dahot M.U., Ahmed Naqvi S.H., Ujjan I.U. Study of Vitamin D deficiency and contributing factors in the population of Hyderabad, Pakistan. Pak J Pharm Sci. 2019;32(3):1063–1068. [PubMed] [Google Scholar]
- 45.Zainel A.J.A.L., Qotba H., Al Nuaimi A., Syed M. Vitamin D status among adults (18–65 years old) attending primary healthcare centres in Qatar: a cross-sectional analysis of the Electronic Medical Records for the year 2017. BMJ open. 2019;9 doi: 10.1136/bmjopen-2019-029334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Altowijri A., Alloubani A., Abdulhafiz I., Saleh A. Impact of nutritional and environmental factors on vitamin D deficiency. Asian Pac J Cancer Prev APJCP. 2018;19:2569. doi: 10.22034/APJCP.2018.19.9.2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bi X., Tey S.L., Leong C., Quek R., Henry C.J. Prevalence of vitamin D deficiency in Singapore: its implications to cardiovascular risk factors. PloS One. 2016;11 doi: 10.1371/journal.pone.0147616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajatanavin N., Kanokrungsee S., Aekplakorn W. Vitamin D status in Thai dermatologists and working-age Thai population. J Dermatol. 2019;46:206–212. doi: 10.1111/1346-8138.14742. [DOI] [PubMed] [Google Scholar]
- 49.Göktaş O., Ersoy C., Ercan I., Can F.E. Vitamin D status in the adult population of Bursa-Turkey. Eur J Gen Pract. 2020;26:156–162. doi: 10.1080/13814788.2020.1846712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al Zarooni A.A.R., Al Marzouqi F.I., Al Darmaki S.H., Prinsloo E.A.M., Nagelkerke N. Prevalence of vitamin D deficiency and associated comorbidities among Abu Dhabi Emirates population. BMC Res Notes. 2019;12:1–6. doi: 10.1186/s13104-019-4536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho-Pham L., Nguyen N.D., Lai T., Eisman J., Nguyen T. Vitamin D status and parathyroid hormone in a urban population in Vietnam. Osteoporos Int. 2011;22:241–248. doi: 10.1007/s00198-010-1207-4. [DOI] [PubMed] [Google Scholar]
- 52.Semba R.D. The discovery of the vitamins. Int J Vitam Nutr Res. 2012;82:310–315. doi: 10.1024/0300-9831/a000124. [DOI] [PubMed] [Google Scholar]
- 53.Jones G. The discovery and synthesis of the nutritional factor vitamin D. Int J Paleopathol. 2018;23:96–99. doi: 10.1016/j.ijpp.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 54.Chang S.W., Lee H.C. Vitamin D and health-The missing vitamin in humans. Pediatr Neonatol. 2019;60:237–244. doi: 10.1016/j.pedneo.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 55.Roth D.E., Abrams S.A., Aloia J., Bergeron G., Bourassa M.W., Brown K.H. Global prevalence and disease burden of vitamin D deficiency: a roadmap for action in low-and middle-income countries. Ann N Y Acad Sci. 2018;1430:44. doi: 10.1111/nyas.13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathur S., Garza D.E., Smith L.F. Endometrial autoantigens eliciting immunoglobulin (Ig) G, IgA, and IgM responses in endometriosis. Fertil Steril. 1990;54:56–63. [PubMed] [Google Scholar]
- 57.Luo X., Liao Q., Shen Y., Li H., Cheng L. Vitamin D deficiency is inversely associated with covid-19 incidence and disease severity in Chinese people. J Nutr. 2021;151:98–103. doi: 10.1093/jn/nxaa332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mantovani A., Byrne C.D., Zheng M.H., Targher G. Diabetes as a risk factor for greater COVID-19 severity and in-hospital death: a meta-analysis of observational studies. Nutr Metabol Cardiovasc Dis. 2020;30:1236–1248. doi: 10.1016/j.numecd.2020.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Perrotta F., Corbi G., Mazzeo G., Boccia M., Aronne L., D’Agnano V. COVID-19 and the elderly: insights into pathogenesis and clinical decision-making. Aging Clin Exp Res. 2020:1–10. doi: 10.1007/s40520-020-01631-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chaudhry R., Dranitsaris G., Mubashir T., Bartoszko J., Riazi S. A country level analysis measuring the impact of government actions, country preparedness and socioeconomic factors on COVID-19 mortality and related health outcomes. EClinicalMedicine. 2020;25:100464. doi: 10.1016/j.eclinm.2020.100464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallapaty S. Vaccines are curbing COVID: data from Israel show drop in infections. Nature. 2021;590(7845):197. doi: 10.1038/d41586-021-00316-4. [DOI] [PubMed] [Google Scholar]
- 62.Padhi S., Suvankar S., Panda V.K., Pati A., Panda A.K. Lower levels of vitamin D are associated with SARS-CoV-2 infection and mortality in the Indian population: an observational study. Int Immunopharm. 2020;88:107001. doi: 10.1016/j.intimp.2020.107001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yadav D., Birdi A., Tomo S., Charan J., Bhardwaj P., Sharma P. Association of vitamin D status with COVID-19 infection and mortality in the Asia Pacific region: a cross-sectional study. Indian J Clin Biochem. 2021:1–6. doi: 10.1007/s12291-020-00950-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gangloff A., Bergeron J., Lemieux I., Després J.-P. Changes in circulating vitamin D levels with loss of adipose tissue. Curr Opin Clin Nutr Metab Care. 2016;19:464–470. doi: 10.1097/MCO.0000000000000315. [DOI] [PubMed] [Google Scholar]
- 65.Ofuji M., Ota Z. Fever, arthralgia, various neurologic symptoms and uremia: systemic lupus erythematosus. Nihon Rinsho. 1975;754–755:1092. [PubMed] [Google Scholar]