Abstract
Background
The incidence of colorectal cancer in North America is rising among patients younger than 50 years. Available data are conflicting regarding presentation and outcomes in this population. This review aimed to synthesize literature regarding young patients with colorectal cancer with respect to patient demographics, disease extent and survival, compared with patients older than 50 years.
Methods
We searched Medline, Embase, the Cochrane Central Register of Controlled Trials and PubMed for articles published between 1990 and the time of search. Articles comparing North American patients with colorectal cancer younger and older than 50 years were eligible for inclusion. We used a random-effects model to pool odds ratios.
Results
Eight retrospective studies were eligible for inclusion (n = 790 959). Mean age was 42.6 years (standard deviation [SD] 5.07) in the younger group, and 69.1 years (SD 9.25) in the older group. Young patients were more likely to present with regional (odds ratio [OR] 1.27, 95% confidence interval [CI] 1.16–1.40) and distant disease (OR 1.47, 95%CI 1.30–1.67). Considering patients at all stages of disease, differences in 5-year overall survival (OR 1.54, 95%CI 0.96–2.47) and cancer-specific survival (OR 1.01, 95%CI 0.91–1.13) were not statistically significant between groups. However, when controlling for disease extent, 5-year cancer-specific survival was significantly higher among young patients with local (OR 1.69, 95%CI 1.43–1.99), regional (OR 1.37, 95%CI 1.16–1.63) and distant disease (OR 1.79, 95%CI 1.45–2.21).
Conclusion
North American patients presenting with colorectal cancer before the age of 50 years are more likely to have advanced disease. Although overall and cancer-specific survival is not significantly different between these groups, younger patients have improved survival when controlling for cancer stage.
Abstract
Contexte
L’incidence du cancer colorectal en Amérique du Nord est en hausse chez les patients de moins de 50 ans. Les données disponibles quant à la présentation et aux issues de la maladie dans cette population sont contradictoires. La présente revue systématique vise à synthétiser les données de la littérature sur les jeunes patients atteints d’un cancer colorectal, entre autres les caractéristiques démographiques des patients, le stade de la maladie et le taux de survie, et à les comparer aux données des patients de plus de 50 ans.
Méthodes
Nous avons interrogé les bases de données Medline, Embase, PubMed et le Cochrane Central Register of Controlled Trials pour repérer les articles publiés entre 1990 et le moment de la recherche. Les études comparants les patients nord-américains atteints d’un cancer colorectal de moins de 50 ans et ceux de plus de 50 ans ont été incluses. Nous avons utilisé un modèle à effets aléatoires pour regrouper les rapports de cotes.
Résultats
Huit études rétrospectives ont été retenues (n = 790 959). L’âge moyen était de 42,6 ans (écart type [É. T.] 5,07) pour le groupe des moins de 50 ans, et de 69,1 ans (É.-T. 9,25) pour l’autre groupe. Les jeunes patients étaient plus susceptibles de présenter un cancer régional (rapport de cotes [RC] 1,27; intervalle de confiance [IC] à 95 % 1,16–1,40) ou un cancer à distance (RC 1,47; IC à 95 % 1,30–1,67). Si on ne tenait pas compte du stade de la maladie, la différence entre le taux de survie globale à 5 ans (RC 1,54; IC à 95 % 0,96–2,47) et le taux de survie au cancer à 5 ans (RC 1,01; IC à 95 % 0,91–1,13) n’était pas statistiquement significative. Toutefois, si on tenait compte de l’étendue de la maladie, le taux de survie lié au cancer à 5 ans était significativement plus élevé chez les jeunes patients ayant un cancer localisé (RC 1,69; IC à 95 % 1,43–1,99), régional (RC 1,37; IC à 95 % 1,16–1,63) ou à distance (RC 1,79; IC à 95 % 1,45–2,21).
Conclusion
Les patients nord-américains de moins de 50 ans présentant un cancer colorectal sont plus susceptibles d’être à un stade avancé de la maladie. Bien que le taux de survie globale et le taux de survie au cancer ne diffèrent pas de manière significative entre les 2 groupes, les jeunes patients présentaient un meilleur taux de survie lorsqu’on tenait compte du stade de la maladie.
Colorectal cancer remains the second most common cancer and third highest cause of cancer-related death in Canada.1 Screening programs across the country have contributed to earlier diagnoses and a decline in incidence over the past decade.2–4 However, in patients younger than 50 years who are too young to screen, the incidence of colorectal cancer is rising.4,5 Although certain genetic and lifestyle factors have been implicated, strategies to improve identification, surveillance and management of these patients are not yet well understood.6
Younger patients with colorectal cancer more frequently harbour germline mutations.7–9 Concordantly, these patients commonly present with advanced disease, poorly differentiated histology and aggressive pathology at diagnosis.10–14 Screening of younger people is advised in the setting of a positive family history, yet most patients presenting with colorectal cancer before age 50 years have no known relatives affected.9,15 As the most common pattern of presentation among patients younger than 50 years is not well characterized, it may be challenging for physicians to properly identify and investigate young patients presenting with colorectal cancer.16,17
Despite being treated more frequently with surgery and adjuvant therapy, smaller studies suggest that the outcomes in this population are not significantly improved compared with those over the age of 50 years.10,18 Furthermore, treatment of these patients poses additional challenges. Young and older long-term survivors are equally likely to have adverse effects of their therapy that alter quality of life, including bowel, urinary and sexual dysfunction.19 Additionally, the preservation of fertility during chemotherapy and radiotherapy, as well as navigating patients’ roles as primary caregivers, are unique to younger patients.20 These nuances of treating younger patients, particularly those with advanced disease, further emphasize the need for better identification of high-risk patients who warrant both earlier screening and more prompt work-up for concerning symptoms.
The aim of the present study was to synthesize the available literature regarding presentation of colorectal cancer in young adults. We hypothesized that patients presenting before the age of 50 years would be more likely to have advanced disease, poorly differentiated histology and worse overall survival despite more aggressive treatment. Characterizing differences in presentation, pathology and disease course will provide information necessary to better evaluate these trends prospectively, with the goal of guiding future recommendations for the prevention and early diagnosis of colorectal cancer in young adults.
Methods
Search strategy and selection criteria
This systematic review is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (refer to Appendix 1, Table S1, available at canjsurg.ca/013019-a1).21
A search strategy was designed and conducted by a medical research librarian with input from study investigators. Search terms included “colorectal cancer,” “age factors,” “young adult,” “early presentation,” “North American” and more (complete search strategy available in Appendix 1, Figure S1). The references of published studies, as well as grey literature, were searched to ensure that all relevant articles were included. Full texts were not discriminated by language. The following databases were searched: Medline (between Jan. 1, 1990, and Mar. 5, 2019), Embase (between Jan. 1, 1990, and Mar. 5, 2019), Cochrane Central Register of Controlled Trials (between Jan. 1, 2000, and Mar. 5, 2019) and PubMed (between Jan. 1, 1990, and Mar. 5, 2019).
Articles were eligible for inclusion if they were an original work, published in any language in a peer-reviewed journal and compared North American younger patients with colorectal cancer versus patients older than 50years as a primary outcome. We included prospective, retrospective and randomized controlled trials. We limited the population to North American studies to better reflect the environmental and genetic risk factors present in the Canadian population. We excluded relevant studies that did not have both young and old groups, those that used an age cut-off greater than 60 years or less than 40 years and studies that included patients with recurrent colorectal cancer. We excluded unpublished abstracts, posters, opinion pieces, case reports, reviews, meta-analyses, letters to editors and editorials.
Two reviewers (C. G. and T. M.) independently evaluated the systematically searched titles and abstracts using a standardized, pilot-tested form. Discrepancies that occurred at the title and abstract screening stages were resolved by automatic inclusion. Discrepancies at the full-text stage were resolved by consensus between the 2 reviewers, and if disagreement persisted, a third reviewer was consulted.
Data extraction
Two reviewers independently conducted data abstraction using a piloted data collection manual, designed a priori. Discrepancies were discussed by both reviewers and resolved by consulting original articles and contacting study authors, if necessary.
Abstracted data included study characteristics, patient demographics (e.g., author, year of publication, study design, age, sex, ethnicity, smoking status and body mass index), tumour characteristics (e.g., location, size, stage, grade and histology), treatment data (e.g., surgical intervention, extent of nodal harvest and use of adjuvant or neoadjuvant systemic therapy) and survival data (e.g., 5-year overall survival and 5-year cancer-specific survival). We combined studies that evaluated the same population over the same time period.11,12
Outcomes
We included 5-year overall survival and 5-year cancer-specific survival as primary outcomes. We calculated cancer-specific survival for all patients in a given cohort, as well as stratified according to stage of cancer at presentation. Secondary outcomes included demographic features such as sex and ethnicity, as well as tumour characteristics (tumour location, tumour grade and tumour histology), cancer stage and treatment regimens. We stratified by age such that patients were defined as young adults if they presented before the age of 50 years and as old adults if they presented at age 50 years or older. We grouped tumours by location as right-sided and left-sided tumours. Rightsided tumours included ascending and transverse colon primaries, and left-sided tumours included rectal, sigmoid and descending colon primaries. We grouped by cancer stage according to local, regional and distant disease. We defined local disease as stage I and stage II cancers, and defined regional disease as stage III cancers or cancers with nodal involvement. We defined distant disease as stage IV cancers or those with distant organ involvement. We considered tumours to be high grade if they had poorly differentiated or undifferentiated histology (grade III and grade IV). We considered all abdominal surgery aimed at curative resection or diversion to be major abdominal surgery.
Assessment of risk of bias
We assessed risk of bias for each included cohort study using the Newcastle–Ottawa Scale (NOS).22 The NOS uses a star-based system in which a study is scored on the basis of 3 categories: selection of study groups, comparability of study groups and identification and reporting of the outcome of interest. We scored each study to a maximum score of 4, 2 and 3 in the 3 categories, respectively. Two reviewers assessed the studies independently. Discrepancies were discussed among reviewers until consensus was reached.
Statistical analysis
We performed statistical analysis using Microsoft Excel and Cochrane Review Manager 5.3. We calculated interobserver agreement with the Cohen’s kappa coefficient for each step of the screening process, with excellent agreement categorized a priori as a kappa of more than 0.80.23 We included exposures and outcomes in the meta-analysis if at least 3 studies had complete data points.24 We entered dichotomous variables into 2 × 2 tables and compared them using odds ratios (ORs). Given heterogeneity among studies, we pooled ORs using the Mantel-Haenszel statistical method and a random-effects model. We set the threshold for statistical significance a priori at p < 0.05. We derived pooled standard deviations (SDs) according to techniques described by Cohen.25 For studies that did not report SD or interquartile range (IQR), we contacted the authors of the studies for missing data. For outcomes that were unable to be statistically analyzed, we provided a systematic narrative summary of outcomes.
Results
Study characteristics
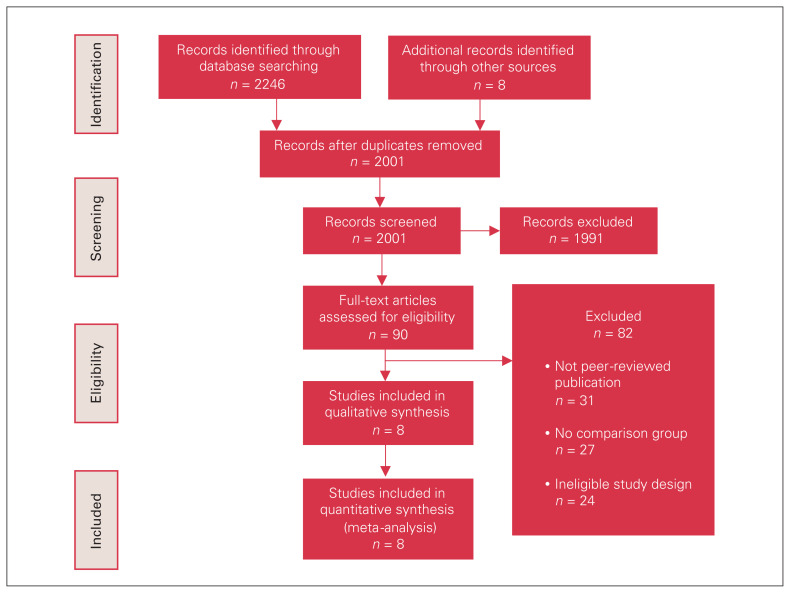
A total of 2254 relevant citations were identified, 8 of which met inclusion criteria.10–14,18,26,27 Study selection showed almost perfect agreement between independent reviewers (Cohen’s kappa 0.82). A PRISMA flow diagram of study selection is illustrated in Figure 1. All included studies were retrospective cohort designs conducted between 2004 and 2018 using population-level databases. The databases included diagnoses of primary colorectal cancer in patients between 1988 and 2013.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) diagram summarizing search strategy.
Seven of the studies used data from the United States, and 1 study used Canadian data. A detailed description of study characteristics from the included studies are featured in Table 1.
Table 1.
Summary of studies in systematic review.
| Author | Study type | Country | Data source | Years of study | Age group, yr | No. of patients | Age, yr, mean ± SD |
|---|---|---|---|---|---|---|---|
| O’Connell et al., 200311 | Retrospective | US | SEER | 1991–1999 | 20–40 | 466 | 34.1 ± 4.5 |
| 60–80 | 11 312 | 70.0 ± 5.5 | |||||
| O’Connell et al., 200412 | Retrospective | US | SEER | 1991–1999 | 20–40 | 1334 | 34.1 ± 4.4 |
| 60–80 | 46 457 | 70.8 ± 5.4 | |||||
| Wang et al., 201013 | Retrospective | US | SEER | 1992–2006 | < 40 | 2642 | — |
| ≥ 40 | 138 769 | — | |||||
| McKay et al., 201427 | Retrospective | Canada | Manitoba Cancer Registry | 2004–2006 | < 45 | 70 | 38.0 ± 4.8 |
| 45–79 | 1459 | 66.0 ± 9.2 | |||||
| ≥ 80 | 557 | 85.0 ± 4.1 | |||||
| Kneuertz et al., 201518 | Retrospective | US | NCDB | 2003–2005 | 18–49 | 13 102 | — |
| 65–75 | 37 007 | — | |||||
| Wang et al., 201514 | Retrospective | US | SEER | 1998–2011 | 20–40 | 6700 | 34.9 ± 4.6 |
| 41–50 | 19 385 | 46.5 ± 2.8 | |||||
| > 50 | 253 538 | 72.0 ± 10.5 | |||||
| Abdelsattar et al., 201610 | Retrospective | US | SEER | 1998–2011 | < 50 | 37 847 | 42.5 ± 6.0 |
| ≥ 50 | 220 177 | 65.3 ± 8.5 | |||||
| Wolbert et al., 201826* | Retrospective | US | Cabell Huntington Hospital Registry | 2003–2016 | < 50 | 24 | 43.1 |
| ≥ 50 | 113 | 64.0 |
NCDB = National Cancer Database Centre; SD = standard deviation; SEER = Surveillance; Epidemiology and End Results; US = United States.
Standard deviations were not available for this study.
Demographics
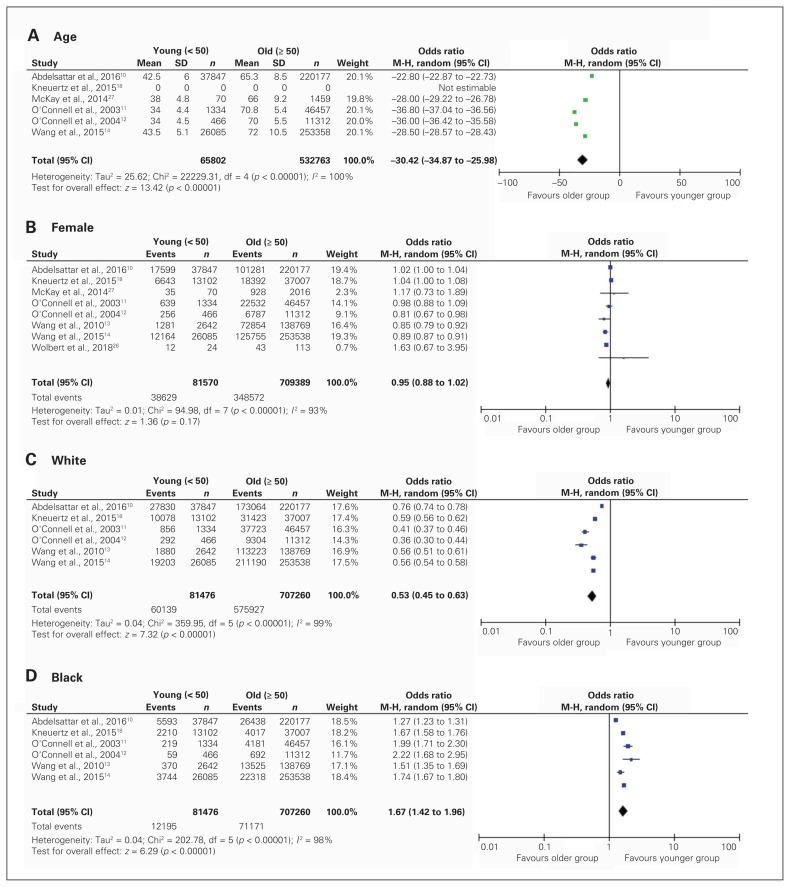
Pooled demographics of patients with colorectal cancer are displayed in Figure 2.
Fig. 2.
Pooled analysis of patient demographics, including age (A), sex (B), White ethnicity (C) and Black ethnicity (D) at time of diagnosis, stratified by age at presentation. Note: The article from Kneuertz and colleagues18 did not have data on the mean age or standard deviation. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel test, SD = standard deviation.
Of the 8 included studies, a total of 81 570 patients were in the young adult group (47.4% female, mean age 42.6 years, SD 5.07) and 709 389 patients were in the older adult group (49.1% female, mean age 69.1 years, SD 9.25). Female sex was balanced between groups (pooled OR 0.95, 95% CI 0.88–1.02). With respect to ethnicity, North American patients who were diagnosed with colorectal cancer before the age of 50 years were less likely to be White, (pooled OR 0.53, 95% CI 0.45–0.63), and more likely to be Black (pooled OR 1.67, 95%CI 1.42–1.96) or of other ethnicity, (pooled OR 1.73, 95%CI 1.48–2.02). Preoperative body mass index was reported in only 1 study, and smoking status was not reported in any studies.
Tumour characteristics
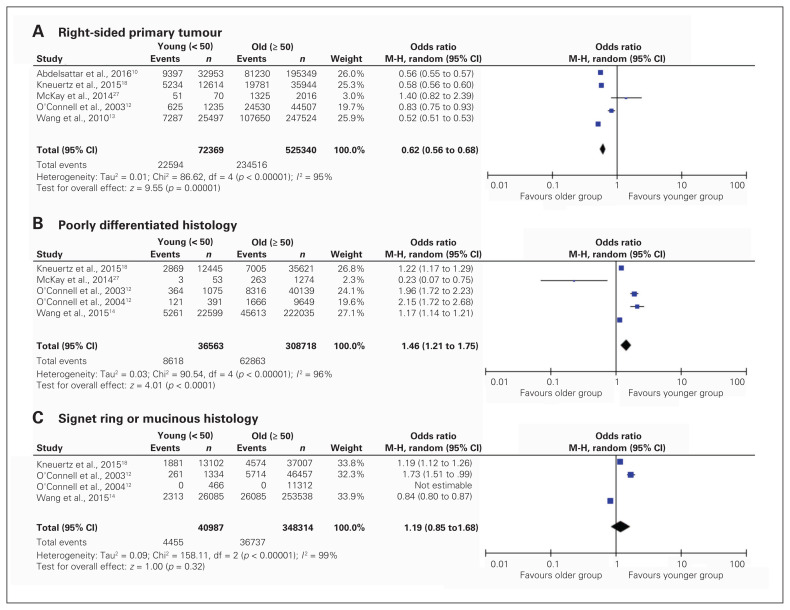
Descriptions of tumour characteristics at disease presentation are displayed in Figure 3. Younger patients were less likely to have right-sided primary tumours (pooled OR 0.62, 95% CI 0.56–0.68). Only 1 study reported median tumour size.14 Younger patients were more likely to present with poorly differentiated pathological features (pooled OR 1.46, 95% CI 1.21–1.75), but both groups were equally as likely to present with signet ring or mucinous histology (pooled OR 1.19, 95% CI 0.85–1.68). Younger patients were more likely to present with regional disease (pooled OR 1.27, 95% CI 1.16–1.40) and distant disease (pooled OR 1.47, 95% CI 1.30–1.67).
Fig. 3.
Pooled analysis of tumour characteristics, including tumour location (A), poorly differentiated histology (B) and signet ring or mucinous histology (C) at time of diagnosis, stratified by age at presentation. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel test.
Patient treatment
Six studies reported rates of procedural (surgical or excisional) intervention; however, there was significant heterogeneity in their reporting that precluded us from including these data in the meta-analysis.10–12,18,26,27 In the 6 included studies, the total population was 369 926 patients, with 52 844 patients younger than 50 and 317 082 patients aged 50 and older. Overall, 331 370 patients (89.7%) underwent resection or excision of their colorectal cancer. In the young adult group, 48 363 patients (91.5%) underwent major abdominal surgery and 3385 patients (6.4%) underwent local excision. In the older adult group, 283 507 patients (89.4%) underwent major abdominal surgery and 18 115 patients (5.7%) underwent local excision. Fewer data were reported pertaining to systemic therapy. Two studies reported rates of neoadjuvant therapy and 3 studies reported rates of adjuvant therapy.10,18,27 Overall, 44 990 patients (16.5%) underwent neoadjuvant therapy and 24 179 patients (46.3%) underwent adjuvant therapy. Neoadjuvant therapy was given to 8911 young adult patients (23.2%) and 36 079 older adult patients (15.5%). Adjuvant therapy was given to 8723 (66.2%) and 15 456 (39.6%) young and old adult patients, respectively. There were no stage-specific treatment data.
Patient outcomes
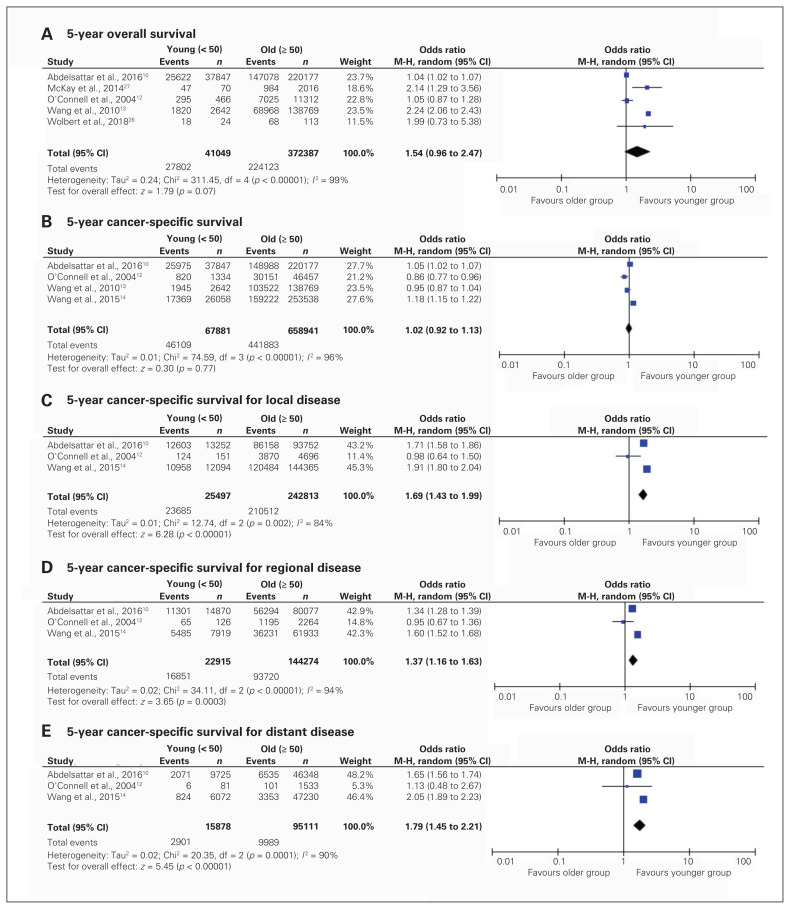
Reported survival data are displayed in Figure 4. Three of the included studies reported 5-year overall survival rate (young adults, n = 358; older adults, n = 703 675). There was no significant difference in 5-year overall survival between the groups (pooled OR 1.54, 95% CI 0.96–2.47). Cancer-specific survival was also similar between age groups at 5 years (pooled OR 1.01, 95% CI 0.91–1.13). When cancer-specific survival was stratified by extent of disease at presentation, younger patients with local (pooled OR 1.69, 95%CI 1.43–1.99), regional (pooled OR 1.37, 95% CI 1.16–1.63) and distant disease (pooled OR 1.79, 95% CI 1.45–2.21) were more likely to be alive at 5 years. Recurrence was reported in only 1 study, revealing higher rates of recurrence among younger patients.26 No included studies reported measures of quality of life.
Fig. 4.
Pooled analysis of patient survival, including 5-year overall survival (A); 5-year cancer-specific survival (B); and 5-year cancer-specific survival for local (C), regional (D) and distant (E) disease, stratified by age at presentation. CI = confidence interval, df = degrees of freedom, M-H = Mantel–Haenszel test.
Risk of bias
The quality assessment of the included studies using the NOS tool is presented in Appendix 1, Table S2. According to the NOS tool, outcome reporting across the included studies was associated with very low risk of bias. All included studies assessed their outcomes with record linkage and had adequate follow-up of cohorts.
There was substantial variability in the reporting of secondary outcomes across studies. Comparability of cohorts across included studies, particularly in terms of treatment data, was poor; 5 (62.5%) of the included studies scored 0 out of a possible 2 according to the NOS. Two (25%) of the included studies had significantly reduced nodal harvest after oncologic operations in the older adult group compared with younger adult group.13,14 Three (37.5%) of the included studies showed that younger adults were significantly more likely to receive neoadjuvant or adjuvant treatment than older adults, independent of the stage of cancer.10,18,27 Comparability of cohorts across studies was limited by different definitions of young and old adult populations. Three (37.5%) studies used 50 years as the cut-off, 1 (12.5%) study used 45 years, 1 (12.5%) used 40 years, 2 (25%) studies used age groups of 20–40 years and 60–80 years, and 1 (12.5%) used age groups of 18–49 years and 65–75 years. Selection of the cohorts was adequate across all of the included studies, as they all relied on long-term, population-level databases.
Discussion
The present review synthesizes the available evidence regarding patients presenting with colorectal cancer before age 50 years, the majority of which comes from large health administrative databases. The findings show that these patients are less commonly White and are more likely to present with poorly differentiated histology and regional or distant disease at diagnosis. Individual studies concluded that younger patients had poorer survival than older patients; however, in aggregate, these data show that when controlling for stage, younger patients have improved overall survival. Our findings suggest that improved overall and cancer-specific survival, when stratified by extent of disease, is because younger patients have a trend toward receiving more treatment than older patients.
Patients presenting with colorectal cancer before the age of 50 years represent a shifting burden on the health care system in North America as the proportion of these patients continues to rise.4,5,28 The present study shows that there is a relative lack of knowledge on how these patients tend to present, as most health administrative databases do not consider presenting complaint at diagnosis, nor commonly cited risk factors such as obesity, smoking, diet and sedentary lifestyles.6,29,30 The results do not allow for novel recommendations on screening guidelines that would uniquely identify younger patients at increased risk beyond family history.
Younger patients presenting with increasingly advanced disease may be reflective of their poorly differentiated histology, delays in diagnosis, or both.31 Increased prevalence of poorly differentiated tumours may be caused by higher rates of germline mutations in this population.8,9,32 However, as delayed diagnosis is likely contributory, patients and primary care providers require more information to guide clinical suspicion of colorectal cancer in patients younger than 50 years presenting with seemingly benign complaints.33 Practically, the finding that younger patients more commonly present with left-sided tumours could encourage physicians to more readily perform flexible sigmoidoscopy in patients below the age of screening with concerning gastrointestinal symptoms.16
Taking into consideration improved stage-specific survival, treatment plans should be individualized in this population to improve quality of life beyond therapy completion. For example, the long-term risks associated with aggressive radiation therapy may be more pronounced in younger patients who have more time to develop secondary malignancies. The treatment of younger patients poses unique challenges not frequently considered in the older adult population, including presentation before the age of retirement, the need to support dependents and infertility.20 Data regarding specific surgical techniques are lacking in this population, suggesting a need to better characterize the use of local resection, minimally invasive techniques, formation of ostomies and the requirement for emergency surgery. Regarding adjuvant therapy, however, trends suggest that younger patients are undergoing more systemic therapy, even in the setting of stage I and II disease.18,34 This may represent an overtreatment in younger patients or undertreatment in older patients. Younger patients may be more able to tolerate aggressive therapy leading to improved relative cancer-specific survival. Although the included studies did not contain specific information about regimen completion or complication rates, younger patients were more likely to receive multi-agent chemotherapy.10,12,18,27 Furthermore, the effects of more aggressive therapy in the available literature suggest that these patients are more likely to have anxiety, poor body image and chronic pain than older patients.19 The effects of different therapies on the ability of younger patients to work and support family is poorly understood.19,35 Furthermore, data are lacking on the financial burden of systemic therapy on young patients, even in a publicly funded, single-payer system, such as Canada. The findings of this systematic review suggest that treatment patterns remain incompletely characterized in younger patients with colorectal cancer, which is particularly relevant given the unique considerations of quality of life that exist in this population.
Limitations
This systematic review of retrospective observational studies has several limitations. There was substantial heterogeneity in reporting of secondary outcomes among the studies that precluded inclusion of secondary outcomes in the meta-analysis. Nonetheless, the main outcomes regarding survival were reported across all studies and were pooled for analysis. Similarly, there was variability in the age groups used to stratify populations within the included studies. Only 3 of the included studies used the exact age 50 years as the demarcation between groups, whereas 1 study used 45 years and 1 study used 40 years. Additionally, most of the included studies used the Surveillance, Epidemiology and End Reporting Database from the United States that may have led to unknown population overlap between studies. Furthermore, there is a lack of detailed reporting in many population-level databases, such that there is limited specific information pertaining to variables such as family history, patient complaint at initial presentation, detection on screening or symptomatic presentation, adjuvant and neoadjuvant treatment and surgical management. As such, treatment data could not be included in the meta-analysis. Finally, these data include patients treated more than 20 years ago and outcomes and treatment strategies may be different in a more modern cohort.
Conclusion
This review represents the largest sample comparing presentation and outcomes of patients presenting with colorectal cancer before and after the age of 50 years. Patients younger than 50 years are more likely to present with more aggressive and advanced disease but have improved stage-specific survival. However, our study shows that information regarding presentation and treatment is lacking. As the incidence of colorectal cancer continues to rise in patients younger than 50 years and countries move to lower the age of screening, this study demonstrates the need for better characterization of those at risk of developing malignant disease.15 Furthermore, as therapy continues to evolve both medically and surgically, there exists an increasing need to include specific variables and outcomes pertaining to the considerations of young patients.
Acknowledgements
The authors thank Jo-Anne Petropoulos, an expert medical librarian, for her assistance with the literature search development.
Footnotes
This work was presented at the 2019 Canadian Surgery Forum. Montreal, Québec.
Competing interests: None declared.
Contributors: C. Griffiths, T. McKechnie, Y. Lee, A. Doumouras, D. Hong, C. Eskicioglu conceived and designed the study. C. Griffiths, T. McKechnie and Y. Lee acquired the data, which all authors analyzed. C. Griffiths, T. McKechnie, Y. Lee, J. Springer and A. Doumouras wrote the manuscript. All authors critically revised the manuscript and gave final approval of the article to be published.
References
- 1.Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2017. Toronto, ON: Canadian Cancer Society; 2017. [Google Scholar]
- 2.Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2018. Toronto, ON: Canadian Cancer Society; 2018. [Google Scholar]
- 3.Austin H, Henley SJ, King J, et al. Changes in colorectal cancer incidence rates in young and older adults in the United States: what does it tell us about screening. Cancer Causes Control. 2014;25:191–201. doi: 10.1007/s10552-013-0321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15–49-year-olds in Canada, 1969–2010. Cancer Epidemiol. 2016;42:90–100. doi: 10.1016/j.canep.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Brenner DR, Ruan Y, Shaw E, et al. Increasing colorectal cancer incidence trends among younger adults in Canada. Prev Med. 2017;105:345–9. doi: 10.1016/j.ypmed.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Arnold M, Sierra MS, Laversanne M, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 7.Khan SA, Morris M, Idrees K, et al. Colorectal cancer in the very young: a comparative study of tumor markers, pathology and survival in early onset and adult onset patients. J Pediatr Surg. 2016;51:1812–7. doi: 10.1016/j.jpedsurg.2016.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Puccini A, Lenz H-J, Marshall JL, et al. Impact of patient age on molecular alterations of left-sided colorectal tumors. Oncologist. 2019;24:319–26. doi: 10.1634/theoncologist.2018-0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stoffel EM, Koeppe E, Everett J, et al. Germline genetic features of young individuals with colorectal cancer. Gastroenterology. 2018;154:897–905. e891. doi: 10.1053/j.gastro.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelsattar ZM, Wong SL, Regenbogen SE, et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer. 2016;122:929–34. doi: 10.1002/cncr.29716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Connell JB, Maggard MA, Liu JH, et al. Rates of colon and rectal cancers are increasing in young adults. Am Surg. 2003;69:866–72. [PubMed] [Google Scholar]
- 12.O’Connell JB, Maggard MA, Liu JH, et al. Do young colon cancer patients have worse outcomes? World J Surg. 2004;28:558–62. doi: 10.1007/s00268-004-7306-7. [DOI] [PubMed] [Google Scholar]
- 13.Wang L, Hollenbeak CS, Stewart DB. Node yield and node involvement in young colon cancer patients: is there a difference in cancer survival based on age? J Gastrointest Surg. 2010;14:1355–61. doi: 10.1007/s11605-010-1275-y. [DOI] [PubMed] [Google Scholar]
- 14.Wang R, Wang M-J, Ping J. Clinicopathological features and survival outcomes of colorectal cancer in young versus elderly: a population-based cohort study of SEER 9 registries data (1988–2011) Medicine (Baltimore) 2015;94:e1402. doi: 10.1097/MD.0000000000001402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schreuders EH, Ruco A, Rabeneck L, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–49. doi: 10.1136/gutjnl-2014-309086. [DOI] [PubMed] [Google Scholar]
- 16.Segev L, Kalady MF, Church JM. Left-sided dominance of early-onset colorectal cancers: a rationale for screening flexible sigmoidoscopy in the young. Dis Colon Rectum. 2018;61:897–902. doi: 10.1097/DCR.0000000000001062. [DOI] [PubMed] [Google Scholar]
- 17.Ambe PC, Jansen S, Zirngibl H. New trend in colorectal cancer in Germany: Are young patients at increased risk for advanced colorectal cancer? World J Surg Oncol. 2017;15:159. doi: 10.1186/s12957-017-1227-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kneuertz PJ, Chang GJ, Hu C-Y, et al. Overtreatment of young adults with colon cancer: more intense treatments with unmatched survival gains. JAMA Surg. 2015;150:402–9. doi: 10.1001/jamasurg.2014.3572. [DOI] [PubMed] [Google Scholar]
- 19.Bailey CE, Tran Cao HS, Hu C-Y, et al. Functional deficits and symptoms of long-term survivors of colorectal cancer treated by multimodality therapy differ by age at diagnosis. J Gastrointest Surg. 2015;19:180–188. doi: 10.1007/s11605-014-2645-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shandley LM, McKenzie LJ. Recent advances in fertility preservation and counseling for reproductive aged women with colorectal cancer: a systematic review. Dis Colon Rectum. 2019;62:762–771. doi: 10.1097/DCR.0000000000001351. [DOI] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess. 2003;7:iii–x. 1–173. doi: 10.3310/hta7270. [DOI] [PubMed] [Google Scholar]
- 23.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–74. [PubMed] [Google Scholar]
- 24.Cochrane handbook for systematic reviews of interventions, version 5.1.0. Vol. 5. The Cochrane Collaboration; 2011. [Google Scholar]
- 25.Cohen J. Statistical power analysis for the behavioral sciences. New York: Academic Press; 1977. [Google Scholar]
- 26.Wolbert T, Leigh EC, Barry R, et al. Later stage disease and earlier onset of rectal cancer: epidemiology and outcomes comparison of rectal cancer in a rural Appalachian area to state and national rates. Am Surg. 2018;84:1229–35. [PubMed] [Google Scholar]
- 27.McKay A, Donaleshen J, Helewa RM, et al. Does young age influence the prognosis of colorectal cancer: a population-based analysis. World J Surg Oncol. 2014;12:370. doi: 10.1186/1477-7819-12-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin. 2018;68:250–81. doi: 10.3322/caac.21457. [DOI] [PubMed] [Google Scholar]
- 29.Joshi AD, Kim A, Lewinger JP, et al. Meat intake, cooking methods, dietary carcinogens, and colorectal cancer risk: findings from the Colorectal Cancer Family Registry. Cancer Med. 2015;4:936–52. doi: 10.1002/cam4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bardou M, Barkun AN, Martel M. Obesity and colorectal cancer. Gut. 2013;62:933–47. doi: 10.1136/gutjnl-2013-304701. [DOI] [PubMed] [Google Scholar]
- 31.Chen FW, Sundaram V, Chew TA, et al. Advanced-stage colorectal cancer in persons younger than 50 Years not associated with longer duration of symptoms or time to diagnosis. Clin Gastroenterol Hepatol. 2017;15:728–737. e723. doi: 10.1016/j.cgh.2016.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mork ME, You YN, Ying J, et al. High prevalence of hereditary cancer syndromes in adolescents and young adults with colorectal cancer. J Clin Oncol. 2015;33:3544–9. doi: 10.1200/JCO.2015.61.4503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hatch QM, Kniery KR, Johnson EK, et al. Screening or symptoms? How do we detect colorectal cancer in an equal access health care system? J Gastrointest Surg. 2016;20:431–8. doi: 10.1007/s11605-015-3042-6. [DOI] [PubMed] [Google Scholar]
- 34.Steele SR, Park GE, Johnson EK, et al. The impact of age on colorectal cancer incidence, treatment, and outcomes in an equal-access health care system. Dis Colon Rectum. 2014;57:303–10. doi: 10.1097/DCR.0b013e3182a586e7. [DOI] [PubMed] [Google Scholar]
- 35.Schneider EC, Malin JL, Kahn KL, et al. Surviving colorectal cancer: patient-reported symptoms 4 years after diagnosis. Cancer. 2007;110:2075–82. doi: 10.1002/cncr.23021. [DOI] [PubMed] [Google Scholar]