Abstract
Background
Repetitive transcranial magnetic stimulation (rTMS) or transcranial direct current stimulation (tDCS) could provide treatment alternatives to stimulant medication for attention-deficit/hyperactivity disorder (ADHD), given some evidence for improvements in cognition and clinical symptoms. However, despite a lack of solid evidence for their use, rTMS and tDCS are already offered clinically and commercially in ADHD. This systematic review and meta-analysis aimed to critically appraise rTMS and tDCS studies in ADHD to inform good research and clinical practice.
Methods
A systematic search (up to February 2019) identified 18 studies (rTMS 4, tDCS 14; 311 children and adults with ADHD) stimulating mainly the dorsolateral prefrontal cortex (dlPFC). We included 12 anodal tDCS studies (232 children and adults with ADHD) in 3 random-effects meta-analyses of cognitive measures of attention, inhibition and processing speed.
Results
The review of rTMS and tDCS showed positive effects in some functions but not others, and little evidence for clinical improvement. The meta-analyses of 1 to 5 sessions of anodal tDCS over mainly the left or bilateral dlPFC showed trend-level improvements in inhibition and processing speed, but not in attention.
Limitations
Heterogeneity in stimulation parameters, patient age and outcome measures limited the interpretation of findings.
Conclusion
The review and meta-analysis showed limited evidence that 1 to 5 sessions of rTMS and tDCS, mostly of the dlPFC, improved clinical or cognitive measures of ADHD. These findings did not support using rTMS or tDCS of the dlPFC as an alternative neurotherapy for ADHD as yet. Larger, multi-session stimulation studies identifying more optimal sites and stimulation parameters in combination with cognitive training could achieve larger effects.
Introduction
Attention-deficit/hyperactivity disorder (ADHD) is defined by age-inappropriate and impairing symptoms of inattention, hyperactivity and impulsivity.1 It is one of the most common childhood disorders, with a prevalence of approximately 7%; problems persist into adulthood in the majority of cases, and ADHD is associated with poor academic and social outcomes.2
Patients with ADHD have cognitive deficits, most prominently in executive functions, such as motor and interference inhibition, selective and sustained attention, working memory and switching, as well as in timing processes and reward-based decision-making.3,4
Furthermore, meta-analyses of functional MRI (fMRI) studies in ADHD show underactivation in different cognitive-domain-dependent frontostriatal and frontocerebellar systems, such as the right inferior and medial prefrontal and striatal regions during cognitive control;5,6 the right dorsolateral prefrontal cortex (dlPFC) and striatal and parietal regions during attention;6 the bilateral superior prefrontal regions during working memory;7 the inferior frontal, parietal and cerebellar regions during timing processes; 8 and the ventromedial frontostriatal areas during reward-related functions.9
The most effective short-term treatment for ADHD is with psychostimulant medications,10 but they have side effects and limited longer-term efficacy.11 Alternative treatments, including behavioural therapies, cognitive training, neurofeedback or dietary interventions, have shown limited efficacy.12–14
Noninvasive brain stimulation treatments, such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), are promising because they can stimulate key brain dysfunctions that have been established in ADHD over the last 2 decades of fMRI research. 9 They are also relatively safe, with minimal side effects; they are cheaper than long-term drug treatments or fMRI neurofeedback, for example; and, more importantly, they can induce neuroplasticity,15 providing hope for longer-term effects, which drugs do not offer.9
In rTMS, rapid magnetic pulses are delivered to the scalp with a wire coil to generate an electric current in the brain via electromagnetic induction. The induced electrical current can trigger action potentials in a focal cortical region under the coil, and when pulses are administered at a particular frequency, rTMS can modulate neural activity with longer-lasting after-effects. In general, high-frequency rTMS (5 to 20 Hz) promotes cortical excitability, and low-frequency rTMS (1 Hz) inhibits cortical excitability.15 Longer-term clinical improvements with rTMS have been demonstrated in several psychiatric disorders: up to 3 months in obsessive–compulsive disorder when stimulating prefrontal, orbitofrontal and supplementary motor regions;16 4 months in schizophrenia when stimulating temporoparietal regions;17 and 12 months in major depressive disorder when stimulating the dlPFC,18–20 supporting its neuroplastic potential. Relative to tDCS, rTMS has greater specificity in targeting neural regions,21 but it is more expensive because of device costs and extensive user-training requirements. 22–24 The most common adverse effects are transient scalp discomfort underneath the coil as a result of stimulation of the pericranial muscles and peripheral nerves.25,26
In tDCS, a weak direct electric current is passed between 2 electrodes (a positive anode and a negative cathode) placed on the scalp. The current modulates spontaneous discharge rates and therefore neuronal network activity by causing subthreshold polarity-dependent shifts in resting membrane potentials, with net increases (anodal stimulation) or decreases (cathodal stimulation) in the excitability of underlying neurons, leading to respective increases or decreases in cortical function and synaptic strength.27 Although tDCS is a relatively new form of noninvasive brain stimulation, there is evidence that it can enhance cognitive functions in healthy controls,28 with longer-term effects of up to 9 or 12 months.29,30 In psychiatric disorders, positive clinical effects have been observed typically up to 1 month after stimulation (for reviews, see Moffa and colleagues,31 Tortella and colleagues32 and Kekic and colleagues),33 although it is possible that effects are longer-term. However, overall, tDCS effects are often small, especially when administered in single sessions in healthy controls.34,35 Relative to rTMS, tDCS is cheaper, easier to use and produces relatively less discomfort; the most common adverse effects are mild transient tingling, itching and reddening of the skin underneath the electrodes.36
Both rTMS and tDCS potentiate cellular and molecular mechanisms involved in use-dependent local and distant synaptic plasticity (e.g., γ-aminobutyric acid [GABA] and glutamate-mediated long-term potentiation), which may lead to longer-term effects.20 This systematic review and meta-analysis focuses on the clinical and cognitive benefits of rTMS and tDCS, because they are the most investigated noninvasive brain stimulation methods in ADHD. To our knowledge, other methods have not been as well investigated or applied in clinical settings in ADHD (e.g., intermittent or continuous theta burst stimulation, transcranial alternating current stimulation or transcranial random noise stimulation).
There has been an increase over the last decade of 18 rTMS and tDCS studies in ADHD. Studies have used relatively heterogenous stimulation protocols, as well as clinical and cognitive outcome measures, and have reported mixed positive and negative effects on cognition and ADHD symptoms.9,37 Furthermore, knowledge is lacking with respect to optimal stimulation protocols for children with ADHD (such as stimulation frequency, intensity or stimulation site), and there are neuroethical concerns about potential costs to nontargeted functions. 38 Despite these shortcomings, however, noninvasive brain stimulation is already offered in private clinics in several countries and is available commercially and online.39,40
A recent meta-analysis of 10 tDCS studies in ADHD (n = 201) found that predominantly anodal tDCS of the dlPFC led to a significant but small improvement in measures of inhibitory control (Hedges’ g = 0.12, 95% confidence interval [CI] 0.01–0.24), and to a significant but moderate improvement in reaction times in n-back tasks in a smaller meta-analysis of 7 effect sizes derived from 3 studies (Hedges’ g = 0.66, 95% CI 0.17–1.25).41 However, effect size estimates may have been inflated, because 2 studies reporting mostly null effects were not included;42,43 multiple dependent effects were clustered in the meta-analyses, unduly reducing variation between effect sizes and overestimating statistical significance;44,45 and the analysis of inhibitory control measures included noninhibitory measures, such as inattention (e.g., omission errors), processing speed (e.g., reaction times to go trials) and reaction time variability, calling into question the specificity of the positive tDCS effects to the domain of inhibitory control.
We therefore considered it paramount and timely to conduct a systematic review of rTMS and tDCS studies and a meta-analysis of tDCS studies in ADHD that included all available empirical studies and controlled for multiple dependent effects and potential bias; that clustered cognitive effects into clearly separated cognitive domains of inhibitory control, attention and processing speed to elucidate effects of tDCS on specific cognitive domains; that tested the replicability of meta-analysis results with jackknife sensitivity analyses; and that included further sensitivity analyses to reduce heterogeneity caused by studies with designs that deviated from the majority.
This review and meta-analysis aimed to provide a critical appraisal of the most consistent clinical and cognitive effects of rTMS and tDCS in ADHD, scrutinizing the quality of the studies, outlining limitations in the field and discussing neuroethical concerns and future directions, with the ultimate aim of guiding clinical practice.
Methods
Eligibility criteria
The systematic review followed Preferred Reporting Items in Systematic Reviews and Meta-Analyses (PRISMA) guidelines46 (Fig. 1; PRISMA checklist, Appendix 1, available at jpn. ca/190179-a1). Inclusion criteria were as follows: empirical studies with sufficient method details that applied rTMS or tDCS in children and/or adults with ADHD confirmed by either a clinical diagnosis (as defined by DSM/ICD criteria) or by meeting cut-off criteria for ADHD on validated ADHD rating scales or research diagnosis questionnaires (e.g., Conners’ Adult ADHD Rating Scale t score > 6547 or the Kiddie Schedule for Affective Disorders and Schizophrenia48). To avoid language bias, we did not exclude studies published in a language other than English, unless the paper could not be accessed and/or translated by the authors of the article. Relevant outcome measures included clinical measures of ADHD and performance measures on cognitive tasks.
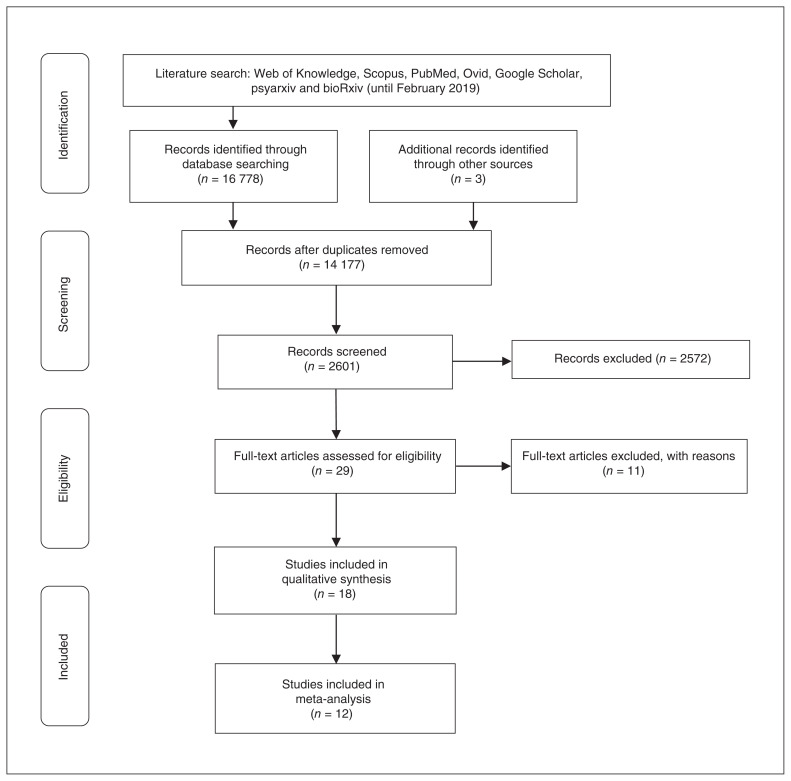
Fig. 1.
Preferred Reporting Items in Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of study selection.
Search strategy
We searched Web of Knowledge, Scopus, PubMed, Ovid, Google Scholar, psyarxiv and bioRxiv (up to the end of February 2019) using the following keywords: “noninvasive brain stimulation,” “transcranial electric stimulation,” “transcranial direct current stimulation,” “tDCS,” “transcranial magnetic stimulation,” “rTMS” or “transcranial electric stimulation,” each in combination with “hyperkinetic disorder,” “attention-deficit/hyperactivity disorder,” “ADHD,” “inattention,” “hyperactivity” or “impulsivity.” We also hand-searched the reference lists of retrieved articles and reviews. One author (S.J.W.) and an additional reviewer (S.W.H.) carried out the search separately; another author (K.R.) crosschecked the results.
Study selection
After removing all duplicates, 1 author (S.J.W.) and an additional reviewer (S.W.H.) independently screened titles and abstracts. The full text of the remaining studies determined final inclusion in accordance with our eligibility criteria. Of the 16 778 studies identified, 14 177 duplicates and 2572 irrelevant papers were excluded after screening titles and abstracts. A full-text review of the remaining 29 studies resulted in the exclusion of a further 11 studies, including 1 rTMS study written in Arabic, because the paper could not be accessed and a translation could not be obtained from the authors49 (Appendix 1, Table S1). This resulted in 18 peer-reviewed, published studies (4 rTMS and 14 tDCS; total ADHD sample = 312), of which 12 anodal tDCS studies (total n = 232) were eligible for meta-analysis (for the list of excluded studies, see Appendix 1, Table S1). The final list of included studies was agreed upon by consensus; any disagreements were resolved by another author (K.R.).
Risk of bias assessment
We assessed risk of bias using the Cochrane Collaboration’s risk of bias tool,50 which rates risk of bias across 5 domains: selection bias, performance bias, detection bias, attrition bias and other biases. Using this tool, S.J.W. and K.R. assessed risk of bias for all sham-controlled studies and resolved any disagreements by consensus. Because the tool is designed for randomized controlled trials, we excluded open-label trials51,52 to avoid undue inflation of bias across domains. We included open-label trials in the systematic review for a complete overview of available empirical studies.
Meta-analysis
Because of the small number of included studies, neither the clinical effects of rTMS and tDCS (n = 2 for each) nor the cognitive effects of rTMS studies (n = 2) and cathodal tDCS (n = 3) could be subjected to meta-analysis. Therefore, we conducted a meta-analysis only on the cognitive effects of anodal tDCS (12 studies) in ADHD. We calculated effect sizes from reported means and standard deviations or t and f values where possible. All data were extracted by S.J.W. We used Plot Digitizer to convert plotted data to numerical values (for an example, see Westwood and Romani35), and we obtained any unreported data by personal communication. All extracted data were cross-checked by K.R.
To reduce large heterogeneity, we clustered cognitive outcome measures into 3 domains and analyzed them in 3 separate meta-analyses. Such clustering of outcome measures into cognitive domains was informed by factor analyses of executive-function measures of ADHD, which typically cluster into factors that comprise measures of attention, inhibition and processing speed.53–56 Accordingly, for attention measures, we included the numbers or percentages of errors or omission errors (or the inversely reported number or percentage of correct trials) and intrasubject reaction time variability or intrasubject coefficient of variation (intrasubject reaction time variability divided by mean reaction time) to go/congruent/ target trials in go/no-go tasks, flanker tasks, Stroop colour and word tasks (Stroop), working memory tasks and the Wisconsin Card Sorting Task (WCST). For inhibition measures, we included the numbers or percentages of commission errors (or the inversely reported number or percentage of correct trials) to no-go trials in the go/no-go task; the number of percentage errors or reaction time to incongruent trials in flanker or Stroop tasks; the number of commission errors in continuous performance tasks (CPTs) or MOXO tasks; stop signal reaction times in the stop task; perseverative errors in the WCST; and multi-button responses in the MOXO task. For processing speed, we included mean reaction times to go/congruent/target trials in alertness, CPT, go/no-go and MOXO tasks, and completion time in the WCST.
We estimated effect sizes using small-sample-corrected standardized mean differences (i.e., Hedges’ g),57 which calculated the difference in performance under sham versus anodal tDCS divided by pooled standard deviation to standardize the effect. We reported effects as positive if a cognitive outcome measure showed improvement with anodal tDCS relative to sham stimulation, and as negative if it showed deterioration. We conducted all meta-analyses using random-effects models to account for heterogeneity (i.e., effect size variation between studies beyond that expected for sampling error alone).58 To provide a measure of heterogeneity, we report the I2 value; I2 values of 75%, 50% and 25% reflect high, moderate and low heterogeneity, respectively.59
When estimating Hedges’ g in crossover designs, we accounted for the correlation between pre and post measures (otherwise, there would have been an underestimation of the effect sizes).57 Where multiple effects were reported from the same sample, we created composite effect sizes by averaging effect sizes and decreasing variances, assuming correlation across the different effects. Because tDCS studies did not report these correlations, we estimated the crossover correlation from reported t values (25 of 60 reported outcomes) derived from analyses comparing anodal tDCS with sham stimulation, and we estimated the correlation across multiple dependent outcomes from an ongoing neurotherapy intervention study in our laboratory with a sample of 74 adolescents with ADHD tested before and after intervention in the Maudsley Attention and Response Suppression task battery, including go/no-go tasks, CPTs, Simon tasks, time estimation tasks54 and the WCST. Specifically, we assumed a correlation of 0.629 between outcome measures for studies with crossover designs, and a correlation of 0.3 between the different effects for composite effects. In addition, we conducted sensitivity analyses assuming crossover correlation of 0.407 and 0.780 (the upper and low 95% CIs of the estimated correlation) and composite correlation of 0.1 and 0.5 to test whether findings depended on our estimates.
Finally, we used jackknife sensitivity analyses (i.e., repeating the same analysis and excluding a different study each time) to establish the replicability of findings. To improve homogeneity, we carried out additional sensitivity analyses that excluded studies with overlapping samples, 60–63 or studies with methods that deviated from the majority of studies, such as those including community participants with high ADHD symptoms on validated ADHD ratings scales but without a clinical ADHD diagnosis;43,64 those including adult samples with ADHD;42,65,66 those targeting the right inferior frontal cortex (IFC);43,67 those reporting change scores (i.e., post minus pre differences) rather than post scores only;66 those using multi-stimulation sessions; 60–62,65 those with effect sizes based on working memory or WCST tasks;61,68 or those using parallel rather than crossover designs.62,66,67 Lastly, given that effect-size estimates might be inflated in studies with a high risk of bias, we conducted meta-regression analyses to compare the effect sizes of studies with high versus low or unclear risk of bias for each risk of bias domain (i.e., selection bias, performance bias, detection bias, attrition bias and other biases). All analyses were conducted by J.R.; meta-analysis calculations were conducted using the function “rma” of the “metafor” package version 2.1 in R version 3.6.69,70
Results
Literature review
rTMS studies
Two double-blind, crossover studies targeted the right dlPFC. In 13 adults with ADHD, 1 session of 20 Hz rTMS relative to sham significantly improved overall self-rated inattention but not hyperactivity symptoms.71 In 9 adults with ADHD, 10 daily sessions of 10 Hz rTMS relative to sham showed no effect on self-rated clinical symptoms, nor on electroencephalography or executive-function measures. 72 In a single-blind study in 21 adolescents with ADHD, 20 daily sessions of 18 Hz deep rTMS over the bilateral dlPFC (n = 13) compared with sham (n = 9) showed no effect on self-rated clinical or cognitive measures of sustained attention.73 An open-label trial in 10 children with ADHD showed fewer teacher-rated inattention and parentrated hyperactivity/impulsivity symptoms 1 week after 5 daily sessions of 1 Hz rTMS of the left dlPFC compared with baseline.52 However, without sham control conditions, placebo effects could not be ruled out (Table 1).
Table 1.
Clinical and cognitive effects of rTMS
| Study | Design | N | Age, yr | Region | Stimulation protocol | Outcome measures | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Sessions, n | Frequency, Hz | Intensity, % of MT | Duration | Clinical* | Cognitive | |||||
| Bloch et al.71 | Single-blind, sham-controlled, crossover | 13 | Adults (age not reported) | Right dlPFC† | 1 | 20 | 100 | 1680 pulses (2 s on, 30 s off) | PANAS (inattention [+], total score [+]); VAS (inattention [+])‡ | Not tested |
| Gomez et al.52 | Open-label | 10 | 7–12 | Left dlPFC | 5 | 1 | 90 | 1500 pulses (on/off not reported) | DSM-IV ADHD symptom checklist (parent-rated hyperactivity/impulsivity [+], teacher-rated inattention [+]) | Not tested |
| Paz et al.73 | Double-blind, sham-controlled, parallel | Active: 9 Sham: 13 |
Active: 32 Sham: 30 |
Bilateral dlPFC§ | 20 | 18 | 120 | 1980 pulses (2 s on, 20 s off) | CAARS | TOVA |
| Weaver et al.72 | Single-blind, sham-controlled, crossover | 9 | 18 | Right dlPFC† | 10 | 10 | 100 | 2000 pulses (4 s on, 26 s off) | CGI-I scale; ADHD-RS-IV scale | WASI/WISC-IV; Conners’ CPT; D-KEFS; Buschke Selective Reminding Test; digit symbol coding test; finger oscillation tasks |
ADHD = attention-deficit/hyperactivity disorder; ADHD-RS-IV = ADHD Rating Scale, Fourth Edition; CAARS = Conners’ Adult ADHD Rating Scales; CGI-I = Clinical Global Impression–Improvement scale; CPT = continuous performance task; D-KEFS = Delis–Kaplan Executive Function System; dlPFC = dorsolateral prefrontal cortex; DSM-IV = Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition; MT = motor threshold; PANAS = Positive and Negative Affect Schedule; rTMS = repetitive transcranial magnetic stimulation; TOVA = Test of Variables of Attention; VAS = visual analogue scale; WASI = Wechsler Abbreviated Scale of Intelligence (selected subtests from the Wechsler Adult Intelligence Scale); WISC-IV = Wechsler Intelligence Scale for Children, Fourth Edition.
Plus sign [+] = statistically significant improvement.
5 cm forward to MT point.
Small change from baseline of 0.25 and 1.16 on 5-point Likert scales.
6 cm rostral to motor cortex.
tDCS studies
Fourteen studies tested tDCS in ADHD; 9 studies were double-blind, 4 studies were single-blind and 1 study was open-label. Ten studies tested children, and 4 studies tested adults. Only 2 studies combined tDCS with cognitive training (Table 2 and Table 3).
Table 2.
Clinical and cognitive effects of tDCS
| Study | Design | N | Mean age, yr | Stimulation protocol | Outcome measures | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Anode/cathode | mA | Sessions, n | Timing* | Duration, min | Clinical† | Cognitive† | ||||
| Children | ||||||||||
| Bandeira et al.51‡ | Open-label | 9 | 11 | Left dlPFC/right SOA | 2 | 5 | Online | 28 | PGI-I | Visual Attention Test (TAVIS-3; omission errors [+]); NEPSY-II-inhibition (switch errors [+]); digit span task; Corsi cube task |
| Breitling et al.67 | Single-blind, sham-controlled, crossover | 21 | 14 | Right IFC/left cheek | 1 | 1 | Online | 20 | Not tested | Flanker task (incongruent trials: errors [+]¶,** and ICV [+]¶,††) |
| Left cheek/right IFC | 1 | 1 | Online | 20 | Not tested | Flanker task | ||||
| Munz et al.60 | Double-blind, sham-controlled, crossover | 14 | 12 | Left dlPFC/right cheek; right dlPFC/left cheek | 0.25 | 1 | Offline | 25 (5 on, 1 off) | Not tested | Go/no-go task (go RT [+] and intrasubject RTV [+]); motor memory task; alertness task |
| Nejati et al.68 (experiment 1) | Double-blind, sham-controlled, crossover | 15 | 10 | Left dlPFC/right dlPFC | 1 | 1 | Offline | 15 | Not tested | Go/no-go task; n-back (accuracy, RT [+]); Stroop task (incongruent trials: errors [+] and RT [+]); WCST (completion time [+]) |
| Nejati et al.68 (experiment 2) | Double-blind, sham-controlled, crossover | 10 | 9 | Left dlPFC/right SOA | 1 | 1 | Offline | 15 | Not tested | Go/no-go task; n-back (accuracy [+],¶ RT [+]**); WCST (total categories completed [+], total errors [+], perseverative errors [+])** |
| Right SOA/left dlPFC | 1 | 1 | Offline | 15 | Not tested | Go/no-go task (no-go accuracy [+])**; n-back; WCST (total categories completed [+], total errors [+] and perseverative errors [+]¶)** | ||||
| Prehn-Kristensen et al.61 | Double-blind, sham-controlled, parallel | 12 | 12 | Left dlPFC/right cheek; right dlPFC/left cheek | 0.25 | 1 | Offline | 25 (5 on, 1 off) | Not tested | Declarative memory task (accuracy [+]); alertness task; digit span task |
| Soff et al.62 | Double-blind, sham-controlled, crossover | 15 | 14 | Left dlPFC/vertex | 1 | 5 | Online | 20 | FBB-ADHD (inattention [+])‡‡,§§,¶¶ | QbTest (inattention [+]††; hyperactivity [+]***)§§,¶¶ |
| Soltaninejad et al.64 | Single-blind, sham-controlled, crossover | 20 | 16 | Left dlPFC/right SOA | 1.5 | 1 | Online | 15 | Not tested | Go/no-go task (go accuracy [+])¶,**; Stroop task |
| Right SOA/left dlPFC | 1.5 | 1 | Online | 15 | Not tested | Go/no-go task (no-go accuracy [+])¶,†††; Stroop task | ||||
| Soltaninejad et al.43§ | Single-blind, sham-controlled, crossover | 20 | 16 | Right IFC/left SOA | 1 | 1 | Online | 15 | Not tested | Go/no-go task (go accuracy [+]); Stroop task |
| Sotnikova et al.63 | Double-blind, sham-controlled, crossover | 13 | 14 | Left dlPFC/vertex | 1 | 1 | Online | 20 | Not tested | QbTest (RT [+], RTV [+],‡‡‡ omission errors [−], accuracy [−])§§§ |
| Adults | ||||||||||
| Allenby et al.65‡ | Double-blind, sham-controlled, crossover | 37 | 32 | Left dlPFC/right SOA | 2 | 3 | Online | 20 | Not tested | Conners’ CPT (commission errors [+]¶¶¶); stop task |
| Cachoeira et al.74 | Double-blind, sham-controlled, parallel | Active: 9 Sham: 8 |
Active: 31 Sham: 34 |
Right dlPFC/left dlPFC | 2 | 5 | Offline | 20 | ADHD checklist (inattention [+], total [+])****; SDS (after tDCS only [+]) | Not tested |
| Cosmo et al.66 | Double-blind, sham-controlled, parallel | Active: 30 Sham: 30 |
Active: 32 Sham: 33 |
Left dlPFC/right dlPFC | 1 | 1 | Offline | 20 | Not tested | Go/no-go task |
| Jacoby et al.42 | Single-blind, sham-controlled, crossover | 20 | 23 | Left and right dlPFC/cerebellum | 1.8 | 1 | Offline | 20 | Not tested | CPT (multi-button presses [+]) |
ADHD = attention deficit/hyperactivity disorder; CPT = continuous performance task; dlPFC = dorsolateral prefrontal cortex; FBB-ADHD = parents’ version of a German adaptive diagnostic checklist for ADHD; ICV = intrasubject coefficient of variation; IFC = inferior frontal cortex; NEPSY-II = Neuropsychological Development Assessment, Second Edition; PGI-I = Patient Global Impression of Improvement; QbTest = Quantitative Behaviour Test; RT = reaction time; RTV = reaction time variability or standard deviation of reaction times; SDS = Sheehan Disability Scale; SOA = supraorbital area; Stroop = Stroop colour and word task; tDCS = transcranial direct current stimulation; WCST = Wisconsin Card Sorting Task.
Refers to whether cognitive performance was during stimulation (online) or after stimulation (offline).
Plus sign [+] = statistically significant improvement; minus sign [−] = statistically significant impairment.
Combined stimulation with cognitive training.
Originally published in Persian; translated by the lead author (ZS).
Would likely not survive multiple comparison correction.
Comparisons between stimulation conditions based on post-hoc least significant difference tests, which do not correct for multiple comparisons.
Based on underpowered analysis focusing on the first session, with 7 participants per condition.
Improvement seen only 7 days after the fifth anodal tDCS session.
Did not survive correction for multiple comparisons.
Based on underpowered analysis focusing on the first 5 sessions, with 7/8 participants per condition.
Improvement seen immediately after the fifth anodal tDCS session and 7 days later.
Significant in comparison to cathodal tDCS only.
Based on a crossover interaction; tDCS reduced RT and RTV in 1 of 4 conditions (2-back tasks), but this did not survive correction for multiple comparisons.
Included carryover effect raised by Soff et al.62
Significant only immediately after anodal tDCS; not significant 3 days later.
Inattention improved immediately after anodal tDCS and after 2 weeks; total score improved only after 2 weeks.
Table 3.
Cognitive task measures (mean ± SD) extracted from studies
| Study | Cognitive task | Cognitive outcome measure | tDCS, mean ± SD | Sham, mean ± SD | Effect* |
|---|---|---|---|---|---|
| Measures of attention | |||||
| Allenby et al.65 | CPT | No. omission errors | 1.9 ± 4.3 | 2.1 ± 2.4 | Single |
| Breitling et al.67 | Flanker task (incongruent trials) | % Omission errors | 2.2 ± 4.6 | 5.8 ± 7.6 | Composite |
| Flanker task (incongruent trials) | Intraindividual coefficient of variation (ms)† | 0.2 ± 0.1 | 0.3 ± 0.1 | ||
| Cosmo et al.66 | Go/no-go task (fruits) | No. omission errors (post/pre)‡ | −2.9 ± 24.5 | −3.7 ± 21.2 | Single |
| Jacoby et al.42 | MOXO task | RT (ms) with 1 distractor | 552.2 ± 53.9 | 558.9 ± 52.1 | Composite |
| MOXO task | RT (ms) with 2 distractors | 556.5 ± 54.4 | 568.9 ± 53.5 | ||
| MOXO task | No. omission errors | 4.2 ± 4.1 | 4.3 ± 4.0 | ||
| Munz et al.60 | Alertness task | Intrasubject RTV (ms) | 69.5 ± 25.1 | 76.1 ± 31.2 | Composite |
| Go/no-go task (go trials) | Intrasubject RTV (ms) | 225.2 ± 246.9 | 379.4 ± 425.3 | ||
| Go/no-go task (go trials) | No. omission errors | 3.0 ± 3.0 | 4.3 ± 4.4 | ||
| Nejati et al.68 (experiment 1) | Go/no-go task (go trials) | % Correct | 93.3 ± 11.4 | 90.9 ± 19.6 | Composite |
| N-back task (1-back) | No. correct | 15.3 ± 8.3 | 14.4 ± 7.4 | ||
| WCST | No. of categories completed | 2.5 ± 0.8 | 2.4 ± 1.1 | ||
| WCST | No. total errors | 29.7 ± 8.3 | 30.7 ± 9.3 | ||
| Nejati et al.68 (experiment 2) | Go/no-go task (go trials) | % Correct | 100 ± 0.0 | 98.5 ± 3.2 | Composite |
| N-back task (1-back) | No. correct | 21.0 ± 2.3 | 17.9 ± 2.9 | ||
| WCST | No. of categories completed | 5.1 ± 0.7 | 3.9 ± 0.7 | ||
| WCST | No. total errors | 11.0 ± 2.9 | 21.6 ± 5.4 | ||
| Prehn-Kristensen et al.61 | Digit span task | No. correct | 9.6 ± 2.4 | 10.8 ± 2.1 | Single |
| Soff et al.62 | QbTest (inattention) | z-scores (omission errors, RT and intrasubject RTV) | 0.1 ± 1.2 | −0.1 ± 0.4 | Single |
| Soltaninejad et al.64 | Go/no-go task (go trials) | % Correct | 98.8 ± 3.6 | 98.9 ± 1.9 | Single |
| Soltaninejad et al.43 | Go/no-go task (go trials)§ | % Correct | — | — | Single |
| Sotnikova et al.63 | QbTest (overall) | Intrasubject RTV (ms) | 214.3 ± 97.2 | 235.2 ± 122.7 | Composite |
| QbTest (overall) | No. omission errors | 38.6 ± 22.8 | 22.5 ± 15.3 | ||
| QbTest (overall) | % Correct¶ | 31.7 ± 5.1 | 43.5 ± 6.9 | ||
| Measures of inhibition | |||||
| Allenby et al.65 | CPT | No. commission errors | 17.1 ± 9.1 | 19.8 ± 10.9 | Composite |
| Stop task | Stop signal RT | 288.4 ± 76.0 | 291.5 ± 68.1 | ||
| Breitling et al.67 | Flanker task (incongruent trials) | RT (ms) | 581.0 ± 43.0 | 585.0 ± 38.0 | Composite |
| Flanker task (incongruent trials) | % Errors | 9.8 ± 7.2 | 20.6 ± 9.2 | ||
| Cosmo et al.66 | Go/no-go task (fruits) | No. commission errors (post/pre)‡ | −5.5 ± 10.0 | −6.9 ± 10.4 | Single |
| Jacoby et al.42 | MOXO task | Multi-button responses | 4.7 ± 4.9 | 7.0 ± 6.3 | Composite |
| MOXO task | No. commission errors | 11.3 ± 11.2 | 11.7 ± 12.1 | ||
| Munz et al.60 | Go/no-go task (no-go trials) | No. commission errors | 15.7 ± 10.3 | 12.6 ± 8.2 | Single |
| Nejati et al.68 (experiment 1) | Go/no-go task (no-go trials) | % correct | 19.9 ± 7.6 | 19.0 ± 7.8 | Composite |
| Stroop task (incongruent trials) | RT (ms) | 2870 ± 2210 | 1390 ± 440 | ||
| Stroop task (incongruent trials) | % Errors | 24.9 ± 12.0 | 34.9 ± 15.5 | ||
| WCST | No. perseverative errors | 17.6 ± 3.6 | 18.0 ± 9.0 | ||
| Nejati et al.68 (experiment 2) | Go/no-go task (no-go trials) | % Correct | 22.7 ± 1.3 | 20.7 ± 4.4 | Composite |
| WCST | No. perseverative errors | 7.8 ± 2.4 | 14.8 ± 3.7 | ||
| Soff et al.62 | QbTest (impulsivity) | z-scores (commission errors, multi-button press per stimulus, anticipatory button press) | 0.2 ± 1.2 | −0.2 ± 0.7 | Single |
| Soltaninejad et al.64 | Go/no-go task (no-go trials) | % Correct | 96.2 ± 8.2 | 95.8 ± 6.9 | Composite |
| Stroop task (incongruent trials) | % Correct | 98.3 ± 2.9 | 96.4 ± 3.6 | ||
| Stroop task (incongruent trials) | RT (ms) | 1080 ± 180 | 1130 ± 220 | ||
| Soltaninejad et al.43 | Go/no-go task (no-go trials)§ | % Correct | — | — | Composite |
| Stroop task (incongruent trials)§ | % Correct | — | — | ||
| Stroop task (incongruent trials)§ | RT (ms) | — | — | ||
| Sotnikova et al.63 | QbTest (overall) | No. commission errors | 6.0 ± 5.1 | 6.0 ± 4.2 | Single |
| Measures of processing speed | |||||
| Allenby et al.65 | CPT | RT (ms) to target | 420.9 ± 63.3 | 419.7 ± 73.0 | Single |
| Jacoby et al.42 | MOXO task | RT (ms) to target | 541.5 ± 50.8 | 547.0 ± 53.5 | Composite |
| Munz et al.60 | Alertness task | RT (ms) | 309.6 ± 51.8 | 302.4 ± 44.3 | Composite |
| Go/no-go task (go trials) | RT (ms) | 453.2 ± 131.3 | 566.9 ± 234.1 | ||
| Nejati et al.68 (experiment 1) | Go/no-go task (go trials) | RT (ms) | 1080 ± 210 | 1030 ± 170 | Composite |
| N-back task (1-back) | RT (ms) | 120.2 ± 22.5 | 175.7 ± 55.4 | ||
| WCST | Completion time (ms) | 237 300 ± 79 800 | 291 100 ± 106 700 | ||
| Nejati et al.68 (experiment 2) | Go/no-go task (go trials) | RT (ms) | 1330 ± 900 | 1230 ± 120 | Composite |
| N-back task (1-back) | RT (ms) | 103.4 ± 24.2 | 162.9 ± 94.4 | ||
| WCST | Completion time (ms) | 123 200 ± 16 900 | 170 300 ± 85 900 | ||
| Soltaninejad et al.64 | Go/no-go task (go trials) | RT (ms) | 830 ± 290 | 910 ± 350 | Single |
| Soltaninejad et al.43 | Go/no-go task (go trials)§ | RT (ms) | — | — | Single |
| Sotnikova et al.63 | QbTest (overall) | RT (ms) | 555.3 ± 116.2 | 564.2 ± 130.8 | Single |
CPT = continuous performance task; QbTest = Quantitative Behaviour Test; RT = reaction time; RTV = reaction time variability; SD = standard deviation of the mean; Stroop = Stroop colour word task; WCST = Wisconsin Card Sorting Task.
For multiple effects from the sample, we created composite effect-size estimates; single effects otherwise.
Intrasubject reaction time variability divided by mean reaction time.
Only change scores (post – pre/baseline) were reported.
The author could not provide raw means and standard deviations for each stimulation condition, so to calculate Hedges’ g we converted the reported t-statistics.57
The % correct was reported as: hits (total no. of target trials – no. of omission errors errors) + correct rejections (total no. of no-go trials – no. of commissions errors)/total number of stimuli63
tDCS studies in children with ADHD
Two single-blind, sham-controlled crossover studies in 20 high school students who scored above the clinical cut-off for ADHD on validated ADHD questionnaires applied a single session of anodal tDCS over the left dlPFC64 or the right IFC.43 Anodal relative to cathodal tDCS of the left dlPFC improved go accuracy, and cathodal tDCS improved no-go accuracy compared with anodal tDCS and sham treatment, but none improved performance on the Stroop task.64 Right IFC anodal tDCS relative to sham treatment improved go accuracy, but there were no equivalent improvements on any other go/no-go or Stroop task measures.43
Two double-blind, sham-controlled, crossover studies applied single-session stimulation over the dlPFC. In 15 adolescents with ADHD, tDCS over the bilateral dlPFC (anode left/cathode right) improved WCST completion time and n-back and Stroop reaction times and errors to incongruent trials, but had no effects on n-back accuracy or go/no-go measures. Furthermore, errors and reaction times to incongruent trials in the Stroop task were used as main outcome measures, rather than the Stroop interference or error effect (reaction time/errors on Stroop – reaction time/errors on congruent trials) — the established key outcome measure of the Stroop task. In 10 adolescents with ADHD, anodal tDCS of the left dlPFC improved n-back working memory accuracy and reaction times compared to both sham treatment and cathodal tDCS. Both anodal and cathodal tDCS over the left dlPFC improved WCST performance; anodal tDCS had stronger effects. Cathodal tDCS of the left dlPFC also improved no-go accuracy, presumably via interhemispheric inhibition increasing right prefrontal regions,68 which have been associated with motor response inhibition in children and adults.54,75 This explanation is partly supported by a single-blind crossover study in 21 adolescents with ADHD, which found that 1 session of anodal (but not cathodal) tDCS over the right IFC versus sham treatment reduced errors (trend level) and reaction time variability in a flanker task.67 However, as in the previous paper,68 findings were based on flanker incongruent trials rather than on flanker interference reaction time or error effect, and the analysis included only the first session to remove a practice effect, reducing the sample size to 7 participants per condition.
One double-blind, crossover study applied 5 daily sessions of anodal versus sham tDCS over the left dlPFC in 15 adolescents with ADHD. The results showed improvements relative to sham treatment in parent-rated clinical measures of inattention and on cognitive measures of attention (as assessed using the QbTest, a combined working memory and go/no-go task) 7 days after anodal tDCS but not immediately after, and improvements in QbTest measures of hyperactivity both immediately after anodal tDCS and 7 days later. Clinical and QbTest impulsiveness measures showed no effects. However, findings were based on the first 5 sessions to remove a carryover effect, reducing the sample to 7 or 8 participants per condition. 62 In the same study, 13 of the 15 adolescents with ADHD showed reduced reaction time variability but increased errors on the QbTest after a single session of anodal tDCS. However, this analysis included a carryover effect.63
A double-blind, sham-controlled crossover study found that relative to sham treatment, overnight slow-wave oscillatory anodal tDCS over the left and right dlPFC improved declarative memory in 12 children with ADHD61 and go reaction time and its intrasubject variability in 14 children with ADHD,60 but had no effects on no-go accuracy, or on measures of alertness, digit span or motor memory.
The only open-label trial in 9 children with ADHD found that 5 daily sessions of anodal tDCS to the left dlPFC combined with a picture association cognitive training task reduced errors on attention (omission) and switch tasks but did not improve working memory. Parents reported improvements in some of their children’s behaviour except for one, who reported their child was “much worse.” However, without a sham control condition, findings could have been confounded by placebo, test–retest or cognitive training effects.51
tDCS studies in adults with ADHD
A double-blind, parallel study found no effects of a single session of anodal tDCS over the left dlPFC relative to sham treatment on go/no-go performance in 60 adults with ADHD.66 A single-blind, crossover study in 20 undergraduates with ADHD found that 1 session of bifrontal anodal tDCS over the left and right dlPFC relative to sham treatment improved hyperactivity measures in a sustained attention task (i.e., multiple/random responses), but not omission errors or reaction times.42 A double-blind, crossover study in 37 adults with ADHD found that 3 sessions over alternate days of visual working memory training combined with anodal tDCS relative to sham tDCS of the left dlPFC reduced commission errors in a sustained attention task immediately after anodal tDCS, but not at the 3-day follow-up; there were no effects on omission errors, reaction times or stop task performance immediately after anodal tDCS or 3 days later.65 Finally, a double-blind, parallel study in 17 adults with ADHD reported that 5 daily sessions of anodal right tDCS (n = 9) relative to sham treatment (n = 8) improved inattention but not hyperactivity/impulsive symptoms immediately after stimulation and 2 weeks later, at which point the total ADHD score was also improved.
Safety in tDCS studies
Brain stimulation was well tolerated overall; the most commonly reported side effects were mild tingling and itching. One study reported dropouts because of tingling sensations and headache43 (Table 4). There was 1 case of an adverse effect, where 1 patient reported hypobulia after a single session of anodal tDCS over the right dlPFC, which persisted in a milder form the following day.74
Table 4.
Side effects and adverse effects reported in studies using rTMS and tDCS
| Study | Side and adverse effects |
|---|---|
| rTMS studies | |
| Bloch et al.71 | Not tested, but authors observed differences in the somatosensory experience of real rTMS and sham, which limited true blinding |
| Gomez et al.52 | Majority reported transient mild headache and scalp discomfort; a minority reported neck pain. No seizure-like cortical activity was found. One case of slight and brief dizziness |
| Paz et al.73 | Not tested |
| Weaver et al.72 | Minority reported transient mild headaches and scalp discomfort |
| tDCS studies | |
| Allenby et al.65 | Burning, itching, pain and tingling were rated significantly higher during anodal tDCS compared with sham |
| Bandeira et al.51 | Majority reported mild to moderate headache, tingling, itching, burning sensation and redness of the skin. Minority reported mild neck pain, sleepiness and static shock. One parent reported child was “much worse” after stimulation |
| Breitling et al.67 | Skin sensations were rated higher during cathodal tDCS relative to sham |
| Cachoeira et al.74 | Comparable reports of headache, tingling, itching, burning sensation and tiredness were found in both sham and anodal tDCS. One patient withdrew after the first sessions because of an “acute mood change, feeling sad, hypobulia, tension … 5 hours after stimulation and persisted in a milder form into the next day” |
| Cosmo et al.66 | None reported |
| Jacoby et al.42 | Not tested, but authors reported that stimulation was well tolerated and no side effects were reported |
| Munz et al.60 | Not tested, but no side effects were reported |
| Nejati et al.68 | Reports of mild itching or tingling under electrodes |
| Prehn-Kristensen et al.61 | Not tested. No side effects reported |
| Soff et al.62 | About half of participants reported tingling and itching sensations under electrode during sham and anodal tDCS |
| Soltaninejad et al.64* | Not reported |
| Soltaninejad et al.43* | Several participants dropped out because of tingling sensation and headache |
| Sotnikova et al.63 | About half of participants reported tingling and itching sensations under electrode during sham and anodal tDCS |
rTMS = repetitive transcranial magnetic stimulation; tDCS = transcranial direct current stimulation.
Personal communication, 2019.
Summary of findings of tDCS studies
Findings were mixed. With respect to the clinical effects of tDCS, only 2 sham-controlled studies tested and found improvement in behavioural symptoms of inattention but not in impulsiveness/hyperactivity symptoms, and an openlabel trial found improvements in parent-rated impression of improvement in global functioning. Of 14 studies that tested cognitive effects, 13 observed positive effects on some cognitive functions but not others, and 1 reported worse performance in a sustained attention task.63 Moreover, 7 sham-controlled studies did not correct for multiple comparisons, 43,62–64,67,68 and the majority of their findings would not have survived correction.
Risk of bias
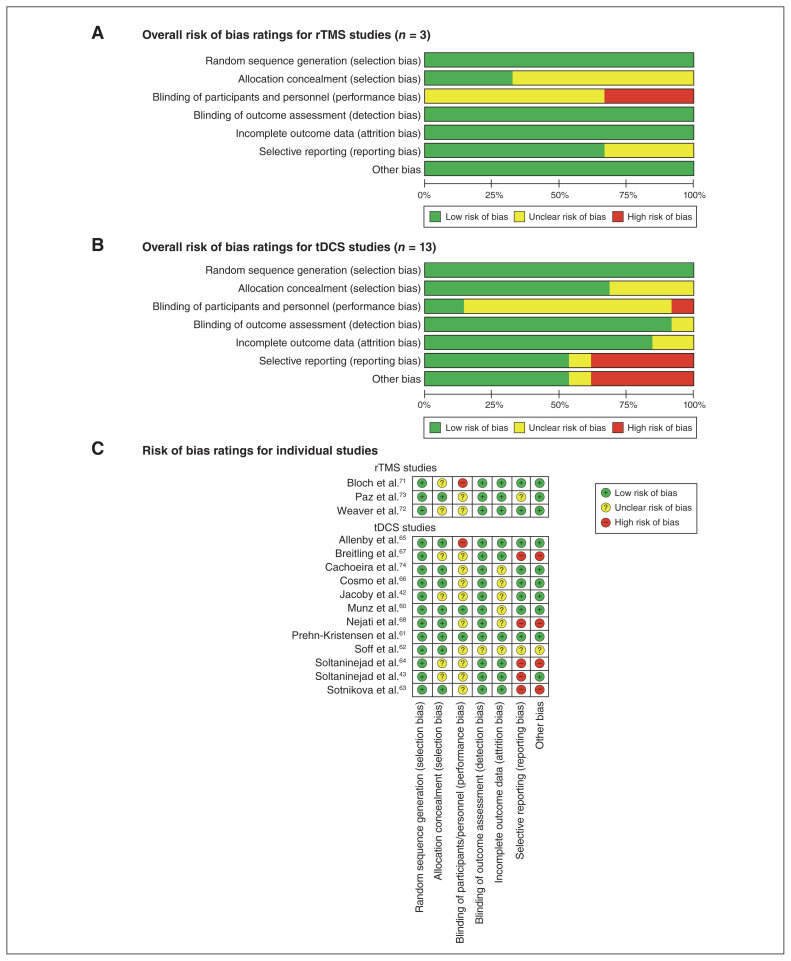
A minority of studies indicated unclear bias for selection (n = 6), detection (n = 1) and attrition (n = 2), but on balance bias was low in these domains. Performance bias (i.e., blinding participants and personnel) was unclear in 11 studies and high in 2 studies; selective reporting bias and other biases (e.g., nonstandard outcome measures, no correction for multiple testing, lenient significance threshold) were high in 4 studies (Fig. 2; Appendix 1, Table S2).
Fig. 2.
Risk of bias ratings for (A) repetitive transcranial magnetic stimulation (rTMS), (B) transcranial direct current stimulation (tDCS) and (C) individual studies. Note: risk of bias ratings were the same for both studies reported in Nejati et al.68
Meta-analysis of anodal tDCS studies
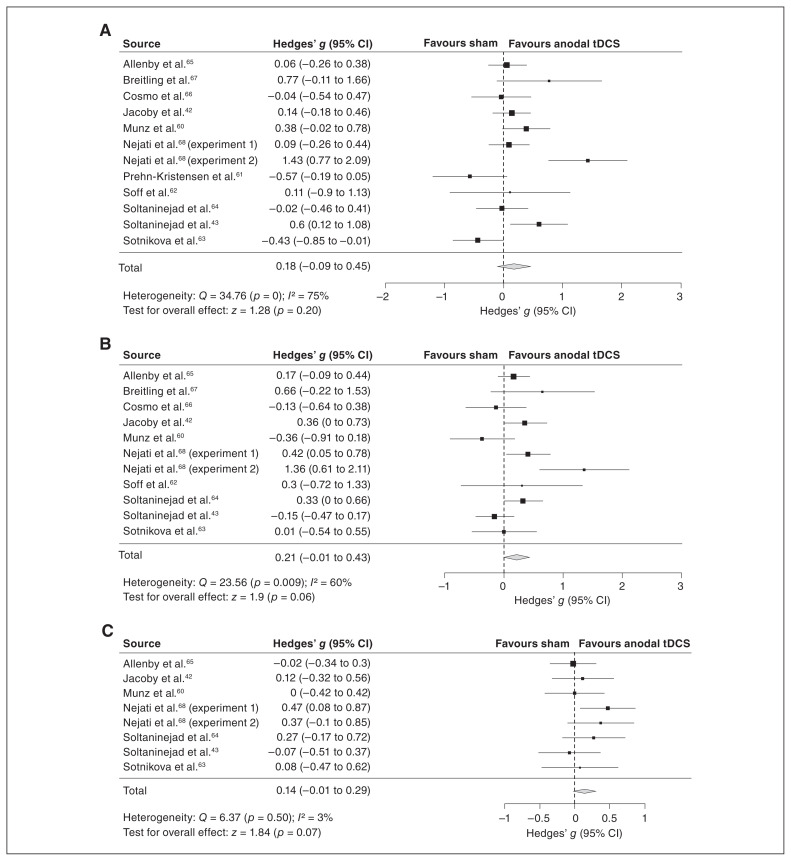
The majority of the studies included in the meta-analysis (10 of 12) used anodal stimulation of mostly left dlPFC regions (unilateral left, n = 5; bifrontal anode left and right, n = 3; bilateral anode left/cathode right, n = 2); only 2 studies involved unilateral stimulation of the right IFC (Table 2). The meta-analyses showed no significant effect in measures of attention (Hedges’ g = 0.18, 95% CI −0.19 to 0.45, p = 0.20) with high heterogeneity (I2 = 75%, p < 0.001) and trend-level improvements in measures of inhibition (Hedge’s g = 0.21, 95% CI −0.01 to 0.43, p = 0.06) with moderate heterogeneity (I2 = 60%, p = 0.01) and of processing speed (Hedges’ g = 0.14, 95% CI −0.01 to 0.29, p = 0.07) with low heterogeneity (I2 = 3%, p = 0.50; Fig. 3). Sensitivity analyses confirmed that these findings did not systematically rely on our estimated correlations for effects from crossover studies or for composite effect-size estimates (Appendix 1, Table S3). Further, we replicated these findings in the jackknife sensitivity analyses (i.e., repeating the main analyses but excluding a different study with each repetition), with only minor exceptions: excluding a minority of individual studies led to a significant but still small effect in measures of inhibition (excluding Cosmo and colleagues,66 Munz and colleagues60 and Soltaninejad and colleagues43) and in processing speed (excluding Allenby and colleagues65 and Soltaninejad and colleagues;43 Table 5).
Fig. 3.
Meta-analysis of measures of (A) attention, (B) inhibition and (C) processing speed. CI = confidence interval; tDCS = transcranial direct current stimulation.
Table 5.
Results of jackknife sensitivity analysis
| Meta-analysis | Studies excluded | Effect size | Heterogeneity | |||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Hedges’ g* | 95% CI | p value | I2 | p value | ||
| Attention | Allenby et al.65 | 0.19 | −0.11 to 0.50 | 0.22 | 77 | < 0.001 |
| Breitling et al.67 | 0.14 | −0.13 to 0.42 | 0.31 | 76 | < 0.001 | |
| Cosmo et al.66 | 0.20 | −0.10 to 0.50 | 0.19 | 78 | < 0.001 | |
| Jacoby et al.42 | 0.19 | −0.12 to 0.49 | 0.24 | 77 | < 0.001 | |
| Munz et al.60 | 0.16 | −0.14 to 0.46 | 0.30 | 77 | < 0.001 | |
| Nejati et al.68 (experiment 1) | 0.19 | −0.12 to 0.50 | 0.22 | 77 | < 0.001 | |
| Nejati et al.68 (experiment 2) | 0.08 | −0.12 to 0.27 | 0.43 | 49 | 0.040 | |
| Prehn-Kristensen et al.61 | 0.23 | −0.03 to 0.50 | 0.09 | 72 | 0.001 | |
| Soff et al.62 | 0.18 | −0.11 to 0.47 | 0.22 | 78 | < 0.001 | |
| Soltaninejad et al.64 | 0.20 | −0.10 to 0.50 | 0.19 | 77 | < 0.001 | |
| Soltaninejad et al.43 | 0.14 | −0.15 to 0.42 | 0.35 | 75 | 0.001 | |
| Sotnikova et al.63 | 0.24 | −0.03 to 0.50 | 0.08 | 70 | 0.002 | |
| Inhibition | Allenby et al.65 | 0.22 | −0.04 to 0.48 | 0.09 | 65 | 0.010 |
| Breitling et al.67 | 0.19 | −0.03 to 0.41 | 0.10 | 62 | 0.010 | |
| Cosmo et al.66 | 0.24 | 0.01 to 0.48 | 0.040 | 63 | 0.010 | |
| Jacoby et al.42 | 0.20 | −0.05 to 0.44 | 0.12 | 65 | 0.010 | |
| Munz et al.60 | 0.25 | 0.05 to 0.46 | 0.020 | 53 | 0.020 | |
| Nejati et al.68 (experiment 1) | 0.19 | −0.06 to 0.43 | 0.13 | 63 | 0.010 | |
| Nejati et al.68 (experiment 2) | 0.15 | −0.03 to 0.32 | 0.10 | 37 | 0.12 | |
| Soff et al.62 | 0.21 | −0.02 to 0.44 | 0.07 | 65 | 0.010 | |
| Soltaninejad et al.64 | 0.20 | −0.05 to 0.45 | 0.12 | 65 | 0.010 | |
| Soltaninejad et al.43 | 0.26 | 0.04 to 0.48 | 0.020 | 52 | 0.030 | |
| Sotnikova et al.63 | 0.23 | −0.01 to 0.47 | 0.06 | 66 | 0.010 | |
| Processing speed | Allenby et al.65 | 0.19 | 0.02 to 0.35 | 0.030 | 0 | 0.53 |
| Jacoby et al.42 | 0.15 | −0.02 to 0.32 | 0.09 | 13 | 0.38 | |
| Munz et al.60 | 0.16 | 0.00 to 0.33 | 0.06 | 10 | 0.44 | |
| Nejati et al.68 (experiment 1) | 0.09 | −0.08 to 0.25 | 0.29 | 0 | 0.79 | |
| Nejati et al.68 (experiment 2) | 0.12 | −0.04 to 0.28 | 0.15 | 2 | 0.50 | |
| Soltaninejad et al.64 | 0.13 | −0.04 to 0.29 | 0.14 | 9 | 0.42 | |
| Soltaninejad et al.43 | 0.17 | 0.01 to 0.33 | 0.040 | 5 | 0.50 | |
| Sotnikova et al.63 | 0.15 | −0.02 to 0.31 | 0.08 | 11 | 0.40 | |
CI = confidence interval.
Effect size estimates assumed a correlation of 0.629 for crossover studies and a correlation of 0.3 for multiple dependent effects.
To improve homogeneity, we carried out additional sensitivity analyses to exclude studies with overlapping samples, methods that departed from the majority of studies or studies that were of lower methodological quality (Table 6). For inhibition measures, these analyses revealed significant but small effects when the analysis was limited to studies using single-session tDCS (Hedges’ g = 0.28, 95% CI 0.01 to 0.56, p = 0.04); reported only post-stimulation scores (Hedges’ g = 0.24, 95% CI 0.01 to 0.48, p = 0.04); or stimulated mainly the left dlPFC (Hedges’ g = 0.24, 95% CI 0.01 to 0.47, p = 0.04), all of which were associated with significant moderate heterogeneity (I2 = 66, p = 0.01; I2 = 63, p = 0.01; I2 = 56, p = 0.02, respectively). For processing speed measures, we found significant but small effects when the analysis was limited to studies that used single- session tDCS (Hedges’ g = 0.22, 95% CI 0.04 to 0.41, p = 0.02), used child samples (Hedges’ g = 0.20, 95% CI 0.01 to 0.39, p = 0.04) or stimulated mainly the left dlPFC (Hedges’ g = 0.17, 95% CI 0.01 to 0.33, p = 0.04), all of which were associated with low heterogeneity (I2 = 0, p = 0.51; I2 = 9, p = 0.41; I2 = 5, p = 0.50, respectively).
Table 6.
Results for sensitivity analysis of the meta-analyses of attention, inhibition and processing speed
| Included studies | Excluded studies | Studies | Effect sizes | Heterogeneity | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Hedges’ g | 95% CI | p value | I2 | p value | |||
| Attention | |||||||
| All | None | 12 | 0.18 | −0.19 to 0.45 | 0.20 | 75 | < 0.001 |
| Studies with crossover design | Allenby et al.65; Breitling et al.67*; Soff et al.62 | 9 | 0.17 | −0.17 to 0.50 | 0.32 | 82 | < 0.001 |
| Patients with clinical ADHD diagnosis | Soltaninejad et al.43; Soltaninejad et al.64† | 10 | 0.16 | −0.16 to 0.48 | 0.33 | 78 | < 0.001 |
| No WCST and working memory tasks | Nejati et al.68‡; Prehn-Kristensen et al.61 | 11 | 0.14 | −0.04 to 0.32 | 0.13 | 39 | 0.12 |
| No overlapping samples§ | Munz et al.60; Soff et al.62 | 10 | 0.16 | −0.16 to 0.49 | 0.32 | 80 | < 0.001 |
| Munz et al.60; Sotnikova et al.63 | 10 | 0.22 | −0.08 to 0.53 | 0.15 | 74 | 0.002 | |
| Prehn-Kristensen et al.61; Soff et al.62 | 10 | 0.24 | −0.04 to 0.52 | 0.09 | 76 | < 0.001 | |
| Prehn-Kristensen et al.61; Sotnikova et al.63 | 10 | 0.29 | 0.05 to 0.54 | 0.020 | 62 | 0.010 | |
| Only single-session tDCS | Allenby et al.65¶; Munz et al.60; Prehn-Kristensen et al.61; Soff et al.62 | 8 | 0.26 | −0.11 to 0.64 | 0.17 | 81 | < 0.001 |
| Report of post scores only | Cosmo et al.66** | 11 | 0.20 | −0.10 to 0.50 | 0.19 | 78 | < 0.001 |
| Target site of dlPFC only | Breitling et al.67††; Soltaninejad et al.43 | 10 | 0.10 | −0.19 to 0.39 | 0.51 | 76 | 0.001 |
| Only studies in children | Allenby et al.65; Cosmo et al.66‡‡; Jacoby et al.42 | 9 | 0.24 | −0.16 to 0.63 | 0.24 | 80 | < 0.001 |
| Inhibition | |||||||
| All | None | 11 | 0.21 | −0.01 to 0.43 | 0.06 | 60 | 0.01 |
| Studies with crossover design | Breitling et al.67*; Cosmo et al.66; Soff et al.62 | 8 | 0.22 | −0.04 to 0.48 | 0.09 | 71 | 0.004 |
| Patients with clinical ADHD diagnosis | Soltaninejad et al.43; Soltaninejad et al.64† | 9 | 0.26 | −0.02 to 0.53 | 0.07 | 62 | 0.02 |
| No WCST and working memory tasks | Nejati et al.68‡ | 11 | 0.17 | 0.00 to 0.35 | 0.06 | 41 | 0.09 |
| Only single-session tDCS | Allenby et al.65¶; Munz et al.60; Soff et al.62 | 8 | 0.28 | 0.01 to 0.56 | 0.040 | 66 | 0.01 |
| Report of post scores only | Cosmo et al.66** | 10 | 0.24 | 0.01 to 0.48 | 0.040 | 63 | 0.01 |
| Target site of dlPFC only | Breitling et al.67††; Soltaninejad et al.43 | 9 | 0.24 | 0.01 to 0.47 | 0.040 | 56 | 0.02 |
| Studies in children only | Allenby et al.65; Cosmo et al.66‡‡; Jacoby et al.42 | 8 | 0.26 | −0.08 to 0.60 | 0.13 | 72 | 0.004 |
| Processing speed | |||||||
| All | None | 8 | 0.14 | −0.01 to 0.29 | 0.07 | 3 | 0.50 |
| Patients with clinical ADHD diagnosis | Soltaninejad et al.43; Soltaninejad et al.64† | 6 | 0.16 | −0.02 to 0.34 | 0.09 | 13 | 0.40 |
| No WCST and working memory tasks | Nejati et al.68‡ | 8 | 0.00 | −0.16 to 0.16 | 1.00 | 0 | 0.78 |
| Only single-session tDCS | Cosmo et al.66¶; Munz et al.60 | 6 | 0.22 | 0.04 to 0.41 | 0.020 | 0 | 0.51 |
| Target site of dlPFC | Soltaninejad et al.43†† | 7 | 0.17 | 0.01 to 0.33 | 0.040 | 5 | 0.50 |
| Studies in children | Allenby et al.65‡‡; Jacoby et al.42 | 6 | 0.20 | 0.01 to 0.39 | 0.040 | 9 | 0.41 |
ADHD = attention deficit/hyperactivity disorder; CI = confidence interval; dlPFC = dorsolateral prefrontal cortex; IFC = inferior frontal cortex; tDCS = transcranial direct current stimulation; WCST = Wisconsin Card Sorting Task.
These sudies used a parallel design; all others used crossover designs.
Participants did not have a clinical diagnosis of ADHD.
Only WCST and working memory data excluded.
To remove overlapping samples, we excluded 1 study at a time.
These studies applied tDCS over multiple sessions; all others used one session.
The only studies to use change scores (post/pre stimulation scores), all other used post-tDCS scores.
The only studies stimulated the right IFC; all other targeted the dlPFC.
Included adults; all others included children and adolescents.
Finally, meta-regression analyses compared the effect sizes from studies with a high risk of reporting bias versus the effect sizes from studies with low or unclear risk of such bias. At a descriptive level, the effect sizes from studies with high risk of reporting bias or “other” biases (e.g., no correction to multiple testing, lenient α level) were larger than the effect sizes from studies with low or unclear risk. These differences were not statistically significant in any of the outcomes for reporting bias (lowest p = 0.14), but “other” biases yielded a significant effect in measures of inhibition (p = 0.03) and processing speed (p = 0.03). Because none of the studies showed high risk of selection, detection or attrition bias, and only 1 study showed high risk of performance bias, we did not carry out meta-regression analyses for these biases.
Discussion
There has been a recent proliferation of noninvasive brain stimulation studies in ADHD, with large heterogeneity in methodology and outcome measures. We therefore conducted a systematic review of rTMS and tDCS studies. Furthermore, we conducted a rigorous meta-analysis of tDCS studies in ADHD that clustered cognitive effects into clearly separated cognitive domains to increase homogeneity, controlled for multiple dependent effects and potential bias of studies, and tested the replicability of meta-analysis results with jackknife and other sensitivity analyses. The meta-analysis of tDCS studies of mostly the dlPFC showed only trend-level effects on improvement of inhibition and processing speed, but not on attention, suggesting limited effects of tDCS on cognition in children and adults with ADHD.
The findings of the systematic review revealed that for rTMS, of the 4 included studies, only 2 measured and found clinical effects, while the other 2 measured and found no effects on cognitive functions. For tDCS, 12 of the 14 studies included in the systematic review stimulated the dlPFC with anodal tDCS, mostly over the left hemisphere (unilateral left, n = 6; bilateral anode left/cathode right, n = 2; bifrontal left and right, n = 3), and 1 study applied bilateral cathodal tDCS over the left dlPFC (anode right/ cathode left), and 2 studies used unilateral anodal tDCS over the right IFC. Only 2 sham-controlled tDCS studies measured clinical outcomes and found that anodal tDCS over the left or right dlPFC improved ADHD inattention, but not impulsiveness/hyperactivity symptoms, immediately after tDCS and 1 week62,74 or 2 weeks later.74 This could suggest that dlPFC stimulation can improve clinical inattention in ADHD, but this finding will need to be confirmed by future, larger studies.
Importantly, the rigorous meta-analysis of 12 tDCS studies applying 1 to 5 sessions of anodal tDCS of mostly left or bilateral/bifrontal dlPFC (with the exception of Soltaninejad and colleagues43 and Breitling and colleagues67) showed only trend-level improvements with small effect sizes in inhibition and processing speed, but no effects on attention.
This systematic review of tDCS and rTMS studies showed that there is very limited evidence that rTMS and tDCS can have an effect on clinical symptoms based on a small sample of 3 sham-controlled studies. The meta-analyses showed — albeit with small effect sizes — that 1 to 5 sessions of anodal tDCS over mostly the left (but also bilateral and bifrontal) dlPFC improved performance on cognitive measures of inhibition and processing speed, but there was no evidence for improvement in attention measures.
Given that 11 of 12 sham-controlled studies in the systematic review tested and found tDCS-induced improvement in 1 or more executive-function measures,42,43,60–65,67,68 the findings of the meta-analysis may seem disappointing. However, the lack of positive overall findings, despite positive findings in individual studies, was likely due to underpowered small study effects that were uncorrected for multiple testing43,62– 64,67,68 and did not survive the scrutiny of meta-analysis. The decision to cluster cognitive outcome measures into domains for the meta-analysis introduced additional heterogeneity, particularly in the attention and inhibition domains; the processing speed domain was more homogeneous.
The relatively small trend-level effect on inhibition may have been related to the fact that predominantly the right hemispheric IFC mediates inhibitory functions in children and adults,74,76–84 and that meta-analyses of fMRI studies in ADHD show underactivation of the right IFC during inhibition tasks.5,6 Stimulation of the right IFC may be more effective for improving inhibitory functions in ADHD. This hypothesis is supported by studies in healthy adults showing that right IFC stimulation improves inhibitory performance. 85–88 The meta-analysis of Salehinejad and colleagues41 reported a similarly small, albeit significant, benefit (Hedges’ g = 0.12) with anodal tDCS of mostly the dlPFC on inhibitory measures, which survived when the analysis focused on accuracy measures or only on studies stimulating the dlPFC. However, Salehinejad and colleagues41 included multiple dependent effects in their meta-analyses, which could have reduced variation between effect sizes and therefore overestimated significance.44,45 Further, their meta-analysis included noninhibitory measures of attention (e.g., CPT omission errors), processing speed (e.g., reaction times to go trials) and reaction time variability,41 and therefore was not specific to inhibitory control, which could explain the differences in findings compared to our meta-analysis.
Although right IFC has been more clearly implicated in motor response inhibition, the small, trend-level improvement in inhibition in our meta-analyses, in line with the small significant effect of Salehinejad and colleagues,41 may have been due to the fact that a majority of inhibitory measures were derived from interference inhibition tasks, which have been shown to be co-mediated by the left dlPFC and IFC89 — in particular the Stroop task.90–93 Further, tDCS studies in healthy adults have reported improved performance on interference inhibition tasks following anodal tDCS of the left dlPFC.94–97 Given that only 2 studies stimulated the right IFC, future studies will have to test whether inhibition functions can be enhanced with anodal tDCS over the right IFC, which would be in line with neuroimaging evidence of a role of the right IFC in inhibitory control76,78,79,81,82,98–101 and evidence of its underactivation in ADHD,5,6,9 as well as with tDCS studies in healthy adults showing that right IFC stimulation improves motor inhibitory performance.85–88
The trend-level positive effect of tDCS of mostly the left dlPFC on processing speed is in line with fMRI evidence indicating that the left dlPFC is a key mediating region of processing speed.102–104 Interestingly, when the processing speed analysis excluded the only study stimulating the right IFC43 — and therefore included only studies stimulating mainly the left dlPFC (unilateral left, n = 5; bilateral, n = 1; bifrontal anodal left and right, n = 2)—the improvement in processing speed became significant, in line with neuroimaging evidence that the left dlPFC mediates processing speed and suggesting that the left dlPFC is an optimal site for enhancing processing speed in ADHD. These findings are partly in line with those of the meta-analysis by Salehinejad and colleagues, 41 which showed that predominantly anodal tDCS over the left dlPFC improved reaction times and its intrasubject variability in n-back tasks.
The lack of systematic positive effects on attention measures may have been because predominantly the right dlPFC and IFC mediate attention, as evidenced by individual studies and meta-analyses of fMRI studies in children and adults,77–79,84,105–107 and in meta-analyses of fMRI studies in ADHD, which show functional underactivation of the right dlPFC/IFC during attention tasks.6 It is therefore possible that stimulation of the right dlPFC and/or right IFC may be more effective for improving attention functions in ADHD.
This meta-analysis of tDCS studies shows that anodal tDCS of mostly left dlPFC has only very limited, trend-level effects on improving inhibition and processing speed, with no evidence for attention improvement. However, we cannot rule out the possibility that stimulation of other prefrontal regions (such as the right hemispheric IFC or dlPFC or parietal regions), multiple session tDCS or tDCS in combination with cognitive training could improve clinical or cognitive functions in ADHD.
With respect to safety, stimulation was well tolerated overall, but 1 tDCS study reported higher errors on a sustained attention task63 and another study reported a hypobulia episode in 1 patient,74 raising neuroethical concerns of potential costs to nontargeted functions.38,108 It has been shown that stimulation of a particular region could impair functions mediated by other regions such as the homologue contralateral region via interhemispheric inhibition or other regions that are top-down controlled by the stimulated region.109,110
Future studies need to address the lack of knowledge about optimal stimulation protocols for children with ADHD. Current knowledge about stimulation effects on the brain and standard protocols are largely derived from adult samples and are therefore not appropriate for children.108,111 In healthy adults, multi-session stimulation combined with cognitive training may lead to longer-term effects,30,112 but we do not know whether this protocol can lead to maladaptive plasticity in the developing brain, especially during “sensitivity periods,” where use-dependent plasticity changes are strongest113 and the possibility of longer-term side effects when using noninvasive brain stimulation in pediatric samples needs more empirical investigation. Future studies should heed recommendations to comprehensively assess effects in children to capture possible unintended outcomes.110,114
It should also be noted that the current findings refer only to studies using 1 to 20 Hz rTMS and conventional tDCS of dlPFC in ADHD. Other noninvasive brain stimulation protocols may be effective in ADHD, such as theta burst stimulation, 115 transcranial alternating current stimulation,116,117 transcranial random noise stimulation118,119 or trigeminal nerve stimulation (TNS).120,121
Limitations
Conclusive evidence of this systematic review of rTMS and tDCS and meta-analysis of anodal tDCS in ADHD is limited by the large heterogeneity between studies with respect to stimulation protocols (coil/electrode placement, number of sessions, stimulation intensity, crossover/parallel design), sample age and cognitive outcome measures.
Furthermore, limitations in individual studies were also present, such as tDCS electrode placement, low power, small effect sizes, biased reporting or insufficient blinding. Specifically, although all but 2 of 12 tDCS studies included in the meta-analysis stimulated the left dlPFC, electrode placement was bilateral (anode left/cathode right) in 2 studies66,68 and bifrontal (anode left and right) in 3 studies.42,60,61 Bilateral or bifrontal tDCS could have had a neutral effect, given that the short inter-electrode distance causes greater current shunting across the scalp and cerebral spinal fluid, resulting in only an estimated 35% of current reaching the brain.122 In fact, it has been shown that bilateral electrode montage over the primary motor cortex can have neurologically neutral effects. 123 With regards to low power, only 2 studies65,66 had more than 30 participants; the sample size across all other studies was relatively small, with an average of 14 participants (with an n range of 7 to 20). Key outcome measures in interference inhibition tasks such as the Stroop and flanker reaction time or error interference effects (incongruent – congruent reaction times/errors) were not reported; instead, reaction times to incongruent trials were used to measure cognitive effects, meaning that effects on interference inhibition measures were confounded by processing speed.64,67,68 Blinding integrity was not reported in 2 studies,43,64 failed in 3 studies65,67,71 and was potentially compromised in 3 studies, where at least 60% of participants correctly identified the stimulation type;62,63,74 thus, placebo effects cannot be excluded. The meta-analysis of tDCS studies was hampered by the fact that only a minority of included studies controlled for baseline differences by analyzing change scores (i.e., post-treatment minus baseline;62,65,66), while other studies analyzed only post-measurement scores to establish effects. Finally, the meta-regression analysis showed that studies with a high risk of “other” biases (e.g., no correction for multiple testing, selective reporting of outcome measures or using a lenient significance threshold43,62–64,67,68) reported larger effect sizes than studies with low or unclear risk of this bias, meaning that summary effect size estimates might have been overestimated. In early rTMS studies, the same research practices unduly inflated positive effects and slowed its uptake as an effective treatment of depression; 124,125 the field risks doing the same with rTMS and tDCS in ADHD. Moreover, with tDCS there is an added danger that children and parents — faced with apparent positive findings — will self-administer given the widely available “do-it-yourself tDCS” material online or commercial devices, one of which has been shown to impair working memory.40 Given the neuroethical concerns of brain stimulation with respect to potential negative effects on nontargeted functions,38,108,114 future researchers are duty-bound to report results to the highest possible standard.114
A final limitation of this meta-analysis is that it was not preregistered.
Conclusion
Based on current evidence, neither rTMS nor tDCS of the dlPFC can be recommended as an alternative neurotherapy for ADHD as yet. More studies are needed to assess clinical efficacy, and the demonstrated cognitive effects have been small and nonsignificant. However, we cannot rule out the possibility that rTMS or tDCS or other stimulation modalities of other regions — or even of the same region but using a larger number of sessions, different amplitude or other parameters, or combined with cognitive training — may be more effective. Furthermore, conclusive evidence from this systematic review of rTMS and tDCS studies and meta-analysis of tDCS studies in ADHD was hampered by heterogeneity in stimulation protocols, sample age and cognitive outcome measures. Larger, double-blind, randomized controlled trials with homogeneous protocols testing systematically for more optimal designs (e.g., multi-session stimulation combined with cognitive training, targeting right dorsal and ventral frontal or inferior parietal regions), 9,126 and testing both clinical and cognitive outcomes, are needed to provide better insights into clinical and cognitive effects, and to provide clear guidance on optimal stimulation protocols. Future studies should also be wary of overstating positive effects and account for possible cognitive costs of tDCS and rTMS in children.
Acknowledgements
The authors thank Sophie Wallace Hanlon (S.W.H.) for assisting S.J.W. with the literature search and study identification. We also thank Zahra Soltaninejad43,64 for providing additional and original data from her study for the meta-analysis, and for translating her paper into English.
Footnotes
Competing interests: K. Rubia has received a grant from Shire pharmaceuticals (now Takeda) for another project not relevant to this work on the effects of guanfacine and lisdexamfetamine on fMRI brain function in ADHD. No other competing interests were declared.
Funding: S. Westwood is supported by Action Medical Research (GN2426), the Garfield Weston Foundation and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and the Maudsley NHS Foundation Trust and King’s College London. K. Rubia receives research support from the Medical Research Council (MR/P012647/1) and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and the Maudsley NHS Foundation Trust and King’s College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care. J. Radua is supported by Miguel Servet Research Contract MS14/00041 and CPII19/00009, and Research Projects PI14/00292 and PI19/00394 from the Plan Nacional de I+D+i 2013–2016 and and 2017–2020, the Instituto de Salud Carlos III-Subdirección General de Evaluación y Fomento de la Investigación and the European Regional Development Fund (FEDER).
Contributors: S. Westwood, J. Radua and K. Rubia designed the study. S. Westwood and K. Rubia acquired the data, which all authors analyzed. S. Westwood and K. Rubia wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
Meeting presentations: An oral presentation of this systematic review and meta-analysis was given by S. Westwood at the European Network for Hyperkinetic Disorders (Eunethydis) International Conference, Edinburgh on Sept. 23, 2018, and at the 3rd International Brain Stimulation Conference, Vancouver, Canada, and the World Federation of ADHD (WFA) 2019. In 2019, the meta-analysis results were presented by K. Rubia at the WFA in Lisbon, at the Eunethydis conference in Nijmegen and with S. Westwood at the National Institute for Health Research Maudsley Biomedical Research Council Conference in London.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. American Psychiatric Association; Washington (DC): 2000. [Google Scholar]
- 2.Cazzoli D, Jung S, Nyffeler T, et al. The role of the right frontal eye field in overt visual attention deployment as assessed by free visual exploration. Neuropsychologia. 2015;74:37–41. doi: 10.1016/j.neuropsychologia.2015.01.027. [DOI] [PubMed] [Google Scholar]
- 3.Noreika V, Falter CM, Rubia K. Timing deficits in attention-deficit/ hyperactivity disorder (ADHD): evidence from neurocognitive and neuroimaging studies. Neuropsychologia. 2013;51:235–66. doi: 10.1016/j.neuropsychologia.2012.09.036. [DOI] [PubMed] [Google Scholar]
- 4.Coghill D, Toplak M, Rhodes S, et al. Cognitive functioning in ADHD: inhibition, memory, temporal discounting, decision-making, timing and reaction time variability. In: Banaschewski T, Coghill D, Zuddas A, editors. Oxford textbook of attention deficit hyperactivity disorder. chapter 10 Oxford, United Kingdom: Oxford University Press; 2018. [Google Scholar]
- 5.Norman LJ, Carlisi C, Lukito S, et al. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 2016;73:815–25. doi: 10.1001/jamapsychiatry.2016.0700. [DOI] [PubMed] [Google Scholar]
- 6.Hart H, Radua J, Nakao T, et al. Meta-analysis of functional magnetic resonance imaging studies of inhibition and attention in attention-deficit/hyperactivity disorder: exploring task-specific, stimulant medication, and age effects. JAMA Psychiatry. 2013;70:185–98. doi: 10.1001/jamapsychiatry.2013.277. [DOI] [PubMed] [Google Scholar]
- 7.McCarthy H, Skokauskas N, Frodl T. Identifying a consistent pattern of neural function in attention deficit hyperactivity disorder: a meta-analysis. Psychol Med. 2014;44:869–80. doi: 10.1017/S0033291713001037. [DOI] [PubMed] [Google Scholar]
- 8.Hart H, Radua J, Mataix-Cols D, et al. Meta-analysis of fMRI studies of timing in attention-deficit hyperactivity disorder (ADHD) Neurosci Biobehav Rev. 2012;36:2248–56. doi: 10.1016/j.neubiorev.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Rubia K. Cognitive neuroscience of attention deficit hyperactivity disorder (ADHD) and its clinical translation. Front Hum Neurosci. 2018;12:100. doi: 10.3389/fnhum.2018.00100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortese S, Adamo N, Del Giovane C, et al. Comparative efficacy and tolerability of medications for attention-deficit hyperactivity disorder in children, adolescents, and adults: a systematic review and network meta-analysis. Lancet Psychiatry. 2018;5:727–38. doi: 10.1016/S2215-0366(18)30269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swanson JM, Arnold LE, Jensen PS, et al. Long-term outcomes in the Multimodal Treatment study of children with ADHD (the MTA): from beginning to end. In: Banaschewski T, Coghill D, Zuddas A, editors. Oxford textbook of attention deficit hyperactivity disorder. chapter 34 Oxford, United Kingdom: Oxford University Press; 2018. [Google Scholar]
- 12.Catala-Lopez F, Hutton B, Nunez-Beltran A, et al. The pharmacological and non-pharmacological treatment of attention deficit hyperactivity disorder in children and adolescents: a systematic review with network meta-analyses of randomised trials. PLoS One. 2017;12:e0180355. doi: 10.1371/journal.pone.0180355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sonuga-Barke EJ, Brandeis D, Cortese S, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275–89. doi: 10.1176/appi.ajp.2012.12070991. [DOI] [PubMed] [Google Scholar]
- 14.Cortese S, Ferrin M, Brandeis D, et al. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54:164–74. doi: 10.1016/j.jaac.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dayan E, Censor N, Buch ER, et al. Noninvasive brain stimulation: from physiology to network dynamics and back. Nat Neurosci. 2013;16:838–44. doi: 10.1038/nn.3422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cocchi L, Zalesky A, Nott Z, et al. Transcranial magnetic stimulation in obsessive-compulsive disorder: a focus on network mechanisms and state dependence. Neuroimage Clin. 2018;19:661–74. doi: 10.1016/j.nicl.2018.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cole JC, Green Bernacki C, Helmer A, et al. Efficacy of transcranial magnetic stimulation (TMS) in the treatment of schizophrenia: a review of the literature to date. Innov Clin Neurosci. 2015;12:12–9. [PMC free article] [PubMed] [Google Scholar]
- 18.Perera T, George MS, Grammer G, et al. The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul. 2016;9:336–46. doi: 10.1016/j.brs.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janicak PG, Dokucu ME. Transcranial magnetic stimulation for the treatment of major depression. Neuropsychiatr Dis Treat. 2015;11:1549–60. doi: 10.2147/NDT.S67477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demirtas-Tatlidede A, Vahabzadeh-Hagh AM, Pascual-Leone A. Can noninvasive brain stimulation enhance cognition in neuropsychiatric disorders? Neuropharmacology. 2013;64:566–78. doi: 10.1016/j.neuropharm.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parkin BL, Ekhtiari H, Walsh VF. Non-invasive human brain stimulation in cognitive neuroscience: a primer. Neuron. 2015;87:932–45. doi: 10.1016/j.neuron.2015.07.032. [DOI] [PubMed] [Google Scholar]
- 22.Sauvaget A, Tostivint A, Etcheverrigaray F, et al. Hospital production cost of transcranial direct current stimulation (tDCS) in the treatment of depression. Neurophysiol Clin. 2019;49:11–8. doi: 10.1016/j.neucli.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 23.Etcheverrigaray F, Bulteau S, Machon LO, et al. [Hospital production cost of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression] Rev Epidemiol Sante Publique. 2015;63:268–74. doi: 10.1016/j.respe.2015.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Zaghi S, Heine N, Fregni F. Brain stimulation for the treatment of pain: a review of costs, clinical effects, and mechanisms of treatment for three different central neuromodulatory approaches. J Pain Manag. 2009;2:339–52. [PMC free article] [PubMed] [Google Scholar]
- 25.Meteyard L, Holmes NP. TMS SMART—scalp mapping of annoyance ratings and twitches caused by transcranial magnetic stimulation. J Neurosci Methods. 2018;299:34–44. doi: 10.1016/j.jneumeth.2018.02.008. [DOI] [PubMed] [Google Scholar]
- 26.Rossi S, Hallett M, Rossini PM, et al. Safety of TMSCG. Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol. 2009;120:2008–39. doi: 10.1016/j.clinph.2009.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashkan K, Shotbolt P, David AS, et al. Deep brain stimulation: a return journey from psychiatry to neurology. Postgrad Med J. 2013;89:323–8. doi: 10.1136/postgradmedj-2012-131520. [DOI] [PubMed] [Google Scholar]
- 28.Dedoncker J, Brunoni AR, Baeken C, et al. A systematic review and meta-analysis of the effects of transcranial direct current stimulation (tDCS) over the dorsolateral prefrontal cortex in healthy and neuropsychiatric samples: influence of stimulation parameters. Brain Stimul. 2016;9:501–17. doi: 10.1016/j.brs.2016.04.006. [DOI] [PubMed] [Google Scholar]
- 29.Ruf SP, Fallgatter AJ, Plewnia C. Augmentation of working memory training by transcranial direct current stimulation (tDCS) Sci Rep. 2017;7:876. doi: 10.1038/s41598-017-01055-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Katz B, Au J, Buschkuehl M, et al. Individual differences and long-term consequences of tDCS-augmented cognitive training. J Cogn Neurosci. 2017;29:1498–508. doi: 10.1162/jocn_a_01115. [DOI] [PubMed] [Google Scholar]
- 31.Moffa AH, Brunoni AR, Nikolin S, et al. Transcranial direct current stimulation in psychiatric disorders: a comprehensive review. Psychiatr Clin North Am. 2018;41:447–63. doi: 10.1016/j.psc.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Tortella G, Casati R, Aparicio LVM, et al. Transcranial direct current stimulation in psychiatric disorders. World J Psychiatry. 2015;5:88–102. doi: 10.5498/wjp.v5.i1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kekic M, Boysen E, Campbell IC, et al. A systematic review of the clinical efficacy of transcranial direct current stimulation (tDCS) in psychiatric disorders. J Psychiatr Res. 2016;74:70–86. doi: 10.1016/j.jpsychires.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 34.Horvath JC, Forte JD, Carter O. Quantitative review finds no evidence of cognitive effects in healthy populations from single-session transcranial direct current stimulation (tDCS) Brain Stimul. 2015;8:535–50. doi: 10.1016/j.brs.2015.01.400. [DOI] [PubMed] [Google Scholar]
- 35.Westwood SJ, Romani C. Transcranial direct current stimulation (tDCS) modulation of picture naming and word reading: a meta-analysis of single session tDCS applied to healthy participants. Neuropsychologia. 2017;104:234–49. doi: 10.1016/j.neuropsychologia.2017.07.031. [DOI] [PubMed] [Google Scholar]
- 36.Krishnan C, Santos L, Peterson MD, et al. Safety of noninvasive brain stimulation in children and adolescents. Brain Stimul. 2015;8:76–87. doi: 10.1016/j.brs.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rubia K. ADHD brain function. In: Banaschewski T, Coghill D, Zuddas A, editors. Oxford textbook of attention deficit hyperactivity disorder. chapter 7 Oxford, United Kingdom: Oxford University Press; 2018. [Google Scholar]
- 38.Brem AK, Fried PJ, Horvath JC, et al. Is neuroenhancement by noninvasive brain stimulation a net zero-sum proposition? Neuroimage. 2014;85:1058–68. doi: 10.1016/j.neuroimage.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fitz NS, Reiner PB. The Perils of using electrical stimulation to change human brains. In: Cohen Kadosh R, editor. The stimulated brain. San Diego (CA): Academic Press; 2014. pp. 61–83. [Google Scholar]
- 40.Steenbergen L, Sellaro R, Hommel B, et al. “Unfocus” on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res. 2016;234:637–43. doi: 10.1007/s00221-015-4391-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salehinejad MA, Wischnewski M, Nejati V, et al. Correction. Transcranial direct current stimulation in attention-deficit hyperactivity disorder: a meta-analysis of neuropsychological deficits. PLoS One. 2019;14:e0221613. doi: 10.1371/journal.pone.0221613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacoby N, Lavidor M. Null tDCS effects in a sustained attention task: the modulating role of learning. Front Psychol. 2018;9:476. doi: 10.3389/fpsyg.2018.00476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soltaninejad Z, Nejati V, Ekhtiari H. Effect of transcranial direct current stimulation on remediation of inhibitory control on right inferior frontal gyrus in attention deficit and hyperactivity symptoms. J Rehabil Med. 2015;3:1–9. [Google Scholar]
- 44.Becker BJ. Handbook of applied multi-variate statistics and mathematical modeling. San Diego (CA): Academic Press; 2000. Multivariate meta-analysis; pp. 499–525. [Google Scholar]
- 45.Lipsey MW, Wilson DB. Practical meta-analysis. Thousand Oaks, (CA): Sage Publications, Inc; 2001. [Google Scholar]
- 46.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–12. doi: 10.1016/j.jclinepi.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Conners CK, Pitkanen J, Rzepa SR. Conners. 3rd edition (Conners 3; Conners 2008) In: Kreutzer JS, DeLuca J, Caplan B, editors. Encyclopedia of clinical neuropsychology. New York: Springer New York; 2011. pp. 675–678. [Google Scholar]
- 48.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school-age children — present and lifetime version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–8. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 49.Bayoumy IM, Khaleel SH, Nada M, et al. Efficacy and attributes of repetitive transcranial magnetic stimulation (rTMS) in treatment of a sample of children with attention deficit hyperactivity disorder (ADHD) Egypt J Neurol Psychiat Neurosurg. 2014;51:361–7. [Google Scholar]
- 50.Higgins JPT, Altman DG, G⊘tzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bandeira ID, Guimaraes RS, Jagersbacher JG, et al. Transcranial direct current stimulation in children and adolescents with attention- deficit/hyperactivity disorder (ADHD): a pilot study. J Child Neurol. 2016;31:918–24. doi: 10.1177/0883073816630083. [DOI] [PubMed] [Google Scholar]
- 52.Gomez L, Vidal B, Morales L, et al. Low frequency repetitive transcranial magnetic stimulation in children with attention deficit/hyperactivity disorder: preliminary results. Brain Stimul. 2014;7:760–2. doi: 10.1016/j.brs.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 53.Kuntsi J, Wood AC, Rijsdijk F, et al. Separation of cognitive impairments in attention-deficit/hyperactivity disorder into 2 familial factors. Arch Gen Psychiatry. 2010;67:1159–67. doi: 10.1001/archgenpsychiatry.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubia K, Smith A, Taylor E. Performance of children with attention deficit hyperactivity disorder (ADHD) on a test battery of impulsiveness. Child Neuropsychol. 2007;13:276–304. doi: 10.1080/09297040600770761. [DOI] [PubMed] [Google Scholar]
- 55.Coghill DR, Seth S, Matthews K. A comprehensive assessment of memory, delay aversion, timing, inhibition, decision making and variability in attention deficit hyperactivity disorder: advancing beyond the three-pathway models. Psychol Med. 2014;44:1989–2001. doi: 10.1017/S0033291713002547. [DOI] [PubMed] [Google Scholar]
- 56.Cheung CH, Rijsdijk F, McLoughlin G, et al. Cognitive and neurophysiological markers of ADHD persistence and remission. Br J Psychiatry. 2016;208:548–55. doi: 10.1192/bjp.bp.114.145185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Higgins JPT, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc. 2009;172:137–59. doi: 10.1111/j.1467-985X.2008.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Borenstein M, Higgins JPT, Hedges LV, et al. Basics of meta-analysis: I2 is not an absolute measure of heterogeneity. Res Synth Methods. 2017;8:5–18. doi: 10.1002/jrsm.1230. [DOI] [PubMed] [Google Scholar]
- 60.Munz MT, Prehn-Kristensen A, Thielking F, et al. Slow oscillating transcranial direct current stimulation during non-rapid eye movement sleep improves behavioral inhibition in attention-deficit/ hyperactivity disorder. Front Cell Neurosci. 2015;9:307. doi: 10.3389/fncel.2015.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prehn-Kristensen A, Munz M, Goder R, et al. Transcranial oscillatory direct current stimulation during sleep improves declarative memory consolidation in children with attention-deficit/hyperactivity disorder to a level comparable to healthy controls. Brain Stimul. 2014;7:793–9. doi: 10.1016/j.brs.2014.07.036. [DOI] [PubMed] [Google Scholar]
- 62.Soff C, Sotnikova A, Christiansen H, et al. Transcranial direct current stimulation improves clinical symptoms in adolescents with attention deficit hyperactivity disorder. J Neural Transm (Vienna) 2017;124:133–44. doi: 10.1007/s00702-016-1646-y. [DOI] [PubMed] [Google Scholar]
- 63.Sotnikova A, Soff C, Tagliazucchi E, et al. Transcranial direct current stimulation modulates neuronal networks in attention deficit hyperactivity disorder. Brain Topogr. 2017;30:656–72. doi: 10.1007/s10548-017-0552-4. [DOI] [PubMed] [Google Scholar]
- 64.Soltaninejad Z, Nejati V, Ekhtiari H. Effect of anodal and cathodal transcranial direct current stimulation on DLPFC on modulation of inhibitory control in ADHD. J Atten Disord. 2019;23:325–32. doi: 10.1177/1087054715618792. [DOI] [PubMed] [Google Scholar]
- 65.Allenby C, Falcone M, Bernardo L, et al. Transcranial direct current brain stimulation decreases impulsivity in ADHD. Brain Stimul. 2018;11:974–81. doi: 10.1016/j.brs.2018.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cosmo C, Baptista AF, de Araujo AN, et al. A randomized, double-blind, sham- controlled trial of transcranial direct current stimulation in attention-deficit/hyperactivity disorder. PLoS One. 2015;10:e0135371. doi: 10.1371/journal.pone.0135371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Breitling C, Zaehle T, Dannhauer M, et al. Improving interference control in ADHD patients with transcranial direct current stimulation (tDCS) Front Cell Neurosci. 2016;10:72. doi: 10.3389/fncel.2016.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nejati V, Salehinejad MA, Nitsche MA, et al. Transcranial direct current stimulation improves executive dysfunctions in ADHD: implications for inhibitory control, interference control, working memory, and cognitive flexibility. J Atten Disord. 2017 doi: 10.1177/1087054717730611. 1087054717730611. [DOI] [PubMed] [Google Scholar]
- 69.Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;1:2010. [Google Scholar]
- 70.RStudio: integrated development for R. Version 3.6. Boston (MA): RStudio, Inc; 2015. [Google Scholar]
- 71.Bloch Y, Harel EV, Aviram S, et al. Positive effects of repetitive transcranial magnetic stimulation on attention in ADHD subjects: a randomized controlled pilot study. World J Biol Psychiatry. 2010;11:755–8. doi: 10.3109/15622975.2010.484466. [DOI] [PubMed] [Google Scholar]
- 72.Weaver L, Rostain AL, Mac EW, et al. Transcranial magnetic stimulation (TMS) in the treatment of attention-deficit/hyperactivity disorder in adolescents and young adults: a pilot study. J ECT. 2012;28:98–103. doi: 10.1097/YCT.0b013e31824532c8. [DOI] [PubMed] [Google Scholar]
- 73.Paz Y, Friedwald K, Levkovitz Y, et al. Randomised sham-controlled study of high-frequency bilateral deep transcranial magnetic stimulation (dTMS) to treat adult attention hyperactive disorder (ADHD): negative results. World J Biol Psychiatry. 2018;19:561–6. doi: 10.1080/15622975.2017.1282170. [DOI] [PubMed] [Google Scholar]
- 74.Cachoeira CT, Leffa DT, Mittelstadt SD, et al. Positive effects of transcranial direct current stimulation in adult patients with attention-deficit/hyperactivity disorder: a pilot randomized controlled study. Psychiatry Res. 2017;247:28–32. doi: 10.1016/j.psychres.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 75.Rubia K, Smith AB, Brammer MJ, et al. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–8. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 76.Rubia K, Lim L, Ecker C, et al. Effects of age and gender on neural networks of motor response inhibition: from adolescence to mid-adulthood. Neuroimage. 2013;83:690–703. doi: 10.1016/j.neuroimage.2013.06.078. [DOI] [PubMed] [Google Scholar]
- 77.Sebastian A, Jung P, Neuhoff J, et al. Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: a combined task-specific and coordinate-based meta-analytic fMRI study. Brain Struct Funct. 2016;221:1635–51. doi: 10.1007/s00429-015-0994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Manly T, Owen AM, McAvinue L, et al. Enhancing the sensitivity of a sustained attention task to frontal damage: convergent clinical and functional imaging evidence. Neurocase. 2003;9:340–9. doi: 10.1076/neur.9.4.340.15553. [DOI] [PubMed] [Google Scholar]
- 79.Smith AB, Halari R, Giampetro V, et al. Developmental effects of reward on sustained attention networks. Neuroimage. 2011;56:1693–704. doi: 10.1016/j.neuroimage.2011.01.072. [DOI] [PubMed] [Google Scholar]
- 80.Zhang R, Geng X, Lee TMC. Large-scale functional neural network correlates of response inhibition: an fMRI meta-analysis. Brain Struct Funct. 2017;222:3973–90. doi: 10.1007/s00429-017-1443-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rae CL, Hughes LE, Weaver C, et al. Selection and stopping in voluntary action: a meta-analysis and combined fMRI study. Neuroimage. 2014;86:381–91. doi: 10.1016/j.neuroimage.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dambacher F, Sack AT, Lobbestael J, et al. A network approach to response inhibition: dissociating functional connectivity of neural components involved in action restraint and action cancellation. Eur J Neurosci. 2014;39:821–31. doi: 10.1111/ejn.12425. [DOI] [PubMed] [Google Scholar]
- 83.Criaud M, Boulinguez P. Have we been asking the right questions when assessing response inhibition in go/no-go tasks with fMRI? A meta-analysis and critical review. Neurosci Biobehav Rev. 2013;37:11–23. doi: 10.1016/j.neubiorev.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 84.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–24. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Campanella S, Schroder E, Vanderhasselt MA, et al. Short-term impact of tDCS over the right inferior frontal cortex on impulsive responses in a go/no-go task. Clin EEG Neurosci. 2018;49:398–406. doi: 10.1177/1550059418777404. [DOI] [PubMed] [Google Scholar]
- 86.Cunillera T, Brignani D, Cucurell D, et al. The right inferior frontal cortex in response inhibition: a tDCS–ERP co-registration study. Neuroimage. 2016;140:66–75. doi: 10.1016/j.neuroimage.2015.11.044. [DOI] [PubMed] [Google Scholar]
- 87.Jacobson L, Javitt DC, Lavidor M. Activation of inhibition: diminishing impulsive behavior by direct current stimulation over the inferior frontal gyrus. J Cogn Neurosci. 2011;23:3380–7. doi: 10.1162/jocn_a_00020. [DOI] [PubMed] [Google Scholar]
- 88.Hogeveen J, Grafman J, Aboseria M, et al. Effects of high-definition and conventional tDCS on response inhibition. Brain Stimul. 2016;9:720–9. doi: 10.1016/j.brs.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 89.Christakou A, Halari R, Smith AB, et al. Sex-dependent age modulation of frontostriatal and temporo-parietal activation during cognitive control. Neuroimage. 2009;48:223–36. doi: 10.1016/j.neuroimage.2009.06.070. [DOI] [PubMed] [Google Scholar]
- 90.van’t Ent D, van Beijsterveldt CEM, Derks EM, et al. Neuroimaging of response interference in twins concordant or discordant for inattention and hyperactivity symptoms. Neuroscience. 2009;164:16–29. doi: 10.1016/j.neuroscience.2009.01.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hwang S, White SF, Nolan ZT, et al. Executive attention control and emotional responding in attention-deficit/hyperactivity disorder: a functional MRI study. Neuroimage Clin. 2015;9:545–54. doi: 10.1016/j.nicl.2015.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chou T-L, Chia S, Shang C-Y, et al. Differential therapeutic effects of 12-week treatment of atomoxetine and methylphenidate on drug-naïve children with attention deficit/hyperactivity disorder: a counting Stroop functional MRI study. Eur Neuropsychopharmacol. 2015;25:2300–10. doi: 10.1016/j.euroneuro.2015.08.024. [DOI] [PubMed] [Google Scholar]
- 93.Peterson BS, Potenza MN, Wang Z, et al. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. Am J Psychiatry. 2009;166:1286–94. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Loftus AM, Yalcin O, Baughman FD, et al. The impact of transcranial direct current stimulation on inhibitory control in young adults. Brain Behav. 2015;5:e00332. doi: 10.1002/brb3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Frings C, Brinkmann T, Friehs MA, et al. Single session tDCS over the left DLPFC disrupts interference processing. Brain Cogn. 2018;120:1–7. doi: 10.1016/j.bandc.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 96.Jeon SY, Han SJ. Improvement of the working memory and naming by transcranial direct current stimulation. Ann Rehabil Med. 2012;36:585–95. doi: 10.5535/arm.2012.36.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baumert A, Buchholz N, Zinkernagel A, et al. Causal underpinnings of working memory and Stroop interference control: testing the effects of anodal and cathodal tDCS over the left DLPFC. Cogn Affect Behav Neurosci. 2020;20:34–48. doi: 10.3758/s13415-019-00726-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dambacher F, Schuhmann T, Lobbestael J, et al. Reducing proactive aggression through non-invasive brain stimulation. Soc Cogn Affect Neurosci. 2015;10:1303–9. doi: 10.1093/scan/nsv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dambacher F, Schuhmann T, Lobbestael J, et al. No effects of bilateral tDCS over inferior frontal gyrus on response inhibition and aggression. PLoS One. 2015;10:e0132170. doi: 10.1371/journal.pone.0132170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubia K, Smith AB, Brammer MJ, et al. Right inferior prefrontal cortex mediates response inhibition while mesial prefrontal cortex is responsible for error detection. Neuroimage. 2003;20:351–8. doi: 10.1016/s1053-8119(03)00275-1. [DOI] [PubMed] [Google Scholar]
- 101.Sebastian A, Jung P, Neuhoff J, et al. Dissociable attentional and inhibitory networks of dorsal and ventral areas of the right inferior frontal cortex: a combined task-specific and coordinate-based meta-analytic fMRI study. Brain Struct Funct. 2016;221:1635–51. doi: 10.1007/s00429-015-0994-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Motes MA, Biswal BB, Rypma B. Age-dependent relationships between prefrontal cortex activation and processing efficiency. Cogn Neurosci. 2011;2:1–10. doi: 10.1080/17588928.2010.512974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Motes MA, Yezhuvath US, Aslan S, et al. Higher-order cognitive training effects on processing speed–related neural activity: a randomized trial. Neurobiol Aging. 2018;62:72–81. doi: 10.1016/j.neurobiolaging.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacobs HI, Leritz EC, Williams VJ, et al. Association between white matter microstructure, executive functions, and processing speed in older adults: the impact of vascular health. Hum Brain Mapp. 2013;34:77–95. doi: 10.1002/hbm.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lawrence NS, Ross TJ, Hoffmann R, et al. Multiple neuronal networks mediate sustained attention. J Cogn Neurosci. 2003;15:1028–38. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- 106.Hampshire A, Chamberlain SR, Monti MM, et al. The role of the right inferior frontal gyrus: inhibition and attentional control. Neuroimage. 2010;50:1313–9. doi: 10.1016/j.neuroimage.2009.12.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Langner R, Eickhoff SB. Sustaining attention to simple tasks: a meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013;139:870–900. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davis NJ. Transcranial stimulation of the developing brain: a plea for extreme caution. Front Hum Neurosci. 2014;8:600. doi: 10.3389/fnhum.2014.00600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Iuculano T, Cohen Kadosh R. The mental cost of cognitive enhancement. J Neurosci. 2013;33:4482. doi: 10.1523/JNEUROSCI.4927-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cohen Kadosh R, Levy N, O’Shea J, et al. The neuroethics of non-invasive brain stimulation. Curr Biol. 2012;22:R108–11. doi: 10.1016/j.cub.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kessler SK, Minhas P, Woods AJ, et al. Dosage considerations for transcranial direct current stimulation in children: a computational modeling study. PLoS One. 2013;8:e76112. doi: 10.1371/journal.pone.0076112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Berryhill ME. Longitudinal tDCS: Consistency across working memory training studies. AIMS Neuroscience. 2017;4:71–86. [Google Scholar]
- 113.Knudsen EI. Sensitive periods in the development of the brain and behavior. J Cogn Neurosci. 2004;16:1412–25. doi: 10.1162/0898929042304796. [DOI] [PubMed] [Google Scholar]
- 114.Baldwin T, Cole J, Fitzgerald M, et al. Novel neurotechnologies. London: Nuffield Council on Bioethics; 2013. [Google Scholar]
- 115.Lowe CJ, Manocchio F, Safati AB, et al. The effects of theta burst stimulation (TBS) targeting the prefrontal cortex on executive functioning: a systematic review and meta-analysis. Neuropsychologia. 2018;111:344–59. doi: 10.1016/j.neuropsychologia.2018.02.004. [DOI] [PubMed] [Google Scholar]
- 116.Alexander ML, Alagapan S, Lugo CE, et al. Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD) Transl Psychiatry. 2019;9:106. doi: 10.1038/s41398-019-0439-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Iuculano T, Cohen Kadosh R. Preliminary evidence for performance enhancement following parietal lobe stimulation in developmental dyscalculia. Front Hum Neurosci. 2014;8:38. doi: 10.3389/fnhum.2014.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mulquiney PG, Hoy KE, Daskalakis ZJ, et al. Improving working memory: exploring the effect of transcranial random noise stimulation and transcranial direct current stimulation on the dorsolateral prefrontal cortex. Clin Neurophysiol. 2011;122:2384–9. doi: 10.1016/j.clinph.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 119.Terney D, Chaieb L, Moliadze V, et al. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. 2008;28:14147–55. doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McGough JJ, Loo SK, Sturm A, et al. An eight-week, open-trial, pilot feasibility study of trigeminal nerve stimulation in youth with attention-deficit/hyperactivity disorder. Brain Stimul. 2015;8:299–304. doi: 10.1016/j.brs.2014.11.013. [DOI] [PubMed] [Google Scholar]
- 121.McGough JJ, Sturm A, Cowen J, et al. Double-blind, sham-controlled, pilot study of trigeminal nerve stimulation for attention-deficit/ hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2019;58:403–411.e3. doi: 10.1016/j.jaac.2018.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Faria P, Hallett M, Miranda PC. A finite element analysis of the effect of electrode area and inter-electrode distance on the spatial distribution of the current density in tDCS. J Neural Eng. 2011;8:066017–066017. doi: 10.1088/1741-2560/8/6/066017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Parkin BL, Bhandari M, Glen JC, et al. The physiological effects of transcranial electrical stimulation do not apply to parameters commonly used in studies of cognitive neuromodulation. Neuropsychologia. 2019;128:332–9. doi: 10.1016/j.neuropsychologia.2018.03.030. [DOI] [PubMed] [Google Scholar]
- 124.Walsh VQ. Ethics and social risks in brain stimulation. Brain Stimul. 2013;6:715–7. doi: 10.1016/j.brs.2013.08.001. [DOI] [PubMed] [Google Scholar]
- 125.Amad A, Jardri R, Rousseau C, et al. Excess significance bias in repetitive transcranial magnetic stimulation literature for neuropsychiatric disorders. Psychother Psychosom. 2019;88:363–70. doi: 10.1159/000502805. [DOI] [PubMed] [Google Scholar]
- 126.Sathappan AV, Luber BM, Lisanby SH. The dynamic duo: combining noninvasive brain stimulation with cognitive interventions. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:347–60. doi: 10.1016/j.pnpbp.2018.10.006. [DOI] [PubMed] [Google Scholar]