Abstract
Background
Reductions in total hippocampus volume have frequently been reported in MRI studies in major depressive disorder (MDD), but reports of differences in total amygdala volume have been inconsistent. Childhood maltreatment is an important risk factor for MDD in adulthood and may affect the volume of the hippocampus and amygdala. In the present study, we examined associations between the volumes of the amygdala subnuclei and hippocampal subfields and history of childhood maltreatment in participants with MDD.
Methods
We recruited 35 patients who met the DSM-IV criteria for MDD and 35 healthy controls. We acquired MRI data sets on a 4.7 T Varian Inova scanner. We manually delineated the amygdala subnuclei (lateral, basal and accessory basal nuclei, and the cortical and centromedial groups) and hippocampal subfields (cornu ammonis, subiculum and dentate gyrus) using reliable volumetric methods. We assessed childhood maltreatment using the Childhood Trauma Questionnaire in participants with MDD.
Results
In participants with MDD, a history of childhood maltreatment had significant negative associations with volume in the right amygdala, anterior hippocampus and total cornu ammonis subfield bilaterally. For volumes of the amygdala subnuclei, such effects were limited to the basal, accessory basal and cortical subnuclei in the right hemisphere, but they did not survive correction for multiple comparisons. We did not find significant effects of MDD or antidepressant treatment on volumes of the amygdala subnuclei.
Limitations
Our study was a cross-sectional study.
Conclusion
Our results provide evidence of negative associations between history of childhood maltreatment and volumes of medial temporal lobe structures in participants with MDD. This may help to identify potential mechanisms by which maltreatment leads to clinical impacts.
Introduction
Major depressive disorder (MDD) causes disability around the world,1 and its etiology remains uncertain, although changes in the hippocampus and amygdala have been suggested as potentially important.2 Volumetric reductions in the hippocampus have often been reported in MRI studies of MDD.2–5 Although the amygdala is an important structure in neuronal circuits of emotion, fear and stress,6,7 the results of volumetric MRI studies of the amygdala in MDD have been inconsistent.2,8–10 The amygdala consists of several functionally different subnuclei groups7: the basolateral complex, including the lateral, basal and accessory basal nuclei; the centromedial group, including the central and medial nuclei; and the cortical group. Generally, the basolateral amygdala is involved in learning and memory through its connections with the prefrontal cortex and the hippocampus.11 Animal studies indicate that during chronic stress, the basolateral amygdala undergoes adaptive plastic changes.12 The centromedial amygdala is involved in the regulation of behavioural, autonomic and hormonal responses to emotional stimuli via its connections with the hypothalamus.11 The cortical group is involved in olfactory-related responses.6,13 Until recently, MRI studies of the amygdala have examined the amygdala only at the level of its total volume,2 but recent advances in MRI have enabled researchers to measure individual amygdala subnuclei in vivo.14
Childhood adversity, including trauma and maltreatment, has been recognized as an important risk factor for developing MDD in adulthood.15 It can induce biological changes in stress-related brain structures, which may then become maladaptive in adult environments and make people more vulnerable to psychiatric disorders.16 Previous MRI studies have found that childhood adversity is associated with smaller volumes in the anterior cingulate, dorsolateral prefrontal and orbitofrontal cortices, and in the hippocampus.17 However, the effects of childhood adversity might not be uniform across the entire structure of the hippocampus.18,19 The hippocampus can be divided into subregions along its anteroposterior axis (head, body and tail), and into cellular subfields along its cross-sectional axis, including the dentate gyrus, the cornu ammonis (CA1–3) and the subiculum.20
Recent MRI studies that measured the volumes of hippocampal subfields reported equivocal findings: patients with MDD had smaller volumes in the dentate gyrus,21 CA1–321,22 or all subfields.23 In contrast, other studies found no differences24 or reported that volume reductions were present only in unmedicated patients.21 The volumetric results for hippocampal subfields from the MDD cohort in the present study have been reported elsewhere.21,25
Animal models of adult chronic stress indicate that the CA3 subfield is most susceptible to cellular changes associated with prolonged stressors and glucocorticoid exposure.26 Additional changes include dendritic retraction and suppressed adult neurogenesis in the dentate gyrus subfield.27,28
Previous human studies have suggested that the adverse effects of psychological stress and childhood adversity were more pronounced in the anterior hippocampus18,29 and the CA2–3 and CA4–dentate gyrus subfields.19 Although the results of individual studies for the relationship between total amygdala volume and childhood adversity have been inconsistent, 30–34 2 meta-analyses showed that people who experienced maltreatment, regardless of the absence or presence of psychiatric disorders, had significantly smaller total amygdala volumes than people who were not maltreated.35,36
Most of the work on the effect of stress on the amygdala and hippocampal substructures has been conducted in animals; 12,26 direct testing of preclinical stress models in humans has been impossible to date. However, recent advances in high-resolution MRI of the hippocampal subfields20 and amygdala subnuclei14 have allowed researchers to test these models in vivo in humans for the first time.
In this study we aimed to investigate volumetric differences in amygdala subnuclei between participants with MDD and healthy controls; and to determine the relationships between childhood maltreatment (as measured using the Childhood Trauma Questionnaire [CTQ]) and the volumes of the amygdala subnuclei and hippocampal subfields in people with MDD using ultra-high-resolution 4.7 T MRI methods developed by our group.14,20
Considering the findings of 2 previous meta-analyses on total amygdala volumes in MDD2,9 that did not show a significant effect of MDD, we did not expect to find significant differences in amygdala volume. However, based on previous animal12,26,37 and human studies,17,18,29,35,36 we hypothesized that childhood adversity would be associated with volumetric reductions in the basolateral amygdala and in the CA1–3 and dentate gyrus subfields — especially in the anterior hippocampus — in participants with MDD. Furthermore, considering the results of previous studies34–36,38 showing that the effects of childhood maltreatment were more pronounced in the right amygdala, we hypothesized that the effects of childhood adversity on the basolateral amygdala would be more pronounced in the right hemisphere.
Methods
Participants
A total of 35 participants with MDD were included in the study: 12 males and 23 premenopausal females aged 18 to 49 years who met the DSM-IV criteria for MDD with moderate or severe episodes, based on a full clinical assessment and the Anxiety Disorders Interview Schedule for DSM-IV–Lifetime Version.39 We also recruited 35 age-, sex- and education-matched healthy controls (12 males, 23 premenopausal females) through local advertisements. Written informed consent was obtained, and the study was approved by the University of Alberta Health Research Ethics Board.
Participants with MDD were recruited from local notices or referred directly from the outpatient psychiatry department by the authors (N.J.C. and P.H.S.). Among the participants with MDD, 25 reported continuous use of antidepressant treatment for more than 6 months, and 10 were either antidepressant-naïve or medication-free for 6 months or more.
Exclusion criteria for participants with MDD were mild depressive episodes; psychotic or atypical features; seasonal affective disorder; lifetime schizophrenia, bipolar disorder, alcohol or substance dependence, anorexia nervosa, or predominant personality or anxiety disorders; antipsychotic or mood stabilizer treatment; corticosteroid use; or significant medical or neurologic diseases. Healthy controls had no lifetime psychiatric disorders, as assessed with the Anxiety Disorders Interview Schedule for DSM-IV–Lifetime Version, and no reported psychosis or mood disorders in first-degree relatives.
We assessed the severity of depressive symptoms using the 17-item Hamilton Depression Rating Scale. We assessed childhood maltreatment in participants with MDD using the 28-item CTQ, which includes 5 subscales of 5 items each (emotional abuse, physical abuse, sexual abuse, emotional neglect and physical neglect) and 3 validity items.40
MRI data acquisition and analysis
We acquired images using a 4.7 T MRI system (Varian). We used a T2-weighted fast spin echo MRI sequence (echo/repetition time: 39/11 000 ms; field of view: 20 × 20 cm; acquisition time: 13.5 minutes; native resolution: 0.52 × 0.68 × 1.0 mm3) to obtain 90 contiguous coronal slices perpendicular to the anterior–posterior commissure. To estimate intracranial volume (ICV), we acquired a whole-brain T1-weighted 3D magnetization-prepared rapid gradient echo (MPRAGE) sequence (axial, echo/repetition time: 8.5 ms/4.5 ms; field of view: 256 × 200 × 180 mm3; voxel size: 1 × 1 × 1 mm3). We used the program DISPLAY (Montreal Neurological Institute) to manually trace ICV using the method of Eritaia41 on the T1-weighted MPRAGE images.
We have published detailed protocols for the manual delineation of the total amygdala,42 amygdala subnuclei,14 hippocampal subregions42 and hippocampal subfields (CA1–3, dentate gyrus and subiculum;20 Fig. 143 and Appendix 1, available at jpn.ca/200034-a1). Two raters measured the amygdala (AAS) and hippocampus (YH) in the T2-weighted fast spin echo images using DISPLAY. Inter- and intra-rater reliabilities and Dice’s kappa coefficients for volumetric measurements have been reported previously (Table 1; see Appendix 1 for more details).14,20,42 We then normalized the raw volumetric measurements of the amygdala and hippocampus to ICV using a proportional method: normalized volume = (raw volume [mm3]/ICV [cm3]) × sample average ICV (cm3).
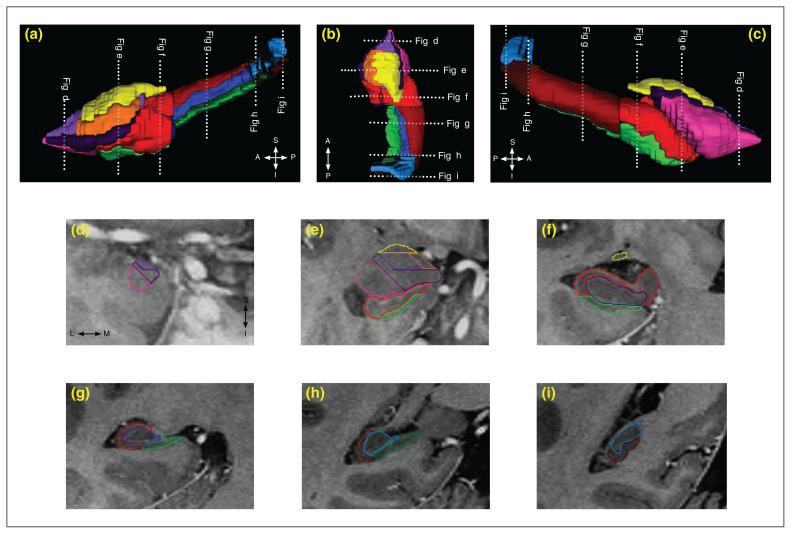
Fig. 1.
Three-dimensional reconstructions of the hippocampal subfields and subregions, as well as the amygdala and its subnuclei, from a healthy individual. Three-dimensional images from the (a) medial, (b) superior and (c) lateral views were created using ITK-SNAP software (version 3.8.0).43 (d–i) Segmentation of the hippocampal subfields and subregions, as well as the amygdala subnuclei on coronal views, are shown on T2-weighted fast spin echo images with inverted contrast. For the hippocampal subfields, the cornu ammonis (CA 1–3) is outlined in red, the dentate gyrus is outlined in blue and the subiculum is outlined in green; hippocampal subfields along the anteroposterior axis of the hippocampus (head, body and tail) are shown with different colour intensities. For the amygdala subnuclei, the lateral nucleus is outlined in pink, the basal nucleus is outlined in dark purple, the accessory basal nucleus is outlined in light purple, the cortical group is outlined in orange and the centromedial group is outlined in yellow. Dotted lines indicate the locations of corresponding MRI slices.
Table 1.
Reliability results
| Region | Intraclass correlation coefficients | Dice’s kappa | ||
|---|---|---|---|---|
|
| ||||
| Intra-rater | Inter-rater | Intra-rater | Inter-rater | |
| Lateral nucleus | 0.95 | 0.93 | 0.86 | 0.82 |
| Basal nucleus | 0.94 | 0.87 | 0.81 | 0.76 |
| Accessory basal nucleus | 0.96 | 0.95 | 0.77 | 0.71 |
| Cortical group | 0.97 | 0.87 | 0.72 | 0.72 |
| Centromedial group | 0.86 | 0.85 | 0.79 | 0.76 |
| Total amygdala | 0.93 | 0.95 | 0.93 | 0.91 |
| Hippocampus head | 0.92 | 0.95 | 0.90 | 0.89 |
| Hippocampus body | 0.93 | 0.83 | 0.87 | 0.86 |
| Hippocampus tail | 0.88 | 0.95 | 0.82 | 0.80 |
| Cornu ammonis | 0.92 | 0.92 | 0.75 | 0.73 |
| Dentate gyrus | 0.84 | 0.86 | 0.81 | 0.81 |
| Subiculum | 0.95 | 0.87 | 0.74 | 0.74 |
| Total hippocampus | 0.97 | 0.95 | 0.90 | 0.89 |
Statistical analysis
All analyses were carried out in SPSS Statistics 25.0 (IBM). We used 1-way analysis of variance to compare age, education, MDD characteristics and ICV in participants with MDD and controls. We used analysis of covariance (ANCOVA) to compare amygdala volumes between participants with MDD and healthy controls, and between participants with medicated and unmedicated MDD and healthy controls. The ANCOVAs included volume as the dependent variable, diagnosis (or treatment) as a between-subject factor, hemisphere as a within-subject factor, interaction between diagnosis or treatment and hemisphere, and ICV as covariates. For diagnosis, analyses were 2-group; for treatment, analyses were 3-group, followed by pair-wise ANCOVA of subgroups. We tested correlations between normalized volumetric measurements, CTQ scores and clinical characteristics in participants with MDD using Pearson or Spearman correlations, as appropriate. Because of our a priori hypotheses, for the amygdala we examined effects of childhood adversity first on the volume of the total amygdala and its subnuclei (hypothesis-driven analysis) and second on the volumes of the individual basolateral amygdala subnuclei (exploratory analysis). Similarly for the hippocampus, we examined the effects of childhood adversity first on the volume of the total hippocampus and the total volume of its subfields (CA1–3, dentate gyrus, subiculum) and subregions (head v. body and tail; hypothesis-driven analysis), and second on the volume of subfields in the hippocampal subregions (exploratory analysis). We also conducted exploratory analyses for all CTQ factors with individual regions of interest. We used Holm–Bonferroni correction for type I error inflation because of multiple hypothesis testing: for all amygdala groups including centromedial, cortical and basolateral (n = 3); for basolateral subnuclei (n = 3); for bilateral hippocampi (n = 2); for total hippocampal subfields (n = 3); for hippocampal subfields in each hippocampal subregion (n = 3); and for all CTQ factors (n = 5). We used Levene’s test to check homogeneity of variance; significance was set at p < 0.05 using a 2-tailed test for all analyses, except for associations between childhood adversity and amygdala-and hippocampus-related volumes, for which we used p < 0.05, 1-tailed (i.e., negative correlations were hypothesized).
Results
Demographic and clinical characteristics
We found no significant differences in age, sex, education or ICV between participants with MDD and healthy controls, or in demographic and clinical characteristics between unmedicated and medicated participants with MDD (Table 2). Analysis of variance showed no significant association between sex and CTQ measurements (all p > 0.1; Table 3), indicating that male and female participants with MDD did not differ from each other.
Table 2.
Demographic and clinical characteristics
| Characteristics | Healthy controls (n = 35) | MDD | F | p values | |||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Total (n = 35) | Medicated (n = 25) | Unmedicated (n = 10) | Controls v. MDD | 3 groups* | |||
| Demographic characteristics | |||||||
| Female/male, n | 23/12 | 23/12 | 17/8 | 6/4 | — | 1.0 | 0.91 |
| Age, yr | 32.3 ± 10.0 | 34.9 ± 8.7 | 36.1 ± 7.7 | 31.9 ± 10.8 | — | 0.25 | 0.25 |
| Education, yr | 15.7 ± 1.7 | 15.4 ± 1.8 | 15.7 ± 1.5 | 14.6 ± 2.2 | — | 0.55 | 0.19 |
| Intracranial volume, cm3 | 1599 ± 172 | 1561 ± 154 | 1537 ± 156 | 1622 ± 141 | — | 0.33 | 0.24 |
| MDD clinical characteristics | |||||||
| HAM-D score | — | — | 17.0 ± 8.4 | 20.0 ± 2.6 | 3.04 | 0.09† | |
| Duration of MDD, yr | — | — | 4.6 ± 3.7 | 4.5 ± 4.7 | 0.004 | 0.95 | |
| Recurrent/nonrecurrent, n | — | — | 20/5 | 7/3 | NA | 0.66‡ | |
| Major depressive episode, n | — | — | 2.5 ± 1.1 | 2.3 ± 1.1 | 0.30 | 0.59 | |
HAM-D = Hamilton Depression Rating Scale; MDD = major depressive disorder; NA = not applicable.
Values are mean ± standard deviation unless otherwise indicated.
Three-group comparison between healthy controls, medicated MDD and unmedicated MDD.
Adjusted for Welch’s F-test because of violation of the homogeneity of variance.
Adjusted for Fisher’s exact test because of violation of Pearson’s χ2 assumption.
Table 3.
Childhood Trauma Questionnaire scores
| Total CTQ and CTQ factors | Patients with MDD | p values* | ||
|---|---|---|---|---|
|
| ||||
| Total (n = 33) | Male (n = 12) | Female (n = 21) | ||
| Total | 50.1 ± 19.0 | 44.7 ± 17.8 | 53.2 ± 19.4 | 0.18 |
| Emotional abuse | 11.7 ± 5.4 | 9.7 ± 5.8 | 12.9 ± 5.0 | 0.08 |
| Physical abuse | 8.3 ± 4.1 | 7.4 ± 3.3 | 8.8 ± 4.5 | 0.35 |
| Sexual abuse | 8.4 ± 6.2 | 7.8 ± 5.8 | 8.8 ± 6.5 | 0.65 |
| Emotional neglect | 13.5 ± 5.4 | 12.9 ± 4.9 | 13.8 ± 5.8 | 0.66 |
| Physical neglect | 8.2 ± 3.8 | 6.9 ± 3.3 | 9.0 ± 3.9 | 0.06 |
CTQ = Childhood Trauma Questionnaire; MDD = major depressive disorder.
Total scores and scores for all factors on the CTQ but emotional neglect were not normally distributed; we compared findings for males and females using the Mann–Whitney U test.
Amygdala volumetric analyses
Diagnosis (participants with MDD v. controls), hemisphere, treatment (unmedicated MDD v. medicated MDD v. controls) and hemisphere interactions were not significant for any amygdala measurement (all p > 0.23), indicating that any group differences were not lateralized. We found no main effects of diagnosis in the 2-group analyses for total amygdala volume or the total volume of each subnucleus group (Table 4). Exploratory analysis of the individual basolateral subnuclei also revealed no significant effect of diagnosis or treatment on the total volume of each subnucleus (all p > 0.16). Correlational analyses revealed no significant relationships between the volumes of the amygdala or its subnuclei and Hamilton Depression Rating Scale score, MDD duration or number of major depressive episodes (all p > 0.16).
Table 4.
Analysis of covariance and descriptive statistics for amygdala measurements*
| Region of interest | Healthy controls v. MDD | Healthy controls v. unmedicated MDD v. medicated MDD | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Healthy controls (n = 35) | MDD (n = 35) | F1,67 | p value† | Unmedicated MDD (n = 10) | Medicated MDD (n = 25) | F2,66 | p value‡ | Unmedicated MDD v. healthy controls | Medicated MDD v. healthy controls | Unmedicated MDD v. medicated MDD | ||||
|
|
|
|
||||||||||||
| F1,42 | p value | F1,57 | p value | F1,32 | p value | |||||||||
|
|
|
|||||||||||||
| Total amygdala | 1994 ± 330 | 1880 ± 307 | 1.35 | 0.25 | 1933 ± 285 | 1858 ± 319 | 0.67 | 0.52 | 0.88 | 0.35 | 1.10 | 0.30 | 0.02 | 0.90 |
| Centromedial group | 287 ± 59 | 278 ± 71 | 0.06 | 0.81 | 306 ± 101 | 267 ± 54 | 0.77 | 0.47 | 0.45 | 0.51 | 1.04 | 0.31 | 0.81 | 0.37 |
| Cortical group | 157 ± 29 | 144 ± 33 | 1.03 | 0.31 | 149 ± 38 | 142 ± 32 | 0.51 | 0.60 | 0.69 | 0.41 | 1.00 | 0.32 | 0.02 | 0.90 |
| Basolateral complex | 1552 ± 282 | 1457 ± 238 | 1.45 | 0.23 | 1477 ± 205 | 1448 ± 253 | 0.78 | 0.46 | 1.41 | 0.24 | 0.83 | 0.37 | 0.01 | 0.94 |
| Lateral nucleus | 642 ± 120 | 598 ± 94 | 2.02 | 0.16 | 596 ± 90 | 599 ± 98 | 1.17 | 0.32 | 2.22 | 0.14 | 0.91 | 0.34 | 0.01 | 0.92 |
| Basal nucleus | 618 ± 122 | 581 ± 105 | 1.08 | 0.30 | 597 ± 100 | 574 ± 108 | 0.54 | 0.59 | 0.64 | 0.43 | 0.84 | 0.36 | 0.00 | 0.96 |
| Accessory basal nucleus | 291 ± 57 | 277 ± 59 | 0.38 | 0.54 | 283 ± 53 | 274 ± 62 | 0.23 | 0.80 | 0.54 | 0.47 | 0.24 | 0.63 | 0.05 | 0.83 |
MDD = major depressive disorder.
Analysis of covariance of volumes (mm3) of the total amygdala, basolateral, cortical and centromedial groups of the amygdala, as well as the lateral, basal and accessory basal nuclei of the basolateral group (covariate, intracranial volumes). Group (diagnosis or treatment) × hemisphere interactions were not significant; volumes are mean ± standard deviation of both hemispheres.
Diagnosis main effect in 2-group comparison: healthy controls and MDD.
Treatment main effect in 3-group comparison: healthy controls, unmedicated MDD and medicated MDD.
Childhood adversity and amygdala volumes in MDD
The volume of the right total amygdala (but not the left; p > 0.35) showed a significant negative association with total CTQ-25 score (Table 5). Similarly, the volume of the right basolateral amygdala showed a negative association with total CTQ-25 score, but this finding did not survive Holm–Bonferroni correction for multiple comparisons. The volumes of the centromedial and cortical groups in the left and right hemispheres (all p > 0.09) and left basolateral amygdala (p > 0.29) were not correlated with total CTQ-25 score.
Table 5.
Summary of p values for relationships between Childhood Trauma Questionnaire scores and volumetric measurements in participants with MDD (n = 33)
| Score | Amygdala | Hippocampus | Subfields within hippocampal subregions | ||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Total | Subregions | Subfields | Head | Body | Tail | ||
| Total score* | Right amygdala: rs: −0.324, p < 0.033 Right basolateral complex: rs: −0.311, p < 0.040 Right accessory basal nucleus: rs: −0.361, p < 0.020 Right basal nucleus: rs: −0.288, p < 0.05 |
Left hippocampus: rs: −0.347, p < 0.024; p < 0.048† Right hippocampus: rs: −0.283, p < 0.06 |
Left head: rs: −0.518, p < 0.001; p < 0.002† Right head: rs: −0.369, p < 0.018; p < 0.018† |
Left cornu ammonis: rs: −0.560, p < 0.001; p < 0.002† Right cornu ammonis: rs: −0.395, p < 0.012 |
Left cornu ammonis: rs: −0.633, p < 0.001; p < 0.001† Right cornu ammonis: rs: −0.412, p < 0.009; p < 0.043† Right dentate gyrus: rs: −0.299, p < 0.046 Left subiculum: rs: −0.319, p < 0.036 |
— | — |
| Emotional abuse* | Right amygdala: rs: −0.359, p < 0.021 Right basolateral complex: rs: −0.343, p < 0.026 Right accessory basal nucleus: rs: −0.448, p < 0.005 Right basal nucleus: rs: −0.375, p < 0.016 Left basal nucleus: rs: −0.293, p < 0.049 Right cortical group: rs: −0.383, p < 0.015 |
Left hippocampus: rs: −0.246, p < 0.09 Right hippocampus: rs: −0.236, p < 0.09 |
Left head: rs: −0.419, p < 0.008 Right head: rs: −0.311, p < 0.040 |
Left cornu ammonis: rs: −0.423, p < 0.007 Right cornu ammonis: rs: −0.381 p < 0.015 |
Left cornu ammonis: rs: −0.557, p < 0.001; p < 0.012† Right cornu ammonis: rs: −0.386, p < 0.014 Left subiculum: rs: −0.254, p < 0.08 |
— | — |
| Physical abuse* | — | Left hippocampus: rs: −0.229, p < 0.1 Right hippocampus: rs: −0.386, p < 0.014 |
Left head: rs: −0.396, p < 0.012 Right head: rs: −0.453, p < 0.004; p < 0.037† |
Left cornu ammonis: rs: −0.393, p < 0.012 Right cornu ammonis: rs: −0.479 p < 0.002 |
Left cornu ammonis: rs: −0.403, p < 0.011 Right cornu ammonis: rs: −0.476, p < 0.003 Right dentate gyrus: rs: −0.481 p < 0.002 Left subiculum: rs: −0.248, p < 0.08 |
— | — |
| Sexual abuse* | — | Left hippocampus: rs: −0.288, p < 0.053 | Left head: rs: −0.245, p < 0.09 | NS | Right dentate gyrus: rs: −0.432, p < 0.006 | Left dentate gyrus: rs: −0.345, p < 0.025 | — |
| Emotional neglect‡ | — | Left hippocampus: rs: −0.367, p < 0.018 Right hippocampus: r: −0.295, p < 0.049 |
Left head: r: −0.439, p < 0.005; p < 0.043† Right head: r: −0.280, p < 0.06 |
Left cornu ammonis: r: −0.539, p < 0.001; p < 0.018† Right cornu ammonis: r: −0.258, p < 0.07 |
Left cornu ammonis: r: −0.536, p < 0.001; p < 0.018† Right cornu ammonis: r: −0.277, p < 0.06 Left subiculum: r: −0.281, p < 0.06 |
Right dentate gyrus: r: −0.248, p < 0.08 | — |
| Physical neglect* | — | — | Left head: rs: −0.519, p < 0.001; p < 0.01† Right head: rs: −0.334, p < 0.029 |
Left cornu ammonis: rs: −0.536, p < 0.001; p < 0.019† Right cornu ammonis: rs: −0.330, p < 0.031 |
Left cornu ammonis: rs: −0.600, p < 0.001; p < 0.004† Right cornu ammonis: rs: −0.410, p < 0.009 Left subiculum: rs: −0.298, p < 0.046 |
— | — |
MDD = major depressive disorder.
Spearman’s correlation.
Corrected p values for those that survived Holm–Bonferroni correction; p value for total amygdala was not adjusted for multiple comparisons because of our initial hypothesis.
Pearson’s correlation.
Exploratory analysis in the basolateral group showed that total CTQ-25 score had negative correlations with the right accessory basal and basal nuclei (Table 5), but not with the left nuclei (both p > 0.11).
Exploratory analysis of CTQ factors showed that only emotional abuse was negatively correlated with the volumes of the total amygdala and the accessory basal, basal and cortical nuclei, mainly in the right hemisphere. However, the exploratory correlations for the amygdala subnuclei did not survive Holm–Bonferroni correction.
Childhood adversity and hippocampal volumes
We found correlations between CTQ scores and hippocampal measurements in both hemispheres (Table 5).
Total hippocampal volume showed a negative relationship with total CTQ-25 score. Among hippocampal subregions, only the head was negatively correlated with total CTQ-25 score, but not the body or tail (both p > 0.13). Analysis of the hippocampal subfields showed that CA1–3 had a significant negative correlation with total CTQ-25 score (Table 5), but not the total dentate gyrus or subiculum (both p > 0.17).
Exploratory analysis of the subfields in the hippocampal head showed that the bilateral CA1–3, right dentate gyrus and left subiculum had a significant negative correlation with total CTQ-25 score (Table 5), but not the left dentate gyrus and right subiculum volumes (both p > 0.31).
Exploratory analysis for the CTQ factors showed that emotional abuse, physical abuse, emotional neglect and physical neglect had strong negative relationships with the volume of the hippocampal head and CA1–3 in the hippocampal head. We found weaker negative associations between physical and sexual abuse and the dentate gyrus of the hippocampal head and body, and between physical neglect and the subiculum in the hippocampal head, but these findings did not survive Holm–Bonferroni correction.
Discussion
The current volumetric in vivo MRI study examined the effects of childhood adversity on the amygdala subnuclei and hippocampal subfields in MDD and the effects of MDD on volumes of the amygdala subnuclei. Although we did not find any significant effect of MDD or long-term antidepressant treatment on the amygdala subnuclei, we did find that childhood adversity was negatively associated with both hippocampal and amygdala volumes. The negative effects of childhood adversity as measured by the CTQ-25 were observed bilaterally in the volumes of the anterior hippocampus (i.e., the hippocampal head) and the total CA1–3 subfield; in the amygdala, these effects were limited to the basolateral amygdala in the right hemisphere. These are structures shown to be affected by chronic stress in preclinical studies.12,26
Effects of adverse experiences in childhood/adolescence and MDD on amygdala subnuclei
Both the amygdala and the hippocampus are regarded as targets of childhood adversity17,44,45 because they exhibit protracted postnatal development, a high density of glucocorticoid receptors and postnatal neurogenesis.44,45
The present study confirmed the negative effects of childhood adversity on the right amygdala and suggested that these effects might also affect the basolateral amygdala.
Our results also suggested that the accessory basal, basal and cortical nuclei could be potential targets for these effects in the amygdala. These results could be explained by differences in the connectivity profile and functions of the amygdala subnuclei. Previous studies have shown that childhood adversity induces abnormal changes in frontolimbic regions. 35,36 The majority of the amygdala reciprocal connections with other frontolimbic regions — including the CA1–3 hippocampal subfield, the caudate, the nucleus accumbens and the orbitofrontal, medial prefrontal and anterior cingulate cortices — run mainly through the accessory basal and basal nuclei and only to a lesser degree through the cortical group.11,13,46 In addition, differences in functional specialization of the amygdala subnuclei could explain their differential vulnerability. Our previous high-resolution functional MRI study of emotional processing by the amygdala subnuclei demonstrated that both the centromedial and basal amygdala groups were most sensitive to negative emotional stimuli.47 In contrast, we reported that the lateral nucleus was not particularly sensitive to negative emotions, suggesting that this structure might play a less important role in this process. Previous functional MRI studies have consistently shown hyperactivity of the amygdala in response to threatening stimuli in maltreated individuals,17 but which subnuclei are responsible for these functional changes remains to be determined. In contrast to functional studies, the findings of structural studies in the amygdalae of maltreated individuals have been heterogenous. Some studies have reported volumetric reduction35,36 or volumetric increase,33,34 but others found no significant effects of maltreatment on amygdala volume in healthy people30,31 or in psychiatric patients.32 Nevertheless, the timing and type of exposure to the early adverse environment could account for these heterogeneous results. 17 It has been suggested that volumetric enlargement has been reported by studies that investigated the effects of early maltreatment (mainly emotional and/or physical neglect) on the amygdala, and volumetric decrease has been reported by studies that investigated older adolescents or adults with more psychopathological symptoms exposed to different types of maltreatment across their development.17 As a result, an early adverse environment might increase amygdala volume during childhood, but further exposure to a stressful environment during adulthood could cause a volumetric reduction because of the biological embedding induced by exposure to an early adverse environment.17
Our findings related to the negative relationship between amygdala volume and childhood adversity were in line with those of our previous study,48 which showed that amygdala volumes in participants with MDD with a history of childhood sexual or physical abuse were smaller than in participants with MDD who did not have such a history.
Stress-integrative functions are also localized in the amygdala subnuclei.6 The centromedial amygdala is the primary regulator of emotional and physiologic responses to acute stressors, and the basolateral amygdala plays a role in the integration of chronic stress.6 Animal studies have linked chronic stress to depression-related behaviours.12 During stress, the basolateral amygdala undergoes plastic changes, with increased dendritic spine density in response to acute stress and increased length and arborization of pyramidal cell dendrites following chronic stress.12,37,49 In contrast, chronic immobilization stress did not change the dendrites of the central amygdala,37,49 and in the medial amygdala it was associated with both a reduction in dendritic spine density50 and no significant change.51 Although our findings on the negative associations between basolateral volume and CTQ scores differed from animal studies, most of those studies used rodents whose ages were relevant to human adulthood and not to childhood. Therefore, future animal studies for comparison could investigate the effects of chronic stress in adult animals exposed to stress during their weaning and adolescent periods.
In the present study the negative associations between total CTQ score, emotional abuse (and total abuse history) and amygdala volumes were observed mainly in the right hemisphere. This finding was in line with a recent structural MRI study in emerging adults that reported negative correlations between adverse childhood experience and the volume of the basolateral and centromedial amygdala (including central, medial and cortical nuclei) in the right hemisphere only.38 Furthermore, basolateral volumetric reduction in the right hemisphere was associated with an increase in symptoms of anxiety and depression, and of alcohol use.38 Moreover, in response to sad stimuli, activation of the right amygdala was positively correlated with CTQ factors, especially physical abuse.52 A recent meta-analysis demonstrated that those who experienced childhood maltreatment had smaller amygdalae in both hemispheres, but the effect sizes for the right amygdala were larger in men.36 Our findings were consistent with a meta-analysis that showed that people who experienced childhood maltreatment had significantly smaller right amygdala volumes than people who were not exposed to childhood maltreatment.35
In line with these findings, previous studies reported hemispheric asymmetries for emotional processing in the amygdala53 and suggested that the right amygdala is involved in the rapid detection of threatening stimuli or the early processing of affective stimuli via right hemisphere subcortical circuits, and the left amygdala is preferentially activated during decoding of cognitive-related emotional stimuli via the left hemisphere’s slower cortical feedback mechanisms.53 Therefore, we speculate that the observed negative relationships between right basal and accessory basal nuclei volumes and a history of abuse that is considered a threat54 might be due to the critical role of the right amygdala in processing threat-related stimuli.53 Such stimuli sensitize the right amygdala to stressful situations later in life, causing volumetric reduction.17,36
Although previous meta-analyses found no significant differences in amygdala volume between participants with MDD and controls,2,3,8–10 individual studies have reported an increase in total amygdala volume with MDD,48 a decrease55 and no effect.56 Similarly, postmortem studies have reported no volumetric or neuronal density difference in the amygdala between participants with MDD and controls,57 a trend toward amygdala volume reduction in people with MDD,58 or a modest (11%) volumetric increase in the lateral nucleus with an increase in the number of neurovascular cells in the basolateral group in people with MDD, without significant differences in the total number and densities of neurons and glia in the basolateral group.59 Some of the confounding variables that might contribute to these conflicting results include the demographic and clinical characteristics of the people with MDD, as well as the anatomic definition of the amygdala.2–4,9,10 We have found larger amygdalae in participants with MDD.48 However, the results of the present study were in agreement with previous studies that demonstrated similar amygdala volumes in participants with MDD and healthy controls.2,9 Although in the present study we employed an identical anatomic definition of the amygdala and used the same criteria to recruit participants with MDD, differences in the field strength of the MRI scanners between our 2 studies (4.7 T v. 1.5 T), and the sex ratio of participants with MDD might have contributed to the discrepancies. Similar to previous studies,2,55,56 we found no correlations between amygdala volume and MDD duration or severity. Finally, we found no associations between amygdala volumes and antidepressant treatment, in agreement with our previous findings.48 Meta-analyses2,9 and a postmortem study59 found no association between medication status and amygdala volume or effects of antidepressant treatment on the basolateral group, respectively.
Effects of adverse experiences in childhood/adolescence on hippocampal subfields
It has been suggested that the hippocampus might be the brain region most vulnerable to stress.17 Our findings indicated that of all hippocampal subfields and subregions, the CA1–3 and the anterior hippocampus showed the strongest negative associations with childhood adversity. In contrast to our findings for the amygdala, which were limited to a history of emotional abuse, our findings for the effects of childhood adversity on the hippocampus include histories of emotional and physical abuse and neglect.
Previous studies have reported hippocampal volumetric reductions in maltreated healthy individuals,30 participants with MDD60 and maltreated individuals regardless of the absence or presence of psychiatric disorders.17,29,36 It is important to note that the values of the CTQ scores in our MDD sample were similar to those reported previously in participants with MDD.48,52,61 The CTQ scores of participants with MDD were generally much higher than those of healthy participants in studies that compared these 2 groups to each other.48,52,61 Because hippocampal volume can be affected by childhood adversity, MDD itself or both, future neuroimaging studies in MDD need to include a control group with a similar level of exposure to adverse childhood experiences to determine if any observed volumetric differences are associated with chronic stress or with MDD diagnosis.
The results of the present study agree with those of previous studies in MDD participants29 and healthy controls18 that suggested that childhood adversity affects the anterior hippocampus. 5,18,29 In addition, our previous study showed that cortisol levels were negatively correlated with anterior hippocampus and CA1–3 volumes in both participants with MDD and healthy controls.62
Our results were also in agreement with those of a previous study that reported negative relationships between childhood maltreatment and volumes of the CA2–3 subfield.19 Although we did not find statistically significant associations between the dentate gyrus and total CTQ-25 score, we believe that these differences could be partially explained by different sample characteristics and the effects of antidepressant treatment on the dentate gyrus in our MDD cohort.21
Animal models of chronic stress have demonstrated that although chronic stress induced dendritic atrophy in both the dorsal and ventral hippocampus, the CA3 was the most vulnerable hippocampal subfield.26 These findings are in agreement with our results. Dendritic retraction in CA1 and the dentate gyrus in animals occurred in response to more severe chronic stress.26 We have demonstrated that the largest proportion of the CA1–3 subfield is in the anterior hippocampus, likely making this subregion a main target of chronic stress.20 In contrast, the largest proportion of the dentate gyrus is in the posterior hippocampus (i.e., the body), which is particularly affected by MDD,21 suggesting that the effects of MDD diagnosis and stress history may be distinct.
In contrast to our findings for the amygdala, our findings of hippocampal changes related to childhood adversity were in accordance with preclinical studies. Explanations for these findings might arise from the different vulnerability windows in postnatal development of these structures. Although the hippocampus achieves dramatic growth in the first 2 years of life, the amygdala continues to develop until young adulthood.44 Therefore, exposure to childhood adversity likely does not correspond to the postnatal window of hippocampal vulnerability but could occur simultaneously with amygdala development.
Limitations
Our study sample was cross-sectional. Although the significant negative associations we found between childhood maltreatment and the volume of the right amygdala were in accordance with the findings of previous studies,35,38 our results for the amygdala subnuclei should be interpreted with caution, because none of those findings remained significant after Holm–Bonferroni correction. Studies that incorporate a treatment-naïve group of MDD patients with and without history of childhood adversity are needed to separate the effects of MDD, history of abuse and antidepressant treatment on hippocampal subfields and amygdala subnuclei. In general, methods used in volumetric studies of human hippocampal subfields63 and amygdala subnuclei14 in vivo do not exactly match the intra-anatomic boundaries of these structures; instead, different geometrical rules are employed that approximately match the location and the orientation of these structures based on histological references.
Conclusion
This study provides in vivo evidence that childhood adversity might have negative effects on specific brain subregions in participants with MDD, namely the basolateral amygdala and the CA1–3 hippocampal subfield.
Acknowledgements
This work was supported by the Canadian Institutes of Health Research operating grant (MOP111049 to NVM). The authors are thankful to all individuals who participated in this research.
Footnotes
Competing interests: None declared.
Contributors: N. Coupland, P. Silverstone, K. Hegadoren and N. Malykhin designed the study. A. Aghamohammadi-Sereshki, P. Silverstone, Y. Huang, R. Carter, P. Seres and N. Malykhin acquired the data, which A. Aghamohammadi-Sereshki, N. Coupland, P. Silverstone, Y. Huang, K. Hegadoren and N. Malykhin analyzed. A. Aghamohammadi-Sereshki, P. Silverstone and N. Malykhin wrote the article, which all authors reviewed. All authors approved the final version to be published and can certify that no other individuals not listed as authors have made substantial contributions to the paper.
References
- 1.Global health estimates 2016: disease burden by cause, age, sex, by country and by region, 2000–2016. Geneva: World Health Organization; 2018. [Google Scholar]
- 2.Schmaal L, Veltman DJ, van Erp TG, et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. 2016;21:806–12. doi: 10.1038/mp.2015.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koolschijn PC, van Haren NE, Lensvelt-Mulders GJ, et al. Brain volume abnormalities in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Hum Brain Mapp. 2009;30:3719–35. doi: 10.1002/hbm.20801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lorenzetti V, Allen NB, Fornito A, et al. Structural brain abnormalities in major depressive disorder: a selective review of recent MRI studies. J Affect Disord. 2009;117:1–17. doi: 10.1016/j.jad.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 5.Malykhin NV, Coupland NJ. Hippocampal neuroplasticity in major depressive disorder. Neuroscience. 2015;309:200–13. doi: 10.1016/j.neuroscience.2015.04.047. [DOI] [PubMed] [Google Scholar]
- 6.Davis M, Whalen PJ. The amygdala: vigilance and emotion. Mol Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- 7.Sah P, Faber ES, Lopez De Armentia M, et al. The amygdaloid complex: anatomy and physiology. Physiol Rev. 2003;83:803–34. doi: 10.1152/physrev.00002.2003. [DOI] [PubMed] [Google Scholar]
- 8.Campbell S, Marriott M, Nahmias C, et al. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607. doi: 10.1176/appi.ajp.161.4.598. [DOI] [PubMed] [Google Scholar]
- 9.Hajek T, Kopecek M, Kozeny J, et al. Amygdala volumes in mood disorders–meta-analysis of magnetic resonance volumetry studies. J Affect Disord. 2009;115:395–410. doi: 10.1016/j.jad.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Hamilton JP, Siemer M, Gotlib IH. Amygdala volume in major depressive disorder: a meta-analysis of magnetic resonance imaging studies. Mol Psychiatry. 2008;13:993–1000. doi: 10.1038/mp.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. Nat Rev Neurosci. 2009;10:423–33. doi: 10.1038/nrn2651. [DOI] [PubMed] [Google Scholar]
- 12.Qiao H, Li MX, Xu C, et al. Dendritic spines in depression: what we learned from animal models. Neural Plast. 2016:8056370. doi: 10.1155/2016/8056370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yilmazer-Hanke DM. Amygdala. In: Mai JK, Paxinos G, editors. The human nervous system. 3rd ed. London: Elsevier Academic Press; 2012. pp. 759–834. [Google Scholar]
- 14.Aghamohammadi-Sereshki A, Huang Y, Olsen F, et al. In vivo quantification of amygdala subnuclei using 4.7 T fast spin echo imaging. Neuroimage. 2018;170:151–63. doi: 10.1016/j.neuroimage.2017.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Lindert J, von Ehrenstein OS, Grashow R, et al. Sexual and physical abuse in childhood is associated with depression and anxiety over the life course: systematic review and meta-analysis. Int J Public Health. 2014;59:359–72. doi: 10.1007/s00038-013-0519-5. [DOI] [PubMed] [Google Scholar]
- 16.McCrory EJ, Viding E. The theory of latent vulnerability: reconceptualizing the link between childhood maltreatment and psychiatric disorder. Dev Psychopathol. 2015;27:493–505. doi: 10.1017/S0954579415000115. [DOI] [PubMed] [Google Scholar]
- 17.Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry. 2016;57:241–66. doi: 10.1111/jcpp.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Szeszko PR, Betensky JD, Mentschel C, et al. Increased stress and smaller anterior hippocampal volume. Neuroreport. 2006;17:1825–8. doi: 10.1097/01.wnr.0000246322.58814.b8. [DOI] [PubMed] [Google Scholar]
- 19.Teicher MH, Anderson CM, Polcari A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc Natl Acad Sci U S A. 2012;109:E563–72. doi: 10.1073/pnas.1115396109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malykhin NV, Lebel RM, Coupland NJ, et al. In vivo quantification of hippocampal subfields using 4.7 T fast spin echo imaging. Neuroimage. 2010;49:1224–30. doi: 10.1016/j.neuroimage.2009.09.042. [DOI] [PubMed] [Google Scholar]
- 21.Huang Y, Coupland NJ, Lebel RM, et al. Structural changes in hippocampal subfields in major depressive disorder: a high-field magnetic resonance imaging study. Biol Psychiatry. 2013;74:62–8. doi: 10.1016/j.biopsych.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 22.Han KM, Kim A, Kang W, et al. Hippocampal subfield volumes in major depressive disorder and bipolar disorder. Eur Psychiatry. 2019;57:70–7. doi: 10.1016/j.eurpsy.2019.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Roddy DW, Farrell C, Doolin K, et al. The hippocampus in depression: more than the sum of its parts? Advanced hippocampal substructure segmentation in depression. Biol Psychiatry. 2019;85:487–97. doi: 10.1016/j.biopsych.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Wisse LE, Biessels GJ, Stegenga BT, et al. Major depressive episodes over the course of 7 years and hippocampal subfield volumes at 7 tesla MRI: the PREDICT-MR study. J Affect Disord. 2015;175:1–7. doi: 10.1016/j.jad.2014.12.052. [DOI] [PubMed] [Google Scholar]
- 25.Travis S, Coupland NJ, Silverstone PH, et al. Dentate gyrus volume and memory performance in major memory depressive disorder. J Affect Disord. 2015;172:159–64. doi: 10.1016/j.jad.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 26.Conrad CD, Ortiz JB, Judd JM. Chronic stress and hippocampal dendritic complexity: methodological and functional considerations. Physiol Behav. 2017;178:66–81. doi: 10.1016/j.physbeh.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 27.Leuner B, Gould E. Structural plasticity and hippocampal function. Annu Rev Psychol. 2010;61:111–40. C1–3. doi: 10.1146/annurev.psych.093008.100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33:88–109. doi: 10.1038/sj.npp.1301574. [DOI] [PubMed] [Google Scholar]
- 29.Vythilingam M, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am J Psychiatry. 2002;159:2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calem M, Bromis K, McGuire P, et al. Meta-analysis of associations between childhood adversity and hippocampus and amygdala volume in non-clinical and general population samples. Neuroimage Clin. 2017;14:471–9. doi: 10.1016/j.nicl.2017.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cohen RA, Grieve S, Hoth KF, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biol Psychiatry. 2006;59:975–82. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 32.De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biol Psychiatry. 2002;52:1066–78. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- 33.Lupien SJ, Parent S, Evans AC, et al. Larger amygdala but no change in hippocampal volume in 10-year-old children exposed to maternal depressive symptomatology since birth. Proc Natl Acad Sci U S A. 2011;108:14324–9. doi: 10.1073/pnas.1105371108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta MA, Golembo NI, Nosarti C, et al. Amygdala, hippocampal and corpus callosum size following severe early institutional deprivation: the English and Romanian Adoptees study pilot. J Child Psychol Psychiatry. 2009;50:943–51. doi: 10.1111/j.1469-7610.2009.02084.x. [DOI] [PubMed] [Google Scholar]
- 35.Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiatry. 2014;171:854–63. doi: 10.1176/appi.ajp.2014.13101427. [DOI] [PubMed] [Google Scholar]
- 36.Paquola C, Bennett MR, Lagopoulos J. Understanding heterogeneity in grey matter research of adults with childhood maltreatment: a meta-analysis and review. Neurosci Biobehav Rev. 2016;69:299–312. doi: 10.1016/j.neubiorev.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 37.Vyas A, Mitra R, Shankaranarayana Rao BS, et al. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oshri A, Gray JC, Owens MM, et al. Adverse childhood experiences and amygdalar reduction: high-resolution segmentation reveals associations with subnuclei and psychiatric outcomes. Child Maltreat. 2019;24:400–10. doi: 10.1177/1077559519839491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown TA, DiNardo PA, Barlow DH. Anxiety disorders interview schedule for DSM-IV: adult and lifetime version, clinician manual. Albany (NY): Graywind Publications; 1994. [Google Scholar]
- 40.Bernstein DP, Stein JA, Newcomb MD, et al. Development and validation of a brief screening version of the Childhood Trauma Questionnaire. Child Abuse Negl. 2003;27:169–90. doi: 10.1016/s0145-2134(02)00541-0. [DOI] [PubMed] [Google Scholar]
- 41.Eritaia J, Wood SJ, Stuart GW, et al. An optimized method for estimating intracranial volume from magnetic resonance images. Magn Reson Med. 2000;44:973–7. doi: 10.1002/1522-2594(200012)44:6<973::aid-mrm21>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 42.Malykhin NV, Bouchard TP, Ogilvie CJ, et al. Three-dimensional volumetric analysis and reconstruction of amygdala and hippocampal head, body and tail. Psychiatry Res. 2007;155:155–65. doi: 10.1016/j.pscychresns.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 43.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage. 2006;31:1116–28. doi: 10.1016/j.neuroimage.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 44.Lupien SJ, McEwen BS, Gunnar MR, et al. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 45.Teicher MH, Andersen SL, Polcari A, et al. The neurobiological consequences of early stress and childhood maltreatment. Neurosci Biobehav Rev. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- 46.Freese JL, Amaral DG. Neuroanatomy of the primate amygdala. In: Whalen PJ, Phelps EA, editors. The human amygdala. New York: Guilford Press; 2009. 2009. pp. 3–42. [Google Scholar]
- 47.Hrybouski S, Aghamohammadi-Sereshki A, Madan CR, et al. Amygdala subnuclei response and connectivity during emotional processing. Neuroimage. 2016;133:98–110. doi: 10.1016/j.neuroimage.2016.02.056. [DOI] [PubMed] [Google Scholar]
- 48.Malykhin NV, Carter R, Hegadoren KM, et al. Fronto-limbic volumetric changes in major depressive disorder. J Affect Disord. 2012;136:1104–13. doi: 10.1016/j.jad.2011.10.038. [DOI] [PubMed] [Google Scholar]
- 49.Vyas A, Bernal S, Chattarji S. Effects of chronic stress on dendritic arborization in the central and extended amygdala. Brain Res. 2003;965:290–4. doi: 10.1016/s0006-8993(02)04162-8. [DOI] [PubMed] [Google Scholar]
- 50.Bennur S, Shankaranarayana Rao BS, Pawlak R, et al. Stress-induced spine loss in the medial amygdala is mediated by tissue-plasminogen activator. Neuroscience. 2007;144:8–16. doi: 10.1016/j.neuroscience.2006.08.075. [DOI] [PubMed] [Google Scholar]
- 51.Marcuzzo S, Dall’oglio A, Ribeiro MF, et al. Dendritic spines in the posterodorsal medial amygdala after restraint stress and ageing in rats. Neurosci Lett. 2007;424:16–21. doi: 10.1016/j.neulet.2007.07.019. [DOI] [PubMed] [Google Scholar]
- 52.Grant MM, Cannistraci C, Hollon SD, et al. Childhood trauma history differentiates amygdala response to sad faces within MDD. J Psychiatr Res. 2011;45:886–95. doi: 10.1016/j.jpsychires.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gainotti G. Emotions and the right hemisphere: can new data clarify old models? Neuroscientist. 2019;25:258–70. doi: 10.1177/1073858418785342. [DOI] [PubMed] [Google Scholar]
- 54.McLaughlin KA, Sheridan MA, Lambert HK. Childhood adversity and neural development: deprivation and threat as distinct dimensions of early experience. Neurosci Biobehav Rev. 2014;47:578–91. doi: 10.1016/j.neubiorev.2014.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kronenberg G, Tebartz van Elst L, Regen F, et al. Reduced amygdala volume in newly admitted psychiatric in-patients with unipolar major depression. J Psychiatr Res. 2009;43:1112–7. doi: 10.1016/j.jpsychires.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Frodl T, Jäger M, Smajstrlova I, et al. Effect of hippocampal and amygdala volumes on clinical outcomes in major depression: a 3-year prospective magnetic resonance imaging study. J Psychiatry Neurosci. 2008;33:423–30. [PMC free article] [PubMed] [Google Scholar]
- 57.Bowley MP, Drevets WC, Ongür D, et al. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–12. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 58.Bielau H, Trübner K, Krell D, et al. Volume deficits of subcortical nuclei in mood disorders: a postmortem study. Eur Arch Psychiatry Clin Neurosci. 2005;255:401–12. doi: 10.1007/s00406-005-0581-y. [DOI] [PubMed] [Google Scholar]
- 59.Rubinow MJ, Mahajan G, May W, et al. Basolateral amygdala volume and cell numbers in major depressive disorder: a postmortem stereological study. Brain Struct Funct. 2016;221:171–84. doi: 10.1007/s00429-014-0900-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis. 2016;2:16065. doi: 10.1038/nrdp.2016.65. [DOI] [PubMed] [Google Scholar]
- 61.Opel N, Redlich R, Zwanzger P, et al. Hippocampal atrophy in major depression: a function of childhood maltreatment rather than diagnosis? Neuropsychopharmacology. 2014;39:2723–31. doi: 10.1038/npp.2014.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Travis SG, Coupland NJ, Hegadoren K, et al. Effects of cortisol on hippocampal subfields volumes and memory performance in healthy control subjects and patients with major depressive disorder. J Affect Disord. 2016;201:34–41. doi: 10.1016/j.jad.2016.04.049. [DOI] [PubMed] [Google Scholar]
- 63.Yushkevich PA, Amaral RS, Augustinack JC, et al. Quantitative comparison of 21 protocols for labeling hippocampal subfields and parahippocampal subregions in in vivo MRI: towards a harmonized segmentation protocol. Neuroimage. 2015;111:526–41. doi: 10.1016/j.neuroimage.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]