Abstract
The World Health Organization nominated SARS-CoV-2 as the cause of the Coronavirus Disease 2019 (COVID-19) and has been granted as a pandemic. COVID-19 is an emerging threat due to the risk of microvascular, venous, and arterial thrombosis, thereby exacerbating organ injury and mortality. Although the exact mechanism of extensive thromboembolism and myocardial injury caused by SARS-CoV-2 is not illuminated, it is clear that COVID-19 related hypercoagulation increasing the fatality of the disease. Herein, we reported a patient with extensive biventricular thrombi along with the new-onset severe systolic dysfunction as an unusual catastrophic presentation of COVID-19. In our patient, there was both a right atrial "clot in transit" from his DVT as well as extensive muralized biventricular thrombus from severe global hypokinesis. We believe that the hypercoagulable state of his COVID-19 infection, along with severe systolic dysfunction, caused this unusual presentation. Although the hypercoagulable state of COVID-19 is well recognized, there have not been any reported cases of extensive de-novo intracardiac thrombus as of yet. We urge awareness of severe and potentially fatal extensive thrombosis and cardiac failure as the initial clinical presentation of possible SARS-CoV-2.
<Learning objective: Thrombotic manifestations are correlated with the high mortality rate in COVID-19; thus, strategies to prevent thrombosis have critical importance. The hypercoagulable state of COVID-19, along with cardiac injury, can lead to an extensive intracardiac thrombus and severe systolic dysfunction even in young patients who don't have previous cardiovascular comorbidities. We urge awareness of severe and potentially fatal extensive thrombosis and cardiac failure as the initial clinical presentation of COVID-19.>
Keywords: COVID-19, Biventricular thrombi, New-onset heart failure, Thromboembolism
Introduction
The novel severe acute respiratory syndrome coronavirus-2 (SARS–CoV-2), first detected in December 2019, has spread to almost every country globally, causing extensive multiorgan failure and mortality [1]. The World Health Organization nominated SARS–CoV-2 as the cause of the Coronavirus Disease 2019 (COVID-19) and has been deemed a pandemic [2]. To date, little is known about the virus's pathologic features and causes of death. One of the more interesting aspects of COVID-19 infection is the unprecedented hypercoagulable state that it renders upon patients and is thought to be a fundamental aspect of mortality and morbidity. Recent clinical data showed that COVID-19 is associated with a hypercoagulable state and predisposes thrombotic complications, both in the venous and arterial circulations [3,4]. COVID-19 infection activates an inflammatory response that releases inflammatory markers. The initiation of immune responses (both innate and adaptive) can lead to immune-thrombosis, complement activation, and endothelial inflammation. Notably, distinct coagulation activation is correlated with multiorgan failure and increases mortality. Given thrombotic manifestations have a crucial role in the high mortality rate in COVID-19, strategies to prevent thrombosis have critical importance. Although the hypercoagulable state of COVID-19 is well recognized, there have not been any reported cases of extensive de-novo intracardiac thrombus as of yet. Herein, we report extensive biventricular thrombi along with the new-onset severe systolic dysfunction as an unusual catastrophic presentation of COVID-19.
Case presentation
A 58-year-old African-American male with a history of diabetes mellitus and hypertension was brought to the emergency room after syncopized at home. He had been taking care of his two parents, who were both ill and confirmed positive with COVID-19 for the past four weeks. He had been experiencing malaise, subjective fever and chills, and shortness of breath, along with right lower extremity swelling. Upon arrival, he was found to be profoundly hypoxic with a saturation of 60% on room air and was intubated for hypoxic respiratory failure.
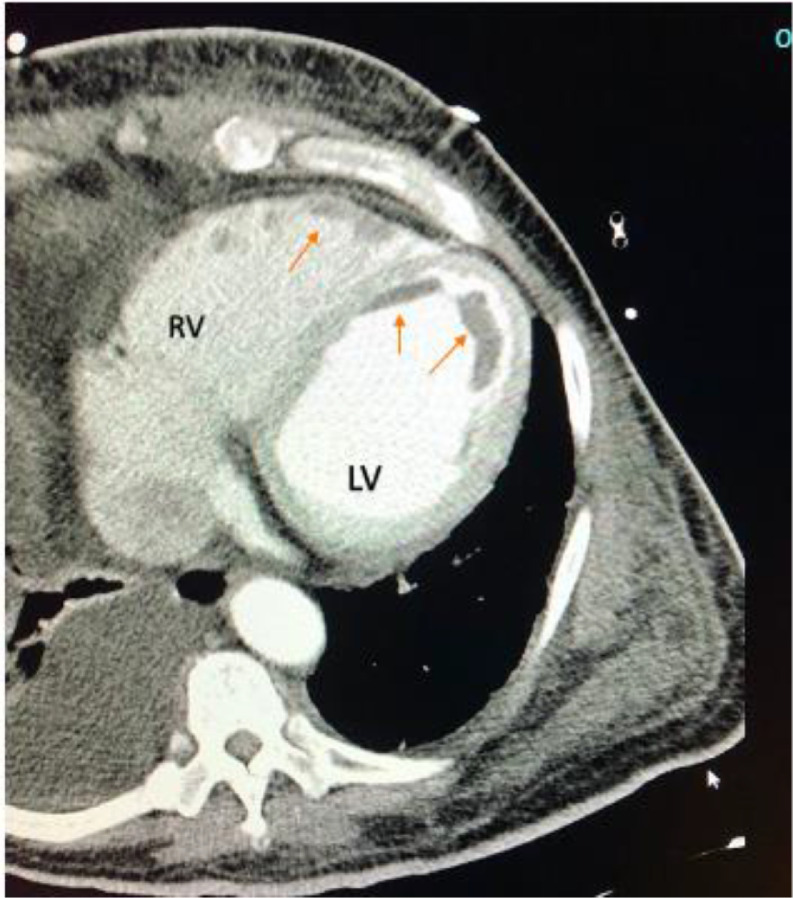
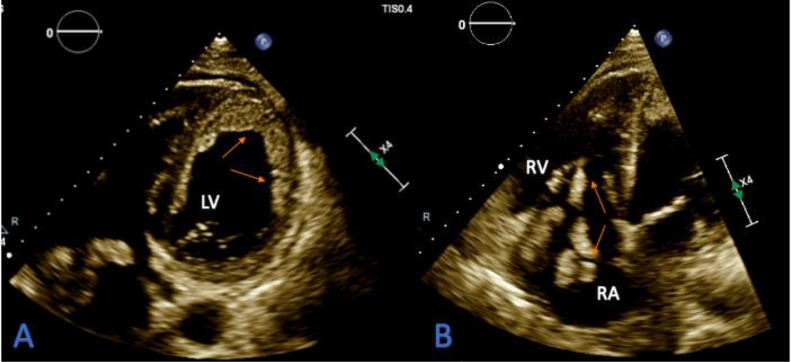
Initial lab work had multiple derangements; complete blood count showed mild leukocytosis with lymphopenia (Table 1). The coagulation profile showed PT 36 s, INR 3.6, and aPTT of 100 s. Fibrinogen was low at 173 mg/dL, the D-dimer level was > 20 ug/mL consistent with coagulopathy. He also had elevated liver enzymes with bilirubinemia, elevated procalcitonin, LDH, and proBNP levels. The troponin I, CK, CK-MB levels were within normal limits (Table 1). Electrocardiogram (ECG) showed normal sinus rhythm with no evidence of previous or new ischemic heart disease. The chest X-ray demonstrated bilateral patchy infiltrates left greater than the right. Given profound hypoxic respiratory failure and strong suspicion for COVID-19 pneumonia, the patient underwent CT Angiogram imaging of the chest. It showed severe bilateral airspace disease consistent with COVID-19 pneumonia, bilateral pleural effusion right greater than left, left-sided congestion, and left and right ventricular thrombi (Fig. 1). There was no pulmonary embolism. Subsequent transthoracic echocardiography was done. It showed an ejection fraction of 10% to 15% with biventricular failure and severe global hypokinesis. An extensive mural thrombus was seen along all walls of the left ventricle (Fig. 2A). Also, in the right atrium was a highly mobile thrombus with extension across the tricuspid valve into the right ventricle (Fig. 2B). Meanwhile, a duplex ultrasound revealed extensive right lower extremity deep vein thrombosis (DVT).
Table 1.
Laboratory values from the initial blood work.
| Parameters | Result | Normal range |
|---|---|---|
| Sodium | 135 | 137–145 mmol/L |
| Potassium | 4.5 | 3.5–5.1 mmol/L |
| Glucose | 93 | 70–100 mg/dL |
| Blood urea nitrogen | 32 | 9–20 mg/dL |
| Creatinine | 1.05 | 0.66–1.25 mg/dL |
| Aspartate transaminase | 122 | 17–59 U/L |
| Alanine aminotransferase | 111 | 0–49 U/L |
| Bilirubin, Total | 4.7 | 0.2–1.3 mg/dL |
| Bilirubin, Direct | 2.8 | 0–0.4 mg/dL |
| Ferritin | 943 | 17.90–464 ng/mL |
| Lactate dehydrogenase | 622 | 120–246 U/L |
| Troponin | 0.05 | 0–0.08 ng/mL |
| Creatine kinase | 75 | 55–170 U/L |
| Creatine kinase-MB | 10 | 5–25 IU/L |
| proB-type natriuretic peptide | 34,824 | 0–899 pg/mL |
| C-reactive protein | 19.7 | Less than 1.0 mg/dL |
| Procalcitonin | 0.13 | 0–0.50 ng/mL |
| Hemoglobin A1c (%) | 7 | 4–6% NGSP |
| D-dimer | Greater than 20 | 0–0.45 ug/mL FEU |
| Fibrinogen | 173 | 214–453 mg/dL |
| International normalized ratio | 3.6 | 0.8–1.1 |
| Prothrombin time | 36 | 12.3–14.7 s |
| Activated partial thromboplastin time | 100 | 24–36 s |
| White blood cells | 10.34 | 4–10.10 103/uL |
| Hemoglobin | 13.2 | 13.7–17.5 g/dL |
| Hematocrit | 42.4 | 40–51% |
| Platelet count | 163 | 150–400 103/uL |
| Segmented Neutrophils | 81% | 35–70% |
| Lymphocytes | Less than 1% | 20–53% |
Fig. 1.
CT Angiogram imaging of the chest showing filling defects along right ventricle (RV) and left ventricle (LV) representing thrombus (arrows).
Fig. 2.
A-B: Transthoracic echocardiography (TTE) showing; A: An extensive mural thrombus (arrows) seen along all walls of the left ventricle (LV). B: Mobile right atrial (RA) thrombus (arrows) extending across the tricuspid valve and extending into the right ventricle (RV).
Initial SARS-CoV-2 PCR testing was negative in nasopharyngeal swab; however, given the strong suspicion of COVID-19 pneumonia, the patient remained isolated and transferred to the ICU in critical condition. He was started on antibiotics and anticoagulation. There was consideration for possible tissue plasminogen activator (tPA) or mechanical thrombectomy, but due to severe coagulopathy and extensive clotting, he was deemed not a candidate. He deteriorated over the next several hours from multifactorial shock, developed ventricular fibrillation, and was unable to be resuscitated. He expired within 24 h of admission. Unfortunately, we couldn't have an opportunity to check serial SARS–CoV-2 PCR from nasopharyngeal swab or COVID-19 antibody testing. Other causes of hypercoagulability were sent off and within normal limits, including anti-cardiolipin antibody, lupus anticoagulant, factor V Leiden, prothrombin, protein S, and C activity.
Discussion
COVID-19 is an emerging threat due to the risk of microvascular, venous, and arterial thrombosis, thereby exacerbating organ injury and mortality. Previous studies suggested a close relationship between the innate immune response and thrombotic complications, contributing to COVID-19 associated hypercoagulability [5]. In particular, coronavirus infections may trigger endothelial dysfunction, systemic inflammation, and an increased pro-coagulatory state by tissue factor pathway activation. The direct activation of the coagulation cascade by a cytokine storm has an essential role in the pro-coagulation state.
In addition to VTE, information on cardiac injury in patients with COVID-19 is limited. There have been various purported mechanisms of cardiac dysfunction in COVID 19. Myocardial injury is a common thread and is evident from elevated troponin, which is ubiquitously seen but not specific for etiology. The various causative mechanisms identified are demand ischemia from acute hemodynamic stresses commonly seen in infections such as fever, tachycardia and adrenergic surge, and hypotension. Acute coronary syndrome, stress cardiomyopathy, and fulminant myocarditis have also been described. Another putative mechanism is the resultant sequelae of cytokine storm which has been a hallmark of SARS-CoV-2 infection [6]. This is similar to sepsis cardiomyopathy, a previously well-described phenomenon, where overwhelming inflammatory cytokine surge leads to nitric oxide-mediated cardiac myocyte dysfunction, diminished myofibril response to calcium and mitochondrial dysfunction [7]. Given our patient's clinical presentation of severe global dysfunction with fever and elevated inflammatory markers, his systolic dysfunction was presumed to be either myocarditis or cytokine related.
To our best of knowledge, this is the first reported case in the literature of extensive biventricular thrombus and acute biventricular heart failure in a suspected COVID-19 patient. Right atrial and right ventricular thrombus are uncommonly seen but previously well described in patients with acute deep vein thrombus presenting as a "clot in transit" en route to the pulmonary artery. These are seen in approximately 5–10% of pulmonary embolism [8]. Separately, ventricular thrombi are also well described and most commonly seen in ischemic heart disease cases with systolic dysfunction and regional wall motion abnormalities leading to de-novo clot formation [9]. Less commonly, they may also be seen associated with a ventricular aneurysm or eosinophilic myocarditis [10]. In our patient, there was both a right atrial "clot in transit" from his DVT as well as extensive muralized biventricular thrombus from severe global hypokinesis. We believe that the hypercoagulable state of his COVID-19 infection, along with severe systolic dysfunction, combined to create the perfect storm needed for this unusual presentation. In this regard, there is an urgent need to understand how and to which extend SARS-CoV-2 provokes a prothrombotic state to develop practical therapeutic approaches for COVID-19-associated thrombosis.
Funding
No funding is reported for this study.
Declaration of Competing Interest
There is no conflict of interest to declare.
Acknowledgments
None.
References
- 1.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J. A Novel Coronavirus from patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [Internet]Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223) doi: 10.1016/S0140-6736(20)30183-5. http://www.sciencedirect.com/science/article/pii/S0140673620301835 497–06Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi M., Thachil J., Iba T., Levy J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–e440. doi: 10.1016/S2352-3026(20)30145-9. http://www.sciencedirect.com/science/article/pii/S2352302620301459 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han H., Yang L., Liu R., Liu F., Liu F., Wu K.L. Prominent changes in blood coagulation of patients with SARS-CoV-2 infection. Clin Chem Lab Med. 2020;58(7):1116–1120. doi: 10.1515/cclm-2020-0188. [DOI] [PubMed] [Google Scholar]
- 5.Maier C.L., Truong A.D., Auld S.C., Polly D.M., Tanksley C.-.L., Duncan A. COVID-19-associated hyperviscosity: a link between inflammation and thrombophilia? Lancet. 2020;395(10239):1758–1759. doi: 10.1016/S0140-6736(20)31209-5. https://pubmed.ncbi.nlm.nih.gov/32464112 2020/05/25Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bader F., Manla Y., Atallah B., Starling R.C. Heart failure and COVID-19. Heart Fail Rev. 2021;26(1):1–10. doi: 10.1007/s10741-020-10008-2. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sato R., Nasu M. A review of sepsis-induced cardiomyopathy. J Intensive Care. 2015;3(1):48. doi: 10.1186/s40560-015-0112-5. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrari E., Benhamou M., Berthier F., Baudouy M. Mobile thrombi of the right heart in pulmonary embolism: delayed disappearance after thrombolytic treatment. Chest. 2005;127(3):1051–1053. doi: 10.1378/chest.127.3.1051. http://www.sciencedirect.com/science/article/pii/S001236921531120X Available from. [DOI] [PubMed] [Google Scholar]
- 9.Delewi R., Zijlstra F., Piek J.J. Left ventricular thrombus formation after acute myocardial infarction. Heart. 2012;98(23):1743–1749. doi: 10.1136/heartjnl-2012-301962. https://pubmed.ncbi.nlm.nih.gov/23151669 Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mariotti R., Petronio A.S., Robiglio L., Balbarini A., Mariani M. Left ventricular aneurysm: clinical and hemodynamic data. Clin Cardiol. 1990;13(12):845–850. doi: 10.1002/clc.4960131207. Available from. [DOI] [PubMed] [Google Scholar]