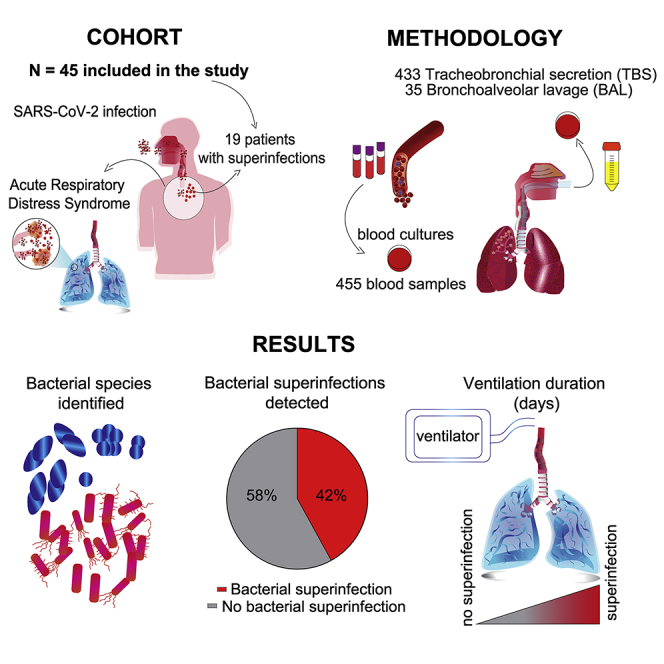
Summary
The impact of secondary bacterial infections (superinfections) in coronavirus disease 2019 (COVID-19) is not well understood. In this prospective, monocentric cohort study, we aim to investigate the impact of superinfections in COVID-19 patients with acute respiratory distress syndrome. Patients are assessed for concomitant microbial infections by longitudinal analysis of tracheobronchial secretions, bronchoalveolar lavages, and blood cultures. In 45 critically ill patients, we identify 19 patients with superinfections (42.2%). Superinfections are detected on day 10 after intensive care admission. The proportion of participants alive and off invasive mechanical ventilation at study day 28 (ventilator-free days [VFDs] at 28 days) is substantially lower in patients with superinfection (subhazard ratio 0.37; 95% confidence interval [CI] 0.15–0.90; p = 0.028). Patients with pulmonary superinfections have a higher incidence of bacteremia, virus reactivations, yeast colonization, and required intensive care treatment for a longer time. Superinfections are frequent and associated with reduced VFDs at 28 days despite a high rate of empirical antibiotic therapy.
Keywords: severe acute respiratory syndrome coronavirus 2, SARS-CoV-2, coronavirus disease 19, COVID-19, acute respiratory distress syndrome, ARDS, bacterial superinfection, longitudinal sampling, invasive mechanical ventilation, antibiotic therapy, co-infection, ventilator free at 28 days
Graphical abstract
Highlights
Secondary bacterial infections (superinfections) are found in 42% of patients
Bacterial superinfections occur on day 10 after intensive care admission
Bacterial superinfections are associated with longer duration of ventilation
Bacterial superinfections are mostly caused by Gram-negative bacteria
Buehler et al. show that detection of bacterial pulmonary superinfection is associated with a more severe disease course in COVID-19 patients, especially a lower likelihood of being alive and off invasive mechanical ventilation at study day 28 (ventilator-free days at 28 days).
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has evolved as the most relevant pandemic of modern history, challenging health care systems all over the world. The clinical characteristics of coronavirus disease 2019 (COVID-19) patients have been thoroughly described in recent studies.1, 2, 3, 4, 5 The triggers for acute respiratory distress syndrome (ARDS) in COVID-19 are virus initiated, subsequently leading to inflammation-mediated lung damage and endotheliitis.5 Although primarily a viral disease, antibiotics are empirically used in over 70% of cases in addition to experimental antiviral and immunomodulatory treatments.1,4, 5, 6, 7 Secondary bacterial and/or fungal infections are a well-described phenomenon in viral illnesses, such as influenza, and are associated with increased morbidity and mortality in viral ARDS, as illustrated during previous pandemics.8 Secondary bacterial infections are typically referred to as superinfections, whereas co-infection is mainly used to describe simultaneous virus infection. Both co- and superinfections have been described in COVID-19 patients.6,9 Data regarding bacterial superinfections in COVID-19 pneumonia are limited and still emerging.10,11 A recent systematic review has concluded that the rate of bacterial/fungal superinfections is low, arguing against the frequent use of broad-spectrum antimicrobials in patients with COVID-19.11,12 However, COVID-19-associated pulmonary aspergillosis (CAPA) has been reported in several cohorts of critically ill patients.13,14 Still, there is a lack of knowledge about the frequency and significance of bacterial, fungal, and viral concomitant infections in critically ill COVID-19 patients.12 Additionally, in most studies performed so far, no thorough and systematic sampling for concomitant infections was performed. The high mortality in severely ill COVID-19 patients is thought to be at least in part due to secondary infections in addition to viral replication in the lower respiratory tract leading to severe lung injury and ARDS.6,15,16
Superinfection seems to represent a major risk factor for mortality in COVID-19 patients.7,17, 18, 19 However, the risk of superinfection in mechanically ventilated patients with severe COVID-19 remains poorly described.
Currently, the diagnostic and treatment approach for superinfections remains unclear and the classical criteria for the detection of superinfections are often of limited use in COVID-19 patients. Clinical symptoms are an expression of the underlying disease of COVID-19 and cannot be used to reliably distinguish between patients presenting with or without relevant superinfections. For this reason, several authors have argued in favor of an empirical antibiotic treatment with a focus on streptococci and staphylococci in severe courses.16 Other opinion leaders recommend (longitudinal) sampling of severely ill patients for early detection and treatment during the entire course of the disease.6,20
Rapid diagnosis of co- and superinfections may not only help to improve survival but would also allow targeted antimicrobial therapy, improving antimicrobial stewardship throughout the course of the pandemic.20,21
The aim of our study was to assess the burden of superinfections and the association with clinical outcomes in critically ill patients with COVID-19 ARDS (CARDS) in a tertiary care intensive care unit (ICU) with highly regulated antibiotic prescription.
Results
Cohort characteristics
A total of 48 critically ill COVID-19 patients with ARDS were screened in the ICU at the University Hospital Zurich between April and June 2020. Three patients had to be excluded from the analysis because patients or relatives denied informed consent (Figure S1). 45 patients with a median age of 60 (54–69) years were included in this study. Most of them were male (35/45; 77.8%). Of the 45 patients, 19 (42.2%) were diagnosed with a superinfection. The median of ventilation duration was 15 days and length of ICU stay was 14 days overall. The median length of hospital stay was 24 days. Baseline characteristics are summarized in Table 1. In general, both groups of patients with and without superinfections were similar with regards to demographics and clinical characteristics. In particular, there were no differences in the severity of the disease and organ dysfunction as assessed by simplified acute physiology score (SAPS) II and sepsis-related organ failure assessment score (SOFA). Intensive care rescue therapies, such as prone position (42% versus 90%) and tracheotomy (23% versus 74%), were required more frequently and/or for longer periods in the superinfection group (Table 1).
Table 1.
Baseline characteristics and study population
| Overall (N = 45) | No bacterial superinfection (n = 26) | Bacterial superinfection (n = 19) | p value | |
|---|---|---|---|---|
| Baseline characteristics | ||||
| Age (years) | 60 (54–69) | 61.5 (54–71) | 59 (54–69) | 0.654 |
| Male sex (n/%) | 35 (77.8%) | 19 (73.1%) | 16 (84.2%) | 0.375 |
| Weight (kg) | 83 (75–99) | 80 (72.5–90) | 92 (78–100) | 0.049 |
| Height (cm) | 175 (165–182) | 172 (160–180) | 176 (169–185) | 0.112 |
| Body mass index (kg/m2) | 27.8 (25.7–31.6) | 27.5 (25.6–30.6) | 27.8 (26.8–35) | 0.346 |
| Comorbidity and other clinical conditions | ||||
| Myocardial infarction/ischemic heart disease | 6 (13.3%) | 5 (19.2%) | 1 (5.3%) | 0.222 |
| Arterial hypertension | 26 (57.8%) | 16 (61.5%) | 10 (52.6%) | 0.550 |
| Chronic kidney disease | 12 (26.7%) | 10 (38.5%) | 2 (10.5%) | 0.047 |
| Dialysis | 1 (2.2%) | 1 (3.8%) | 0 (0%) | 0.999 |
| Diabetes, no insulin therapy | 5 (11.1%) | 3 (11.5%) | 2 (10.5%) | 0.999 |
| Diabetes, insulin therapy | 14 (31.1%) | 7 (26.9%) | 7 (36.8%) | 0.528 |
| Asthma | 1 (2.2%) | 1 (3.8%) | 0 (0%) | 0.999 |
| Chronic obstructive pulmonary disease (COPD) | 5 (11.1%) | 4 (15.4%) | 1 (5.3%) | 0.378 |
| Renal or liver transplantation | 5 (11.1%) | 4 (15.4%) | 1 (5.3%) | 0.378 |
| Immunosuppression | 7 (15.6%) | 5 (19.2%) | 2 (10.5%) | 0.222 |
| Cancer | 4 (8.9%) | 2 (7.7%) | 2 (10.5%) | 0.999 |
| Smoking | 14 (31.1%) | 7 (26.9%) | 7 (36.8%) | 0.625 |
| Alcohol abuse | 1 (2.2%) | 0 (0%) | 1 (5.3%) | 0.999 |
| Drug abuse | 0 (0%) | 0 (0%) | 0 (0%) | 1.000 |
| Medical therapy before admission | ||||
| Statin | 10 (22.7%) | 6 (24%) | 4 (21.1%) | 0.999 |
| Angiotensin-converting enzyme (ACE) inhibitor | 7 (15.9%) | 4 (16%) | 3 (15.8%) | 0.999 |
| Scores/index | ||||
| Sepsis-related organ failure assessment score (SOFA) | 8 (5–10) | 8 (3–10) | 9 (7–10) | 0.480 |
| Simplified acute physiology score (SAPS) II | 36 (25–50) | 32.5 (24–50) | 42 (28–51) | 0.275 |
| Lowest PaO2/FiO2 ratio at admission | 122 (94–177) | 129 (97–200) | 108 (85–163.5) | 0.228 |
| Organ failure during ICU stay | ||||
| Acute kidney injury | 27 (60%) | 15 (57.7%) | 12 (63.2%) | 0.712 |
| Dialysis | 18 (40%) | 9 (34.6%) | 9 (47.4%) | 0.388 |
| Invasive mechanical ventilation | 40 (88.9%) | 21 (80.8%) | 19 (100%) | 0.043 |
| Extracorporeal life support (ECLS) | 8 (17.8%) | 3 (11.5%) | 5 (26.3%) | 0.253 |
| Rescue therapies | ||||
| Prone position | 28 (62.2%) | 11 (42.3%) | 17 (89.5%) | 0.001 |
| Inhaled nitric oxide (iNO) | 11 (24.4%) | 5 (19.2%) | 6 (31.6%) | 0.341 |
| Tracheotomy | 20 (44.4%) | 6 (23.1%) | 14 (73.7%) | 0.001 |
| Timing (days) | ||||
| Time to ICU admission | 2 (1–6) | 2 (1–6) | 3 (1–6) | 0.636 |
| Duration prone position | 6 (1–10) | 1.5 (1–7) | 6 (4–10) | 0.022 |
| Duration iNO therapy | 3 (1–6) | 1 (1–3) | 4.5 (1–22) | 0.349 |
| Duration intubation to tracheotomy | 20 (10–33) | 10.5 (9–21) | 27 (15–33) | 0.114 |
| Laboratory values at admission | ||||
| White blood cell (WBC) count (G/L) | 7.7 (5.7–10.7) | 7.5 (5.7–10) | 8 (5.3–13.4) | 0.515 |
| Hemoglobin (gr/L) | 118.5 (101.5–133) | 117 (107–132) | 126 (98–134) | 0.896 |
| Hematocrit (%) | 0.4 (0.3–0.4) | 0.4 (0.3–0.4) | 0.4 (0.3–0.4) | 0.619 |
| Platelet count (G/L) | 199 (169.5–272) | 200 (177–271) | 190 (154–297) | 0.776 |
| Alanine transaminase (ALT) (U/L) | 42.5 (25.5–65.5) | 31 (24–60) | 56 (33–72) | 0.008 |
| Lactate dehydrogenase (LDH) (U/L) | 676 (527–842.5) | 619 (471–742) | 772 (626–876) | 0.144 |
| Alkaline phosphatase (U/L) | 74.5 (53.5–103.5) | 77.5 (56–108) | 61 (53–98) | 0.308 |
| Urea (mmol/L) | 6 (4.3–10.3) | 6.2 (4.1–7.9) | 6 (4.4–12.2) | 0.651 |
| Creatinine (μmol/L) | 92.5 (67–138.5) | 95 (70–128) | 91 (57–149) | 0.387 |
| Serum sodium (mmol/L) | 138 (134–141) | 137 (135–140) | 141 (134–146) | 0.203 |
| Serum potassium (mmol/L) | 3.9 (3.7–4.5) | 4.1 (3.7–4.4) | 3.8 (3.5–4.7) | 0.601 |
| C-reactive protein (CRP) (mg/L) | 168.5 (83.5–276.5) | 124 (62–238) | 255 (102–301) | 0.034 |
| Procalcitonin (PCT) (mcg/L) | 0.3 (0.2–1.2) | 0.2 (0.1–1.7) | 0.4 (0.2–1.2) | 0.060 |
| Interleukin-6 (IL-6) (ng/L) | 127 (71.2–454) | 122 (84–697) | 127 (62.7–263) | 0.554 |
| COVID-19 targeted drug therapy and antimicrobials | ||||
| Steroids | 21 (46.7%) | 8 (30.8%) | 13 (68.4%) | 0.012 |
| Hydroxychloroquine | 27 (61.4%) | 13 (52%) | 14 (73.7%) | 0.143 |
| Lopinavir/ritonavir | 7 (15.9%) | 4 (16%) | 3 (15.8%) | 0.999 |
| Remdesivir | 8 (18.2%) | 4 (16%) | 4 (21.1%) | 0.704 |
| Tocilizumab | 2 (4.7%) | 2 (8.3%) | 0 (0%) | 0.501 |
| Empiric antimicrobial therapy | 40 (8,839%) | 22 (88%) | 18 (94.7%) | 0.441 |
| Sample size overall | ||||
| TBS | 433 | 114 | 319 | |
| BAL | 35 | 12 | 23 | |
| Blood cultures | 455 | 152 | 303 | |
Demographic and clinical characteristics as well as risk factors of COVID-19 patients stratified according to presence or absence of pulmonary relevant pathogens in tracheobronchial secretions (TBSs) and bronchioalveolar lavages (BALs) reflecting superinfection. The data are presented as median (interquartile range (IQR)) or number and percentage (%). The two groups were compared using chi-square test/Fisher exact or the Mann-Whitney test as appropriate.
Microbiological sampling and superinfections
Overall, 433 tracheobronchial secretions (TBSs), 35 bronchoalveolar lavages (BALs) samples, and 455 blood culture pairs were analyzed for the presence of microorganisms (Figure S1). The range of respiratory samples per patient was 1–36 with a median of 6 per patient.
In nineteen patients (42.2%), at least one clinically relevant bacterium or fungus was detected in TBSs/BALs during the study period, whereas in 26 patients (57.8%), no relevant microorganisms were detected in TBSs/BALs.
A total of 342 TBSs/BALs were collected in the superinfection group, and 169 pulmonary relevant microorganisms were detected in these samples. Only in two cases, results did not match between BALs and TBSs. TBSs became negative for detected pathogens within a median of 12 days. Despite high frequency of positive TBSs, blood cultures showed only seven different bacterial species in 14 positive blood culture pairs (Table 2).
Table 2.
Clinical outcomes and microorganisms detected
| No bacterial superinfection (n = 26) | Bacterial superinfection (n = 19) | p value | |
|---|---|---|---|
| Outcomes and superinfection data | |||
| Duration of ventilation (days) | 8 (5.9–15.1) | 37 (22.2–43.7) | <0.001 |
| Length of ICU stay (days) | 9 (7.0–14.9) | 39 (28.5–57.0) | <0.001 |
| Length of hospital stay (days) | 17 (14.4–26.4) | 44 (34.2–63.3) | <0.001 |
| Patients died | 6 (23.1%) | 4 (21.1%) | 0.999 |
| Patients with bacterial respiratory superinfection | 0 (0%) | 19 (100%) | |
| Patients with superinfection detected in BAL | 0 (0%) | 6 (31.6%) | |
| Patients with bloodstream infection | 2 (7.7%) | 9 (47.4%) | 0.004 |
| Patients with Aspergillus detection | 2 (7.7%) | 3 (15.8%) | 0.636 |
| Patients with yeast colonization | 12 (46.2%) | 17 (89.5%) | 0.004 |
| Patients with multidrug resistant (MDR) pathogens | 0 (0%) | 10 (52.6%) | <0.001 |
| Causative microorganisms | |||
| Microbiology of superinfections | |||
| Overall pathogen detection in TBS/BALa | 83 | 375 | |
| Relevant pulmonary pathogen detection in TBS/BALa | 0 (0%) | 169 (45.1%) | |
| Citrobacter freundii, koseri | 0 (0%) | 11 (2.9%) | |
| Enterobacter cloacae | 0 (0%) | 14 (3.7%) | |
| Escherichia coli | 0 (0%) | 2 (0.5%) | |
| Klebsiella aerogenes | 0 (0%) | 7 (1.9%) | |
| Klebsiella pneumoniae | 0 (0%) | 27 (7.2%) | |
| Legionella pneumophila | 0 (0%) | 2 (0.5%) | |
| Pseudomonas aeruginosa | 0 (0%) | 64 (17.1%) | |
| Acinetobacter spp. | 0 (0%) | 2 (0.5%) | |
| Burkholderia cepacia | 0 (0%) | 30 (8.0%) | |
| Morganella morganii | 0 (0%) | 3 (0.8%) | |
| Streptococcus pneumoniae | 0 (0%) | 2 (0.5%) | |
| Streptococcus anginosus | 0 (0%) | 3 (0.8%) | |
| Staphylococcus aureus | 0 (0%) | 2 (0.5%) | |
| Microbiology of bloodstream infections | |||
| Overall | 2/152 (1.3%) | 12/303 (4.0%) | |
| Citrobacter spp. | 0 (0%) | 2 (16.7%) | |
| Enterococcus faecalis and E. faecium | 1 (50%) | 6 (50%) | |
| Klebsiella aerogenes | 0 (0%) | 1 (8.3%) | |
| Klebsiella pneumoniae | 0 (0%) | 1 (8.3%) | |
| Moraxella spp. | 1 (50%) | 0 (0%) | |
| Candida glabrata | 0 (0%) | 1 (8.3%) | |
| Pseudomonas aeruginosa | 0 (0%) | 1 (8.3%) | |
| Viral detection | |||
| Overall | 2 | 9 | |
| Influenza A virus | 0 (0%) | 1 (11.1%) | |
| Influenza B virus | 0 (0%) | 0 (0%) | |
| Respiratory viruses, multiplex PCRb (n = 34) | 0 (0%) | 0 (0%) | |
| Herpes simplex virus type 1 (HSV-1) (PCR in blood) | 0 (0%) | 5 (55.5%) | |
| Herpes simplex virus type 2 (HSV-2) (PCR in blood) | 0 (0%) | 0 (0%) | |
| Human herpesvirus 6 (HHV-6) (PCR in blood) | 0 (0%) | 2 (22.2%) | |
| Cytomegalovirus (CMV) (PCR in blood) | 1 (50%) | 0 (0%) | |
| Epstein-Barr virus (EBV) (PCR in blood) | 1 (50%) | 1 (11.1%) | |
The data are presented as median (95% confidence interval [CI] of median) or number and percentage (%). The two groups were compared using chi-square test/Fisher exact or the Mann-Whitney test as appropriate.
Overall detected microorganisms, including repetitive detection in the same patient
Multiplex PCR assay for respiratory syncytial virus (RSV) A/B; influenza A/B virus; adenovirus; coronaviruses 229E, HKU1, NL63, and OC43; human bocavirus; human metapneumovirus (hMPV); rhino/enterovirus; and parainfluenza virus 1-4
In the group without superinfections, 83/114 TBS samples showed growth but without recovery of clinically relevant lung pathogens. Candida albicans was the most frequently isolated non-relevant organism. In the 12 BAL samples, there was also no evidence of pulmonary relevant microorganisms. However, bacteremia was detected twice in a total of 152 blood culture pairs (Table 2).
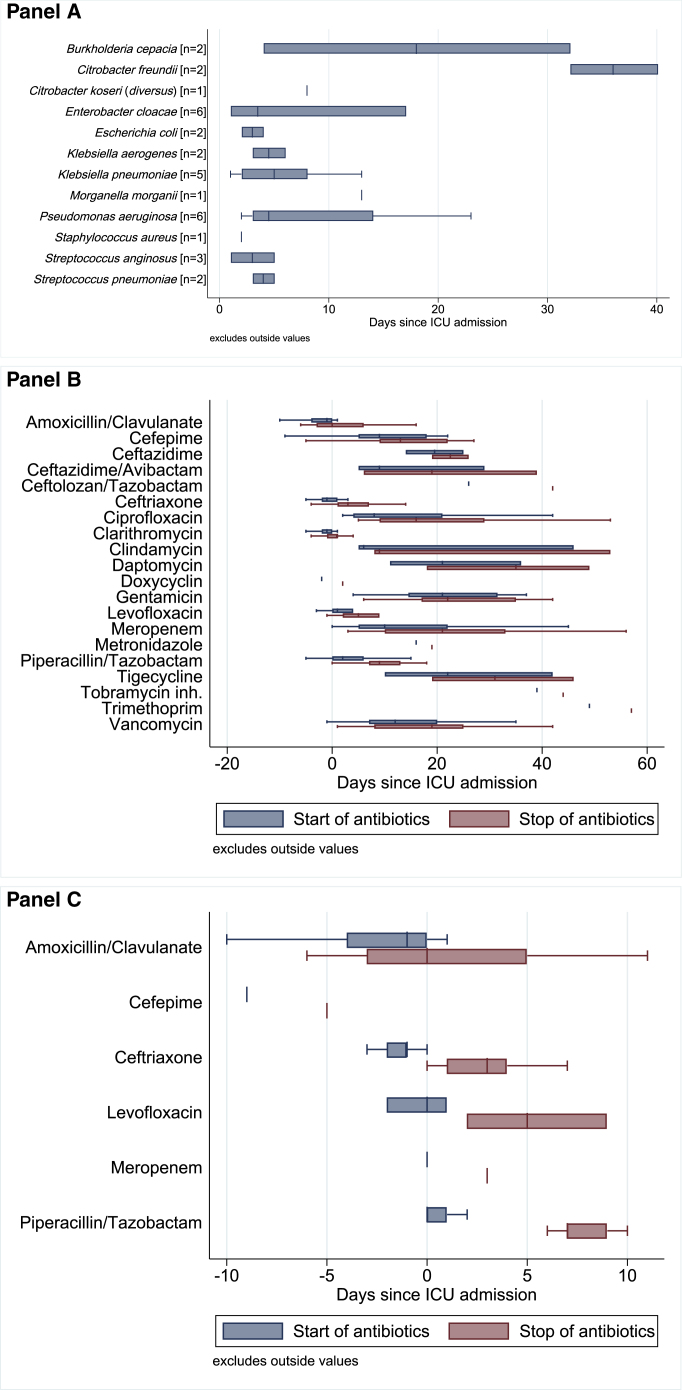
The detection time points of pulmonary relevant and non-relevant microorganisms are depicted in Figures 1 and S2, respectively. On average, relevant pulmonary pathogens were detected on day 10 after ICU admission and reflect the hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) spectrum (Figure 1A). Non-relevant pulmonary pathogens22 were detected on average on day 3 post-ICU admission (Figure S2). The most frequently isolated bacteria per patient were Enterococcus spp. (15/45), Enterobacter/Citrobacter (8/45), and Klebsiella spp. (7/45). Additionally, Streptococcus pneumoniae (2/45), Streptococcus anginosus (3/45), Escherichia coli (2/45), Enterobacter spp. (6/45), Citrobacter spp. (3/45), Pseudomonas aeruginosa (6/45), Burkholderia cepacia (2/45), and coagulase-negative staphylococci (13/45) were found.
Figure 1.
Spectrum and isolation time points in TBSs and BALs reflect hospital-acquired pneumonia (HAP)/ventilator-associated pneumonia (VAP) spectrum
(A) First detection time points of the most frequently cultured respiratory pathogens censored at 42 days (detection of Acinetobacter baumannii at day 77 and A. bereziniae at day 66). The n refers to the number of patients with a first detection of the respective pathogen. A total of 468 respiratory specimens from 45 patients were analyzed. Only the first detection event of a relevant respiratory pathogen in a given patient is reported.
(B) Antibiotics used for treatment of superinfections.
(C) Empiric antibiotic therapy used during first course of antibiotic treatment (before or at admission onto ICU).
See also Figures S1 and S2.
Empirical antimicrobial therapy was given to 40/45 (88.9%) patients, antifungal therapy to 10/45 (22.2%) patients, and antiviral therapy to treat concomitant viral infections to 9/45 (20%) patients. Figures 1B and 1C summarize the antibiotic treatment received by the patients.
In ten patients (22.2%), multi-drug-resistant (MDR) bacteria (Pseudomonas aeruginosa, Enterobacter cloacae, and Burkholderia cepacia) were detected.
Serum reactivation of herpes simplex type 1 and 2 (HSV-1 and -2) was detected in 5 out of 45 patients. Human herpes virus 6 (HHV-6) was detected twice, cytomegalovirus (CMV) reactivation occurred once, and Epstein-Barr virus (EBV) reactivation occurred twice. One patient had a co-infection with influenza A virus (Table 2).
Furthermore, colonizations with fungi were detected, and the isolated organisms included Candida spp. (29/45 patients), non-Candida yeast (21/45 patients), and Aspergillus spp. (5/45 patients). A detailed overview of relevant respiratory pathogens detected in TBSs/BALs and blood cultures is shown in Table 2. Colonizing microorganisms not considered as being relevant pulmonary pathogens are depicted in Figure S2.
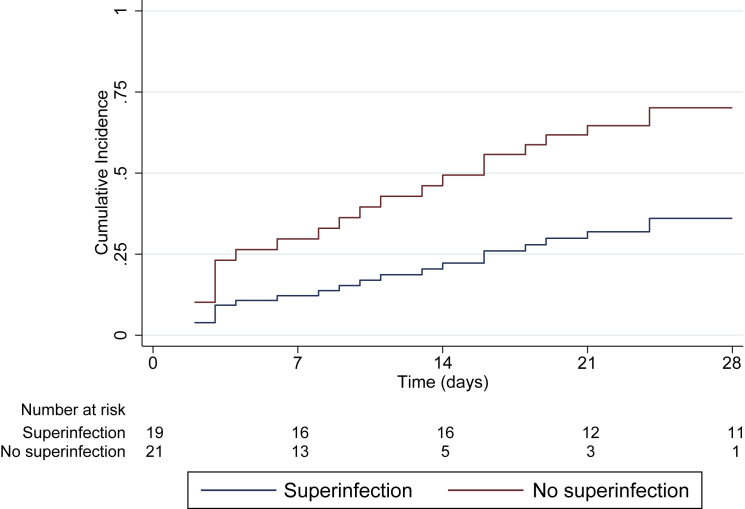
Proportion of participants alive and off invasive mechanical ventilation at study day 28 (ventilator-free days at 28 days)
COVID-19 patients with pulmonary superinfections had substantially lower ventilator-free days (VFDs) at 28 days than those without superinfections (Figure 2), with a subhazard ratio of 0.37 (95% confidence interval 0.15–0.90; p = 0.028).
Figure 2.
Proportion of participants alive and off invasive mechanical ventilation at study day 28 (ventilator-free days [VFDs] at 28 days)
Cumulative incidence curves for proportion of participants alive and off invasive mechanical ventilation at study day 28 (VFDs at 28 days). Only patients receiving invasive mechanical ventilation (N = 40) were included in this analysis. See also Figure S3 and Table S1.
Secondary outcomes
Patients with superinfections detected in respiratory specimens were ventilated for significantly longer time periods (8 versus 37 days; p < 0.001) and had a significantly longer duration of stay in the ICU (9 versus 39 days; p < 0.001) and overall hospitalization time (17 versus 44 days; p < 0.001) as compared to patients without superinfections (Table 2).
Further outcomes
Patients with pulmonary superinfections had significantly more bacteremia (p = 0.004), virus co-infections/reactivations other than SARS-CoV-2 (p = 0.001), colonization with yeasts (p = 0.004), and infections with MDR pathogens (p < 0.001; Table 2).
Longitudinal laboratory inflammation parameters (leukocytes, C-reactive protein [CRP], procalcitonin [PCT], and neutrophil/lymphocyte ratio) for days 1–16 are shown in Figure S3 and Table S1. Only for CRP, the mixed-model evaluation showed a significant difference between the groups with increased CRP in the superinfection group (p < 0.001), whereas for leucocytes, there was a significant increase in numbers over time in both groups (Figure S3).
Discussion
In this prospective cohort study of critically ill, ventilated COVID-19 patients, the presence of superinfection was associated with extended ventilation times, increased duration of intensive care and hospitalization, and increased need for intensive-care rescue therapies, such as proning and steroid use. Bacterial superinfections were detected in 42.2% of patients in our cohort, which is slightly higher than reported in previous studies.1,2,5,6,15,16,23 This discrepancy with other studies might be mainly due to the nature of our cohort consisting of severely ill patients with CARDS. In addition, differing from other studies, sampling was prospectively and repetitively scheduled and not only performed at admission as in other studies, which may account for underreporting of superinfections. Regional differences can also play an important role in bacterial superinfections and spectrum of resistance. This could explain in part the increased rate of superinfections compared to previous literature.11,24,25
Although some studies have concluded that bacterial superinfections do not play a major role in disease severity and treatment choices, the results of the present study challenge the generalizability to severely ill CARDS patients.6,11 In our cohort, isolation of relevant respiratory bacteria was associated with more-severe COVID-19 disease courses with significantly longer duration of invasive mechanical ventilation and prolonged ICU and hospital stays. Compared to other studies investigating the role of superinfection in COVID-19, duration of ICU stay and length of ventilation was high, reflecting the disease severity of patients included in this study.7,11 Additionally, data on the duration of ventilation and ICU stay are often missing in other studies, making comparisons difficult.4,11,15,21,26 Furthermore, due to the comparatively high SOFA score upon admission but moderate mortality, as in our cohort, long-term ICU-treatment complications, such as nosocomial infections, become more frequent.
Relevant respiratory bacteria were isolated on average on day 10 after ICU admission in our cohort, suggesting mainly nosocomial infections. In contrast to bacterial superinfections observed in influenza pneumonia, COVID-19 superinfections with Gram-positive bacteria, such as pneumococci or staphylococci, were rare in this study. It has to be mentioned that, in critically ill influenza patients, also infections with Gram-negative bacteria (such as P. aeruginosa) have been reported.27 Similar observations were made for Middle East respiratory syndrome coronavirus (MERS-CoV)- and SARS-CoV-1-associated superinfections.28, 29, 30 In this study, mainly Gram-negative pathogens, such as Pseudomonas and Enterobacteriaceae, including MDR bacteria, were isolated, which is in line with previous reported studies.11
Based on the findings that pulmonary bacterial superinfections seem to be mostly nosocomial and were associated with receipt of empiric broad-spectrum antibiotic therapy, use of antimicrobials may be more appropriately guided by the detection of pathogenic bacteria in longitudinal, high-quality respiratory samples. Patients could only be treated if pathogenic bacteria were detected in longitudinal samplings in line with antimicrobial stewardship interventions.12,26 Future prospective, randomized trials to investigate the efficacy of targeted antimicrobial therapy should be conducted to define best practice regarding prevention and treatment of bacterial superinfections in COVD-19. The isolation of mainly Gram-negative rods, including MDR, led to the use of third-line antibiotics, such as tigecycline, ceftazidime/avibactam, or ceftolozane/tazobactam, after initial empirical therapy of nosocomial pneumonia (Figure 1). It is important to consider the short- and long-term consequences that the use of antimicrobials, especially broad-spectrum, may have on drug resistance. A worrisome potential consequence of the COVID-19 pandemic might be the long-term spread of antimicrobial resistance (AMR) due to increased exposure of patients to antimicrobial agents that may have been used inappropriately.31 In this framework, employment of standardized longitudinal screening with early detection and susceptibility testing before establishment of antimicrobial therapy could minimize the use of broad-spectrum, second- and third-line antibiotics, thus reducing AMR.
The high rate of yeast detection might be associated with the widespread use of broad-spectrum empirical antimicrobial therapy.32 Invasive aspergillosis was not detected by standard methods. However, molecular detection methods might be an important tool in future studies.33,34 So far, only few studies have investigated fungal superinfections in COVID-19 patients.13, 14, 15,17,35, 36, 37 The significance of viral reactivation remains unclear.11 In our study, reactivations of HSV-1 and HHV-6 in the serum occurred in patients with bacterial superinfections. These findings support the hypothesis that superinfections associated with increased COVID-19 disease severity might enhance susceptibility to viral reactivations. Further studies with higher participant numbers should clarify the significance of this finding.
Prone positioning was more frequently performed in patients with superinfections. However, as patients received continuous subglottic suctioning, we believe that reflux during proning should not be an explanation for the observed superinfection differences. Rather, proning might reflect disease severity in the superinfection group. No selective digestive decontamination was performed.
In line with previous studies, conventional clinical laboratory tests, such as leukocytes, PCT, and neutrophil/lymphocyte ratio progressions, were not associated with pulmonary superinfections and therefore do not seem very useful for the detection of bacterial superinfections in COVID-19 patients on mechanical ventilation. Although CRP values differed significantly, the wide range observed in both groups did not allow the identification of a threshold value that clearly distinguishes patients with superinfection. This complicates the diagnosis of bacterial superinfections and emphasizes the importance of longitudinal microbiological diagnostics.
Advantages of this study are the prospective longitudinal monitoring of respiratory materials with concomitant recording of demographic data, microbiological evaluations, and antimicrobial therapy in a tertiary care center in a high-resource setting that did not experience health-care shortage during the first pandemic wave. Furthermore, this study used strict definitions for relevant respiratory pathogens. The diagnosis of superinfections was performed prospectively based on longitudinal sampling comprising detection of not only bacterial but also viral and fungal agents.
In summary, the detection of bacterial pulmonary superinfection was associated with a more-severe disease course in COVID-19 patients, especially a lower likelihood of being alive and off invasive mechanical ventilation at study day 28 (VFDs at 28 days). Future trials should investigate the effect of tailored antimicrobial therapy on outcome, antibiotic resistance, and drug use based on longitudinal assessment of respiratory tract cultures.
Limitations of study
Limitations of the study are the single-center design, small number of patients, and the high number of patients with empirical broad-spectrum antibiotic therapy (>90% of cases) at admission. Another limitation is the lack of a uniform, internationally valid definition of a bacterial infection of the lower respiratory tract and the inclusion of five patients that did not end up needing mechanical ventilation. However, patients not on mechanical ventilation were not included in the analysis of the primary outcome. Finally, as with all observational studies, causality cannot be inferred from reported associations.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Biological samples | ||
| Tracheobronchial Secretions | University Hospital Zürich, Institute of Intensive Care Medicine | MicrobiotaCOVID biobank |
| Bronchoalveolar Lavages | University Hospital Zürich, Institute of Intensive Care Medicine | MicrobiotaCOVID biobank |
| Blood cultures | University Hospital Zürich, Institute of Intensive Care Medicine | MicrobiotaCOVID biobank |
| Software and algorithms | ||
| STATA version 15 | StataCorp, College Station, TX, USA | https://www.stata.com/stata15/ |
| R version 3.6.3 | R project | https://www.r-project.org/ |
| SPSS Version 23 | SPSS Science, Chicago, IL, USA | https://www.ibm.com/analytics/spss-statistics-software |
| Graphpad Prism 7 | Graphpad, San Diego, CA, USA | https://www.graphpad.com/scientific-software/prism/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to the lead contacts, Philipp K. Buehler (philipp.buehler@usz.ch) or Silvio D. Brugger (silvio.brugger@usz.ch).
Materials availability
This study did not generate new unique materials.
Data and code availability
The data and analysis scripts used in this study are available upon request to the Lead author.
No new code was generated.
Experimental model and subject details
Study design, ethics and population
The study was conducted as part of the MicrobiotaCOVID cohort study, a single-center, prospective observational study conducted at the Institute of Intensive Care Medicine of the University Hospital Zurich (Zurich, Switzerland) registered at clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT04410263). The study was approved by the Local Ethics Committee of the Canton of Zurich, Switzerland (Kantonale Ethikkommission Zurich BASEC ID 2020 - 00646).
Patients with confirmed SARS-CoV-2 infection and CARDS requiring ICU support and mainly invasive mechanical ventilation hospitalized between April 2020 and June 2020 during the first COVID-19 wave in Switzerland were eligible.
Inclusion criteria were age > 18 years, SARS-CoV-2 infection as determined by real-time reverse transcriptase-polymerase chain reaction (RT-PCR) positivity of nasopharyngeal and/or pharyngeal swabs, TBS or BAL and hospitalization in the ICU for moderate or severe ARDS according to the Berlin criteria38.
Exclusion criteria were patients or relatives denying informed consent and patients still being treated in the ICU when the study period ended.
Study outcomes
The primary outcome was proportion of participants alive and off invasive mechanical ventilation at study day 28, i.e., ventilator-free days (VFDs) at 28 days39. Patients not on mechanical ventilation were excluded for the primary outcome analysis. Secondary outcomes were length of hospital stay, ICU stay and duration of mechanical ventilation. Further outcomes included the association of pulmonary superinfection and bacteraemia, other virus co-infections/reactivations, colonization with yeast, bacterial infections with multidrug resistance (MDR), and longitudinal laboratory inflammation parameters.
Method details
Sample collection, processing and testing
If the respiratory situation, as assessed by the ICU attending physician in charge, allowed bronchoscopy, BAL (10ml of saline) was collected by the ICU personnel upon ICU admission and during the later course of the disease. TBS was collected from each ventilated patient at least on day 0 (i.e., upon ICU admission), day 1, day 2, day 3, day 5 and henceforth every 5 days. If the clinical situation did not allow TBS collection, no sampling was performed.
Samples were processed at the Institute for Medical Microbiology and at the Institute for Medical Virology of the University of Zurich. Standard clinical microbiology techniques were used for culturing, isolation and identification of bacterial and fungal microorganisms as previously described40. SARS-CoV-2 was detected by real-time RT-PCR as previously described41. Aspergillus spp. was primarily identified in culture of TBS and BAL and also Galactomannan screening was performed in selected patients at physician’s discretion.
At admission, multiplex PCR for respiratory syncytial virus (RSV) A/B, influenza A/B virus, adenovirus, coronaviruses 229E, HKU1, NL63 and OC43, human bocavirus, human metapneumovirus (hMPV), rhino/enterovirus and parainfluenzavirus 1-4 was performed in nasopharyngeal swabs. Multiplex PCR for the detection of atypical respiratory bacteria (Legionella pneumophila, Chlamydophilia spp., Bordetella spp. and Mycoplasma spp.) was performed on pharyngeal swabs at ICU admission.
Moreover, we assessed serum detection and viral load of the following viruses: herpesviruses type 1 and 2 (HSV-1 and −2), Cytomegalovirus (CMV), Epstein–Barr virus (EBV) and human herpesvirus 6 (HHV-6). Additional virus diagnostics, blood and urine cultures were initiated by the treating physicians according to the clinical situation.
Data collection and covariates
Clinical and laboratory data were obtained from electronic health records and included demographics, comorbidities / risk factors, medication, ICU scores, laboratory values, organ failure, need for invasive ventilation, need for extracorporeal life support (ECLS), rescue therapies, length of ICU/hospital stay, COVID-19 targeted experimental therapy (steroids, hydroxychloroquine, lopinavir/ritonavir, remdesivir, tocilizumab) and empiric antibiotic therapy.
Daily measurements of inflammatory parameters C-reactive protein and procalcitonin (CRP/PCT), leukocyte count and the neutrophil/lymphocyte ratio were routinely performed over the first 16 days after ICU admission.
Definition of superinfection
A multidisciplinary panel of ICU and infectious diseases consultants (unrelated to the study group) assessed the clinical status of the patients on a daily basis. Superinfection was diagnosed according to the panel’s judgement of clinical deterioration and routine laboratory assessment as well as microbiological results. In more detail, the isolation of microorganisms from respiratory specimen cultures (TBS and/or BAL) regarded as clinically relevant by the panel was used as antimicrobial treatment guidance and the first specimen without pathogen growth was considered as the end of an episode in concordance with the clinical course. In case of isolation of more than one respiratory pathogen (i.e., in the bacterial and/or fungal analysis sample) in a given respiratory sample all were included in this study.
Organisms with low pathogenicity for lung infections such as Enterococcus spp., Candida spp., coagulase-negative staphylococci and non-pneumococcal streptococci were reported but not considered a relevant clinical pathogen of the airways in accordance with the literature42.
Detection of the HSV-1 and −2 as well as CMV, EBV, HHV-6 in blood were also reported but were considered reactivations. Viral co-infections/reactivations were only diagnosed if clinical signs of tracheitis or pathological signs of viral co-infection in cytology were observed.
Quantification and statistical analysis
Statistical analyses
Due to the unknown rate of concomitant infections in severely ill COVID-19 patients a power calculation was not feasible. Comparisons of population characteristics were performed using Mann-Whitney U tests and the Chi-square/Fisher exact test for categorical variables, as appropriate. For longitudinal analysis of laboratory parameters, differences between time points and superinfection status were tested using linear mixed effects models. To estimate the effect of superinfections on ventilator-free days (VFDs) at 28 days (proportion of participants alive and off invasive mechanical ventilation at study day 28), we used a competing risk regression model according to Fine & Gray censored at 28 days, with the event of extubation as outcome event and death as the competing risk. An alpha level of 0.05 was considered statistically significant. Statistical analysis was performed using STATA version 15 (StataCorp, College Station, TX), R version 3.6.3 (http://www.r-project.org/), SPSS Version 23 (SPSS Science, Chicago, IL, USA) and Graphpad Prism 7 (San Diego, CA, USA).
Additional resources
This study has been registered at clinicaltrials.gov (ClinicalTrials.gov Identifier: NCT04410263, https://clinicaltrials.gov/ct2/show/NCT04410263). We followed the STROBE statement checklist in reports of cohort studies (https://www.strobe-statement.org/index.php?id=available-checklists).
Acknowledgments
Funding was provided from Promedica Foundation 1449/M to S.D.B., University of Zurich CRPP Precision medicine for bacterial infections to A.S.Z., and unrestricted funds to R.A.S. The funders had no role in study design, performance, analysis, and interpretation of findings.
Author contributions
Conceptualization, funding acquisition, and resources, P.K.B., A.S.Z., D.A.H., R.A.S., and S.D.B.; project administration, P.K.B. and S.D.B.; investigation, formal analysis, data curation, and visualization, P.K.B., P.D.W.G., D.A.H., P.M.F., S.M.S., A.G.-M., and S.D.B.; writing – original draft, P.K.B., D.A.H., S.D.B., P.M.F., and F.A.; writing – review and editing, P.K.B., A.S.Z., D.A.H., P.D.W.G., C.T.A., A.G.-M., S.M.S., F.A., M.A.M., J.B., M.P.H., P.M.F., R.A.S., and S.D.B. All authors revised the manuscript and approved the final version.
Declaration of interests
The authors declare no competing interests.
Published: March 14, 2021
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xcrm.2021.100229.
Contributor Information
Philipp K. Buehler, Email: philipp.buehler@usz.ch.
Silvio D. Brugger, Email: silvio.brugger@usz.ch.
Supplemental information
References
- 1.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A., Cereda D., Coluccello A., Foti G., Fumagalli R., COVID-19 Lombardy ICU Network Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wendel Garcia P.D., Fumeaux T., Guerci P., Heuberger D.M., Montomoli J., Roche-Campo F., Schuepbach R.A., Hilty M.P., RISC-19-ICU Investigators Prognostic factors associated with mortality risk and disease progression in 639 critically ill patients with COVID-19 in Europe: Initial report of the international RISC-19-ICU prospective observational cohort. EClinicalMedicine. 2020;25:100449. doi: 10.1016/j.eclinm.2020.100449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., China Novel Coronavirus Investigating and Research Team A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and fungal coinfection in individuals with coronavirus: a rapid review to support COVID-19 antimicrobial prescribing. Clin. Infect. Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martín-Loeches I., Sanchez-Corral A., Diaz E., Granada R.M., Zaragoza R., Villavicencio C., Albaya A., Cerdá E., Catalán R.M., Luque P., H1N1 SEMICYUC Working Group Community-acquired respiratory coinfection in critically ill patients with pandemic 2009 influenza A(H1N1) virus. Chest. 2011;139:555–562. doi: 10.1378/chest.10-1396. [DOI] [PubMed] [Google Scholar]
- 9.Kreitmann L., Monard C., Dauwalder O., Simon M., Argaud L. Early bacterial co-infection in ARDS related to COVID-19. Intensive Care Med. 2020;46:1787–1789. doi: 10.1007/s00134-020-06165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hughes S., Troise O., Donaldson H., Mughal N., Moore L.S.P. Bacterial and fungal coinfection among hospitalized patients with COVID-19: a retrospective cohort study in a UK secondary-care setting. Clin. Microbiol. Infect. 2020;26:1395–1399. doi: 10.1016/j.cmi.2020.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lansbury L., Lim B., Baskaran V., Lim W.S. Co-infections in people with COVID-19: a systematic review and meta-analysis. J. Infect. 2020;81:266–275. doi: 10.1016/j.jinf.2020.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Coppola S., Ciabattoni A., Pozzi T., Castagna V., Bassi G.L., Chiumello D. Hazardous mismatch between pulmonary pathogens and antibiotic treatments in COVID-19 patients. Br. J. Anaesth. 2020;125:e380–e382. doi: 10.1016/j.bja.2020.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir. Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellière S., Dudoignon E., Fodil S., Voicu S., Collet M., Oillic P.-A., Salmona M., Dépret F., Ghelfenstein-Ferreira T., Plaud B. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin. Microbiol. Infect. 2020 doi: 10.1016/j.cmi.2020.12.005. Published online December 13, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., Wu Y., Zhang L., Yu Z., Fang M. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir. Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J., Zhou L., Yang Y., Peng W., Wang W., Chen X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir. Med. 2020;8:e11–e12. doi: 10.1016/S2213-2600(20)30071-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He Y., Li W., Wang Z., Chen H., Tian L., Liu D. Nosocomial infection among patients with COVID-19: A retrospective data analysis of 918 cases from a single center in Wuhan, China. Infect. Control Hosp. Epidemiol. 2020;41:982–983. doi: 10.1017/ice.2020.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cox M.J., Loman N., Bogaert D., O’Grady J. Co-infections: potentially lethal and unexplored in COVID-19. Lancet Microbe. 2020;1:e11. doi: 10.1016/S2666-5247(20)30009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of co-infection between SARS-CoV-2 and other respiratory pathogens. JAMA. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones R.N. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin. Infect. Dis. 2010;51(Suppl 1):S81–S87. doi: 10.1086/653053. [DOI] [PubMed] [Google Scholar]
- 23.Clancy C.J., Nguyen M.H. COVID-19, superinfections and antimicrobial development: What can we expect? Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa524. Published online May 1, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrasa H., Rello J., Tejada S., Martín A., Balziskueta G., Vinuesa C., Fernández-Miret B., Villagra A., Vallejo A., San Sebastián A., Alava COVID-19 Study Investigators SARS-CoV-2 in Spanish intensive care units: early experience with 15-day survival in Vitoria. Anaesth. Crit. Care Pain Med. 2020;39:553–561. doi: 10.1016/j.accpm.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tagarro A., Epalza C., Santos M., Sanz-Santaeufemia F.J., Otheo E., Moraleda C., Calvo C. Screening and severity of coronavirus disease 2019 (COVID-19) in children in Madrid, Spain. JAMA Pediatr. 2020 doi: 10.1001/jamapediatrics.2020.1346. Published online April 8, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X., Ge Y., Wu T., Zhao K., Chen Y., Wu B., Zhu F., Zhu B., Cui L. Co-infection with respiratory pathogens among COVID-2019 cases. Virus Res. 2020;285:198005. doi: 10.1016/j.virusres.2020.198005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin-Loeches I., van Someren Gréve F., Schultz M.J. Bacterial pneumonia as an influenza complication. Curr. Opin. Infect. Dis. 2017;30:201–207. doi: 10.1097/QCO.0000000000000347. [DOI] [PubMed] [Google Scholar]
- 28.Arabi Y.M., Balkhy H.H., Hayden F.G., Bouchama A., Luke T., Baillie J.K., Al-Omari A., Hajeer A.H., Senga M., Denison M.R. Middle East respiratory syndrome. N. Engl. J. Med. 2017;376:584–594. doi: 10.1056/NEJMsr1408795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Assiri A., Al-Tawfiq J.A., Al-Rabeeah A.A., Al-Rabiah F.A., Al-Hajjar S., Al-Barrak A., Flemban H., Al-Nassir W.N., Balkhy H.H., Al-Hakeem R.F. Epidemiological, demographic, and clinical characteristics of 47 cases of Middle East respiratory syndrome coronavirus disease from Saudi Arabia: a descriptive study. Lancet Infect. Dis. 2013;13:752–761. doi: 10.1016/S1473-3099(13)70204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod. Pathol. 2005;18:1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rawson T.M., Moore L.S.P., Castro-Sanchez E., Charani E., Davies F., Satta G., Ellington M.J., Holmes A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020;75:1681–1684. doi: 10.1093/jac/dkaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.de Oliveira Santos G.C., Vasconcelos C.C., Lopes A.J.O., de Sousa Cartágenes M.D.S., Filho A.K.D.B., do Nascimento F.R.F., Ramos R.M., Pires E.R.R.B., de Andrade M.S., Rocha F.M.G., de Andrade Monteiro C. Candida infections and therapeutic strategies: mechanisms of action for traditional and alternative agents. Front. Microbiol. 2018;9:1351. doi: 10.3389/fmicb.2018.01351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caméléna F., Moy A.C., Dudoignon E., Poncin T., Deniau B., Guillemet L., Le Goff J., Budoo M., Benyamina M., Chaussard M. Performance of a multiplex polymerase chain reaction panel for identifying bacterial pathogens causing pneumonia in critically ill patients with COVID-19. Diagn. Microbiol. Infect. Dis. 2021;99:115183. doi: 10.1016/j.diagmicrobio.2020.115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dudoignon E., Caméléna F., Deniau B., Habay A., Coutrot M., Ressaire Q., Plaud B., Berçot B., Dépret F. Bacterial pneumonia in COVID-19 critically ill patients: a case series. Clin. Infect. Dis. 2021;72:905–906. doi: 10.1093/cid/ciaa762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., Hallek M., Jung N., Klein F., Persigehl T. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang J., Yang Q., Zhang P., Sheng J., Zhou J., Qu T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID-19 in Zhejiang, China: a retrospective case series. Crit. Care. 2020;24:299. doi: 10.1186/s13054-020-03046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Yang B., Li Q., Wen L., Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin. Infect. Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ranieri V.M., Rubenfeld G.D., Thompson B.T., Ferguson N.D., Caldwell E., Fan E., Camporota L., Slutsky A.S., ARDS Definition Task Force Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 39.Yehya N., Harhay M.O., Curley M.A.Q., Schoenfeld D.A., Reeder R.W. Reappraisal of ventilator-free days in critical care research. Am. J. Respir. Crit. Care Med. 2019;200:828–836. doi: 10.1164/rccm.201810-2050CP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Frey P.M., Marti G.R., Droz S., de Roche von Arx M., Suter-Riniker F., Aujesky D., Brugger S.D. Bacterial colonization of handheld devices in a tertiary care setting: a hygiene intervention study. Antimicrob. Resist. Infect. Control. 2019;8:97. doi: 10.1186/s13756-019-0546-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Corman V.M., Landt O., Kaiser M., Molenkamp R., Meijer A., Chu D.K.W., Bleicker T., Brünink S., Schneider J., Schmidt M.L. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chastre J., Fagon J.Y. Ventilator-associated pneumonia. Am. J. Respir. Crit. Care Med. 2002;165:867–903. doi: 10.1164/ajrccm.165.7.2105078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data and analysis scripts used in this study are available upon request to the Lead author.
No new code was generated.