Abstract
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections present with increased disease severity and poor clinical outcomes in diabetic patients compared with their nondiabetic counterparts. Diabetes/hyperglycemia-triggered endothelial dysfunction and hyperactive inflammatory and immune responses are correlated to twofold to threefold higher intensive care hospitalizations and more than twice the mortality among diabetic coronavirus disease 2019 (COVID-19) patients. While comorbidities such as obesity, cardiovascular disease, and hypertension worsen the prognosis of diabetic COVID-19 patients, COVID-19 infections are also associated with new-onset diabetes, severe metabolic complications, and increased thrombotic events in the backdrop of aberrant endothelial function. While several antidiabetic medications are used to manage blood glucose levels, we discuss the multifaceted ability of metformin to control blood glucose levels and possibly attenuate endothelial dysfunction, inhibit viral entry and infection, and modify inflammatory and immune responses during SARS-CoV-2 infections. These actions make metformin a viable candidate drug to be considered for repurposing and gaining ground against the SARS-CoV-2-induced tsunami in diabetic COVID-19 patients.
Keywords: blood glucose control, coronavirus, COVID-19, diabetes, endothelial dysfunction, metformin, SARS-CoV-2
Diabetes and COVID-19: Reciprocity at Play
On January 7, 2020, a novel beta-coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (see Glossary), was identified as the causative agent of the ‘atypical pneumonia’ that surfaced in Wuhan, China, on December 30, 2019 [1]. Since the World Health Organization (WHO) declared the SARS-CoV-2-caused coronavirus disease 2019 (COVID-19) outbreak a pandemic on March 11, 2020, to date (March 5, 2021), over 116 million COVID-19 cases have been reported, and more than 2.5 million people have lost their lives globallyi [1]. Finding an effective way to control the rapid spread of the disease and a cure for COVID-19 remains at the forefront of the battle against this pandemic even as the vaccines – Tozinameran/BNT162b2/Comirnaty (Pfizer/BioNTech), mRNA-1273 (Moderna), AZD1222/Covishield (University of Oxford/AstraZeneca), and Ad26.COV2.S/JNJ-78436735 (Janssen Vaccines and Prevention) – are being approved and authorized for emergency use in several countries [2., 3., 4., 5.]. However, the emergence of highly transmissible SARS-CoV-2 mutants have raised concerns that these variants could evade the body’s immune response, threaten vaccine efficacy, and may cause a resurgence of highly transmissible cases of COVID-19 [6].
The highly contagious SARS-CoV-2 virus can affect all individuals irrespective of age, gender, and ethnicity, but to varying degrees [7]. Most COVID-19 cases remain asymptomatic or present with relatively mild flu-like symptoms, increasing the risk of transmission and the significant spread of SARS-CoV-2 [8]. Nevertheless, the vulnerability, aggressiveness/severity of the disease, hospitalization rates, and mortality is significantly higher in men, among the elderly, and in those with one or more comorbidities/pre-existing conditions such as hypertension, cardiovascular or cerebrovascular diseases, diabetes, cancers, and renal damage [1,9]. In the face of pre-existing/comorbid conditions, severe COVID-19 cases can rapidly progress into acute respiratory distress syndrome (ARDS), septic shock, multiple-organ dysfunction syndrome (MODS), and organ failure [10].
The ability of SARS-CoV-2 to infect and damage multiorgan systems is dependent on the expression/distribution pattern of the host angiotensin-converting enzyme 2 receptor (ACE2; which binds to the viral spike protein) and the transmembrane serine protease 2 (TMPRSS2; which cleaves and primes the viral spike protein), in various organs and tissues, facilitating viral activation and entry in the host cell [11,12]. Comorbidities, such as diabetes and obesity, upregulate ACE2, leading to an increase in viral load within various tissues and organs [13]. Furthermore, increased ACE2 shedding from the cell surface in diabetic and obese subjects facilitates redistribution of ACE2 in the body and its accumulation in the lungs [13]. Conversely, the ACE2 receptor plays a crucial role in regulating the renin–angiotensin–aldosterone system (RAAS) and hence supports and protects cardiovascular and pulmonary function [14., 15., 16.]. ACE2 maintains a crucial balance by downregulating the levels of angiotensin II (AngII) by activating the ACE2/Ang(1-7)/MAS axis of RAAS, thereby conferring protective vasodilatory, vascular protective, antifibrotic, antiproliferative, and anti-inflammatory effects [17]. Therefore, the depletion of ACE2 by SARS-CoV-2 binding can lead to adverse cardiopulmonary events and multiorgan injury [12]. Interestingly, the ACE2-expressing endothelial cells (ECs) of the vasculature are crucial targets for SARS-CoV-2 infection [18., 19., 20.]. In diabetic and obese individuals, a decrease in the baseline expression of ACE2 in the vasculature leads to endothelial dysfunction (ED), contributing to the higher incidence of thrombotic events in COVID-19 patients [13,19,20].
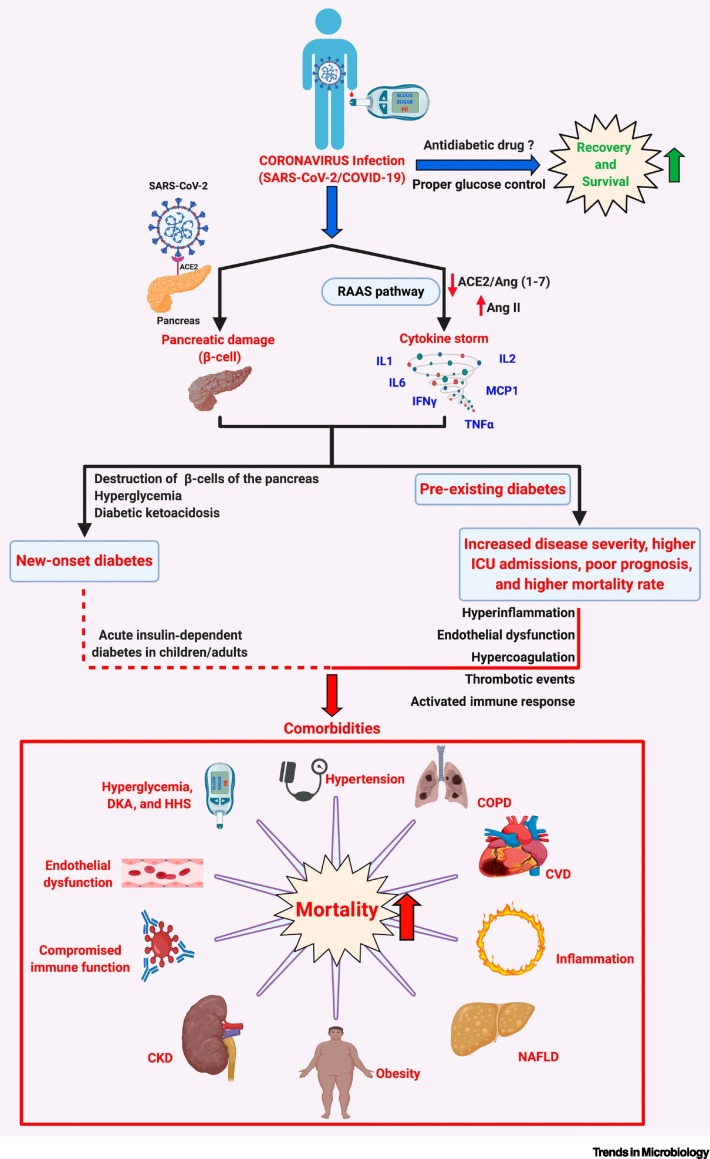
Previously, in coronaviral infections caused by SARS-CoV-1 and the Middle East respiratory syndrome coronavirus (MERS-CoV), pre-existing diabetes was identified as an independent risk factor contributing to increased disease severity and higher mortality among affected individuals [21]. Similarly, COVID-19 patients with pre-existing diabetes with/without one or more comorbidities such as obesity, hypertension, cardiovascular disease, and chronic kidney disease (CKD) require in-hospital intensive care unit (ICU) care/treatment and tracheal intubation/mechanical ventilation and correlate with poor prognosis and increased risk of all-cause mortality when compared with their nondiabetic counterparts (Figure 1 ) [1]. Interestingly, the occurrence of new-onset diabetes and related severe metabolic complications (diabetic ketoacidosis and hyperosmolarity) in COVID-19 patients indicate a bidirectional relationship between diabetes and COVID-19 (Figure 1) [22].
Figure 1.
Diabetes/Hyperglycemia and Possible Outcomes in COVID-19 Patients.
SARS-CoV-2 infection causes activation of RAAS that can result in a ‘cytokine storm’ via the AngII/AT1R axis, resulting in the synthesis and secretion of proinflammatory cytokines/chemokines such as tumor necrosis factor-α (TNFα), interleukins (ILs)1/2/6, interferon-γ (IFNγ), and monocyte chemoattractant protein-1 (MCP1). In SARS-CoV-2-infected individuals with pre-existing diabetes, the diabetes-associated proinflammatory status and endothelial dysfunction, and the incidence of one or more comorbidities – such as obesity, hypertension, CVD, NAFLD, and CKD, and the hyperglycemia-induced DKA and HHS – can lead to an increase in disease severity, higher rates of intensive care unit (ICU) admissions, and may be responsible for the poor prognosis and higher mortality rates in diabetic COVID-19 patients. Interestingly, reports also suggest that SARS-CoV-2 infects the β-islets of the pancreas, causing β-cell damage and subsequent new-onset diabetes, severe hyperglycemia and DKA in COVID-19 patients. Treatment using appropriate glucose-lowering agents and proper management of blood glucose levels aids recovery and survival among affected diabetic COVID-19 patients. Various aspects – such as benefits, contraindications, and limitations of using certain combinations of glucose-lowering agents and antiviral treatments that could affect the outcome of the disease in a diabetic COVID-19 patient – must be carefully analyzed. Abbreviations: ACE2, angiotensin-converting enzyme 2; AngII, angiotensin II; AT1R, angiotensin II type I receptor; CKD, chronic kidney disease, COPD, chronic obstructive pulmonary disorder; COVID-19, coronavirus disease 2019; CVD, cardiovascular disease; DKA, diabetic ketoacidosis; HHS, hyperglycemic hyperosmolar syndrome; NAFLD, nonalcoholic fatty liver disease; RAAS, renin–angiotensin–aldosterone system; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Created with BioRender.com.
Reports suggest that the proper management of blood glucose levels reduced disease severity, the incidence of ARDS, the requirement for ICU admissions, and ventilator support, and it promoted recovery in COVID-19 patients with pre-existing diabetes (Figure 1) [10]. While several antihyperglycemic medications are available to manage blood glucose levels, it is necessary to carefully ascertain the best possible antidiabetic medication that can be safely administered to COVID-19 patients to control hyperglycemia and to suppress related adverse events. In this regard, metformin treatment was beneficial in treating diabetic COVID-19 patients since it reduced disease severity and mortality rates in COVID-19 patients [16,23]. This opinion article highlights the multifaceted benefits of metformin and investigates the possibility of repurposing metformin as an effective drug for the treatment of diabetic COVID-19 patients.
Diabetes and COVID-19: Increased Disease Severity and Mortality
Diabetic patients have a compromised immune response and are prone to severe bacterial and viral infections/diseases, require more recovery time, and present longer-lasting adverse effects than their nondiabetic counterparts [24]. Proper management and maintenance of controlled blood glucose levels (avoiding consistent hyperglycemia) are closely related to the body’s ability to regulate immune and inflammatory responses and fight infections in chronic diabetic patients [10]. An aggravated inflammatory and immune response-related severe course of the disease and higher mortality was observed during various bacterial/viral infections in hyperglycemic/diabetic COVID-19 patients that could be significantly reversed by maintaining controlled blood glucose levels.
COVID-19 and Pre-existing Diabetes
Plotting glycated hemoglobin (HbA1c) against the risk of SARS-CoV-2 infection/COVID-19-related hospitalization shows a characteristic J-curve, which indicates that diabetes is associated with a higher risk of infections (respiratory infections in particular) [1]. Although pre-existing diabetes did not increase the risk of occurrence of COVID-19, there was a significant increase in the severity of SARS-CoV-2 infection (COVID-19) among diabetic individuals, thereby increasing their risk of hospitalization and requirement for emergency care [1]. Elderly severely ill diabetic COVID-19 patients exhibited an exaggerated inflammatory response and were more likely to require mechanical ventilation and ICU support, with a markedly higher risk of mortality than COVID-19 patients without diabetes [25]. Worldwide studies corroborated severe pneumonia cases, increased risk of ICU admissions, and higher mortality rates in COVID-19 patients with diabetes (Table 1 ).
Table 1.
Prevalence of Pre-existing Diabetes and COVID-19 Outcomes (Adapted from [1])
| Study type | Study origin | Study population | Prevalence of diabetes (%) | Outcome | Refs |
|---|---|---|---|---|---|
| Retrospective | China | 258 | 24 | ↑ Mortality | [26] |
| Meta-analysis | India | 16 003 (from 33 studies) | 9.8 | ↑ Disease severity ↑ Mortality |
[27] |
| Retrospective | China | 1590 | NA | ↑ ICU admission, or invasive ventilation, or death | [28] |
| Meta-analysis | China | 1527 (from 6 studies) | 9.7 | ↑ ICU admissions | [29] |
| Meta-analysis | Italy | 1687 (from 6 studies) | NA | ↑ Disease severity | [30] |
| Meta-analysis | Italy | 355 (from 6 studies) | 35.5 | ↑ Mortality | [30] |
| Retrospective | USA | 5279 | 22.6 | ↑ Hospitalization | [31] |
| Meta-analysis | Italy | 1382 (from 4 studies) | NA | ↑ ICU admissions | [32] |
| Meta-analysis | Italy | 471 (from 4 studies) | NA | ↑ Mortality | [32] |
| Retrospective | China | 191 | 19 | ↑ Mortality | [9] |
| Retrospective | China | 7337 | 13 | ↑ Mortality | [10] |
| Retrospective | China | 193 | 25 | ↑ Mortality | [25] |
| Retrospective | Italy | 59 | 44 | ↑ Disease severity ↓ Survival |
[33] |
| Meta-analysis | China | 1576 (from 7 studies) | NA | ↑ Disease severity | [34] |
| Cohort | UK | 61 414 470 | 0.4 (type 1 diabetes) | ↑ Mortality | [35] |
| Cohort | UK | 61 414 470 | 4.7 (type 2 diabetes) | ↑ Mortality | [35] |
| Retrospective | France | 1317 | 88.5 | ↑ Tracheal intubation for mechanical ventilation and/or mortality | [36] |
COVID-19 and New-Onset Diabetes
Viral infections, such as those caused by enterovirus, rotavirus, and mumps virus, could lead to acute type 1 diabetes [37]. Upon SARS-CoV-1 infection, individuals with no previous diagnosis or history of diabetes developed acute hyperglycemia, an independent indicator for higher mortality among such individuals [38]. This was associated with the ability of the SARS-CoV-1 virus to bind to the pancreatic islet ACE2 receptors, causing acute islet damage [38].
Similarly, despite the absence of pre-existing diabetes, the occurrence of new-onset hyperglycemia in COVID-19 patients could result from SARS-CoV-2 viral infection [1,22]. Evidence suggests that the pancreatic ACE2 receptor facilitates SARS-CoV-2 binding and entry, and the ensuing cellular damage should explain the new-onset of diabetes in COVID-19 patients [1,39]. SARS-CoV-2 binding to its ACE2 receptor on the pancreas downregulates ACE2 activity and creates an imbalance in RAAS [40]. The subsequent accumulation of AngII, and the overactivation of the AngII/ angiotensin II type I receptor (AT1R)-axis, triggers macrophage activation and triggers NF-κB signaling. This, in turn, leads to the excessive synthesis and secretion of several inflammatory cytokines (hypercytokinemia/cytokine storm), resulting in pancreatic damage, and partially explains new-onset diabetes in COVID-19 patients [17,40,41]. Significant increases in the levels of several proinflammatory cytokines/markers [interleukin (IL)-1β, IL-6, IL-10, and tumor necrosis factor alpha (TNFα)] were reported in severely ill COVID-19 patients and COVID-19 patients admitted to the ICU, while higher levels of IL-6 were correlated with higher mortality rates [41,42]. An in vitro model that studied virus tropism using pseudoviruses for SARS-CoV-2 entry in human pancreatic α- and β-cells showed that the pancreatic cells were highly permissive to SARS-CoV-2 entry and mimicked the chemokine induction that is typical of COVID-19 patients [37].
A study involving 33 children (who were previously exposed to SARS-CoV-2 or had active SARS-CoV-2 infection) reported the occurrence of new-onset type 1 diabetes in 30 of them (aged 23 months to 16.8 years) [43]. Twenty-one children (70%) developed diabetic ketoacidosis (DKA), while 11 of the 21 children reported severe DKA [43]. Despite a direct link, the study postulated that SARS-CoV-2 exposure caused an increase in new-onset type 1 diabetes in children [43]. A single-case study reported high blood glucose concentration (552 mg/dl) and HbA1c (16.8%) levels in a 19-year-old male who presented with DKA and tested positive for antibodies against SARS-CoV-2, indicating a possible COVID-19 infection 5–7 weeks before hospitalization [44]. Interestingly, autoimmune factors that could lead to the development of a type 1 diabetic condition were ruled out, suggesting a causal link between COVID-19 and the development of diabetes possibly due to SARS-CoV-2 infection of the β-cells via the ACE2 receptors and a direct cytolytic effect of the virus on the cells [44]. Remarkably, reports suggest a significant increase in mortality among COVID-19 patients diagnosed with new-onset hyperglycemia (without diabetes) compared with patients with pre-existing diabetes [45,46].
Health authorities must make general health recommendations that require COVID-19 patients to monitor their blood glucose levels frequently and remain vigilant regarding possible signs/symptoms of hyperglycemia. Therapeutic recommendations must be made based on initial and continuously monitored blood glucose measurements and the patient’s medical history. Evaluation of markers of inflammation, coagulation factors, acute-phase reactants, hepatic and renal function will help to identify hypercytokinemia and predict prothrombotic events. Thus, necessary adjustments in the treatment plan will improve the prognosis and survival of high-risk diabetic COVID-19 patients.
Endothelial Dysfunction: The Common Denominator in Diabetes and COVID-19
The vasculature is most vulnerable to SARS-CoV-2 infections [18]. ECs express ACE2 receptors through which SARS-CoV-2 enters the cells [19]. SARS-CoV-2 particles and host inflammatory cells found inside ECs with evidence of endothelial and inflammatory cell death suggest that the endothelium and alterations in its function may contribute to disease progression and outcome among COVID-19 patients [19,20]. COVID-19 is associated with ED, hyperviscosity/coagulation, higher incidence of thrombotic events, and microvascular complications as evidenced by elevated levels of D-dimer, von Willebrand factor (VWF), fibrinogen, and soluble P-selectin, and an increase in the activity of VWF and factor VIII activity in critically ill COVID-19 ICU patients when compared with their non-ICU counterparts [18,47,48]. An increase in the incidence of venous thromboembolism, microvascular lung thrombosis, arterial events, and disseminated intravascular damage was linked to higher ICU admissions, the occurrence of terminal events, and mortality among severe COVID-19 patients [1,18,48,49].
In diabetic COVID-19 patients, it is still too early to precisely state whether diabetes-associated ED exacerbates COVID-19 severity or whether COVID-19 infection accentuates diabetes-associated ED. In diabetes, the endothelium is exposed to hyperinsulinemia, hyperglycemia, and an excess of free fatty acids, and a consequent array of adverse molecular events – in response to various triggers such as increased reactive oxygen species (ROS) and decreased endothelial nitric oxide (NO) levels – causing ED [50]. Diabetes-associated ED, and a consequent prothrombotic state, likely increases the risk of thromboembolic events in diabetic COVID-19 patients [1,25,51]. Furthermore, an increase in the levels of advanced glycation end-products (AGEs), and subsequent activation of the receptors of AGEs (RAGEs), contribute to ED and chronic vascular complications that promote coagulation, supported by an increase in vascular hyperpermeability, increased leukocyte adhesion, and extravasation in diabetes [52,53]. While the lungs’ type 1 alveolar epithelial cells constitutively express RAGE, its activation in ECs, smooth muscle cells, neurons, and immune cells depends on the local expression of RAGE ligands [53,54]. Ligands (other than AGEs), such as high-mobility group box 1 protein (HMGB1), S100 calcium-binding protein A12 (S100A12), other danger-associated molecular patterns (DAMPs), and exogenous pathogen-associated molecular patterns (PAMPs), released from SARS-CoV-2 infected damaged/dying cells, can activate the innate immune system via RAGE activation [53]. The activation of RAGE, in turn, creates a feed-forward loop that exacerbates inflammatory responses via the RAGE-mediated transcription of NF-κB-dependent proinflammatory genes that code for inflammatory cytokines and cell adhesion molecules [53,55].
Constant clinical evaluation of ED and markers of thrombotic events, radiological assessment, and thromboprophylaxis/anticoagulant therapy is recommended and encouraged as a part of standard care for all COVID-19 patients, especially those with diabetes [48,56]. Therapeutic intervention(s) that support restoration and stabilization of the normal endothelial apparatus and function, and target inflammation, may improve prognosis and survival of COVID-19 patients [57]. Currently, studies are ongoing to investigate the possible beneficial effects of anticoagulant therapy and potential interventions that improve endothelial function, such as RAS inhibitors, statins, and antioxidants, to decrease disease severity and mortality among COVID-19 patients [57].
Hyperglycemia and COVID-19 Outcome: The Need for Glycemic Control
Irrespective of whether a COVID-19 patient has pre-existing diabetes, was diagnosed with new-onset diabetes, or is nondiabetic, the available evidence points to the fact that blood glucose level is a crucial factor that would determine (i) susceptibility to a COVID-19 infection in the event of an exposure, (ii) severity of the disease, (iii) treatment strategies, (iv) recovery, and (v) outcomes (measured in terms of disease severity/ARDS/ICU admissions/cardiac injury/renal damage/survival/mortality) among COVID-19 patients [10,46,58,59]. Hyperglycemia and higher fasting blood/plasma glucose (FBG/FPG) levels (Table 2 ) unarguably increased disease severity, the incidence of ARDS, and cardiac and renal damage, and were correlated with higher ICU admissions and mortality among COVID-19 patients. Hence, blood glucose measurements are crucial and must be frequently monitored and managed efficiently under strictly supervised treatment.
Table 2.
Hyperglycemia and COVID-19 Outcomes (Adapted from [1])
| Study type | Study origin | Study population | Prevalence of diabetes (%) | Parameter | Outcome | Refs |
|---|---|---|---|---|---|---|
| Retrospective | China | 810 | 100 | Median blood glucose during hospital stay (6.4 mmol/l; well controlled vs 10.6 mmol/l; poorly controlled) | ↑ Mortality ↑ ARDS ↑ Cardiac injury ↑ Renal damage |
[10] |
| Retrospective | Italy | 59 | 42.4 | Blood glucose at the time of hospital admission (>7.7 mmol/l) | ↑ Disease severity ↑ Mortality ↓ Survival |
[33] |
| Cohort | UK | 17 278 392 | 9.9 | HbA1c (≥7.5%) | ↑ Mortality | [60] |
| Retrospective | China | 904 | 15 | Hyperglycemia | ↑ Mortality | [61] |
| Retrospective | China | 269 | 19.3 | Hyperglycemia | ↑ Mortality | [62] |
| Retrospective | China | 28 | 100 | Random hyperglycemia | ↑ Disease severity ↑ ICU admissions ↑ ARDS ↑ Mortality |
[63] |
| Retrospective | USA | 1122 | 40.2 | HbA1c (≥6.5%) and uncontrolled hyperglycemia (more than two blood glucose measurements ≥180 mg/dl within any 24 h period) | ↑ Mortality ↑ Median length of stay at hospital |
[45] |
| Cohort | Kuwait, USA | 417 | 23.3 | Fasting blood glucose levels (1 mmol/l or 5 mmol/l increase in FBG; FBG ≥7 mmol/l) | ↑ ICU admissions | [59] |
| Retrospective | China | 605 | No previous diagnosis of diabetes | FBG <6.1 mmol/l FBG –6.1 to 6.9 mmol/l FBG >7.0 mmol/l |
↑ Mortality (in patients with FBG > 7.0 mmol/l) | [58] |
| Retrospective | China | 166 | 36.7 (diabetic) 12.7 (FPG >7.0 mmol/l) |
FPG >7.0 mmol/l, but HbA1c (<6.5%) | ↑ ICU admissions ↑ Mechanical ventilation ↑ Mortality |
[64] |
Repurposing Metformin: Potential Impact on COVID-19 Treatment Strategy
Several antihyperglycemic drugs are routinely used to manage blood glucose levels in diabetic patients. The question remains as to which medication/drug a clinician would recommend (in combination with other required drugs such as antivirals) to effectively reduce blood glucose levels and adverse long-term post-COVID-19 effects and ultimately save the lives of SARS-CoV-2-infected diabetic patients. Glucose-lowering agents, such as insulin and sitagliptin, decreased disease severity and mortality rates in diabetic COVID-19 patients [33,65]. The beneficial effects observed when using insulin could be correlated to its anti-inflammatory and immunomodulatory effects and the achievement of glycemic control in the patients [66]. Conversely, the inhibitory effect of insulin on disintegrin and metalloproteinase domain-containing protein-17 (ADAM17) facilitates the proteolytic cleavage and shedding of the active ectodomain of ACE2 and, in turn, increases the availability and activity of ACE2 for SARS-CoV-2 infection, which in part may explain the worsening clinical profile and poor prognosis in insulin-administered COVID-19 patients [61,67]. On the other hand, metformin, the most prescribed oral antihyperglycemic drug, could be beneficial in treating COVID-19 at multiple levels. With its well-studied and documented benefits, metformin may hold a possible answer to blood glucose management and attenuation of COVID-19 complications.
Meta-analysis studies reported significant metformin treatment-associated reduction in COVID-19 infection-related mortality [68,69]. Reports from retrospective studies suggest a significant metformin treatment-associated reduction in mortality among high-risk diabetic COVID-19 patients [23]. Interestingly, a retrospective cohort study, involving 6256 type 2 diabetic or obese [body mass index (BMI) at least 30 kg/m2] COVID-19 participants – among whom 2333 patients were on a metformin treatment plan before their COVID-19 diagnosis – found a gender-dependent effect in which metformin treatment was associated with a reduction in disease severity and mortality among women, but not in men [70]. A retrospective study, among 1213 COVID-19 patients (678 were using metformin), showed that metformin treatment was associated with an increased incidence of acidosis (but not mortality) in type 2 diabetic COVID-19 participants, which was correlated to high metformin dosage, compromised renal function, and severe COVID-19 illness [71]. However, owing to the ability of metformin to reduce inflammation and confer cardioprotection among type 2 diabetic COVID-19 patients, the researchers recommended the continuation of metformin therapy while continually monitoring the patients for acidosis and deterioration of renal function [71]. Crouse et al. reaffirmed the role of diabetes as a prominent independent risk factor that contributed to a higher mortality rate among diabetic COVID-19 patients when compared with nondiabetic COVID-19 patients [72]. They reported a threefold decrease in mortality among diabetic COVID-19 patients on a metformin treatment regimen before their COVID-19 diagnosis, while prior insulin use did not affect mortality [72]. This beneficial effect of metformin was observed even after correcting for other COVID-19 risk factors such as age, sex, race, obesity, and hypertension, or CKD and heart failure [72].
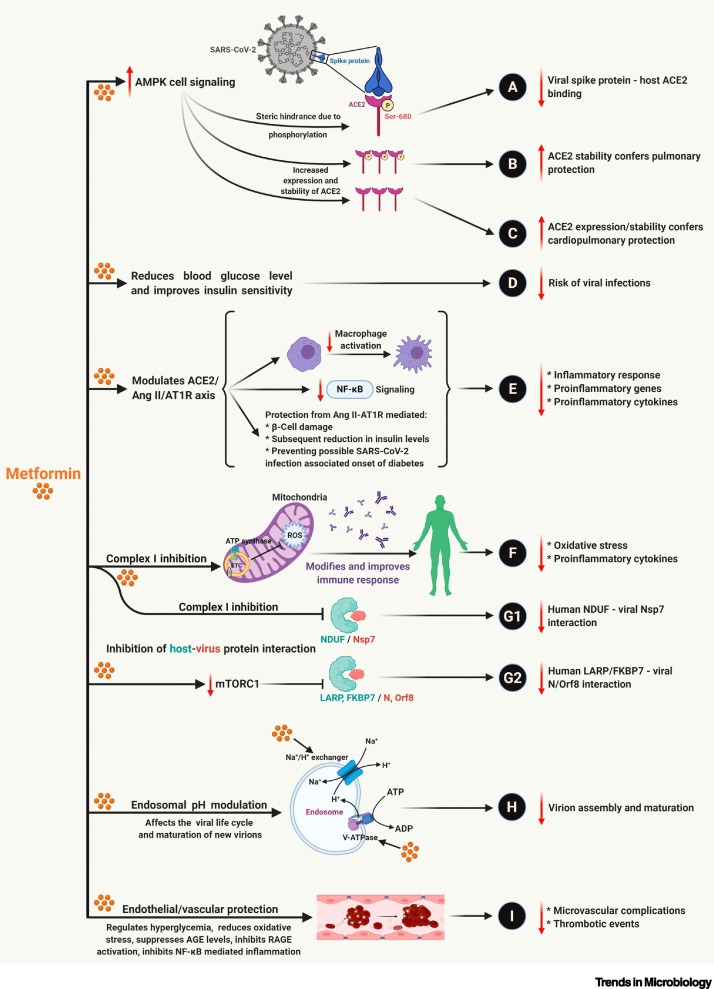
In addition to lowering blood glucose levels and increasing insulin sensitivity, metformin has documented molecular effects that suggest its therapeutic efficacy against COVID-19 (please refer to Figure 2 for details).
Figure 2.
Multiple Benefits of Metformin Treatment against SARS-CoV-2 Infection.
Metformin treatment-associated activation of AMPK-mediated signaling mechanisms is well studied and documented [79]. The AMPK-dependent increase in (A): ACE2 receptor phosphorylation (Ser680) causes a conformational change that inhibits ACE2 – viral spike protein binding and reduction of viral entry into the cell [15,16]. AMPK-mediated increase in (B): ACE2 phosphorylation (ACE2 phosphorylation prevents polyubiquitination and subsequent 26-proteasome-mediated degradation of ACE2). (C) ACE2 expression increases its half-life/stability and offers cardiopulmonary protection via RAAS regulation [14., 15., 16.]. The ability of metformin to reduce blood glucose levels and improve insulin stability (D) reduces the risk of SARS-CoV-2 infections [15]. Metformin treatment-associated increase in ACE2 levels and stability, in turn, regulates the ACE2/AngII/AT1R axis and suppresses (E) inflammatory response and release of proinflammatory cytokines by inhibiting macrophage activation and NF-κB signaling [16]. Metformin targets complex I of the mitochondrial electron transport chain (ETC), inhibits the generation of reactive oxygen species (ROS), and (F) suppresses the oxidative stress-mediated release of proinflammatory cytokines and attenuates inflammatory immune response [15,80]. Inhibition of ETC and mTORC1 signaling (via AMPK or PI3K/Akt) by metformin (G1 and G2) contributes to the suppression of host–viral protein interactions, such as NDUF (human)–Nsp7 (viral) and LARP/FKBP7 (human)–N/ORF8 (viral) interactions [81]. The suppression of the host–virus protein interactions inhibits host-dependent viral replication, synthesis of viral proteins, virion maturation, and release. Metformin, a strong base, targets the vacuolar ATPase (V-ATPase) and endosomal Na+/H+ exchangers (eNHEs) (H), increasing the cellular and endosomal pH and suppressing the endocytotic cycle and virion assembly and maturation [15,82]. The antihyperglycemic, antioxidant, immunomodulatory, and anti-inflammatory effects of metformin attenuate endothelial dysfunction and confer vascular protection, thus (I) reducing microvascular complications and thrombotic events during SARS-CoV-2 infection. Abbreviations: ACE2, angiotensin-converting enzyme 2; AGE, advanced glycation end-product; AMPK, AMP-activated protein kinase; AngII, angiotensin II; AT1R, AT1R, angiotensin II type I receptor; mTORC1, mammalian target of rapamycin complex 1; RAAS, renin–angiotensin–aldosterone system; RAGE, receptor of AGE; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. Created with BioRender.com.
The use of metformin in type 2 diabetic patients is associated with a reduced risk of deep-vein thrombosis, as reported by a nonrandomized, pair-matched cohort study [73]. Other studies suggest that metformin prevents platelet activation and extracellular mitochondrial DNA release, thereby preventing venous and arterial thrombosis without a significantly prolonged bleeding time [74]. Metformin confers multiple protective effects on the endothelium, improves endothelium-dependent vascular response, and attenuates ED in diabetes through several well studied mechanisms such as the activation of AMP-activated protein kinase (AMPK), Sirt1, and endothelial nitric oxide synthase (eNOS) [75]. Besides, metformin can protect the endothelium by reducing oxidative stress, inhibiting endothelial inflammation, and suppressing leukocyte–endothelium interactions while also attenuating EC senescence and apoptosis and preserving the endothelial glycocalyx that shields against ED [75,76]. Furthermore, metformin attenuates RAGE overexpression, inhibits hyperglycemia-induced NF-κB activation and subsequent gene expression of several proinflammatory cytokines and cell adhesion molecules in vascular wall cells (ECs and smooth muscle cells) and macrophages, and confers vascular protection [77,78].
In chronic diabetic patients with multiple comorbidities, such as obesity, hypertension, and cardiovascular diseases, the aberrant activation of RAAS, increase in oxidative stress and inflammation, and activation of the immune system can exacerbate the clinical complications during SARS-COV-2 infection and thus contribute to mortality in COVID-19 patients. Metformin may be recommended (in the absence of severe kidney ailments) as a preventive drug to protect type 2 diabetic and obese patients from severe illness and increase survival among COVID-19 patients. The protective effects of metformin go beyond its antihyperglycemic effect and ability to increase insulin sensitivity in chronic diabetic patients with multiple comorbidities. Studies are warranted to explore the antihyperglycemic, antiviral, anti-inflammatory, immunomodulatory, and antithrombotic effects of metformin that may contribute to its protective effects in diabetic COVID-19 patients in the absence/presence of one or more comorbidities.
Metformin Efficacy in COVID-19 Treatment – Clinical Trials
Available data support the repurposing and use of metformin as an effective therapeutic in the treatment of COVID-19. Data from ClincalTrials.gov (https://www.clinicaltrials.gov/) [as of January, 20 2020; primary search keyword (condition/disease): COVID-19; secondary search keyword (other terms): diabetes] showed 159 clinical trials that are related to COVID-19 and diabetes. However, only four of these studies were specific to metformin [secondary search keyword (other terms): metformin] in COVID-19ii.
The ‘COVIDOUT – Outpatient Treatment of COVID-19 with Metformin’ (NCT04510194; Phase II/III; 750 participants; age 30–85 years) intends to investigate whether metformin (1500 mg; daily) treatment in nonhospitalized adults with SARS-CoV-2 can (i) prevent hypoxia and emergency department utilization, (ii) prevent disease progression in COVID-19, and (iii) improve viral load and C-reactive protein (CRP)iii. A Phase II trial ‘Pilot Study into the Use of Metformin and Low Dose Naltrexone (LDN) for Patients with Coronavirus Disease 2019 (COVID-19) – Assessment of Short and Long Term Effects’ (NCT04604678; 80 participants, age 30–70 years) aims to study the effect of a combination of metformin (1500 mg/day) and LDN (4.5 mg/day) on the attenuation of symptoms and disease severity, recovery time, hospitalization rates, and mortality in COVID-19 patients at definite intervals over 4 weeksiv.
In a 20-participant (age 18 years and older) Phase II trial (NCT04626089), the ‘Adaptive Study for Efficacy and Safety of Metformin Glycinate for the Treatment of Patients with Metabolic Syndrome (MS) and Type-2 Diabetes Mellitus (DM2), Hospitalized with Severe Acute Respiratory Syndrome Secondary to SARS-CoV-2 (randomized, double-blind)’ the investigators intend to evaluate the efficacy and safety of metformin glycinate (620 mg; twice daily) plus standard treatment in COVID-19 patients (who have metabolic syndrome or type 2 diabetes) with ARDS (secondary to SARS-CoV-2 infections) in comparison to similar patients who receive the standard treatment alonev. A similar trial (NCT04625985; Phase II; enrolling an estimated 20 participants, 18 years and older) investigates the efficacy and safety of metformin glycinate (620 mg; twice daily) and standard treatment in hospitalized COVID-19 patients with ARDS (secondary to SARS-CoV-2 infection)vi.
Some of the clinical trials may include nondiabetic COVID-19 patients, which will help to determine whether metformin would offer protection in nondiabetic SARS-CoV-2-infected patients. More studies and clinical trials are warranted to elucidate the molecular mechanisms that support the use of metformin in COVID-19 and answer the outstanding questions (see Outstanding Questions) regarding metformin and its therapeutic potential in COVID-19 treatment.
Outstanding Questions.
Are diabetic patients on a metformin treatment regimen more resistant to SARS-CoV-2 infection than diabetic patients on other antihyperglycemic medications?
Is metformin treatment beneficial in ‘nondiabetic’ COVID-19 patients who develop acute new-onset hyperglycemia?
Can the beneficial effects of metformin observed in diabetic or obese patients taking metformin before their COVID-19 diagnosis be replicated if patients are administered metformin after COVID-19 diagnosis, irrespective of their diabetic status (both nondiabetic and diabetic) and BMI (both nonobese and obese)?
How does metformin influence endothelial function/dysfunction, inflammation, and thrombotic events in diabetic/hyperglycemic COVID-19 patients?
Will metformin therapy attenuate proinflammatory and prothrombotic events in nondiabetic COVID-19 patients?
Does metformin have any contraindications when used in combination with other drugs used in the treatment of COVID-19?
What are the long-term effects of metformin treatment in COVID-19 patients?
Alt-text: Outstanding Questions
Metformin: Adverse Effects and Possible Contraindications in COVID-19
Most studies have outlined the beneficial effects of metformin in COVID-19 patients, while some studies have reported an increased risk of acidosis (but not mortality) and disease severity in COVID-19 patients using metformin [71,83]. This suggests that metformin is not an appropriate choice in patients with severe respiratory distress, renal impairment, or heart failure [1], and highlights the importance of paying attention to pre-existing conditions and comorbidities in drug selection. Furthermore, contraindications to metformin must be addressed before administration.
Concluding Remarks and Future Perspectives
The world looks forward to the safety and efficacy of the approved vaccines for protection against new SARS-CoV-2 infections even as stringent measures (lockdowns/masking/social distancing) are being enforced to reduce the spread of the virus. Although crucial information regarding SARS-CoV-2/COVID-19 was collected in record time, a lot about how the viral infection contributes to disease progression and varying severity in different individuals remains unknown. It is, however, evident that hyperglycemia and diabetes-related comorbidities significantly increase disease severity and mortality in COVID-19 patients. Therefore, blood glucose levels must be properly monitored and managed to improve prognosis and survival rates in diabetic COVID-19 patients. The antidiabetic drug metformin, in addition to its glucose-lowering effect, has potential antiviral, cardioprotective, vasculoprotective, immunomodulatory, and anti-inflammatory effects and hence could be repurposed for the treatment of COVID-19. Furthermore, metformin is considered to be safe, is well-tolerated, has minimal side effects, is off-patent since 2002 (hence economical), and can be made readily available to COVID-19 patients who may benefit from metformin intervention [79].
The immediate need in this battle against COVID-19 is an efficacious drug/therapeutic that can successfully treat disease, improve recovery and prognosis, and attenuate the long-term adverse effects of COVID-19 in the potential ‘long-haulers’ of the disease. While effective vaccine(s) and efficient vaccination programs are at the forefront in the fight against COVID-19, drugs routinely used for other pathological conditions must not be overlooked in terms of their potential efficacy to treat this disease. Host-directed therapies utilizing one or more of the currently marketed drugs with reasonable safety profiles are an excellent approach to combat this pandemic.
Author Contributions
The authors contributed as follows. Conceptualization: S.M.S. and D.B.; literature review and resources: S.M.S. and E.V.; writing: original draft preparation S.M.S. and E.V., review and editing S.M.S., revisions and addressing reviewers’ comments S.M.S., D.B., and E.V.; figure preparation and editing: S.M.S. and E.V.; visualization: S.M.S. and D.B.; supervision: D.B.; project administration: D.B.; funding acquisition: D.B.; all authors have read and agreed to the published version of the manuscript.
Acknowledgments
Acknowledgments
This work was supported by a National Priorities Research Program grant (NPRP 11S-1214-170101; awarded to Professor Dr Dietrich Büsselberg, June 2019–present) from the Qatar National Research Fund (QNRF, a member of Qatar Foundation). The statements made herein are solely the responsibility of the authors. We thank Kevin Zhai (Weill Cornell Medicine-Qatar) for language editing and corrections.
Declaration of Interests
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the article.
Glossary
- ARDS
acute respiratory distress syndrome – a life-threatening respiratory failure following a rapidly progressing widespread inflammation of the lungs and fluid accumulation in the lungs. Symptoms of ARDS include severe shortness of breath, laborious rapid breathing, low blood pressure, confusion, and extreme fatigue.
- COVID-19
coronavirus disease 2019 – the severe acute respiratory syndrome illness, first reported in December 2019 in Wuhan, China, caused by the novel SARS-CoV-2 virus.
- DKA
diabetic ketoacidosis – a serious complication associated with diabetes where there is excessive breakdown of fat, and the liver converts fat to ketones, making the blood more acidic.
- Host-directed therapies
therapies directed towards host-mediated immune response rather than directly targeting the pathogen itself.
- Hypercytokinema/cytokine storm
an immune response which results in the excessive synthesis and secretion of proinflammatory cytokines into the blood.
- MERS-CoV
Middle East respiratory syndrome coronavirus, the novel virus, identified in Saudi Arabia in 2012, that causes MERS.
- MODS
multiple organ dysfunction syndrome – infection- or injury-induced tissue/organ damage caused by unbalanced immune and unregulated systemic inflammatory response.
- Repurposing (drug)
using a drug (in the same form or modified form) for the treatment of a disease different from its original intended use, OR the process of identifying new therapeutic uses for already-available/existing routinely used drugs.
- SARS-CoV-1
severe acute respiratory syndrome coronavirus, the virus, identified in China in 2003, that causes SARS.
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2, the novel virus, identified in China in 2020, that causes COVID-19.
Resources
ihttps://coronavirus.jhu.edu/?utm_source=jhu_properties&utm_medium=dig_link&utm_content=ow_jhuhomepage&utm_campaign=jh20iihttps://clinicaltrials.gov/ct2/results?recrs=&cond=COVID&term=metformin&cntry=&state=&city=&dist=iiihttps://clinicaltrials.gov/ct2/show/NCT04510194?term=metformin&cond=COVID-19&draw=2&rank=2ivhttps://clinicaltrials.gov/ct2/show/NCT04604678?term=NCT04604678&draw=2&rank=1vhttps://clinicaltrials.gov/ct2/show/NCT04626089?term=NCT04626089&draw=2&rank=1vihttps://clinicaltrials.gov/ct2/show/NCT04625985?term=NCT04625985&draw=2&rank=1References
- 1.Apicella M., et al. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack F.P., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baden L.R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2020;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Voysey M., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sadoff J., et al. Interim results of a phase 1–2a Trial of Ad26.COV2.S Covid-19 vaccine. N. Engl. J. Med. 2021 doi: 10.1056/NEJMoa2034201. Published online January 13, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kupferschmidt K. New mutations raise specter of ‘immune escape’. Science. 2021;371:329. doi: 10.1126/science.371.6527.329. [DOI] [PubMed] [Google Scholar]
- 7.Chakravarty D., et al. Sex differences in SARS-CoV-2 infection rates and the potential link to prostate cancer. Commun. Biol. 2020;3:374. doi: 10.1038/s42003-020-1088-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Oran D.P., Topol E.J. The proportion of SARS-CoV-2 infections that are asymptomatic. Ann. Intern. Med. 2021 doi: 10.7326/M20-6976. Published online January 22, 2021. [DOI] [PubMed] [Google Scholar]
- 9.Zhou F., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu L., et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068–1077.e1063. doi: 10.1016/j.cmet.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffmann M., et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ashraf U.M., et al. SARS-CoV-2, ACE2 expression, and systemic organ invasion. Physiol. Genomics. 2020;53:51–60. doi: 10.1152/physiolgenomics.00087.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kruglikov I.L., et al. Obesity and diabetes as comorbidities for COVID-19: Underlying mechanisms and the role of viral–bacterial interactions. eLife. 2020;9 doi: 10.7554/eLife.61330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malhotra A., et al. ACE2, Metformin, and COVID-19. iScience. 2020;23:101425. doi: 10.1016/j.isci.2020.101425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Scheen A.J. Metformin and COVID-19: From cellular mechanisms to reduced mortality. Diabetes Metab. 2020;46:423–426. doi: 10.1016/j.diabet.2020.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma S., et al. Metformin in COVID-19: A possible role beyond diabetes. Diabetes Res. Clin. Pract. 2020;164:108183. doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banu N., et al. Protective role of ACE2 and its downregulation in SARS-CoV-2 infection leading to macrophage activation syndrome: therapeutic implications. Life Sci. 2020;256:117905. doi: 10.1016/j.lfs.2020.117905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goshua G., et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol. 2020;7:e575–e582. doi: 10.1016/S2352-3026(20)30216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar A., et al. SARS-CoV-2 cell entry receptor ACE2 mediated endothelial dysfunction leads to vascular thrombosis in COVID-19 patients. Med. Hypotheses. 2020;145:110320. doi: 10.1016/j.mehy.2020.110320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Varga Z., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williams D.M., et al. Diabetes and novel coronavirus infection: implications for treatment. Diabetes Ther. 2020;11:1915–1924. doi: 10.1007/s13300-020-00858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubino F., et al. New-onset diabetes in Covid-19. N. Engl. J. Med. 2020;383:789–790. doi: 10.1056/NEJMc2018688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo P., et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am. J. Trop. Med. Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toniolo A., et al. The diabetes pandemic and associated infections: suggestions for clinical microbiology. Rev. Med. Microbiol. 2019;30:1–17. doi: 10.1097/MRM.0000000000000155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Y., et al. Clinical characteristics and outcomes of patients with severe covid-19 with diabetes. BMJ Open Diabetes Res. Care. 2020;8 doi: 10.1136/bmjdrc-2020-001343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y., et al. Association of diabetes mellitus with disease severity and prognosis in COVID-19: A retrospective cohort study. Diabetes Res. Clin. Pract. 2020;165:108227. doi: 10.1016/j.diabres.2020.108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kumar A., et al. Is diabetes mellitus associated with mortality and severity of COVID-19? A meta-analysis. Diabetes Metab. Syndr. 2020;14:535–545. doi: 10.1016/j.dsx.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guan W.-j., et al. Comorbidity and its impact on 1590 patients with Covid-19 in China: a nationwide analysis. Eur. Respir. J. 2020;55:2000547. doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li B., et al. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020;109:531–538. doi: 10.1007/s00392-020-01626-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fadini G.P., et al. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J. Endocrinol. Investig. 2020;43:867–869. doi: 10.1007/s40618-020-01236-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrilli C.M., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369 doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roncon L., et al. Diabetic patients with COVID-19 infection are at higher risk of ICU admission and poor short-term outcome. J. Clin. Virol. 2020;127:104354. doi: 10.1016/j.jcv.2020.104354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sardu C., et al. Outcomes in patients with hyperglycemia affected by Covid-19: can we do more on glycemic control? Diabetes Care. 2020;43:1408–1415. doi: 10.2337/dc20-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang J., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barron E., et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cariou B., et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang L., et al. A human pluripotent stem cell-based platform to study SARS-CoV-2 tropism and model virus infection in human cells and organoids. Cell Stem Cell. 2020;27:125–136.e127. doi: 10.1016/j.stem.2020.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang J.K., et al. Binding of SARS coronavirus to its receptor damages islets and causes acute diabetes. Acta Diabetol. 2010;47:193–199. doi: 10.1007/s00592-009-0109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alves A.M., et al. SARS-CoV-2 leading to acute pancreatitis: an unusual presentation. Braz. J. Infect. Dis. 2020;24:561–564. doi: 10.1016/j.bjid.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ni W., et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care. 2020;24:422. doi: 10.1186/s13054-020-03120-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hojyo S., et al. How COVID-19 induces cytokine storm with high mortality. Inflam. Regenerat. 2020;40:37. doi: 10.1186/s41232-020-00146-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ragab D., et al. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Unsworth R., et al. New-onset type 1 diabetes in children during COVID-19: multicenter regional findings in the U.K. Diabetes Care. 2020;43:e170–e171. doi: 10.2337/dc20-1551. [DOI] [PubMed] [Google Scholar]
- 44.Hollstein T., et al. Autoantibody-negative insulin-dependent diabetes mellitus after SARS-CoV-2 infection: a case report. Nat. Metab. 2020;2:1021–1024. doi: 10.1038/s42255-020-00281-8. [DOI] [PubMed] [Google Scholar]
- 45.Bode B., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 2020;14:813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singh A.K., Singh R. Hyperglycemia without diabetes and new-onset diabetes are both associated with poorer outcomes in COVID-19. Diabetes Res. Clin. Pract. 2020;167:108382. doi: 10.1016/j.diabres.2020.108382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jin Y., et al. Endothelial activation and dysfunction in COVID-19: from basic mechanisms to potential therapeutic approaches. Signal Transd. Target. Ther. 2020;5:293. doi: 10.1038/s41392-020-00454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribes A., et al. Thromboembolic events and Covid-19. Adv. Biol. Regul. 2020;77:100735. doi: 10.1016/j.jbior.2020.100735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gavriilaki E., et al. Endothelial dysfunction in COVID-19: lessons learned from coronaviruses. Curr. Hypertens. Rep. 2020;22:63. doi: 10.1007/s11906-020-01078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Triggle C.R., et al. Why the endothelium? The endothelium as a target to reduce diabetes-associated vascular disease. Can. J. Physiol. Pharmacol. 2020;98:415–430. doi: 10.1139/cjpp-2019-0677. [DOI] [PubMed] [Google Scholar]
- 51.Pechlivani N., Ajjan R.A. Thrombosis and vascular inflammation in diabetes: mechanisms and potential therapeutic targets. Front. Cardiovasc. Med. 2018;5:1. doi: 10.3389/fcvm.2018.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan K.C.B., et al. Advanced glycation end products and endothelial dysfunction in type 2 diabetes. Diabetes Care. 2002;25:1055–1059. doi: 10.2337/diacare.25.6.1055. [DOI] [PubMed] [Google Scholar]
- 53.De Francesco E.M., et al. COVID-19 and diabetes: the importance of controlling RAGE. Front. Endocrinol. 2020;11:526. doi: 10.3389/fendo.2020.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kierdorf K., Fritz G. RAGE regulation and signaling in inflammation and beyond. J. Leukoc. Biol. 2013;94:55–68. doi: 10.1189/jlb.1012519. [DOI] [PubMed] [Google Scholar]
- 55.van Zoelen M.A.D., et al. The role of receptor for advanced glycation endproducts (RAGE) in infection. Crit. Care. 2011;15:208. doi: 10.1186/cc9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tang N., et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nägele M.P., et al. Endothelial dysfunction in COVID-19: Current findings and therapeutic implications. Atherosclerosis. 2020;314:58–62. doi: 10.1016/j.atherosclerosis.2020.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang S., et al. Fasting blood glucose at admission is an independent predictor for 28-day mortality in patients with COVID-19 without previous diagnosis of diabetes: a multi-centre retrospective study. Diabetologia. 2020;63:2102–2111. doi: 10.1007/s00125-020-05209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alahmad B., et al. Fasting blood glucose and COVID-19 severity: nonlinearity matters. Diabetes Care. 2020;43:3113–3116. doi: 10.2337/dc20-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Williamson E.J., et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen Y., et al. Clinical characteristics and outcomes of patients with diabetes and COVID-19 in association with glucose-lowering medication. Diabetes Care. 2020;43:1399–1407. doi: 10.2337/dc20-0660. [DOI] [PubMed] [Google Scholar]
- 62.Li X., et al. Risk factors for severity and mortality in adult COVID-19 inpatients in Wuhan. J. Allergy Clin. Immunol. 2020;146:110–118. doi: 10.1016/j.jaci.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang F., et al. Clinical characteristics of 28 patients with diabetes and COVID-19 in Wuhan, China. Endocr. Pract. 2020;26:668–674. doi: 10.4158/EP-2020-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhang Y., et al. The clinical characteristics and outcomes of patients with diabetes and secondary hyperglycaemia with coronavirus disease 2019: A single-centre, retrospective, observational study in Wuhan. Diabetes Obes. Metab. 2020;22:1443–1454. doi: 10.1111/dom.14086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Solerte S.B., et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43:2999–3006. doi: 10.2337/dc20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sun Q., et al. New insights into insulin: The anti-inflammatory effect and its clinical relevance. World J. Diabetes. 2014;5:89–96. doi: 10.4239/wjd.v5.i2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Salem E.S.B., et al. Insulin treatment attenuates renal ADAM17 and ACE2 shedding in diabetic Akita mice. Am. J. Physiol. Renal Physiol. 2014;306:F629–F639. doi: 10.1152/ajprenal.00516.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hariyanto T.I., Kurniawan A. Metformin use is associated with reduced mortality rate from coronavirus disease 2019 (COVID-19) infection. Obes. Med. 2020;19:100290. doi: 10.1016/j.obmed.2020.100290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lukito A.A., et al. The effect of metformin consumption on mortality in hospitalized COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab. Syndr. 2020;14:2177–2183. doi: 10.1016/j.dsx.2020.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bramante C.T., et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2:e34–e41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng X., et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;32:537–547 e533. doi: 10.1016/j.cmet.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Crouse A.B., et al. Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front. Endocrinol. 2021;11:1081. doi: 10.3389/fendo.2020.600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lu D.-Y., et al. Metformin use in patients with type 2 diabetes mellitus is associated with reduced risk of deep vein thrombosis: a non-randomized, pair-matched cohort study. BMC Cardiovasc. Disord. 2014;14:187. doi: 10.1186/1471-2261-14-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xin G., et al. Metformin uniquely prevents thrombosis by inhibiting platelet activation and mtDNA release. Sci. Rep. 2016;6:36222. doi: 10.1038/srep36222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Triggle C.R., Ding H. Metformin is not just an antihyperglycaemic drug but also has protective effects on the vascular endothelium. Acta Physiol. 2017;219:138–151. doi: 10.1111/apha.12644. [DOI] [PubMed] [Google Scholar]
- 76.Yamaoka-Tojo M. Endothelial glycocalyx damage as a systemic inflammatory microvascular endotheliopathy in COVID-19. Biomed. J. 2020;43:399–413. doi: 10.1016/j.bj.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Isoda K., et al. Metformin inhibits proinflammatory responses and nuclear factor-κB in human vascular wall cells. Arterioscler. Thromb. Vasc. Biol. 2006;26:611–617. doi: 10.1161/01.ATV.0000201938.78044.75. [DOI] [PubMed] [Google Scholar]
- 78.Chen X.F., et al. Metformin corrects RAGE overexpression caused signaling dysregulation and NF-κB targeted gene change. Int. J. Clin. Exp. Med. 2016;9:2913–2920. [Google Scholar]
- 79.Samuel S.M., et al. Metformin: the answer to cancer in a flower? Current knowledge and future prospects of metformin as an anti-cancer agent in breast cancer. Biomolecules. 2019;9:846. doi: 10.3390/biom9120846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen X., et al. Immunomodulatory and antiviral activity of metformin and its potential implications in treating coronavirus disease 2019 and lung injury. Front. Immunol. 2020;11:2056. doi: 10.3389/fimmu.2020.02056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gordon D.E., et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature. 2020;583:459–468. doi: 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Esam Z. A proposed mechanism for the possible therapeutic potential of Metformin in COVID-19. Diabetes Res. Clin. Pract. 2020;167:108282. doi: 10.1016/j.diabres.2020.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gao Y., et al. Risk of metformin in patients with type 2 diabetes with COVID-19: a preliminary retrospective report. Clin. Transl. Sci. 2020;13:1055–1059. doi: 10.1111/cts.12897. [DOI] [PMC free article] [PubMed] [Google Scholar]