Abstract
Since the beginning of the COVID-19 outbreak, attention has gradually moved from the respiratory manifestations of the disease toward its dermatologic aspects. The need for wearing personal protective measures and their cutaneous side effects, detection of related or specific COVID-19 skin eruptions, and the evaluation of certain risk groups of immunosuppressed dermatologic patients have initiated significant discussions about various therapeutic interventions and, in particular, about biologic therapy for psoriasis and for autoinflammatory, orphan, or malignant cutaneous disorders. Autoimmune bullous dermatoses have been of concern due to their chronic course, at times life-threatening prognosis, and the need for prolonged and often aggressive immunomodulatory therapy. We have summarized the current knowledge regarding the impact of COVID-19 infection on autoimmune bullous dermatoses, including recommendations for the main treatment strategies, available patient information, and the registries organized for documentation during the COVID-19 pandemic.
Introduction
The current coronavirus disease (COVID-19) is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that spreads through droplets and aerosols and causes fever, fatigue, dry cough, and subsequent dyspnea.1 Headache, myalgia, arthralgia, and gastrointestinal symptoms occur in 5%-15% of cases.2 In approximately 20% of patients, the infection progresses to severe interstitial pneumonia and may induce a life-threatening uncontrolled host-immune response known as the cytokine release syndrome.3 In addition to these, numerous maculopapular, urticarial, vesicular, pseudo-chilblain, or livedoid nonspecific and specific skin manifestations of COVID-19 have been reported.4 Evidence is increasing regarding the important impact of SARS-CoV-2 infection on the care and treatment of various dermatologic entities, especially for several high-risk patient groups.5
Autoimmune bullous dermatoses (AIBD) comprise heterogeneous disorders including the intraepidermal pemphigus group, namely pemphigus vulgaris and foliaceus, and the subepidermal pemphigoid group, namely bullous and mucous membrane pemphigoid, epidermolysis bullosa acquisita, linear IgA disease, and dermatitis herpetiformis. Skin and mucosal blistering is their common clinical feature, which may lead to significant cutaneous damage with vast erosions formation. Impaired wound healing requires specific topical care, avoidance of alcohol containing agents or adhesive wound dressings, application of topical immunomodulators, and prevention of secondary infections. Their chronic and potentially life-threatening course is often controlled by aggressive and prolonged treatment with immunosuppressive drugs that may increase the risk of systemic infections, vascular incidents, and a related mortality rate.6
We present an overview on the impact of the current COVID-19 pandemic on AIBD: Their course and risk evaluation, expert recommendations on the treatment strategies, available patient information, and registries for documentation of SARS-CoV-2 infected cases. For this purpose, we performed a PubMed search using the following keywords: COVID-19, SARS-CoV-2, coronavirus, autoimmune bullous diseases, autoimmune blistering diseases, pemphigus, pemphigoid, epidermolysis bullosa, linear IgA dermatosis, and dermatitis herpetiformis. Additional manual searches of reference lists have included available letters to the editor, case reports and series, reviews, and data registries covering the topic. The search was updated on November 10, 2020. We found published papers that exclusively discussed pemphigus, bullous and mucous membrane pemphigoid, but there seems to have been a lack of published data on the other entities from the AIBD spectrum (Table 1 ).
Table 1.
Reports on the different AIBD entities during COVID-19 pandemic
| Autoimmune bullous dermatoses | Author/journal/reference | Type of communication |
|---|---|---|
| AIBD in general | Drenovska K et al. Int J Dermatol. 20204 Di Altobrando A et al. J Eur Acad Dermatol Venereol. 202013 Carugno A et al. Br J Dermatol. 202017 De Fata Salvatores G et al. Dermatol Ther. 202041 Anuragi RP et al. Dermatol Ther. 202046 Kasperkiewicz M. J Am Acad Dermatol. 202056 Brunasso AMG et al. Dermatol Ther. 202057 Chen P et al. J Dermatolog Treat. 202058 Kasperkiewicz M et al. J Eur Acad Dermatol Venereol. 202060 EADV Task Force61 IPPF62 |
Review Letter to the editor Research letter Letter to the editor Letter to the editor Review Original report Original report Letter, recommendations Recommendations Patient information |
| Pemphigus | Diotallevi F et al. Australas J Dermatol. 202012 Balestri R et al. J Eur Acad Dermatol Venereol. 202014 Ghalamkarpour F et al. Dermatol Ther. 202015 Abdollahimajd F et al. Arch Acad Emerg Med. 202019 Shakshouk H et al. J Am Acad Dermatol. 202020 Pathania YS et al. J Dermatolog Treat. 202021 Shakshouk H et al. J Am Acad Dermatol. 202025 Mahmoudi H et al. Dermatol Ther. 202028 Schultz B et al. J Am Acad Dermatol. 202030 Shahidi-Dadras M et al. J Dermatolog Treat. 202031 Waldman RA et al. J Am Acad Dermatol. 202032 Beyzaee AM et al. Dermatol Ther. 202033 Elmas ÖF et al. Dermatol Ther. 202034 |
Letter to the editor Letter, Case report Letter Review Letter, recommendations Letter to the editor Reply to comment Letter to the editor Comment Letter to the editor Letter in reply Review Review |
| Bullous pemphigoid | Azimi SZ et al. Dermatol Ther. 202044 | Short paper |
| Mucous membrane pemphigoid | Daneshpazhooh M et al. J Dermatolog Treat. 202049 Amir Dastmalchi D et al. J Dermatolog Treat. 202050 |
Case report Retrospective study |
| Epidermolysis bullosa acquisita | NF | NF |
| Linear IgA dermatosis | NF | NF |
| Dermatitis herpetiformis | NF | NF |
AIBD, autoimmune bullous dermatoses; NF, not found.
The pemphigus group and COVID-19
Pemphigus vulgaris (PV) is a life-threatening autoimmune blistering disease mediated by autoantibodies directed against two desmosomal adhesion proteins, desmoglein 1 and desmoglein 3, which are expressed in the skin and mucous membranes. PV is characterized by flaccid bullae and painful mucosal and cutaneous erosions.7
PV patients, for the most part those who have moderate-to-severe disease, are often subjected to aggressive immunosuppressive treatment regimens limiting the pathogenic process. High-dose and long-term treatment with systemic corticosteroids are associated with numerous side effects, including suppressed immunity and increased susceptibility to infections. Concomitant “corticosteroid-sparing” agents, such as azathioprine, cyclophosphamide, and mofetil mycophenolate (MMF), which are classic cytotoxic agents or immunomodulators, increase immunosuppression.8 In some pemphigus cases, the anti-CD-20 monoclonal antibody rituximab is used, as well as high doses of intravenous immunoglobulins (IVIG) or immunoadsorption.9 , 10 Bruton tyrosine kinase (BTK) inhibitors and other treatment options may also potentiate immune dysregulation.11 In addition, damaged skin and mucosal surfaces, together with prolonged topical use of corticosteroids, may impair local immunity, thus favoring secondary infections.
Since the outbreak of COVID-19 pandemic, multiple reports have focused on the follow-up of PV patients and registration of SARS-CoV-2–suspicious or SARS-CoV-2–positive cases; however, the data are controversial. A regional dermatology center in Ancona, Italy, contacted 72 PV patients and found no COVID-19–related complaints or deaths to have been registered. In this situation, a local task force group recommended maintenance of systemic corticosteroids <20 mg of prednisone per day, a reduction of doses >20 mg/d, discontinuation of corticosteroid-sparing agents, and a postponement of rituximab application.12
In opposition to this approach, other Italian centers have observed only isolated pemphigus patients with proven COVID-19, namely a 53-year-old man on combination azathioprine-systemic corticosteroids and a 65-year-old woman on MMF, respectively.13 , 14 In both cases, immunosuppressive therapy was discontinued and reintroduction was advised only after complete healing, supported by a negative swab result for SARS-CoV-2, despite the antiviral potential of MMF investigated in Middle East respiratory syndrome (MERS) coronavirus.
An observation from Iran reported the severe course of PV in a 40-year-old woman following rituximab administration and consequent SARS-CoV-2 positivity. Her treatment was switched to IVIG.15 The exacerbation of her symptoms could be attributed to eitherrituximab or COVID-19.16
Contrary to these isolated cases, experience from Bergamo, Italy, an area with a high incidence of COVID-19, demonstrated that 7 of 31 (22.6%) PV patients developed COVID-19–suspected manifestations after contact with potential or known COVID-19–affected individuals. A total of 6 PV patients (19.4%) experienced mild to moderate symptoms, while a 69-year-old PV patient (3.2%) with previous breast cancer developed severe disease requiring hospitalization, but later recovered. All patients were in remission and therapeutic regimens were preserved, except for hospitalized cases in which immunosuppressive treatment was discontinued.17
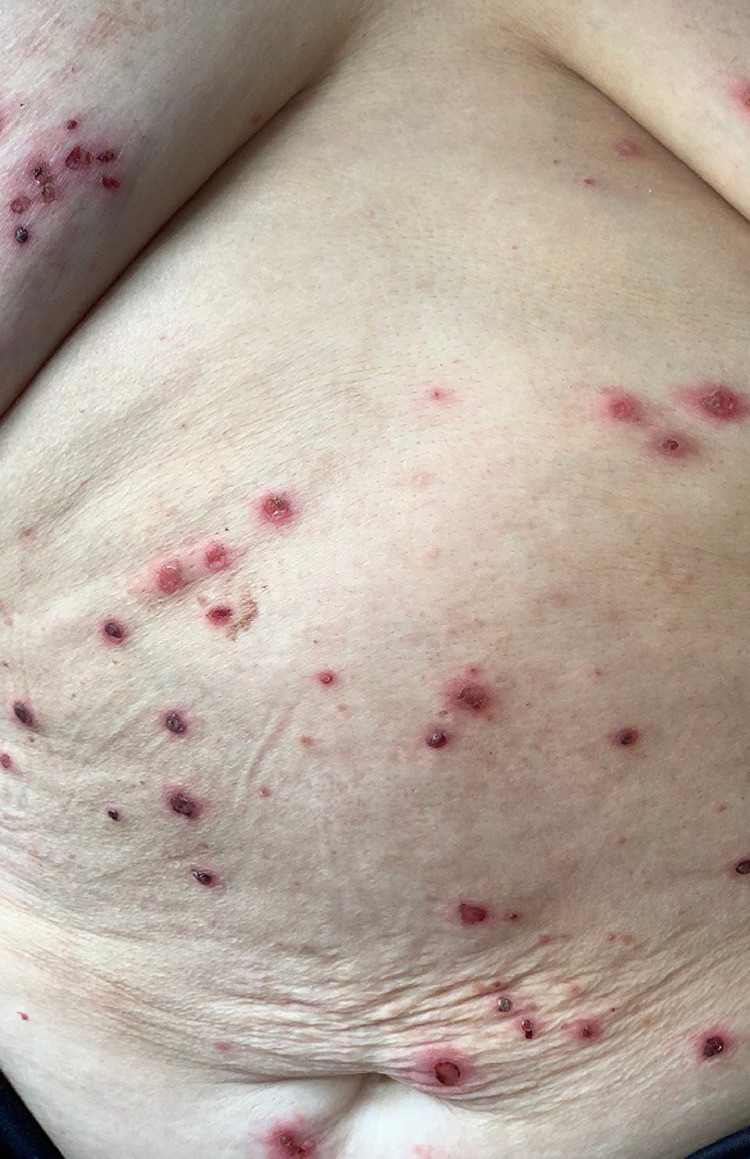
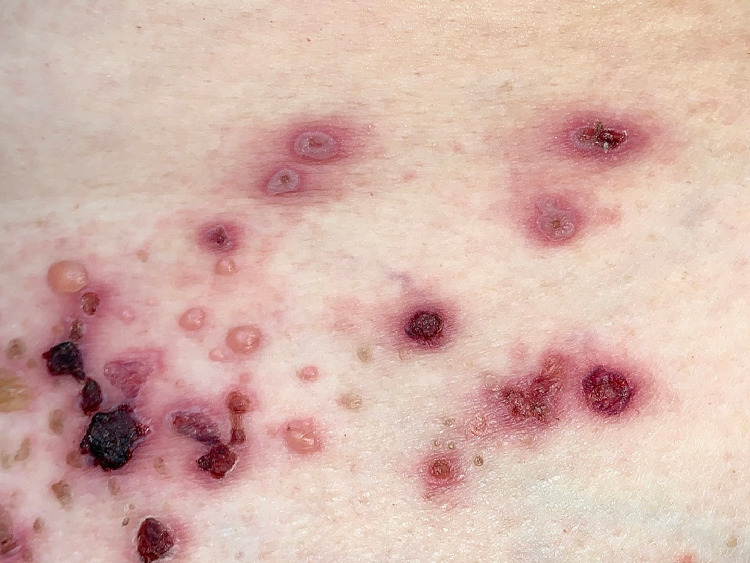
Reporting our personal experience, at the Department of Dermatology, Medical University-Sofia, Bulgaria, we observed only one pemphigus patient suspicious of being infected with SARS-CoV-2. A 79-year-old woman initially presented with a pruritic widespread varicelliform eruption (Figure 1 ), positive IgM blood test, negative IgG test, and negative polymerase chain reaction (PCR) test. The patient had a history of chronic lympholeukosis, recent Herpes zoster infection, and other comorbidities. Laboratory findings, including histology, immunofluorescent and immunoserologic methods, confirmed the diagnosis of PV. In the course of treatment with systemic corticosteroids, antibiotics, and acyclovir, her general condition worsened, a dry cough developed, and the varicelliform eruption was replaced by flaccid bullae and erosions typical for PV (Figure 2 ). Soon after discharge, she died of unknown causes. It may be speculated that this patient was affected by COVID-19; moreover, varicella-like exanthema has already been reported as a specific COVID-19–associated skin manifestation.18 In addition, the eventual herpetic or paraneoplastic nature of her initial varicelliform eruption or triggering of the pemphigus by SARS-CoV-2 should be considered.
Fig. 1.
Varicelliform eruption with multiple umbilicated vesicles in a patient who tested positive for IgM-SARS-CoV-2. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Fig. 2.
Varicelliform vesicles and flaccid bullae on normal skin typical for PV in a patient who tested positive for IgM-SARS-CoV-2. (Same patient as in Figure 1.) SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Multiple reviews have analyzed the appropriate general and treatment measures for pemphigus patients during the COVID-19 era. The Iranian authors utilized their experience from previous SARS and MERS epidemics. They proposed an algorithm for management of pemphigus depending on positivity or negativity of COVID-19 tests, in addition to strict adherence to health principles, stress avoidance, regular teledermatology visits, a less aggressive approach for mild cases, utilizing topical or intralesional corticosteroids, dapsone, or doxycycline, and caution with emerging anti–COVID-19 therapies such as antimalarial drugs or IL-6 inhibitors.19 Tapering of systemic corticosteroids to the lowest effective dose was advised to reduce the risk of infection and COVID-19–associated mortality. In an active COVID-19 infection, steroid-sparing immunosuppressive agents, such as azathioprine and MMF, were tapered or discontinued. Hydroxychloroquine, especially for the elderly, IVIG, or plasmapheresis may be useful in both pemphigus and COVID-19, but the effect of convalescent plasma on pemphigus is unknown. The new oral BTK inhibitor (PRN1008, currently in phase 3 clinical trials; NCT02704429), being tested for the treatment of pemphigus, might be promising due to its self-limited immunomodulatory effect. Tocilizumab, a humanized anti-IL-6 monoclonal antibody used in the inflammatory phase of COVID-19, has been reported as being helpful in the treatment of pemphigus.20 , 21
The use of rituximab, a chimeric mouse/human anti-CD-20 monoclonal antibody approved as first-line treatment for moderate-to-severe pemphigus due to its efficacy and safety, became widely questionable during COVID-19.22, 23, 24 Concerns were related to its significant effect on B-cells, leading to slow recovery of B-cell immunity, which often takes more than 6 months. In addition, because rituximab requires intravenous administration, visits to hospitals or medical centers are necessary, increasing the patient's risk of acquiring SARS-CoV-2 infection. To avoid concurrent peaks of immunosuppression with peaks of COVID-19, some authors have proposed postponing rituximab infusions until the screening for SARS-CoV-2 infection by PCR prior to its administration has been completed.20 , 25 In agreement with this recommendation, data from an ongoing French study suggest rituximab treatment as major risk factor for COVID-19, so the postponement of maintenance infusions in patients in clinical remission is advised. Rituximab infusions remain acceptable in young patients who have severe pemphigus but no comorbidities.26 Other authors have considered continuing planned rituximab infusions in patients who have no infection, similar to treatments with other biologics. Prompt discontinuation was advised in patients with respiratory symptoms until complete remission or in those who have been in contact with proved COVID-19 patients until a swab confirmed negativity for SARS-CoV-2.27, 28, 29 Opponents of the delay of rituximab infusion supported case-by-case evaluation and unrestricted application of rheumatologic rather than hematologic doses, especially in severe or polymorbid cases to avoid general immunosuppression from the alternative, systemic corticosteroids.30 Some reassuring data came from a teledermatologic study in Iran on 167 pemphigus patients treated with rituximab during the period 2014-2019. Only 5 SARS-CoV-2–infected patients were detected, 1 asymptomatic and 4 mild cases who did not require hospitalization. No relapses of pemphigus or cutaneous manifestations of COVID-19 were detected. None of the patients had received rituximab within the year preceding the pandemic. Of the 45 patients who had received rituximab within the past year, none developed COVID-19.31
Rituximab therapy gave rise also to the question regarding the vaccine response during routine and anti–COVID-19 vaccination of pemphigus patients. Based on vaccination experience, when vaccines for COVID-19 are available, dermatologists may advise vaccination 12-20 weeks after completion of a treatment cycle with rituximab or extend dosing intervals so that a minimum time of 4 weeks precede the next drug infusion.32
Despite the controversial data in the literature, the final decision on the administration of rituximab should belong to the treating physician who may precisely evaluate the severity of the disease, the drugs’ adverse effects, results of continuing the previous treatments, and the risk of changing the patient's therapeutic regimen.33
Pandemic-related lockdown measures have contributed also to delayed pemphigus diagnosis and treatment and therefore to a higher risk of poor prognosis, patients’ disability, and important economic effects. In addition, the great psychological burden of COVID-19, together with high rates of psychiatric morbidity and impaired quality of life, may require online or face-to-face psychological support programs for pemphigus patients.34
Pemphigus foliaceus (PF) is characterized by exclusive cutaneous involvement, better prognosis, and less aggressive treatment than needed for PV. No cases of PF and COVID-19 have been observed. No reports on PF during the pandemic were found in the literature.
The pemphigoid group and COVID-19
Bullous pemphigoid (BP) is the most common subepidermal autoimmune blistering disease. It is clinically characterized by pruritus and blister formation on an erythematous base with predominantly cutaneous involvement and symmetric distribution on the arms, thighs, and trunk.35 BP typically affects the elderly who are twice as likely to develop serious illness when infected with COVID-19 according to the US Centers for Disease Control and Prevention.36 , 37 In addition, BP patients may suffer from multiple comorbidities, such as hypertension, diabetes, chronic obstructive pulmonary disease, history of heart surgery, or malignancy. In terms of prognosis quo ad vitam, BP may be compared with end-stage heart failure, with more than 40% of patients’ fatal outcome within 12 months.38
Mortality is closely related to patients’ age and general condition. In a retrospective study from Scotland, 48% of BP patients died within 2 years of diagnosis, especially from respiratory diseases.39 Similarly, poor prognosis in COVID-19 is for the most part caused by sepsis, multiorgan failure, or acute respiratory distress syndrome (ARDS).40
Although most BP patients are in long-term care facilities and nursing homes that are often targeted by SARS-CoV-2, data on BP-COVID-19 are considerably lacking. An isolated case series from Bergamo, Italy, reported suspected COVID-19 symptoms in 10 out of 62 (16.1%) BP patients which for the most part appeared after contact with suspected or known SARS-CoV-2–positive individuals. A total of 6 BP patients (9.7%) experienced mild-to-moderate symptoms, such as flu-like complaints, cough, low-grade fever, anosmia, and ageusia, which resolved without hospitalization. A total of 4 BP patients (6.5%) experienced severe symptoms, such as pneumonia with respiratory failure, and needed hospitalization. All hospitalized patients had positive COVID-19 nasal swab and received hydroxychloroquine. Of these patients, 3 who died (4.8%, mean age 85.0 years), had severe cognitive impairment.17
In another report from Italy41 that followed 10 BP patients (median age 68.5 years) with comorbidities such as diabetes, arterial hypertension or cancer, no COVID-19 manifestations have been registered, despite prolonged treatment with either topical and systemic corticosteroids or azathioprine. The doses had been adjusted, but the agents were not discontinued.41
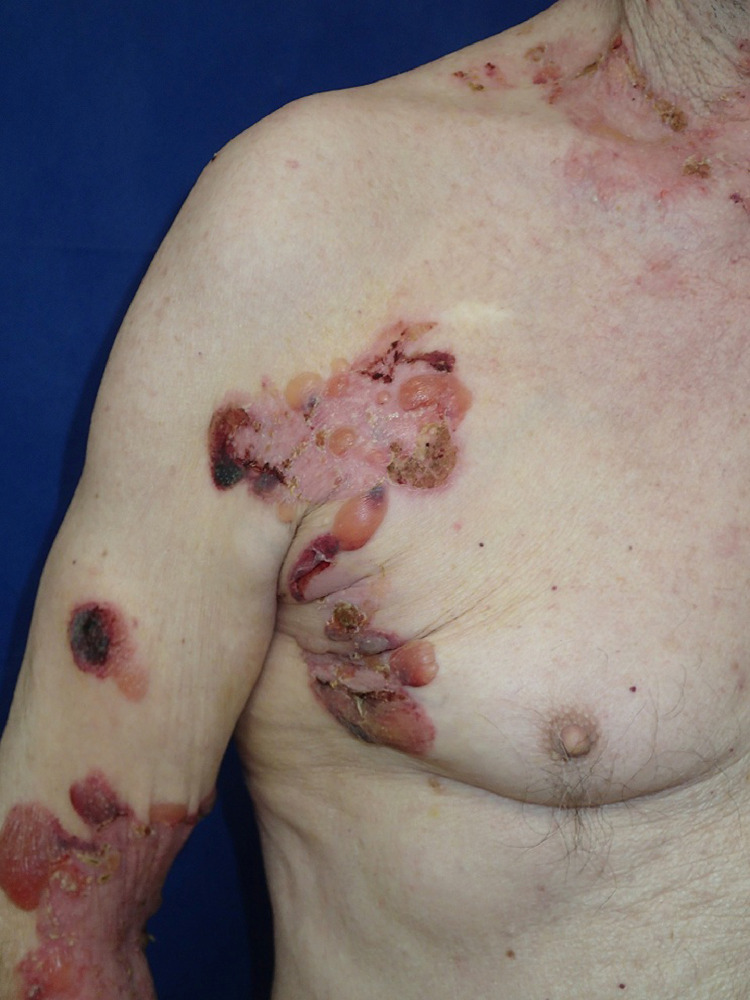
At the Department of Dermatology, Medical University-Sofia, Bulgaria, for the period of the pandemic, we observed only 1 BP patient, suspected of having been in contact with a family member affected by affected COVID-19. This was a 72-year-old man with newly diagnosed BP, having typical clinical characteristics (Figure 3 ) that followed pembrolizumab administration for metastatic melanoma. He was living in close contact with his daughter who was IgM-SARS-CoV-2 positive. The patient presented in good general condition, with a normal chest x-ray and negative SARS-CoV-2 tests. Treatment with topical and systemic corticosteroids was well tolerated and resulted in rapid BP control.
Fig. 3.
Tense blisters and multiple erosions in a patient with BP who was the contact person to an IgM-SARS-CoV-2 positive relative. BP, bullous pemphigoid; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
The COVID-19 pandemic has raised additional concerns related to the immunosuppressive nature of BP therapy. Topical and systemic corticosteroids, methotrexate, anti-interleukin 17, dupilumab or rituximab, together with the impaired skin barrier function, make BP patients potential victims of SARS-CoV-2 and opportunistic infections, thus increasing the mortality risk especially attributable to pneumonia.42 Potent topical corticosteroids have been widely accepted as being safer than systemic therapy, especially for extensive disease.43 Based on these observations, during the pandemic, most BP patients in Iran were initially treated with topical clobetasol due to their advanced age and comorbidities.44 In new and relapsing cases, clobetasol was effectively combined with doxycycline or oral corticosteroids up to 0.5 mg/kg, methotrexate was discontinued and prednisone >10 mg/d was avoided or reduced when possible. In confirmed BP-COVID-19 cases, IVIG administration for parallel control of both conditions was suggested despite the lack of clear evidence on their effect on COVID-19 and the poor tolerability in elderly patients.
Discontinuation of immunomodulatory therapy should follow detailed evaluation and individual risk-benefit analysis.44 Plasmapheresis is generally anecdotal in BP and COVID-19 but may also be considered.20 Dapsone, another treatment option in BP, together with doxycycline, was effective ini both BP and COVID-19.45 In BP, as in pemphigus, prophylactic anticoagulation has been suggested to reduce the mortality risk during the current pandemic. The two conditions were previously estimated as prothrombotic states with approximately 15-fold increased risk of venous thromboembolism in the acute phase of BP and about two-fold risk of pulmonary thromboembolism in pemphigus. Together with the cytokine storm, coagulopathy and venous thromboembolism are the main factors for a fatal outcome in patients with severe COVID-19.46
Mucous membrane pemphigoid (MMP) is a rare subepidermal blistering disease with predominant mucosal involvement and a tendency to aggressive scarring that may result in blindness, esophageal strictures, hoarseness, or respiratory failure.47 MMP usually requires early and often aggressive combined corticosteroid and immunosuppressive therapy, which increases the risk of infection.48 Since the beginning of the pandemic, only anecdotal reports have appeared regarding MMP cases affected by COVID-19. A 43-year-old Iranian MMP patient with concomitant COVID-19, who had been treated with prednisolone, rituximab, and MMF, was changed to IVIG.49 Both the clinical presentation of MMP and the patient's general condition improved significantly, which was explained as being the possible effect of IVIG on the so-called cytokine storm.49
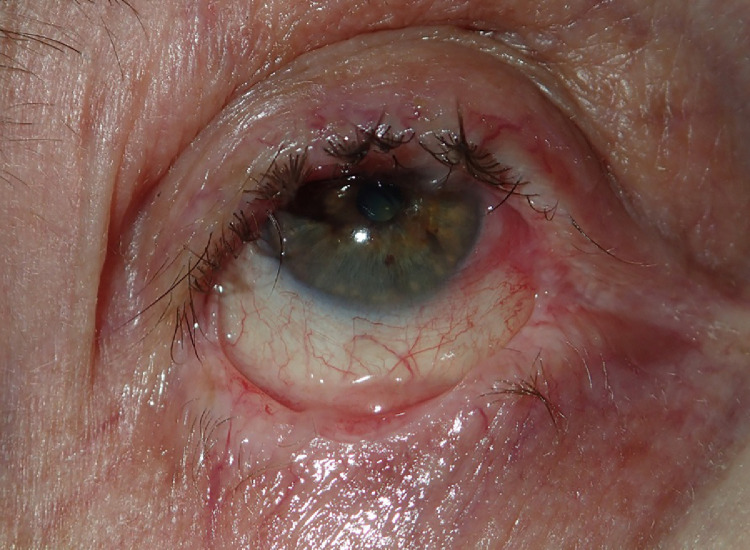
At the very beginning of the pandemic, we diagnosed an 85-year-old man with MMP, presenting with oral-pharyngeal erosions and severe ocular scarring (Figure 4 ). The patient was in good general condition with no past pulmonary pathology. He did well with methylprednisolone, dapsone, and azathioprine. A month after discharge, he was hospitalized for severe pneumonia and died from respiratory insufficiency. Although the autopsy and SARS-CoV-2 tests were not performed, COVID-19 is suspected.
Fig. 4.
Severe ocular scarring in a patient with MMP who later developed pneumonia and died from pulmonary insufficiency. MMP, mucous membrane pemphigoid.
Regarding treatment options for MMP in the context of the COVID-19 outbreak, it has been suggested that mild forms may be safely controlled with topical corticosteroids, prednisolone 10 mg/d, doxycycline/tetracycline, dapsone/sulphapyridine, or colchicine. Cyclophosphamide was discouraged, and caution was recommended with azathioprine, MMF, and methotrexate for their increased risk during the COVID-19 pandemic. In severe, rapidly progressing, or recalcitrant MMP, additional immunosuppressors or early administration of rituximab could prevent debilitating scarring, although low-dose cycles for the latter may be considered.50 IVIG was demonstrated as being safe in the treatment of AIBD patients with concomitant COVID-19 who require a rapid result with minimal immunosuppression.49
Epidermolysis bullosa acquisita (EBA), linear IgA dermatosis (LAD), and dermatitis herpetiformis (DH) have not been reported so far in the context of the COVID-19 pandemic, most likely due to their low incidence. In DH patients, it may be interesting to investigate the potential protective effect of dapsone therapy in the context of the current situation, as for a time, dapsone was considered as a possible treatment regimen for COVID-19 and even a preventive measure for COVID-19–related ARDS due to its capacity to inhibit neutrophil chemotaxis.51 Contrary to EBA, hereditary epidermolysis bullosa which is characterized by genetically caused skin fragility, was paid more attention by specialists and institutions. This resulted in the workup of respective patient information, registries, expert recommendations, and consensus statements for prevention and treatment.52, 53, 54, 55
General overview and expert recommendations on treatment strategies
Evidence-based information on the impact of the COVID-19 crisis on AIBD continues to rely on telephone calls and telemedicine sessions that collect data on isolated case series most likely attributable to their low incidence and difficulty in obtaining large patients’ cohorts. In an attempt to evaluate all AIBD patients with suspicious or proven COVID-19, a rapid systematic review was conducted in July 2020, which detected a total of 732 AIBD patients (211 pemphigus cases, 112 pemphigoid cases, 409 not specified).56 COVID-19 symptoms were observed in 70 (9.5%) patients, and the diagnosis was confirmed in 16 (2.1%) patients. A total of 6 (0.8%) patients had severe symptoms that required hospitalization, and 3 (0.4%) patients died due to COVID-19, all of whom were elderly or had comorbidities. Only 3 (0.4%) patients demonstrated limited control of their AIBD.14 , 17 , 57 Results from this preliminary systematic analysis suggest that AIBD patients on immunomodulatory therapies are basically not at an increased risk for severe or fatal COVID-19, as in the general population severe COVID-19 was about 15% and the overall COVID-19 mortality rate was estimated 1.5-3.6%. In conclusion, the pandemic generally does not have a negative effect on have the course of AIBD; therefore, delays or discontinuation of important immunomodulatory treatment should be avoided.
These findings support other reports recommending that, if a patient has definitely not been exposed to COVID-19, immunomodulatory therapy should be continued as withdrawal might lead to uncontrolled disease activity and increased morbidity and mortality. For patients with an autoimmune disease who have been in contact with COVID-19 positive individuals or who are under quarantine or experience COVID-19 symptoms, it may be prudent to pause maintenance immunosuppression treatment for 2 weeks after COVID-19 exposure or travel. Other authors have suggested that corticosteroids and immune suppressants, such as azathioprine and MMF, should be reduced to the lowest effective dose.20 Chinese dermatologists who were the first to face SARS-CoV-2 shared their experience during the worst 3 months of the pandemic in China.58 Of their 38 AIBD patients, 17 had discontinued their treatment without consulting their physicians, and 21 developed severe psychologic issues. The authors therefore suggested regular telecontacts between doctors and patients, close monitoring, and psychologic support.58 The patients were encouraged to consult with their dermatologist before discontinuing any medications.59
AIBD experts’ recommendations have focused on efficient but less aggressive and safer therapeutic agents and regimens during the COVID-19 pandemic. In summary, they have suggested maintaining immunomodulatory therapy as uncontrolled AIBD activity and elevated morbidity and mortality may follow withdrawal. Patients with confirmed COVID-19 should be evaluated for their level of risk. Azathioprine, MMF, cyclophosphamide, cyclosporine, and methotrexate may be paused for the duration of COVID-19 symptoms, and topical corticosteroids, prednis(ol)one 10 mg/d, dapsone/sulfapyridine, doxycycline/tetracycline, colchicine, or IVIG may be continued. It was advised that prednis(ol)one >10 mg/d may be reduced depending on the activity of the AIBD, age, comorbidities, and severity of COVID-19 in collaboration between the dermatologist and physician in charge of COVID-19. Important dose reduction or abrupt discontinuation of systemic corticosteroids should be discouraged, particularly for patients with severe AIBD. In addition, some evidence demonstrates that prednis(ol)one may have a beneficial effect on COVID-19. Immunosuppressive drugs may be helpful in combatting overactivation of the immune system, which is responsible for the pulmonary injury caused by SARS-CoV-2.60
Information for AIBD patients and healthcare professionals during the COVID-19 pandemic
AIBD patients, especially those suffering from PV and MMP, have been advised by the European Academy of Dermatology and Venereology (EADV) task force, the European Reference Networks, the International Pemphigus & Pemphigoid Foundation, and other organizations regarding general protective measures, the effects of systemic corticosteroids, steroid-sparing agents, rituximab, and other treatment options during the COVID-19 pandemic.61 , 62 Adherence to general precautions for protection of the elderly and immunosuppressed AIBD patients has been recommended, including social distancing, avoiding gatherings, application of teledermatology and screening of the patients for COVID-19 symptoms, as well as evaluating the psychological tolerance of patients during quarantine and developing supportive strategies, especially for those on high doses of corticosteroids.44 Alternatively, because they are in closed contact with large wound areas and body fluids, health care professionals (HCP) who treat AIBD patients have been advised to respect three levels of personal protective measures (PPM) depending on SARS-CoV-2 negativity or positivity and severity of AIBD-COVID-19 symptoms.58 These precautions are in addition to other COVID-19 recommendations for caregivers in dermatology.63 Updates regarding dermatologic PPM during the COVID-19 pandemic have also been posted on the World Health Organization and Centers for Disease Control home pages. Available online information is summarized in Table 2 .
Table 2.
Online information for AIBD patients and health care professionals during the COVID-19 pandemic
| Organization/source | Patient information available online | Subject of information |
|---|---|---|
| EADV Task Force on AIBD | https://www.eadv.org/covid-19/task-force | PPM, therapy |
| ERN | https://ern-skin.eu/covid-19/ | PPM, therapy |
| IPPF | http://www.pemphigus.org/information-for-pemphigus-and-pemphigoid-patients-related-to-coronavirus-disease-covid-19/ | PPM, therapy |
| NICE guideline | https://www.nice.org.uk/guidance/ng169 | Biologic/immunomodulatory therapy |
| BAD | http://www.bad.org.uk/healthcare-professionals/covid-19 | Biologic/immunomodulatory therapy |
| AAD | https://www.aad.org/member/practice/coronavirus/clinical-guidance/biologics | Biologic therapy |
| WHO | www.who.int | General situation and precautions |
| CDC | www.cdc.gov | General situation and precautions |
AAD, American Academy of Dermatology; AIBD, autoimmune bullous dermatoses; BAD, British Association of Dermatologists; CDC, Centers for Disease Control and Prevention; EADV, European Academy of Dermatology and Venereology; ERN, European Reference Networks for rare diseases; IPPF, International Pemphigus & Pemphigoid Foundation; NICE, National Institute for Health and Care Excellence; PPM, personal protective measures; WHO, World Health Organization.
AIBD registries during COVID-19 pandemic
Registries regarding COVID-19–related skin manifestations have been launched by the American Academy of Dermatology, the International League of Dermatological Societies, and the French and Spanish dermatologic societies in an attempt to collect and quickly disseminate knowledge among HCP.4 , 64
In addition, a disease-specific registry on AIBD cases affected by COVID-19 has been initiated by the corresponding EADV task force, similar to other registries that focus on diseases such as psoriasis, atopic dermatitis, connective tissues diseases, and others. Registry results were reported during the annual meeting of the AIBD task force held during the last virtual EADV Congress in the autumn of 2020. The AIBD registry is available at the following URL: https://recovab.umcg.nl.
Future perspectives and conclusions
Knowledge of the impact of COVID-19 on AIBD is still based on anecdotal case reports or small case series and on isolated experts’ recommendations. The available information is useful but is often controversial and requires constant updating. To date, most reports covering the impact of COVID-19 on AIBD conclude that larger prospective studies are necessary. In response to this recommendation, a multicenter study has already been initiated in France, aiming to analyze the incidence and severity of COVID-19 among AIBD patients compared with the general population, focusing on patients treated with rituximab.26
In addition, the careful study of the interactions between SARS-CoV-2 and the different types of immune response and immune modulation of conventional and new drugs have demonstrated that many treatments may be safely carried out during the COVID-19 pandemic. Some of the treatments for AIBD may even alleviate COVID-19 symptoms. Data on existing and emerging therapies are constantly evolving, with a continuous reassessment of the possible management strategies for AIBD during COVID-19.65
Conflict of interest
Dr. Drenovska reports personal fees from Roche, Abbvie, Johnson & Johnson, Novartis, and Eli Lilly, outside the submitted work. Dr. Vassileva reports personal fees from Abbvie, Novartis, Johnson & Johnson, Eli Lilly, Sanofi, Roche, UCB, and Berlin Chemie, outside of the submitted work. Dr. Tanev has no conflicts of interest to declare. Dr. Joly is a consultant for Roche, Amgen, Principia Biopharma, Argenx, Astra Zeneca, Sanofi-Regeneron, Innovaderm, and Thermofisher.
References
- 1.Zhang M.Q., Wang X.H., Chen Y.L., et al. Clinical features of 2019 novel coronavirus pneumonia in the early stage from a fever clinic in Beijing [in Chinese] Zhonghua Jie He He Hu Xi Za Zhi. 2020;43:E013. doi: 10.3760/cma.j.issn.1001-0939.2020.0013. [DOI] [PubMed] [Google Scholar]
- 2.Guan W., Ni Z., Yu H.u., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Picchianti Diamanti A., Rosado M.M., Pioli C., Sesti G., Laganà B. Cytokine release syndrome in COVID-19 patients, A new scenario for an old concern: the fragile balance between infections and autoimmunity. Int J Mol Sci. 2020;21:3330. doi: 10.3390/ijms21093330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Drenovska K, Schmidt E, Vassileva S. Covid-19 pandemic and the skin. Int J Dermatol. 2020;59:1312–1319. doi: 10.1111/ijd.15189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wollina U. Challenges of COVID-19 pandemic for dermatology. Dermatol Ther. 2020;33:e13430. doi: 10.1111/dth.13430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duvert-Lehembre S., Joly P. Autoimmune blistering diseases [in French] Rev Med Interne. 2014;35:166–173. doi: 10.1016/j.revmed.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt E., Kasperkiewicz M., Pemphigus Joly P. Lancet. 2019;394:882–894. doi: 10.1016/S0140-6736(19)31778-7. [DOI] [PubMed] [Google Scholar]
- 8.Drenovska K., Manuelyan K., Vassileva S. New strategies in the treatment of pemphigus [in Bulgarian] Medinfo. 2011;5:1–4. [Google Scholar]
- 9.Murrell D.F., Peña S., Joly P., Marinovic B., et al. Diagnosis and management of pemphigus: recommendations of an international panel of experts. J Am Acad Dermatol. 2020;82:575. doi: 10.1016/j.jaad.2018.02.021. el-585.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joly P., Horwath B., Patsatsi Α., et al. Updated S2 K guidelines on the management of pemphigus vulgaris and foliaceus initiated by the European academy of dermatology and venereology (EADV) J Eur Acad Dermatol Venereol. 2020;34:1900–1913. doi: 10.1111/jdv.16752. [DOI] [PubMed] [Google Scholar]
- 11.Bilgic A., Murrell D.F. What is novel in the clinical management of pemphigus. Expert Rev Clin Pharmacol. 2019;12:973–980. doi: 10.1080/17512433.2019.1670059. [DOI] [PubMed] [Google Scholar]
- 12.Diotallevi F., Simonetti O., Radi G., et al. Management of patients with pemphigus vulgaris during the COVID-19 pandemic: experience of a second level dermatology center. Australas J Dermatol. 2021;62:e158–e159. doi: 10.1111/ajd.13414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Di Altobrando A., Patrizi A., Bardazzi F. Should SARS-CoV-2 influence immunosuppressive therapy for autoimmune blistering diseases? J Eur Acad Dermatol Venereol. 2020;34:e295–e297. doi: 10.1111/jdv.16491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balestri R., Rech G., Girardelli C.R. Occurrence of SARS-CoV-2 during mycophenolate mofetil treatment for pemphigus. J Eur Acad Dermatol Venereol. 2020;34:e435–e436. doi: 10.1111/jdv.16578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ghalamkarpour F., Pourani M.R. Aggressive course of pemphigus vulgaris following COVID-19 infection. Dermatol Ther. 2020;33:e14398. doi: 10.1111/dth.14398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Feldman R.J. Paradoxical worsening of pemphigus vulgaris following rituximab therapy. Br J Dermatol. 2015;173:858–859. doi: 10.1111/bjd.13823. [DOI] [PubMed] [Google Scholar]
- 17.Carugno A., Sena P., Raponi F., Robustelli Test E., Vezzoli P. Patients with bullous skin disease in a high-epidemic COVID-19 area, Bergamo, Italy. Br J Dermatol. 2020;183:589–591. doi: 10.1111/bjd.19266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marzano A.V., Genovese G., Fabbrocini G., et al. Varicella-like exanthem as a specific COVID-19-associated skin manifestation: multicenter case series of 22 patients. J Am Acad Dermatol. 2020;83:280–285. doi: 10.1016/j.jaad.2020.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdollahimajd F., Shahidi-Dadras M., Robati R.M., Dadkhahfar S. Management of pemphigus in COVID-19 pandemic era; a review article. Arch Acad Emerg Med. 2020;8:e51. [PMC free article] [PubMed] [Google Scholar]
- 20.Shakshouk H., Daneshpazhooh M., Murrell D.F., Lehman J.S. Treatment considerations for patients with pemphigus during the COVID-19 pandemic. J Am Acad Dermatol. 2020;82:e235–e236. doi: 10.1016/j.jaad.2020.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pathania Y.S., Bhardwaj A. Treatment of severe pemphigus vulgaris during COVID-19 pandemic. J Dermatolog Treat, in press. doi: 10.1080/09546634.2020.1801971. [DOI] [PubMed]
- 22.Joly P., Maho-Vaillant M., Prost-Squarcioni C., et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux 3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031–2040. doi: 10.1016/S0140-6736(17)30070-3. [DOI] [PubMed] [Google Scholar]
- 23.Schmidt E. Rituximab as first-line treatment of pemphigus. Lancet. 2017;389:1956–1958. doi: 10.1016/S0140-6736(17)30787-0. [DOI] [PubMed] [Google Scholar]
- 24.Hebert V., Joly P. Rituximab in pemphigus. Immunotherapy. 2018;10:27–37. doi: 10.2217/imt-2017-0104. [DOI] [PubMed] [Google Scholar]
- 25.Shakshouk H., Daneshpazhooh M., Murrell D.F., Lehman J.S. Authors’ reply to the comment “Treatment considerations for patients with pemphigus during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84:e61–e62. doi: 10.1016/j.jaad.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aouar K., Guillibert A., Bohelay G., et al. Incidence and prognosis of COVID-19 in patients with autoimmune bullous diseases [in French] Ann Dermatol Venereol. 2020;147(12):A75. [Google Scholar]
- 27.Di Altobrando A, Patrizi A, Abbenante D, Bardazzi F. Rituximab: a safe therapeutic option during the COVID-19 pandemic?J Dermatolog Treat, in press. doi: 10.1080/09546634.2020.1800565. [DOI] [PubMed]
- 28.Mahmoudi H., Tavakolpour S., Nili A., Salehi Farid A., Daneshpazhooh M., Rashidian M. Treatment of pemphigus patients in the COVID-19 era: a specific focus on rituximab. Dermatol Ther. 2020;33:e14188. doi: 10.1111/dth.14188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.American Academy of Dermatology . 2020. Guidance on the use of biologic agents during COVID-19 outbreak. Available at: https://www.aad.org/member/practice/coronavirus/clinical-guidance/biologics. Accessed November 10, 2020. [Google Scholar]
- 30.Schultz B., Pearson D.R., Mansh M. Reply to “Treatment considerations for patients with pemphigus during the COVID-19 pandemic. J Am Acad Dermatol. 2021;84:e59–e60. doi: 10.1016/j.jaad.2020.07.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shahidi-Dadras M, Abdollahimajd F, Ohadi L, et al. COVID-19 in pemphigus vulgaris patients with previous rituximab therapy: a tele-medicine experience. Dermatolog Treat, in press. doi: 10.1080/09546634.2020.1789041. [DOI] [PubMed]
- 32.Waldman RA, Creed M, Sharp K, et al. Letter in reply: Toward a COVID-19 vaccine strategy for pemphigus patients on rituximab. J Am Acad Dermatol, in press. doi: 10.1016/j.jaad.2020.10.075. [DOI] [PMC free article] [PubMed]
- 33.Beyzaee AM, Rahmatpour Rokni G, Patil A, Goldust M. Rituximab as the treatment of pemphigus vulgaris in the COVID-19 pandemic era: a narrative review. Dermatol Ther, in press. doi: 10.1111/dth.14405. [DOI] [PMC free article] [PubMed]
- 34.Elmas Ö.F, Demirbaş A, Türsen Ü, Atasoy M, Lotti T. Pemphigus and COVID-19: critical overview of management with a focus on treatment choice. Dermatol Ther, in press. doi: 10.1111/dth.14265. [DOI] [PubMed]
- 35.Schmidt E., Zillikens D. Pemphigoid diseases. Lancet. 2013;381:320–332. doi: 10.1016/S0140-6736(12)61140-4. [DOI] [PubMed] [Google Scholar]
- 36.Hébert V., Joly P. Bullous pemphigoid, a dermatosis of the elderly [in French] Rev Prat. 2017;67:1080–1083. [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. Coronavirus disease 2019. Available at: https://www.tandfonline.com/doi/pdf/10.1080/09546634.2020.1800565?needAccess=true. Accessed March 23, 2020.
- 38.Vassileva S. In: Emergency Dermatology. 2nd ed. Wolf R, Davidovici B, Parish JL, Parish LC, editors. CRC Press, Taylor & Francis Group; New York, NY: 2016. Acute, severe bullous dermatoses; pp. 210–225. [Google Scholar]
- 39.Vassileva S., Drenovska K., Manuelyan K. Autoimmune blistering diseases as systemic diseases. Clin Dermatol. 2014;32:364–375. doi: 10.1016/j.clindermatol.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.De Fata Salvatores G., Villani A., Fabbrocini G., Di Guida A. Patients with bullous disorders during COVID-19 period: management and adherence to treatment. Dermatol Ther. 2020;33:e13697. doi: 10.1111/dth.13697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Patel P.M., Jones V.A., Murray T.N., et al. A review comparing international guidelines for the management of bullous pemphigoid, pemphigoid gestationis, mucous membrane pemphigoid, and epidermolysis bullosa acquisita. Am J Clin Dermatol. 2020;21:557–565. doi: 10.1007/s40257-020-00513-3. [DOI] [PubMed] [Google Scholar]
- 43.Joly P., Roujeau J.C., Benichou J., et al. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med. 2002;346:321–327. doi: 10.1056/NEJMoa011592. [DOI] [PubMed] [Google Scholar]
- 44.Azimi S.Z., Firooz A., Murrell D.F., Daneshpazhooh M. Treatment concerns for bullous pemphigoid in the COVID-19 pandemic era. Dermatol Ther. 2020;33:e13956. doi: 10.1111/dth.13956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Farouk A., Salman S. Dapsone and doxycycline could be potential treatment modalities for COVID-19. Med Hypotheses. 2020;140 doi: 10.1016/j.mehy.2020.109768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anuragi R.P., Kansal N.K. Immunobullous diseases, prothrombotic state, and COVID-19: role of prophylactic anticoagulation in bullous pemphigoid and pemphigus. Dermatol Ther. 2020;33:e14361. doi: 10.1111/dth.14361. [DOI] [PubMed] [Google Scholar]
- 47.Holtsche M.M., Zillikens D., Schmidt E. Mucous membrane pemphigoid [in German] Hautarzt. 2018;69:67–83. doi: 10.1007/s00105-017-4089-y. [DOI] [PubMed] [Google Scholar]
- 48.Manuelyan K., Drenovska K., Vassileva S. Pemphigoid—bullous, cicatricial and gestationis [in Bulgarian] Medicart. 2013;3:49–52. [Google Scholar]
- 49.Daneshpazhooh M., Soori T., Isazade A., Noormohammadpour P. Mucous membrane pemphigoid and COVID-19 treated with high-dose intravenous immunoglobulins: a case report. J Dermatolog Treat. 2020;31:446–447. doi: 10.1080/09546634.2020.1764472. [DOI] [PubMed] [Google Scholar]
- 50.Amir Dastmalchi D., Moslemkhani S., Bayat M., et al. The efficacy of rituximab in patients with mucous membrane pemphigoid. J Dermatolog Treat, in press. doi: 10.1080/09546634.2020.1801974. [DOI] [PubMed]
- 51.Altschuler E.L., Kast R.E. Dapsone, colchicine and olanzapine as treatment adjuncts to prevent COVID-19 associated adult respiratory distress syndrome (ARDS) Med Hypotheses. 2020;141 doi: 10.1016/j.mehy.2020.109774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abdollahimajd F., Youssefian L., Pourani M.R., Vahidnezhad H., Uitto J. Novel coronavirus 2019 (COVID-19) and epidermolysis bullosa: report of three cases. Dermatol Ther. 2020;33:e14194. doi: 10.1111/dth.14194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bodemer C, Bauer J, Bolling MC, et al. Patients with rare skin diseases and COVID-19. European Reference Networks. Available at: https://ern-skin.eu/covid-19/. Accessed November 10, 2020.
- 54.Vahidnezhad H., Moravvej H., Bahmanjahromi A., Youssefian L., Abdollahimajd F. Epidermolysis bullosa and the COVID-19 pandemic: challenges and recommendations. J Dermatolog Treat, in press. doi: 10.1080/09546634.2020.1788701. [DOI] [PubMed]
- 55.Murrell D.F., Lucky A.W., Salas-Alanis J.C., et al. Multidisciplinary care of epidermolysis bullosa during the COVID-19 pandemic-consensus: recommendations by an international panel of experts. J Am Acad Dermatol. 2020;83:1222–1224. doi: 10.1016/j.jaad.2020.06.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kasperkiewicz M. COVID-19 outbreak and autoimmune bullous diseases: a systematic review of published cases. J Am Acad Dermatol. 2021;84:563–568. doi: 10.1016/j.jaad.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brunasso A.M.G., Massone C. Teledermatologic monitoring for chronic cutaneous autoimmune diseases with smartworking during COVID-19 emergency in a tertiary center in Italy. Dermatol Ther. 2020;33:e13495. doi: 10.1111/dth.13695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen P, Zhang G, Zhan Y. Management strategies of autoimmune bullous diseases during the outbreak of 2019 novel coronavirus disease (COVID-19). J Dermatolog Treat, in press. doi: 10.1080/09546634.2020.1771261. [DOI] [PubMed]
- 59.Askanase A.D., Khalili L., Buyon J.P. Thoughts on COVID-19 and autoimmune diseases. Lupus Sci Med. 2020;7:e000369. doi: 10.1136/lupus-2020-000396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kasperkiewicz M., Schmidt E., Fairley J.A., et al. Expert recommendations for the management of autoimmune bullous diseases during the COVID-19 pandemic. J Eur Acad Dermatol Venereol. 2020;34:e302–e303. doi: 10.1111/jdv.16525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.European Academy of Dermatology and Venereology. Guidance from the EADV Task Force Autoimmune Blistering Diseases during the COVID-19 pandemic. Available at: https://www.eadv.org/covid-19/task-force. Accessed November 10, 2020.
- 62.International Pemphigus & Pemphigoid Foundation. Information for pemphigus and pemphigoid patients related to coronavirus disease (COVID-19). Available at: http://www.pemphigus.org/information-for-pemphigus-and-pemphigoid-patients-related-to-coronavirus-disease-covid-19/. Accessed November 10, 2020.
- 63.National Institute for Health and Care Excellence. COVID-19 rapid guideline: dermatological conditions treated with drugs affecting the immune response. Available at: https://www.nice.org.uk/guidance/ng169. Accessed November 10, 2020. [PubMed]
- 64.Freeman E.E., McMahon D.E., Fitzgerald M.E., et al. The American Academy of Dermatology COVID-19 registry: crowdsourcing dermatology in the age of COVID-19. J Am Acad Dermatol. 2020;83:509–510. doi: 10.1016/j.jaad.2020.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schön M.P., Berking C., Biedermann T., et al. COVID-19 and immunological regulations-from basic and translational aspects to clinical implications. J Dtsch Dermatol Ges. 2020;18:795–807. doi: 10.1111/ddg.14169. [DOI] [PMC free article] [PubMed] [Google Scholar]