Abstract
Functional outcome following glioma surgery is defined as how the patient functions or feels. Functional outcome is a coprimary end point of surgery in patients with diffuse glioma, together with oncological outcome. In this review, we structure the functional outcome measurements following glioma surgery as reported in the last 5 yr. We review various perspectives on functional outcome of glioma surgery with available measures, and offer suggestions for their use. From the recent neurosurgical literature, 160 publications were retrieved fulfilling the selection criteria. In these publications, neurological outcomes were reported most often, followed by activities of daily living, seizure outcomes, neurocognitive outcomes, and health-related quality of life or well-being. In more than a quarter of these publications functional outcome was not reported. A minimum essential consensus set of functional outcome measurements would benefit comparison across neurosurgical reports. The consensus set should be based on a combination of clinician- and patient-reported outcomes, assessed at a predefined time before and after surgery. The selected measurements should have psychometric properties supporting the intended use including validity-related evidence, reliability, and sensitivity to detect meaningful change with minimal burden to ensure compliance. We circulate a short survey as a start towards reporting guidelines. Many questions remain to better understand, report, and improve functional outcome following glioma surgery.
Keywords: Glioma, Neurosurgical procedures, Craniotomy, Treatment outcome, Patient outcome assessment, Patient-reported outcome measures, Quality of life
ABBREVIATIONS
- ECOG
Eastern Cooperative Oncology Group
- FACT-Br
Functional Assessment of Cancer Treatment-Brain
- FACT-G
Functional Assessment of Cancer Treatment-General
- HRQoL
health-related quality of life
- KPS
Karnofsky Performance Score
- MDASI-BT
MD Anderson Symptom Inventory Brain Tumor
- MUIS-BT
Mishel Uncertainty in Illness Scale-Brain Tumor
- NANO
Neurologic Assessment in Neuro-Oncology
- NIHSS
National Institutes of Health Stroke Scale
- PCI
Patient Concerns Inventory
- PROMIS
Patient-Reported Outcomes Measurement Information System
- QLQ-C30
Quality of Life Questionnaire-C30
- SNAS
Sherbrooke Neuro-Oncology Assessment Scale
- WHO
World Health Organization
Diffuse gliomas invade the brain, relentlessly recur, transform into higher grade gliomas, and are invariably lethal.1-5 Glioma surgery aims to extensively remove tumor tissue infiltrating the brain while preserving brain functions by avoiding damage to critical brain structures, except in case the tumor is considered to be unresectable. The more extensive the resection of glioma tissue, the longer patient survival is prolonged and the greater symptoms and seizures are reduced.6-10 Nevertheless, when critical structures are compromised, the patient's condition worsens permanently with shortened survival as a consequence.11-14 This dilemma is sometimes referred to as the oncofunctional balance in glioma surgery.15 This is not necessarily a trade-off between living longer or living better, as surgery could at the same time serve both end points. If these end points of cancer treatment are presented to patients as a trade-off, patients generally prioritize a better life over a longer life, in particular when facing an incurable malignancy, in less than optimal condition and at older age,16 including patients with a glioblastoma.17,18
Oncological outcome is typically measured as overall or progression free survival or time to malignant transformation. Residual tumor volume and extent of resection are surrogate markers of oncological outcome available immediately after surgery. Minimum thresholds for extent of resection and maximum residual volumes have been suggested,19,20 while others have argued a continuous positive relationship between extent of resection and survival.21-23
Functional outcome of glioma surgery is defined as the alterations in how the patient functions or feels. Multiple perspectives on functional integrity exist depending on who weighs the outcome: the patient, the patient's proxy, the neurosurgeon, the neuropsychologist, or another observer. These perspectives are seldom identical and can be either subjective, which measure how patients feel about their condition, or objective, which measure how patients perform on a specific task. These clinical outcome assessments have been categorized by the rater: patient-reported indicating information directly from the patient without interpretation, such as fatigue; clinician-reported based on an interpretation by a medical professional, such as muscle strength examination; observer-reported by someone else, such as a partner questionnaire; or rater-independent performance outcomes according to standardized objective tests administered by trained professionals, such as the Trail Making Test.24-26
In this review, we structure the functional outcome measurements following glioma surgery as reported in the last 5 yr. We review meaningful measures from various perspectives on functional outcome of glioma surgery. Studies of particular interest are marked by [•], and key literature by [••]. As part of this review, we circulate a survey to reach consensus on reporting guidelines. Consensus among neurosurgeons would facilitate comparisons and pooling of outcomes across surgical cohorts and thus development of evidence-based surgical decision algorithms to improve functional outcome.
SYSTEMATIC REVIEW OF FUNCTIONAL OUTCOME MEASUREMENTS
To determine the practice of reporting in the last 5 yr, we extracted the functional outcome measurements in glioma surgery cohorts according to the PRISMA statement.27 We retrieved citations from PubMed by using these search terms: “Glioma”[MeSH] AND (“Surgical Procedures, Operative”[MeSH] OR “resection”[tiab]) AND (“Patient Outcome Assessment”[MeSH] OR “outcome”[tiab]). The search was conducted on April 2, 2019. The set was restricted to publications from January 1, 2014 in any language. We included cohorts of 20 or more adults, reporting on surgery for supratentorial diffuse glioma (WHO grade II-IV). A meta-analysis of the functional outcomes and risk of bias assessment was not performed, because the diverse outcome measurements and patient eligibility criteria precluded quantitative data synthesis. The main reasons for exclusion were opinioned reviews, case reports, pediatrics, and epidemiological studies from registries. The titles and abstracts of identified studies were reviewed, and any study reporting on surgical outcome was included for full-text review. The search strategy retrieved 2779 unique publications. After screening of titles and abstracts, 294 eligible publications were reviewed in full text.
The inclusion criteria were met in 160 publications from which we extracted the reported measurements and the timing of assessment in relation to surgery, as summarized in Table and detailed in the Datatable, Supplemental Digital Content 1. Of these studies, neurological outcome was reported most often (58%), followed by activities of daily living (25%), seizure outcome (13%), neurocognitive outcome (8%), and health-related quality of life (HRQoL, 6%). No functional outcome was addressed in 27% of these studies. This indicates an opportunity to standardize reporting of functional outcomes after glioma surgery.
TABLE.
Distribution of Functional Outcome Measurements After Glioma Surgery in Publications From 5 Years (2014-2018)
| Publications on functional outcome in glioma surgery | n = 160 |
|---|---|
| Neurological examination | 92 (58%) |
| Muscle strength | 40 (25%) |
| Language | 31 (19%) |
| Vision | 9 (6%) |
| Unspecified | 48 (30%) |
| NIH Stroke Scale | 3 (2%) |
| Neurological Assessment Neuro-Oncology scale | 1 (1%) |
| Activities of daily living | 40 (25%) |
| Karnofsky Performance Scale | 34 (21%) |
| Eastern Cooperative Oncology Group/WHO scale | 4 (3%) |
| Barthel index | 1 (1%) |
| Modified Rankin Scale | 2 (1%) |
| Seizure outcome | 21 (13%) |
| Engel | 8 (5%) |
| Neurocognitive performance | 12 (8%) |
| Well-being/health-related quality of life | 9 (6%) |
| QLQ-C30/BN20 | 6 (4%) |
| EuroQol-5D | 4 (3%) |
| No functional outcome | 43 (27%) |
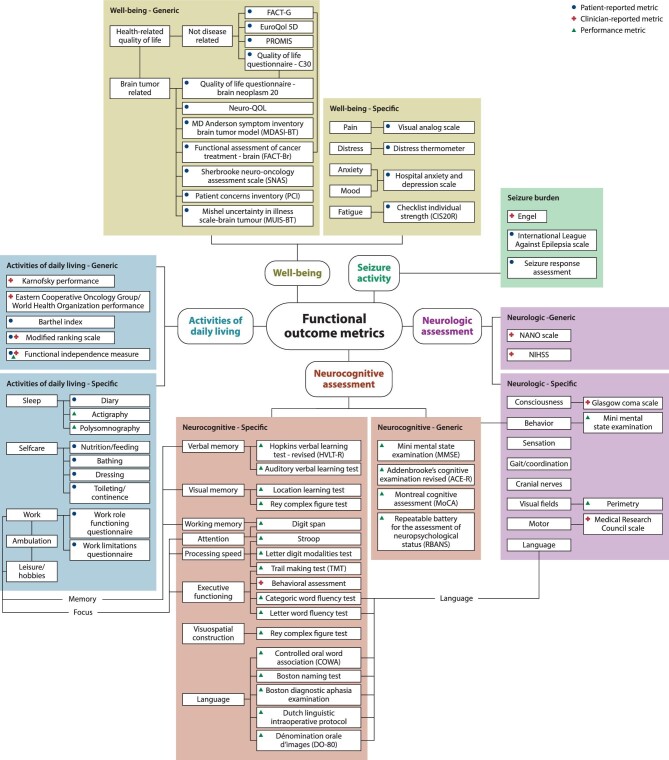
Functional outcome of glioma surgery can therefore be categorized in at least 5 contexts (Figure). Neurological, neurocognitive performance, and seizure activity are direct indicators of brain function. Activities in daily life and HRQoL are higher order aggregates of objective consequences and subjective perceptions of brain function.
FIGURE.
Infographic on functional outcome measurements following glioma surgery.
In the following sections, for each functional outcome category we consecutively describe the applicable measures, review the recent literature, and offer suggestions for the use of measurements.
Neurological Outcome
Neurological examination before and after glioma surgery is standard care. Variation in reported outcomes can be explained by differences in patient selection, in criteria for the severity of deficits, in timing of assessment and in applied surgical techniques to minimize neurological deficits. Furthermore, the neurological examination findings are also known to vary between neurologists.28,29 This is not surprising, because many elements of neurological examination are indiscrete or descriptive observations. An exception is muscle strength that is usually measured in 6 Medical Research Council grades,30 although its reliability has been criticized.31 Language examination in standard care is typically based on self-report or history taking. Structured language assessments are discussed in the section on neurocognitive outcome. Visual field examination is measured with technician-reported perimetry based on patient responses to stimuli. Two examples of generic scales for neurological assessment are the Neurologic Assessment in Neuro-Oncology (NANO) scale and the National Institutes of Health Stroke Scale (NIHSS). The NANO scale has been developed for response assessment as a clinician-reported measurement covering 9 neurological domains: gait, muscle strength, ataxia, sensation, visual fields, facial muscle strength, language, consciousness, and behavior.32 The NANO scale has discrete levels of functioning, high interobserver agreement, and can be assessed in 4 min by neurologists and non-neurologists. The NIHSS is designed to quantify impairment from stroke in subdomains: level of consciousness, gaze, visual fields, facial palsy, motor arm and leg, ataxia, sensory, language, articulation, extinction, and inattention.33 It is reasonably reliable and can be administered in 10 min.34,35
Many neurosurgical reports have included clinician-reported measures, typically muscle strength and language. New long-term motor deficits after glioma surgery were observed in 3% of 591 patients [•],36 4% of 207,37 4% of 648,38 5% of 294 [•],39 6% of 306,12 7% of 309 [•],40 8% of 222 [•],41 9% of 1229 [•],42 and 11% of 734.43 New long-term language deficits were observed in 2% of 250 patients [••],44 2% of 306,12 2% of 207,37 4% of 648,38 5% of 222 [•],41 6% of 1229 [•],42 and 14% of 309 [•].40 None of these reports used the NIHSS or NANO scale. The NIHSS group average was unchanged in 22 patients 3 mo following glioma surgery, while 26% of patients had a neurological deterioration.45 Others used the NIHSS to define severity as more than one point change compared to preoperative, in 2% of 288 patients 3 mo after low-grade glioma surgery,46 and in 26% of 110 patients 1 mo after recurrent glioblastoma resection [•].47 The NANO score group average was unchanged after surgery in 342 patients with glioblastoma.48 In a prospective database from 52 hospitals in North America, worse neurological status was observed in 10% of 499 patients.49 In a meta-analysis on neurological outcome after glioma surgery severe deficits were observed at 3 mo postoperative in 4.6% (95% CI, 3.3%-6.1%) and deficits of any severity were observed in 7.1% (95% CI, 5.3%-9.0%) of 6095 patients from 75 publications [••].50 These consisted of 254 hemipareses, 28 severe monopareses, 38 aphasias, 107 dysphasias, and 40 combined motor and language deficits. Other less often reported deficits included hemianopia, facial palsy, somatosensory syndrome, parietal syndrome, dysnomia, and cranial nerve deficits. It is unclear whether less often reported neurological events have a lower incidence after glioma surgery or whether these events are missed because not systematically examined. The use of intraoperative stimulation mapping reduced the late severe deficit rate from 8.3% without mapping to 3.4% with mapping (odds ratio: 0.39; 95% CI 0.23-0.64).
For outcomes research, neurological outcome should be standardized. Criteria for severity could be guided by the NANO or NIHSS scale. Standardized baseline assessment should be shortly before surgery, ideally on the day of hospital admission. Standardized follow-up assessment after surgery should not be too early, because many patients will recover from transient neurological deficits in the first weeks to months. Timing should also not be too late, because the neurological condition could have declined from other treatments or glioma progression. Three months postoperative is probably optimal for recovery of most transient neurological deficits, in absence of clinical and radiological progression. Furthermore, observer bias could be diminished by a baseline and follow-up examination by an (oncological) neurologist or another team member other than the neurosurgeon who performed the surgery.
Activities of Daily Living
Activities of daily living reflect the ability in everyday tasks for the patient due to a change in general condition, neurological, or neurocognitive deficits. Daily activities include bathing, feeding, dressing, functional transfers, ambulation, and continence. More complex instrumental activities are included as well, such as transportation, meal preparation, household and financial management, medication management, companionship, and social interaction. Generic measurements include the Karnofsky Performance Score (KPS) and the Eastern Cooperative Oncology Group or World Health Organization (ECOG/WHO) score. The KPS was designed to evaluate the effect of chemotherapeutics in 11 grades.51 The ECOG/WHO consists of 6 grades.52 Both scales have overall good inter-rater agreement in the existing literature,53-58 although some have found poor agreement.59 Alternative measurements include the Barthel index, the modified Rankin Scale, and the Functional Independence Measure. The Barthel index is based on 10 items (feeding, bathing, grooming, dressing, bowel and bladder control, toilet use, transfers, mobility on level surface, and on stairs) with a sum score ranging from 100 to 0,60 which has been shown to be reliable in stroke patients.61-63 The modified Rankin Scale has 7 grades, was designed for stroke, and has good reliability.64-67 The Functional Independence Measure has 18 items measured on a 7-point scale with a sum score ranging from 18 to 126, was designed for inpatient rehabilitation, and has good reliability.68,69 The Lawton Instrumental Activities of Daily Living Scale for older adults indexes the ability to perform tasks in 8 domains: telephone use, shopping, food preparation, housekeeping, laundry, transportation, responsibility of own medications, and handling finances. Administration takes 15 min.70 Detailed activities of daily living can be measured as specific items from the generic metrics. In addition, for employment status, the Work Role Functioning Questionnaire is available consisting of 27 self-reporting items with 5-point scales,71 and the Work Limitations Questionnaire consisting of 25 self-reporting items in 4 subscales: time management, physical demands, mental demands, and output demands.72 To measure sleep, a number of approaches are available including diaries,73 questionnaires,74 actigraphy,75 or polysomnography. Many questionnaires for patients with brain tumors include items on activities of daily living covering a wide range of content.76
Activities of daily living have been reported before and after glioma surgery mainly as KPS, and less often as ECOG/WHO score. The median KPS after surgery has been reported to be similar to the median KPS before surgery.48,77-79 Alternatively, the percentage of KPS decline was reported to be 5% of 292 patients [•],19 24% of 330,80 and 13% of 250 at 3 mo.81 The KPS improved in 53% of 330 patients,80 and in 13% of 250.81 The Barthel index,82 the modified Rankin score,83 and the Functional Independence Measure84 were each reported in single publications. Employment status after lower grade glioma surgery has recently been described as functional outcome measure in several reports demonstrating return to work in 74% of 78 patients [•],85 80% of 20,86 82% of 34,87 85% of 39,88 and 91% of 68 [•].89
Activities of daily living provide a perspective on everyday tasks as a consequence of neurological and/or neurocognitive dysfunction, mediated by compensatory strategies and environmental factors. Efforts strive towards objective standardized measures that test patients in the absence of mediating factors in a controlled environment. The measure should be standardized in timing of assessment before and after surgery and in observer, in parallel with neurological examination. Of note, the ADL measurements are ordinal categories and not linear continuous measurements, which are inadvertently summarized as average of study populations in many reports. It would be more informative to report the incidence per grade before and after surgery and as individual difference scores. For instance, a threshold for improved or declined KPS was proposed with difference scores of over 20 or under 20.19,90 Furthermore, distinction should be made between ability to return to work and ability to return to work full time in the same capacity as prior to surgery.
Seizure Outcome
Seizure outcome according to the Engel classification is customarily used in the literature on epilepsy surgery outcomes.91 Four classes are distinguished of which class I is considered seizure freedom without auras. An alternative measure is the classification from the International League Against Epilepsia consisting of 6 classes,92 which aims to avoid some of the ambiguities in the Engel classes. The inter-rater reliability and correlation of both scales were demonstrated to be very good.93 Seizure status is also queried in questionnaires on well-being.
The benefit of glioma resections on seizures has been well documented. Seizure freedom was reported after surgery in 68% of 40 patients,94 79% of 57,95 80% of 15,96 84% of 74,97 84% of 105,98 89% of 335,99 90% of 107 [•],85 97% of 73,100 and 100% of 25.86 The timing of postoperative assessment was not always mentioned, but usually occurred at 6 mo.85,86 Seizure outcome has sometimes been reported as Engel I classification consisting of seizure freedom at 1 yr postoperative in 36% of 147 patients,101 65% of 65,102 66% of 53,103 67% of 52,104 77% of 47,105 78% of 40,106 81% of 150,107 and 86% of 51.88 Clearly, seizure outcome depends on the preoperative seizure types, frequencies and durations, and the antiepileptic drug dosing changes, which partly explains this variation. Some authors describe new postoperative seizures as complication.97,108 The median seizure freedom rate after low-grade glioma resections was 71% in recent meta-analyses, with gross total resection as the main predictor [••].9,10
Comparison between surgical cohorts would be possible with reporting of seizure freedom at 1 yr postoperative for patients with preoperative seizures despite medication.
Neurocognitive Outcome
Generic measures are in use to screen for overall neurocognitive outcome, typically designed to detect dementia. The Mini Mental State Examination consists of 11 questions resulting in a maximum score of 30, which can be administered in 5 to 10 min.109 A score below 22 is considered clinically significant neurocognitive impairment. The Mini Mental State Examination has however very poor sensitivity to detect less than severe neurocognitive impairment, limiting its utility in glioma patients.110 The test-retest reliability is high in several patient populations.111 Alternative generic measures with potentially better sensitivity are the Addenbrooke's Cognitive Examination-Revised,112 the Montreal Cognitive Assessment,113 and the Repeatable Battery for the Assessment of Neuropsychological Status.114 These alternatives have so far not been validated in glioma patients. Neurocognitive tests can be summarized in cognitive domains, including attention/concentration, receptive and expressive language, memory/learning, visual-perceptual/spatial skills, and executive functions. Mood and personality variables as confounders of neurocognitive functioning are often also indexed. Usually the tests selected by the neuropsychologist depend on the referral question, for instance to direct a rehabilitation program. For language, several structured assessments are available. The Boston Diagnostic Aphasia Examination includes subtests of conversation, auditory comprehension, oral expression, reading, and writing; has excellent reliability; and is available in many languages.115 The extended version lasts 2 h, the shortened version 45 min. The Boston Naming Test has 60 items for picture naming and administration takes 20 min.116 The Dutch Linguistic Intraoperative Protocol comprises phonological, semantic, syntactic, naming, and articulatory tasks in 90 min and has been translated in other languages.117 The Dénomination Orale d’Images (DO–80) consists of picture naming with an 80-item set based on word frequency in French.118
Descriptions in reports on glioma surgery outcome have so far included qualitative summaries,86 quantitative summaries limited to one domain, such as detailed language assessment,85,119,120 memory,102 executive functions,121 or a combination of separate subtests45,122 and domain summaries [•]123-125 during the perioperative period.126 Some reports have described group means only.125,126 The timing of neurocognitive assessment varies from the point of hospital discharge119 to longer postoperative follow-up at 3 wk,124 3 mo,45,86,120-122 1 yr,102,123,125 and 40 mo.126 One study compared neurocognitive follow-up at 3 and 12 mo postoperative and observed further improvement in language, although the effects were small [•].125 In a recent meta-analysis on neurocognitive outcome, only 11 (10%) of 115 identified publications met the PRISMA criteria [••].127 Attention, language, and executive function were the most frequently reported cognitive domains. A positive impact of surgery was observed at 1 wk postoperative and at 6 mo follow-up for most cognitive domains, except for executive functions. This meta-analysis may have been positively skewed, because several studies were excluded that used z-score standardization and demonstrated less favorable outcome.128 In another meta-analysis, the level of neurocognitive outcome reporting of randomized controlled trials in brain tumor patients was of “high quality” in 20 (31%) of 65 studies [••].129 Key common shortcomings were unclear processing of missing data and not discussing the limitations and the generalizability of the tests.
Comprehensive testing of neurocognitive performance in all domains in standard care is likely unnecessary and unrealistic. Shorter assessment may improve compliance of patients and avoid selection bias. Reviews on neurocognitive outcome after glioma surgery have been descriptive and concluded that the test battery and the timing of baseline and follow-up assessment should be standardized for comparison between study cohorts [•].130-132 Specific testing focused on the domains of greatest importance remains the best clinical option. The neurocognitive domains deemed essential to be evaluated include attention, executive functions, verbal memory, and psychomotor speed. Such a standard test battery would ideally meet the following criteria: measuring the neurocognitive domains that are most vulnerable for tumor effects and treatment; standardization of test materials and procedures for administration; availability of normative data; sufficient test-retest reliability; limitation of practice effects by alternate versions of test material; availability in several languages; and an administration time within 40 min. A test battery meeting these criteria has also been recommended for brain tumor cohorts, the general cancer population, and multicenter clinical studies [••].133-137 This clinical trial core test battery covers learning and memory by the Hopkins Verbal Learning Test-Revised, verbal fluency by the Controlled Oral Word Association test, visual-motor scanning speed by the Trail Making Test part A, and executive function using the Trail Making Test part B.138 This test set can be administered by trained team members.
Health-Related Quality of Life
HRQoL is defined as a multidimensional concept consisting of at least physical, psychological, and social capacity as reported by the patient, which is distinct from objective patient performance.139 The generic EORTC Quality of Life Questionnaire-C30 (QLQ-C30) provides 15 scores from 6 single-item questions, consisting of dyspnea, insomnia, anorexia, constipation, diarrhea, and financial impact, and 9 scales with multiple items, covering global health/quality of life, physical functioning, role functioning, emotional functioning, cognitive functioning, social functioning, fatigue, nausea/vomiting, and pain.140 The brain neoplasm specific module EORTC QLQ-BN20 provides 11 scores consisting of 7 single items, consisting of general condition, headache, seizures, fatigue, hair loss, pruritus, and bladder control, and 4 multi-item scales, covering future uncertainty, visual disorder, motor dysfunction, and communication deficit.141,142 The EuroQol-5D is a measure of health status that consists of 6 items and has been used in many conditions and treatments. Based on 5 single-item scales, covering mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, a single score is produced with 0 representing dead, 1 perfect health, and negative values for states worse than death.143 A 3-level and a 5-level answer version are available on paper, electronic, or by telephone. Administration takes 5 min. The EuroQol-5D has been extensively studied in many healthy and diseased populations in various countries, and was shown to be reliable and sensitive.144 The MD Anderson Symptom Inventory Brain Tumor Module (MDASI-BT) provides a composite score for symptom severity and for symptom interference. The 21 symptom items and 7 interference items take less than 5 min to complete on paper, electronic or by telephone, and has been shown to be reliable and sensitive.145 The Functional Assessment of Cancer Treatment-Brain (FACT-Br) includes 27 items measuring general (FACT-G) cancer-related physical, social, emotional, and functional well-being, and a 23-item scale for symptoms and problems specific to brain tumors.146 Administration takes 15 min, and it has been shown to be reliable and sensitive. Alternative brain tumor specific scales that measure well-being are the Sherbrooke Neuro-Oncology Assessment Scale (SNAS),147 the Patient Concerns Inventory (PCI),148 as a holistic needs assessment tool, and the Mishel Uncertainty in Illness Scale-Brain Tumor (MUIS-BT),149 which indexes disease-related uncertainties. The Patient-Reported Outcomes Measurement Information System (PROMIS) was established by the National Institutes of Health and measures key patient-reported health indicators and symptoms covering several domains: pain, fatigue, emotional distress, physical functioning, and social role participation.150 NeuroQOL is a PROMIS-based measurement system for patients with neurological disease covering 13 domains, each consisting of 8 to 9 questions.151
The most frequently used generic HRQoL measures for well-being after glioma surgery are the EORTC QLQ-C30 and the QLQ-BN20.45,152 Improvement and deterioration of these measurements after glioma resection occurred in 29% and 35% of patients [•].45 Some publications only report the group average before and after surgery.152-155 Others have used the EuroQol-5D measurement. Improvement and deterioration were respectively observed in 27% and 14% of patients,156 17% and 13%,157 and 20% and 25% [•].158
DISCUSSION
For functional outcome assessment following glioma surgery, the aim is to measure the functional integrity compared to the situation before surgery on a scale that captures how the patient functions or feels. The measure of assessment should be valid in content, be reliable, and be able to detect change over time.24
A valid metric measures what it is supposed to measure. For glioma surgery, the measurement would be meaningful to the patient and reflect how the patient functions or feels, such as return to work.
A reliable metric provides consistent results. For glioma surgery, consistent measurements would be robust against timing, environment, and rater. Timing of assessment is critical because brain functions can fluctuate after surgery due to other treatments and medication. Measurements should be robust against measurement variation by the environment, such as a walking test providing similar results on the hospital ward, at home, or in a rehabilitation facility. Furthermore, some measurements have better agreement between raters than others.
The metric should detect meaningful changes. In essence, the research question determines what is meaningful and should guide a selection of generic and specific measures. Generic measurements, such as the NIHSS, may deviate only little on the dynamic range, if one item has deteriorated. For instance, a global aphasia would be measured as 3 on the NIHSS with a maximum sum of 32, which might not adequately reflect the meaning of this change to a patient.
Reporting Guidelines With a Selection of Functional Outcome Measurements
As research questions are diverse, so are selections of functional outcome measurements. For outcomes research on functional assessments in glioma surgery cohorts, however, a minimum essential standard set of functional measurements should be available from reports. This set would follow clinical practice closely, have minimal burden from measurement, and encompass the most important components for comparison across surgical cohorts. As a first step towards consensus on a standard set in the neurosurgical community, we provide a short survey in the Survey, Supplemental Digital Content 2. Supporting materials with details on the functional outcome measurements and scales are available from the author by request.
For studies with more specific purposes, new metrics, subscales, or individual items within measures can be added to the minimum essential standard set. For instance, in a study evaluating a Stroop test for intraoperative stimulation mapping, it naturally follows to compare baseline and longitudinal Stroop test assessments in addition to the standard outcome set. This would be a highly sensitive measure for the study purpose and at the same time serve to correlate changes in the new measure with the standard outcomes.
Practical Implication
A practical implication from systematic preoperative neurocognitive screening is to identify patients unable to contribute to shared decision making due to reduced mental capacity from tumor effects.159 This is important to recognize for informed consent for surgery or for study participation. Preoperative mental incapacity is common (25%) in glioma patients and often underestimated by clinicians.160
Future Directions
Several open questions may direct future efforts to better understand functional outcome after glioma surgery and to improve reporting.
Inter-Rater Agreement in Functional Outcomes
In recent publications of glioma surgery, clinician-reported outcomes of neurological examination are most frequently reported. Usually, the raters and their expertise have not been described. Likely, the attending neurosurgeons, residents, or nurse practitioners accounted for the examinations. What is the agreement among raters and between disciplines, eg, neurosurgeons versus neurologists, in commonly used measurements, such as neurological examination or activities of daily living?
Correlation Between Functional Outcomes
Furthermore, the correlation between functional outcomes has seldom been studied, in particular perioperatively. If 2 measures would be confirmed to correlate well, then one score could be converted into another to enable comparison among cohorts, such as the KPS and the ECOG/WHO performance.161 If an absence of association between measures would be established, this may indicate distinct sources of information with mutually contributing perspectives on functional outcome.
Response Shift and Noncompliance
Response shift is the longitudinal change in the patient's perception of their self-reported outcomes related to an internally shifting reference. By nature, people can lower their reference when facing terminal disease or meeting others with a lower perceived performance. As a consequence, patients can self-report their health status as good, not because it was unaffected by surgery, but because of their adaptation to new life circumstances with limitations, which may have been worse. Response shift can be measured by a so-called then test to correct self-reported outcomes.162,163 In one study, a relevant response shift for the EuroQol-5D was documented for patients with a decline or improvement in their reported quality of life at 6 mo after surgery, but absent on average [•].164 In patients with cancer other than glioma, response shift for self-reported outcomes was frequently detected, but the effect sizes were generally small.162,163
Noncompliance is also important for interpretation of functional outcomes. If patient competence becomes compromised for completion of questionnaires, then data would not be missing at random and missing outcomes of the sickest patients could lead to too favorable interpretation of results. Additionally, completion of burdensome questionnaires is oftentimes restricted for ethical reasons in patients near the end of life.165 The EuroQol-5D 6 mo after glioma surgery was completed by 62% of patients, with dropout mainly due to patient death, reporting by proxies, patient withdrawal, and nonresponsiveness [•].164 The EORTC QLQ-C30/BN20 was completed by 82% at hospital discharge after glioma surgery, and by 74% patients at 3 mo postoperative,45 and by only 28% of patients at 1 yr after second-line therapy.152 A potential direction for a solution is to replace reported outcomes of patients by proxies, such as a relative. However, the level of agreement between patient and patient-by-proxy ratings of HRQOL tends to be lower in patients with more severe neurocognitive deficits, limiting its validity.166 Another solution is to keep the measurement as short and simple as possible for a responsive and valid measurement.
Objective Versus Subjective Outcome
Should functional outcomes be objective measures of performance, or subjective perceptions of condition and capacity, or a combination? Objective deficits or lower performance following glioma surgery can be acceptable for some patients, but others without deficit and unchanged capacity can be unsatisfied with poor quality as they refer their new situation to their premorbid status. To some patients any change in the type of work or in professional efficiency may be unacceptable, but to others not returning to their same job or to work at a different capacity could be acceptable. Some patients may prefer more on the quantity of life versus the quality and to function reasonably well for many years, while others prefer to live a shorter life at full capacity. Consequently, the patient's personal goals are the main determinants of what is an acceptable dysfunction following glioma surgery. And personal goals are diverse: between cultures, between continents, between countries, between teams, between patients, and importantly within patients due to response shift. Subjective patient-reported outcome is undoubtedly most important for individualized patient care. But comparison across cohorts would become meaningless, if subjective measurements would be the only source of information. Perhaps subjective measurements should be corrected for their most important, yet unknown, drivers? Perhaps we should develop ‘interventions’ to enforce patients to shift from one perspective to another to improve how they feel about their situation?
As far as we are aware, only 2 interventions have shown to improve some aspects of functional outcome in glioma patients.167 Neurocognitive performance and fatigue improved in patients with stable gliomas after a cognitive rehabilitation program for 6 wk [••].168 Memory and information speed improved moderately in patients with brain tumors using Donepezil.169 Apart from efforts towards new interventions to improve functional outcome after surgery, a greater impact can be expected from neurosurgeons preoperatively identifying the patients prone to decline, and better understanding how to avoid deterioration, as neurosurgical complications seem to be closely related to functional outcomes.124,156,170
Predictors and Confounders of Functional Outcome
We would like to better understand why some patients improve and others deteriorate from glioma surgery. Reporting of changes in group averages may mask important individual changes [•].171 Patients can improve in outcome due to effects other than surgery, such as practice effects from serial testing, seizure reduction due to medication, initiation or discontinuation of corticosteroids, mood altering medication, analgesics, or other treatments. Patients can on the other hand also decline due to effects other than surgery, such as early tumor progression, increased seizure burden, or toxicity from other treatments and medication. And patient-related factors modulate how they function and feel, such as age, education, employment, social interactions, anxiety, distress, fatigue, depression, impaired judgment, coping strategies, and prior medical experiences. In addition, noise inherent to the measure will result in measurement error. How can measurement changes due to surgery be distinguished from other effects? Theoretically by randomizing surgery, but ethical concerns, patient preference, and perceived absence of equipoise by clinicians have precluded success. When does a measure detect a meaningful change? A potential solution for this is the Reliable Change Index, for which the difference between measurements before and after surgery is corrected for practice effects and measurement error, as measured in test-retest variation.172 It is common practice to refer neurocognitive tests results to a healthy population.173 Instead, for the purpose of isolating glioma surgery as causative factor for functional outcome, the reference population should probably be glioma patients whose measurements are subject to all mentioned effects, except for the surgery.
In a simplified causative model with surgery causing a change in functional outcome, all mentioned factors could be considered response modifiers or confounders of functional outcome, not unlike prognostic factors in survival analysis. Another approach, however, may be able to capture the complexity of the interdependent components to perioperative changes in functional measurements as symptom cluster or to use network analysis on the multiple dimensions of functional outcome.174-176
Ultimately, accurate predictions on functional outcome would be modeled based on a standardized data collection of outcome measurements and confounders in a large representative population with pooled data from many neurosurgical teams. After validation of these predictive models, neurosurgeons and their patients will be able to make surgical decisions guided by patient-specific risk estimates on the multiple dimensions of functional outcome according to individualized patient goals.
CONCLUSION
Functional outcome is a coprimary end point of glioma surgery, together with oncological outcome. In the most recent neurosurgical literature, neurological outcome was reported most often, followed by activities of daily living, seizure outcome, neurocognitive outcome, and HRQoL. A minimum essential consensus set of functional outcome measurements would benefit comparison between neurosurgical reports, based on a combination of clinician- and patient-reported outcomes and performances, subjective and objective, measured with measurements that are valid, reliable, and able to detect meaningful change. Many questions remain to better understand, report, and improve functional outcome following glioma surgery.
Funding
This study did not receive any funding or financial support.
Disclosures
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Supplementary Material
Acknowledgments
We thank Kenneth Probst and Noel Sirivansanti, medical illustrators at UCSF, for preparing the figure artwork.
Contributor Information
Philip C De Witt Hamer, Amsterdam UMC, Vrije Universiteit, Department of Neurosurgery, Cancer Center, Amsterdam, Netherlands.
Martin Klein, Amsterdam UMC, Vrije Universiteit, Department of Medical Psychology, Neuroscience Campus, Amsterdam, Netherlands.
Shawn L Hervey-Jumper, University of California San Francisco, Department of Neurological Surgery, San Francisco, California.
Jeffrey S Wefel, University of Texas MD Anderson Cancer Center, Department of Neuro-Oncology and Department of Radiation Oncology, Houston, Texas.
Mitchel S Berger, University of California San Francisco, Department of Neurological Surgery, San Francisco, California.
Supplemental Digital Content 1. Datatable.
Supplemental Digital Content 2. Survey on functional outcome metrics.
REFERENCES
- 1. Berger MS, Hervey-Jumper S, Wick W. Astrocytic gliomas WHO grades II and III. In: Berger MS, Weller M, eds. Handbook of Clinical Neurology. Vol. 134. Amsterdam, The Netherlands: Elsevier; 2016:345-360. [DOI] [PubMed] [Google Scholar]
- 2. Sanai N, Berger MS.. Surgical oncology for gliomas: the state of the art. Nat Rev Clin Oncol. 2018;15(2):112-125. [DOI] [PubMed] [Google Scholar]
- 3. Wirsching H-G, Galanis E, Weller M. Glioblastoma. In: Berger MS, Weller M, eds. Handbook of Clinical Neurology Vol. 134. Amsterdam, The Netherlands: Elsevier; 2016:381-397. [DOI] [PubMed] [Google Scholar]
- 4. Van Den Bent MJ, Bromberg JEC, Buckner J. Low-grade and anaplastic oligodendroglioma. In: Berger MS, Weller M, eds. Handbook of Clinical Neurology. Vol. 134. Amsterdam, The Netherlands: Elsevier; 2016:361-380. [DOI] [PubMed] [Google Scholar]
- 5. Weller M, Wick W, Aldape Ket al. Glioma. Nat Rev Dis Prim. 2015;1(1):15017. [DOI] [PubMed] [Google Scholar]
- 6. Brown TJ, Brennan MC, Li Met al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol. 2016;2(11):1460-1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Almenawer SA, Badhiwala JH, Alhazzani Wet al. Biopsy versus partial versus gross total resection in older patients with high-grade glioma: a systematic review and meta-analysis. Neuro Oncol. 2015;17(6):868-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hervey-Jumper SL, Berger MS.. Evidence for improving outcome through extent of resection. Neurosurg Clin N Am. 2019;30(1):85-93. [DOI] [PubMed] [Google Scholar]
- 9.[••] Englot DJ, Berger MS, Barbaro NM, Chang EF. Predictors of seizure freedom after resection of supratentorial low-grade gliomas: a review. J Neurosurg. 2011;115(2):240-244. [DOI] [PubMed] [Google Scholar]
- 10.[••] Bonney PA, Boettcher LB, Burks JDet al. Rates of seizure freedom after surgical resection of diffuse low-grade gliomas. World Neurosurg. 2017;106:750-756. [DOI] [PubMed] [Google Scholar]
- 11. Bette S, Wiestler B, Kaesmacher Jet al. Infarct volume after glioblastoma surgery as an independent prognostic factor. Oncotarget. 2016;7(38):61945-61954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McGirt MJ, Mukherjee D, Chaichana KL, Than KD, Weingart JD, Quinones-Hinojosa A. Association of surgically acquired motor and language deficits on overall survival after resection of glioblastoma multiforme. Neurosurgery. 2009;65(3):463-469; discussion 469-470. [DOI] [PubMed] [Google Scholar]
- 13. Gorlia T, Wu W, Wang Met al. New validated prognostic models and prognostic calculators in patients with low-grade gliomas diagnosed by central pathology review: a pooled analysis of EORTC/RTOG/NCCTG phase III clinical trials. Neuro Oncol. 2013;15(11):1568-1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rahman M, Abbatematteo J, De Leo EKet al. The effects of new or worsened postoperative neurological deficits on survival of patients with glioblastoma. J Neurosurg. 2016;127(1):123-131. [DOI] [PubMed] [Google Scholar]
- 15. Mandonnet E, Duffau H.. An attempt to conceptualize the individual onco-functional balance: why a standardized treatment is an illusion for diffuse low-grade glioma patients. Crit Rev Oncol Hematol. 2018;122:83-91. [DOI] [PubMed] [Google Scholar]
- 16. Shrestha A, Martin C, Burton M, Walters S, Collins K, Wyld L. Quality of life versus length of life considerations in cancer patients: a systematic literature review. Psychooncology. 2019;28(7):1367-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brom L, Pasman HRW, Widdershoven GAMet al. Patients’ preferences for participation in treatment decision-making at the end of life: qualitative interviews with advanced cancer patients. PLoS One. 2014;9(6):e100435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gabel N, Altshuler DB, Brezzell Aet al. Health related quality of life in adult low and high-grade glioma patients using the National Institutes of Health Patient Reported Outcomes Measurement Information System (PROMIS) and Neuro-QOL assessments. Front Neurol. 2019;10:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.[•]Chaichana KL, Cabrera-Aldana EE, Jusue-Torres Iet al. When gross total resection of a glioblastoma is possible, how much resection should be achieved? World Neurosurg. 2014;82(1-2):e257-e265. [DOI] [PubMed] [Google Scholar]
- 20. Sanai N, Polley M-YY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3-8. [DOI] [PubMed] [Google Scholar]
- 21. Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE. Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol. 2014;32(8):774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ellingson BM, Abrey LE, Garcia Jet al. Post-chemoradiation volumetric response predicts survival in newly diagnosed glioblastoma treated with radiation, temozolomide, and bevacizumab or placebo. Neuro Oncol. 2018;20(11):1525-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Solheim O, Gulati S, Jakola AS. Glioblastoma resection: in search of a threshold between worthwhile and futile. Neuro Oncol. 2014;16(4):610-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Walton MK, Powers JH, Hobart Jet al. Clinical outcome assessments: conceptual foundation-report of the ISPOR clinical outcomes assessment - emerging good practices for outcomes research task force. Value Health. 2015;18(6):741-752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blakeley JO, Coons SJ, Corboy JR, Leidy NK, Mendoza TR, Wefel JS. Clinical outcome assessment in malignant glioma trials: measuring signs, symptoms, and functional limitations. Neuro Oncol. 2016;18:ii13-ii20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Armstrong TS, Gilbert MR.. Patient reported endpoints for measuring clinical benefit in (high grade glioma) primary brain tumor patients. Curr Treat Options Oncol. 2014;15(4):519-528. [DOI] [PubMed] [Google Scholar]
- 27. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336-341. [DOI] [PubMed] [Google Scholar]
- 28. Shinar D, Gross CR, Mohr JPet al. Interobserver variability in the assessment of neurologic history and examination in the stroke data bank. Arch Neurol. 1985;42(6):557-565. [DOI] [PubMed] [Google Scholar]
- 29. Thaller M, Hughes T.. Inter-rater agreement of observable and elicitable neurological signs. Clin Med. 2014;14(3):264-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Dyck PJ, Boes CJ, Mulder Det al. History of standard scoring, notation, and summation of neuromuscular signs. A current survey and recommendation. J Peripher Nerv Syst. 2005;10(2):158-173. [DOI] [PubMed] [Google Scholar]
- 31. Vanhoutte EK, Faber CG, Van Nes SIet al. Modifying the Medical Research Council grading system through Rasch analyses. Brain. 2012;135(5):1639-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nayak L, Deangelis LM, Brandes AAet al. The Neurologic Assessment in Neuro-Oncology (NANO) scale: a tool to assess neurologic function for integration into the Response Assessment in Neuro-Oncology (RANO) criteria. Neuro Oncol. 2017;19(5):625-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. NIH Stroke Scale International . http://www.nihstrokescale.org/. Accessed February 24, 2020.
- 34. Brott T, Adams HP, Olinger CPet al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke. 1989;20(7):864-870. [DOI] [PubMed] [Google Scholar]
- 35. Goldstein LB, Bertels C, Davis JN. Interrater reliability of the NIH stroke scale. Arch Neurol. 1989;46(6):660-662. [DOI] [PubMed] [Google Scholar]
- 36.[•] Bello L, Riva M, Fava Eet al. Tailoring neurophysiological strategies with clinical context enhances resection and safety and expands indications in gliomas involving motor pathways. Neuro Oncol. 2014;16(8):1110-1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vorster SJ, Barnett GH.. A proposed preoperative grading scheme to assess risk for surgical resection of primary and secondary intraaxial supratentorial brain tumors. Neurosurg Focus. 1998;4(6):e2. [DOI] [PubMed] [Google Scholar]
- 38. Chaichana KL, Parker SL, Olivi A, Quiñones-Hinojosa A. Long-term seizure outcomes in adult patients undergoing primary resection of malignant brain astrocytomas. Clinical article. J Neurosurg. 2009;111(2):282-292. [DOI] [PubMed] [Google Scholar]
- 39.[•] Keles GE, Lundin DA, Lamborn KR, Chang EF, Ojemann G, Berger MS. Intraoperative subcortical stimulation mapping for hemispherical perirolandic gliomas located within or adjacent to the descending motor pathways: evaluation of morbidity and assessment of functional outcome in 294 patients. J Neurosurg. 2004;100(3):369-375. [DOI] [PubMed] [Google Scholar]
- 40.[•] Kim SS, McCutcheon IE, Suki Det al. Awake craniotomy for brain tumors near eloquent cortex. Neurosurgery. 2009;64(5):836-846. [DOI] [PubMed] [Google Scholar]
- 41.[•] Duffau H, Lopes M, Arthuis Fet al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985-96) and with (1996-2003) functional mapping in the same institution. J Neurol Neurosurg Psychiatry. 2005;76(6):845-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.[•] Li YM S D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: can we do better than gross-total resection? J Neurosurg. 2015;124(4):977-988. [DOI] [PubMed] [Google Scholar]
- 43. Zhang JS, Qu L, Wang Qet al. Intraoperative visualisation of functional structures facilitates safe frameless stereotactic biopsy in the motor eloquent regions of the brain. Br J Neurosurg. 2018;32(4):372-380. [DOI] [PubMed] [Google Scholar]
- 44.[••] Sanai N, Mirzadeh Z, Berger MS. Functional outcome after language mapping for glioma resection. N Engl J Med. 2008;358(1):18-27. [DOI] [PubMed] [Google Scholar]
- 45.[•] Wolf J, Campos B, Bruckner T, Vogt L, Unterberg A, Ahmadi R. Evaluation of neuropsychological outcome and “quality of life” after glioma surgery. Langenbeck's Arch Surg. 2016;401(4):541-549. [DOI] [PubMed] [Google Scholar]
- 46. Coburger J, Merkel A, Scherer Met al. Low-grade glioma surgery in intraoperative magnetic resonance imaging: results of a multicenter retrospective assessment of the German study group for intraoperative magnetic resonance imaging. Neurosurgery. 2016;78(6):775-785. [DOI] [PubMed] [Google Scholar]
- 47.[•] Oppenlander ME, Wolf AB, Snyder LAet al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg. 2014;120(4):846-853. [DOI] [PubMed] [Google Scholar]
- 48. Heiland DH, Haaker G, Watzlawick Ret al. One decade of glioblastoma multiforme surgery in 342 elderly patients: what have we learned? J Neurooncol. 2018;140(2):385-391. [DOI] [PubMed] [Google Scholar]
- 49. Chang SM, Parney IF, McDermott Met al. Perioperative complications and neurological outcomes of first and second craniotomies among patients enrolled in the glioma outcome project. J Neurosurg. 2003;98(6):1175-1181. [DOI] [PubMed] [Google Scholar]
- 50.[••] De Witt Hamer PC, Robles SG, Zwinderman AH, Duffau H, Berger MS. Impact of intraoperative stimulation brain mapping on glioma surgery outcome: a meta-analysis. J Clin Oncol. 2012;30(20):2559-2565. [DOI] [PubMed] [Google Scholar]
- 51. Karnofsky D, Burchenal J. The evaluation of chemotherapeutic agents in cancer. In: Macleod C, ed. Evaluation Chemotherapy Agents. New York, NY: Columbia University Press; 1949:191-205. [Google Scholar]
- 52. Oken MM, Creech RH, Tormey DCet al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649-655. [PubMed] [Google Scholar]
- 53. Yates JW, Chalmer B, McKegney FP. Evaluation of patients with advanced cancer using the Karnofsky Performance Status. Cancer. 1980;45(8):2220-2224. [DOI] [PubMed] [Google Scholar]
- 54. Mor V, Laliberte L, Morris JN, Wiemann M. The Karnofsky Performance Status scale. An examination of its reliability and validity in a research setting. Cancer. 1984;53(9):2002-2007. [DOI] [PubMed] [Google Scholar]
- 55. Schag CC, Heinrich RL, Ganz PA. Karnofsky Performance Status revisited: reliability, validity, and guidelines. J Clin Oncol. 1984;2(3):187-193. [DOI] [PubMed] [Google Scholar]
- 56. Taylor AE, Olver IN, Sivanthan T, Chi M, Purnell C. Observer error in grading performance status in cancer patients. Support Care Cancer. 1999;7(5):332-335. [DOI] [PubMed] [Google Scholar]
- 57. Zimmermann C, Burman D, Bandukwala Set al. Nurse and physician inter-rater agreement of three performance status measures in palliative care outpatients. Support Care Cancer. 2010;18(5):609-616. [DOI] [PubMed] [Google Scholar]
- 58. Myers J, Gardiner K, Harris Ket al. Evaluating correlation and interrater reliability for four performance scales in the palliative care setting. J Pain Symptom Manage. 2010;39(2):250-258. [DOI] [PubMed] [Google Scholar]
- 59. Chow R, Chiu N, Bruera Eet al. Inter-rater reliability in performance status assessment among health care professionals: a systematic review. Ann Palliat Med. 2016;5(2):83-92. [DOI] [PubMed] [Google Scholar]
- 60. Mahoney F, Barthel D. Functional evaluation: the Barthel index. Md State Med J. 1965;14:61-65. [PubMed] [Google Scholar]
- 61. Collin C, Wade DT, Davies S, Horne V. The Barthel ADL index: a reliability study. Int Disabil Stud. 1988;10(2):61-63. [DOI] [PubMed] [Google Scholar]
- 62. Lee Y-C, Yu W-H, Hsueh I-P, Chen S-S, Hsieh C-L. Test-retest reliability and responsiveness of the Barthel Index-based supplementary scales in patients with stroke. Eur J Phys Rehabil Med. 2017;53(5):710-718. [DOI] [PubMed] [Google Scholar]
- 63. Richards SH, Peters TJ, Coast J, Gunnell DJ, Darlow MA, Pounsford J. Inter-rater reliability of the Barthel ADL index: how does a researcher compare to a nurse? Clin Rehabil. 2000;14(1):72-78. [DOI] [PubMed] [Google Scholar]
- 64. Rankin J. Cerebral vascular accidents in patients over the age of 60. II. Prognosis. Scott Med J. 1957;2(5):200-215. [DOI] [PubMed] [Google Scholar]
- 65. Van Swieten JC, Koudstaal PJ, Visser MC, Schouten H, Van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19(5):604-607. [DOI] [PubMed] [Google Scholar]
- 66. Farrell B, Godwin J, Richards S, Warlow C. The United Kingdom Transient Ischaemic Attack (UK-TIA) aspirin trial: final results. J Neurol Neurosurg Psychiatry. 1991;54(12):1044-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wilson JT, Hareendran A, Hendry A, Potter J, Bone I, Muir KW. Reliability of the modified Rankin scale across multiple raters: benefits of a structured interview. Stroke. 2005;36(4):777-781. [DOI] [PubMed] [Google Scholar]
- 68. Keith RA, Granger CV, Hamilton BB, Sherwin FS. The functional independence measure: a new tool for rehabilitation. Adv Clin Rehabil. 1987;1:6-18. [PubMed] [Google Scholar]
- 69. Ottenbacher KJ, Hsu Y, Granger CV, Fiedler RC. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. 1996;77(12):1226-1232. [DOI] [PubMed] [Google Scholar]
- 70. Lawton MP, Brody EM.. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist. 1969;9(3):179-186. [PubMed] [Google Scholar]
- 71. Dorland HF, Abma FI, Roelen Cet al. Work functioning trajectories in cancer patients: results from the longitudinal work life after cancer (WOLICA) study. Int J Cancer. 2017;141(9):1751-1762. [DOI] [PubMed] [Google Scholar]
- 72. Feuerstein M, Hansen JA, Calvio LC, Johnson L, Ronquillo JG. Work productivity in brain tumor survivors. J Occup Environ Med. 2007;49(7):803-811. [DOI] [PubMed] [Google Scholar]
- 73. Carney CE, Buysse DJ, Ancoli-Israel Set al. The consensus sleep diary: standardizing prospective sleep self-monitoring. Sleep. 2012;35(2):287-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Monk TH, Buysse DJ, Kennedy KS, Pods JM, DeGrazia JM, Miewald JM. Measuring sleep habits without using a diary: the sleep timing questionnaire. Sleep. 2003;26(2):208-212. [DOI] [PubMed] [Google Scholar]
- 75. Carney CE, Lajos LE, Waters WF. Wrist actigraph versus self-report in normal sleepers: sleep schedule adherence and self-report validity. Behav Sleep Med. 2004;2(3):134-143. [DOI] [PubMed] [Google Scholar]
- 76. Oort Q, Taphoorn MJB, Sikkes SAM, Uitdehaag BMJ, Reijneveld JC, Dirven L. Evaluation of the content coverage of questionnaires containing basic and instrumental activities of daily living (ADL) used in adult patients with brain tumors. J Neurooncol. 2019;143(1):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ringel F, Pape H, Sabel Met al. Clinical benefit from resection of recurrent glioblastomas: results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18(1):96-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Sang S, Wanggou S, Wang Zet al. Clinical long-term follow-up evaluation of functional neuronavigation in adult cerebral gliomas. World Neurosurg. 2018;119:e262-e271. [DOI] [PubMed] [Google Scholar]
- 79. Roux A, Peeters S, Zanello Met al. Extent of resection and carmustine wafer implantation safely improve survival in patients with a newly diagnosed glioblastoma: a single center experience of the current practice. J Neurooncol. 2017;135(1):83-92. [DOI] [PubMed] [Google Scholar]
- 80. Awad AW, Karsy M, Sanai Net al. Impact of removed tumor volume and location on patient outcome in glioblastoma. J Neurooncol. 2017;135(1):161-171. [DOI] [PubMed] [Google Scholar]
- 81. Frey D, Schilt S, Strack Vet al. Navigated transcranial magnetic stimulation improves the treatment outcome in patients with brain tumors in motor eloquent locations. Neuro Oncol. 2014;16(10):1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lu CY, Chen XLL, Chen XLL, Fang XJ, Zhao YL. Clinical application of 3.0 T intraoperative magnetic resonance combined with multimodal neuronavigation in resection of cerebral eloquent area glioma. Medicine (Baltimore). 2018;97(34):e11702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Spena G, D’Agata F, Panciani PP, Buttolo L, di Monale Bastia MB, Fontanella MM. Practical prognostic score for predicting the extent of resection and neurological outcome of gliomas in the sensorimotor area. Clin Neurol Neurosurg. 2018;164:25-31. [DOI] [PubMed] [Google Scholar]
- 84. Roberts PS, Nuño M, Sherman Det al. The impact of inpatient rehabilitation on function and survival of newly diagnosed patients with glioblastoma. PM R. 2014;6(6):514-521. [DOI] [PubMed] [Google Scholar]
- 85. Pallud J, Dezamis E. Functional and oncological outcomes following awake surgical resection using intraoperative cortico-subcortical functional mapping for supratentorial gliomas located in eloquent areas. Neurochirurgie. 2017;63(3):208-218. [DOI] [PubMed] [Google Scholar]
- 86. Mandonnet E, De Witt Hamer P, Poisson Iet al. Initial experience using awake surgery for glioma: oncological, functional, and employment outcomes in a consecutive series of 25 cases. Neurosurgery. 2015;76(4):382-389. [DOI] [PubMed] [Google Scholar]
- 87. Muto J, Dezamis E, Rigaux-Viode Oet al. Functional-based resection does not worsen quality of life in patients with a diffuse low-grade glioma involving eloquent brain regions: a prospective cohort study. World Neurosurg. 2018;113:e200-e212. [DOI] [PubMed] [Google Scholar]
- 88. Bai SC, Xu BN, Wei SHet al. Intraoperative high-field magnetic resonance imaging combined with functional neuronavigation in resection of low-grade temporal lobe tumors. World J Surg Oncol. 2015;13(1):1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Ng S, Herbet G, Moritz-Gasser S, Duffau H. Return to work following surgery for incidental diffuse low-grade glioma: a prospective series with 74 patients. Neurosurgery. 2020;87(4):720-729. [DOI] [PubMed] [Google Scholar]
- 90. Chambless LB, Kistka HM, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC. The relative value of postoperative versus preoperative Karnofsky Performance Scale scores as a predictor of survival after surgical resection of glioblastoma multiforme. J Neurooncol. 2015;121(2):359-364. [DOI] [PubMed] [Google Scholar]
- 91. Engel J, Van Ness P, Rasmussen T, Ojemann L. Outcome with respect to epileptic seizures. In: Engel J Jr, ed. Surgical Treatment of the Epilepsies. 2nd edn. New York, NY: Raven Press; 1993. [Google Scholar]
- 92. Wieser HG, Blume WT, Fish Det al. Proposal for a new classification of outcome with respect to epileptic seizures following epilepsy surgery. Epilepsia. 2001;42(2):282-286. [PubMed] [Google Scholar]
- 93. Durnford AJ, Rodgers W, Kirkham FJet al. Very good inter-rater reliability of Engel and ILAE epilepsy surgery outcome classifications in a series of 76 patients. Seizure. 2011;20(10):809-812. [DOI] [PubMed] [Google Scholar]
- 94. Eseonu CI, Rincon-Torroella J, Refaey K, Quiñones-Hinojosa A. The cost of brain surgery: awake vs asleep craniotomy for perirolandic region tumors. Neurosurgery. 2017;81(2):307-314. [DOI] [PubMed] [Google Scholar]
- 95. Eseonu CI, Rincon-Torroella J, Lee YM, Refaey K, Tripathi P, Quinones-Hinojosa A. Intraoperative seizures in awake craniotomy for perirolandic glioma resections that undergo cortical mapping. J Neurol Surgery, Part A Cent Eur Neurosurg. 2018;79(3):239-246. [DOI] [PubMed] [Google Scholar]
- 96. Blumenthal DT, Kanner AA, Aizenstein Oet al. Surgery for recurrent high-grade glioma after treatment with bevacizumab. World Neurosurg. 2018;110:e727-e737. [DOI] [PubMed] [Google Scholar]
- 97. Eseonu CI, ReFaey K, Garcia O, Raghuraman G, Quinones-Hinojosa A. Volumetric analysis of extent of resection, survival, and surgical outcomes for insular gliomas. World Neurosurg. 2017;103:265-274. [DOI] [PubMed] [Google Scholar]
- 98. Eyüpoglu IY, Hore N, Merkel A, Buslei R, Buchfelder M, Savaskan N. Supra-complete surgery via dual intraoperative visualization approach (DiVA) prolongs patient survival in glioblastoma. Oncotarget. 2016;7(18):25755-25768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Dewan MC, White-Dzuro GA, Brinson PR, Thompson RC, Chambless LB. Perioperative seizure in patients with glioma is associated with longer hospitalization, higher readmission, and decreased overall survival. J Neurosurg. 2016;125(4):1033-1041. [DOI] [PubMed] [Google Scholar]
- 100. Chen LF, Yang Y, Ma XDet al. Optimizing the extent of resection and minimizing the morbidity in insular high-grade glioma surgery by high-field intraoperative MRI guidance. Turk Neurosurg. 2017;27(5):696-706. [DOI] [PubMed] [Google Scholar]
- 101. Yang P, Liang T, Zhang Cet al. Clinicopathological factors predictive of postoperative seizures in patients with gliomas. Seizure. 2016;35:93-99. [DOI] [PubMed] [Google Scholar]
- 102. Meguins LC, Adry RA, da Silva Júnior SCet al. Gross-total resection of temporal low grade gliomas is a critically important factor in achieving seizure-freedom. Arq Neuropsiquiatr. 2015;73(11):924-928. [DOI] [PubMed] [Google Scholar]
- 103. Kemerdere R, Yuksel O, Kacira Tet al. Low-grade temporal gliomas: surgical strategy and long-term seizure outcome. Clin Neurol Neurosurg. 2014;126:196-200. [DOI] [PubMed] [Google Scholar]
- 104. Ius T, Pauletto G, Isola Met al. Surgery for insular low-grade glioma: predictors of postoperative seizure outcome. J Neurosurg. 2013;120(1):12-23. [DOI] [PubMed] [Google Scholar]
- 105. Kim J, Radjadurai S, Rahman Zet al. Outcomes of tumour related epilepsy in a specialised epilepsy surgery unit. J Clin Neurosci. 2019;59:265-269. [DOI] [PubMed] [Google Scholar]
- 106. Tanriverdi T, Kemerdere R, Baran Oet al. Long-term surgical and seizure outcomes of frontal low-grade gliomas. Int J Surg. 2016;33:60-64. [DOI] [PubMed] [Google Scholar]
- 107. Devaux B, Chassoux F, Landré Eet al. Surgery for dysembryoplastic neuroepithelial tumors and gangliogliomas in eloquent areas. Functional results and seizure control. Neurochirurgie. 2017;63(3):227-234. [DOI] [PubMed] [Google Scholar]
- 108. Ening G, Osterheld F, Capper D, Schmieder K, Brenke C. Risk factors for glioblastoma therapy associated complications. Clin Neurol Neurosurg. 2015;134:55-59. [DOI] [PubMed] [Google Scholar]
- 109. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 110. Meyers CA, Wefel JS.. The use of the mini-mental state examination to assess cognitive functioning in cancer trials: no ifs, ands, buts, or sensitivity. J Clin Oncol. 2003;21(19):3557-3558. [DOI] [PubMed] [Google Scholar]
- 111. Tombaugh TN, McIntyre NJ.. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922-935. [DOI] [PubMed] [Google Scholar]
- 112. Mioshi E, Dawson K, Mitchell J, Arnold R, Hodges JR. The Addenbrooke's Cognitive Examination Revised (ACE-R): a brief cognitive test battery for dementia screening. Int J Geriatr Psychiatry. 2006;21(11):1078-1085. [DOI] [PubMed] [Google Scholar]
- 113. Nasreddine ZS, Phillips NA, Bédirian Vet al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695-699. [DOI] [PubMed] [Google Scholar]
- 114. Randolph C, Tierney MC, Mohr E, Chase TN. The Repeatable Battery for the Assessment of Neuropsychological Status (RBANS): preliminary clinical validity. J Clin Exp Neuropsychol. 1998;20(3):310-319. [DOI] [PubMed] [Google Scholar]
- 115. Roth C. Boston diagnostic aphasia examination. In: Kreutzer J, Deluca J, Caplan B, eds. Encyclopedia of Clinical Neuropsychology. 3rd edn. New York, NY: Springer; 2011:428-430. [Google Scholar]
- 116. Nicholas LE, Brookshire RH, MacLennan DL, Schumacher JG, Porrazzo SA. The Boston Naming Test: revised administration and scoring procedures and normative information for non-brain-damaged adults. Clin Aphasiology. 1988;18:103-115. [Google Scholar]
- 117. De Witte E, Satoer D, Robert Eet al. The dutch linguistic intraoperative protocol: a valid linguistic approach to awake brain surgery. Brain Lang. 2015;140:35-48. [DOI] [PubMed] [Google Scholar]
- 118. Metz M, Metz-Lutz M, Kremin Het al. Standardisation d’un test de dénomination orale: contrôle des effets de l’âge, du sexe et du niveau de scolarité chez les sujets adultes normaux. Rev Neuropsychol. 1991;1:73-95. [Google Scholar]
- 119. D’Andrea G, Familiari P, Di Lauro A, Angelini A, Sessa G. Safe resection of gliomas of the dominant angular gyrus availing of preoperative fMRI and intraoperative DTI: preliminary series and surgical technique. World Neurosurg. 2016;87:627-639. [DOI] [PubMed] [Google Scholar]
- 120. Antonsson M, Jakola A, Longoni Fet al. Post-surgical effects on language in patients with presumed low-grade glioma. Acta Neurol Scand. 2018;137(5):469-480. [DOI] [PubMed] [Google Scholar]
- 121. Puglisi G, Sciortino T, Rossi M, Bello L. Preserving executive functions in non-dominant frontal lobe glioma surgery: an intraoperative tool. J Neurosurg. 2018;131(2):474-480. [DOI] [PubMed] [Google Scholar]
- 122. Hoffermann M, Bruckmann l, Mahdy Ali K, Zaar K, Avian A, von Campe G. Pre- and postoperative neurocognitive deficits in brain tumor patients assessed by a computer based screening test. J Clin Neurosci. 2017;36:31-36. [DOI] [PubMed] [Google Scholar]
- 123.[•] Hendriks EJ, Habets EJJ, Taphoorn MJBet al. Linking late cognitive outcome with glioma surgery location using resection cavity maps. Hum Brain Mapp. 2018;39(5):2064-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.[•] Habets EJJ, Kloet A, Walchenbach R, Vecht CJ, Klein M, Taphoorn MJB. Tumour and surgery effects on cognitive functioning in high-grade glioma patients. Acta Neurochir (Wien). 2014;156(8):1451-1459. [DOI] [PubMed] [Google Scholar]
- 125.[•] Satoer D, Visch-Brink E, Smits Met al. Long-term evaluation of cognition after glioma surgery in eloquent areas. J Neurooncol. 2014;116(1):153-160. [DOI] [PubMed] [Google Scholar]
- 126. Campanella F, Palese A, Del Missier Fet al. Long-term cognitive functioning and psychological well-being in surgically treated patients with low-grade glioma. World Neurosurg. 2017;103:799-808.e9. [DOI] [PubMed] [Google Scholar]
- 127.[••] Ng JCH, See AAQ, Ang TY, Tan LYR, Ang BT, King NKK. Effects of surgery on neurocognitive function in patients with glioma: a meta-analysis of immediate post-operative and long-term follow-up neurocognitive outcomes. J Neurooncol. 2019;141(1):167-182. [DOI] [PubMed] [Google Scholar]
- 128. Rijnen SJM, Sitskoorn MM, Gehring K. Comment on: effects of surgery on neurocognitive function in patients with glioma: a meta-analysis of immediate post-operative and long-term follow-up neurocognitive outcomes. J Neurooncol. 2019;143(1):175-176. [DOI] [PubMed] [Google Scholar]
- 129.[••] Habets EJJ, Taphoorn MJB, Klein M, Vissers T, Dirven L. The level of reporting of neurocognitive outcomes in randomised controlled trials of brain tumour patients: a systematic review. Eur J Cancer. 2018;100:104-125. [DOI] [PubMed] [Google Scholar]
- 130.[•] Satoer D, Visch-Brink E, Dirven C, Vincent A. Glioma surgery in eloquent areas: can we preserve cognition? Acta Neurochir (Wien). 2016;158(1):35-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.[•] Klein M, Duffau H, De Witt Hamer PC. Cognition and resective surgery for diffuse infiltrative glioma: an overview. J Neurooncol. 2012;108(2):309-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.[•] Rofes A, Mandonnet E, Godden Jet al. Survey on current cognitive practices within the european low-grade glioma network: towards a european assessment protocol. Acta Neurochir (Wien). 2017;159(7):1167-1178. [DOI] [PubMed] [Google Scholar]
- 133.[••] Lin NU, Wefel JS, Lee EQet al. Challenges relating to solid tumour brain metastases in clinical trials, part 2: neurocognitive, neurological, and quality-of-life outcomes. A report from the RANO group. Lancet Oncol. 2013;14(10):e407-e416. [DOI] [PubMed] [Google Scholar]
- 134.[••] Wefel JS, Vardy J, Ahles T, Schagen SB. International cognition and cancer task force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12(7):703-708. [DOI] [PubMed] [Google Scholar]
- 135.[••] Van den Bent MJ, Wefel JS, Schiff Det al. Response Assessment in Neuro-Oncology (a report of the RANO group): assessment of outcome in trials of diffuse low-grade gliomas. Lancet Oncol. 2011;12(6):583-593. [DOI] [PubMed] [Google Scholar]
- 136.[••] Alexander BM, Brown PD, Ahluwalia MSet al. Clinical trial design for local therapies for brain metastases: a guideline by the Response Assessment in Neuro-Oncology Brain Metastases Working Group. Lancet Oncol. 2018;19(1):e33-e42. [DOI] [PubMed] [Google Scholar]
- 137.[••] Camidge DR, Lee EQ, Lin NUet al. Clinical trial design for systemic agents in patients with brain metastases from solid tumours: a guideline by the response assessment in Neuro-Oncology Brain Metastases working group. Lancet Oncol. 2018;19(1):e20-e32. [DOI] [PubMed] [Google Scholar]
- 138. Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5th edn. Oxford, UK: Oxford University Press; 2012. [Google Scholar]
- 139. Aaronson NK. Quality of life: what is it? How should it be measured? Oncology. 1988;2(5):69-76. [PubMed] [Google Scholar]
- 140. Aaronson NK, Ahmedzai S, Bergman Bet al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. [DOI] [PubMed] [Google Scholar]
- 141. Osoba D, Aaronson NK, Muller Met al. The development and psychometric validation of a brain cancer quality-of-life questionnaire for use in combination with general cancer-specific questionnaires. Qual Life Res. 1996;5(1):139-150. [DOI] [PubMed] [Google Scholar]
- 142. Fayers P, Bottomley A, EORTC Quality of Life Group, Quality of Life Unit. Quality of Life Research within the EORTC-the EORTC QLQ-C30. European Organisation for Research and Treatment of Cancer. Eur J Cancer. 2002;38(Suppl 4):S125-S133. [DOI] [PubMed] [Google Scholar]
- 143. Group EQ. EuroQol–a new facility for the measurement of health-related quality of life. Health Policy. 1990;16(3):199-208. [DOI] [PubMed] [Google Scholar]
- 144. Janssen MF, Pickard AS, Golicki Det al. Measurement properties of the EQ-5D-5L compared to the EQ-5D-3L across eight patient groups: a multi-country study. Qual Life Res. 2013;22(7):1717-1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Armstrong TS, Mendoza T, Gning Iet al. Validation of the M.D. Anderson Symptom Inventory Brain Tumor module (MDASI-BT). J Neurooncol. 2006;80(1):27-35. [DOI] [PubMed] [Google Scholar]
- 146. Weitzner MA, Meyers CA, Gelke CK, Byrne KS, Cella DF, Levin VA. The Functional Assessment of Cancer Therapy (FACT) scale. Development of a brain subscale and revalidation of the general version (FACT-G) in patients with primary brain tumors. Cancer. 1995;75(5):1151-1161. [DOI] [PubMed] [Google Scholar]
- 147. Goffaux P, Boudrias M, Mathieu D, Charpentier C, Veilleux N, Fortin D. Development of a concise QOL questionnaire for brain tumor patients. Can J Neurol Sci. 2009;36(3):340-348. [DOI] [PubMed] [Google Scholar]
- 148. Rooney AG, Netten A, McNamara Set al. Assessment of a brain-tumour-specific patient concerns inventory in the neuro-oncology clinic. Support Care Cancer. 2014;22(4):1059-1069. [DOI] [PubMed] [Google Scholar]
- 149. Lin L, Acquaye AA, Vera-Bolanos E, Cahill JE, Gilbert MR, Armstrong TS. Validation of the Mishel's uncertainty in illness scale-brain tumor form (MUIS-BT). J Neurooncol. 2012;110(2):293-300. [DOI] [PubMed] [Google Scholar]
- 150. Cella D, Yount S, Rothrock Net al. The Patient-Reported Outcomes Measurement Information System (PROMIS): progress of an NIH roadmap cooperative group during its first two years. Med Care. 2007;45(5 Suppl 1):S3-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Cella D, Lai J-S, Nowinski CJet al. Neuro-QOL: brief measures of health-related quality of life for clinical research in neurology. Neurology. 2012;78(23):1860-1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Stöckelmaier L, Renovanz M, König Jet al. Therapy for recurrent high-grade gliomas: results of a prospective multicenter study on health-related quality of life. World Neurosurg. 2017;102:383-399. [DOI] [PubMed] [Google Scholar]
- 153. Nickel K, Renovanz M, König Jet al. The patients’ view: impact of the extent of resection, intraoperative imaging, and awake surgery on health-related quality of life in high-grade glioma patients—results of a multicenter cross-sectional study. Neurosurg Rev. 2018;41(1):207-219. [DOI] [PubMed] [Google Scholar]
- 154. Jakola AS, Unsgård G, Myrmel KSet al. Surgical strategies in low-grade gliomas and implications for long-term quality of life. J Clin Neurosci. 2014;21(8):1304-1309. [DOI] [PubMed] [Google Scholar]
- 155. Suchorska B, Weller M, Tabatabai Get al. Complete resection of contrast-enhancing tumor volume i s associated with improved survival in recurrent glioblastoma - results from the DIRECTOR trial. Neuro Oncol. 2016;18(4):549-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Jakola AS, Sagberg LM, Gulati S, Solheim O. Perioperative quality of life in functionally dependent glioblastoma patients: a prospective study. Br J Neurosurg. 2015;29(6):843-849. [DOI] [PubMed] [Google Scholar]
- 157. Sagberg LM, Solheim O, Jakola AS. Quality of survival the 1st year with glioblastoma: a longitudinal study of patient-reported quality of life. J Neurosurg. 2015;124(4):989-997. [DOI] [PubMed] [Google Scholar]
- 158.[•] Drewes C, Sagberg LM, Jakola AS, Solheim O. Perioperative and postoperative quality of life in patients with glioma—a longitudinal cohort study. World Neurosurg. 2018;117:e465-e474. [DOI] [PubMed] [Google Scholar]
- 159. Hewins W, Zienius K, Rogers JL, Kerrigan S, Bernstein M, Grant R. The effects of brain tumours upon medical decision-making capacity. Curr Oncol Rep. 2019;21(6):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Kerrigan S, Erridge S, Liaquat I, Graham C, Grant R. Mental incapacity in patients undergoing neuro-oncologic treatment: a cross-sectional study. Neurology. 2014;83(6):537-541. [DOI] [PubMed] [Google Scholar]
- 161. Buccheri G, Ferrigno D, Tamburini M. Karnofsky and ECOG Performance Status scoring in lung cancer: a prospective, longitudinal study of 536 patients from a single institution. Eur J Cancer. 1996;32A(7):1135-1141. [DOI] [PubMed] [Google Scholar]
- 162. Schwartz CE, Bode R, Repucci N, Becker J, Sprangers MAG, Fayers PM. The clinical significance of adaptation to changing health: a meta-analysis of response shift. Qual Life Res. 2006;15(9):1533-1550. [DOI] [PubMed] [Google Scholar]
- 163. Ilie G, Bradfield J, Moodie Let al. The role of response-shift in studies assessing quality of life outcomes among cancer patients: a systematic review. Front Oncol. 2019;9:783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.[•] Jakola AS, Skjulsvik AJ, Myrmel KSet al. Surgical resection versus watchful waiting in low-grade gliomas. Ann Oncol. 2017;28(8):1942-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Sizoo EM, Pasman HRW, Buttolo Jet al. Decision-making in the end-of-life phase of high-grade glioma patients. Eur J Cancer. 2012;48(2):226-232. [DOI] [PubMed] [Google Scholar]
- 166. Ediebah DE, Reijneveld JC, Taphoorn MJBet al. Impact of neurocognitive deficits on patient–proxy agreement regarding health-related quality of life in low-grade glioma patients. Qual Life Res. 2017;26(4):869-880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Lonkhuizen PJC, Klaver KM, Wefel JS, Sitskoorn MM, Schagen SB, Gehring K. Interventions for cognitive problems in adults with brain cancer: a narrative review. Eur J Cancer Care (Engl). 2019;28(3):1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.[••] Gehring K, Sitskoorn MM, Gundy CMet al. Cognitive rehabilitation in patients with gliomas: a randomized, controlled trial. J Clin Oncol. 2009;27(22):3712-3722. [DOI] [PubMed] [Google Scholar]
- 169. Rapp SR, Case LD, Peiffer Aet al. Donepezil for irradiated brain tumor survivors: a phase III randomized placebo-controlled clinical trial. J Clin Oncol. 2015;33(15):1653-1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Jakola AS, Unsgård G, Solheim O. Quality of life in patients with intracranial gliomas: the impact of modern image-guided surgery. J Neurosurg. 2011;114(6):1622-1630. [DOI] [PubMed] [Google Scholar]
- 171.[•] van Loenen IS, Rijnen SJM, Bruijn J, Rutten GJM, Gehring K, Sitskoorn MM. Group changes in cognitive performance after surgery mask changes in individual patients with glioblastoma. World Neurosurg. 2018;117:e172-e179. [DOI] [PubMed] [Google Scholar]
- 172. Guhn M, Forer B, Zumbo BD. Reliable change index. In: Michalos A, ed. Encyclopedia of Quality of Life and Well-Being Research. Dordrecht: Springer; 2014:5459-5462. [Google Scholar]
- 173. Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27(3):248-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci. 2014;15(10):683-695. [DOI] [PubMed] [Google Scholar]
- 175. Zhou X, Menche J, Barabási A-L, Sharma A. Human symptoms-disease network. Nat Commun. 2014;5:4212. [DOI] [PubMed] [Google Scholar]
- 176. Barabási A-L. Network medicine—from obesity to the “diseasome”. N Engl J Med. 2007;357(4):404-407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.