Abstract
Objectives
To study the impact of diabetes on the long-term cognitive trajectories of older adults in 2 countries with different socioeconomic and health settings, and to determine whether this relationship differs by cognitive domains. This study uses Mexico and the United States to confirm if patterns hold in both populations, as these countries have similar diabetes prevalence but different socioeconomic conditions and diabetes-related mortality.
Methods
Two nationally representative cohorts of adults aged 50 years or older are used: the Mexican Health and Aging Study for Mexico and the Health and Retirement Study for the United States, with sample sizes of 18,810 and 26,244 individuals, respectively, followed up for a period of 14 years. The outcome is cognition measured as a total composite score and by domain (memory and nonmemory). Mixed-effect linear models are used to test the effect of diabetes on cognition at 65 years old and over time in each country.
Results
Diabetes is associated with lower cognition and nonmemory scores at baseline and over time in both countries. In Mexico, diabetes only predicts lower memory scores over time, whereas in the United States it only predicts lower memory scores at baseline. Women have higher total cognition and memory scores than men in both studies. The magnitude of the effect of diabetes on cognition is similar in both countries.
Discussion
Despite the overall lower cognition in Mexico and different socioeconomic characteristics, the impact of diabetes on cognitive decline and the main risk and protective factors for poor cognition are similar in both countries.
Keywords: Cognitive aging, Cross-cultural study, Longitudinal methods
The risk of cognitive decline increases with age. As the world population older than 60 years is expected to almost double from 12.3% in 2015 to 21.5% by 2050, cognitive health has become a public health challenge (United Nations, 2015). By 2050, the number of people living with dementia is also expected to more than double, from 46.8 million to 131.5 million people (Prince et al., 2015).
There is abundant population-level evidence that diabetes is associated with greater cognitive decline (Biessels et al., 2006; Cheng et al., 2012). Furthermore, there is evidence that the micro- and macrovascular damage caused by hyperglycemia is associated with the brain infarctions and decreased blood flow to the brain that are present in vascular dementia (McCrimmon et al., 2012); and that insulin resistance is associated with the metabolism of β-amyloid plaques present in Alzheimer’s disease (Biessels et al., 2006).
Although there is established evidence that diabetes is associated with poorer cognition, it is important to inquire if this relationship differs depending on the socioeconomic and cultural context in which individuals age. This is because older adults with poor socioeconomic status (SES) across the life course are at a greater risk for poor cognition in later life (Everson-Rose et al., 2003; Singh-Manoux et al., 2005) and the burden of diabetes, especially diabetes-related mortality and disease management are strongly related to the socioeconomic resources, education and access to care, which differ by setting.
Thus, we address the question: How universal is the impact of diabetes on cognition in old age? One way to answer this question is to test this relationship in two countries. Two representative samples in different countries provide a natural experiment to assess if differences in the socioeconomic and health context in which older adults lived over their life course affect this relationship.
Mexico and the United States are good examples of two countries to study this relationship. First, these countries have very different contexts. While they are geographically close, they differ in the current stages of the demographic and epidemiologic transition and in socioeconomic conditions (He et al., 2015). In Mexico, population aging occurs at a faster pace than in the United States. In 2015, almost 7% of the Mexican population and 14.9% of the U.S. population were older than 65 years (He et al., 2015). However, while it took 69 years for the older adult population in the United States to double from 7% to 14%, projections show that it will take less than 35 years for the population in Mexico to do the same, reaching 18% by 2050 (He et al., 2015).
In the United States, the slower and constant pace of aging was accompanied by social development. The slow demographic transition guaranteed time to structure pensions and the health care system to better address the needs of older adults. In contrast, the health care system and pension system in Mexico are not as ready to accommodate a large and fast-growing older population. Furthermore, those in Mexico aged in a context of poor infrastructure for education (Organisation for Economic Cooperation and Development [OECD], 2013), and an increased burden of both chronic and infectious diseases that current older adults in the United States did not experience as they aged (Samper-Ternent et al., 2012).
Second, while diabetes prevalence in Mexico is similar to that in the United States—nearly 25% in 2014 (Centers for Disease Control and Prevention [CDC], 2014; Subsecretaría de Integración y Desarrollo del Sector Salud, 2015), diabetes-related mortality is higher in Mexico. In Mexico, diabetes is the second leading cause of death with 86 deaths per 100,000 in 2017 (Instituto Nacional de Estadistica y Geografia [INEGI], 2018), whereas in the United States it is the seventh leading cause of death with 21.5 per 100,000 in 2017 (Murphy et al., 2018). This difference in the diabetes burden may be associated with late diagnosis and poor disease management in Mexico, as only 9.6% of adults aged 20 years and older with diabetes in Mexico reported having their HbA1C checked in the past year (Gutiérrez et al., 2012), compared to 72.8% in the United States (CDC, 2015).
Given these contexts, we examine if the association between diabetes and cognition holds in both countries, although the possible life course or current contextual factors that may contribute or explain the differences are beyond the scope of the article. The objective of this study was to examine the impact of diabetes on the cognitive trajectories of older adults in Mexico and the United States, and we explore if this relationship differs across cognitive domains. The first hypothesis is that diabetes will be associated with lower baseline cognition and a greater decline over time in both countries. The second hypothesis is that diabetes will be significantly associated with a steeper decline in both verbal memory and nonmemory in both countries.
The third hypothesis refers to the difference between Mexico and the United States in the strength of the association between diabetes and cognitive decline. We expect that older Mexican adults will have steeper cognitive decline associated with diabetes compared to those in the United States. The diabetes burden in Mexico may negatively affect the cognitive trajectory of older adults to a greater extent when compared with the United States. This is because relatively poor diabetes management in the Mexican population also increases vascular risk and the risk for diabetes-related comorbidities, adding to the risk of cognitive decline in the presence of diabetes.
Method
Data Sets
The Health and Retirement Study (HRS) was used to study the United States. This is a nationally representative longitudinal cohort of older Americans 50 years and older. The HRS has a comprehensive questionnaire that covers topics such as demographics, health conditions, cognition, disability, family structure and relationships, widowhood, and socioeconomic factors, among others. HRS participants have been followed up biannually since 1992. For this analysis, the 2000, 2002, 2012, and 2014 HRS waves were used in order to maximize comparability with the Mexican data. The response rates for these waves were 85.4%, 86.6%, 89.6%, and 87.9%, respectively. The HRS adds a new cohort every 6 years. Thus, two cohorts were added between the waves selected: the early baby boomers in 2004, born 1942–1947 and the mid baby boomers in 2010, born 1954–1959 (Sonnega et al., 2014). The 2014 RAND HRS longitudinal file was used in this study. This is a longitudinal file of the HRS merged and managed by RAND Corporation to facilitate data analysis and data set comparability with other HRS sister studies. The RAND HRS fat files for each wave were used to add some variables that were not available in the longitudinal file (Bugliari et al., 2018).
The Mexican Health and Aging Study (MHAS) was used to study Mexico. This study is highly comparable to the HRS in its study design, sampling procedures, and questionnaire, making cross-national comparisons easier. The MHAS is a nationally representative longitudinal cohort of community-dwelling older Mexican adults 50 years and older. The cohort has been followed in 2001, 2003, 2012, and 2015. The response rate for each wave was 91.8%, 93.3%, 88.1%, and 88.3%, respectively (INEGI, 2016; Wong et al., 2017). In 2012, a sample of 5,896 individuals born between 1952 and 1962 was added. All waves were used for this analysis.
Sample Selection Criteria
The sample was restricted to older adults 50 and older in the MHAS and in the HRS, with at least one direct interview, and at least one assessment of cognition and self-reported diabetes. Individuals who required a proxy interview at every wave were excluded because the cognitive measures in the proxy interview are not comparable with those in the direct interview. Furthermore, individuals living in a nursing home at the time of the interview were excluded from the sample. In the MHAS, individuals with all proxy interviews were on average 10 years older and were more likely to have lower levels of education, to self-report diabetes, and to be male. The proxy interviews in the HRS were similar to those in the MHAS. Participants living in nursing homes were older, less educated, and more likely to have diabetes than those living in the community.
The final sample size of individuals followed at least one time was 18,810 in the MHAS and 26,244 in the HRS. See Supplementary Appendix Figures 1 and 2 for sample selection criteria. Given that a complete case analysis was conducted for the longitudinal analysis, some observations that met the sample selection criteria were excluded from the longitudinal analysis as they were missing values for at least one covariate. The sample that was excluded comprised 179 individuals (who contributed 630 observations over time) in the HRS and 281 individuals (who contributed 1,057 observations over time) in the MHAS. In the HRS, those missing did not differ in diabetes status but had lower cognition than those who remained in the sample. In the MHAS, those missing were less likely to have diabetes but had lower cognition than those who remained in the sample.
Dependent Variable
The outcome was cognition, measured in each wave by a total standardized cognition score and by two separate cognitive domains (verbal memory and nonmemory) in each study. Cognitive domains are defined by a grouping of several neuropsychologic tasks to assess cognitive function depending on the well-established brain function and skills they measure, such as orientation, registration, attention and calculation, recall, language, and visuospatial ability There is no specific number of tasks that comprise one domain, but one domain is composed of several tasks.
The cognitive assessments in both studies show good validity and reliability to measure cognitive impairment (Michaels-Obregon et al., 2014; Ofstedal et al., 2005). However, in the HRS, there is evidence that non-Hispanic whites (NHWs) perform the tests better than non-Hispanic blacks (NHBs) and Hispanics, and this difference needs to be considered when interpreting the results (Ofstedal et al., 2005). Missing values and nonresponse for cognition have been imputed by the MHAS and HRS study groups. See Supplementary Appendix Table 1 for the description of the cognitive measures in the HRS and MHAS.
In the HRS, the total cognition score was calculated as the average of four standardized tasks measured with a modified version of the Telephone Interview for Cognitive Status: verbal learning, verbal recall, backward count, and serial 7s. The verbal memory domain score was calculated as the average of two standardized tasks: verbal learning and verbal recall. The nonmemory domain score was calculated as the average of two standardized tasks: backward count and serial 7s scores.
In the MHAS, the total cognition score was calculated as the average of four standardized tasks measured with a modified version of the Cross-Cultural Cognitive Examination: verbal learning, verbal recall, visuospatial ability, and visual scanning. The verbal memory domain score was calculated as the average of two standardized tasks: verbal learning and verbal recall. The nonmemory domain score was calculated as the average of two standardized tasks: visuospatial ability and visual scanning.
In both countries, each task score was standardized due to different ranges of scores across tasks because some tasks would contribute more to the overall score than others, and a lower score in a larger-score task would mean an overall lower cognitive score. Furthermore, the ranges of scores would be different between data sets. Tasks were standardized using the sample mean and standard deviation, so that each task had the same weight to the total cognition score, following other studies with a compositive cognitive score (Kaffashian et al., 2013; Wilson et al., 2015).
Independent Variables
Time-varying self-reported diabetes status was the main independent variable in both studies. Respondents in both studies were asked: “Has a doctor or other medical professional ever/in the past two years told you that you have diabetes?” with answers being yes or no. However, in the MHAS, there were inconsistencies where individuals said they had diabetes in one wave and reported not having diabetes in a subsequent wave. For this reason, individuals who said they had diabetes at least 2 times in the MHAS were considered as having diabetes. As underdiagnosis of diabetes is relatively high (nearly 18% in a subsample of the MHAS 2012; Kumar et al., 2016), we believe that those who said they had diabetes at least 2 times are likely to indeed have diabetes. This recording was only done for a conceptual reason, as a sensitivity analysis revealed that there were no differences in the longitudinal results whether the original diabetes variable or the recorded one was used.
Covariates
Demographic covariates common to both data sets included age and marital status (married, widowed, and other) as time-varying variables and baseline sex. Socioeconomic covariates included insurance status (uninsured and insured) as a time-varying variable and baseline years of education. Health covariates included time-varying chronic diseases such as self-reported stroke, hypertension, and high depressive symptoms. High depressive symptoms were defined as five or more depressive symptoms in the MHAS or four or more depressive symptoms in the HRS; self-reported height and weight for body mass index (BMI: obese if BMI ≥30, not obese if BMI <30); and visits to the doctor in the previous 2 years (yes, no). Death (yes, no) and loss to follow-up not due to death (yes, no) were also included as time-varying covariates. We used the BMI values imputed by the MHAS study group to minimize missing values.
Data set-specific covariates were included to capture additional sources of heterogeneity and socioeconomic or health disparities that are specific to each country. In Mexico, one of the main factors associated with inequalities is differences between rural and urban areas, thus in the country-specific analysis for Mexico, a time-varying variable measuring locality size was included to account for these differences (less urban [population <100,000] or more urban [population ≥100,000]). In the HRS, rural/urban differences are not as pronounced and one of the main sources of disparities occur by race/ethnicity. On the other hand, race/ethnicity is not a factor for heterogeneity in Mexico, and the MHAS does not measure ethnic differences. Thus, baseline race/ethnicity was included as an additional covariate for the United States to account for these differences (NHB, NHW, and Hispanic).
Statistical Analysis
Baseline analysis
Baseline data from MHAS 2001 and HRS 2000 were pooled, and weighted baseline characteristics were compared between data sets using a chi-square test. This was the only pooled analysis conducted in this study. Cognition was not compared directly between data sets in any of the data analyses because the cognition score can not be fully harmonized due to differences in the mode of interview and cognitive measures included in each study. We instead test the same association within each country. Results were weighted using survey weights provided by the MHAS and HRS to account for each country’s sampling design (including stratum and cluster differences in the HRS).
Longitudinal analysis
Unweighted mixed-effects linear regression was used to model the association between self-reported diabetes and cognitive trajectory in each country. This analysis accounts for within-person variation over time and between-person variations. Age, centered at 65 years old, was defined as the time variable because most cognitive decline occurs after this age (Rabe-Hesketh & Skrondal, 2012). All models included a random intercept and random slope for age, and the covariance matrix was unstructured.
First, similar models were fit for the MHAS and the HRS, including common demographic and health covariates available in both studies. These models were conducted for the total standardized cognition score and by domain. The models included all main effects and the interaction between diabetes and age, in order to determine the annual change in the cognitive score by diabetes status.
Because the cognition score is a standardized variable (z-score), the range of scores is very low and the coefficients appear to be very small. Thus, in order to better interpret the relative magnitude of the effect of diabetes on cognition in each country, predictive margins of cognition were estimated, holding the other covariates at their means. These estimates were re-scaled to express the effect of diabetes on cognition in terms of the equivalent years of education. This did not change the results of the analysis but allowed us to reinterpret the effect of diabetes on cognition at 65 years old and over time.
Second, study-specific analyses were conducted using the total standardized cognition score, to examine the effect of locality size in the MHAS and race/ethnicity in the HRS. The MHAS model included all main effects and the three-way interaction between locality size, diabetes, and age. The HRS models included all main effects and the three-way interaction between race/ethnicity, diabetes, and age.
All analyses were conducted with STATA 14 (College Station, TX).
Results
Baseline Characteristics
Older adults in the HRS were more likely to be female and, on average, were 3.1 years older than those in the MHAS (Table 1). Those in the MHAS had, on average, a third the years of education of those in the HRS (4.00 years vs. 12.45 years, p < .001) and were almost 10 times more likely to be uninsured than those in the HRS (45.3% vs. 4.9%, p < .001). There was no difference in diabetes prevalence between the two samples, but those in the HRS were more likely to have two or more comorbidities (21.7% vs. 18.3%, p < .001) and to have obesity (25.6% vs. 19.1%, respectively, p < .001). Older adults in the HRS were also more likely to say they had visited a doctor in the past 2 years (93.9% vs. 63.2%, p < .001; Table 1). The other demographic and health characteristics were largely similar in the two countries. In the MHAS, 53.6% of the older adults lived in less urban areas. In the HRS, 9.3% of older adults were NHB and only 6.5% were Hispanic.
Table 1.
Comparison of Baseline Demographic and Health Characteristics Between the 2001 Mexican Health and Aging Study and the 2000 Health and Retirement Study
| MHAS 2001 (N = 13,186) | HRS 2000 (N = 16,661) | p Valuea | |
|---|---|---|---|
| Sex (%) | |||
| Male | 44.4 | 47.0 | .001 |
| Female | 55.6 | 53.0 | |
| Age, mean (SD) | 62.4 (14.6) | 65.5 (8.2) | <.001 |
| Marital status (%) | |||
| Married/partner | 67.0 | 65.5 | .02 |
| Widowed | 19.0 | 18.4 | |
| Other | 14.0 | 16.1 | |
| Years of education, mean (SD) | 4.00 (6.5) | 12.45 (2.6) | <.001 |
| Insurance status (%) | |||
| Uninsured | 45.4 | 4.9 | <.001 |
| Insured | 54.6 | 95.1 | |
| Diabetes (%) | |||
| No | 87.0 | 87.1 | .9 |
| Yes | 13.0 | 12.9 | |
| Comorbidities (%) | |||
| 0 | 44.1 | 40.7 | <.001 |
| 1 | 37.5 | 37.6 | |
| 2+ | 18.3 | 21.7 | |
| Body mass index (%) | |||
| Not obese | 80.9 | 74.4 | <.001 |
| Obese | 19.1 | 25.6 | |
| Visited the doctor in the past 2 years (%) | |||
| No | 36.8 | 6.1 | <.001 |
| Yes | 63.2 | 93.9 | |
| Place of residence, MHAS (%) | |||
| Less urban | 53.6 | NA | — |
| More urban | 46.4 | ||
| Race and ethnicity, HRS (%) | |||
| Non-Hispanic white | NA | 84.2 | — |
| Non-Hispanic black | 9.3 | ||
| Hispanic | 6.5 |
Notes: HRS = Health and Retirement Study; MHAS = Mexican Health and Aging Study; SD = standard deviation. Results were weighted according to each country’s sampling design.
aDifference in the chi-square test between baseline waves of MHAS and HRS at α ≤ 0.05.
Longitudinal Analysis
In the MHAS, those with diabetes had a significantly lower total standardized cognition score at 65 years old compared to those without diabetes (β: −0.03, 95% confidence interval [CI]: −0.05 to −0.02). Diabetes was also associated with a significantly greater decline over time in the total standardized cognition score (β: −0.004, 95% CI: −0.01 to −0.002; Table 2). The analysis by domain showed that, at 65 years old, diabetes was only associated with lower scores in the nonmemory domain (β: −0.05, 95% CI: −0.06 to −0.03). However, over time, diabetes predicted lower scores in both the verbal memory and nonmemory domains (β: −0.004, 95% CI: −0.01 to −0.002 and β: −0.004, 95% CI: −0.01 to −0.002, respectively; Table 2).
Table 2.
Linear Mixed-Effect Models for the Effect of Diabetes Status on Standardized Cognitive Scores, Total and by Domain, at Baseline and Over Time, Mexican Health and Aging Study and the Health and Retirement Study
| Total cognition, β (95% CI) | p Value | Verbal memory domain, β (95% CI) | p Value | Nonmemory domain, β (95% CI) | p Value | |
|---|---|---|---|---|---|---|
| MHAS | ||||||
| Diabetes at 65 years old | ||||||
| No | Ref. | Ref. | Ref. | |||
| Yes | −0.03 (−0.05 to −0.02) | <.001 | −0.02 (−0.04 to 0.002) | .08 | −0.05 (−0.06 to −0.03) | <.001 |
| Annual decline (Diabetes × Age) | ||||||
| No diabetes | Ref. | Ref. | Ref. | |||
| Yes diabetes | −0.004 (−0.01 to −0.002) | <.001 | −0.004 (−0.01 to −0.002) | <.001 | −0.004 (−0.01 to −0.002) | <.001 |
| HRS | ||||||
| Diabetes at 65 years old | ||||||
| No | Ref. | Ref. | Ref. | |||
| Yes | −0.05 (−0.06 to −0.04) | <.001 | −0.06 (−0.08 to −0.04) | <.001 | −0.04 (−0.06 to −0.03) | <.001 |
| Annual decline (Diabetes × Age) | ||||||
| No diabetes | Ref. | Ref. | Ref. | |||
| Yes diabetes | −0.002 (−0.003 to −0.001) | .002 | −0.002 (−0.044 to 0.00) | .06 | −0.002 (−0.004 to −0.0004) | .02 |
Notes: CI = confidence interval; HRS = Health and Retirement Study; MHAS = Mexican Health and Aging Study. Also adjusted for sex, age centered at 65 years old, marital status, education, insurance status, comorbidities, body mass index, physician visit, death, loss to follow-up, and study cohort. All models included a random intercept and random slope for age.
Old age and having comorbidities such as stroke and high depressive symptoms were associated with lower cognition scores across the three outcomes (total cognition and both domains). On the other hand, having more years of education and health insurance, as well as having obesity were associated with higher cognition scores across the three outcomes. Women were more likely to have higher cognition scores for total cognition and verbal memory, but lower nonmemory scores compared to men (Supplementary Appendix Table 2).
In the HRS, those with diabetes had a significantly lower total standardized cognition score at 65 years old compared to those without diabetes (β: −0.05, 95% CI: −0.06 to −0.04). Diabetes was also associated with a greater decline over time (β: −0.002, 95% CI: −0.003 to −0.001; Table 2). The analysis by domain showed that diabetes was associated with lower scores in both the verbal memory and the nonmemory domains at 65 years old (β: −0.06, 95% CI: −0.08 to −0.04 and β: −0.04, 95% CI: −0.06 to −0.03, respectively). However, the effect of diabetes over time was only significant for the nonmemory domain (β: −0.002, 95% CI: −0.004 to −0.0004; Table 2).
Old age and having comorbidities such as stroke, high depressive symptoms, and hypertension were also associated with lower cognition scores across the three outcomes in the HRS. The same was observed for being widowed or divorced/single compared to those married/in a civil union. Having more years of education, having obesity, and visiting the doctor in the last 2 years were associated with higher cognition scores across the three outcomes in this data set. Women had higher scores than men in the total standardized cognition score and memory domain, but significantly lower scores in the nonmemory domain. Health insurance was not significantly associated with any of the outcomes (Supplementary Appendix Table 3).
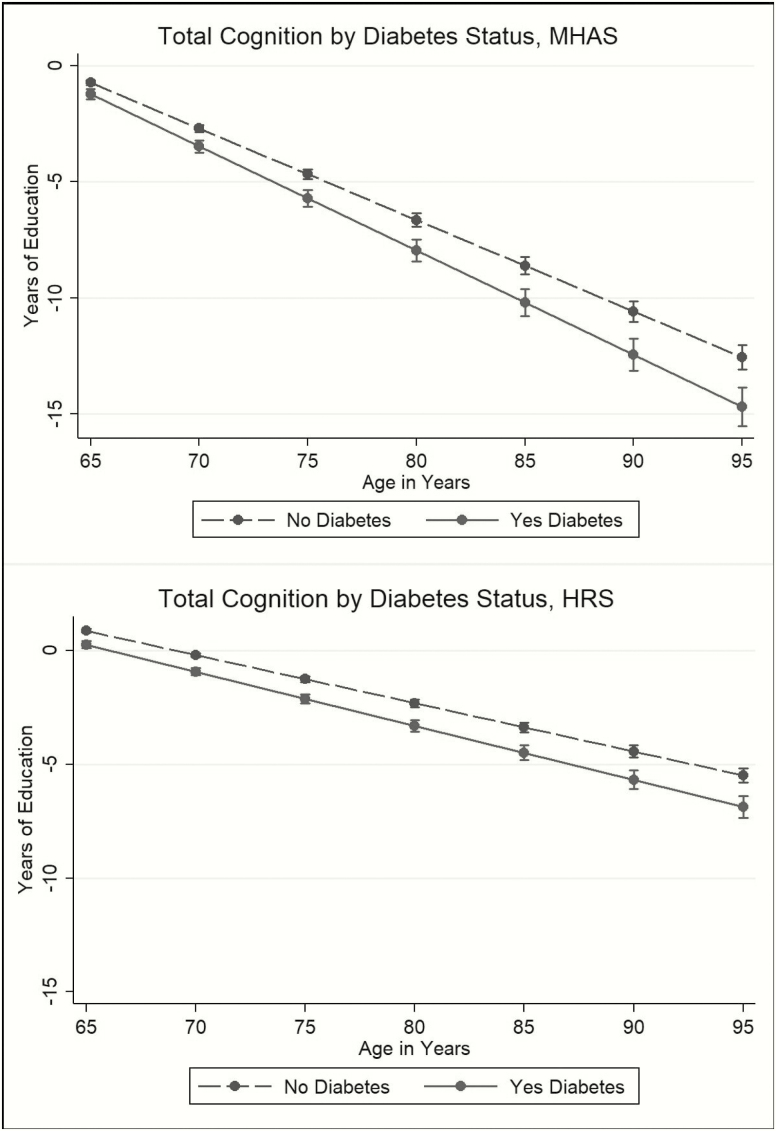
The impact of diabetes on the total standardized cognition score at 65 years old, compared to those without diabetes, was equivalent to having 0.50 fewer years of education in Mexico and 0.60 fewer years in the United States (Figure 1). By age 80, the estimated effect of diabetes was equivalent to having 1.34 fewer years of education in Mexico and 0.99 fewer years of education in the United States (Figure 1).
Figure 1.
The effect of diabetes status on total cognition score in terms of years of education, Mexican Health and Aging Study (MHAS), and the Health and Retirement Study (HRS).
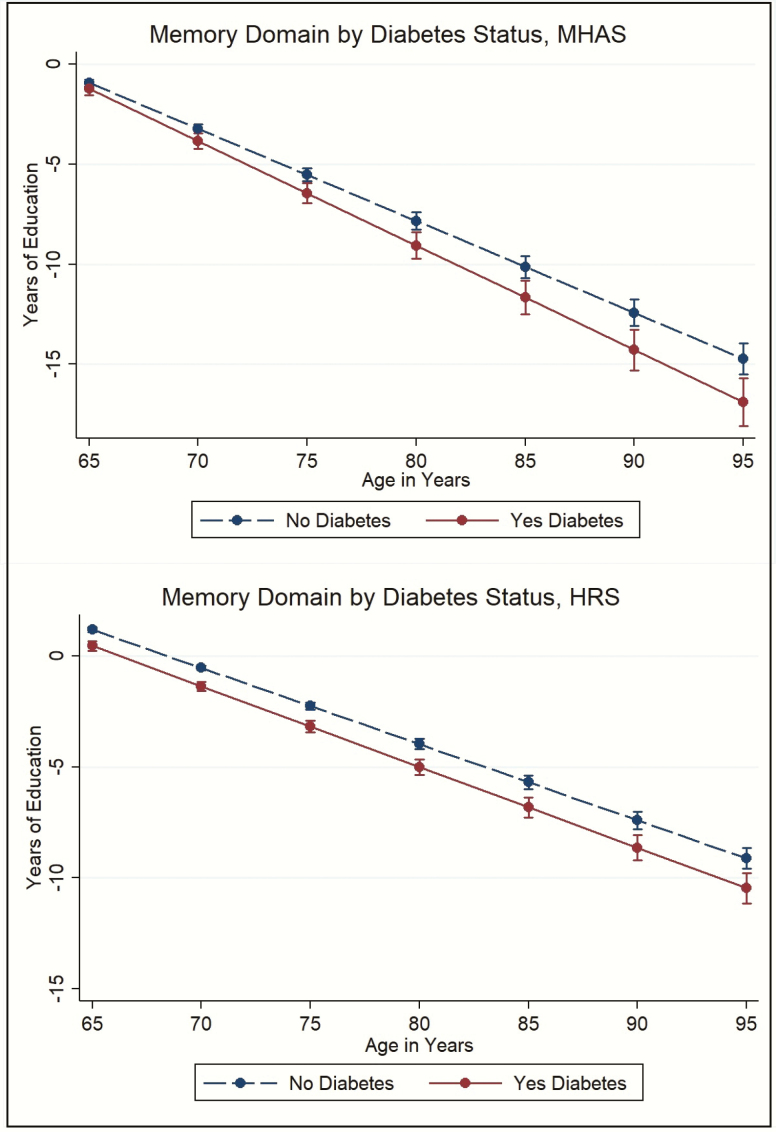
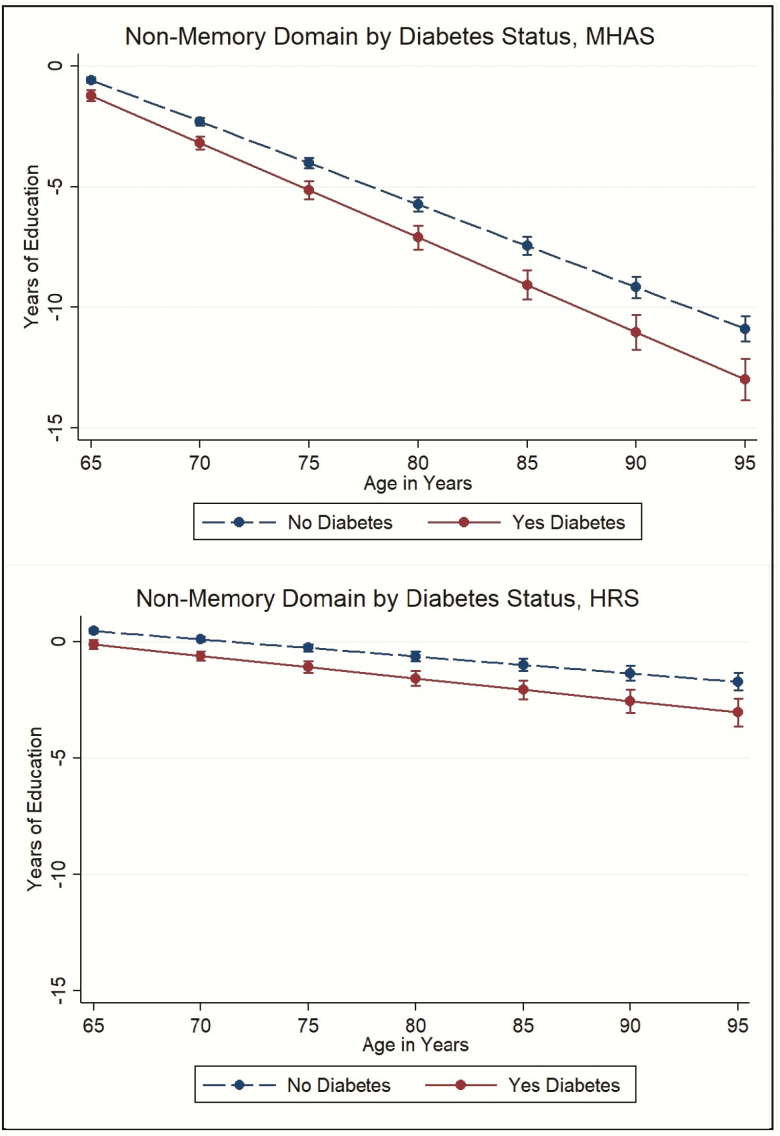
Similar results were observed by domain. For the memory domain, at 65 years old, the effect of diabetes on cognition was equivalent to 0.35 fewer years of education in Mexico (although this effect was not statistically significant) and 0.72 fewer years in the United States. By age 80, the effect of diabetes on cognition was equivalent to having 1.25 fewer years of education in Mexico and 1.03 fewer years of education in the United States (Figure 2). For the nonmemory domain, at 65 years old, the effect of diabetes on cognition was equivalent to having 0.65 fewer years of education in Mexico and 0.58 fewer years in the United States. By age 80, the effect was equivalent to having 1.38 fewer years of education in Mexico and 0.95 fewer years of education in the United States (Figure 3).
Figure 2.
The effect of diabetes status on the verbal memory domain in terms of years of education, Mexican Health and Aging Study (MHAS), and the Health and Retirement Study (HRS).
Figure 3.
The effect of diabetes status on the nonmemory domain in terms of years of education, Mexican Health and Aging Study (MHAS), and the Health and Retirement Study (HRS).Figures footnote: All figures are predictive margins in terms of years of education, based on results from Table 2 for MHAS and HRS. All results are adjusted for covariates included in those models.
Country-specific analysis
The MHAS-specific analysis that included locality size showed that, at 65 years, those living in less urban areas had lower total cognition scores compared to those in more urban areas (β: −0.10, 95% CI: −0.11 to −0.08). This difference was also observed over time, where those living in less urban areas had steeper cognitive decline (β: −0.003, 95% CI: −0.004 to −0.001). The interaction with diabetes at 65 years old was not significant, but the three-way interaction between locality size, diabetes, and age was significant for those who lived in more urban areas. Those who lived in more urban areas and had diabetes had significantly less cognitive decline over time compared to those who lived in the same locality size without diabetes (β: −0.005, 95% CI: −0.01 to −0.003; not reported in tables).
The HRS-specific analysis that included race and ethnicity showed that, at 65 years old, NHBs and Hispanics had significantly lower total standardized cognition scores compared to NHWs (β: −0.37, 95% CI: −0.39 to −0.36 and β: −0.13, 95% CI: −0.16 to −0.11, respectively). However, this difference was not significant over time. The interaction with diabetes at 65 years old showed that only Hispanics with diabetes had significantly lower cognition scores compared to their counterparts without diabetes. The three-way interaction between race and ethnicity, diabetes, and age was not significant (not reported in tables).
Discussion
In this cross-national longitudinal study, we assessed the impact of diabetes on the cognitive trajectories of older adults in Mexico and the United States. The first hypothesis of our study was that diabetes was associated with lower total cognition scores at 65 years old and was also associated with a significant decline in total cognition over time in both countries. This first hypothesis was observed in our study. However, evidence for the second hypothesis that diabetes would be associated with both memory and nonmemory domains was mixed. Self-reported diabetes was associated with lower nonmemory scores at baseline and over time in both countries. However, results for the trajectory in the verbal memory domain were mixed. In Mexico, the effect of diabetes on the memory domain was not observed at 65 years old, whereas in the United States it was not observed over time. Despite the significant difference by diabetes status in the nonmemory domain, the overall magnitude of the decline independent of diabetes status is smaller compared to the memory domain, especially in the HRS.
The mixed evidence regarding the domain-specific effect of diabetes on cognitive function is consistent with previous evidence that diabetes is associated with both vascular dementia and Alzheimer’s disease mechanisms, and that most individuals have multiple symptoms, including memory decline and decline in attention, executive function, and other nonmemory domains. Evidence from a longitudinal study with the HRS showed that self-reported diabetes was associated with greater memory decline compared to those without diabetes, but this effect was not significant after including Hemoglobin A1c levels in the model (Marden et al., 2017). At the same time, another longitudinal study in Sweden showed that diabetes was not associated with memory decline over time, but was associated with significant decline in verbal abilities and perceptual speed domains (Marseglia et al., 2018). Most of the previous population-based evidence shows that diabetes is more strongly associated with symptoms of vascular dementia than Alzheimer’s disease (Cheng et al., 2012). The stronger relationship of diabetes with vascular dementia can be explained by the important micro- and macrovascular damage caused by diabetes that directly affects the brain, increasing the likelihood of other cerebrovascular diseases such as stroke (Biessels et al., 2006; Cheng et al., 2012). It is important to note that, although we denoted this domain as nonmemory in both countries, the specific tasks included in the two studies are different. In the HRS, the nonmemory tasks measure complex attention, working memory, and processing speed, whereas, in the MHAS, these tasks are more related to attention and visuospatial ability. Yet, all are nonmemory in nature.
The third hypothesis of our study referred to the difference in the strength of the association between diabetes and cognitive decline in Mexico and the United States. We hypothesized that older Mexican adults would have a steeper cognitive decline associated with diabetes compared to those in the United States. Our results showed that despite the socioeconomic and health differences between the United States and Mexico, the magnitude of the effect of diabetes on the cognition score appears to be similar in both studies, although the data sets were not statistically compared at this level. However, the apparently similar results have different implications when considered in terms of mean years of education. At first, the magnitude of the diabetes effect in the equivalent number of education years described above seems similar between countries (1.34 fewer years of education in Mexico and 0.99 fewer years of education in the United States at age 80 for total cognition). However, in Mexico, the education mean at baseline is 4.00 years of education (SD: 6.5), whereas in the United States it is 12.45 years (SD: 2.6), demonstrating that losing an equivalent 1.34 years of education in Mexico may mean relatively more than the 0.99 years in the United States.
Furthermore, the relative magnitude of the effect of diabetes on cognition in both studies is considerable. At age 65, the effect of having diabetes on total cognition (vs. not having diabetes) was equivalent to losing near half a year of education in both studies (fewer 0.5 years in the MHAS and 0.6 in the HRS). The relative magnitude of this effect was a quarter of the effect of stroke (equivalent to fewer 2.1 years of education in the MHAS and the HRS) and half the effect of high depressive symptoms (equivalent to fewer 1.1 years of education in the MHAS and 1.5 years in the HRS). Given the high prevalence of diabetes in both samples compared to the other chronic diseases and the consistency of the results, we consider that the magnitude of the effect of diabetes on cognition is substantial.
Although the magnitude of the association between diabetes and cognition appears to be similar in both studies, there are important differences in age trajectories in this association between data sets. In the MHAS, the magnitude of the association appeared to be weaker at 65 years old compared to the HRS, but stronger over time. This difference is likely driven by the domain differences, because the effect of diabetes on the memory domain in the MHAS is not significant at 65 years old whereas in the United States it is not significant over time.
Furthermore, the overall cognitive decline seems to be steeper in the MHAS, independent of diabetes, although the data sets were not statistically compared at this level as seen in Figures 1–3. The steeper decline in cognition across domains in the MHAS may be associated with poor socioeconomic conditions accumulated through the life course, especially in education and access to health care services. As indicated by the cross-national comparison at baseline, older adults in Mexico had a similar prevalence of diabetes as those in the United States but presented different socioeconomic contexts, with 3 times lower education and almost 10 times more uninsured than in the United States. Even though there were important changes in the Mexican health care system in the early 2000s, older adults in Mexico still had a lower proportion of health insurance in the later waves of the data. Several studies have found that early-life conditions affect late-life cognition, and this relationship is mediated by socioeconomic characteristics (e.g., education) in adult life (Singh-Manoux et al., 2005). Furthermore, a cumulative high SES was associated with higher cognitive function over time (Lyu & Burr, 2016), which can explain the overall better cognition among older U.S. adults. It is important to note that these results were likely observed because we accounted for the differential survival in both studies. Although older adults in Mexico are more likely to experience healthy survivor bias than those in the United States because many have survived poor socioeconomic and health conditions in childhood and middle age than older adults in the United States, we accounted for this bias adjusting for all death or loss to follow-up in the study period.
The results also showed that the main risk factors and protective factors for cognitive health are consistent for both data sets. The main risk factors for poor cognition were reporting a stroke or having depressive symptoms, while the main protective factors for poor cognition were having more years of education, having health insurance in the MHAS, having obesity, and visiting the doctor.
A sensitivity analysis to test the effect of obesity over time was also significant in both countries. This result is similar to others observed in the U.S. population (Langa et al., 2017). While some evidence shows that obesity in late life may be protective for poor cognition, and that obesity in middle age is associated with greater risk of poor cognition (Fitzpatrick et al., 2009; Tolppanen et al., 2014), this apparent protective effect may be due to a healthy survival bias because those with obesity in middle life are less likely to survive to old age. Thus, obese individuals who survive to old age could be selected for better cognitive function.
There were important sex differences associated with cognition across domains that were similar in both studies. In the unadjusted results, women in both countries were more likely to have higher mean total cognition scores than men. The same was observed in the longitudinal analysis for total cognition and the memory domain. However, in both studies, women had lower scores than men in the nonmemory domain. The sex differences observed in this study have also been observed in other studies with the MHAS, where women show higher adjusted cognition scores in the tasks of verbal learning and recall (which we used to calculate the memory domain), but lower scores in the other tasks, including visual scanning and visuospatial ability (Díaz-Venegas et al., 2019). However, another study using several tasks showed that women are more likely to have dementia (Mejia-Arango & Gutierrez, 2011). In the HRS, the evidence for sex differences on cognition is not as clear. Several studies measuring dementia and cognitive impairment no dementia did not find a sex difference (Langa et al., 2017), but one study showed women were less likely to have cognitive impairment than men (Langa et al., 2008).
The country-specific analysis for Mexico showed that participants who lived in less urban areas had the lowest cognition scores at 65 years old. Their decline was also significantly steeper over time. However, the difference by diabetes over time was observed only for those who lived in more urban areas. Although these results are adjusted by socioeconomic and health characteristics that could explain the differences by locality size, this coefficient is still significant at 65 years old. Other studies using the MHAS have shown that locality size is strongly associated with disparities in cognition, even after adjusting for factors such as insurance status and education (Saenz et al., 2018).
The country-specific analysis for the United States showed that NHBs and Hispanics have lower cognitive scores than NHWs at 65 years old. However, there was no difference in the trajectory of cognitive decline by race and ethnicity. The poorer cognitive status of NHBs and Hispanics compared to NHWs is commonly observed in the literature, and the difference is mainly associated with differences in education (Díaz-Venegas et al., 2016; Mehta et al., 2004). Previous studies also show that the baseline differences by race and ethnicity tend to be greater than the cognitive decline over time (Gross et al., 2015). Despite the consistencies of these results, it is important to note that the greater cognitive function of NHWs may also be related to their ability to perform better in the cognitive assessments than NHBs and Hispanics due to higher education levels, as described in the Method section.
One of the limitations of our analyses is our inability to directly compare the measures of cognitive functioning between the two countries. This was not possible due to conceptual differences in the mode of interview, differences in survey protocols used, and an overall different number and type of questions between data sets. Nevertheless, this study is the first to identify whether the relationship between diabetes and cognition was present at 65 years old and over time in two countries with different aging contexts and to determine the magnitude of the association in both countries. Another limitation, especially in the MHAS, is the study gap between 2003 and 2012, and the self-reported diagnosis of diabetes, which can introduce bias due to inconsistencies across waves. However, we minimized this bias by adopting the correction that those who reported diabetes at least 2 times were likely to be diagnosed with diabetes. Furthermore, the exclusion of respondents with all proxy interviews is a limitation because these individuals may be more likely to be cognitively impaired. This group could not be included in the study because they do not receive the same cognitive assessment. Future studies can address this limitation by developing harmonized algorithms to categorize cognitive status in studies such as the HRS and the MHAS to include proxy and direct measures of cognition.
In conclusion, this study demonstrated that diabetes negatively affects the long-term cognitive trajectory of older adults in both Mexico and the United States, using large, nationally representative samples of community-dwelling older adults. The impact of diabetes was clearer for the trajectory of the nonmemory domain in both countries. Although the overall decline in cognition was steeper in Mexico, the difference in cognitive decline between those with and without diabetes was similar in both countries.
One of the policy implications of this study is that context-specific cognitive trajectories shown by this approach help identify factors that are common to both populations, and factors that vary across these two societies, pointing to specific and relevant ways to intervene in diabetes care and specific subpopulations to target to delay cognitive decline. Furthermore, an important contribution of this study is the methodological frame that others can adapt for cross-national comparisons of cognitive aging or other health outcomes.
The regularity of our findings helps us come closer to speculate that these patterns may be universal, regardless of the socioeconomic and life course differences experienced by populations of older adults in different countries. We caution, however, that more research with cross-national comparisons is needed to continue to advance our knowledge of global aging patterns.
Supplementary Material
Acknowledgments
We thank the University of Texas Medical Branch and the Sealy Center on Aging for providing the logistic support to conduct the study.
Funding
The Mexican Health and Aging Study is supported by the National Institute of Health/National Institute on Aging in the United States (R01AG018016) and the Instituto Nacional de Estadística y Geografía (INEGI) in Mexico. The Health and Retirement Study is supported by the National Institute of Health/National Institute on Aging in the United States (R01U01AG009740). The authors also acknowledge funding support from the National Institute of Health/National Institute on Aging (Resource Centers for Minority Aging Research (RCMAR), P30AG059301 and K01AG058789) and the University of Texas Medical Branch (Jeane B. Kempner predoctoral fellowship).
Conflict of Interest
None declared.
Author Contributions
J. Avila, D. Jupiter, B. Downer, S. Mejia-Arango, and R. Wong planned the study. J. Avila performed all statistical analyses. D. Jupiter and R. Wong supervised the data analysis. S. Mejia-Arango, B. Downer, and R. Wong supervised the cognitive measures used in both data sets and the data interpretation. J. Avila, D. Jupiter, B. Downer, S. Mejia-Arango, and R. Wong wrote the article. All authors revised the article.
References
- Biessels, G. J., Staekenborg, S., Brunner, E., Brayne, C., & Scheltens, P. (2006). Risk of dementia in diabetes mellitus: A systematic review. The Lancet. Neurology, 5(1), 64–74. doi: 10.1016/S1474-4422(05)70284-2 [DOI] [PubMed] [Google Scholar]
- Bugliari, D., Campbell, N., Chan, C., Hayden, O., Hayes, J., Hurd, M., Main, R., Mallett, J., McCullough, C., Meijer, E., Moldoff, M., Pantoja, P., Rohwedder, S., & St. Clair, P. (2018). RAND HRS longitudinal file 2014 (V2) documentation. Labor & Population Program. RAND Center for the Study on Aging. [Google Scholar]
- Centers for Disease Control and Prevention (CDC) . (2014). National Diabetes Statistics Report, 2014. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. [Google Scholar]
- Centers for Disease Control and Prevention . (2015). Diabetes Report Card 2014. U.S. Department of Health and Human Services. Centers for Disease Control and Prevention. [Google Scholar]
- Cheng, G., Huang, C., Deng, H., & Wang, H. (2012). Diabetes as a risk factor for dementia and mild cognitive impairment: A meta-analysis of longitudinal studies. Internal Medicine Journal, 42(5), 484–491. doi: 10.1111/j.1445-5994.2012.02758.x [DOI] [PubMed] [Google Scholar]
- Díaz-Venegas, C., Downer, B., Langa, K. M., & Wong, R. (2016). Racial and ethnic differences in cognitive function among older adults in the USA. International Journal of Geriatric Psychiatry, 31(9), 1004–1012. doi: 10.1002/gps.4410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Venegas, C., Samper-Ternent, R., Michaels-Obregón, A., & Wong, R. (2019). The effect of educational attainment on cognition of older adults: Results from the Mexican Health and Aging Study 2001 and 2012. Aging & Mental Health, 23(11), 1586–1594. doi: 10.1080/13607863.2018.1501663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everson-Rose, S. A., Mendes de Leon, C. F., Bienias, J. L., Wilson, R. S., & Evans, D. A. (2003). Early life conditions and cognitive functioning in later life. American Journal of Epidemiology, 158(11), 1083–1089. doi: 10.1093/aje/kwg263 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, A. L., Kuller, L. H., Lopez, O. L., Diehr, P., O’Meara, E. S., Longstreth, W. T.Jr, & Luchsinger, J. A. (2009). Midlife and late-life obesity and the risk of dementia: Cardiovascular health study. Archives of Neurology, 66(3), 336–342. doi: 10.1001/archneurol.2008.582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, A. L., Mungas, D. M., Crane, P. K., Gibbons, L. E., MacKay-Brandt, A., Manly, J. J., Mukherjee, S., Romero, H., Sachs, B., Thomas, M., Potter, G. G., & Jones, R. N. (2015). Effects of education and race on cognitive decline: An integrative study of generalizability versus study-specific results. Psychology and Aging, 30(4), 863–880. doi: 10.1037/pag0000032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez, J. P., Rivera-Dommarco, J., Shamah-Levy, T., Villalpando-Hernández, S., Franco, A., Cuevas-Nasu, L., Romero-Martínez, M., & Hernández-Ávila, M. (2012). Encuesta Nacional de Salud y Nutrición 2012. Resultados Nacionales. Instituto Nacional de Salud Pública. Retrieved from http://ensanut.insp.mx/informes/ENSANUT2012ResultadosNacionales.pdf [Google Scholar]
- He, W., Goodkind, D., & Kowa, P. (2015). An aging world: 2015. U.S. Government Publishing Office. [Google Scholar]
- Instituto Nacional de Estadistica Y Geografia (INEGI) . (2016). Informe de resultados del operativo de campo. Retrieved from http://mhasweb.org/Resources/DOCUMENTS/2015/Informe_Operativo_de_Campo_2015.pdf
- Instituto Nacional de Estadistica Y Geografia . (2018). Estadística de defunciones registradas 2017. Retrieved from https://www.inegi.org.mx/contenidos/saladeprensa/boletines/2018/EstSociodemo/DEFUNCIONES2017.pdf
- Kaffashian, S., Dugravot, A., Elbaz, A., Shipley, M. J., Sabia, S., Kivimäki, M., & Singh-Manoux, A. (2013). Predicting cognitive decline: A dementia risk score vs. the Framingham vascular risk scores. Neurology, 80(14), 1300–1306. doi: 10.1212/WNL.0b013e31828ab370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar, A., Wong, R., Ottenbacher, K. J., & Al Snih, S. (2016). Prediabetes, undiagnosed diabetes, and diabetes among Mexican adults: Findings from the Mexican Health and Aging Study. Annals of Epidemiology, 26(3), 163–170. doi: 10.1016/j.annepidem.2015.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa, K. M., Larson, E. B., Crimmins, E. M., Faul, J. D., Levine, D. A., Kabeto, M. U., & Weir, D. R. (2017). A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med, 177(1), 51–58. doi: 10.1001/jamainternmed.2016.6807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa, K. M., Larson, E. B., Karlawish, J. H., Cutler, D. M., Kabeto, M. U., Kim, S. Y., & Rosen, A. B. (2008). Trends in the prevalence and mortality of cognitive impairment in the United States: Is there evidence of a compression of cognitive morbidity? Alzheimer’s & Dementia, 4(2), 134–144. doi: 10.1016/j.jalz.2008.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu, J., & Burr, J. A. (2016). Socioeconomic status across the life course and cognitive function among older adults: An examination of the latency, pathways, and accumulation hypotheses. Journal of Aging and Health, 28(1), 40–67. doi: 10.1177/0898264315585504 [DOI] [PubMed] [Google Scholar]
- McCrimmon, R. J., Ryan, C. M., & Frier, B. M. (2012). Diabetes and cognitive dysfunction. Lancet (London, England), 379(9833), 2291–2299. doi: 10.1016/S0140-6736(12)60360-2 [DOI] [PubMed] [Google Scholar]
- Mehta, K. M., Simonsick, E. M., Rooks, R., Newman, A. B., Pope, S. K., Rubin, S. M., & Yaffe, K. (2004). Black and white differences in cognitive function test scores: What explains the difference? Journal of the American Geriatrics Society, 52(12), 2120–2127. doi: 10.1111/j.1532-5415.2004.52575.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mejia-Arango, S., & Gutierrez, L. M. (2011). Prevalence and incidence rates of dementia and cognitive impairment no dementia in the Mexican population: Data from the Mexican Health and Aging Study. Journal of Aging and Health, 23(7), 1050–1074. doi: 10.1177/0898264311421199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels-Obregon, A., Mejia-Arango, S., & Wong, R. (2014). The Mexican Health and Aging Study: Cognitive function measures, version 3. Retrieved from mhasweb.org
- Murphy, S., Xy, J., Kochanek, M., & Arias, E. (2018). Mortality in the United States, 2017. NCHS Data Brief, no 328. National Center for Health Statistics. [PubMed] [Google Scholar]
- Ofstedal, M. B., Fisher, G. G., & Herzog, A. R. (2005). Documentation of cognitive functioning measures in the Health and Retirement Study. Retrieved from http://hrsonline.isr.umich.edu/sitedocs/userg/dr-006.pdf
- Organisation for Economic Cooperation and Development (OECD) . (2013). Country note: Education at a glance, Mexico. Retrieved from https://www.oecd.org/edu/Mexico_EAG2013%20Country%20Note.pdf
- Prince, M., Wimo, A., Guerchet, M., Ali, G. C., Wu, Y. T., & Prina, M. (2015). The global impact of dementia: Executive summary. World Alzheimer Report. Retrieved from https://www.alz.co.uk/research/world-report-2015 [Google Scholar]
- Rabe-Hesketh, S., & Skrondal, A. (2012). Multilevel and longitudinal modeling using Stata (Vol. I: Continuous Responses). STATA Press. [Google Scholar]
- Saenz, J. L., Downer, B., Garcia, M. A., & Wong, R. (2018). Cognition and context: Rural-urban differences in cognitive aging among older Mexican adults. Journal of Aging and Health, 30(6), 965–986. doi: 10.1177/0898264317703560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samper-Ternent, R., Michaels-Obregon, A., Wong, R., & Palloni, A. (2012). Older adults under a mixed regime of infectious and chronic diseases. Salud Publica de Mexico, 54(5), 487–495. doi: 10.1590/s0036-36342012000500005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux, A., Richards, M., & Marmot, M. (2005). Socioeconomic position across the lifecourse: How does it relate to cognitive function in mid-life? Annals of Epidemiology, 15(8), 572–578. doi: 10.1016/j.annepidem.2004.10.007 [DOI] [PubMed] [Google Scholar]
- Sonnega, A., Faul, J. D., Ofstedal, M. B., Langa, K. M., Phillips, J. W., & Weir, D. R. (2014). Cohort profile: The Health and Retirement Study (HRS). International Journal of Epidemiology, 43(2), 576–585. doi: 10.1093/ije/dyu067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subsecretaría de Integración y Desarrollo del Sector Salud, Dirección General de Evaluación del Desempeño . (2015). Informe sobre la salud de los mexicanos 2015: Diagnóstico general de la salud poblacional. Retrieved from https://www.gob.mx/cms/uploads/attachment/file/64176/INFORME_LA_SALUD_DE_LOS_MEXICANOS_2015_S.pdf [Google Scholar]
- Tolppanen, A. M., Ngandu, T., Kåreholt, I., Laatikainen, T., Rusanen, M., Soininen, H., & Kivipelto, M. (2014). Midlife and late-life body mass index and late-life dementia: Results from a prospective population-based cohort. Journal of Alzheimer’s Disease, 38(1), 201–209. doi: 10.3233/JAD-130698 [DOI] [PubMed] [Google Scholar]
- United Nations . (2015). World Population Ageing 2015. Retrieved from https://www.un.org/en/development/desa/population/publications/pdf/ageing/WPA2015_Report.pdf
- Wilson, R. S., Boyle, P. A., Yu, L., Barnes, L. L., Sytsma, J., Buchman, A. S., Bennett D. A., Schneider, J. A. (2015). Temporal course and pathologic basis of unawareness of memory loss in dementia. Neurology, 85(11), 984–991. doi: 10.1212/WNL.0000000000001935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong, R., Michaels-Obregon, A., & Palloni, A. (2017). Cohort profile: The Mexican Health and Aging Study (MHAS). International Journal of Epidemiology, 46(2), e2. doi: 10.1093/ije/dyu263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marden, J. R., Mayeda, E. R., Tchetgen, E. J. T., Kawachi, I., & Glymour, M. M. (2017). High hemoglobin A1c and diabetes predict memory decline in the Health and Retirement Study. Alzheimer Disease and Associated Disorders, 31(1), 48–54. doi: 10.1097/wad.0000000000000182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseglia, A., Dahl Aslan, A. K., Fratiglioni, L., Santoni, G., Pedersen, N. L., & Xu, W. (2018). Cognitive trajectories of older adults with prediabetes and diabetes: A population-based cohort study. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 73(3), 400–406. doi: 10.1093/gerona/glx112 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.