Abstract
Introduction: Alzheimer disease (AD) is characterized by the decline of cognitive functions such as learning and memory. Scientific society has proposed some non-pharmacological interventions, among which photobiomodulation has gained prominence for its beneficial effects. Therefore, we investigated, through systematic review, the therapeutic potential of photobiomodulation in AD.
Methods: This systematic review was registered under the number CRD42019128416 in the International Prospective Record of Systematic Reviews (PROSPERO). A systematic search was conducted on the bibliographic databases (PubMed and ScienceDirect) with the keywords based on MeSH terms: "photobiomodulation therapy" or "low-level laser therapy" or "LLLT" or "light emitting diode" and "amyloid" or "Alzheimer". The data search was conducted from 2008 to 2019. We follow the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline. The search strategy included experimental in vivo and in vitro studies in the English language and photobiomodulation as a non-pharmacological intervention. We included 10 studies, being 5 in vivo studies, 4 in vitro studies and 1 study using in vivo and in vitro. To evaluate the quality of the studies, we used the Rob tool of the Systematic Review Center for Laboratory Animal Experimentation (SYRLE).
Results: The studies showed that photobiomodulation is able to reduce inflammatory response, oxidative stress and apoptotic effects generated by amyloid beta (Aβ) and restore mitochondrial function and cognitive behavior.
Conclusion: Taken together, these results indicate that photobiomodulation may be a useful tool for treating AD.
Keywords: Photobiomodulation therapy, Low-level laser therapy, LLLT, Light emitting diode, Amyloid, Alzheimer’s disease
Introduction
Worldwide, Alzheimer disease (AD) incidence increases faster than any other age-related dementia.1 It is estimated that approximately 15 million people around the world suffer from this disease. By the year 2050, it is expected that 13 million people in the United States and 16 million people in Europe will be affected by AD.2
AD is a progressive neurodegenerative disease, characterized by gradual loss of memory and motor activities.3-5 The pathophysiology of AD involves two mechanisms. The formation of senile plaques to which the amyloid peptide (Aβ) is considered the main component.6 When accumulated in cortical and limbic regions, the senile plaques induce synaptic and dendritic dysfunctions, also the activation of microglia and astrocytes, triggering inflammatory response. These alterations lead to neuronal death due to cellular and biochemical damage, such as the formation of free radicals and reactive oxygen species.7 Another feature of AD pathophysiology is related to intracellular neurofibrillary tangles, composed of tau protein. Phospho-Tau leads to the development of neurofibrillary tangles, impairing neuronal functioning and structure.8 These mechanisms are involved in the process of cerebral atrophy, especially in the areas of the temporal lobe, such as the hippocampus and entorhinal cortex, thus determining the impairment of cognitive functions as well as behavioral disorders.9,10
Despite the advanced findings in AD, there is still no treatment that can cure the disease. However, some non-pharmacological treatments such as cognitive therapy,11-12 occupational therapy,13 and physical exercise 14-15 have provided benefits to cognitive and behavioral impairments. Recently, photobiomodulation has been of interest to the scientific community because it is a noninvasive therapy that provides interesting results for several tissues, such as wound healing, inflammation in various diseases and tendonitis.16 In addition, promising evidence has emerged about its beneficial effects on the brain.17-20 However, the results are still not sufficient due to methodological differences. Thus, in this systematic review, we analyzed the effect of photobiomodulation in in vivo and in vitro studies to provide general information on all available evidence suggesting photobiomodulation as an efficient non-pharmacological tool in AD. In addition, we will present recommendations for possible research on the use of photobiomodulation and methodologies, and finally, we will discuss the mechanisms involved in the improvement of AD symptoms through photobiomodulation.
Materials and Methods
Data Sources and Searches
This systematic review was registered under the number CRD42019128416 in the International Prospective Record of Systematic Reviews (PROSPERO) and can be accessed in https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=128416. We used the PubMed and ScienceDirect databases with the keywords based on MeSH terms: “photobiomodulation therapy” or “low-level laser therapy” or “LLLT” or “light emitting diode” and “amyloid” or “Alzheimer”. Data search was conducted from 2008 to 2019. To ensure the clarity and transparency of the articles, we followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guideline.21
Selection Criteria
We selected in vivo and in vitro studies to obtain information related to cellular and molecular effects as well as their impact on cognitive function in AD.20 The search strategy included experimental in vivo and in vitro studies and photobiomodulation as a non-pharmacological intervention. In addition, we included literature reviews and experimental studies that addressed our subject without yearly restriction for discussion.
Selection Process
Initially, duplicate articles were excluded. After that, the titles and abstracts of the search results were independently reviewed by two researchers, who applied the inclusion and exclusion criteria (Table 1). The article was considered eligible when the researcher examined the effects of photobiomodulation on brain functions in AD. Studies that included another intervention associated with photobiomodulation were excluded. Next, the results of the selection process were discussed in a consensual way to avoid disagreements regarding the eligibility of the articles. Finally, the articles were read in full to select the final list of articles. At this stage, studies evaluating brain structure (e.g. neurotrophic factors, inflammatory markers, mitochondrial function and neuronal morphology) and cognitive functions (e.g. behavioral analyzes) were included.
Table 1. List of Inclusion and Exclusion Criteria.
| Area | Inclusion Criteria | Exclusion Criteria |
| Subjects | AD models | Models without AD |
| Intervention | Transcranial photobiomodulation | Pharmacological intervention and others |
| Results | ||
| Kind of study | Experimental in vivo and in vitro studies | Clinical studies, case reports and review |
| Language | English | Other languages |
| Year of publication | 2008 until 2019 | Outside this period |
AD: Alzheimer disease
Data Extraction and Data Synthesis
The articles included were divided into in vivo and in vitro studies. After that, both of the groups were subdivided according to the type of model used (cell expressing the AD phenotype and animal model of AD) in the studies. In that way, the comparison between the effects provided by the photobiomodulation in different model types became easier to perform. For data extraction, we used an individualized data form,22 which included information about the reference (author and year), characteristics of the population (sample model), characteristics of the intervention with photobiomodulation (intensity, duration, and frequency), and characteristics of the results (applied analyses and results). The data are presented in the results section as a summary of the study findings. Besides that, a qualitative synthesis of the studies was performed. Due to the variation applied in the intervention, a meta-analysis was not performed.
Quality Appraisal
We used the Rob tool of the Systematic Review Center for Laboratory Animal Experimentation (SYRLE) to evaluate the quality of studies.23 The Rob was developed to study the methodology of experimental animal studies, based on the Cochrane Rob technique, which evaluated randomized clinical trials. The SYRLE Rob checklist consists of 10 items, which are classified in selection bias (item 1 to 3), performance bias (items 4 and 5), detection bias (items 6 and 7), attrition bias (item 8), selective outcome reporting (item 9) and other sources of bias (item 10). Item 1 is related to the description of the methodology. In order to generate a detailed allocation sequence of the sample, which will allow a comparison among the groups. Item 2 relates to the characteristics of the animals that are compared to evaluate the intervention and groups. Item 3 describes the method for concealing the allocation sequence of the intervention which could have taken place before or during the experiment. Item 4 describes all measures used to shelter the animals in the animal room. Item 5 describes all the measures used to blind the researchers, making it impossible for them to know the intervention that each animal received. Item 6 relates to whether the animals were randomly separated for analysis and how the methods were used for the selection of animals. Item 7 describes all the measures used to blind the evaluators about the intervention that each animal received. Item 8 relates to the integrity of results, attritions, or exclusions. Item 9 relates to how the selective results were examined and what was found. Item 10 describes important concerns about biases not covered by other domains in the tool. The evaluations were made by two evaluators, who classified the information as positive (yes) which indicates a low risk of bias, negative (no) which indicates a high risk of bias, and inaccurate (unclear) which indicates an unclear risk of bias. Disagreements were resolved by consensus.
Results
Study Selection
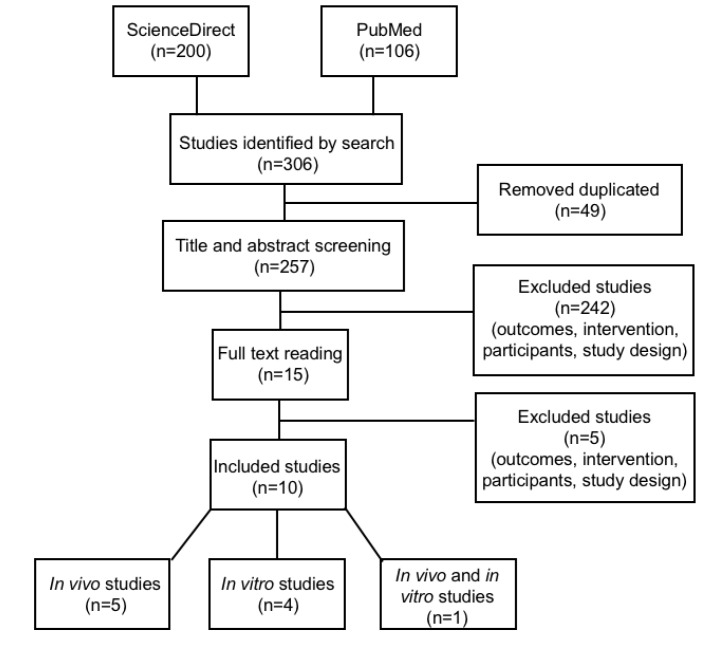
The search in PubMed and ScienceDirect databases resulted in 306 studies. After exclusion by duplicity (49) and screening (242), 15 studies were left for reading in full. After reading, 5 studies were excluded, with 10 studies remaining in the systematic review, 5 in vivo studies, 4 in vitro and 1 study using both in vivo and in vitro. The process of selection of the articles is illustrated in Figure 1.
Figure 1.
Article Search and Selection Process.
Study Characteristics
Characteristics of In Vivo Studies
The total number of studies using rats and mice was 5 articles. The studies used Wistar rats,24 a K369I tau transgenic model,25 an APPswe/PSEN1dE9 transgenic model,18,25 5XFAD mice26 and Sprague Dawley rats.27 The age of the animals varied from 3 to 12 months. Only in the works of Da Luz Eltchechem et al24 and Lu et al,27 the animals were free of disease at the start of the study. It is noteworthy that in all studies, the animals received transcranial photobiomodulation treatment. Most of the studies aimed to analyze the effects of photobiomodulation on damages caused by high levels of Aβ, inflammatory response and oxidative stress; mitochondrial function, signaling pathways and cognitive function were also a target of interest for the authors. The summary of the findings is given in Table 2.
Table 2. Results of In Vivo Studies .
| Autor | Subjects | Intervention | Main Results | Level of Evidence |
| De Taboada et al18 | APPswe/PSEN1dE9 transgenic model |
Parameters: wavelength: 808nm; fluence: 1,2 J/cm2, 6 J/cm2, 12 J/cm2. Frequency: 3 times/week for 6 months. |
- Reduction in amyloid load; - Mitigation of the cognitive effects; - Reduction in the expression of inflammatory markers; - Increase in ATP levels and mitochondrial function. |
50% |
| Purushothuman et al25 | APPswe/PSEN1dE9 and K369I tau transgenic model |
Parameters: wavelength: 670 nm; fluence: 9,6 J/cm2. Frequency: 5 times/week for 4 weeks. |
- Reduction in hyperphosphorylated tau and neurofibrillary tangles - Reduction in oxidative stress markers levels; - Restoration of expression of the function mitochondrial; - Reduction in the size and number of amyloid-β plaques. |
40% |
| Lu et al27 | Sprague-Dawley rats |
Parameters: wavelength: 808 nm; fluence: 15 J/cm2. Frequency: 5 consecutive days |
- Attenuation of the toxic effects of Aβ; - Preservation of mitochondrial activity and integrity; - Suppression of oxidative stress; - Inhibition of inflammatory response; - Memory restoration (spatial and recognition memory). |
40% |
| Da Luz Eltchechem et al24 | Wistar rats |
Parameters: wavelength: 627 nm; fluence: 7 J/cm2. Frequency: 7, 14 and 21 consecutive days |
- Reduction in the presence of Aβ plaques; - Increase in spatial memory and behavioral and motor skills. |
50% |
| Cho et al26 | 5XFAD mice |
Parameters: wavelength: 610 nm; fluence: 2 J/cm2. Frequency: 3 times/week for 14 weeks. |
- Reduction in amyloid accumulation, neuronal loss, and microgliosis; - Mitigation of spatial memory and aversive memory. |
40% |
Characteristics of In Vitro Studies
The total number of studies using cells expressing AD was 4 articles. The studies used PC12 cells28-30 and primary rat astrocytes.31 Following the idea of in vivo studies, in the in vitro studies, the aim was to verify the effects of photobiomodulation on Aβ levels. In addition, the authors gave attention to signaling pathways related to cell survival and death as well as the inflammatory process. The summary of the study findings is given in Table 3.
Table 3. Results of In Vitro Studies .
| Autor | Subjects | Intervention | Main Results | Level of Evidence |
| Zhang et al,29 2008 | PC12 cells | Parameters: wavelength: 632.8 nm; fluences: 0.156 J/cm2 and 1248 J/cm2 | - Inhibition of cell apoptosis via PKC-mediated regulation of bax/bcl-xl mRNA ratio. | 40% |
| Yang et al,31 2010 | primary rat astrocytes | Parameters: wavelength: 632,8nm; fluence: 16.2 J/cm2 |
- Suppression of cellular pathways of oxidative stress; - Reduction in inflammatory response. |
30% |
| Liang et al,28 2012 | PC12 cells | Parameters: wavelength: 632.8 nm; fluence: 2 J/cm2 | - Promotion of prosurvival effects through the Akt/GSK3b/b-catenin pathway. | 40% |
| Zhang et al,30 2012 | PC12 cells | Parameters: wavelength: 632.8 nm; fluence: 2 J/cm2 | - Promotion of prosurvival effects through the Akt/YAP/p73 signaling pathway. | 40% |
Characteristics of the Use of Both In Vivo and In Vitro Model of AD
Only the study of Meng et al17 did both cells (human neuroblastoma cell line SHSY5Y) and AD model animals (APPswe/PSEN1dE9 transgenic model), with 50% of the evidence level.
In the study by Meng et al,17 the cells were irradiated by a red laser, with a wavelength of 632.8 nm, using 4 fluences (0.5 J/cm2, 1 J/cm2, 2 J/cm2and 4 J/cm2). They noted the inhibition of Aβ-generated toxicity as well as the restoration of dendritic atrophy by the activation of ERK/CREB/BDNF signaling pathway.
Quality Evaluation
In Vivo Studies
According to the SYRCLE tool, no study reached the maximum score. The classified items maintained a prevalence of 44% for ‘’yes’’, 44% for “no” and 8% for “unclear”. Items 1 (selection bias) and 9 (reporting bias) had the best ranking, with all studies writing them correctly. In contrast, item 5 (performance bias) was the lowest-ranked item, with all studies writing it incorrectly. The studies with the best evaluation were those by De Taboada et al18 and Da Luz Eltchechem,24 with 5 items classified as “yes”. The other studies had 4 items classified as “yes”.
In Vitro Studies
According to the SYRCLE tool, no study reached the maximum score. The classified items maintained a prevalence of 37.5% for “yes”, 52.5% for “no” and 10% for “unclear”. Items 1, 2 (selection bias) and 9 (reporting bias) had the best ranking, with all studies spelling them correctly. However, items 3 (selection bias), 6, 7 (detection bias) and 8 (attrition bias) had the lowest rating. The studies with the best evaluation were those by Zhang et al,29 Liang et al28 and Zhang et al,30 with 4 items classified as “yes”. The study with the worst evaluation was conducted by Liang et al,28 with 3 items classified as “yes”.
Studies Using Both In Vivo and In Vitro Model of AD
Despite using tact cells as animals, we evaluated them, mainly considering the conditions related to in vitrostudies. According to the SYRCLE tool, the study conducted by Meng et al18 had 4 items classified as ‘’yes”.
Discussion
The objective of this systematic review was to analyze the therapeutic potential of photobiomodulation in AD. In vivo and in vitro studies have provided promising findings on the effects of photobiomodulation in AD.
Amyloid Beta
It was observed that photobiomodulation reduced the accumulation, size and quantity of Aβ in AD model animals.18,24,25,26 Only in the study by Lu et al. (2017), this effect was not observed. Probably, this divergence should be in the protocol used, in which the animals received the highest fluency (15J/cm2) with the shortest treatment period (five consecutive days) between the studies. Nevertheless, the protocol used by the authors inhibited Aβ-induced toxic effects.27 Possibly, this reduction in Aβ levels promoted by photobiomodulation may be related to changes in the activity of BACE1 and cathepsin B, enzymes that cleave amyloid precursor protein (APP), which in turn produces Aβ.32,33
Inflammatory response and oxidative stress
We noted that photobiomodulation reduced inflammatory response and oxidative stress in the in vivo18,25,27 and in vitrostudies.31 For example, it has been observed that AD model animals exposed to photobiomodulation decreased the levels of TNFa, IL-1b e IL-6 inflammatory markers and oxidative stress as NADPH.27 These processes are closely related to AD. Inflammatory and neurotoxic mediators are known to contribute to neuronal degeneration.34,35,36 In addition, it has been noted that these processes occur in senile plaques due to the presence of microglia and astrocytes activated by Aβ in or near these plaques.37,38,39 Given this, photobiomodulation may be a potent non-pharmacological therapy, as it reduces inflammatory response and oxidative stress by reducing Aβ levels.
Mitochondrial function
The studies by De Taboada et al. (2011), Purushothuman et al. (2014) and Lu et al. (2017) revealed beneficial effects on mitochondrial function of AD model animals exposed to photobiomodulation. For example, De Taboada et al. (2011) noted that ATP concentration and oxygen consumption of AD model animals were restored with photobiomodulation treatment three times a week for six months. Lu et al27 observed that 5 consecutive days of photobiomodulation treatment is capable of suppressing fission protein expression and preserving mitochondrial fusion. These effects are promising since patients with AD exhibit impaired ATP levels and synthesis, such as, oxidative phosphorylation.40-42 These effects are possibly related to the ability of photobiomodulation to increase the enzyme cytochrome c oxidase (CCO), which represents the fourth unit of the mitochondrial respiratory chain. The CCO has the function of reducing oxygen and water, facilitating electrons transference in the mitochondrial membrane, and promoting changes in molecular levels due to increased cellular metabolism.43,44 In this sense, these data suggest that photobiomodulation may restore mitochondrial damage observed in AD by increasing CCO levels.
Signaling Pathways
In vitro studies have highlighted the effects of photobiomodulation on signaling pathways.17,28,29,30,31 For example, Zhang et al29 observed that photobiomodulation could promote anti-apoptotic effects by high regulation of bcl-x and low regulation of baxmediated by PKC signaling on PC12 cells. Liang et al28 also observed that photobiomodulation attenuated the pro-apoptotic effects of Aβ through the Akt/GSK3b/b-catenin on PC12 cells. The photobiomodulation inhibits GSK3b, abolishing its effects of b-catenin mediated neuronal degeneration. Zhang et al30 showed that photobiomodulation inhibited the anti-apoptotic effects generated by Aβ via Akt/YAP/p73/Bax signaling activation. They observed that Akt promoted a cytoplasmic distribution of YAP, which interacted with p73, targeted by Bax, thereby inhibiting the apoptotic effects caused by Aβ. In fact, most studies pay attention to the effects of photobiomodulation on signaling pathways related to cell survival, proposing targets for the inhibition of apoptotic effects of Aβ. In the study conducted by Meng et al,18 it was observed that photobiomodulation treatment restored Aβ-induced dendritic atrophy. They identified that the cellular mechanism involved in this phenomenon was in the activation of the ERK/CREB/BDNF signaling.
Cognitive Function
Regarding cognitive function, several studies have shown that photobiomodulation is able to improve the cognitive function of AD model animals.18,24,26,27 For instance, Lu et al27 observed that photobiomodulation was able to restore cognitive impairment of AD model animals in the Barnes maze test and recognition of objects and hippocampal-dependent tasks.45,46 These data are interesting since the cognitive deficit observed in AD is due to an impairment in the CA1 region (Important memory-related hippocampal sub-region), compromising a series of molecular reactions, which trigger synaptic failures.47 Therefore, it is possible to assume that photobiomodulation can improve memory by maintaining hippocampal integrity.24,27
Conclusion
Taken together, these results indicate that photobiomodulation may be a useful tool for treating AD due to its ability to reduce inflammatory response, oxidative stress, and apoptotic effects generated by Aβ and to restore mitochondrial function and cognitive function.
Ethical Considerations
Not applicable.
Conflict of interests
The authors declare that they have no conflicts of interest.
Acknowledgements
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo ao Ensino e Pesquisa (FAEP) and Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; #2017/16443-0). Sérgio Gomes da Silva is a researcher (level 2) of CNPq (PQ #306442/2016-7).
Please cite this article as follows: Cardoso, FDS, Lopes-Martins RAB, Gomes da Silva S. Therapeutic potential of photobiomodulation In Alzheimer’s disease: a systematic review. J Lasers Med Sci. 2020;11(suppl 1):S16-S22. doi:10.34172/jlms.2020.S3.
References
- 1.Forman MS, Trojanowski JQ, Lee VMY. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10(10):1055–63. doi: 10.1038/nm1113. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. Active ageing: A policy framework. No. WHO/NMH/NPH/02.8. World Health Organization, 2002. [PubMed]
- 3.Förstl H, Kurz A. Clinical features of Alzheimer’s disease. Eur Arch Psychiatry Clin Neurosci. 1999;249(6):288–290. doi: 10.1007/s004060050101. [DOI] [PubMed] [Google Scholar]
- 4.Francis PT, Palmer AM, Snape M, Wilcock GK. The cholinergic hypothesis of Alzheimer’s disease: a review of progress. J Neurol, Neurosurg Psychiatry. 1999;66(2):137–147. doi: 10.1136/jnnp.66.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Racchi M, Govoni S. The pharmacology of amyloid precursor protein processing. Exp Gerontol. 2003;38(1-2):145–157. doi: 10.1016/s0531-5565(02)00158-4. [DOI] [PubMed] [Google Scholar]
- 6.Haass C. Take five—BACE and the γ-secretase quartet conduct Alzheimer’s amyloid β-peptide generation. EMBO J. 2004;23(3):483–488. doi: 10.1038/sj.emboj.7600061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Selkoe DJ. Defining molecular targets to prevent Alzheimer disease. Arch Neurol. 2005;62(2):192–195. doi: 10.1001/archneur.62.2.192. [DOI] [PubMed] [Google Scholar]
- 8.Jenkins SM, Johnson GV. Modulation of tau phosphorylation within its microtubule-binding domain by cellular thiols. J Neurochem. 1999;73(5):1843–1850. [PubMed] [Google Scholar]
- 9.Gauthier S, Reisberg B, Zaudig M, Petersen RC, Ritchie K, Broich K. et al. Mild cognitive impairment. Lancet. 2006;367(9518):1262–1270. doi: 10.1016/S0140-6736(06)68542-5. [DOI] [PubMed] [Google Scholar]
- 10.Grossman H, Bergmann C, Parker S. Dementia: a brief review. Mt Sinai J Med. 2006;73(7):985–992. [PubMed] [Google Scholar]
- 11.Olazarán J, Muñiz R, Reisberg B, Peña-Casanova J, del Ser T, Cruz-Jentoft AJ. et al. Benefits of cognitive-motor intervention in MCI and mild to moderate Alzheimer disease. Neurology. 2004;63(12):2348–2353. doi: 10.1212/01.wnl.0000147478.03911.28. [DOI] [PubMed] [Google Scholar]
- 12.Rozzini L, Costardi D, Chilovi BV, Franzoni S, Trabucchi M, Padovani A. Efficacy of cognitive rehabilitation in patients with mild cognitive impairment treated with cholinesterase inhibitors. Int J Geriatr Psychiatry. 2007;22(4):356–360. doi: 10.1002/gps.1681. [DOI] [PubMed] [Google Scholar]
- 13.Ávila A, De-Rosende-Celeiro I, Torres G, Vizcaíno M, Peralbo M, Durán M. Promoting functional independence in people with Alzheimer’s disease: Outcomes of a home-based occupational therapy intervention in Spain. Health Soc Care Community. 2018;26(5):734–743. doi: 10.1111/hsc.12594. [DOI] [PubMed] [Google Scholar]
- 14.Podewils LJ, Guallar E, Kuller LH, Fried LP, Lopez OL, Carlson M. et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161(7):639–651. doi: 10.1093/aje/kwi092. [DOI] [PubMed] [Google Scholar]
- 15.Larson EB. Physical activity for older adults at risk for Alzheimer disease. JAMA. 2008;300(9):1077–1079. doi: 10.1001/jama.300.9.1077. [DOI] [PubMed] [Google Scholar]
- 16.Chung H, Dai T, Sharma SK, Huang YY, Carroll JD, Hamblin MR. The nuts and bolts of low-level laser (light) therapy. Ann Biomed Eng. 2012;40(2):516–533. doi: 10.1007/s10439-011-0454-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng C, He Z, Xing D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: implications for Alzheimer’s disease. J Neurosci. 2013;33(33):13505–13517. doi: 10.1523/JNEUROSCI.0918-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Taboada L, Yu J, El-Amouri S, Gattoni-Celli S, Richieri S, McCarthy T. et al. Transcranial laser therapy attenuates amyloid-β peptide neuropathology in amyloid-β protein precursor transgenic mice. J Alzheimers Dis. 2011;23(3):521–535. doi: 10.3233/JAD-2010-100894. [DOI] [PubMed] [Google Scholar]
- 19.de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low-level light therapy. IEEE J Sel Top Quantum Electron. 2016;22(3):7000417. doi: 10.1109/JSTQE.2016.2561201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamblin MR. Hamblin MRShining light on the head: photobiomodulation for brain disorders. BBA Clin. 2016;6:113–124. doi: 10.1016/j.bbacli.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 22.Wright RW, Brand RA, Dunn W, Spindler KP. How to write a systematic review. Clin OrthopRelat Res. 2007;455:23–9. doi: 10.1097/BLO.0b013e31802c9098. [DOI] [PubMed] [Google Scholar]
- 23.Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW. SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.da Luz Eltchechem C, Salgado ASI, Zângaro RA, da Silva Pereira MC, Kerppers II, da Silva LA. et al. Transcranial LED therapy on amyloid-β toxin 25–35 in the hippocampal region of rats. Lasers Med Sci. 2017;32(4):749–756. doi: 10.1007/s10103-017-2156-3. [DOI] [PubMed] [Google Scholar]
- 25.Purushothuman S, Johnstone DM, Nandasena C, Mitrofanis J, Stone J. Photobiomodulation with near infrared light mitigates Alzheimer’s disease-related pathology in cerebral cortex– evidence from two transgenic mouse models. Alzheimers Res Ther. 2014;6(1):2. doi: 10.1186/alzrt232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho GM, Lee SY, Park JH, Kim MJ, Park KJ, Choi BT. et al. Photobiomodulation using a low-level light-emitting diode improves cognitive dysfunction in the 5XFAD mouse model of Alzheimer’s disease. J GerontolA Biol Sci Med Sci. 2020;75(4):631–9. doi: 10.1093/gerona/gly240. [DOI] [PubMed] [Google Scholar]
- 27.Lu Y, Wang R, Dong Y, Tucker D, Zhao N, Ahmed ME. et al. Low-level laser therapy for beta amyloid toxicity in rat hippocampus. Neurobiol Aging. 2017;49:165–182. doi: 10.1016/j.neurobiolaging.2016.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liang J, Liu L, Xing D. Photobiomodulation by low-power laser irradiation attenuates Aβ-induced cell apoptosis through the Akt/GSK3β/β-catenin pathway. Free Radic Biol Med. 2012;53(7):1459–1467. doi: 10.1016/j.freeradbiomed.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 29.Zhang L, Xing D, Zhu D, Chen Q. Low-power laser irradiation inhibiting Aβ25-35-induced PC12 cell apoptosis via PKC activation. Cell PhysiolBiochem. 2008;22(1-4):215–222. doi: 10.1159/000149799. [DOI] [PubMed] [Google Scholar]
- 30.Zhang H, Wu S, Xing D. Inhibition of Aβ25–35-induced cell apoptosis by low-power-laser-irradiation (LPLI) through promoting Akt-dependent YAP cytoplasmic translocation. Cell Signal. 2012;24(1):224–232. doi: 10.1016/j.cellsig.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 31.Yang X, Askarova S, Sheng W, Chen JK, Sun AY, Sun GY. et al. Low energy laser light (6328 nm) suppresses amyloid-β peptide-induced oxidative and inflammatory responses in astrocytes. Neuroscience. 2010;171(3):859–868. doi: 10.1016/j.neuroscience.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL. et al. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4(3):233–4. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 33.Hook VYH, Kindy M, Reinheckel T, Peters C, Hook G. Genetic cathepsin B deficiency reduces β-amyloid in transgenic mice expressing human wild-type amyloid precursor protein. BiochemBiophys Res Commun. 2009;386(2):284–288. doi: 10.1016/j.bbrc.2009.05.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chao CC, Hu S, Sheng WS, Bu D, Bukrinsky MI, Peterson PK. Cytokine-stimulated astrocytes damage human neurons via a nitric oxide mechanism. Glia. 1996;16(3):276–284. doi: 10.1002/(SICI)1098-1136(199603)16:3<276::AIDGLIA10>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 35.Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to β-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93(1-2):182–193. doi: 10.1016/s0165-5728(98)00226-4. [DOI] [PubMed] [Google Scholar]
- 36.Apelt J, Schliebs R. β-amyloid-induced glial expression of both pro-and anti-inflammatory cytokines in cerebral cortex of aged transgenic Tg2576 mice with Alzheimer plaque pathology. Brain Res. 2001;894(1):21–30. doi: 10.1016/s0006-8993(00)03176-0. [DOI] [PubMed] [Google Scholar]
- 37.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24(3):173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 38.Hu J, Akama KT, Krafft GA, Chromy BA, Van Eldik LJ. Amyloid-β peptide activates cultured astrocytes: morphological alterations, cytokine induction and nitric oxide release. Brain Res. 1998;785(2):195–206. doi: 10.1016/s0006-8993(97)01318-8. [DOI] [PubMed] [Google Scholar]
- 39.Yates SL, Burgess LH, Kocsis-Angle J, Antal JM, Dority MD, Embury PB. et al. Amyloid β and amylin fibrils induce increases in proinflammatory cytokine and chemokine production by THP-1 cells and murine microglia. J Neurochem. 2000;74(3):1017–1025. doi: 10.1046/j.1471-4159.2000.0741017.x. [DOI] [PubMed] [Google Scholar]
- 40.Pettegrew JW, Panchalingam K, Klunk WE, McClure RJ, Muenz LR. Alterations of cerebral metabolism in probable Alzheimer’s disease: a preliminary study. Neurobiol Aging. 1994;15(1):117–132. doi: 10.1016/0197-4580(94)90152-x. [DOI] [PubMed] [Google Scholar]
- 41.Bosetti F, Brizzi F, Barogi S, Mancuso M, Siciliano G, Tendi EA. et al. Cytochrome c oxidase and mitochondrial F1F0-ATPase (ATP synthase) activities in platelets and brain from patients with Alzheimer’s disease. Neurobiol Aging. 2002;23(3):371–376. doi: 10.1016/s0197-4580(01)00314-1. [DOI] [PubMed] [Google Scholar]
- 42.Terni B, Boada J, Portero-Otin M, Pamplona R, Ferrer I. Mitochondrial ATP-synthase in the entorhinal cortex is a target of oxidative stress at stages I/II of Alzheimer’s disease pathology. Brain pathol. 2010;20(1):222–233. doi: 10.1111/j.1750-3639.2009.00266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hatefi Y. The mitochondrial electron transport and oxidative phosphorylation system. Annu Rev Biochem. 1985;54:1015–1069. doi: 10.1146/annurev.bi.54.070185.005055. [DOI] [PubMed] [Google Scholar]
- 44.Ludwig B, Bender E, Arnold S, Hüttemann M, Lee I, Kadenbach B. Cytochrome C oxidase and the regulation of oxidative phosphorylation. Chembiochem. 2001;2(6):392–403. doi: 10.1002/1439-7633(20010601)2:6<392::AIDCBIC392>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 45.Barnes CA, Jung MW, McNaughton BL, Korol DL, Andreasson K, Worley PF. LTP saturation and spatial learning disruption: effects of task variables and saturation levels. J Neurosci. 1994;14(10):5793–5806. doi: 10.1523/JNEUROSCI.14-10-05793.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P. et al. Object recognition test in mice. Nat Protoc. 2013;8(12):2531–7. doi: 10.1038/nprot.2013.155. [DOI] [PubMed] [Google Scholar]
- 47.Counts SE, Alldred MJ, Che S, Ginsberg SD, Mufson EJ. Synaptic gene dysregulation within hippocampal CA1 pyramidal neurons in mild cognitive impairment. Neuropharmacology. 2014;79:172–179. doi: 10.1016/j.neuropharm.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]