Abstract
Introduction: Antimicrobial photodynamic therapy (aPDT) as a supplement to the conventional root canal preparation has shown promising results. Previous studies have adopted various combinations of light sources and photosensitizers, which makes it difficult to compare the disinfection efficacy of different PDT protocols. The aim of the present study was to compare the efficacy of three photosensitizers (toluidine blue, methylene blue, and curcumin) in PDT using LED against Enterococcus faecalis in root canal disinfection.
Methods: Root canals of 54 single-rooted extracted teeth were prepared using the ProTaper Gold rotary system and were incubated with E. faecalis for three weeks. They were then randomly divided into five experimental groups and a control group: (1) Irrigation with 2.5% NaOCl for 30 seconds, (2) NaOCl irrigation followed by TB-PDT, (3) NaOCl irrigation followed by MB-PDT, (4) NaOCL irrigation followed by curcumin-PDT, (5) Curcumin solvent (1% ethanol+1% BSA), (6) Control (irrigation with normal saline). Sampling was done by collecting dentin shavings from the root canals, and colony-forming units were determined for each treatment group. The data were analyzed by Kruskal-Wallis and Mann-Whitney U tests. The significance level was set at P<0.05.
Results: In all treatment groups, the mean values of colony forming unit (CFU) decreased by 99% compared to the control group. The lowest mean values of CFU were observed in groups 2 and 4, followed groups 3, 1, and 5 respectively. The mean CFU count in group 2 was significantly lower than that of group 1 (P value=0.011), while there were no significant differences among groups 1, 3, and 4 (P value >0.05).
Conclusion: The adjunction of toluidine blue-mediated PDT by means of a light-emitting diode to NaOCl irrigation increased its antibacterial efficacy against E. faecalis and could be an effective complementary method in root canal disinfection.
Keywords: Antimicrobial photodynamic therapy, LED, Enterococcus faecalis, Toluidine blue, Methylene blue, Curcumin
Introduction
Microorganisms serve as the main etiology of pulpal and periapical disease. Therefore, the main objective of endodontic treatment is to eliminate the microorganisms from the root canal system. Mechanical debridement, application of chemical irrigants and other antimicrobial protocols are crucial to achieving this purpose.1,2 Due to its antibacterial and tissue dissolving properties, sodium hypochlorite (NaOCl) is considered as the gold standard chemical irrigant.3 However, its limited penetration depth into dentinal tubules prevents direct contact with the bacteria residing in deep layers of dentin.4,5 The penetration depth of NaOCl was reported to be in the range of 40 to 309 µm,6 while Enterococcus faecalis may penetrate up to 1000 µm inside dentinal tubules.7 Novel approaches such as the use of high power lasers and photodynamic therapy (PDT) have been proposed to enhance the efficacy of conventional chemo-mechanical preparation of the root canal.8,9 Light is considered to provide greater access to the areas unreachable by conventional techniques owing to its better penetration into dentin tissue.9,10 The bactericidal effect of high-power lasers has been reported in several studies.11 However, their intra-canal use could be associated with damages to dental and periapical tissues such as carbonization, ankyloses, root resorption, and peri-radicular necrosis.12
Antimicrobial photodynamic therapy (aPDT) involves the activation of a non-toxic dye (photosensitizer) by the light of a specific wavelength in the presence of molecular oxygen which results in oxidative damage to microorganisms by producing reactive oxygen species.13 The lower surface tension of aqueous solutions of photosensitizers may facilitate their diffusion into dentin as compared to NaOCl.14 Unlike high-power lasers that act through photothermal effects,1 the lethal action of PDT is based on photochemical reactions, which allows eliminating the microorganisms without causing thermal damages to the adjacent tissues.15 The main types of light sources used in clinical PDT are lasers, light-emitting diodes (LED), and halogen lamps.16 LED lamps are considered safe light sources due to less heat production. Easy application and cost-effectiveness are some of their advantages over lasers.17
The advantage of photodynamic therapy as a supplement to the conventional chemomechanical preparation of the root canal has been shown in the previous studies,15,18 yet there is no standard protocol for its clinical use.
The outcome of PDT relies on the interactions of the light, photosensitizer (PS) and oxygen.19 The major groups of PSs used in PDT are hematoporphyrin derivatives, phenothiazines, cyanines, phytotherapic agents, phthalocyanines and chlorines.15 Phenothiazines such as methylene blue (MB) and toluidine blue (TB) are the most studied PSs for biofilm inactivation.20 As MB and TB are amphiphilic, they can be used against both gram-positive and gram-negative bacteria present in endodontic infections.15
Curcumin is a natural phenolic compound extracted from the rootstocks of Curcuma longa and is used as a food additive.21,22 Owing to its substantial oxidative properties and high ability of light absorption, curcumin seems to be an appropriate PS for PDT and it has been shown to cause promising results in PDT against oral pathogens.22 A previous study reported that light activation of curcumin using LED resulted in significantly higher antibacterial efficacy compared to ultrasonic activation of NaOCl.23
Previous studies have adopted various combinations of light sources and PSs, as well as different light parameters, PS concentrations,15 and methods for biofilm cultivation,20 which makes it difficult to compare the disinfection efficacy of different PDT protocols. The aim of the present study was to compare the efficacy of three PSs (TB, MB, and curcumin) in PDT using FotoSan® 630 LED against E. faecalis as an adjunct to the conventional root canal disinfection strategies.
Materials and Methods
Sample Preparation
This in vitro study was carried out on 54 human teeth extracted due to periodontal disease or severely carious crowns. The selected teeth were central and lateral incisors and premolars with intact, completely developed roots and <30 degrees of root curvature. The presence of a single canal was confirmed by buccolingual and mesiodistal radiographs. After extraction the root surfaces were cleaned using a curette and the teeth were stored in 5.25% sodium hypochlorite solution for 5 days. The teeth were then decoronated using a disc in order to achieve a standard root length of 12 mm. The working length of the root canals was established at 0.5 mm distance from the apices by inserting a #10 K-file (Dentsply Maillefer, Tulsa, OK, USA) into the canal and observing its tip at the apical foramen. The apical foramina were then sealed with glass ionomer cement (GC Fuji II, Tokyo, Japan). Root canal instrumentation was performed using ProTaper Gold rotary files (Dentsply Maillefer, Tulsa, OK, USA) at 300 rpm driven by the Reciproc motor (VDW, Munich, Germany) according to the sequence recommended by the manufacturer up to #30 file (Master apical file=30). After instrumentation with each file patency was ensured by inserting a #10 K-file 1 mm beyond the working length and the canals were irrigated with 5 mL of 2.5% sodium hypochlorite using a syringe with a 30-gauge needle. After canal preparation irrigation was performed with 1 mL of 17% EDTA for 3 minutes in order to remove the smear layer followed by a final rinse with 5 mL of 2.5% NaOCl and 5 mL saline. The canals were then dried using paper points. Afterwards the specimens were placed in test tubes containing sterile brain heart infusion (BHI) broth (Merck, Darmstadt, Germany) and were sterilized in an autoclave at 121°C for 15 minutes. The tubes were then sealed and incubated at 37°C for 48 hours. To ensure the absence of bacterial contamination, 6 specimens were randomly selected and incubated in a sterile BHI medium. No bacterial growth was detected after 24 hours.
Root Canal Contamination With Enterococcus faecalis
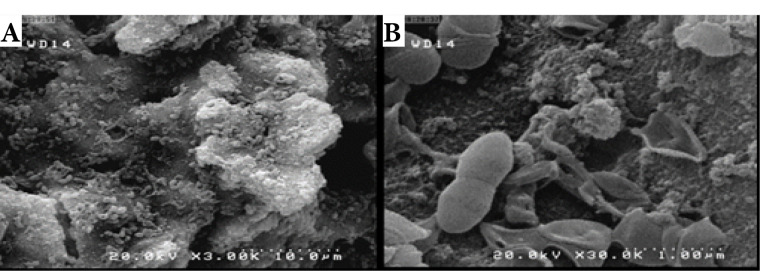
Enterococcus faecalis bacteria (ATCC9854) taken from a frozen stock culture were transferred into the BHI medium and incubated at 37°C for 24 hours. Single colonies were inoculated in 10 ml of BHI broth and were incubated at 37°C for 24 hours. A 1.5×108CFU/mL bacterial suspension equivalent to 0.5 McFarland was prepared. (The optical density (OD600) of the suspension was spectrophotometrically set to 0.08-0.1). The roots were individually placed in sterile test tubes. 1 mL of 0.5 McFarland bacterial suspension was injected into each tube using an insulin syringe. The specimens were then incubated at 37°C under anaerobic conditions for three weeks. The BHI broth media were refreshed on alternate days. Two specimens from the control group were sectioned longitudinally and placed in fixative solution (2.5% glutaraldehyde) at 4°C for 24 hours. The specimens were then dehydrated in ascending graded ethanol (50%, 70%, 85%, 90%, 95%, once each, and twice in 100%) for 20 minutes at each concentration and were dried at room temperature. Subsequently, the dentin sections were sputter-coated with gold under vacuum and examined by a scanning electron microscope to ensure biofilm formation on the root canal surface and in the dentinal tubules (Figure 1).
Figure 1.
SEM Images of Samples After 3 Weeks of Incubation With E. faecalis: A (3000×), B (30000×).
Treatment Groups
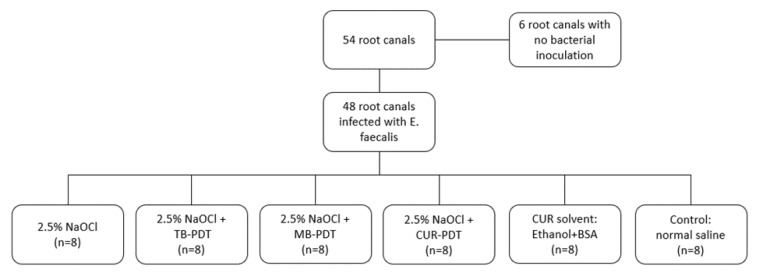
After 3 weeks of incubation the media were evacuated and the roots were rinsed thoroughly with normal saline in order to remove the planktonic bacteria. The outer surfaces of the roots were coated with nail varnish. The specimens were then assigned to 5 treatment groups (n=8) and a control group (n=8) using simple randomization (Figure 2).
Figure 2.
Division of the Groups.
Group 1: Root canal irrigation with sodium hypochlorite (NaOCl)
Group 2: NaOCl irrigation followed by toluidine blue-mediated PDT (NaOCl+TB)
Group 3: NaOCl irrigation followed by methylene blue-mediated PDT (NaOCl+MB)
Group 4: NaOCl irrigation followed by curcumin-mediated PDT (NaOCl+CUR)
Group 5: Application of curcumin solvent (1% ethanol+1% BSA) for 120 seconds (CUR solvent)
Control group: Root canal irrigation with 5 mL of sterile normal saline for 30 seconds using a syringe with a 30-gauge needle inserted 1 mm short of the apices.
NaOCl Irrigation
In groups 1-4, root canal irrigation was performed with 5 mL of 2.5% NaOCl for 30 seconds using a syringe with a 30-gauge needle inserted 1 mm short of the apices. To neutralize NaOCl, the root canals were rinsed with 1 mL of 5% sodium thiosulfate (Na2S2O3) (Merck, Germany) and normal saline for 30 seconds.
Photosensitizers
TB and MB solutions at concentrations of 0.5 mg/mL were prepared using normal saline. Curcumin was dissolved in a solvent containing 1% ethanol and 1% bovine serum albumin (BSA) to a concentration of 0.5 mg/mL.
Photodynamic Therapy
In groups 2-4, the respective photosensitizers were injected into the root canals and remained for 120 seconds prior to irradiation. PDT was carried out for 60 seconds by means of an LED lamp (FotoSan 630, CMS Dental, Copenhagen, Denmark) emitting light in the red spectrum with a power peak at 630nm and output intensity of 2000-4000 mw/cm2. The irradiation was performed using an endodontic tip of 0.5 mm diameter which was introduced into the canal up to ½ of the working length.
Sampling and Colony Counting
The roots were rinsed with normal saline and sampling was done by collecting dentin shavings. Sterile F3 ProTaper Gold rotary files were used in the root canals for 30 seconds. The files were then transferred into test tubes containing 10 ml of normal saline and were vortexed for 1 minute. The bacterial suspensions were diluted using ten-fold serial dilutions and cultured on BHI agar plates using the Spread plate technique. After 24 hours of incubation colony forming units (CFUs) were counted using a colony counter (Teif Azma Teb, Iran). The actual bacterial counts were calculated based on the corresponding dilution factor and the results were reported as CFU/mL.
Statistical Analysis
Data were analyzed using SPSS version 18 (SPSS Inc., IL, USA). The mean and standard deviation of CFU values in the control and treatment groups were reported. The Kruskal-Wallis and Mann-Whitney U tests were used for comparing the disinfection efficacy of the three photosensitizers. The significance level was set at P˂0.05.
Results
The mean CFU count in the control group was considered as the baseline for comparison among the treatment groups. In all treatment groups CFU counts decreased by 99% relative to the control group (Table 1).
Table 1. CFU/mL Counts of Enterococcus faecalis, Percentage of Culture-Negative Samples and Bacterial Reduction After Antibacterial Treatments .
| Group | Incidence of Negative Cultures (%) | CFU/mL | Bacterial Reduction Relative to the Control Group (%) | |
| Mean | SD | |||
| Control | 0 | 4.4×108 | 3.7×107 | - |
| NaOCl | 0 | 1.4×105 | 1.4×105 | 99.97 |
| Curcumin solvent | 0 | 7.9×105 | 9.4×105 | 99.82 |
| NaOCl+CUR | 33.3 | 5.9×103 | 8×103 | 99.99 |
| NaOCl+TB | 66.7 | 4.2×103 | 6.5×103 | 99.99 |
| NaOCl+MB | 33.3 | 1.3×104 | 1.96×104 | 99.99 |
The mean value of CFU in the control group was significantly higher than that of all the treatment groups. Among treatment groups, the lowest mean value of CFU was observed in the NaOCl+TB group followed by the NaOCl+CUR, NaOCl+MB, NaOCl, and CUR solvent groups respectively. The mean CFU count in the NaOCl+TB group was significantly lower than that of the NaOCl group. There were no significant differences among the NaOCl, NaOCl+CUR, and NaOCl+MB groups (Table 2).
Table 2. P Values for Comparison Among CFU Means of Different Experimental Groups.
| Group | Control | NaOCl+CUR | NaOCl+TB | NaOCL+MB | NaOCl | CUR Solvent |
| Control | 0.007 | 0.001 | 0.002 | 0.013 | 0.039 | |
| NaOCl+CUR | 0.007 | 0.443 | 0.904 | 0.078 | 0.006 | |
| NaOCl+TB | 0.001 | 0.443 | 0.203 | 0.011 | 0.002 | |
| NaOCl+MB | 0.002 | 0.904 | 0.203 | 0.067 | 0.006 | |
| NaOCl | 0.013 | 0.078 | 0.011 | 0.067 | 0.201 | |
| CUR solvent | 0.039 | 0.006 | 0.002 | 0.006 | 0.201 |
Discussion
Enterococcus faecalis is the most common bacterial species in the root canals of teeth with persistent periradicular lesions.24 It has been shown that it is resistant to conventional disinfection strategies.25 In this study, a 21-day old E. faecalis biofilm model was used for contaminating the root canals.
Most studies have used paper cones for sampling the root canal which restricts the samples to a portion of planktonic bacteria from the fluid in the root canal and underestimates the bacterial counts.26,27 Filing the root canal wall is needed to improve sampling the bacteria organized in a biofilm structure.28 In the current study, dentin shavings were collected. This method allows sampling the bacteria present in the dentinal tubules.26
Although PDT as a stand-alone treatment may not be sufficient for eliminating the bacteria from the root canal system, it has been shown that it is a promising adjunctive method for further reduction of the microorganisms after the conventional chemo-mechanical preparation.15 In the present study, PDT was carried out using an LED lamp (FotoSan® 630) and three photosensitizers (methylene blue, toluidine blue, and curcumin) after root canal irrigation with 2.5% sodium hypochlorite. All the PSs were used at the same concentration of 0.5 mg/mL to compare their antibacterial efficacy in PDT.
FotoSan 630 is an LED device developed for aPDT for endodontic and periodontal applications emitting light in the red spectrum with peak power at 630 nm.
LED lamps are safer alternative light sources as they do not cause a significant change in temperature.26 The use of LED in aPDT against E. faecalis has shown positive results.17,26
In the previous studies, various organic solvents have been used for dissolving curcumin, including ethanol, n-methyl-glucamine, and DMSO (dimethyl sulfoxide).22,29 The binding of curcumin to BSA increases its solubility in aqueous solutions.30 In the present study, curcumin was dissolved in a solvent containing 1% ethanol and 1% BSA.
The absorption peak of the PS should be in accordance with the wavelength of the light in order to produce reactive oxygen species (ROS) such as singlet oxygen which induce injury or death to microorganisms.31,32 The maximum absorption doses of the PSs MB, TB, and Curcumin are 660, 630, and 450 nm respectively,33 and the wavelength of the LED lamp used in the present study was 630 nm. Evaluating the photochemical and antimicrobial effects of phytotherapeutic PSs, Nardini et al32 reported that curcumin presented similar ROS production to MB when activated at 660 nm. Moreover, the activation of curcumin by a red light LED (660 nm) led to a statistically significant reduction in planktonic cultures and biofilms of E. faecalis.
The results of the present study demonstrated that PDT using all three PSs significantly reduced the E. faecalis CFU/mL. The adjunction of PDT to NaOCl irrigation led to a greater bactericidal effect compared to the conventional endodontic treatment. This finding is consistent with previous studies.3,12,13,15 It should be noted that in the present study, TB-PDT was the only treatment which led to a statistically significant additional bactericidal effect compared to the NaOCl group. Vaziri et al assessed the antibacterial effect of 2.5% NaOCl and the combination of 2.5% NaOCl irrigation with TB-PDT using a 625 nm diode laser against E. faecalis and reported superior bactericidal efficacy of the combination over the sole use of NaOCl.34
Souza et al investigated the antibacterial effect of MB- and TB-PDT using 660 nm diode lasers. The results showed that although PDT enhanced the disinfection efficacy of instrumentation and irrigation with NaOCl, the effect did not reach statistical significance.31 This may be due to the low concentration of the PSs (0.15 μg/mL) used in their study.
Lopez-Jimenez et al evaluated the effect of TBO-mediated PDT using LED (FotoSan 630) and MB-mediated PDT using a 670 nm diode laser on E. faecalis biofilms cultures. The concentrations of TB and MB used in the study were 0.1 mg/mL and 0.05 mg/mL respectively. Confocal laser scanning microscopy results showed a significant increase in bacterial death in PDT-treated samples with no significant difference between the two PDT protocols.35
Pourhajibagher et al compared the antimicrobial and anti-biofilm effects of different PSs in aPDT using a diode laser and an LED. They reported that CUR-PDT led to the highest bactericidal and anti-biofilm activity against E. faecalis, which was significantly more than that of TB- and MB- PDT.33 This may be due to a different wavelength of LED used for activating curcumin in their study (450 nm) which led to greater absorption of light by this PS.
The results of the present study showed that PDT using 0.5 mg/mL TB enhanced the efficacy of NaOCl in reducing the CFUs of E. faecalis. This finding needs to be confirmed by further studies and clinical trials. Although all treatments significantly reduced the CFUs, there was no significant difference among adjunctive MB- and curcumin-mediated PDT and the sole use of sodium hypochlorite in terms of reducing the CFUs. Likewise, Oda et al reported similar disinfection efficacy of MB- and Cur-PDT using a 660 nm diode laser and a blue LED respectively.36 Given the lower cost of curcumin and less discoloration caused by its use compared to other PSs, further research using different concentrations, light sources, pre-irradiation and irradiation times and energy dosages is recommended to determine appropriate parameters for its application in PDT.
Conclusion
Within the limitations of this study, the adjunction of PDT using a 0.5 mg/mL TB photosensitizer and an LED to NaOCl irrigation increased its antibacterial efficacy against E. faecalis and could be an effective complementary method in root canal disinfection.
Ethical Considerations
Ethical approval was obtained from the research ethics committee of Shahid Beheshti University (IR.SBMU.RETECH.REC.1396.322).
Conflict of Interests
The authors declare no conflict of interest.
Acknowledgements
This research was supported by the grant of Laser Application in Medical Sciences Research Center of Shahid Beheshti Medical University.
Please cite this article as follows: Mozayeni MA, Vatandoost F, Asnaashari M, Shokri M, Azari-Marhabi S, Asnaashari N. Comparing the efficacy of toluidine blue, methylene blue and curcumin in photodynamic therapy against Enterococcus faecalis. J Lasers Med Sci. 2020;11(suppl 1):S49-S54. doi:10.34172/jlms.2020.S8.
References
- 1.Chiniforush N, Pourhajibagher M, Shahabi S, Kosarieh E, Bahador A. Can antimicrobial photodynamic therapy (aPDT) enhance the endodontic treatment? J Lasers Med Sci. 2016;7(2):76–85. doi: 10.15171/jlms.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahimi S, Janani M, Lotfi M, Shahi S, Aghbali A, Vahid Pakdel M. et al. A review of antibacterial agents in endodontic treatment. Iran Endod J. 2014;9(3):161–8. doi: 10.22037/iej.v9i3.5086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chrepa V, Kotsakis GA, Pagonis TC, Hargreaves KM. The effect of photodynamic therapy in root canal disinfection: A systematic review. J Endod. 2014;40(7):891–8. doi: 10.1016/j.joen.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 4.Berutti E, Marini R, Angeretti A. Penetration ability of different irrigants into dentinal tubules. J Endod. 1997;23(12):725–7. doi: 10.1016/s0099-2399(97)80342-1. [DOI] [PubMed] [Google Scholar]
- 5.Mohammadi Z. Laser applications in endodontics: an update review. Int Dent J. 2009;59(1):35–46. doi: 10.1922/IDJ_2006Mohammadi12. [DOI] [PubMed] [Google Scholar]
- 6.Ghorbanzadeh A, Aminsobhani M, Sohrabi K, Chiniforush N, Ghafari S, Shamshiri AR. et al. Penetration depth of sodium hypochlorite in dentinal tubules after conventional irrigation, passive ultrasonic agitation and Nd:YAG laser activated irrigation. J Lasers Med Sci. 2016;7(2):105–11. doi: 10.15171/jlms.2016.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haapasalo M, Ørstavik D. In vitro infection and disinfection of dentinal tubules. J Dent Res. 1987;66(8):1375–9. doi: 10.1177/00220345870660081801. [DOI] [PubMed] [Google Scholar]
- 8.Kishen A. Advanced therapeutic options for endodontic biofilms. Endod Topics. 2012;22(1):99–123. doi: 10.1111/j.1601-1546.2012.00284.x. [DOI] [Google Scholar]
- 9.Bago I, Plečko V, Gabrić Pandurić D, Schauperl Z, Baraba A, Anić I. Antimicrobial efficacy of a high-power diode laser, photo-activated disinfection, conventional and sonic activated irrigation during root canal treatment. Int Endod J. 2013;46(4):339–47. doi: 10.1111/j.1365-2591.2012.02120.x. [DOI] [PubMed] [Google Scholar]
- 10.Schoop U, Kluger W, Moritz A, Nedjelik N, Georgopoulos A, Sperr W. Bactericidal effect of different laser systems in the deep layers of dentin. Lasers Surg Med. 2004;35(2):111–16. doi: 10.1002/lsm.20026. [DOI] [PubMed] [Google Scholar]
- 11.Abdo S, Alkaisi A, Saleem M, Zetouni J. Clinical applications of lasers in endodontic. J Dent Res. 2018;1(1):1003. [Google Scholar]
- 12.Trindade AC, De Figueiredo JA, Steier L, Weber JB. Photodynamic therapy in endodontics: A literature review. Photomed Laser Surg. 2015;33(3):175–82. doi: 10.1089/pho.2014.3776. [DOI] [PubMed] [Google Scholar]
- 13.Pourhajibagher M, Bahador A. Adjunctive antimicrobial photodynamic therapy to conventional chemo-mechanical debridement of infected root canal systems: A systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2019;26:19–26. doi: 10.1016/j.pdpdt.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Pileggi G, Wataha JC, Girard M, Grad I, Schrenzel J, Lange N. et al. Blue light-mediated inactivation of Enterococcus faecalis in vitro. Photodiagnosis Photodyn Ther. 2013;10(2):134–40. doi: 10.1016/j.pdpdt.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Plotino G, Grande NM, Mercade M. Photodynamic therapy in endodontics. Int Endod J. 2019;52(6):760–74. doi: 10.1111/iej.13057. [DOI] [PubMed] [Google Scholar]
- 16.Nagata JY, Hioka N, Kimura E, Batistela VR, Terada RS, Graciano AX. et al. Antibacterial photodynamic therapy for dental caries: evaluation of the photosensitizers used and light source properties. Photodiagnosis Photodyn Ther. 2012;9(2):122–31. doi: 10.1016/j.pdpdt.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 17.Asnaashari M, Mojahedi SM, Asadi Z, Azari-Marhabi S, Maleki A. A comparison of the antibacterial activity of the two methods of photodynamic therapy (using diode laser 810 nm and LED lamp 630 nm) against Enterococcus faecalis in extracted human anterior teeth. Photodiagnosis Photodyn Ther. 2016;13:233–37. doi: 10.1016/j.pdpdt.2015.07.171. [DOI] [PubMed] [Google Scholar]
- 18.Firmino RT, Brandt LM, Ribeiro GL, Dos Santos KS, Catão MH, Gomes DQ. Endodontic treatment associated with photodynamic therapy: Case report. Photodiagnosis Photodyn Ther. 2016;15:105–8. doi: 10.1016/j.pdpdt.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 19.Chiniforush N, Pourhajibagher M, Shahabi S, Bahador A. Clinical approach of high technology techniques for control and elimination of endodontic microbiota. J Lasers Med Sci. 2015;6(4):139–50. doi: 10.15171/jlms.2015.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cieplik F, Tabenski L, Buchalla W, Maisch T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front Microbiol. 2014;5:405. doi: 10.3389/fmicb.2014.00405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cieplik F, Deng D, Crielaard W, Buchalla W, Hellwig E, Al-Ahmad A. et al. Antimicrobial photodynamic therapy - what we know and what we don’t. Crit Rev Microbiol. 2018;44(5):571–89. doi: 10.1080/1040841X.2018.1467876. [DOI] [PubMed] [Google Scholar]
- 22.Santezi C, Reina BD, Dovigo LN. Curcumin-mediated photodynamic therapy for the treatment of oral infections-A review. Photodiagnosis Photodyn Ther. 2018;21:409–15. doi: 10.1016/j.pdpdt.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Neelakantan P, Cheng CQ, Ravichandran V, Mao T, Sriraman P, Sridharan S. et al. Photoactivation of curcumin and sodium hypochlorite to enhance antibiofilm efficacy in root canal dentin. Photodiagnosis Photodyn Ther. 2015;12(1):108–14. doi: 10.1016/j.pdpdt.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 24.Arias-Moliz MT, Ferrer-Luque CM, Espigares-García M, Baca P. Enterococcus faecalis biofilms eradication by root canal irrigants. J Endod. 2009;35(5):711–4. doi: 10.1016/j.joen.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 25.Dunavant TR, Regan JD, Glickman GN, Solomon ES, Honeyman AL. Comparative evaluation of endodontic irrigants against Enterococcus faecalis biofilms. J Endod. 2006;32(6):527–31. doi: 10.1016/j.joen.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 26.Rios A, He J, Glickman GN, Spears R, Schneiderman ED, Honeyman AL. Evaluation of photodynamic therapy using a light-emitting diode lamp against Enterococcus faecalis in extracted human teeth. J Endod. 2011;37(6):856–9. doi: 10.1016/j.joen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 27.Tennert C, Drews AM, Walther V, Altenburger MJ, Karygianni L, Wrbas KT. et al. Ultrasonic activation and chemical modification of photosensitizers enhances the effects of photodynamic therapy against Enterococcus faecalis root-canal isolates. Photodiagnosis Photodyn Ther. 2015;12(2):244–51. doi: 10.1016/j.pdpdt.2015.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Sakko M, Tjäderhane L, Rautemaa-Richardson R. Microbiology of root canal infections. Prim Dent J. 2016;5(2):84–9. doi: 10.1308/205016816819304231. [DOI] [PubMed] [Google Scholar]
- 29.Cusicanqui Méndez DA, Gutierres E, José Dionisio E, Afonso Rabelo Buzalaf M, Cardoso Oliveira R, Andrade Moreira Machado MA. et al. Curcumin-mediated antimicrobial photodynamic therapy reduces the viability and vitality of infected dentin caries microcosms. Photodiagnosis Photodyn Ther. 2018;24:102–8. doi: 10.1016/j.pdpdt.2018.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Mitra SP. Binding and stability of curcumin in presence of bovine serum albumin. J Surface Sci Technol. 2007;23(3-4):91–110. [Google Scholar]
- 31.Souza LC, Brito PR, de Oliveira JC, Alves FR, Moreira EJ, Sampaio-Filho HR. et al. Photodynamic therapy with two different photosensitizers as a supplement to instrumentation/irrigation procedures in promoting intracanal reduction of Enterococcus faecalis. J Endod. 2010;36(2):292–6. doi: 10.1016/j.joen.2009.09.041. [DOI] [PubMed] [Google Scholar]
- 32.Nardini EF, Almeida TS, Yoshimura TM, Ribeiro MS, Cardoso RJ, Garcez AS. The potential of commercially available phytotherapeutic compounds as new photosensitizers for dental antimicrobial PDT: A photochemical and photobiological in vitro study. Photodiagnosis Photodyn Ther. 2019;27:248–54. doi: 10.1016/j.pdpdt.2019.05.027. [DOI] [PubMed] [Google Scholar]
- 33.Pourhajibagher M, Kazemian H, Chiniforush N, Hosseini N, Pourakbari B, Azizollahi A. et al. Exploring different photosensitizers to optimize elimination of planktonic and biofilm forms of Enterococcus faecalis from infected root canal during antimicrobial photodynamic therapy. Photodiagnosis Photodyn Ther. 2018;24:206–11. doi: 10.1016/j.pdpdt.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 34.Vaziri S, Kangarlou A, Shahbazi R, Nazari Nasab A, Naseri M. Comparison of the bactericidal efficacy of photodynamic therapy, 25% sodium hypochlorite, and 2% chlorhexidine against Enterococcus faecalis in root canals; an in vitro study. Dent Res J (Isfahan) 2012;9(5):613–8. doi: 10.4103/1735-3327.104882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.López-Jiménez L, Fusté E, Martínez-Garriga B, Arnabat-Domínguez J, Vinuesa T, Viñas M. Effects of photodynamic therapy on Enterococcus faecalis biofilms. Lasers Med Sci. 2015;30(5):1519–26. doi: 10.1007/s10103-015-1749-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oda DF, Duarte MAH, Andrade FB, Moriyama LT, Bagnato VS, de Moraes IG. Antimicrobial action of photodynamic therapy in root canals using LED curing light, curcumin and carbopol gel. Int Endod J. 2019;52(7):1010–19. doi: 10.1111/iej.13092. [DOI] [PubMed] [Google Scholar]