The rising incidence of early-age onset colorectal cancer (EOCRC) first came to widespread attention in 2017 when the American Cancer Society (ACS) published updated age-stratified CRC incidence rates using data from the Surveillance, Epidemiology, and End-Results Program (SEER).1 This research demonstrated that CRC incidence rates among people younger than age 50 in the United States had been rising since 1990. In 2018, the incorporation of these revised CRC incidence rates into microsimulation models led the ACS to issue a qualified recommendation to initiate CRC screening at age 45 instead of 50.2, 3 As this was contrary to other guidelines,4, 5 the ACS recommendation was initially met with skepticism. Some hypothesized that the increase in EOCRC incidence was artifactual from early diagnosis of asymptomatic CRCs secondary to increased utilization of CRC screening tests by young people.6-8 However, subsequent research demonstrated that, on average, the presenting stage of EOCRC is more advanced than that of traditional-onset CRC, thereby rendering the hypothesis of early diagnosis of asymptomatic CRCs unlikely.9-11 Despite these results, acceptance of the ACS recommendation has been limited; it is estimated that only 10% of primary care provides offer CRC screening prior to age 50.12
Low adoption of the ACS recommendation may be secondary to confusion from conflicting guidelines, uncertainty about insurance reimbursement, and status-quo bias.13 For example, the concept that CRC screening starts at age 50 is ingrained for many providers. Students who matriculated into health professions programs in 1997—when the first CRC screening guidelines were published—are now entering the third decade of their careers as medical providers.14 Consequently, healthcare providers have perceived archetypes of which patients develop CRC and how they present; they imagine a 60-year-old man with weight loss who has never had CRC screening or a 70-year-old woman who had a tubular adenoma at age 55 but presents too late for her surveillance colonoscopy. In contrast, due to gaps in the medical literature on patterns of pre-diagnosis symptoms, there is not a standard clinical vignette describing patients who may have EOCRC. This lack of data on EOCRC presenting symptoms combined with emphasis on value-based care may lead providers to limit their evaluation of young patients with abdominal symptoms, thereby missing opportunities to diagnose EOCRC at early stages. These gaps in the medical literature are partly because most prior studies exploring EOCRC in the United States rely on data from SEER,1, 9, 10, 15 which does not report information on symptoms or testing that preceded cancer diagnosis. On the other hand, the availability of administrative health data for clinical research provides an opportunity to study patient cohorts from symptom identification to testing to cancer diagnosis, but these have been minimally utilized thus far.
In October 2020, the U.S. Preventative Services Task Force (USPSTF) released draft guidelines that are consistent with the 2018 ACS recommendation to start CRC screening at age 45 (Grade B).16 Furthermore, because commercial insurers are mandated to provide coverage for USPSTF Grade A and B recommendations without cost sharing,17 these guidelines may ameliorate some of the hypothesized barriers to the adoption of CRC screening starting at age 45. However, without new research on the pre-diagnosis symptomatology of EOCRC, the benefits of EOCRC screening could be blunted by continued uncertainty about appropriate evaluation strategies for patients with gastrointestinal symptoms. To fill these knowledge gaps, we conducted a retrospective cohort study of individuals ages 18 – 49 in the United States using longitudinal patient-level health claims data. Using this cohort, we describe trends in the utilization of tests with the potential to diagnose EOCRC, clinical characteristics of those who undergo EOCRC evaluation, and risk factors and presenting symptoms of those who are ultimately diagnosed with EOCRC. Together, these results may provide context for the changing epidemiology of CRC and lead to additional research that clarifies the timing and modality of appropriate symptomatic evaluation for individuals at risk for EOCRC.
Characteristics of individuals ages 18 – 49
This retrospective cohort study was performed using data from Optum’s de-identified Clinformatics® Data Mart Database (Optum). Optum is a patient-level database consisting of the medical claims of 88 million unique enrollees of large commercial and Medicare Advantage health plans in the United States. The Institutional Review Board of the University of Pennsylvania has classified research using Optum as exempt.
Using Optum, we identified 23,977,025 unique individuals who were 18 – 49 years-old during 2004 – 2018 (Supplemental methods). The median follow-up in the cohort was 2.0 years (IQR 1.0 – 4.0 years). Individuals ages 45 – 49 accounted for 17% of the cohort. Other demographic characteristics stratified by year are presented in Supplemental table 1.
Cross-sectional imaging is the most frequently used test with the potential to diagnose EOCRC
The utilization of tests that can potentially diagnose EOCRC was assessed by identifying procedure claims with Current Procedural Terminology-4, Healthcare Common Procedure Coding System, or International Classification of Diseases (ICD) codes for lower endoscopy (colonoscopy or flexible sigmoidoscopy), fecal occult blood testing (FOBT), fecal immunochemical testing (FIT), FIT-DNA, barium enema, CT colonography, or cross-sectional imaging (CT-abdomen/pelvis or MRI-abdomen/pelvis). The utilization of each test was calculated as the number of tests performed in a given year divided by the number of person-years of follow-up in the general population cohort in that year. Individuals could contribute multiple tests per year in this analysis. Barium enema, CT-colonography, and FIT-DNA accounted for less than 1% of utilization in all years of follow-up, so these trends are not presented. The change of rates over time was assessed by calculating the average annual percent change (AAPC).18
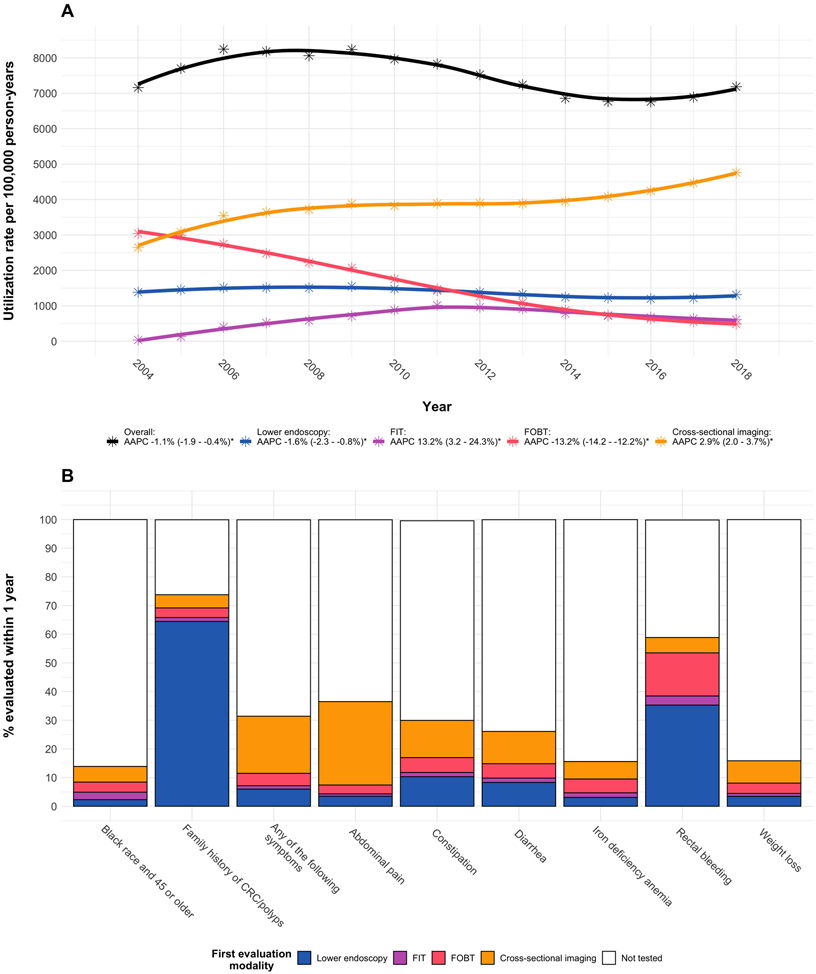
The overall utilization of tests that can potentially diagnose CRC declined by 1.1% per year (Figure 1A). This was largely driven by a 13.2% average annual decline in FOBT utilization that was only partially replaced by FIT. Lower endoscopy utilization declined by 1.6% per year, but cross-sectional imaging utilization increased by 2.9% per year and was the most frequently used modality by 2005. From 2005 – 2018, the percentage of all tests that were cross-sectional imaging grew from 40 – 66%.
Figure 1. Utilization of tests with the potential to diagnose EOCRC.
Panel A: Trends in test utilization stratified by modality
Panel B: Percent of individuals who underwent evaluation within 1 year of risk factor/symptom documentation, stratified by first modality completed
Notes: * Indicates AAPC 95% CI does not cross 0. Smooth trend lines were generated using LOESS.
There is high variability in testing practices for individuals with potential indications for EOCRC evaluation
We identified individuals who were eligible for early CRC screening or diagnostic testing using ICD codes. During the study period, individuals under age 50 with a family history of CRC or advanced polyps were eligible for early CRC screening. Starting in 2009, Black individuals who were age 45 – 49 were also eligible for early CRC screening by some guidelines.5, 19 Additionally, individuals with alarm symptoms (iron deficiency anemia, rectal bleeding, weight loss) or non-alarm gastrointestinal symptoms (e.g. abdominal pain, constipation, diarrhea) were potentially eligible for diagnostic testing that could lead to a purposeful or incidental diagnosis of EOCRC. Among individuals with each indication for testing, we calculated the percentage who completed a test with the potential to diagnose EOCRC within 1 year from the first claim for the risk factor/symptom. This percentage was stratified by first completed test modality.
Figure 1B illustrates the percentage of individuals who underwent evaluation within 1 year following risk factor/symptom documentation. Of potential indications, family history of CRC/polyps was evaluated most frequently at 74% within 1 year (64% by lower endoscopy). Only 14% of Black individuals age 45 were evaluated by age 46 (2% by lower endoscopy, 3% by FIT, 4% by FOBT, and 5% by cross-sectional imaging). When increasing the testing window to age 50, the percentage increased to 39% (8% by lower endoscopy, 7% by FIT, 9% by FOBT, and 15% by cross-sectional imaging). Of symptom indications for diagnostic testing, rectal bleeding was evaluated most often at 59% (35% by lower endoscopy). Iron deficiency anemia and weight loss were evaluated least often at 15% each. Incidence rates of each potential indications for early CRC screening or diagnostic evaluation increased over the study period with the exception of Black individuals turning age 45 and rectal bleeding (Supplemental figure 1).
More than two-thirds of EOCRCs present with gastrointestinal symptoms without another identifiable risk factor
We identified individuals with incident EOCRC if they had two or more ICD claims for CRC at least 60 days apart. Based on ICD-9 codes, this definition has 80% sensitivity and 99% specificity for CRC.20 We chose to use this high-specificity case-finding definition instead of criteria incorporating only one ICD code in order to avoid recording historical CRCs that had previously been treated. To calculate the incidence rate of EOCRC, the number of individuals diagnosed with EOCRC in a given year was divided by the total number of person-years contributed in that year by individuals at risk for EOCRC.
We also categorized EOCRCs by risk factor. EOCRCs were attributed to individuals with inflammatory bowel disease or individuals with a family history of CRC/polyps if the EOCRC was diagnosed after the first inflammatory bowel disease/family history claim. EOCRCs were attributed to individuals who were Black race and age 45 or older if the EOCRC was diagnosed within or after the year that they turned 45. EOCRCs were attributed to individuals with symptoms if they had a claim for the symptom of interest no more than 180 days before the EOCRC. If an EOCRC could be attributed to multiple subgroups, it was allocated to the risk factor based on the following hierarchy: inflammatory bowel disease > family history of CRC/polyps > Black race and age 45 or older > symptoms.
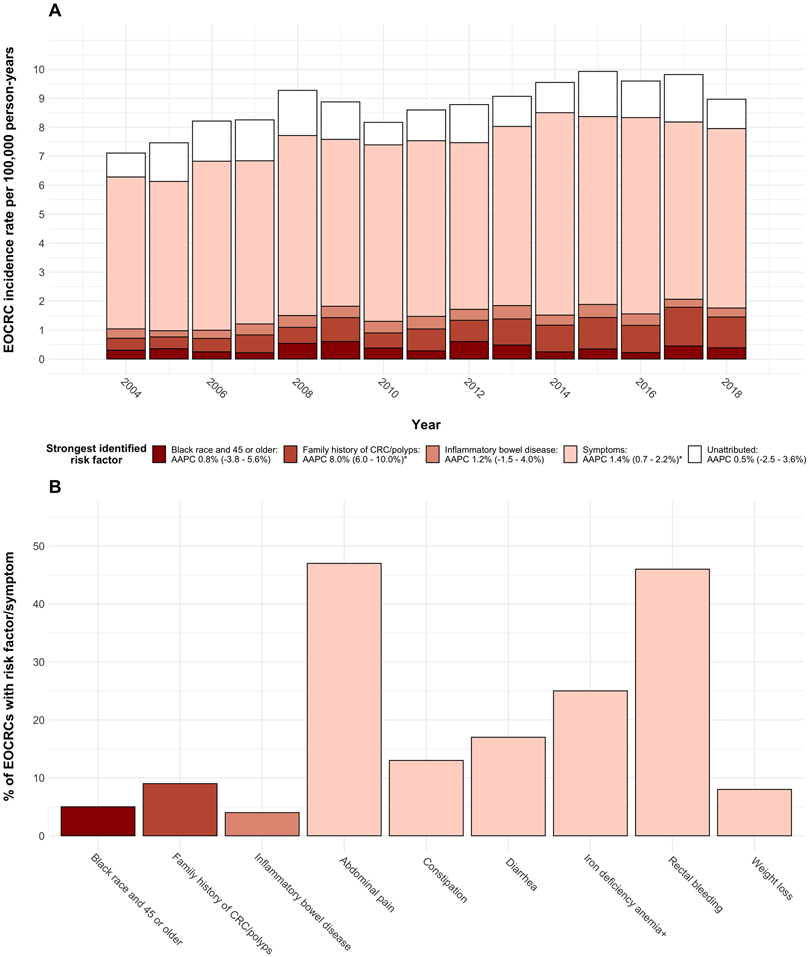
The total number of EOCRCs identified was 6163, and the EOCRC incidence rate rose by 1.8% per year during the study (AAPC 95% CI 1.1 – 2.6%) from 7.8 per 100,000 person-years in 2004 to a peak of 11.0 per 100,000 person-years in 2015 (Figure 2A). The incidence rates of EOCRCs attributed to inflammatory bowel disease or Black race and age older than 45 was stable throughout the study. The incidence rates of EOCRCs attributed to family history of CRC/polyps or symptoms rose throughout the study (AAPC 8.0% and 1.4%, respectively). In each year, symptoms were the most common identified risk factor, accounting for 63 – 75% of EOCRCs. Aggregated from 2004 – 2018, abdominal pain and rectal bleeding were the most common symptoms, contributing to 47% and 46% of EOCRCs, respectively (Figure 2B). Weight loss was the least common symptom at 8%. Black race and age older than 45 accounted for 5% of EOCRCs, family history of CRC/polyps accounted for 9%, and inflammatory bowel disease accounted for 4%.
Figure 2. EOCRC incidence rates and risk factors.
Panel A: Trend in EOCRC incidence rate stratified by strongest identified risk factor
Panel B: Percent of EOCRCs with risk factors, alarm symptoms, or non-alarm symptoms aggregated from 2004 – 2018
Note: * Indicates AAPC 95% CI does not cross 0
Implications for EOCRC diagnostic evaluation
In this cohort study of 18 – 49 year-olds in the United States, we describe utilization patterns of tests with the potential to diagnose EOCRC, clinical characteristics of individuals who undergo testing, and risk factors and presenting symptoms of individuals who are ultimately diagnosed with EOCRC. In particular, this study demonstrated that cross-sectional imaging is the most commonly utilized test among individuals with gastrointestinal symptoms, testing practices are highly variable among potential indications for evaluation, and gastrointestinal symptoms without another identifiable risk factor precede more than two-thirds of EOCRCs.
The results of this study may serve as preliminary data for future studies that investigate strategies for diagnostic evaluation of symptoms that could represent EOCRC. For example, although iron deficiency anemia and weight loss are considered red flag symptoms in individuals who present with gastrointestinal complaints, only 15% of individuals with either of these symptoms underwent evaluation within 1 year. Subtle weight loss has previously been associated with EOCRC,21 so ascertainment and appropriate evaluation of this symptom may be an important factor in improving EOCRC outcomes. Furthermore, among individuals with rectal bleeding 41% were not evaluated and 23% underwent initial evaluation by FIT, FOBT, or cross-sectional imaging. Use of these inappropriate modalities for evaluation of rectal bleeding may cause delays in diagnosis of EOCRC that contribute to advanced stage at diagnosis. As 46% of individuals with EOCRC present with rectal bleeding, diagnostic algorithms that help providers distinguish hemorrhoidal bleeding from potential malignant bleeding may be warranted.22 Similarly, nearly half of all patients who were diagnosed with EOCRC were documented to have abdominal pain prior to EOCRC diagnosis. Because abdominal pain is a common and non-specific symptom among young patients, it is difficult to determine when providers should pursue additional testing versus offer reassurance. Future studies that develop and assess prognostic tools that distinguish EOCRC from benign conditions that present with abdominal pain, such as irritable bowel syndrome, are necessary.
Although CRC screening for Black individuals starting at age 45 was initially recommended by some guidelines starting in 2009,23 this study demonstrated only 9% utilization of lower endoscopy, FIT, and FOBT by age 46 among Black individuals. Because Black individuals in the United States have higher incidence of CRC and higher CRC-related mortality compared to other races,24 improving the implementation of CRC programs targeting Black individuals is crucial to reducing racial disparities in CRC outcomes. In contrast, this study demonstrated high rates of lower endoscopy utilization among individuals with family history of CRC/polyps. However, because we were not able to link individual patients to their family members, we cannot determine what percentage of patients with family history were identified and underwent evaluation within the recommended interval. Because of the reduction of the CRC screening initiation age to 45 among the general population, future studies should evaluate whether modifications to high-risk CRC screening recommendations for individuals with family history of CRC/polyps or Black race are necessary to further improve CRC outcomes in these populations.
Like all observational analyses, our study has potential limitations to consider. These include possible under-ascertainment of risk factors such as family history and confounding of trends by potential changes in symptom reporting and documentation. Despite these theoretical limitations, the results of this study contribute new and complementary findings to prior analyses from SEER. In particular, it highlights opportunities for improving the evaluation of patients with gastrointestinal symptoms. Although the United States is on the verge of a new era in which CRC screening begins at age 45 instead of 50, there is still potential to improve EOCRC morbidity and mortality through timely and appropriate diagnostic evaluation of patients who present with gastrointestinal symptoms. These results describing current clinical practice may help inform future research that develops and evaluates novel diagnostic strategies for young people with symptoms that could represent EOCRC.
Supplementary Material
Acknowledgments
Grant support:
• Ravy K. Vajravelu: NIH/NIDDK – K08-DK119475
• Shivan J. Mehta: NIH/NCI – K08-CA234326
• Thomas B. Karasic: NIH/NCI – U24-CA231858
• Ronac Mamtani: NIH/NCI – P30-CA016520
• Frank I. Scott: NIH/NIDDK – K08-DK095951
Role of the funding source: This research was funded by the National Institutes of Health in the United States. The funders of this study had no role in the study design, data collection, data analysis, data interpretation, or presentation of the results.
Abbreviations:
- AAPC
Average annual percent change
- ACS
American Cancer Society
- CI
Confidence interval
- CRC
Colorectal cancer
- EOCRC
Early-age onset colorectal cancer
- FIT
Fecal immunochemical testing
- FOBT
Fecal occult blood testing
- ICD
International Classification of Diseases
- SEER
Surveillance, Epidemiology, and End-Results Program
- USPSTF
U.S. Preventative Service Task Force
Footnotes
Corporate authors:
Dr. Karasic has served as a consultant for Pfizer. The scope of work was unrelated to this research. He has received research funding from Bristol-Myers Squibb, Celgene, Eli Lilly, H3Biomedicine, Sirtex, Syndax, and Taiho, also unrelated to this research.
Dr. Mamtani has served as a consultant for Genentech-Roche. The scope of work was unrelated to this research.
Dr. Scott has served as a consultant to Janssen, Merck, and Takeda Pharmaceuticals. The scope of work was unrelated to this research. He has received research funding from Takeda, Janssen, and the Crohn’s and Colitis Foundation, also unrelated to this research. He has served as a speaker for PRIME Incorporated, unrelated to this research.
References
- 1.Siegel RL, Fedewa SA, Anderson WF, et al. Colorectal Cancer Incidence Patterns in the United States, 1974-2013. J Natl Cancer Inst 2017;109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf AMD, Fontham ETH, Church TR, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American Cancer Society. CA Cancer J Clin 2018;68:250–281. [DOI] [PubMed] [Google Scholar]
- 3.Peterse EFP, Meester RGS, Siegel RL, et al. The impact of the rising colorectal cancer incidence in young adults on the optimal age to start screening: Microsimulation analysis I to inform the American Cancer Society colorectal cancer screening guideline. Cancer 2018;124:2964–2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bibbins-Domingo K, Grossman DC, Curry SJ, et al. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2016;315:2564–75. [DOI] [PubMed] [Google Scholar]
- 5.Rex DK, Boland CR, Dominitz JA, et al. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol 2017;112:1016–1030. [DOI] [PubMed] [Google Scholar]
- 6.Bretthauer M, Kalager M, Weinberg DS. From Colorectal Cancer Screening Guidelines to Headlines: Beware! Ann Intern Med 2018;169:405–406. [DOI] [PubMed] [Google Scholar]
- 7.Murphy CC, Lund JL, Sandler RS. Young-Onset Colorectal Cancer: Earlier Diagnoses or Increasing Disease Burden? Gastroenterology 2017;152:1809–1812 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Imperiale TF, Kahi CJ, Rex DK. Lowering the Starting Age for Colorectal Cancer Screening to 45 Years: Who Will Come…and Should They? Clin Gastroenterol Hepatol 2018;16:1541–1544. [DOI] [PubMed] [Google Scholar]
- 9.Meester RGS, Mannalithara A, Lansdorp-Vogelaar I, et al. Trends in Incidence and Stage at Diagnosis of Colorectal Cancer in Adults Aged 40 Through 49 Years, 1975-2015. JAMA 2019;321:1933–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abdelsattar ZM, Wong SL, Regenbogen SE, et al. Colorectal cancer outcomes and treatment patterns in patients too young for average-risk screening. Cancer 2016;122:929–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnett-Hartman AN, Powers JD, Chubak J, et al. Treatment patterns and survival differ between early-onset and late-onset colorectal cancer patients: the patient outcomes to advance learning network. Cancer Causes Control 2019;30:747–755. [DOI] [PubMed] [Google Scholar]
- 12.Read AJ, Waljee AK, Saini SD. A National Survey of Adoption of the 2018 American Cancer Society Colorectal Cancer Screening Guideline in Primary Care. Clin Gastroenterol Hepatol 2020;Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mehta SJ, Asch DA. How to help gastroenterology patients help themselves: leveraging insights from behavioral economics. Clin Gastroenterol Hepatol 2014;12:711–4. [DOI] [PubMed] [Google Scholar]
- 14.Winawer SJ, Fletcher RH, Miller L, et al. Colorectal cancer screening: clinical guidelines and rationale. Gastroenterology 1997;112:594–642. [DOI] [PubMed] [Google Scholar]
- 15.Loomans-Kropp HA, Umar A. Increasing Incidence of Colorectal Cancer in Young Adults. J Cancer Epidemiol 2019;2019:9841295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colorectal Cancer: Screening: U.S. Preventative Services Task Force. [Google Scholar]
- 17.Siu AL, Bibbins-Domingo K, Grossman D. Evidence-Based Clinical Prevention in the Era of the Patient Protection and Affordable Care Act: The Role of the US Preventive Services Task Force. JAMA 2015;314:2021–2. [DOI] [PubMed] [Google Scholar]
- 18.Clegg LX, Hankey BF, Tiwari R, et al. Estimating average annual per cent change in trend analysis. Stat Med 2009;28:3670–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shergill AK, Lightdale JR, Bruining DH, et al. The role of endoscopy in inflammatory bowel disease. Gastrointest Endosc 2015;81:1101–21 e1–13. [DOI] [PubMed] [Google Scholar]
- 20.Setoguchi S, Solomon DH, Glynn RJ, et al. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between medicare claims and cancer registry data. Cancer Causes Control 2007;18:561–9. [DOI] [PubMed] [Google Scholar]
- 21.Low EE, Demb J, Liu L, et al. Risk Factors for Early-Onset Colorectal Cancer. Gastroenterology 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lewis JD, Brown A, Localio AR, et al. Initial evaluation of rectal bleeding in young persons: a cost-effectiveness analysis. Ann Intern Med 2002;136:99–110. [DOI] [PubMed] [Google Scholar]
- 23.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol 2009;104:739–50. [DOI] [PubMed] [Google Scholar]
- 24.Ashktorab H, Kupfer SS, Brim H, et al. Racial Disparity in Gastrointestinal Cancer Risk. Gastroenterology 2017;153:910–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.