Abstract
Background:
To evaluate changes of associated markers in neonatal pathological jaundice due to bacterial infection in newborns, to provide an experimental basis for early diagnosis and treatment of neonatal pathological jaundice.
Methods:
A total of 126 newborns with neonatal pathological jaundice in the Pediatrics Department of Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University from Jan 2016 to Jun 2018 were enrolled. The patients were divided into bacterial infection group (76 cases with combined bacterial infection) and non-infection group (50 cases without bacterial infection). Peripheral blood was drawn from patients, and levels of inflammatory factors, levels of indexes of liver function and levels of cardiac markers were detected. Correlation between inflammatory factors and neonatal pathological jaundice was assessed.
Results:
The levels of WBC, hs-CRP and PCT in the bacterial infection group were significantly higher than those in the non-infected group (P<0.05). The level of TRF in the bacterial infection group was significantly lower than that in the non-infection group (P<0.01). In the bacterial infection group, the levels of WBC, hs-CRP, PCT, and TRF were positively correlated with the levels of CK, CKMB, LDH, and α-HBDB, respectively (all P<0.05). The TRF level after treatment was significantly higher than that before treatment (P<0.01).
Conclusion:
Markers such as WBC, hs-CRP, PCT, and TRF can be used as effective indicators in diagnosis of pathological jaundice due to bacterial infection in newborns. The combined testing of WBC, hs-CRP, PCT, and TRF was helpful for early diagnosis and early clinical intervention of neonatal pathological jaundice, which can lower the risk of clinical complications.
Keywords: Pathological jaundice, Newborn, Bacterial infection, Inflammatory factors, Combined testing
Introduction
Neonatal jaundice is a medical condition, in which high serum level of bilirubin was observed in a newborn baby within 28 days after birth. The major clinical symptom was a yellowish discoloration of the white part of the eyes and skin (1). Neonatal jaundice is common in babies. It is a physiological phenomenon and the main manifestation of many diseases as well (2).
Neonatal jaundice can be generally divided into two types: physiological and pathological. Physiological jaundice is caused by abnormal metabolism of bilirubin, which appears after 2–3 days of birth. Without taking medications, it can disappear by itself in about 1–2 weeks after birth. Therefore, physiological jaundice causes a small impact on the baby’s overall health (3). Pathological jaundice, however, appears within 24 h of birth, and the baby’s serum level of bilirubin continues to rise over time. The jaundice can last more than 2 weeks for full-term babies and last more than 4 weeks for premature babies, during which time the jaundice may be recurring or worsening. Causes of pathological jaundice include infections, congenital biliary malformations and neonatal hemolysis. If not diagnosed and treated in a timely manner, it can lead to riboflavin disease, and in rare cases it can lead to irreversible and life-threatening bilirubin encephalopathy due to very high levels of bilirubin. Survivors may have varying degrees of neurological sequelae associated with neurotoxicity of high-level bilirubin.
Clinical statistics showed that the incidence of neonatal pathological jaundice is on the rise, and bacterial infection is the major factor causing neonatal pathological jaundice (4). Therefore, in the diagnosis of neonatal pathological jaundice, in addition to clinical signs, screening of ideal sero-logical markers is of great significance for comprehensive assessment of neonatal pathological jaundice. In this study, alterations in levels of inflammatory factors, including WBC, CRP, PCT, and TRF, and levels of cardiac markers were evaluated in newborn patients with neonatal jaundice, aimed to provide a theoretical basis for diagnosis and treatment of the disease.
Materials and Methods
Subjects
A total of 126 newborns who were diagnosed with neonatal pathological jaundice and received treatment in the pediatrics department of Qilu Hospital (Qingdao), Cheeloo College of Medicine, Shandong University China, from Jan 2016 to Jun 2018 were enrolled as subjects in this study. The patients were divided into two groups, i.e. bacterial infection group (76 cases with combined bacterial infection) and non-infection group (50 cases without bacterial infection). Causes of the pathological jaundice included neonatal hemolysis, biliary atresia, tumor or congenital anomalies. Inclusion criteria were the diagnostic criteria for neonatal pathological jaundice (5), including: 1) Jaundice appeared within 24 h of birth; 2) Total bilirubin (TBIL) level >221.00 μM for full-term babies, >257.00 μM for premature babies, or daily increase in the TBIL level >85.00 μM for newborn babies; 3) Jaundice lasted more than 4 weeks for premature babies or more than 2 weeks for full-term babies; 4) Patients with recurrent jaundice; and 5) Serum direct bilirubin (DBIL) >34.00 μM.
Patients matching at least one of above criteria had pathological jaundice, otherwise had physiological jaundice. Infections were confirmed by a comprehensive assessment of the patient's condition combined with a pathological test. There were 39 males and 37 females in the bacterial infection group. Their gestational age was 35–41 weeks with an average of (38.23±1.35) weeks. Their birth age was 1–28 days with an average of (15.65±4.16) days. Their birth weight was 2167–4359 g with an average of (3564.28±636.97) g. In terms of delivery method, there were 56 cases of natural delivery and 20 cases of cesarean section. There were 26 males and 24 females in the non-infection group. Their gestational age was 35–41 weeks with an average of (38.45±1.54) weeks. Their birth age was 1–28 days with an average of (14.97±4.86) days. Their birth weight was 2184–4332 g with an average of (3578.51±640.21) g. In terms of delivery method, there were 37 cases of natural delivery and 13 cases of cesarean section. Patients’ basic data in the two groups were comparable, and the differences were not statistically significant (P>0.05).
This study was approved by the hospital Ethics Committee. A consent form was signed by the patient family indicating voluntary participation in the study.
Methods
A fasting venous blood sample was collected from each infant patient in the early morning on the day following admission. K2-EDTA anticoagulated blood was used for routine blood test. Serum samples were obtained through centrifugation of self-coagulated venous blood at 3000 rpm, and were used for tests including high-sensitivity C-reactive protein (hs-CRP), procalcitonin (PCT), transferrin (TRF), total bilirubin (TBIL), direct bilirubin (DBIL), indirect bilirubin (IBIL), alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), creatine kinase (CK), creatine kinase myocardial band (CKMB), lactate dehydrogenase (LDH), and α-hydroxybutyrate dehydrogenase (α-HBDB). A fully automated hematology analyzer (SYSMEX XS-1000i) was used for the routine blood test. Tests of PCT and TRF were performed on a Roche E601 fully automated analyzer based on the electrochemiluminescence immunoassay method. Tests of hs-CRP, TRF, TBIL, DBIL, IBIL, ALT, AST, GGT, ALP, CK, CKMB, LDH, and α-HBDB were carried out using a fully automated biochemistry analyzer (Siemens ADVIA 2400). Original reagents coming with the instruments were used in the tests. The operating manuals were strictly followed. Quality controls met requirements. The tested markers had following reference range: (15.00 to 20.00)x109 for WBC, (0.00 to 5.00) mg/L for hs-CRP, (0.00 to 0.25) ng/mL for PCT, (28.60 to 51.90) μM for TRF, (1.70 to 21.00) μM for TBIL, (0.00 to 5.60) μM for DBIL, (0.00 to 19.00) μM for IBIL, (0.00 to 40.00) U/L for ALT, (0.00 to 40.00) U/L for AST, (0.00 to 50.00) U/L for GGT, (36.00 to 150.00) U/L for ALP, (0.00 to 171.00) U/L for CK, (0.00 to 24.00) U/L for CKMB, (135.00 to 225.00) U/L for LDH, and (0.00 to 182.00) U/L for α-HBDB.
Statistical method
The SPSS 22.0(Chicago, IL, USA) statistics software was used in data processing and analysis. The t test and χ2 test were used in data analysis. Measurement data were expressed as x¯±s. The independent sample t test was used in comparison of means between groups. The χ2 test was used in comparison of rates of count data. Pearson’s correlation was used in the correlation analysis. A difference was statistically significant when P<0.05.
Results
Comparison of levels of inflammatory markers
Levels of WBC, hs-CRP and PCT in peripheral blood of patients with neonatal pathological jaundice in the bacterial infection group were significantly higher than those in the non-infection group, and the differences were statistically significant (P<0.05). The level of TRF in the bacterial infection group was significantly lower than that in the non-infection group (P<0.01) (Table 1).
Table 1:
Comparison of levels of inflammatory markers (x¯±s)
| Group | WBC (x109/L) | hs-CRP (mg/L) | PCT (ng/mL) | TRF (μM) |
|---|---|---|---|---|
| Infection | 25.35±3.42a | 16.53±2.81a | 5.69±1.36a | 12.34±2.74a |
| Non-infection | 13.21±1.98 | 3.26±0.78 | 0.15±0.04 | 40.56±5.65 |
| t | 3.365 | 4.985 | 4.460 | −4.923 |
| P | 0.015 | 0.002 | 0.004 | 0.003 |
Note:
P<0.05, compared with the non-infection group
Comparison of levels of indexes of liver function
As shown in Table 2, there were no significant differences in serum levels of TBIL, DBIL, IBIL, ALT, AST, GGT, and ALP between the bacterial infection group and the non-infection group (P>0.05). The serum levels of CK, CKMB, LDH, and α-HBDB in the bacterial infection group were significantly higher than those in the non-infection group, and the differences were statistically significant (P<0.05).
Table 2:
Comparison of levels of indexes of liver function (x¯±s)
| Marker | Infection group | Non-infection group | t | P |
|---|---|---|---|---|
| TBIL (μM) | 352.51±26.74b | 325.22±23.43 | 0.841 | 0.433 |
| DBIL (μM) | 28.05±4.81b | 17.27±5.52 | 1.613 | 0.158 |
| IBIL (μM) | 324.46±27.61b | 307.95±21.38 | 0.518 | 0.623 |
| ALT (U/L) | 31.25±2.56b | 30.79±3.31 | 0.120 | 0.908 |
| AST (U/L) | 27.62±4.56b | 21.83±5.26 | 0.911 | 0.379 |
| GGT (U/L) | 38.76±4.12b | 32.56±3.78 | 1.215 | 0.270 |
| ALP (U/L) | 125.34±6.51b | 133.16±7.01 | 0.895 | 0.405 |
| CK (U/L) | 375.69±32.64c | 250.23±17.61 | 3.712 | 0.010 |
| CKMB (U/L) | 46.25±5.52c | 18.36±2.75 | 4.954 | 0.003 |
| LDH (U/L) | 405.73±46.82c | 289.67±23.54 | 2.427 | 0.049 |
| α-HBDB (U/L) | 312.54±28.69c | 139.67±15.66 | 5.782 | 0.001 |
Note:
P>0.05;
P<0.05, compared with the non-infection group
Correlation analysis of WBC, hs-CRP, PCT, and TRF with cardiac markers (CK, CKMB, LDH, and α-HBDB)
In the bacterial infection group, the levels of WBC, hs-CRP, PCT, and TRF were positively correlated with the levels of CK, CKMB, LDH, and α-HBDB, respectively ((r=0.979, 0.821, 0.964, and 0.976, respectively; P<0.05), (r=0.980, 0.902, 0.966, and 0.977, respectively; P<0.01), (r=0.981, 0.954, 0.967, and 0.979, respectively; P<0.01), and (r=0.981, 0.926, 0.966, and 0.798, respectively; P<0.01), respectively), and the differences were statistically significant (all P<0.05) (Table 3).
Table 3:
Correlation analysis of WBC, hs-CRP, PCT, and TRF with cardiac markers (CK, CKMB, LDH, and α-HBDB)
| Item | CK | CKMB | LDH | α-HBDB | ||||
|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | |
| WBC | 0.979 | 0.000 | 0.821 | 0.012 | 0.964 | 0.000 | 0.976 | 0.000 |
| hs-CRP | 0.980 | 0.000 | 0.902 | 0.002 | 0.966 | 0.000 | 0.977 | 0.000 |
| PCT | 0.981 | 0.000 | 0.954 | 0.000 | 0.967 | 0.000 | 0.979 | 0.000 |
| TRF | 0.981 | 0.000 | 0.926 | 0.001 | 0.966 | 0.000 | 0.798 | 0.000 |
Note: P<0.05
Comparison of levels of inflammatory markers WBC, hs-CRP, PCT, and TRF before and after treatment in the bacterial infection group
As shown in Fig. 1, in the bacterial infection group, the serum levels of WBC, hs-CRP and PCT were significantly lower after treatment than those before treatment (t=−3.345, −5.112 and −4.475, respectively; P=0.015, 0.002 and 0.004, respectively), and the differences were statistically significant (P> 0.05). However, the TRF level was significantly higher after treatment than that before treatment (t=4.655; P=0.003), and the difference was statistically significant (P<0.01).
Fig. 1:
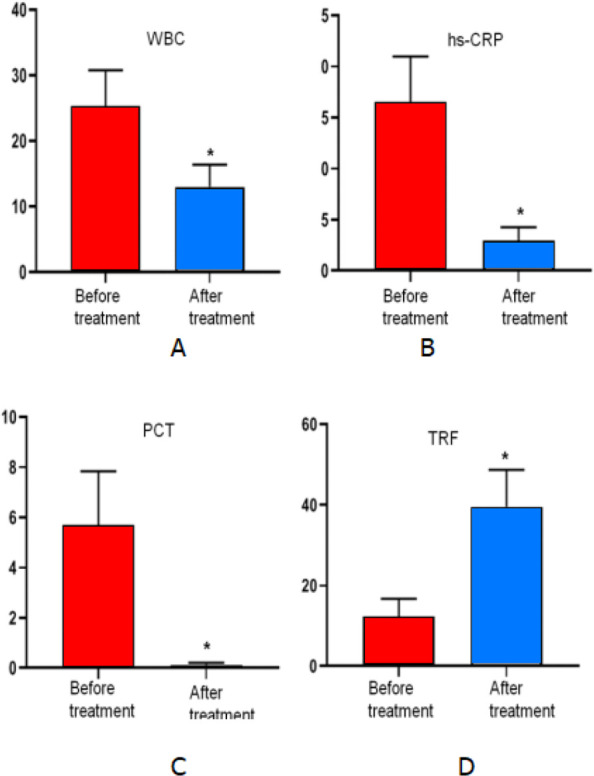
A. The WBC level after treatment was compared with that before treatment, P<0.05. B. The hs-CRP level after treatment was compared with that before treatment, P<0.05. C. The PCT level after treatment was compared with that before treatment, P<0.05. D. The TRF level after treatment was compared with that before treatment, P<0.05
Discussion
Neonatal physiological jaundice may disappear without treatment. Neonatal pathological jaundice is divided into a few types such as jaundice due to bacterial infection, breast milk jaundice, obstructive jaundice, and hemolytic jaundice, etc. Among them, bacterial infection is the major cause leading to jaundice (6). Neonatal bacterial infection can be triggered by a couple of high-risk factors such as premature rupture of membranes, contamination of amniotic fluid, and maternal vaginal infections during delivery (7). Infection can cause premature destruction of red blood cells of the newborn to varying degrees, resulting in hemolytic symptoms. In addition, catalytic activities of enzymes in the liver of the infant patient are greatly inhibited, causing disorders of bilirubin metabolism and eventually leading to a significant increase in bilirubin levels in the child's blood system (8). There is a high incidence of jaundice due to bacterial infection and it often starts with jaundice only without other clinical manifestations such as fever and cough in newborn patients. The cause of the disease is often hidden, and the degree of the disease varies. Excessive bilirubin in the body can lead to brain damage. Mild pathological jaundice can affect the baby’s growth and development, while in severe cases it can cause bilirubin encephalopathy, leading to disability and even death (9). Therefore, screening of ideal serological markers is of great significance for early diagnosis, condition evaluation and treatment guidance.
WBC count and classification were typical markers for bacterial infection used in routine blood test. White blood cells participate in the body's defensive response in different ways. Despite limited diagnostic value, WBC is an essential and routinely obtained marker in clinic. WBC count can be used as a clinical marker of neonatal infections, and therefore it can be used as a useful reference to justify testing items in a clinical laboratory. WBC count plays a valuable role in estimating the degree of infection and evaluating the immune status of the body. However, there are defects such as susceptibility to internal and external factors and wide fluctuation range; therefore, in some cases it is not reliable in estimating the infection status of the body. C-reactive protein (CRP) is an acute phase protein synthesized by the liver. It is an important marker of the body's inflammatory response. Due to relatively low level in a healthy person, CRP has been widely used in clinical practice as a monitoring marker of bacterial infection, demonstrating certain values in assisting the diagnosis of neonatal infections (10). However, the CRP test exhibits unsatisfactory sensitivity due to individual differences. The high-sensitivity C-reactive protein (hs-CRP) test is a sensitive technique for detecting low levels of CRP to identify low but persistent levels of infection (11, 12). Hs-CRP, as one of the sensitive markers of acute phase response proteins, showed a sudden increase during the inflammatory response process, and the increase in its serum level precedes other clinical manifestations including elevated body temperature (13). The hs-CRP level increased significantly in serum of patients with jaundice due to bacterial infection. Therefore, it can be used as an important indicator for detection of a bacterial infection. However, it is not an appropriate indicator for detection of a viral infection due to its minimal change. Due to above reasons, the hs-CRP test can be used to differentiate between bacterial infection and viral infection in newborns, thus providing a valuable tool in diagnosis of neonatal pathological jaundice due to bacterial infection. Procalcitonin (PCT) is a glycoprotein with no hormonal activity. It shares an identical amino acid sequence with calcitonin. Under normal physiological conditions, PCT is produced by thyroid C cells, and its normal level in human blood is <0.25 ng/mL. When a viral infection occurs, the PCT level maintains in its normal range. However, when a bacterial infection occurs, cells other than the thyroid cells such as macrophages in the liver and neuroendocrine cells can also secrete PCT, causing a rapid and significant rise in its serum level up to 20–200 ng/mL. The increase in the PCT level can be detected in the early stage of infection (2–3 h), indicating that it can be used as an ideal indicator for early diagnosis of bacterial infection. PCT is an important serum marker found in recent years for diagnosis of bacterial infection. It has good correlation with the degree of inflammation. The PCT level is associated with the jaundice index (14). PCT is not only a sensitive indicator for predicting neonatal infections, but also a good indicator for guiding clinical use of antibiotics. In babies with neonatal infection, the PCT level was significantly reduced after effective antibiotic treatment (15), suggesting that it can be used as an indicator of treatment outcome.
Transferrin (TRF) is a β globulin synthesized mainly by liver cells with a half-life of 7 days. It was found in recent years that TRF can be used not only to assist in diagnosis of iron deficiency diseases, but also as a marker of bacterial infection (16). The TRF serum level decreases in acute phase response, thus it is a negative acute phase response protein. TRF can maintain the stability of red blood cell membranes, thus it plays a protective role in red blood cell damage when infection occurs (16). TRF can also promote renal bili-rubin clearance by combining with free bilirubin. TRF expression is significantly down regulated in pathological jaundice and its serum level decreases as jaundice worsens (17). TRF is associated with occurrence of bilirubin encephalopathy (18). The inflammation markers discussed above are of great significance for diagnosis of neonatal pathological jaundice. However, due to the variety of pathogens and individual variation of immune status, it is often difficult to diagnose the disease using one marker. Combined testing of a few markers often improves diagnostic sensitivity and accuracy.
Combined detection of serum myocardium enzymogram can inform the status of myocardial function to some extent. The increase of levels of myocardial enzymes in newborns with neonatal jaundice is due to the toxicities of high concentrations of bilirubin, and especially indirect bilirubin, which causes changes of some important enzymes, disturbs membrane potential and disrupts energy metabolism, inhibits the process of cell glycogenolysis, and causes damage to mitochondria in cardiac muscle cells (19).
Cardiac markers CK, LDH and HBDB are often used as diagnostic indicators of myocardial injury. CK is found in the mitochondria and cytoplasm of skeletal muscle, cardiac muscle, brain, and other visceral tissues. It can reversibly catalyze the reaction of creatine and adenosine triphosphate to produce creatine phosphate and adenosine diphosphate. LDH is an important enzyme that catalyzes the redox reaction between lactic acid and pyruvate in glycolysis and gluconeogenesis. LDH is rich in tissues such as myocardium, skeletal muscle and kidney. HBDB is an important enzyme in the oxidative utilization of ketone bodies. It is mainly distributed in cardiac muscle, brain, kidney, and other visceral tissues. Elevated HBDB level often indicates damage to the heart, brain, kidney and other organs. Markers CK, LDH and HBDB lack tissue specificity. CK-MB is mainly found in cardiac muscle cells, and its level is very low in normal human serum. Therefore, CK-MB is a specific enzyme to myocardial cells. When the myocardium is damaged, a large amount of CK-MB is released into blood, so it is highly specific for diagnosis of myocardial damage. At present, markers LDH, CK, HBDB, and CK-MB are still commonly used in enzymatic examinations related to myocardial damage (20). Elevated serum levels of LDH, CK, HBDB, and CK-MB often indicate a possible damage to myocardial cells (21).
Among the many factors that are associated with neonatal pathological jaundice, infection is the most important and common one (14). We found that levels of inflammatory factors WBC, hs-CRP and PCT in peripheral blood of patients with neonatal pathological jaundice in the bacterial infection group were significantly higher than those in the non-infection group, while the level of TRF was significantly reduced. Spearman’s correlation analysis showed that the serum levels of LDH, CK, HBDB, and CK-MB in patients with neonatal pathological jaundice in the bacterial infection group were significantly increased, and they were positively correlated with the inflammatory factors WBC, hs-CRP and PCT, while were negatively correlated with TRF. The above findings suggested that myocardial function might be impaired in neonatal pathological jaundice due to bacterial infection. In such cases, health care providers need to be vigilant. It is necessary to carry out a comprehensive assessment of the condition and provide timely diagnosis and treatment to avoid any delay. Dynamic detection of serum WBC, sh-CRP, PCT, TRF can be used to monitor the condition of disease, guide the treatment, and evaluate the prognosis. The above inflammatory indicators are of great significance in the diagnosis of neonatal pathological jaundice. However, the pathogens and the immune status of the body are different; each diagnostic indicator has its own clinical and laboratory characteristics. It is difficult to diagnose diseases with single indicator. Joint detection can improve the value of diagnosis.
A limitation of the study is that the number of enrolled cases in this study was relatively small. In the future, more laboratories could work together to increase the sample size.
Conclusion
The combined detection of serum inflammatory factors WBC, sh-CRP, PCT and TRF can be used as a basis for early diagnosis of pathological jaundice, disease evaluation, treatment guidance and efficacy prediction.
Acknowledgements
No funding was received in this study.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical considerations
Ethical issues (Including plagiarism, misconduct, data fabrication and/or falsification, double publication and/or submission, redundancy, etc.) have been completely observed by the authors.
References
- 1.Scrafford CG, Mullany LC, Katz J, et al. (2013). Incidence of and risk factors for neonatal jaundice among newborns in southern Nepal. Trop Med Int Health, 18(11): 1317–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong T, Chen T, White RA, et al. (2018). Meconium microbiome associates with the development of neonatal jaundice. Clin Transl Gastroenterol, 9(9): 182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olusanya BO, Slusher TM, Imosemi DO, et al. (2017). Maternal detection of neonatal jaundice during birth hospitalization using a novel two-coloricterometer. PLoS One, 12(8): e0183882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiao Y, Jin Y, Meng H, et al. (2018). An analysis on treatment effect of blue light phototherapy combined with Bifico in treatingneonatal hemolytic jaundice. Exp Ther Med, 16(2): 1360–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wan A, Mat Daud S, Teh SH, et al. (2016). Management of neonatal jaundice in primary care. Malays Fam Physician, 11(2–3): 16–19. [PMC free article] [PubMed] [Google Scholar]
- 6.Bassari R, Koea JB. (2015). Jaundice associated pruritis: a review of pathophysiology and treatment. World J Gastroenterol, 21(5): 1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Panahi R, Jafari Z, Sheibanizade A, et al. (2013). The Relationship between the Behavioral Hearing Thresholds and Maximum Bilirubin Levels at Birth in Children with a History of Neonatal Hyperbilirubinemia. Iran J Otorhinolaryngol, 25(72): 127–134. [PMC free article] [PubMed] [Google Scholar]
- 8.Siu SL, Chan LW, Kwong AN. (2018). Clinical and biochemical characteristics of infants with prolonged neonatal jaundice. Hong Kong Med J, 24(3): 270–276. [DOI] [PubMed] [Google Scholar]
- 9.Llorente AM, Castillo CL. (2012). Congenital cytomegalovirus infection in fraternal twins: a longitudinal case study examiningneurocognitive and neurobehavioral correlates. Appl Neuropsychol Child, 1(1): 63–73. [DOI] [PubMed] [Google Scholar]
- 10.Volanakis JE. (2001). Human C-reactive protein: expression, structure, and function. Mol Immunol, 38(2–3): 189–197. [DOI] [PubMed] [Google Scholar]
- 11.Yang C, Yang Y, Li B, et al. (2016). The diagnostic value of high-sensitivity C-reactive protein/albumin ratio in evaluating early-onsetinfection in premature. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue, 28(2): 173–177. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Zhong X, Cheng G, et al. (2017). Hs-CRP and all-cause, cardiovascular, and cancer mortality risk: A meta-analysis. Atherosclerosis, 259: 75–82. [DOI] [PubMed] [Google Scholar]
- 13.Kosińska-Kaczyńska K, Szymusik I, Kaczyński B, et al. (2013). Iatrogenic and spontaneous late preterm twins--which are at higher risk of neonatalcomplications. Ginekol Pol, 84(6): 430–435. [DOI] [PubMed] [Google Scholar]
- 14.Lencot S, Cabaret B, Sauvage G, et al. (2014). A new procalcitonin cord-based algorithm in early-onset neonatal infection: for a change ofparadigm. Eur J Clin Microbiol Infect Dis, 33(7): 1229–1238. [DOI] [PubMed] [Google Scholar]
- 15.Cottineau M, Launay E, Branger B, et al. (2014). Diagnostic value of suspicion criteria for early-onset neonatal bacterial infection: report tenyears after the Anaes recommendations. Arch Pediatr, 21(2): 187–193. [DOI] [PubMed] [Google Scholar]
- 16.Lee BK, Le Ray I, Sun JY, et al. (2016). Haemolytic and nonhaemolytic neonatal jaundice have different risk factor profiles. Acta Paediatr, 105(12): 1444–1450. [DOI] [PubMed] [Google Scholar]
- 17.Christensen RD, Yaish HM, Lemons RS. (2014). Neonatal hemolytic jaundice: morphologic features of erythrocytes that will help you diagnosethe underlying condition. Neonatology, 105(4): 243–249. [DOI] [PubMed] [Google Scholar]
- 18.Lamola AA, Bhutani VK, Wong RJ, et al. (2013). The effect of hematocrit on the efficacy of phototherapy for neonatal jaundice. Pediatr Res, 74(1): 54–60. [DOI] [PubMed] [Google Scholar]
- 19.Bhutani VK. (2001). Neonatal hyperbilirubinemia and the potential risk of subtle neurological dysfunction.. Pediatr Res, 50(6): 679–680. [DOI] [PubMed] [Google Scholar]
- 20.Wang XF, Hong JG. (2011). Management of severe asthma exacerbation in children. World J Pediatr, 7(4): 293–301. [DOI] [PubMed] [Google Scholar]
- 21.Ricci F, De Caterina R. (2011). Isolated creatine kinase-MB rise with normal cardiac troponins: a strange occurrence withdifficult interpretation. J Cardiovasc Med (Hagerstown), 12(10): 736–740. [DOI] [PubMed] [Google Scholar]


