Abstract
Background & Aims
The colon is innervated by intrinsic and extrinsic neurons that coordinate functions necessary for digestive health. Sympathetic input suppresses colon motility by acting on intrinsic myenteric neurons, but the extent of sympathetic-induced changes on large-scale network activity in myenteric circuits has not been determined. Compounding the complexity of sympathetic function, there is evidence that sympathetic transmitters can regulate activity in non-neuronal cells (such as enteric glia and innate immune cells).
Methods
We performed anatomical tracing, immunohistochemistry, optogenetic (GCaMP calcium imaging, channelrhodopsin), and colon motility studies in mice and single cell RNA sequencing in human colon to investigate how sympathetic postganglionic neurons modulate colon function.
Results
Individual neurons in each sympathetic prevertebral ganglion innervated the proximal or distal colon, with processes closely opposed to multiple cell types. Calcium imaging in semi-intact mouse colon preparations revealed changes in spontaneous and evoked neural activity, as well as activation of non-neuronal cells, induced by sympathetic nerve stimulation. The overall pattern of response to sympathetic stimulation was unique to the proximal or distal colon. Region-specific changes in cellular activity correlated with motility patterns produced by electrical and optogenetic stimulation of sympathetic pathways. Pharmacology experiments (mouse) and RNA sequencing (human) indicated that appropriate receptors were expressed on different cell types to account for the responses to sympathetic stimulation. Regional differences in expression of alpha-1 adrenoceptors in human colon emphasize the translational relevance of our mouse findings.
Conclusions
Sympathetic neurons differentially regulate activity of neurons and non-neuronal cells in proximal and distal colon to promote distinct changes in motility patterns, likely reflecting the distinct roles played by these two regions.
Keywords: gastrointestinal, interstitial cells of Cajal, platelet-derived growth factor receptor-alpha, GCaMP
INTRODUCTION
The gastrointestinal tract executes complex functions to facilitate digestion. The colon in particular is responsible for formation, storage and expulsion of fecal matter, all while maintaining a balanced microbiome, proper epithelial barrier and appropriate immune state. To accomplish this, the enteric nervous system (ENS) initiates reflexes and coordinates activity between neuronal and non-neuronal cell types, including interstitial cells, smooth muscle cells and epithelial cells1. For example, interstitial cells of Cajal (ICC) are electrically coupled to smooth muscle and induce pace-making waves of depolarization that contribute to motility, the overall pattern of which is influenced by neuromodulation2–4. Epithelial cells maintain a protective barrier, interact with the microbiome and innate immune cells, secrete mucins, and directly communicate with sensory neurons, including intrinsic primary afferents that initiate local motility reflexes and extrinsic primary afferents that activate spinal/vagal reflexes5–7.
In addition to the ENS, the colon is innervated by extrinsic sympathetic, parasympathetic and sensory neurons, allowing bidirectional communication with the central nervous system. The importance of sympathetic activity to intestinal function is most readily manifest in the key role it plays in regulating motility, secretion, and blood flow during different phases of digestion8–10. Changes in blood flow are mediated by sympathetic regulation of vascular smooth muscle, whereas regulation of motility and secretion has been attributed to sympathetic actions on myenteric11 and submucosal neurons12,13, respectively, through alpha-2 adrenoceptor signaling14–17. Sympathetic nerve stimulation inhibits spontaneous and evoked potentials in myenteric neurons18, and suppresses reflexive and rhythmic motility patterns in the guinea-pig and mouse distal colon11,19. However, it is unknown whether this also applies to the proximal colon, and direct recordings of sympathetic-induced effects on large-scale, network activity in myenteric neurons are lacking.
An emerging role has also developed for sympathetic neurons as regulators of non-neuronal cells involved in colon function. For example, sympathetic transmitters activate enteric glia in isolated mouse colon20, as well as ICC21 and epithelial cells in culture5. However, the sympathetic nervous system can exert its effects through epinephrine and norepinephrine released into circulation from the adrenal gland (i.e., hormonally-mediated) or through neurotransmitters released from sympathetic postganglionic neurons (SPNs) (i.e., neutrally-mediated). Pharmacological experiments are unable to determine if the observed changes in target-cell activity are due to hormonal or neural mechanisms and cannot establish a direct relationship between activation of sympathetic neurons and real-time responses of cells receiving this input. Therefore, it is currently unknown to what extent SPNs activate non-neuronal cell types, and importantly, whether these mechanisms also exist in the human colon.
Here we used ex vivo preparations with semi-intact circuits to determine the impact of increasing sympathetic drive on colon motility and on the activity of myenteric neurons, colon epithelium, and interstitial cells. Using mice that genetically express GCaMP (a calcium indicator) in neurons and non-neuronal cells enabled real-time measurement of calcium transients in multiple cell types in response to SPN stimulation. SPN activation directly influenced multiple cell types to alter local and pan-colonic motility patterns in a region-specific manner, with different responses in proximal and distal colon. Remarkably, RNA sequencing provided molecular evidence that some aspects of sympathetic input to human colon are also region-specific, emphasizing the translational relevance of regional differences in sympathetic modulation of colon function.
MATERIALS AND METHODS
Animals
Male and female mice aged 3–6 months were used; information on mouse strains can be found in Supplemental Methods. Mice were housed in an AAALAC-approved facility with a 12-hour light/dark cycle and free access to water and standard chow. Animal use protocols were approved by Institutional Animal Care and Use Committees at University of Pittsburgh and University of Toledo.
Anatomical mapping of sympathetic prevertebral ganglia
NPY-GFP mice were euthanized with isoflurane and transcardially perfused with saline. A midline laparotomy was performed and organs were moved to the side to expose the abdominal aorta and sympathetic prevertebral ganglia (SPG). Anatomical mapping of SPG was performed using an Olympus MVX10 Macro Zoom microscope equipped with X-cite™ 120 Fluorescence Illumination System and Photometrics® CoolSNAP™ ES camera using appropriate filters.
Immunohistochemistry of sympathetic ganglia
See Supplemental Table S1 for all antibody information. For immunolabeling of tyrosine hydroxylase (TH), SPG from NPY-GFP mice were collected, immersion fixed in 4% paraformaldehyde for 30 min, cryoprotected in 25% sucrose in 0.01M PBS at 4°C for 24 hrs, sectioned (14μm) on a cryostat, and mounted on Superfrost Plus microscope slides (Fisher Scientific, Pittsburgh, PA). Slides were washed (3 × 5 min) in 0.01M PBS and incubated in 0.01M PBS containing 10% donkey serum, 0.3% Triton, and a rabbit anti-mouse TH antibody for 24 hours at room temperature. Slides were washed in 0.01M PBS, incubated (90 min; room temperature) with a donkey anti-rabbit Cy™3 antibody and coverslipped. Two fields per section, 5 sections, >50μm apart, per animal were imaged using a Leica DM4000B microscope with Leica EL6000 external light source and Leica DFC7000 T digital camera using 20x objective with appropriate filters.
Whole-mount colon immunohistochemistry
For immunolabeling, full-length colons were dissected, cut along the anti-mesenteric border, pinned flat, and fixed for 2 hours in 4% paraformaldehyde in 0.01M phosphate-buffered saline (PBS). Tissue was washed in buffer containing 0.1M Tris HCl, 1.5% NaCl, and 0.3% Triton X-100 at pH 7.4 (TNT) three times for 30 min, then incubated overnight at room temperature in TNT containing 20% normal donkey serum and primary antibodies. Colons were washed in TNT (4 hours) and incubated overnight at room temperature in TNT containing secondary antibodies. Tissue was imaged as described above.
Back-labeling of sympathetic neurons that innervate the colon
C57BL/6J mice were anesthetized with isoflurane (2%) and laparotomy was performed to access pelvic viscera. Fluorescent retrograde dyes (cholera toxin subunit beta) were injected (5–10μl) into the wall of the proximal (below cecum) or distal colon (base of bladder). After 3 days, SPGs were dissected and processed for immunohistochemistry, as described above. Colons were visualized to confirm successful injections.
Ex vivo colon preparations with intact extrinsic sympathetic input
NPY-GFP or NPY-ChR2 mice were euthanized with isoflurane and transcardially perfused with carbogenated (95% O2 and 5% CO2) artificial cerebrospinal fluid (ACSF), containing (in mM): 117.9 NaCl, 4.7 KCL, 25 NaHCO3, 1.3 NaH2PO4, 1.2 MgSO4-7H2O, 2.5 CaCl2, 11.1 D-glucose, 2 sodium butyrate, 20 sodium acetate. The colon and SPG were isolated, removed with nerves intact, and pinned to a Sylgard-lined dish while superfused with ACSF at 35°C (Supplemental Figure S1). Fecal contents were removed by gently flushing the lumen with ACSF. Sympathetic-induced changes in motility were measured using electrical or optogenetic stimulation using a 473 nm wavelength laser (Laserglow Technologies, Toronto, Canada) and 1.5 mm optical fiber (ThorLabs, Newton, NJ) placed on or directly above the inferior mesenteric ganglion. For local contractions, colon tissue movement was recorded before, during, and after SPN stimulation using a CMOS camera (Prime 95B Photometrics; Roper Scientific, Tuscon, AZ) under 10x objective. Images were collected with Metamorph software (Molecular Devices, San Jose, CA) at 20Hz sampling rate, 50ms exposure. Tissue movement was quantified using the Template Matching ImageJ plugin. Pan-colonic motility patterns were video recorded (Sony, HDR-CX440) for 20 minutes without stimulation and 20 minutes during SPN stimulation.
For GCaMP imaging, the ex vivo dissection was performed in E2aCre-GCaMP6s mice; the colon was cut adjacent to the mesentery, taking caution not to sever SPN-containing lumbar colonic nerves, and pinned flat. Nifedipine (1μM, Sigma), an L-type calcium channel blocker, was added to ACSF to improve stability for calcium imaging. In full thickness preparations, this concentration is sufficient to eliminate most spontaneous contractions but does not suppress calcium signaling in ICC22. Recordings of SPN responses were imaged during electrical stimulation of nerve fibers. Because of the varying amount and location of adipose tissue surrounding nerve fibers, the electrode was placed along the mesenteric nerve in a position that activated the most cell bodies (SPNs). Calcium signals from various cell types were imaged in different colon layers before, during and after stimulation. Transients with a signal amplitude >4 standard deviations above baseline were considered to be “activity.” GCaMP imaging files were processed and analyzed as previously described22. See Supplemental Methods for additional details.
Drug preparation
Solutions containing yohimbine (100nM), guanethidine (3μM), or prazosin (1μM) (all from Sigma) were prepared with fresh ACSF on the day of the experiment.
Data analysis and statistics for mouse colon experiments
Microscopy images, video recordings of colon motility, and GCaMP imaging files were de-identified and coded for blinded analysis. Data is represented as mean ± SEM, where n=number of mice. Statistical tests used included paired and unpaired Student’s t-tests, one-way and two-way analysis of variance (ANOVA) with post-hoc tests for multiple comparisons as indicated in the figure legends. Differences were considered significant if p<0.05.
For methods regarding human colon samples please see Supplemental Methods.
RESULTS
Distribution of sympathetic postganglionic neurons (SPNs) innervating proximal or distal colon
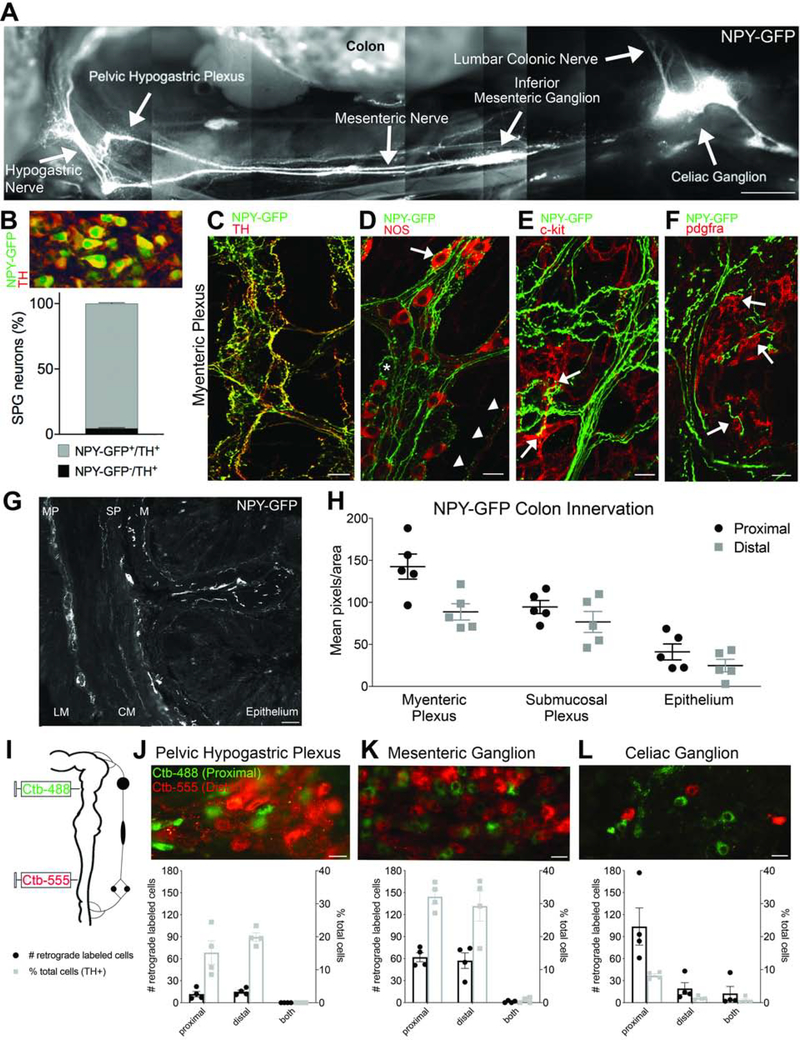
To visualize sympathetic inputs in a semi-intact model, we used an NPY-GFP reporter mouse to create a montage image of SPN innervation from sympathetic prevertebral ganglia (SPG) to the colon (Figure 1A). In the NPY-GFP reporter mouse, almost all of the TH-immunoreactive (TH+) SPG neurons were NPY-GFP+ (96%; Figure 1B) confirming the utility of this mouse line. Whole-mount immunostaining revealed dense sympathetic innervation within myenteric ganglia, with the majority of NPY-GFP+ fibers colocalized with TH (Figure 1C). NPY-GFP+ fibers in the myenteric plexus were closely associated with NOS+ and NOS− myenteric neurons (Figure 1D), as well as interstitial cells that express c-kit (Figure 1E) or platelet-derived growth factor receptor-alpha (pdgfra; Figure 1F). NPY-GFP+ terminals innervated all layers of the colon (Figure 1G), but the density of expression was region- (p=0.0025) and layer-dependent (p<0.0001, n=5; Figure 1H; there was no significant interaction between colon region and layer). When the hypogastric nerve was ligated and cut, allowing extrinsic fibers to degenerate, the expression of NPY-GFP+ diminished in all layers of the distal colon (n=3; Supplementary Figure S2), confirming that the majority of NPY-GFP+ axons in this reporter line arise from extrinsic sympathetic neurons.
Figure 1. Individual sympathetic postganglionic neurons (SPNs) innervate proximal or distal colon.
(A) Montage shows innervation from sympathetic prevertebral ganglia to colon in NPY-GFP reporter mouse. (B) Quantification of sympathetic ganglion neurons that express NPY-GFP and/or TH (all NPY-GFP+ cells expressed TH). (C) Whole-mount immunolabeling reveals colocalization of TH and NPY-GFP+ fibers. (D) NPY-GFP+ fibers appear to innervate NOS+ (arrow) and NOS- myenteric neurons (asterisk); NPY-GFP+ varicosities are also closely opposed to NOS+ terminals within smooth muscle (arrowheads). (E-F) NPY-GFP+ fibers make close contacts to c-kit+ interstitial cells of Cajal (E) and PDGFRA+ cells (F) in the myenteric plexus. (G) Cross section of NPY-GFP mouse colon shows innervation to all layers; LM, longitudinal muscle, MP, myenteric plexus, CM, circular muscle, SP, submucosal plexus, M, mucosa, E, epithelium. (H) Comparison of NPY-GFP fiber density in proximal and distal colon. (I) Colon back-labeling experimental design. (J-L) Individual sympathetic postganglionic neurons in the pelvic hypogastric plexus (J), inferior mesenteric ganglion (K), and celiac ganglion (L) project to proximal (green) or distal (red) colon regions. Bar graphs show total number (left axis, black) and percentage (right axis, gray) of labeled cells. Scale bars, 1mm (A), 100μm (G), 20μm (C-F,J-L).
To identify the source of sympathetic innervation to proximal and distal colon, two different colors of fluorescently-tagged cholera toxin subunit beta (CTB, 488nm and 555nm) were used to back-label SPNs (n=8; Figure 1I). Tracer spread was comparable for proximal and distal injections and did not result in overlap in the colon (Supplemental Figure S3). Examination of sympathetic ganglia revealed distinct populations of SPNs that innervated either proximal or distal colon, based on green or red labeling, respectively (Figure 1G–1I). Whereas most labeled neurons in the celiac ganglion (CG) projected to proximal colon, the inferior mesenteric ganglion (IMG) and pelvic hypogastric plexus (PHP) had equal proportions of neurons innervating proximal and distal regions, with no apparent topographic organization. The CG contained the greatest number of colon-projecting neurons, but the IMG contained the highest proportion of colon-projecting neurons (~60% versus 10% of TH+ neurons in IMG and CG, respectively).
Sympathetic stimulation suppresses local contractions and alters pan-colonic motility patterns
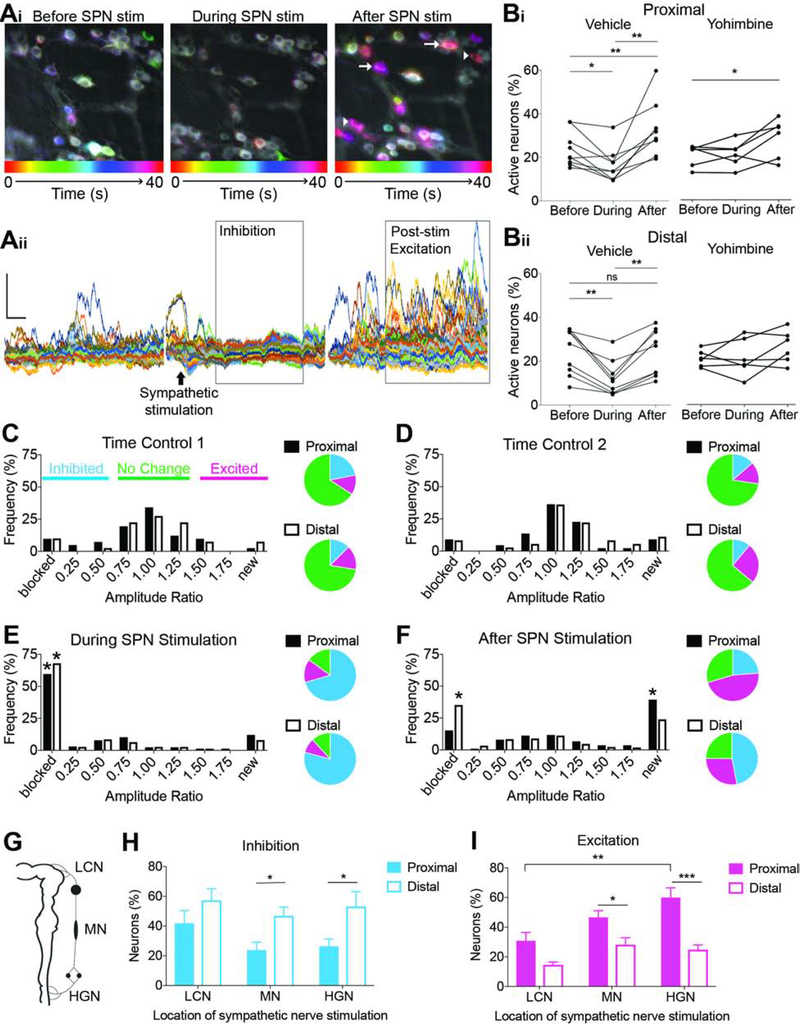
The effect of sympathetic stimulation on colon motility was examined in an ex vivo mouse preparation that maintains SPG continuity with the colon by preserving the lumbar colonic, mesenteric, and hypogastric nerves (LCN, MN, and HGN). Using E2aCre-GCaMP mice, which express GCaMP globally, we characterized spontaneous and electrically-evoked activity in SPNs of the IMG (Figure 2A). Without electrical stimulation, ongoing GCaMP activity was visualized in 10.5±1.9% of SPNs (n=5) and different patterns of GCaMP signals were observed (Figure 2B), reflecting different activity levels. When spontaneous smooth muscle contractions were blocked with nifidepine, the average amplitude and number of calcium transients produced by SPNs decreased (p=0.0105 and p=0.0439, respectively; n=4), suggesting some activity depended on sensory input from the colon presumably via intestinofugal neurons23–26. Electrical stimulation (20Hz,5s) of the LCN, MN, or HGN antidromically activated many SPN neurons in the IMG regardless of which nerve was stimulated, whereas activation of CG and PHP neurons was location-dependent (Figure 2C). Stimulation of the MN was most effective for activating SPNs in all ganglia and used for the remaining experiments, unless indicated. When different frequencies of stimulation were applied to the same location, the amplitude of GCaMP response from individual neurons increased with higher stimulation frequencies (p=0.0014, n=5 mice, 5–10 cells per experiment; Figure 2D), suggesting a relationship between level of activation and GCaMP signal.
Figure 2. Electrical and optogenetic stimulation of SPNs produce local and pan-colonic motility changes.
(A) Maximum intensity projection of GCaMP fluorescence of SPNs. (B) Example traces of spontaneous activity in SPNs. (C) Electrical stimulation (20Hz) of LCN, MN, and HGN differentially activated neurons in sympathetic prevertebral ganglia; note that only MN stimulation was effective at producing responses (ΔF) in all three ganglia. (D) Amplitude of SPN responses increased as stimulation frequency increased. Inset, example GCaMP traces from an individual SPN to electrical stimulation of the MN at 1–20 Hz for 5 s (black bar); (E) Average tissue movement before, during, and after nerve stimulation at 1–20 Hz. Movement was significantly decreased during electrical stimulation (ES). (F) Experimental design to measure motility changes produced by optogenetic (blue laser) stimulation of SPNs using NPY-ChR2 mice. (G) Laser also significantly decreased local motility during stimulation. (H-J) Effects of ES on pan-colonic motility patterns. (K-M) Effects of laser on pan-colonic motility patterns. *p<0.05, **p<0.01, ***p<0.001, repeated measures (rm) two-way ANOVA, Tukey’s post-hoc correction for multiple comparisons (D,E,G); paired Student’s t-test (H-M). Scale bars, 20ΔF, 1s (B,D).
We have previously shown that tissue movement within an imaging field is directly correlated to local changes in smooth muscle tension in the colon22 and used this technique to confirm that activation of SPNs suppressed colon motility. Electrical stimulation of SPNs inhibited ongoing local contractions to 30.5±10.8% of baseline (p=0.0037, n=8; Figure 2E), and after 10–20 sec spontaneous contractions resumed. Post-stimulation movement was often greater than before stimulation (9/16 trials, 56%), suggesting “rebound” contractions were produced as a result of temporary suppression of colon motility induced by sympathetic stimulation. Because electrical stimulation is non-specific and activates all fibers of passage (e.g., spinal sensory and preganglionic sympathetic neurons), we used a mouse model that expresses the blue-light activated channelrhodopsin (ChR2) in NPY neurons to selectively activate SPNs and measure local motility changes (Figure 2F). To restrict laser-stimulation to SPNs, we made a custom “shield” that prevented light from hitting the colon directly. Similar to electrical stimulation, optogenetic activation (10s) of SPNs decreased local contractions to 36.7±2.0% of baseline (p=0.0238, n=4; Figure 2G), and post-stimulation movement was greater than before stimulation in 50% of trials (8/16). The pattern of contractions produced after optogenetic stimulation were rhythmic and occurred at a similar frequency as ICC depolarization (11.4±0.62 cycles/min), suggesting that SPN activation may facilitate motor patterns generated by ICC. Importantly, these data show that both electrical and optogenetic stimulation of SPNs produce comparable changes in local motility.
Colonic migrating motor complexes (CMMCs) are migrating contractions that require neurogenic (i.e., myenteric neurons) and myogenic (i.e., ICC) mechanisms for their initiation, propagation, and rhythmic nature27. The presence or absence of intact SPG circuits affected CMMC characteristics (Supplemental Figure S4), suggesting that peripheral neuron connections to the colon modulate ongoing function in ex vivo preparations. We applied electrical (20Hz for 5s) or laser stimulation (10s) to the MN every two minutes to determine whether increasing sympathetic input disrupted CMMCs. Electrical, but not laser, stimulation decreased the frequency of CMMCs (electrical: p=0.0450, n=4; Figure 2H; laser: p=0.7220, n=4; Figure 2K), and the length of CMMC propagation was decreased by both electrical and laser (electrical: p=0.0321, n=4; Figure 2I; laser: p=0.0088, n=4; Figure 2L), such that migrating contractions rarely reached the distal colon. Ripples are myogenic motility patterns characterized by low amplitude, rapid, non-propagating contractions27. Ripple-like contractions were easily visualized in the proximal colon and increased in response to electrical and laser stimulation (electrical: p=0.0252, n=4; Figure 2J; laser: p=0.0495, n=4; Figure 2M). These data suggest that sympathetic input decreases CMMC propagation to the distal colon while promoting myogenic contractions in the proximal colon.
Sympathetic input differentially influences myenteric neuron activity in proximal and distal colon
To understand the cellular mechanisms underlying sympathetic-induced motility patterns, we measured myenteric neuron activity using GCaMP imaging. SPN stimulation had biphasic effects on GCaMP activity in myenteric neurons (Figure 3A). As a population, the percentage of neurons with spontaneous GCaMP activity decreased during SPN stimulation in proximal (p=0.0204, n=8; Figure 3Bi) and distal (p=0.0030, n=8; Figure 3Bii) colon. Sympathetic-induced inhibition was often followed by a coordinated increase in GCaMP activity (“post-stimulation excitation,” Figure 3Aii). Although occasionally observed in distal colon (8/26 trials, 31%), increases in neural activity after stimulation were only significant in proximal regions (proximal: 23/28 trials, 82%, p=0.0067, n=8; distal: p=0.8890, n=8; Figure 3B). The alpha-2 adrenoceptor antagonist, yohimbine, blocked SPN-induced inhibition in proximal and distal colon (proximal: p=0.9492, n=6; distal: p=0.9809, n=6; Figure 3B); however, post-stimulation excitation still occurred (p=0.0261, n=5; Figure 3B). Interestingly, yohimbine increased the occurrence of post-stimulation excitation in distal colon from 31% (8/26 trials) to 61% of trials (11/18). Blocking norepinephrine release with guanethidine also prevented inhibition of myenteric neurons, but not post-stimulation excitation. In fact, in many instances, the post-stimulation excitatory phase in distal colon occurred earlier in the presence of guanethidine (data not shown), suggesting that alpha-2 adrenergic signaling may suppress excitatory actions of SPN stimulation in distal colon regions. Overall, these results confirm that SPN-release of norepinephrine inhibits myenteric neuron activity via alpha-2 adrenoceptors, and experiments revealed a delayed, excitatory response in the proximal colon that was not dependent on norepinephrine-mediated inhibition.
Figure 3. Sympathetic input differentially influences myenteric neuron activity in proximal and distal colon partially mediated via alpha-2 adrenergic receptors.
(A) Time-lapse color-coded images (Ai) and F/F traces (Aii) of GCaMP activity in myenteric neurons before, during, and after SPN stimulation (black arrow). Gray boxes highlight two phases of response (inhibition and post-stimulation excitation) to sympathetic activation. White arrows indicate neurons with increased spontaneous activity and arrowheads indicate neurons with “new” activity. (B) Percentage of myenteric neurons in proximal (Bi) and distal (Bii) colon with GCaMP activity before, during, and after SPN stimulation in the presence of vehicle (left) or yohimbine (right). (C-F) Histograms and corresponding pie charts showing the relative frequency of neurons in proximal (black) and distal (white) colon exhibiting changes in amplitude of GCaMP signals over time (C and D), during SPN stimulation (E), and after SPN stimulation (F); frequency of blocked neurons during stimulation was significantly higher compared to time-controls in both regions, but after stimulation, proximal colon had more “new” active neurons, whereas more neurons in the distal colon had sustained inhibition compared to time-controls. (G) Schematic illustrates nerves from SPGs to the colon. (H-I) Comparison of the frequency of neurons in the proximal and distal colon that are inhibited (H) or excited (I) after LCN, MN, and HGN stimulation. *p<0.05, **p<0.01, ***p<0.001; rm one-way ANOVA (B) and rm two-way ANOVA (H,I), Tukey’s post-hoc correction for multiple comparisons.
Analysis of changes in GCaMP signals from individual myenteric neurons revealed additional insights into sympathetic-induced changes in neural activity. In control experiments (no stimulation), the majority of myenteric neurons did not exhibit significant changes in GCaMP signal amplitude over two or even three consecutive 40-s recordings (Figure 3C–D). By contrast, GCaMP signals were completely blocked in significantly more neurons during SPN stimulation compared to control experiments (p<0.0001, Figure 3E), and as predicted by our population analysis, SPN-induced responses in proximal and distal colon diverged after this inhibitory phase. In distal colon, more neurons were still “blocked” compared to control experiments (p=0.008), whereas more “new” neurons (not active prior to stimulation) began to exhibit GCaMP signals in the proximal colon after SPN stimulation compared to time controls (p<0.0001, Figure 3F). Therefore, sympathetic input to the colon reduces ongoing activity in myenteric neurons, but also recruits activity in a new population of neurons selectively in the proximal colon, presumably causing the observed sequence of changes in smooth muscle contractions.
To test whether region-specific effects observed after SPN stimulation depended on the nerve stimulated, we compared activity changes in response to LCN, MN, and HGN stimulation (Figure 3G). There were more neurons inhibited by SPN stimulation in the distal versus proximal colon, regardless of the nerve stimulated (main effect of colon region, p=0.0005; no effect of stimulus location, p=0.1215; Figure 3H). In proximal colon, there were more neurons excited by SPN stimulation compared to distal colon, especially when the HGN was stimulated (main effect of colon region, p<0.0001; main effect of stimulus location, p=0.0013; Figure 3I). Overall, these data reveal that post-stimulation excitation is specific to the proximal colon, does not depend on alpha-2 adrenoreceptor activation, and may involve components unique to pelvic (HGN) sympathetic circuits.
To determine whether different myenteric neuron subtypes are targeted by SPNs in proximal versus distal colon, we repeated experiments using more specific GCaMP models (NOS- and VGLUT2-GCaMP). Inhibitory motor neurons and interneurons express NOS, whereas VGLUT2-expressing neurons are thought to be ascending interneurons or a subtype of sensory afferent. Interestingly, a higher proportion of NOS-GCaMP+ neurons in proximal colon were inhibited with SPN stimulation compared to distal colon (p=0.0018, n=3) whereas more VGLUT2-GCaMP+ neurons were inhibited in distal versus proximal regions (p=0.0004, n=3; Supplemental Figure S5). In the NOS1-GCaMP mouse model, only 34% of GCaMP+ neurons were immunoreactive for NOS1, suggesting that many GCaMP+ neurons expressed NOS1 sometime during development but not in adulthood. VGLUT2-GCaMP+ myenteric neurons represented a more homogenous population, and we verified that no VGLUT2-GCaMP+ neurons were NOS-immunoreactive. Although we cannot confirm whether NOS1-GCaMP+ and VGLUT2-GCaMP+ neurons represent nitrergic and glutamatergic myenteric neurons, respectively, these data support the concept that sympathetic input to proximal and distal regions is functionally unique.
Sympathetic input has region-specific effects on ascending and descending myenteric circuits
To understand the effects of sympathetic input on myenteric neurons that make up descending and ascending circuits involved in reflexive peristalsis, we compared responses to direct colon stimulation before, during, and after activation of SPNs (Figure 4A). Even without SPN stimulation, evoked responses in proximal, but not distal, colon were significantly decreased in colon preparations with intact SPG compared to isolated colons (main effect of intact SPG in proximal (p=0.0023, n=5, Figure 4B), no effect in distal colon (p=0. 6914, n=5, Figure 4C)), indicating that ongoing SPN activity is sufficient to inhibit evoked activity of neurons in proximal colon regions. Further increasing SPN activity with electrical stimulation produced region-specific changes. In proximal colon, myenteric neuron responses to anal stimulation (i.e., those that receive ascending input) were significantly decreased during SPN stimulation (p=0.0070, n=5; Figure 4Dii), but responses to oral stimulation (i.e., those that receive descending input) were not altered (Figure 4Di). In middle and distal colon, myenteric neuron responses to oral stimulation were significantly decreased after SPN activation (middle: p=0.0461, n=5, Figure 4Ei; distal: p=0.0055, Figure 4Fi). Decreases to anal stimulation did not reach significance (Figure 4Eii and 4Fii). These results suggest that sympathetic input suppresses myenteric reflexes in a region-dependent manner; descending myenteric circuits in middle and distal colon regions are targeted to prevent relaxation necessary for anterograde movement of fecal contents, whereas ascending input to the proximal colon is inhibited, likely decreasing reflex-initiated propulsive contractions.
Figure 4. Sympathetic input has region-specific effects on ascending and descending myenteric neuron circuits.
(A) Diagram illustrating experimental setup. (B-C) Percentage of myenteric neurons in proximal (B) and distal (C) colon that responded to colon stimulation in isolated colon preparations (circles) and colon preparations with SPG intact (without external SPN activation; squares). (D-F) Percentage of neurons in proximal (D), middle (E), and distal (F) colon regions that responded to oral (i) or anal (ii) stimulation during and immediately following SPN stimulation. *p<0.05, **p<0.01; two-way ANOVA (B,C) and one-way ANOVA (D-F) with Tukey’s post hoc test for multiple comparisons.
Sympathetic input increases calcium transients in epithelial cells of proximal colon
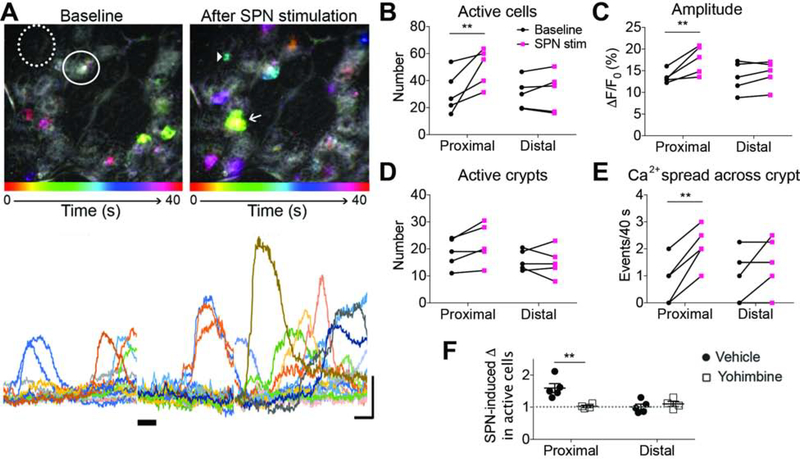
The colon epithelium contains a heterogenous population of cells that interact with other cell types to regulate colon functions. Sympathetic input has been reported to influence epithelial cell proliferation28, and studies of intestinal organoids indicate that epithelial enterochromaffin cells have functional alpha-2 adrenoceptors that elicit calcium transients when activated by norepinephrine5. To investigate whether sympathetic-to-epithelial communication exists in intact colon preparations, we examined the effects of sympathetic activation on colon epithelial cells using E2a-GCaMP mice (Figure 5A). About half of the colonic crypts per imaging field contained epithelial cells with ongoing GCaMP calcium transients (1–3 cells per “active” crypt). In proximal colon, SPN stimulation increased the number of epithelial cells with GCaMP activity (p=0.0078, n=5; Figure 5B) and increased the amplitude of GCaMP signal (p=0.0034, n=5; Figure 5C). The number of crypts with active epithelial cells did not increase (p=0.1010, n=5; Figure 5D), but SPN stimulation increased the spread of calcium signals between cells within a crypt in proximal colon (p=0.0023, n=5; Figure 5E). The addition of yohimbine prevented SPN-induced responses (p=0.0025, n=5; Figure 5F), suggesting that sympathetic input activates proximal colon epithelium via alpha-2 adrenoceptors.
Figure 5. Sympathetic input selectively increases GCaMP activity in proximal colon epithelium.
(A) Time-lapse color-coded images (top) and traces (bottom) of GCaMP activity in epithelial cells before (left) and after (right) SPN stimulation. Solid circle, active crypt; dotted circle, inactive crypt; arrowhead, active epithelial cell; arrow, “calcium spread” across >2 cells. (B-E) The number of active epithelial cells (B), amplitude of GCaMP signal (C), number of active crypts (D), and number of ”calcium spread” events were measured before and after SPN stimulation and compared between the proximal and distal colon. (F) Effects of yohimbine on SPN-induced changes in active epithelial cells in proximal and distal colon; dotted at y=1 represents no change in activity. **p<0.01; rm two-way ANOVA (B,C,E), two-way ANOVA (F) with Tukey’s post hoc test for multiple comparisons.
SPN stimulation had no effect on GCaMP activity in distal colon epithelium using the parameters above. However, norepinephrine (10μM) applied to isolated distal and proximal colon tissue increased the number of active cells from both colon regions (Supplemental Figure S6). Epithelial cells in distal colon, therefore, appear to express functional adrenoceptors that are not sufficiently activated with nerve stimulation, suggesting that neural and systemic release of norepinephrine produce unique effects with respect to sympathetic actions on colon epithelium via synaptic and volume transmission.
Sympathetic input alters activity of interstitial cells in proximal colon
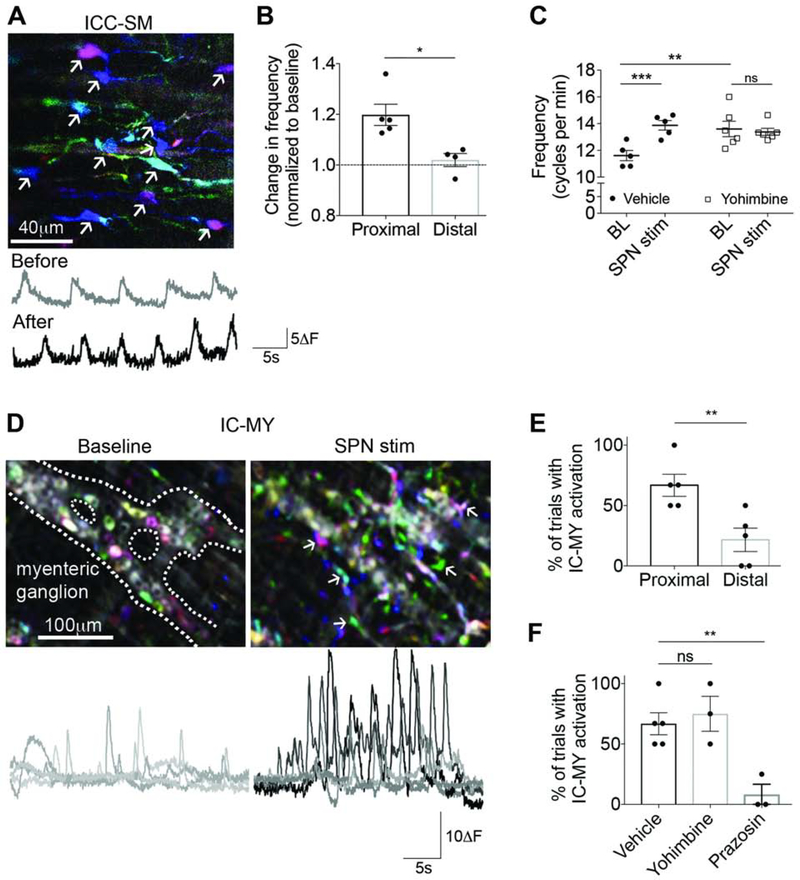
The syncytium of smooth muscle, c-kit-expressing interstitial cells of Cajal (ICC), and cells that express platelet-derived growth factor receptor-alpha (PDGFRA+ cells), are known as the SIP syncytium, and play an important role in the production of colon motility patterns29–30. To determine whether sympathetic-induced changes in motility were modulated in part by interstitial network activity in submucosa (Figure 6A) and/or myenteric plexus (Figure 6D), calcium transients were studied in response to SPN stimulation in E2a-GCaMP mice. Once again, SPN stimulation caused region-specific changes (Figure 6B,6E). In proximal colon, the frequency of calcium transients in submucosal interstitial “pace-making” cells (ICC-SM) was significantly increased (p=0.0048, n=5; Figure 6C). Yohimbine blocked this response (p=0.6006; Figure 6C), but the baseline frequency was increased compared to vehicle, suggesting that ongoing signals from SPNs modulate ICC-SM in the absence of stimulation. Because of the known alpha-2 adrenergic mediated effects on myenteric neurons and the influence they have on ICC3–4, the effects of SPN stimulation on ICC-SM may be an indirect consequence of neuron activity changes.
Figure 6. Sympathetic input influences interstitial cells in proximal colon.
(A) Time-lapse color-coded images (left) and traces (right) of GCaMP activity from interstitial cells of Cajal in the submucosal plexus (ICC-SM) (arrows) before and after SPN stimulation. (B) SPN-induced changes in ICC-SM frequency were significantly different in proximal and distal colon. (C) In proximal colon regions, SPN stimulation significantly increased ICC-SM frequency (filled circles, vehicle); yohimbine increased baseline frequency but blocked the response to SPN stimulation (open squares). (D) Time-lapse color-coded images (top) and traces (bottom) of GCaMP activity from interstitial cells in the myenteric plexus (IC-MY) (arrows) at baseline and with SPN stimulation. (E) Percentage of trials with SPN-induced activation of IC-MY in proximal colon was significantly greater than distal colon. (F) Yohimbine did not prevent IC-MY activation, but alpha-1 receptor antagonist, prazosin, significantly decreased IC-MY activation due to SPN stimulation. *p<0.05, **p<0.01, ***p<0.001; Student’s unpaired t-test (B,E), two-way (C) and one-way (F) ANOVA with Tukey’s post hoc test for multiple comparisons.
SPN stimulation also induced activity in interstitial cells located in the myenteric plexus (IC-MY) that consisted of an increase in: 1) the number of cells with calcium transients, 2) the number of transients evoked, and 3) the amplitude of GCaMP signal (Figure 6D). The percentage of trials that successfully produced activation was higher in proximal versus distal colon (p=0.0093, n=5; Figure 6E). Responses from IC-MY induced by SPN activation were not blocked by yohimbine (p=0.8097; n=3), but were suppressed when prazosin, an alpha-1 adrenoreceptor antagonist, was added (p=0.0080, n=3; Figure 6F). Although we were unable to confirm the identity of IC-MY activated by SPNs using the non-specific E2a-GCaMP model, RNA sequencing data from mouse intestine show alpha-1 adrenoceptor expression exclusively in PDGFRA+ cells31–33, and a recent study reports norepinephrine-induced calcium transients via alpha-1 adrenoceptors in this specific cell population34. Combined, these data suggest that alpha-1 adrenoceptor activation by norepinephrine released from SPNs directly increases calcium transients in PDGFRA+ cells in the myenteric plexus (IC-MY) of proximal mouse colon, but not in distal regions when all SPG input to the colon is intact.
Molecular evidence that sympathetic neurons regulate human interstitial networks in a region-dependent manner
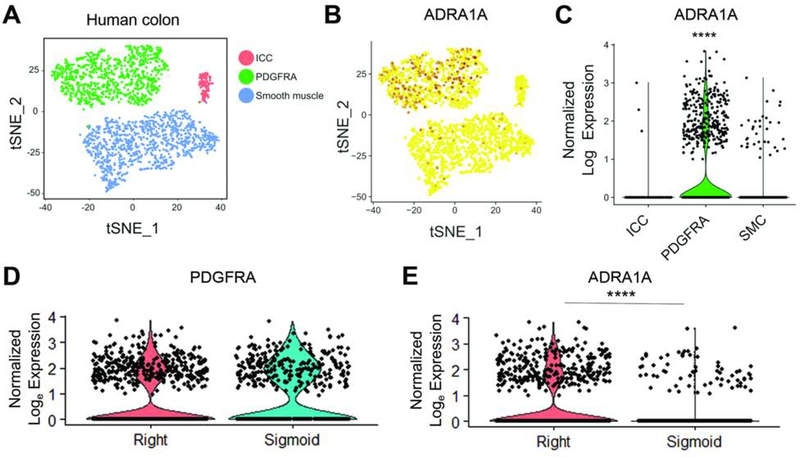
To determine whether human interstitial cells also express receptors implicated in sympathetic neuromodulation of mouse interstitial networks, we retrieved nuclei of ICC (gene markers KIT and ANO1), smooth muscle (MYH11 and ACTG2), and PDGFRA+ cells (PDGFRA) (Figure 7A; Supplementary Figure S7) from human colon tissue (Supplemental Table S2) and performed RNA sequencing. The gene that encodes stimulatory alpha-1 adrenoceptors, ADRA1A, was significantly expressed only in the PDGFRA+ population (p<0.0001; Figure 7B and 7C), and no other adrenergic receptor genes were highly expressed in cells of the SIP syncytium (Supplementary Figure S8). Figure 7D shows that PDGFRA+ cells are comparable between the two colon regions, but remarkably, ADRA1A expression was significantly greater in right colon compared to sigmoid colon (p<0.0001; Figure 7E), emphasizing the translational relevance of region-specific sympathetic input to the colon.
Figure 7. RNA sequencing of human interstitial cells provides molecular evidence that region-specific responses to sympathetic input exist in human colon.
(A) T-SNE plot showing 1684 SIP syncytium nuclei from human colon; ICC, PDGFRA+, and smooth muscle cells (SMC) formed distinct clusters, identified using canonical markers (Supplementary Figure S7). (B-C) T-SNE and violin plots showing the adrenoceptor, ADRA1A, was highly expressed in PDGFRA+ cells, but not in other groups. (D-E) ADRA1A expression was significantly higher in right colon compared to sigmoid colon, despite no difference in PDGFRA. ***p< 0.0001; Wilcoxon rank sum test with Bonferroni correction (C,E).
DISCUSSION
The ability to visualize sympathetic neuron-mediated, real-time responses across numerous cell types in the colon painted a holistic picture of the cellular mechanisms underlying the well-established effects of adrenergic or sympathetic nerve stimulation on motility. Here, through the use of GCaMP imaging in a mouse colon preparation that keeps sympathetic circuits intact, we revealed a more complex population response of myenteric neurons, the drivers of colon motility, that differed between proximal and distal colon regions. Region-specificity of responses to sympathetic neuron activation extended to epithelial and interstitial cells. Results indicated that sympathetic neurons activate alpha-2 adrenoceptors on epithelial cells and alpha-1 adrenoceptors on interstitial cells (putative PDGFRα+ cells) in the myenteric plexus, specifically in proximal colon. Importantly, RNA sequencing of right and sigmoid human colon provided molecular evidence that sympathetic input affects these two regions differently, thus emphasizing the translational and clinical relevance of regional differences in cellular responses to sympathetic input.
The unique effects of sympathetic activity on proximal and distal colon support the concept that these two regions have distinct functions and characteristics. The diversity of responses and cell types receiving sympathetic input in proximal colon supports the idea of increased circuit complexity compared to distal colon35. For example, in proximal colon regions, sympathetic activation transiently reduced activity in a subpopulation of myenteric neurons, but also increased activity in interstitial cells, epithelial cells, and a new population of myenteric neurons that were not previously active. These changes in cellular activity resulted in increased ripples and non-propagating contractions conducive for mixing of fecal contents. The same stimulation suppressed motility to distal colon by inhibiting ongoing and evoked activity of myenteric neurons, as previously described11,19. Whereas Spencer et al. (1999) reported that sympathetic stimulation inhibited both ascending and descending reflexive pathways11, the data presented here showed inhibition only of distal myenteric neurons that received descending input in response to oral stimulation. A number of differences could account for the conflicting results. For example, muscle contractions evoked by mucosal stimulation were measured in the previous study, whereas GCaMP calcium imaging was used here to measure responses in myenteric neurons, rather than muscle activity. We used a shorter electrical stimulus to activate sympathetic neurons, and it is possible that longer stimulation is required for inhibition of ascending pathways. Importantly, the preparation used here contained input from additional sympathetic prevertebral ganglia that may influence responses in the distal colon.
It is possible that some of our results were confounded by the fact that electrical stimulation of the LCN, MN, or HGN does not selectively activate sympathetic postganglionic neurons; rather, activation of pre- and postganglionic neurons, and antidromic stimulation of sensory afferents likely occurred. We reported recently that antidromic stimulation of lumbosacral extrinsic primary afferents with electrical or chemical (capsaicin) stimulation does not directly affect activity in myenteric neurons22, and we did not observe the changes in activity in non-neuronal cells as we report here. However, we cannot rule out the possibility that direct changes occur with antidromic stimulation of thoracolumbar extrinsic primary afferents, especially given the functional and molecular differences of afferents at different spinal levels36,37. Importantly, most of the changes produced by SPN stimulation were blocked with adrenoceptor antagonists, and optogenetic stimulation of SPNs (using NPY-ChR2 mice) and electrical stimulation produced comparable changes in local and pan-colonic motility. Thus, we are confident that the majority of cellular responses described here are in fact due to release of norepinephrine from sympathetic neurons.
Our findings combined with previous studies on sympathetic input to glia20 and immune cells38 support the concept that sympathetic neurons directly impact diverse cell types to modulate functions critical for colon health. Maintenance of ongoing, integrated colon function is complicated by the need to monitor, regulate, and rid the body of an ever-changing luminal content that includes an interdependent microbiome. To do this, the colon must coordinate epithelial/endocrine secretions, a highly reactive immune system, and overall motility to ensure that luminal contents move at the appropriate rate required for waste removal and microbiome balance. Sympathetic postganglionic neurons are anatomically and functionally positioned to influence all of these processes. More generally, sympathetic input appears to act as a moderator between the proximal and distal colon to ensure timely formation, transport, storage and expulsion of fecal contents. Although speculative, one would expect that abnormal sympathetic activity, and the resulting dysmotility, would cause profound changes in the microbiome and immune status of the colon. Future preclinical studies would benefit from characterizing the dysregulation of sympathetic input to different cell types in mouse models of GI disorders (e.g., colon cancer, chemotherapy-induced neuropathy, colitis). Such detailed understanding of the impact of sympathetic signaling would improve treatment of GI disorders that have associated sympathetic dysfunction.
Supplementary Material
BACKGROUND AND CONTEXT
Sympathetic input is thought to regulate colon function by influencing enteric neurons and non-neuronal cells (e.g., enteric glia, epithelium, immune cells), but real-time recordings of cellular responses to direct sympathetic nerve stimulation are lacking. It is also unknown whether sympathetic neurons utilize the same mechanisms to modulate proximal and distal colon function.
NEW FINDINGS
Sympathetic-induced responses in diverse cell types and changes in motility patterns are unique to proximal and distal colon in mice. Molecular evidence from human colon samples highlights the translational relevance of region-specific sympathetic input to the colon.
LIMITATIONS
Clinical studies are required to confirm that sympathetic activity produces unique functional responses in different regions of human colon. Additional studies should also investigate how input from the central nervous system via sympathetic preganglionic neurons influence colon responses.
IMPACT
Sympathetic input differentially regulates activity in proximal and distal colon to maintain ongoing, integrated functions critical for colon health. Dysregulation of sympathetic activity (either pathological or medication-induced) should be considered as a source of dysmotility, microbiome or immune changes associated with gastrointestinal disorders.
Acknowledgements
The authors acknowledge and thank Chris Sullivan for expert technical support and mouse husbandry, as well as Vijay Jain for assistance during his student internship. The authors also thank Dr. Emma Furth, Dr. Federico Valdivieso, Dr. Michael D. Feldman, Caitlin Feltcher, Andrew Kromer, Lauren Schmucker, Silvia H. López, and Jennifer Finan for help coordinating tissue acquisition.
Supported by NIH grants: OT2-OD023859 (MJH), T32DK063922-GI, T32NS073548-PCPR, R01AR069951 (KMA, BMD), R01DK122798 (BMD), R01DK124955 (BMD), F32 DK120115 (KMSE), Irma and Norman Braman Endowment (ROH), the Suzi and Scott Lustgarten Center Endowment (ROH), The Children’s Hospital of Philadelphia Research Institute (ROH) and REACHirschsprung’s Foundation (KMSE).
Abbreviations
- SPN
sympathetic postganglionic neuron
- ENS
enteric nervous system
- ICC
interstitial cells of Cajal
- TH
tyrosine hydroxylase
- NPY
neuropeptide Y
- GFP
green fluorescent protein
- SPG
sympathetic prevertebral ganglia
- CG
celiac ganglion
- IMG
inferior mesenteric ganglion
- PHP
pelvic hypogastric plexus
- LCN
lumbar colonic nerve
- MN
mesenteric nerve
- HGN
hypogastric nerve
- PDGFRA
platelet-derived growth factor receptor alpha
- CMMC
colonic migrating motor complex
- IC-MY
interstitial cells in myenteric plexus
- IC-SM
interstitial cells in submucosal plexus
Footnotes
All authors have no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Schneider S, Wright CM, Heuckeroth RO. Unexpected Roles for the Second Brain: Enteric Nervous System as Master Regulator of Bowel Function. Annu Rev Physiol 2019;81:235–259. [DOI] [PubMed] [Google Scholar]
- 2.Iino S, Ward SM, Sanders KM. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J Physiol 2004;556:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker SA, Drumm BT, Cobine CA, et al. Inhibitory Neural Regulation of the Ca (2+) Transients in Intramuscular Interstitial Cells of Cajal in the Small Intestine. Front Physiol 2018;9:328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker SA, Drumm BT, Skowronek KE, et al. Excitatory Neuronal Responses of Ca(2+) Transients in Interstitial Cells of Cajal in the Small Intestine. eNeuro 2018;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellono NW, Bayrer JR, Leitch DB, et al. Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell 2017;170:185–198 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaelberer MM, Buchanan KL, Klein ME, et al. A gut-brain neural circuit for nutrient sensory transduction. Science 2018;361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makadia PA, Najjar SA, Saloman JL, et al. Optogenetic Activation of Colon Epithelium of the Mouse Produces High-Frequency Bursting in Extrinsic Colon Afferents and Engages Visceromotor Responses. J Neurosci 2018;38:5788–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janig W Integration of gut function by sympathetic reflexes. Baillieres Clin Gastroenterol 1988;2:45–62. [DOI] [PubMed] [Google Scholar]
- 9.Lundgren O Sympathetic input into the enteric nervous system. Gut 2000;47 Suppl 4:iv33–5; discussion iv36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lomax AE, Sharkey KA, Furness JB. The participation of the sympathetic innervation of the gastrointestinal tract in disease states. Neurogastroenterol Motil 2010;22:7–18. [DOI] [PubMed] [Google Scholar]
- 11.Spencer N, McCarron SL, Smith TK. Sympathetic inhibition of ascending and descending interneurones during the peristaltic reflex in the isolated guinea-pig distal colon. J Physiol 1999;519 Pt 2:539–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.North RA, Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol 1985;358:17–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bornstein JC, Costa M, Furness JB. Intrinsic and extrinsic inhibitory synaptic inputs to submucous neurones of the guinea-pig small intestine. J Physiol 1988;398:371–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty NS, Hancock AA. Role of alpha-2 adrenergic receptors in the control of diarrhea and intestinal motility. J Pharmacol Exp Ther 1983;225:269–74. [PubMed] [Google Scholar]
- 15.Scheibner J, Trendelenburg AU, Hein L, et al. Alpha 2-adrenoceptors in the enteric nervous system: a study in alpha 2A-adrenoceptor-deficient mice. Br J Pharmacol 2002;135:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stebbing M, Johnson P, Vremec M, et al. Role of alpha(2)-adrenoceptors in the sympathetic inhibition of motility reflexes of guinea-pig ileum. J Physiol 2001;534:465–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nasser Y, Ho W, Sharkey KA. Distribution of adrenergic receptors in the enteric nervous system of the guinea pig, mouse, and rat. J Comp Neurol 2006;495:529–53. [DOI] [PubMed] [Google Scholar]
- 18.Hirst GD, McKirdy HC. Presynaptic inhibition at mammalian peripheral synapse? Nature 1974;250:430–1. [DOI] [PubMed] [Google Scholar]
- 19.Spencer NJ, Bywater RA, Klemm MF. Effects of sympathetic nerve stimulation on membrane potential in the circular muscle of mouse distal colon. Neurogastroenterol Motil 1998;10:543–552. [DOI] [PubMed] [Google Scholar]
- 20.Gulbransen BD, Bains JS, Sharkey KA. Enteric glia are targets of the sympathetic innervation of the myenteric plexus in the guinea pig distal colon. J Neurosci 2010;30:6801–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sergeant GP, Thornbury KD, McHale NG, et al. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol Cell Physiol 2002;283:C885–94. [DOI] [PubMed] [Google Scholar]
- 22.Smith-Edwards KM, Najjar SA, Edwards BS, et al. Extrinsic primary afferent neurons link visceral pain to colon motility through a spinal reflex in mice. Gastroenterology 2019;157:522–536 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messenger JP, Furness JB. Distribution of enteric nerve cells projecting to the superior and inferior mesenteric ganglia of the guinea-pig. Cell Tissue Res 1993;271:333–9. [DOI] [PubMed] [Google Scholar]
- 24.Parkman HP, Ma RC, Stapelfeldt WH, et al. Direct and indirect mechanosensory pathways from the colon to the inferior mesenteric ganglion. Am J Physiol 1993;265:G499–505. [DOI] [PubMed] [Google Scholar]
- 25.Sharkey KA, Lomax AE, Bertrand PP, et al. Electrophysiology, shape, and chemistry of neurons that project from guinea pig colon to inferior mesenteric ganglia. Gastroenterology 1998;115:909–18. [DOI] [PubMed] [Google Scholar]
- 26.Szurszewski JH, Ermilov LG, Miller SM. Prevertebral ganglia and intestinofugal afferent neurones. Gut 2002;51 Suppl 1:i6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Corsetti M, Costa M, Bassotti G, et al. First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques. Nat Rev Gastroenterol Hepatol 2019;16:559–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kennedy MF, Tutton PJ, Barkla DH. Adrenergic factors involved in the control of crypt cell proliferation in jejunum and descending colon of mouse. Clin Exp Pharmacol Physiol 1983;10:577–586. [DOI] [PubMed] [Google Scholar]
- 29.Sanders KM, Koh SD, Ro S, et al. Regulation of gastrointestinal motility--insights from smooth muscle biology. Nat Rev Gastroenterol Hepatol 2012;9:633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev 2014;94:859–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ha SE, Lee MY, Kurahashi M, et al. Transcriptome analysis of PDGFRalpha+ cells identifies T-type Ca2+ channel CACNA1G as a new pathological marker for PDGFRalpha+ cell hyperplasia. PLoS One 2017;12:e0182265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee MY, Ha SE, Park C, et al. Transcriptome of interstitial cells of Cajal reveals unique and selective gene signatures. PLoS One 2017;12:e0176031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeisel A, Hochgerner H, Lonnerberg P, et al. Molecular Architecture of the Mouse Nervous System. Cell 2018;174:999–1014 e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kurahashi M, Kito Y, Baker SA, et al. A novel postsynaptic signal pathway of sympathetic neural regulation of murine colonic motility. FASEB 2020;34(4):5563–5577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Hao MM, Van den Haute C, et al. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine. Elife 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hockley JRF, Taylor TS, Callejo et al. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut 2019;68:633–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meerschaert KA, Adelman PC, Friedman RL, et al. Unique molecular characteristics of visceral afferents arising from different levels of the neuraxis: location of afferent somata predicts fuction and stimulus detection modalities. J Neuro (accepted for publication). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matheis F, Muller PA, Graves CL, et al. Adrenergic Signaling in Muscularis Macrophages Limits Infection-Induced Neuronal Loss. Cell 2020;180:64–78 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.