Abstract
Background
Meat-products are considered an enriched media for mycotoxins. This study aimed to investigate the prevalence of toxigenic Aspergillus species in processed meat samples, HPLC-quantitative measurement of aflatoxin B1 and ochratoxin A residues, and molecular sequencing of aflR1 and pks genes. One hundred and twenty processed beef meat specimens (basterma, sausage, and minced meat; n = 40 for each) were collected from Ismailia Province, Egypt. Samples were prepared for total mold count, isolation, and identification of Aspergillus species. All samples were analyzed for the production of both Aflatoxin B1 and Ochratoxin A mycotoxins by HPLC. Molecular identification of Aspergillus flavus and Aspergillus ochraceus was performed using PCR amplification of the internal transcribed spacer (ITS) region; furthermore, the aflR1 and pks genes were sequenced.
Results
The total mold count obtained from sausage samples was the highest one, followed by minced meat samples. The prevalence of A. flavus was (15%), (7.5%), and (10%), while the prevalence of A. ochraceus was (2.5%), (10%), and (0%) in the examined basterma, sausage, and minced meat samples, respectively. Using PCR, the ITS region was successfully amplified in all the tested A. flavus and A. ochraceus strains. Aflatoxin B1 was detected in six basterma samples (15%). Moreover, the ochratoxin A was detected only in four sausage samples (10%). The aflR1 and pks genes were amplified and sequenced successfully and deposited in the GenBank with accession numbers MF694264 and MF694264, respectively.
Conclusions
To the best of our knowledge, this is the first report concerning the HPLC-Molecular-based approaches for the detection of aflatoxin B1 and ochratoxin A in processed beef meat in Egypt. The production of aflatoxin B1 and ochratoxin A in processed meat constitutes a public health threat. Aflatoxin B1 is commonly associated with basterma samples. Moreover, ochratoxin A was detected frequently in sausage samples. The routine inspection of mycotoxins in processed meat products is essential to protect human consumers.
Keywords: A. flavus; A. ochraceus; Meat-products, HPLC; Aflatoxin B1; Ochratoxin A; Gene sequencing
Background
Beef meat and meat products are considered the most desirable and favorable food in Egypt. Beef meat is characterized by a high nutritional value due to its high content of essential amino acids, minerals, fats, and vitamins. In 2019, Egypt imported beef meat with the following values: 982 million US$ from Brazil (65% market share), 466 million US$ from India (31% market share), 16.2 million US$ from Colombia, 8.25 million US$ from Australia, 5.08 million US$ from New Zealand, and 2.4 million US$ from the USA [1, 2]. Basterma and sausage are processed meat products prepared from meat with the addition of food additives. The contamination of beef meat and meat products with mycotoxins constitutes public health hazards to the consumers. Depending on the type and predilection sites of the mycotoxins, different symptoms in humans have been determined including hepatotoxicity, liver cancer, nephrotoxicity, immunosuppression, mutagenicity, nervous and hormonal system disturbance [3].
Mycotoxins are commonly produced by the following species: Aspergillus, Fusarium, and Penicillium [4]. Many studies on mammals and poultry reported that: mycotoxins have mutagenic, hepatotoxic, carcinogenic, teratogenic, immunosuppressive, nephrotoxic, and embryotoxic effects [5]. Although single mold species could release more than one type of mycotoxin, one mycotoxin could be produced by different mold species. Globally, aflatoxins (AFs) are the most medically significant mycotoxins that contaminate human and animal foodstuff. According to the International Agency for Research on Cancer (IARC), aflatoxins, produced primarily by A. flavus have been confirmed as carcinogenic agents that mainly affects liver causing hepatocellular carcinoma [6, 7]. The treatment of aflatoxins with ultra-high temperature (UHT), roasting, pasteurization, baking, and cold storage cannot destruct them because aflatoxins are heat-stable. Numerous types of AFs exist naturally; but, the most potent types are; B1, B2, G1, and G2 [8]. Ochratoxin A (OTA) is a secondary metabolite produced mainly by Aspergillus species especially, A. ochraceus when the environmental and storage conditions are optimum for the growth and multiplication of these fungi, such as in tropical and subtropical regions [9, 10]. The major types of OTA are A, B, and C, that pose a significant threat to human and animal health [11].
Contamination of meat and meat products with molds commonly results from contaminated equipment or air. The mold contamination results in unfavorable alterations in the meat and meat products that causes severe infections, mycotoxicosis, and allergic reactions to consumers [12]. Humans are subjected to OTA either by ingestion, inhalation, or skin contact. Certain types of foods are considered the main source for OTA such as; coffee beans, grapes, wine, beef meat, beef meat products, poultry, pork, fish, cheese, and eggs [13].
Processed meat products are considered excellent substrate for mycotoxigenic fungal proliferation/colonization and the subsequent mycotoxin production; due to the mold contamination that occurs during the handling, manufacturing, and storage [14]. Various species of fungi produce hundreds of toxic metabolites. Globally, meat products are considered a common source of mycotoxins [15–19]. The mold contamination of meat and meat products results in severe illness in humans and animals due to the production of mycotoxins such as aflatoxins and ochratoxin A (OTA) [7]. Several studies from different countries have reported the occurrence of mycotoxins in both fresh and processed meat. Despite this topic has gained much attention in the last years, the European Regulation does not set specific limits for aflatoxins and ochratoxins in meat and processed meat products. The existence of mycotoxins in meat and meat products is considered a public health threat that magnifiy the need for more investigations concerning the detection of mycotoxins in such type of food [20–26].
This study aimed to investigate the prevalence of toxigenic Aspergillus species in processed meat samples, HPLC-quantitative measurement of aflatoxin B1 and ochratoxin A residues, and molecular sequencing of aflatoxin regulatory gene (aflR1) and polyketide synthase gene (pks). To the best of our knowledge, this is first study combined HPLC and Molecular assays for the detection of aflatoxin B1 and ochratoxin A in processed beef meat in Egypt.
Materials and methods
Samples collection and processing
A total of 120 processed beef meat specimens (basterma, sausage, and minced meat; n = 40 for each type) were collected randomly from licensed retail markets that gained good hygiene practice (GHP) in 4 different localities in Ismailia Province, Egypt. The collected specimens were labeled, placed into polyethylene sterile bags, and rapidly transported under complete aseptic conditions in an icebox to the Microbiology laboratory, Animal Health Research Institute, Egypt. For each specimen, 25 g were minced aseptically in a grinder through a 4 mm sterilized plate diameter (AC110V, China). In Egypt, the basterma is prepared from beef meat (3–5 cm thickness), salt, and other additives (ground fenugreek seed, ground paprika, cumin, black pepper, cayenne pepper, and garlic). Moreover, beef sausage is mostly produced from beef meat, fat tissues, salt, spices mixture (fennel, black pepper, cubeb, Nutmeg, cinnamon, cumin, and clovers), garlic, starch, and sodium glutamate. Besides, minced meat is prepared by grinding beef meat and fat (75%:25%).
Mold enumeration, isolation, and identification of Aspergiluus spp.
For propagation of mold associated with food spoilage, each minced sample was mixed with 225 mL of sterile peptone water (0.1%), then ten-fold serial dilutions were performed as previously reported by Downes and Ito [27]. Briefly, 1 mL of the processed dilution was poured into duplicated sterile Petri dishes, and then gently mixed with Dichloran Rose Bengal Chloramphenicol agar (Oxoid, UK). The inoculated plates were incubated up to 1 week at 25 °C and then examined for the mold growth and enumeration expressed as CFU/g. The suspected colonies were inoculated onto Sabouraud Dextrose slant agar (Oxoid, UK), and incubated at room temperature for up to 5 days for further mycological examination. The identification of suspected colonies was carried out depending upon the macroscopical examination; growth rate, texture, diameter, color, and characters of examined colonies, and the microscopical examination using lactophenol blue staining to investigate the morphological characters including; the conidial stage and head, sclerotia production, conidia, and conidiophore as previously described by Pitt and Hocking [28].
Extraction and HPLC-quantitative measurement of aflatoxin B1 and ochratoxin A residues
The chemicals and reagents used for extraction and HPLC-quantitative measurement of mycotoxins in the processed meat samples were purchased from Sigma (Sigma, Germany). Phosphate-buffered saline (PBS) was prepared by dissolving 8 g of NaCl, 0.2 g of KCl, 0.2 g of KH2PO4, and 1.2 g of Na2HPO4 in 1000 mL of water. The pH for PBS was adjusted to 7.0 with 0.1 M HCl.
Ten grams of each examined specimen were homogenized with 40 mL of acetonitrile: water (60:40, v/v) and 0.2 g NaCl for 90 s, then blended by a magnetic stirrer for 10 min. The filtration of the mixture was carried out through fast filtering Whatman No. 1 filter paper (Whatman Inc., Clifton, NJ, USA). Four mL of the filtrate were diluted with 44 mL of 2% tween-20-PBS solution in a 50 mL Erlenmeyer flask. Then, the filtrate was cleaned up using liquid/liquid extraction method as follows: 0.5 mL aliquot of filtrate was mixed with 0.5 mL acetonitrile, then 0.5 mL of the mixture was pipetted into an Alltech 1.5 mL Extract-Clean reservoir packed with 200 mg basic aluminum oxide (9 mm high-layer adsorbent). The quantitative detection of the mycotoxins was performed by HPLC system (Thermo Fisher Scientific, Waltham, MA 02451, USA) using 100 μL of the extract as previously described by Herzallah [29].
The fluorescence-detector was adjusted to an excitation and an emission wavelength of 365 nm and 435 nm, respectively. Concerning the validation and quality assurance of the quantitative measurement of aflatoxins; a seven-point standardization curve was conducted using the following concentrations: (0.1, 0.5, 1, 2, 5, 10, and 20 μg/kg) for aflatoxin B1. Moreover, the signal-to-noise approach was used to detect the limits of quantification (LOQ) and the limits of detection (LOD).
To ensure the accuracy of the test, approximately 25 g aflatoxins-free sample (for each sample type) was spiked with aflatoxin B1 at levels of 3, 5, and 10 μg/ kg. The spiked samples were examined using the HPLC, followed by the estimation of both the recovery and standard deviation. The protocol was performed in three replicates. For the validation and quality assurance of the quantitative measurement of ochratoxins, a five-point standardization curve was conducted using the following concentrations: 0.5, 2, 5, 10, and 30 μg/kg.
Besides, the signal-to-noise approach was used to detect the limits of quantification (LOQ) and the limits of detection (LOD). To ensure the accuracy of the test, about 25 g ochratoxin A-free sample (for each sample type) was spiked with ochratoxin A at the levels of 1, 5, and 20 μg/kg. The assay was performed in three replicates. The recovery rate for aflatoxin B1 and ochratoxin A was 90 and 92%, respectively [29, 30].
Molecular identification of A. ochraceus and A. flavus by amplification of internal transcribed spacer (ITS) region
Colonies with distinct phenotypic characters were used for DNA extraction. The DNA extraction was performed using the Patho Gene-SpinTM DNA/RNA Extraction kit (iNtRON cat. No. 17154, Korea). ITS region was amplified by PCR using the forward primer ITS1 5′-TCCGTAGGTGAACCTGCGG-3′ and the reverse primer ITS4 5′-TCCTCCGCTTTATTGATATG − 3′ (Sigma, Germany) with variable expected amplicon size (700–800 bp) [31]. PCR mixtures consisted of 50 μL containing; 25 μL master mix (Bioline, cat. BIO-25049, England), one μL each primer, and 30 to 80 ng of genomic DNA or distilled water (as a negative control). PCR was programmed as follow: 94 °C for 4 min followed by 35 cycles of 94 °C for 1 min, 56 °C for 1 min and 72 °C for 1 min. A final extension step was done at 72 °C for 10 min. PCR amplicons were visualized by gel electrophoresis (1.5%).
Amplification and sequence analysis of aflatoxin regulatory gene (aflR1) of A. flavus
PCR amplification for the aflR1 gene was performed in six toxigenic isolates of A.flavus using the forward primer AflR-1F 5′-AAGCTCCGGGATAGCTGTA-3′ and the reverse primer AflR-2R 5′-AGGCCACTAAACCCGAGTA − 3′ for the identified isolates with an expected amplicon size of 1079 bp [32]. Fifty μL volume of PCR was done as follow: initial denaturation at 95 °C for 10 min followed by 30 cycles of 94 °C for 30 s, 55 °C for 45 s, and 72 °C for 75 s. A final extension step was carried out at 72 °C for 10 min. As the retrieved A. flavus isolates revealed harmony in their phenotypic characteristics, the PCR product of one randomly selected isolate was purified with the Gene JETPCR purification kit (Thermo Scientific, Cat. K0701). PCR amplicons were sequenced in both directions using the Applied-Biosystem Automated 3730XL DNA sequencer (Macrogen, Seoul, South Korea). The obtained sequences were deposited in GenBank with accession No. MF094441, and then analyzed using the BLASTn tool at the National Center of Biotechnology Information. The evolutionary history was inferred by using the Maximum Likelihood method based on the Tamura-Nei model [33]. The evolutionary analyses were conducted using MEGA6 software (http://www.megasoftware.net/) [34].
Amplification and sequence analysis of polyketide synthase gene (pks) of A. ochraceus
Two sets of primers were used for PCR amplification of the pks gene (Table 1) in 4 toxigenic isolates of A.ochraceus. Fifty μL volume PCR was carried out as follows: initial denaturation at 94 °C for 4 min followed by 35 cycles of 94 °C for 40 s, 58 °C for 40 s, and 72 °C for 40 s. A final extension step was carried out at 72 °C for 10 min. Meanwhile, the recovered A. ochraceus isolates showed harmony in their phenotypic characteristics: the PCR product of one randomly selected isolate was purified with was purified by the Gene JETPCR purification kit (Thermo Scientific, Cat. K0701). PCR amplicons were sequenced in both directions using the Applied-Biosystem Automated 3730XL DNA sequencer (Macrogen, Seoul, South Korea). The obtained sequences were deposited in GenBank with accession No. MF694264, and then were analyzed using the BLASTn tool at the National Center of Biotechnology Information. The evolutionary history was inferred by using the Maximum Likelihood method based on the General Time Reversible model [36]. The evolutionary analyses were performed using MEGA6 software (http://www.megasoftware.net/) [34].
Table 1.
Primers used for the amplification of pks gene of A. ochraceus
| Primer | Oligonucleotide sequence | Expected amplicon Size (pb) | Reference |
|---|---|---|---|
| AoLc35-12 L | 5′-GCCAGACCATCGACACTGCATGCTC-3’ | 520 | [35] |
| AoLc35-12R | 5′-CGACTGGCGTTCCAGTACCATGAGCC-3’ | ||
| AoOTA-L | 5′-CATCCTGCCGCAACGCTCTATCTTTC-3’ | 690 | |
| AoOTA-R | 5′-CAATCACCCGAGGTCCAAGAGCCTCG-3’ |
Statistical analysis
The statistical analyses carried out using GraphPad Prism version 8.0.1 (244) (San Diego, CA, USA). All results were elaborated as mean together with standard deviation (SD). The Chi-square was implemented to analyze the data; the significance level was (P < 0.05).
Results
Total mold counts (CFU/g) in meat products samples
Total mold count (CFU/g) obtained from sausage samples was the highest one (2.9 × 102 ± 0.91 × 102), followed by minced meat samples (1.74 × 102 ± 0.52 × 102), and basterma samples (0.79 × 102 ± 0.31 × 102). Statistically, there is a significant difference in the total mold count among various examined samples (P < 0.0001) (Table 2).
Table 2.
Total mold count in examined meat products specimens (CFU/g)
| Meat products | Total mold count (CFU/g) | |||
|---|---|---|---|---|
| Minimum | Maximum | Mean(±SD) | P value | |
| Basterma | 36 | 140 | 0.79 × 102 ± 0.31 × 102 | *P < 0.0001 |
| Sausage | 103 | 380 | 2.9 × 102 ± 0.91 × 102 | |
| Minced meat | 70 | 249 | 1.74 × 102 ± 0.52 × 102 | |
*Significant, (P < 0.05)
The phenotyic chracterstics and prevalence of A. flavus and A. ochraceus in the examined meat products samples
Concerning the phenotypic characteristics of the recovered isolates, the colonies of A. flavus are characterized by a white soft velvety surface that becomes raised and turned floccose at the center after few days. The colonies produced yellowish-green and olive conidia during the sporulation. The conidia cover the entire surface of the colonies except for the edges, where a white border was produced. Sclerotia produced in white color then became deep brown. The diameter of A. flavus colonies ranged from 50 to 70 mm. Moreover, the colonies of A. ochraceus grow rapidly and are characterized with white soft velvety surfaces then turned in a characteristic yellow-gold color and have distinct globose conidial heads. The conidiophores have a powdery form that could be observed by the naked eye. Furthermore, the mycelium is submerged mainly in the agar media, and the conidial heads are commonly arranged in zones. The reverse appearance of the petri dish is mainly brownish. The diameter of A. ochraceus colonies ranged from 45 to 55 mm. (Figs. 1 and 2).
Fig. 1.
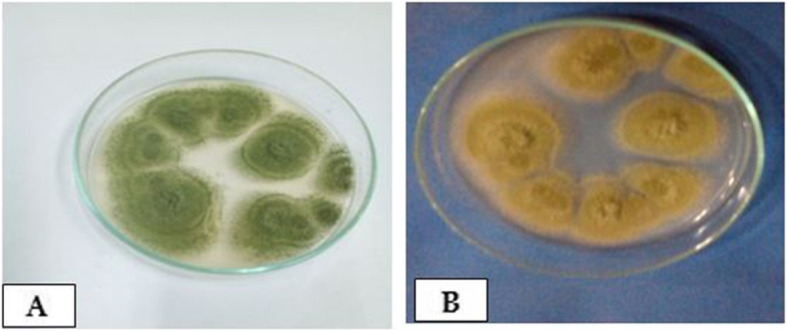
A. flavus and A. ochraceus colonies on SDA:. a. A. flavus: white soft velvety colonies that turn yellowish-green, a pigment of the conidial spores. b. A. ochraceus: white soft velvety colonies that turn yellow-gold conidia, a pigment of the conidial spores
Fig. 2.
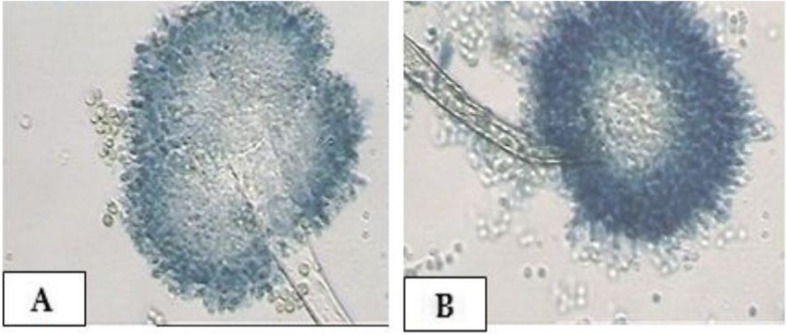
Microscopical examination of A. flavus and A. ochraceus (40×): a. A. flavus: conidial heads are radiate to loosely columnar with age. b. A. ochraceus: distinct globose conidial head
In the present study, the prevalence of A. flavus was (15%), (7.5%), and (10%) in the examined basterma, sausage, and minced meat samples, respectively. Moreover, the prevalence of A. ochraceus was (2.5%), (10%), and (0%) in the examined basterma, sausage, and minced meat samples, respectively (Table 3).
Table 3.
The prevalence of A. flavus and A. ochraceus isolates in the examined meat product samples
| Isolated Aspergillus species | Basterma (n = 40) | Sausage (n = 40) | Minced meat (n = 40) | |||
|---|---|---|---|---|---|---|
| Number of isolates | % | Number of isolates | % | Number of isolates | % | |
| A. flavus | 6 | 15 | 3 | 7.5 | 4 | 10 |
| A. ochraceus | 1 | 2.5 | 4 | 10 | 0 | 0 |
Prevalence of aflatoxin B1 and ochratoxin A in the examined meat products samples
Concerning the occurrence of aflatoxin B1 in the examined samples, it could be detected and quantified only in six basterma samples (15%). The minimum detected level was 16.5 μg/kg, while the maximum level of detection was 26.6 μg/kg with a mean value of 21.80 ± 3.823 μg/kg. Moreover, the examined minced meat and sausage samples were negative for aflatoxin B1.
Regarding the occurrence of ochratoxin A in the examined samples, it was detected only in four sausage samples (10%). The minimum detected level was 3.8 μg/kg, while the maximum level of detection was 17 μg/kg with a mean value of 10 ± 2.9 μg/kg. Furthermore, the examined basterma and minced meat samples were negative for ochratoxin A. Statistically, the production of aflatoxin B1 in basterma samples is significantly different from sausage and minced meat (P < 0.0001). Moreover, the production of ochratoxin A in sausage samples is significantly different from basterma and minced-meat (P = 0.0041; P < 0.05) (Table 4).
Table 4.
Prevalence of aflatoxin-B1 and ochratoxin A in the examined meat product samples (μg/kg)
| Meat Products | Aflatoxin B1 | Ochratoxin A | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of + ve samples | % | Range (μg/kg) | Mean ± SD | P value | No of + ve samples | % | Range (μg/kg) | Mean ± SD | P value | |
|
Basterma N = 40 |
6 | 15 | 16.5–26.6 | 21.80 ± 3.823 | *P < 0.0001 | 0 | 0 | 0 | 0.0 ± 0.0 | *P = 0.0044 |
|
Sausage N = 40 |
0 | 0 | 0 | 0.0 ± 0.0 | 4 | 10 | 3.8–17 | 10 ± 2.9 | ||
|
Minced meat N = 40 |
0 | 0 | 0 | 0.0 ± 0.0 | 0 | 0 | 0.0 ± 0.0 | |||
*Significant, (P < 0.05)
Molecular identification of A. flavus and A. ochraceus by PCR amplification of internal transcribed spacer (ITS) region
The genetic identification of the recovered A. ochraceus and A. flavus isolates was carried out usig PCR amplification of internal transcribed spacer (ITS) region. The PCR revealed that the ITS region was successfully amplified in all tested A. flavus and A. ochraceus isolates and giving the specific molecular size.
Amplification and sequence analysis of aflatoxin regulatory gene (aflR1) of A. flavus
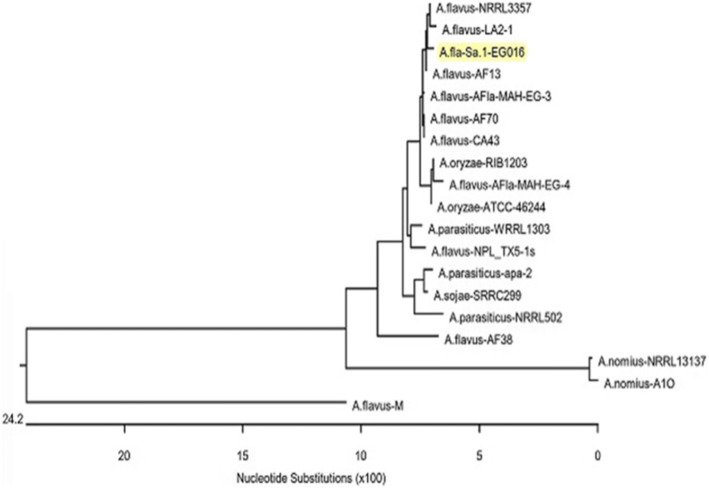
Using PCR, the aflR1 gene was amplified successfully in six A. flavus strains isolated from the examined basterma samples (~ 1079 bp). The PCR products were sent for sequencing, and the retrieved sequences were deposited in the GenBank with accession number: MF094441. According to the blastn tool at the NCBI, the identity was 99.73 with query covered 100% percentage to the other aflR1 genes deposited in the Genebank database (Fig. 3).
Fig. 3.
Phylogenetic analysis of the aflR1 gene using maximum likelihood method. The tree was generated based on the aflR gene nucleotide sequence; it illustrates the phylogenetic position of the retrieved strain (A.fla-Sa.1-EG016) with respect to other strains deposited in Genbank where the topology of the joining tree of the aflR gene sequence is almost the same. The phylogenetic tree was created by MEGA6 (http://www.megasoftware.net/)
Amplification and sequence analysis of polyketide synthase gene (pks) for the of A. ochraceus
The PCR proved that the pks gene was amplified successfully in four A. ochraceus strains isolated from sausage samples. The PCR products were sent for sequencing, and the recovered sequences were deposited in the GenBank with accession number: MF694264. According to the blastn tool, the similarity was 100 with query covered 100% percentage to other pks genes deposited in the Genebank database (Fig. 4).
Fig. 4.
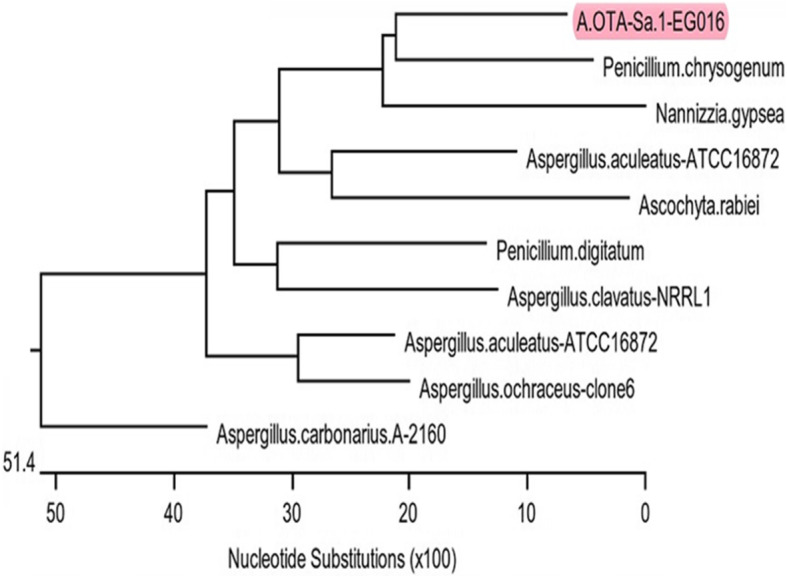
The phylogenetic analysis of pks gene using the maximum likelihood method. The trees was generated based on pks gene nucleotide sequence; it illustrates the phylogenetic position of the retrieved strain (AOTA-Sa.1-EGO16) with respect to other strains deposited in Genbank where the topology of the joining tree of the pks gene sequence is almost the same. The phylogenetic tree was created by MEGA6 (http://www.megasoftware.net/)
Discussion
Our results showed that the highest mean count (CFU/g) was in sausage (2.9 × 10 2 ± 0.91 × 102), followed by 1.74 × 102 ± 0.52 × 102 for minced meat, and 0.79 × 102 ± 0.31 × 102 for basterma. A previous study reported that the count was 2.8 × 102 ± 37.4 for basterma samples [37]. Moreover, Mousa et al. [38], and Ebraheem and Mohamed [39] reported that the mold count was 1.22 × 102 ± 0.49 × 102 and 2.4 × 102 ± 0.27 × 102 in basterma samples, respectively. Besides, a previous investigation [40] reported that the mean total mold count was 2.26 × 102 ± 0.58 × 102 in sausage samples. Molds are commonly detected inside or on the surface of definite aged, preserved meats, especially fermented sausage and could be metabolically active during the prolonged curing time and the ripening time of sausage. Therefore, fungi extremely affect the appearance, flavor, and quality of these meats. Moreover, the presence of cured salts, low water activity condition (aw), pH falls due to fermentation processes in sausage samples, and the ability of molds to withstand high concentrations of cured meat make sausage a favorable environment for mold growth [41–43].
In the present study, ten retrieved isolates were toxigenic (10/18, 55.55%) including six A. flavus strains (originated from the basterma samples) harbored the aflatoxin B1 and four A. ochraceus strains (isolated from sausage samples) harbored the ochratoxin A. Besides, A. parasiticus and A. nomius were not detected during the isolation and identification. In Egypt, A. flavus and A. ochraceus are the most predominant species [38]. Our findings revealed that A. flavus is the most predominant mold species isolated from the processed meat samples. Ebraheem and Mohamed [39] reported that the most predominant isolated mold species from basterma and luncheon samples was A. flavus. Besides, Makhlouf et al. [44] reported that Aspergillus was the chief genus found in the examined spices samples that used in the meat processing; A. flavus and A. ochraceus were the most predominant species, which is also in agreement with the findings reported by previous studies in Morocco, India, and Brasil [45–48]. In the current study, the prevalence of A. flavus was higher than A. ochraceus in the examined processed beef meat samples. A.flavus is a ubiquitous microorganism widely distributed in nature, soil, and different types of foods. A. flavus is an opportunistic microorganism characterized by a broad host range. Besides, A. flavus commonly contaminates most of the food additives used in meat processing [39, 49].
In the present study, the highest percentage of toxigenic isolated Aspergillus species was recorded in basterma samples followed by sausage samples. Moreover, no toxigenic Aspergillus species was detected in minced meat samples. Zohri et al. reported that two out of the four strains of A. flavus could produce aflatoxin B1 in the examined sausage samples [50]. Besides, a previous study reported that aflatoxins were detected in 15% of minced meat samples and 10% of fresh sausage [51]. Mycotoxins production is mainly affected by the type and composition of meat products, feed additives, the kind of nutrient contents, and the mechanism of its processing. Furthermore, numerous factors affecting both the growth of different types of molds and their synthesis for mycotoxins involve humidity, temperature, environment, water activity (aw), pH, nutrients, fungal load, physiological state, nature of the substrate, and microbial interaction. Molds commonly gained access to the preserved meats such as basterma and sausage that become active with the prolonged ripening time of such types of processed meat with subsequent mycotoxins production [43, 52].
Our findings evident that the level of aflatoxin B1 recovered from examined basterma samples exceeded the international regulatory limits for meat products (> 20 μg/kg). The estimation of aflatoxin B1 production is essential during processing and storage. The food-additives, especially spices, are considered a common source of contamination with mycotoxins during meat processing [53]. A previous study reported that more than 80% of the isolated A. flavus strains are toxigenic, and 47% of these strains produced aflatoxin B1 (chemotypes I and III) [44]. In the present study, the prevalence of aflatoxigenic strains is consistent with the findings of several previous studies [45, 47, 54, 55]. In vitro, A. flavus produces pronounced levels of aflatoxins. The emergence of toxigenic A. flavus strains calls the need for the application of strict hygienic measures during meat processing and storage [44]. In light of our results, the ochratoxin A detected in four isolated A. ochraceus (80%), which is considered a high percentage. However, Zohri et al., 2014 reported that A. ochraceus did not produce any detectable amounts of mycotoxins from sausage samples [50]. In the current study, the detected level of ochratoxin A in sausage samples is relatively high and exceeded the legal limits suggested by Italy. Until now, both European and American regulations didn’t set an international legal limit of ochratoxin A in meat and meat products. Only Italy set the legal limit of ochratoxin A as 1 μg/kg meat [56, 57].
In the present study, the macroscopic and microscopic examinations and PCR amplification of the ITS region were used to confirm the diagnosis of the retrieved A. flavus and A. ochraceus isolates. Furthermore, PCR was performed successfully for the amplification of aflR1 and pks genes. The aflR1 gene was detected in six A. flavus strains isolated from basterma with subsequent sequencing. According to the blastn tool at the NCBI, the identity was 99.73 with query covered 100% percentage to the deposited aflR1gene in the Genebank database (accession number MF094441). Besides, The pks gene was detected in four A. ochraceus strains retrieved from sausage samples and sequenced. According to the blastn tool, the similarity was 100 with a query covered 100% percentage to other pks genes deposited in the Genebank database (accession number MF694264). Sequences diversity and omissions in different genes/regions of the aflatoxins and ochratoxins biosynthetic-clusters could be used to detect the polyphyletic grouping of A. flavus and A. ochraceus [58]. Aflatoxin B is a common carcinogenic mycotoxin commonly produced by A. flavus. The aflatoxin regulatory gene (aflR) is mainly involved in the regulation of aflatoxins-biosynthesis. The aflR gene is encoded for the AflR protein that is responsible for the activation of the functional genes controlling the aflatoxin production pathway. The down-regulation of the aflR gene inhibits the expression of other genes and adversely affects the production pathway [59].
Ochratoxin A produced by A. ochraceus is known as a potent carcinogenic mycotoxin that is commonly incriminated in chronic interstitial nephritis in humans. Ochratoxin A is a polyketide-derived secondary metabolite. Therefore the polyketide synthase gene (pks) is mainly involved in the biosynthesis of ochratoxin A. The molecular detection of the pks gene plays a vital role in the demonstration of the ochratoxigenic strains of A. ochraceus. The mutant strains of A. ochraceus in which the pks gene is disturbed or down-regulated were found to miss their ability to produce the ochratoxin A [60].
In conclusion, to the best of our knowledge, this is the first report regarding the HPLC-Molecular-based approaches for the detection of aflatoxin B1 and ochratoxin A in processed beef meat in Egypt. The present study emphasized the contamination of processed beef meat products by the toxigenic A. flavus and A. ochraceus strains that lead to their spoilage. The production of aflatoxin B1 and ochratoxin A in processed meat constitutes a public health threat. Aflatoxin B1 is commonly associated with basterma samples; furthermore, the ochratoxin A is detected frequently in sausage samples. The routine inspection of mycotoxins in processed meat products is essential to protect human consumers. HPLC is a reliable quantitative assay for the investigation of mycotoxins residues in processed meat products. The combination of both phenotypic and molecular characterization is a reliable epidemiological tool for the identification of the toxigenic A. flavus and A. ochraceus in meat products. It is necessary to adopt proper hygienic measures during the production and storage of processed meat.
Acknowledgements
Not applicable.
Authors’ contributions
A.M.A. and M.E.E. conceived and designed the experiments. A.M.A., M.E.E., E.M.E., S.M.A, H.R.H., H.R., N.S.S., and H.F.H. performed the experiments. A.M.A., M.E.E., E.M.E., H.R., N.S.S., S.M.A, H.R.H., and H.F.H. performed the data analysis, data accuracy, data validation, statistical analysis, investigation, and supervision. A.M.A, M.E.E., H.R., and H.F.H. drafted the manuscript. A.M.A. writing, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Abdelazeem M. Algammal, Email: abdelazeem.algammal@vet.suez.edu.eg
Mahmoud E. Elsayed, Email: mahmoud_elsayed@vet.suez.edu.eg
References
- 1.Motarjemi Y, Moy G, Todd E. Encyclopedia of food safety. Waltham: Academic Press; 2013.
- 2.Shaltout FA, Amin RA, Nassif MZ, Abd-Elwahab SA. Detection of aflatoxins in some meat products. Benha Vet Med J. 2014;27(2):368–374. [Google Scholar]
- 3.Gagaoua M, Boudechicha H-R. Ethnic meat products of the north African and Mediterranean countries: an overview. J Ethn Foods. 2018;5(2):83–98. doi: 10.1016/j.jef.2018.02.004. [DOI] [Google Scholar]
- 4.Luo Y, Liu X, Li J. Updating techniques on controlling mycotoxins-a review. Food Control. 2018;89:123–132. doi: 10.1016/j.foodcont.2018.01.016. [DOI] [Google Scholar]
- 5.da Rocha MEB, Freire FCO, Maia FEF, Guedes MIF, Rondina D. Mycotoxins and their effects on human and animal health. Food Control. 2014;36(1):159–165. doi: 10.1016/j.foodcont.2013.08.021. [DOI] [Google Scholar]
- 6.Kurtzman C, Horn B, Hesseltine C. Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie Van Leeuwenhoek. 1987;53(3):147–158. doi: 10.1007/BF00393843. [DOI] [PubMed] [Google Scholar]
- 7.Al-Jaal BA, Jaganjac M, Barcaru A, Horvatovich P, Latiff A. Aflatoxin, fumonisin, ochratoxin, zearalenone and deoxynivalenol biomarkers in human biological fluids: a systematic literature review, 2001–2018. Food Chem Toxicol. 2019;129:211–228. doi: 10.1016/j.fct.2019.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Shahbazi Y. Aflatoxin M1 contamination in milk and dairy products: implications on human health. In: Nutrients in dairy and their implications on health and disease. Waltham: Academic Press; 2017. p. 237–50.
- 9.Yeni F, Yavaş S, Alpas H, Soyer Y. Most common foodborne pathogens and mycotoxins on fresh produce: a review of recent outbreaks. Crit Rev Food Sci Nutr. 2016;56(9):1532–1544. doi: 10.1080/10408398.2013.777021. [DOI] [PubMed] [Google Scholar]
- 10.Chrevatidis A. Mycotoxins|Occurrence and determination. In: Caballero B, editor. Encyclopedia of food sciences and nutrition. 2. Oxford: Academic; 2003. pp. 4089–4096. [Google Scholar]
- 11.Cinar A, Onbaşı E. Mycotoxins: the hidden danger in foods. In: Mycotoxins and food safety: IntechOpen.5 Princes Gate Court, London, SW7 2QJ, UK; 2019.
- 12.Stagnitta PV, Micalizzi B, De Guzmán AMS. Prevalence of some bacteria yeasts and molds in meat foods in San Luis, Argentina. Cent Eur J Public Health. 2006;14(3):141-4. [DOI] [PubMed]
- 13.Leitão AL. Occurrence of ochratoxin A in coffee: threads and solutions-a mini-review. Beverages. 2019;5(2):36. doi: 10.3390/beverages5020036. [DOI] [Google Scholar]
- 14.Ferrão J, Bell V, Chabite I, Fernandes T. Mycotoxins, food and health mycotoxins, food and health. J Nutr Health Food Sci. 2017;5(7):1–10. [Google Scholar]
- 15.Tola M, Kebede B. Occurrence, importance and control of mycotoxins: a review. Cogent Food Agric. 2016;2(1):1191103. [Google Scholar]
- 16.Degirmencioglu N, Esecali H, Cokal Y, Bilgic M. From safety feed to safety food: the application of HACCP in mycotoxin control. Arch Zootech. 2005;8:19–32. [Google Scholar]
- 17.Deligöz E, Bilge N. Threat coming with milk: aflatoxin. Turk J Agric Food Sci Technol. 2017;5(8):846–857. doi: 10.24925/turjaf.v5i8.846-857.1111. [DOI] [Google Scholar]
- 18.Science CfA . Mycotoxins: risks in plant, animal, and human systems. Ames: Council for Agricultural Science and Technology; 2003. [Google Scholar]
- 19.Escrivá L, Font G, Manyes L, Berrada H. Studies on the presence of mycotoxins in biological samples: an overview. Toxins. 2017;9(8):251. doi: 10.3390/toxins9080251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dada TA, Ekwomadu TI, Mwanza M. Multi mycotoxin determination in dried beef using liquid chromatography coupled with triple quadrupole mass spectrometry (LC-MS/MS) Toxins. 2020;12(6):357. doi: 10.3390/toxins12060357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Montanha FP, Anater A, Burchard JF, Luciano FB, Meca G, Manyes L, Pimpão CT. Mycotoxins in dry-cured meats: a review. Food Chem Toxicol. 2018;111:494–502. doi: 10.1016/j.fct.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Aziz NH, Youssef YA. Occurrence of aflatoxins and aflatoxin-producing moulds in fresh and processed meat in Egypt. Food Addit Contam. 1991;8(3):321–331. doi: 10.1080/02652039109373981. [DOI] [PubMed] [Google Scholar]
- 23.Iqbal SZ, Nisar S, Asi MR, Jinap S. Natural incidence of aflatoxins, ochratoxin A and zearalenone in chicken meat and eggs. Food Control. 2014;43:98–103. doi: 10.1016/j.foodcont.2014.02.046. [DOI] [Google Scholar]
- 24.Gareis M, Scheuer R. Ochratoxin A in meat and meat products. Arch Leb. 2000;51(4/5):102–104. [Google Scholar]
- 25.Chain EPoCitF. Schrenk D, Bodin L, Chipman JK, del Mazo J, Grasl-Kraupp B, Hogstrand C, Hoogenboom L, Leblanc JC, Nebbia CS. Risk assessment of ochratoxin A in food. EFSA J. 2020;18(5):e06113. doi: 10.2903/j.efsa.2020.6113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elzupir AO, Abdulkhair BY. Health risk from aflatoxins in processed meat products in Riyadh, KSA. Toxicon. 2020;181:1–5. doi: 10.1016/j.toxicon.2020.04.092. [DOI] [PubMed] [Google Scholar]
- 27.Downes F, Ito K. Compendium of methods for the microbiological examination of foods. Washington DC: American Public Health Association; 2001. [Google Scholar]
- 28.Pitt JI, Hocking AD. Fungi and food spoilage. New York: Springer; 2009. p. 388. [Google Scholar]
- 29.Herzallah SM. Determination of aflatoxins in eggs, milk, meat and meat products using HPLC fluorescent and UV detectors. Food Chem. 2009;114(3):1141–1146. doi: 10.1016/j.foodchem.2008.10.077. [DOI] [Google Scholar]
- 30.Zareshahrabadi Z, Bahmyari R, Nouraei H, Khodadadi H, Mehryar P, Asadian F, Zomorodian K. Detection of aflatoxin and ochratoxin A in spices by high-performance liquid chromatography. J Food Qual. 2020;2020:1–8. doi: 10.1155/2020/8858889. [DOI] [Google Scholar]
- 31.Bellemain E, Carlsen T, Brochmann C, Coissac E, Taberlet P, Kauserud H. ITS as an environmental DNA barcode for fungi: an in silico approach reveals potential PCR biases. BMC Microbiol. 2010;10(1):189. doi: 10.1186/1471-2180-10-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallo A, Stea G, Battilani P, Logrieco AF, Perrone G. Molecular characterization of an Aspergillus flavus population isolated from maize during the first outbreak of aflatoxin contamination in Italy. Phytopathol Mediterr. 2012;51(1):198–206. [Google Scholar]
- 33.Tamura K, Nei M. Estimation of the number of nucleotide substitutions in the control region of mitochondrial DNA in humans and chimpanzees. Mol Biol Evol. 1993;10(3):512–526. doi: 10.1093/oxfordjournals.molbev.a040023. [DOI] [PubMed] [Google Scholar]
- 34.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dao HP, Mathieu F, Lebrihi A. Two primer pairs to detect OTA producers by PCR method. Int J Food Microbiol. 2005;104(1):61–67. doi: 10.1016/j.ijfoodmicro.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Nei M, Kumar S. Molecular evolution and phylogenetics. New York: Oxford University Press; 2000.
- 37.Ouf JM, Khafaga N, Shabana E. Incidence of proteolytic and lipolytic moulds and yeasts in some ready to eat meat products. Assiut Vet Med J. 2010;56(126):132–143. [Google Scholar]
- 38.Mousa MM, Ahmed AA, El-Shamy SY. Microbiological criteria of some meat products. Alex J Vet Sci. 2014;42(1):83-9.
- 39.Ebraheem LM, Mohamed GM. Evaluation of mycological status and detection of its toxinsin basterma and luncheon in assuit city. Assiut Vet Med J. 2015;58(133):1–10. [Google Scholar]
- 40.El-Tabiy A. Mycological study on some processed meat products exposed for sale in markets. Assiut Vet Med J. 2006;52(110):121–131. [Google Scholar]
- 41.Afshar P, Shokrzadeh M, Raeisi SN, Ghorbani-HasanSaraei A, Nasiraii LR. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon. 2020;178:50–58. doi: 10.1016/j.toxicon.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Yang C, Song G, Lim W. Effects of mycotoxin-contaminated feed on farm animals. J Hazard Mater. 2020;389:122087. doi: 10.1016/j.jhazmat.2020.122087. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Sun Y, Huo B, Zhao X, Huang H, Li S, Bai J, Liang J, Gao Z. A copper monosulfide-nanoparticle-based fluorescent probe for the sensitive and specific detection of ochratoxin A. Talanta. 2020;222:121678. doi: 10.1016/j.talanta.2020.121678. [DOI] [PubMed] [Google Scholar]
- 44.Makhlouf J, Carvajal-Campos A, Querin A, Tadrist S, Puel O, Lorber S, Oswald IP, Hamze M, Bailly J-D, Bailly S. Morphologic, molecular and metabolic characterization of Aspergillus section Flavi in spices marketed in Lebanon. Sci Rep. 2019;9(1):1–11. doi: 10.1038/s41598-019-41704-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Mahgubi A, Puel O, Bailly S, Tadrist S, Querin A, Ouadia A, Oswald I, Bailly J-D. Distribution and toxigenicity of Aspergillus section Flavi in spices marketed in Morocco. Food Control. 2013;32(1):143–148. doi: 10.1016/j.foodcont.2012.11.013. [DOI] [Google Scholar]
- 46.Garcia MV, Parussolo G, Moro CB, Bernardi AO, Copetti MV. Fungi in spices and mycotoxigenic potential of some Aspergilli isolated. Food Microbiol. 2018;73:93–98. doi: 10.1016/j.fm.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 47.Jeswal P, Kumar D. Mycobiota and natural incidence of aflatoxins, ochratoxin A, and citrinin in Indian spices confirmed by LC-MS/MS. Int J Microbiol. 2015;2015:1–8. doi: 10.1155/2015/242486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aiko V, Mehta A. Prevalence of toxigenic fungi in common medicinal herbs and spices in India. 3 Biotech. 2016;6(2):159. doi: 10.1007/s13205-016-0476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klich MA. Aspergillus flavus: the major producer of aflatoxin. Mol Plant Pathol. 2007;8(6):713–722. doi: 10.1111/j.1364-3703.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 50.Zohri A, Moharram A, Refaie R. Mycobiota contaminating beef burger and sausage with reference to their toxins and enzymes. J Basic Appl Mycol (Egypt) 2014;5:61. [Google Scholar]
- 51.Gaber GA. Mycotoxin residues in meat and meat products. Vet Med J. 1996;44(2):181–187. [Google Scholar]
- 52.Aldars-García L, Berman M, Ortiz J, Ramos AJ, Marín S. Probability models for growth and aflatoxin B1 production as affected by intraspecies variability in Aspergillus flavus. Food Microbiol. 2018;72:166–175. doi: 10.1016/j.fm.2017.11.015. [DOI] [PubMed] [Google Scholar]
- 53.Van Egmond H, Jonker M. Worldwide regulations for mycotoxins in food and feed: the situation in 2003. Draft FAO Food and Nutrition Paper. Bilthoven: National Institute for Public Health and the Environment; 2004. [Google Scholar]
- 54.Prencipe S, Siciliano I, Contessa C, Botta R, Garibaldi A, Gullino ML, Spadaro D. Characterization of Aspergillus section Flavi isolated from fresh chestnuts and along the chestnut flour process. Food Microbiol. 2018;69:159–169. doi: 10.1016/j.fm.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 55.Riba A, Bouras N, Mokrane S, Mathieu F, Lebrihi A, Sabaou N. Aspergillus section Flavi and aflatoxins in Algerian wheat and derived products. Food Chem Toxicol. 2010;48(10):2772–2777. doi: 10.1016/j.fct.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 56.Berni E, Montagna I, Restivo FM, Degola F. Ochratoxin A control in meat derivatives: intraspecific biocompetition between Penicillium nordicum strains. J Food Qual. 2017;2017:8370106. doi: 10.1155/2017/8370106. [DOI] [Google Scholar]
- 57.European Union Commission Regulation (EC) No 105/2010 of 5 February 2010 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards ochratoxin A. Off J Eur Union. 2010;L35:7–8. [Google Scholar]
- 58.Chang P-K, Ehrlich KC, Hua S-ST. Cladal relatedness among Aspergillus oryzae isolates and Aspergillus flavus S and L morphotype isolates. Int J Food Microbiol. 2006;108(2):172–177. doi: 10.1016/j.ijfoodmicro.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 59.Wang B-S, Zhao Q, Wang Y-Z. The uncertainty of assessing aflatoxin B1-producing ability using aflR gene in Aspergillus species. Afr J Microbiol Res. 2011;5(31):5603–5606. [Google Scholar]
- 60.O’Callaghan J, Caddick M, Dobson A. A polyketide synthase gene required for ochratoxin a biosynthesis in Aspergillus ochraceus. Microbiology. 2003;149(12):3485–3491. doi: 10.1099/mic.0.26619-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.