Abstract
Rationale and objectives:
Fibrosis is characterized by progressive replacement of normal tissue by extracellular matrix. Diagnosis relies on biopsies as noninvasive methods for detection and quantification of fibrosis are still limited. This work aimed to address the ability of two molecular MR probes, EP-3533 and Gd-Hyd, to identify fibrosis and fibrogenesis, respectively, independently of the presence of underlying inflammation in a mouse model of chronic liver disease caused by infection with Schistosoma mansoni.
Methods:
Three groups of mice that develop either mild type-2 inflammation and fibrosis (wild-type), severe fibrosis with exacerbated type-2 inflammation (Il10−/−Il12b−/−Il13ra2−/−), or minimal fibrosis with marked type-1 inflammation (Il4ra∂/∂) following infection with S. mansoni were imaged using both probes for determination of signal enhancement. S. mansoni-infected wild-type mice developed chronic liver fibrosis.
Results:
The liver MR signal enhancement after either probe administration was significantly higher in S. mansoni-infected wild-type mice compared to naïve animals. The S. mansoni-infected Il4ra∂/∂ mice presented with little liver signal enhancement after probe injection despite the presence of substantial inflammation. S. mansoni-infected Il10−/−Il12b−/−Il13ra2−/− mice presented with marked fibrosis, which correlated to increased signal enhancement after injection of either probe.
Conclusion:
Both MR probes, EP-3533 and Gd-Hyd, were specific for fibrosis in this model of chronic liver disease regardless of the presence or severity of the underlying inflammation. These results, in addition to previous findings, show the potential application of both molecular MR probes for detection and quantification of fibrosis from various etiologies.
Keywords: liver, inflammation, fibrosis, fibrogenesis, Gd-Hyd, EP-3533, molecular imaging, staging, Schistosoma mansoni, MRI
Introduction
Fibrosis is characterized by the excessive accumulation of extracellular matrix as a consequence of chronic tissue injury.(1) Diseases characterized by tissue fibrosis account for nearly half of the deaths in the industrialized world and represent an enormous unmet medical challenge.(2) Management of patients with fibrosis is difficult due to the lack of specific and noninvasive methods for detection and quantification of this condition. The development of molecular magnetic resonance (MR) probes capable of specifically detecting fibrosis is an innovative strategy for fulfilling this largely unmet clinical need.(3)
The liver is one of the most common sites for development of fibrosis often due to alcohol consumption, hepatitis B or C infection, diabetes, and fatty liver disease.(4) Fibrosis tends to progress and can lead to cirrhosis, hepatic dysfunction, portal hypertension, and/or hepatocellular carcinoma. It is crucial to quantify liver fibrosis stages since treatment decision and monitoring algorithms are related to the degree of fibrosis.(5–7) Liver fibrosis is characterized by increased collagen deposition, especially type I, and upregulation of lysyl oxidase (LOX) and LOX-like enzymes, which are responsible for oxidizing the collagen lysine ε-amino groups to aldehydes (allysine) to form collagen crosslinks.(8) Both characteristics have been exploited for detection and quantification of fibrosis.(9, 10)
The type I collagen targeting probe EP-3533 has been widely studied to detect and stage liver fibrosis induced by diet, chemical toxin, or surgical injury in mice and rats.(11–17). EP-3533 comprises a 16 amino acid cyclic peptide identified by a phage display screen against type I collagen that is conjugated to 3 gadolinium chelates for MR signal enhancement. EP-3533 has a blood half-life of 19 min in mice, minimal uptake in normal liver, and is excreted into the urine. It was reported that EP-3533 did not inhibit receptor binding or enzymatic activity in 33 different in vitro assays, and has showed no evidence of toxicity in a wide range of animal studies. A newer probe Gd-Hyd, targeting allysine as a marker of fibrogenesis has been investigated in dietary and chemical toxin models.(11, 13, 18) Gd-Hyd utilizes the stable Gd-DOTA chelate conjugated to a hydrazide moiety which enables reversible covalent binding to extracellular allysine. Gd-Hyd was reported to have a blood half-life of 5 minutes in mice, no uptake in normal liver, and that >99.5% of the injected dose was eliminated from mice within 24 hours.
Although the animal models used to evaluate Gd-Hyd and EP-3533 are commonly used for modeling fibrosis, they also present with a marked inflammatory response. The presence of inflammation could potentially be a confounding factor for the specific detection of fibrosis and fibrogenesis due to vascular and cellular alterations associated with this condition.(19, 20) A key question in the development of these promising molecular MR probes is whether they can specifically detect fibrosis and distinguish fibrosis/fibrogenesis from inflammation. The purpose of this study was to determine whether EP-3533 and/or Gd-Hyd could correctly detect liver fibrosis/fibrogenesis in the presence of strong hepatic inflammation, i.e. demonstrate high sensitivity, and conversely whether these probes would not show liver enhancement in the presence of liver inflammation but without liver fibrosis present, i.e. demonstrate high specificity.
Murine liver fibrosis induced by infection with Schistosoma mansoni is initiated by entrapment of parasite eggs in hepatic portal venules leading to severe vascular obstruction and induction of granulomatous inflammation. Infection is characterized by an early CD4+ T-helper (Th) 1 response that transitions to a Th2 response as egg laying commences, resulting in robust production of the cytokines interleukin (IL)-4, IL-5, and IL-13, which in particular has been shown to elicit potent tissue remodeling response.(21–23) Several features of the murine disease, including liver fibrosis, hepatosplenomegaly, portal hypertension, ascites formation, and gastrointestinal bleeding closely resemble those observed in human schistosomiasis.(24) In addition, mice can be genetically engineered to modulate the intensity of the Th1 or Th2 response upon infection with S. mansoni.(25–27) In this study, we used two such models: (i) S. mansoni-infected Il4ra∂/∂ mice (4R-Delta), which develop less fibrosis than wild-type mice despite severe type 1 polarized inflammation;(27) and (ii) S. mansoni-infected Il10−/−Il12b−/−Il13ra2−/− mice (TKO), which develop rapidly accelerated liver fibrosis and highly polarized type 2 inflammation. We first examined whether EP-3533 and Gd-Hyd could detect liver fibrosis and fibrogenesis induced by infection with S. mansoni in wild-type mice, and then analyzed the ability of the probes to specifically detect fibrosis and fibrogenesis independent of underlying inflammation using the 4R-Delta and TKO mice.
Materials and Methods
Animal model
All experiments and procedures were performed in accordance with the National Institutes of Health’s “Guide for the Care and Use of Laboratory Animals” and were approved by the [redacted].
4R-Delta mice were obtained from Taconic Farms Inc. (Derwood, MD, USA) and breeding colonies were maintained at [redacted]. TKO (backcrossed ≥10 generations to BALB/c) breeding colonies were maintained at [redacted].
Mice between 15–16 weeks of age were infected percutaneously by suspending tails in water containing 35 S. mansoni cercariae for 45 minutes. Cerceriae were obtained by shedding infected Biomphalaria glabrata snails (Biomedical Research Institute; Rockville, MD, USA). Mice were then transferred and housed under specific pathogen-free conditions at [redacted]
A total of 33 mice were included in this study. Healthy wild-type mice (control, n = 6) and S. mansoni-infected wild-type mice (WT, n = 9) were imaged 10–11 weeks after infection, while S. mansoni-infected 4R-Delta (n = 9) and TKO (n = 9) mice were imaged 6–7 weeks after infection. Half of the animals were randomly assigned to be imaged with Gd-Hyd (100 μmol/kg) and the other half with EP-3533 (10 μmol/kg). 24 h after the first scan, the animals underwent an additional scan with the other probe and then were sacrificed one hour after the imaging for biochemical analysis and histological evaluations. At the time of euthanasia, livers were perfused and removed for subsequent analyses.
Probes
EP-3533 is a 10–amino acid cyclic peptide conjugated to three gadopentetate (Gd) moieties via a thiourea linkage. EP-3533 has a relaxivity (r1) of 48.3 mM−1s−1 at 1.4T (16.1 mM−1s−1 per Gd ion) and binds specifically to type I collagen with Kd = 1.8 μM. Gd-Hyd is a 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetate gadolinium chelate (Gd-DOTA) conjugated to a hydrazide moiety. Gd-Hyd has a r1 = 4.1 mM−1s−1 at 1.4T and binds specifically to the allysine residues on oxidized collagen. EP-3533 and Gd-Hyd were prepared according to previously reported procedures.(18, 28)
Imaging protocol
Animals were anesthetized with 1–2% isoflurane with body temperature maintained at 37 °C. The tail vein was cannulated for intravenous delivery of the contrast agent while the animal was positioned in the scanner in a custom designed cradle. Respiration rate was monitored with a small animal physiological monitoring system (SA Instruments, Inc., Stony Brook, NY, USA), and anesthesia was adjusted to maintain 60 ± 5 breaths per minute. Imaging was performed at 9.4 T using a small-bore animal MRI scanner (Bruker, Billerica, MA, USA) with a custom-built volume coil.
After a localizer image to position the animal, a 2D fast low angle shot (FLASH) sequence was acquired and used to position the single slice for the T1 map sequence, and to guide placement of a high temporal resolution FLASH sequence. The probe (Gd-Hyd or EP-3533) was injected intravenously and 20 minutes after injection, the high temporal resolution FLASH sequence was acquired again. If EP-3533 had been injected, the T1 map was repeated 30 minutes after injection.
Images were acquired with the following sequences and parameters: 2D FLASH with respiratory gating (matrix 90 × 140, repetition time (TR)/echo time (TE)/flip angle (FA) = 150 ms/3.5 ms/40°; field of view (FOV) 18 × 28 mm; slice thickness 0.5mm; 5 slices; 8 averages). T1 map sequence with respiratory gating (Matrix 96 × 128; variable inversion time (TI)= 1000, 600, 400, 200,100 ms; TE/FA = 2.75 ms/20°; the effective repetition time was 1000 ± 90 ms; FOV 24 × 32 mm, slice thickness 0.75 mm, one slice), and 2D FLASH sequence with high temporal resolution (Matrix = 106 × 64; TR/TE/FA = 30 ms/2.47 ms/20°; FOV 28 mm × 18 mm; slice thickness 0.75 mm; 5 slices; 20 repetitions). Alternate methods for respiratory gating, e.g. retrospective gating in small animal imaging of the liver could also be employed.(29–31)
Image analysis
For the 2D FLASH sequence, regions of interest (ROIs) were drawn in five liver slices, covering the whole liver, and also the muscle as a control using FreeView (FreeSurfer, Massachusetts General Hospital Corporation, Boston, MA, USA). Blood vessels were excluded from the liver ROI via a signal threshold method. The average signal intensity (SI) was measured in liver and muscle. Noise was estimated by placing an ROI in the air outside the animal and measuring the standard deviation (SD) of the SI in the air signal. Liver-to-muscle contrast to noise ratios (CNR) were calculated as CNR = (SIliver – SImuscle)/SDair. The change in CNR was calculated as a difference: ΔCNR = (CNRpost-CNRpre) where “post” refers to a time point after probe injection and “pre” refers to the image taken before probe injection. For T1 map sequence, ROIs comprising roughly half of the liver area were drawn in the central slice using Osirix (Pixmeo SARL, Geneva, Switzerland). The SI was plotted versus each TI value and T1 was calculated from a 3-paramater fit using the equation SI = S0 (1 - A•e−TI/T1 + e−TR/T1), where S0 represents the maximum SI, and A is the cosine of the FA (typically 180°). Relaxation rate (R1) = (1/T1) was then calculated and changes in R1 was measured as a difference ΔR1 = (R1post-R1pre).
Gene expression
RNA was isolated from frozen liver tissue using TRIzol (Life Technologies, Grand Island, NY, USA) according to the manufacturer’s instructions followed by DNAse I (Promega, Madison, WI, USA) treatment. Complementary DNA was synthesized using total RNA (1 μg) from each sample by single-strand reverse transcription (SuperScript III First- Strand Synthesis SuperMix; Life Technologies). Quantitative real-time polymerase chain reaction (PCR) using TaqMan gene expression assays (Thermo Fisher Scientific, Waltham, MA, USA) were used to analyze the expression of interferon gamma (Ifng); collagen, type I, alpha1 (Col1a1); and collagen, type VI, alpha1 (Col6a1) in liver tissue on an Applied Bioscience 7900HT Fast Real Time PCR system using 96- well plates with a reaction volume of 20 μL. The 2−ΔCT method was used for relative quantification of messenger RNA (mRNA) with normalization to 18S. The TaqMan assays used were as follows: 18S, Hs03003631_g1; Ifng, Mm01168134_m1; Col1a1, Mm00801666_g1; Col6a1, Mm00487160_m1.
Western blot
Livers were homogenized in radioimmunoprecipitation assay buffer (Boston BioProducts, Ashland, MA, USA) containing protease and phosphatase inhibitors (Sigma Aldrich, St. Louis, MO) to extract proteins. Protein concentrations were normalized to 40 μg using the bicinchoninic acid method (Pierce Chemical Company, Rockford, IL, USA). Samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA, USA). Immunoblotting for resistin-like molecule alpha (RELM-α) (PeproTech) was performed with β-actin (Abcam) as control. Blots were incubated with anti-rabbit secondary antibodies conjugated to horseradish peroxidase (HRP) (Cell Signaling Technology) and developed with a chemiluminescent HRP substrate (Perkin-Elmer, Waltham, MA, USA). Western blots were repeated 3 times to ensure reproducibility.
Histological analyses
The gold standard for measuring liver fibrosis in this study was histology. Formalin-fixed samples were embedded in paraffin, cut into 5 μm-thick-sections, and stained with Sirius red according to standard procedures. The collagen proportional area (CPA), as determined by the % area stained with Sirius red, was morphometrically quantified using image-processing software (ImageJ; National Institutes of Health, Bethesda, MD, USA).
Egg counting
Approximately 100–200 mg of liver tissue was weighed and recorded prior to digestion in 10 mL 4% KOH for 12 hours at 37°C. Tissue digests were homogenized by vortexing and eggs were counted by brightfield microscopy.
Hydroxyproline analyses
Liver hydroxyproline was quantified using a HPLC-based assay described previously.(32)
Statistical Analysis
All data are reported as the mean ± standard error. Differences among groups were evaluated with either paired or unpaired Student’s t-test. In all tests, a P value < 0.05 was considered to be significant.
Results
Infection with S. mansoni induces robust liver fibrosis
Mice infected with S. mansoni develop many features similar to the human disease.(33) In particular, the infection is characterized by significant liver fibrosis and portal hypertension, which can lead to pathological sequelae, including hepatosplenomegaly, ascites, and gastrointestinal varices that can rupture causing internal bleeding.(34)
The egg burden in the infected wild-type mice 9–11 weeks post-infection was equal to 8300 ± 4000 (mean ± S.D.) eggs per liver. These animals also presented with significant hepatosplenomegaly (Figure 1A,B). Their livers were characterized by a grayish color, with yellow-white, pinpoint sized, punctate areas visible on all serosal surfaces, which presented with a nodular texture. Blood was detected in the stool of 3 of 8 animals in this group, indicative of gastrointestinal bleeding. Collagen deposition was confirmed by quantification of tissue hydroxyproline levels (Figure 1C), and confirmed by histology with Sirius red-staining (Figure 1D). Sirius-red stained liver sections exhibited large deposits of collagen around the granulomas and throughout the liver parenchyma, which was quantitated by CPA (Figure 1E). Presence of inflammatory infiltrate was confirmed by Giemsa-staining, characterized by a substantial accumulation of eosinophils and macrophages (Figure 1F).
Figure 1. S. mansoni-infected mice develop severe liver fibrosis.

Liver and spleens from naïve mice (n = 6) or WT mice infected with S. mansoni for 9–11 weeks (n = 8) were harvested (A) and weighed (B). Collagen content of the liver was assessed by hydroxyproline levels (C). Liver sections were stained with Sirius red (D) and collagen content was quantified by measuring the collagen proportional area (E). The arrow in D shows the presence of an egg trapped in a liver sinusoid and the concentric deposition of extracellular matrix. Giemsa-staining showing inflammatory infiltrate in infected animals (magnification = 10x) (F). The inserts show the presence of macrophages and eosinophils in S. mansoni-infected animals (magnification = 40x). Results are expressed as average ± SEM and the statistical significance was assessed by two-tailed Student’s t-test with Welch’s correction.
Molecular MR imaging of collagen allows for detection of S. mansoni-induced liver fibrosis
S. mansoni-infected mice underwent abdominal MRI at 9 – 11 weeks after infection and were compared to age-matched naïve mice (Figure 2A). Images were acquired pre- and post-contrast injection with the type I collagen–targeted molecular probe EP-3533. Based on the established pharmacokinetics of EP-3533 in mice, quantitative analysis was performed at 20 and 30 minutes post injection to allow time for blood background signal to diminish. Analyses of the liver-to-muscle CNR were performed pre- and 20 min post EP-3533 injection. Infected animals presented a 2-fold increment in the CNR after probe injection, while for the naïve animals these values remained constant (Figure 2B). ΔCNR was strongly correlated to the hydroxyproline content in the infected animals with the highest ΔCNR values observed in the most fibrotic animals (Figure 2C).
Figure 2. Molecular imaging of Type I collagen and allysine detect liver fibrosis in S. mansoni-infected mice.

Representative images of EP-3533 signal enhancement at 15 minutes post probe injection in naïve and infected mice (A). T1-weighed gradient echo sequences were performed pre and 20 min post EP-3533 injection in naïve (n = 5) or infected WT (n = 8) mice to determine changes in contrast to noise ratio (ΔCNR) (B). The values of ΔCNR for EP-3533 directly correlates to the hydroxyproline content in infected animals (C). Representative images of Gd-Hyd signal enhancement at 15 minutes post probe injection in naïve and infected WT mice (D). T1-weighed gradient echo sequences were also performed pre and 15 min post Gd-Hyd injection in naïve or infected mice to determine ΔCNR (E). The values of ΔCNR for Gd-Hyd directly correlates to the hydroxyproline content in infected animals (F). Pearson correlation coefficient (r) was calculated for (C) and (F). Other results are expressed as average ± SEM and the statistical significance was assessed by two-tailed Student’s t-test with Welch’s correction.
The injection of EP-3533 causes an increase in the longitudinal relaxation rate, R1, in both normal and fibrotic livers, but the probe clears at a rate inversely proportional to the degree of tissue fibrosis. The change in R1 at 30 min after probe administration is directly proportional to probe concentration, therefore, we measured R1 values both pre- and 30 min post-injection, and computed the change in relaxation rate (ΔR1) on a voxelwise basis throughout the liver. Consistent with the more fibrotic tissue, S. mansoni infection was associated with increased ΔR1 in the liver compared to naïve control mice (ΔR1 = 0.42 ± 0.05 vs. 0.15 ± 0.07 for infected and naïve mice, p = 0.012, respectively).
S. mansoni induced fibrosis is confirmed by molecular MR imaging of fibrogenesis
S. mansoni-infected wild-type mice were also imaged with a T1-weighed gradient echo sequence pre- and 15 min post administration of Gd-Hyd. This time point was chosen based on the pharmacokinetics previously established for this probe in mice.(18) Post Gd-Hyd axial liver images shows a clear signal enhancement, compared to pre-injection for animals infected with S. mansoni (Figure 2D). ΔCNR in the liver were calculated for each animal (Figure 2E) and no significant changes in the overall ΔCNR values were observed for naïve animals 15 min post-contrast, while an overall 3-fold increase was observed for infected animals. The values for ΔCNR positively correlated with the hydroxyproline content (Figure 2F) for the infected animals. These findings indicate that Gd-Hyd imaging of oxidized collagen correlates with the biology of the disease and provides a noninvasive marker for measuring S. mansoni-induced hepatic fibrogenesis.
TKO mice develop severe fibrosis and 4R-delta mice develop severe inflammation with minimal fibrosis
Previous reports demonstrate that disease progression in TKO and 4R-delta mice is faster than in wild-type mice but driven by opposing mechanisms,(25, 33) with 4R-Delta succumbing to a highly polarized type 1 inflammatory response and TKO mice developing polarized type 2 inflammation with lethal fibrosis. Based on that, these transgenic animals were imaged 6–7 weeks post-infection.
The egg burden in TKO mice was significantly higher than for 4R-Delta mice (14100 ± 5000 vs 6500 ± 4300 eggs/liver, p = 0.0045). 4R-Delta animals presented similar liver weight to naïve animals (4.95 ± 0.22 vs 5.23 ± 0.16 % of body weight, p = 0.354) while increased spleen weight was observed (0.94 ± 0.04 vs 0.41 ± 0.03 % of body weight, p < 0.0001) (Figure 3A). By comparison, marked hepatosplenomegaly was observed in TKO mice (18-fold and 3.2-fold increase for liver and spleen weight, respectively). Blood in stool, associated with gastrointestinal bleeding, was noted in 4 out of 9 TKO mice and 2 out of 9 4R-Delta mice.
Figure 3. TKO mice develop severe fibrosis whereas 4R-delta mice develop severe inflammation with minimal fibrosis.
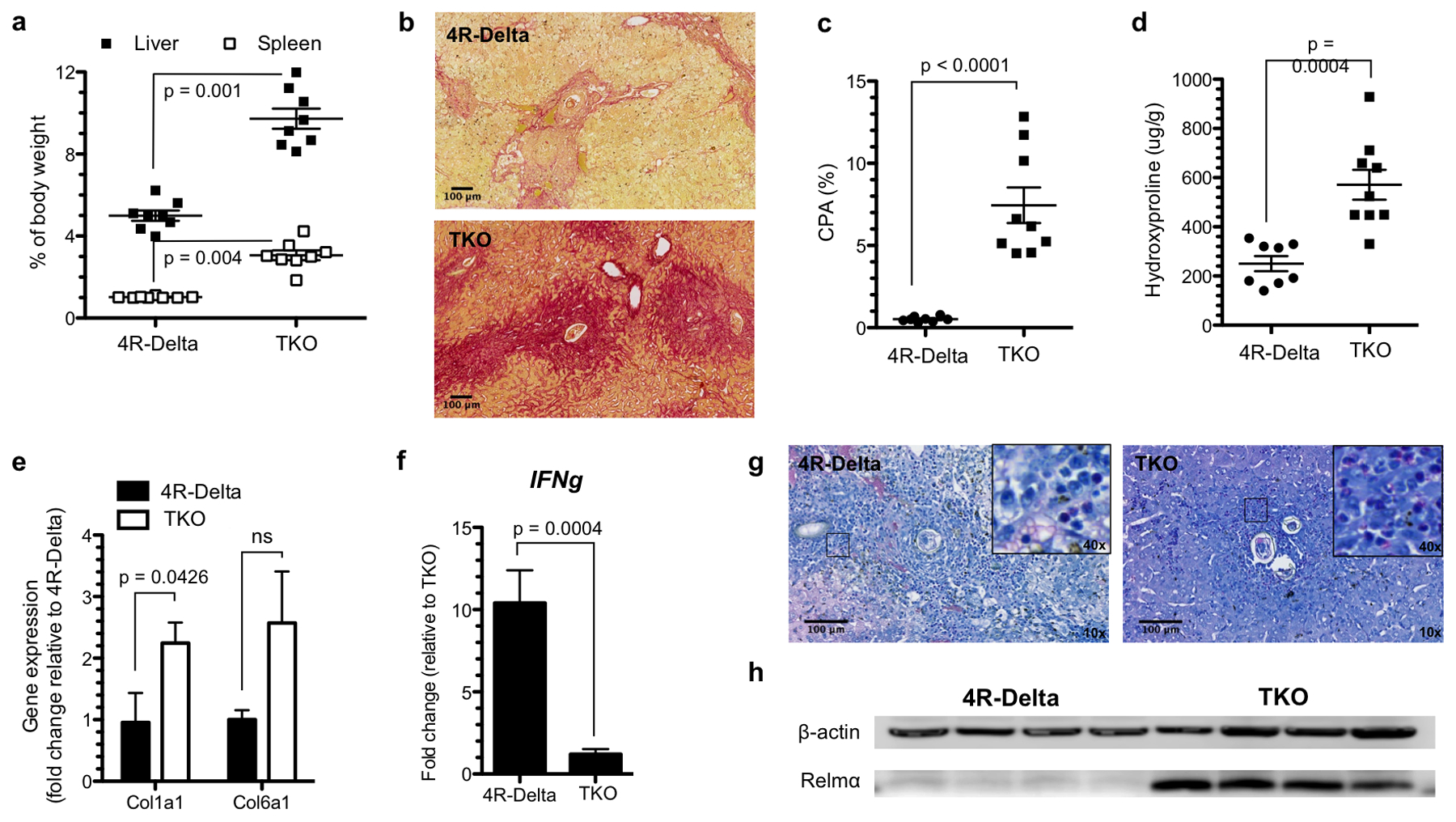
Liver and spleens from 4R-Delta and TKO mice infected with S. mansoni for 6–7 weeks (n = 8 per group) were harvested and weighed (A). Liver sections were stained with Sirius-red (B) and collagen content was quantified by measuring the collagen proportional area (C) and by hydroxyproline analysis (D). Expression of Col1a1 and Col6a1 mRNA (E) and type-1 response Ifng mRNA (F) was analyzed by qRT-PCR. Enhanced inflammatory response in 4R-Delta mice was also observed by Giemsa-staining of liver sections (magnification = 10x) (G). The inserts show the presence of macrophages and eosinophils (magnification = 40x). Type-2 response gene expression (Relmα) was assessed by Western blotting (H). Results are expressed as average ± SEM and the statistical significance was assessed by two-tailed Student’s t-test with Welch’s correction.
Sirius red stained liver sections exhibited larger deposits of collagen around the granulomas and throughout the liver parenchyma in TKO mice compared to 4R-Delta mice, as assessed by CPA quantification (Figure 3B,C). The extent of fibrosis was also shown by increased hydroxyproline levels in livers of TKO mice (Figure 3D). The hydroxyproline content in 4R-Delta mice was similar to naïve mice while for TKO mice it increased approximately 2·5-fold.
Expression of Col1a1 mRNA was increased in TKO animals (Figure 3E) and the expression of Col6a1 mRNA, was also increased in most of the TKO mice but no statistical difference was observed between the groups. In contrast, markers of Th1-type inflammation, such as Ifng, were highly expressed in 4R-Delta mice (Figure 3F). This was associated with increased inflammatory cell infiltration into the liver parenchyma as assessed by Giemsa-staining (Figure 3G). Relmα, an IL-4 responsive gene,(35) was highly expressed in TKO mice, as measured by western blot. The expression of this protein was not observed in 4R-Delta mice (Figure 3H).
EP-3533, can specifically detect liver fibrosis independent of the presence of underlying inflammation
TKO and 4R-Delta mice infected with S. mansoni underwent abdominal MRI 6–7 weeks after infection. Images were acquired pre- and post- EP-3533 injection, and demonstrated stronger liver signal enhancement in the more fibrotic animals (TKO) compared to the 4R-Delta mice, which presented with minimal fibrosis (Figure 4A). Analysis of the liver-to-muscle CNR was performed pre- and 20 min post EP-3533 injection. TKO mice presented a 3-fold increase in CNR values after probe injection, while no changes were observed for 4R-Delta (Figure 4B). ΔCNR was directly correlated to hydroxyproline content in TKO mice (Figure 4C), confirming the specific binding of EP-3533 to areas of increased expression of collagen. In addition, we also measured R1 values both pre- and 30 min post-injection, and computed the ΔR1 values. Consistent with the biological findings, ΔR1 values were larger for TKO mice compared to 4R-Delta mice (ΔR1 = 0.44 ± 0.15 s−1 vs 0.21 ± 0.10 s−1 for 4R-Delta and TAC313, p = 0.02, respectively).
Figure 4. Molecular imaging of collagen and allysine can specifically detect liver fibrosis independent of the presence of underlying inflammation.
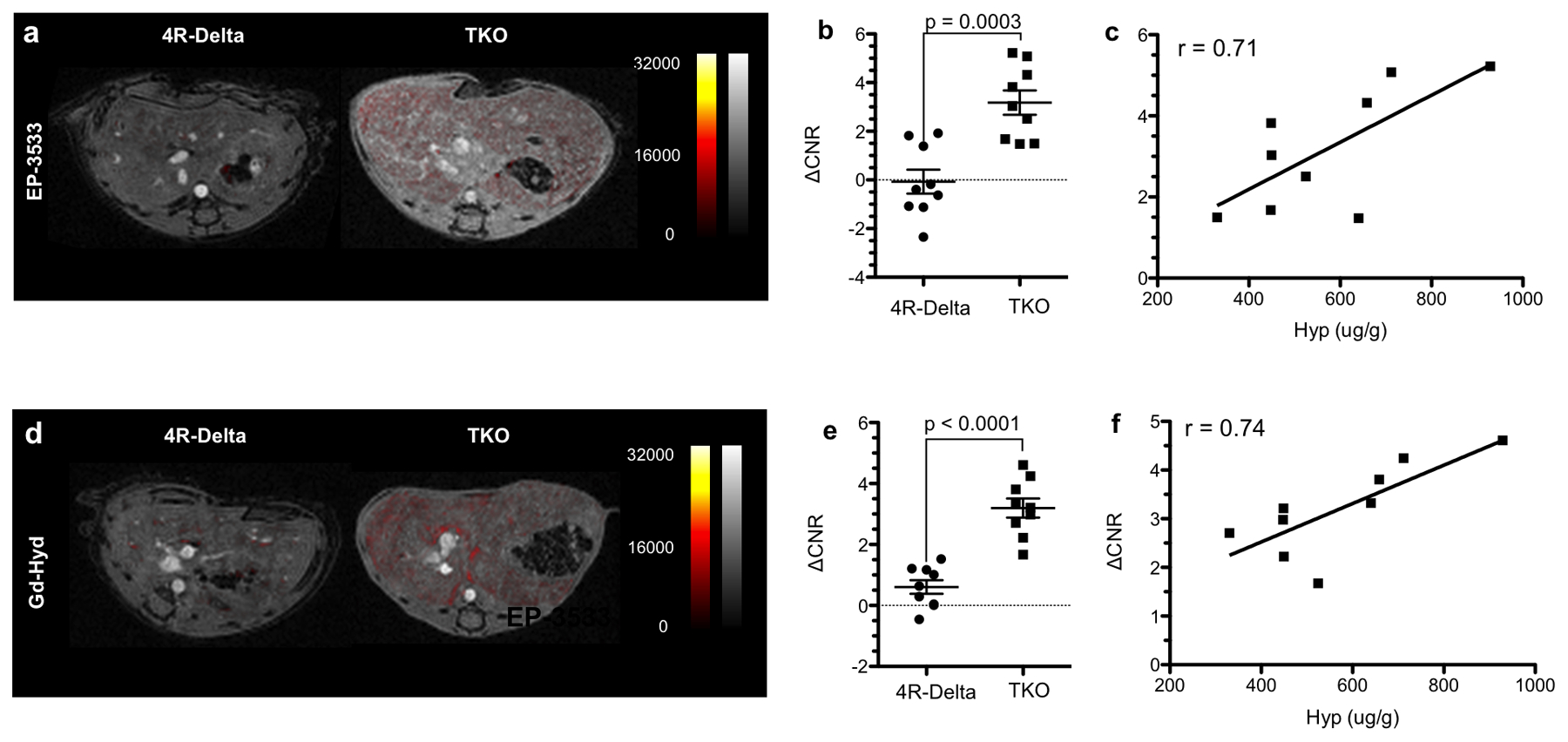
Representative images of EP-3533 signal enhancement at 15 minutes post probe injection in 4R-Delta and TKO mice (A). T1-weighed gradient echo sequences were performed pre and 20 min post EP-3533 injection in both groups (n = 9 per group) to determine changes in contrast to noise ratio (ΔCNR) (B). The values of ΔCNR for EP-3533 directly correlate to the hydroxyproline content in the fibrotic animals (TKO) (C). Representative images of Gd-Hyd signal enhancement at 15 minutes post probe injection in 4R-Delta and TKO mice (D). T1-weighed gradient echo sequences were also performed pre and 15 min post Gd-Hyd injection to determine ΔCNR (E). The values of ΔCNR for Gd-Hyd directly correlate to the hydroxyproline content in TKO mice (F). Pearson correlation coefficient (r) was calculated for (C) and (F). Other results are expressed as average ± SEM and the statistical significance was assessed by two-tailed Student’s t-test with Welch’s correction.
Gd-Hyd can specifically detect liver fibrogenesis
Similar to the results for EP-3533, mice imaged with Gd-Hyd demonstrated increased signal intensity in pinpoint-sized punctuated areas corresponding to the granulomas (Figure 4D). The low signal enhancement observed for 4R-Delta mice, consistent with absence of fibrosis in this group, was confirmed by ΔCNR assessment. ΔCNR 15 min post-probe injection in 4R-Delta mice was similar to the values for naïve animals, which suggests that the enhanced inflammatory response in these animals does not lead to any unspecific signal enhancement in conditions where fibrogenesis is absent (Figure 4E). By comparison, ΔCNR increased in TKO mice and was directly correlated to the hydroxyproline content (Figure 4F).
Discussion
Current noninvasive methods to assess tissue fibrosis and fibrogenesis are limited. Serum markers of fibrosis have been shown to be adequate to identify advanced fibrosis but have low accuracy on staging the disease.(36, 37) Imaging techniques, such as ultrasound (US), computed tomography (CT) and MR imaging have been used to identify morphologic alterations in fibrotic livers, but similarly to the serological analyses, these methods are also better able to identify fibrosis in advanced stages.(38) US elastography has shown great accuracy for identifying cirrhosis but intermediate accuracy for distinguishing between earlier stages.(39) In addition, the ability to identify fibrosis is impaired in obese and ascites-bearing patients, which are common features of non-alcoholic steatohepatitis (NASH) patients, due to the low penetration of the US beams,(40) and could be confounded by the presence of other hepatic and extra-hepatic conditions, such as inflammation, that affect liver stiffness.(38) MR elastography (MRE) is currently the most accurate imaging method to diagnose and stage liver fibrosis. It presents high accuracy even for mild fibrosis.(41) Although less affected by obesity, this condition can negatively impact the image readouts. Iron deposition in liver as well as other diseases that can affect liver stiffness also reduces the accuracy of this technique.(42) As a consequence of the limitations of the current noninvasive methods, novel imaging methods that can detect and quantify liver fibrosis in a sensitive and specific manner are necessary.
Liver fibrosis is characterized by the increased deposition of extracellular matrix, especially type1 collagen. The Gd-based peptide probe EP-3533 can interact with type 1 collagen specifically and sensitively detect liver fibrosis progression. Another important characteristic during the fibrogenic process is the upregulation of LOX and LOX-like enzymes. These are quinone-containing, copper dependent amine oxidases that catalyze collagen oxidation, which leads to cross-linking. LOX enzymes function by oxidizing the terminal amino group of lysine to a reactive aldehyde. The subsequent product, allysine, forms covalent bonds with other allysines or unmodified lysine terminal amino groups, forming links between collagen helices. LOX activity has been shown to be increased in liver fibrosis, and contributes to disease pathogenesis.(43) The small molecule contrast agent Gd-Hyd comprises the stable Gd-DOTA chelate and a hydrazide functional group that binds LOX-generated allysine residues on collagen.(43)
In the current work, we tested both probes to detect liver fibrosis and fibrogenesis in a S. mansoni-infected mouse model, which mimics chronic liver fibrosis and its complications in humans.(25) This model is characterized by intense granulomatous response that evolves from an early type-1 to a sustained and dominant type-2 cytokine response, leading to increased collagen deposition in the perisinusoidal and periportal areas, associated with vascular obstruction caused by egg deposition.(44) Since the adult worms constantly lay eggs, the fibrotic process remains activated unless disease resolution occurs.(45) Therefore, this model is ideal for testing both probes that can detect active fibrosis (Gd-Hyd) as well as stable collagen deposition (EP-3533).
Compared to traditional liver fibrosis models, infection with S. mansoni leads to a more localized and heterogeneous fibrosis development, which is associated with vascular obstruction. Therefore, we first evaluated the ability of these MR probes to detect and quantify the fibrosis in wild-type infected animals. We observed a strong correlation between the signal enhancement and the hydroxyproline content in these animals for both probes, which suggests that they can identify and quantify fibrosis even in conditions of vascular impairment. Further studies correlating liver blood flow, fibrosis level and signal enhancement would be useful to consolidate this hypothesis.
Like other animal models of liver fibrosis, S. mansoni-infected animals also present with an intense underlying inflammation (Figure 1F), which could skew the probe readouts. Cytokines released in inflamed tissue will lead to stimulation of macrophages and cytotoxic T cells and alteration in vascular permeability and distribution.(46, 47) To rule out that the enhancement observed for the infected wild-type mice is a consequence of greater extracellular volume and/or enhanced vascular permeability due to inflammation we have tested both probes in two genetically modified mouse models infected with S. mansoni: TKO and 4R-Delta. TKO mice are over-responsive to IL-13, the principal effector of Th2 response following S. mansoni infection, which will ultimately lead to fibrosis. These animals are knockouts for the receptor IL13Rα2, a high affinity decoy receptor for IL-13;(48) IL-12p40, an activator of the type 1 inflammatory response and counter-regulator of type 2 immunity;(49) and IL-10, a potent immunosuppressive cytokine.(43) The other model, 4R-Delta, is a knockout for the receptor IL4Rα, which is the signaling receptor for the Type 2 effector responses triggered by both IL-4 and IL-13.(23) This model is non-responsive to these pro-fibrotic cytokines and instead presents with an enhanced pro-inflammatory cytokine response.(50) 4R-Delta mice imaged 20 min-post EP-3533 injection demonstrated similar ΔR1 and ΔCNR values to the naïve mice. Similarly, ΔCNR assessed 15 min post Gd-Hyd injection was close to zero, the same as observed for naïve mice. Meanwhile, TKO mice presented strong signal enhancement, associated to increased ΔCNR and ΔR1 upon injection of EP-3533, and similar results were observed for Gd-Hyd. The main phenotypic difference between these two groups is the enhanced type 1 inflammatory response associated with reduced fibrosis in 4R-Delta mice, which suggests that the presence of general inflammation does not interfere in the ability of both probes to identify fibrosis.
Conclusion
Gd-Hyd and EP-3533 could specifically detect fibrogenesis and collagen deposition, respectively, in the S. mansoni model of chronic liver disease. Both probes detected fibrosis irrespective of the underlying inflammation, a common feature in human disease that could confound imaging. In addition to these results, Gd-Hyd and EP-3533 have been tested in several other models of liver fibrosis.(11–13, 15–17, 51) Although there is no perfect animal model that completely mimics liver fibrosis and cirrhosis in humans, these probes have now been shown to detect and stage disease in toxin, biliary stasis, diet, and infection models of liver fibrosis providing confidence that they will perform well in humans. Imaging efficacy has also been shown in cardiac,(52) pulmonary,(53) and muscle fibrosis models,(54) as well as in tumor stroma in cancers such as pancreatic cancer.(55) Thus, these probes should have broad applicability in the clinic where they could be used to identify patients with fibrosis and active fibrogenesis in several disease settings.
Sources of Support:
This work was supported by grants from the National Institutes of Health (DK104956, DK104302, DK121789, EB009062, OD025234, and OD010650), and by the by the National Institute of Allergy and Infectious Diseases intramural research program.
References
- 1.Rockey DC, Bell PD, Hill JA. Fibrosis--A Common Pathway to Organ Injury and Failure. N Engl J Med. 2015;373(1):96. [DOI] [PubMed] [Google Scholar]
- 2.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214(2):199–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montesi SB, Desogere P, Fuchs BC, Caravan P. Molecular imaging of fibrosis: recent advances and future directions. J Clin Invest. 2019;129(1):24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paik JM, Golabi P, Younossi Y, et al. Changes in the Global Burden of Chronic Liver Diseases From 2012 to 2017: The Growing Impact of Nonalcoholic Fatty Liver Disease. Hepatology. 2020. [DOI] [PubMed] [Google Scholar]
- 5.Bruix J, Sherman M, Practice Guidelines Committee AAftSoLD. Management of hepatocellular carcinoma. Hepatology. 2005;42(5):1208–36. [DOI] [PubMed] [Google Scholar]
- 6.Friedman SL. Liver fibrosis -- from bench to bedside. J Hepatol. 2003;38 Suppl 1:S38–53. [DOI] [PubMed] [Google Scholar]
- 7.Puoti C, Guarisco R, Bellis L, Spilabotti L. Diagnosis, management, and treatment of hepatitis C. Hepatology. 2009;50(1):322; author reply 4–5. [DOI] [PubMed] [Google Scholar]
- 8.Chen W, Yang A, Jia J, et al. Lysyl oxidase (LOX) family members: rationale and their potential as therapeutic targets for liver fibrosis. Hepatology. 2020. [DOI] [PubMed] [Google Scholar]
- 9.Akam EA, Abston E, Rotile NJ, et al. Improving the reactivity of hydrazine-bearing MRI probes for in vivo imaging of lung fibrogenesis. Chemical Science. 2020;11(1):224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salarian M, Turaga RC, Xue S, et al. Early detection and staging of chronic liver diseases with a protein MRI contrast agent. Nat Commun. 2019;10(1):4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou IY, Clavijo Jordan V, Rotile NJ, et al. Advanced MRI of Liver Fibrosis and Treatment Response in a Rat Model of Nonalcoholic Steatohepatitis. Radiology. 2020;296(1):67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atanasova I, Sojoodi M, Leitao HS, et al. Molecular Magnetic Resonance Imaging of Fibrin Deposition in the Liver as an Indicator of Tissue Injury and Inflammation. Invest Radiol. 2020;55(4):209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erstad DJ, Farrar CT, Ghoshal S, et al. Molecular magnetic resonance imaging accurately measures the antifibrotic effect of EDP-305, a novel farnesoid X receptor agonist. Hepatol Commun. 2018;2(7):821–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu B, Wei L, Rotile N, et al. Combined magnetic resonance elastography and collagen molecular magnetic resonance imaging accurately stage liver fibrosis in a rat model. Hepatology. 2017;65(3):1015–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farrar CT, DePeralta DK, Day H, et al. 3D molecular MR imaging of liver fibrosis and response to rapamycin therapy in a bile duct ligation rat model. J Hepatol. 2015;63(3):689–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs BC, Wang H, Yang Y, et al. Molecular MRI of collagen to diagnose and stage liver fibrosis. J Hepatol. 2013;59(5):992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polasek M, Fuchs BC, Uppal R, et al. Molecular MR imaging of liver fibrosis: a feasibility study using rat and mouse models. J Hepatol. 2012;57(3):549–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen HH, Waghorn PA, Wei L, et al. Molecular imaging of oxidized collagen quantifies pulmonary and hepatic fibrogenesis. JCI insight. 2017;2(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolin CE, Arteel GE. The Matrisome, Inflammation, and Liver Disease. Semin Liver Dis. 2020;40(2):180–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosseini N, Shor J, Szabo G. Alcoholic Hepatitis: A Review. Alcohol Alcohol. 2019;54(4):408–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gieseck RL 3rd, Wilson MS, Wynn TA , Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol. 2018;18(1):62–76. [DOI] [PubMed] [Google Scholar]
- 22.Gieseck RL 3rd , Ramalingam TR, Hart KM, et al. Interleukin-13 Activates Distinct Cellular Pathways Leading to Ductular Reaction, Steatosis, and Fibrosis. Immunity. 2016;45(1):145–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wynn TA. IL-13 effector functions. Annu Rev Immunol. 2003;21:425–56. [DOI] [PubMed] [Google Scholar]
- 24.Liu Y, Meyer C, Xu C, et al. Animal models of chronic liver diseases. Am J Physiol Gastrointest Liver Physiol. 2013;304(5):G449–68. [DOI] [PubMed] [Google Scholar]
- 25.Mentink-Kane MM, Cheever AW, Wilson MS, et al. Accelerated and progressive and lethal liver fibrosis in mice that lack interleukin (IL)-10, IL-12p40, and IL-13Ralpha2. Gastroenterology. 2011;141(6):2200–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramalingam TR, Pesce JT, Sheikh F, et al. Unique functions of the type II interleukin 4 receptor identified in mice lacking the interleukin 13 receptor alpha1 chain. Nat Immunol. 2008;9(1):25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jankovic D, Kullberg MC, Noben-Trauth N, et al. Schistosome-infected IL-4 receptor knockout (KO) mice, in contrast to IL-4 KO mice, fail to develop granulomatous pathology while maintaining the same lymphokine expression profile. J Immunol. 1999;163(1):337–42. [PubMed] [Google Scholar]
- 28.Caravan P, Das B, Dumas S, et al. Collagen‐Targeted MRI Contrast Agent for Molecular Imaging of Fibrosis. Angewandte Chemie International Edition. 2007;46(43):8171–3. [DOI] [PubMed] [Google Scholar]
- 29.Fries P, Massmann A, Robert P, et al. Evaluation of Gadopiclenol and P846, 2 High-Relaxivity Macrocyclic Magnetic Resonance Contrast Agents Without Protein Binding, in a Rodent Model of Hepatic Metastases: Potential Solutions for Improved Enhancement at Ultrahigh Field Strength. Invest Radiol. 2019;54(9):549–58. [DOI] [PubMed] [Google Scholar]
- 30.Muller A, Hochrath K, Stroeder J, et al. Effects of Liver Fibrosis Progression on Tissue Relaxation Times in Different Mouse Models Assessed by Ultrahigh Field Magnetic Resonance Imaging. Biomed Res Int. 2017;2017:8720367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fries P, Seidel R, Muller A, et al. Comparison of self-gated and prospectively triggered fast low angle shot (FLASH) sequences for contrast-enhanced magnetic resonance imaging of the liver at 9.4 T in a rat model of colorectal cancer metastases. Invest Radiol. 2013;48(10):738–44. [DOI] [PubMed] [Google Scholar]
- 32.Hutson PR, Crawford ME, Sorkness RL. Liquid chromatographic determination of hydroxyproline in tissue samples. Journal of Chromatography B. 2003;791(1):427–30. [DOI] [PubMed] [Google Scholar]
- 33.Henderson GS, Nix NA, Montesano MA, et al. Two distinct pathological syndromes in male CBA/J inbred mice with chronic Schistosoma mansoni infections. Am J Pathol. 1993;142(3):703–14. [PMC free article] [PubMed] [Google Scholar]
- 34.Cheever AW, Andrade ZA. Pathological lesions associated with Schistosoma mansoni infection in man. Trans R Soc Trop Med Hyg. 1967;61(5):626–39. [DOI] [PubMed] [Google Scholar]
- 35.Vannella KM, Barron L, Borthwick LA, et al. Incomplete deletion of IL-4Ralpha by LysM(Cre) reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis. PLoS Pathog. 2014;10(9):e1004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Moreno C, Mueller S, Szabo G. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J Hepatol. 2019;70(2):273–83. [DOI] [PubMed] [Google Scholar]
- 37.Motola DL, Caravan P, Chung RT, Fuchs BC. Noninvasive biomarkers of liver fibrosis: clinical applications and future directions. Current pathobiology reports. 2014;2(4):245–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horowitz JM, Venkatesh SK, Ehman RL, et al. Evaluation of hepatic fibrosis: a review from the society of abdominal radiology disease focus panel. Abdom Radiol (NY). 2017;42(8):2037–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Degos F, Perez P, Roche B, et al. Diagnostic accuracy of FibroScan and comparison to liver fibrosis biomarkers in chronic viral hepatitis: a multicenter prospective study (the FIBROSTIC study). J Hepatol. 2010;53(6):1013–21. [DOI] [PubMed] [Google Scholar]
- 40.Myers RP, Pomier-Layrargues G, Kirsch R, et al. Discordance in fibrosis staging between liver biopsy and transient elastography using the FibroScan XL probe. J Hepatol. 2012;56(3):564–70. [DOI] [PubMed] [Google Scholar]
- 41.Park CC, Nguyen P, Hernandez C, et al. Magnetic Resonance Elastography vs Transient Elastography in Detection of Fibrosis and Noninvasive Measurement of Steatosis in Patients With Biopsy-Proven Nonalcoholic Fatty Liver Disease. Gastroenterology. 2017;152(3):598–607 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner M, Corcuera-Solano I, Lo G, et al. Technical Failure of MR Elastography Examinations of the Liver: Experience from a Large Single-Center Study. Radiology. 2017;284(2):401–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ikenaga N, Peng ZW, Vaid KA, et al. Selective targeting of lysyl oxidase-like 2 (LOXL2) suppresses hepatic fibrosis progression and accelerates its reversal. Gut. 2017;66(9):1697–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wynn TA, Thompson RW, Cheever AW, Mentink-Kane MM. Immunopathogenesis of schistosomiasis. Immunol Rev. 2004;201:156–67. [DOI] [PubMed] [Google Scholar]
- 45.Hoffmann KF, Cheever AW, Wynn TA. IL-10 and the dangers of immune polarization: excessive type 1 and type 2 cytokine responses induce distinct forms of lethal immunopathology in murine schistosomiasis. J Immunol. 2000;164(12):6406–16. [DOI] [PubMed] [Google Scholar]
- 46.Andrade G, Bertsch DJ, Gazzinelli A, King CH. Decline in infection-related morbidities following drug-mediated reductions in the intensity of Schistosoma infection: A systematic review and meta-analysis. PLoS Negl Trop Dis. 2017;11(2):e0005372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheever AW, Mosimann JE, Deb S, et al. Natural history of Schistosoma mansoni infection in mice: egg production, egg passage in the feces, and contribution of host and parasite death to changes in worm numbers. Am J Trop Med Hyg. 1994;50(3):269–80. [DOI] [PubMed] [Google Scholar]
- 48.Asadzadeh Z, Mohammadi H, Safarzadeh E, et al. The paradox of Th17 cell functions in tumor immunity. Cell Immunol. 2017;322:15–25. [DOI] [PubMed] [Google Scholar]
- 49.Swiderska M, Jaroszewicz J, Stawicka A, et al. The interplay between Th17 and T-regulatory responses as well as adipokines in the progression of non-alcoholic fatty liver disease. Clin Exp Hepatol. 2017;3(3):127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104(6):777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou IY, Catalano OA, Caravan P. Advances in Functional and Molecular MRI Technologies in Chronic Liver Diseases. J Hepatol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Helm PA, Caravan P, French BA, et al. Postinfarction myocardial scarring in mice: molecular MR imaging with use of a collagen-targeting contrast agent. Radiology. 2008;247(3):788–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Caravan P, Yang Y, Zachariah R, et al. Molecular magnetic resonance imaging of pulmonary fibrosis in mice. Am J Respir Cell Mol Biol. 2013;49(6):1120–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Murphy AP, Greally E, O’Hogain D, et al. Noninvasive quantification of fibrosis in skeletal and cardiac muscle in mdx mice using EP3533 enhanced magnetic resonance imaging. Magn Reson Med. 2019;81(4):2728–35. [DOI] [PubMed] [Google Scholar]
- 55.Polasek M, Yang Y, Schühle DT, et al. Molecular MR imaging of fibrosis in a mouse model of pancreatic cancer. Scientific Reports. 2017;7(1):8114. [DOI] [PMC free article] [PubMed] [Google Scholar]


