Abstract
Zebrafish have a remarkable ability to regenerate the myocardium after injury by proliferation of pre-existing cardiomyocytes. Fibroblast growth factor (FGF) signaling is known to play a critical role in zebrafish heart regeneration through promotion of neovascularization of the regenerating myocardium. Here, we define an additional function of FGF signaling in the zebrafish myocardium after injury. We find that FGF signaling is active in a small fraction of cardiomyocytes before injury, and that the number of FGF signaling-positive cardiomyocytes increases after amputation-induced injury. We show that ERK phosphorylation is prominent in endothelial cells, but not in cardiomyocytes. In contrast, basal levels of phospho-AKT positive cardiomyocytes are detected before injury, and the ratio of phosphorylated AKT-positive cardiomyocytes increases after injury, indicating a role of AKT signaling in cardiomyocytes following injury. Inhibition of FGF signaling reduced the number of phosphorylated AKT-positive cardiomyocytes and increased cardiomyocyte death without injury. Heart injury did not induce cardiomyocyte death; however, heart injury in combination with inhibition of FGF signaling caused significant increase in cardiomyocyte death. Pharmacological inhibition of AKT signaling after heart injury also caused increased cardiomyocyte death. Our data support the idea that FGF-AKT signaling-dependent cardiomyocyte survival is necessary for subsequent heart regeneration.
Keywords: Zebrafish, Cardiomyocytes, cell survival, heart regeneration, Fibroblast growth factor, AKT pathway
INTRODUCTION
Adult mammalian heart tissue regenerates poorly following injury, which instead leads to fibrosis, scar formation and death (Ahuja et al., 2007; Finegold et al., 2013). Although recent studies have provided evidence that postnatal mammalian cardiomyocytes (CMs) slowly turn over (Bergmann et al., 2009; Senyo et al., 2013), the turnover rate is too low to repair the heart after injury. In contrast, lower vertebrates, including newts and zebrafish, possess significant ability to regenerate the injured heart in the adult stage. Remarkably, zebrafish can regenerate lost heart tissue following resection of up to 20% of the myocardium (Poss et al., 2002; Raya et al., 2003). Additionally, zebrafish can initiate regeneration following other types of injury such as cryoinjury to the ventricle as well as genetic ablation of more than half of CMs (Chablais et al., 2011; Gonzalez-Rosa et al., 2011; Schnabel et al., 2011; Wang et al., 2011). It has been demonstrated that neonatal mice also possess significant cardiac regenerative ability during the 1st week of the postnatal life (Notari et al., 2018; Porrello et al., 2011). In both zebrafish and neonatal mice, genetic lineage-tracing approaches that label CMs before injury led to the conclusion that heart regeneration occurs by de-differentiation and proliferation of pre-existing CMs (Jopling et al., 2010; Kikuchi et al., 2010; Porrello et al., 2011). Therefore, understanding the mechanisms of heart regeneration is relevant to biomedical research due to the possibility of developing new therapeutic targets to enhance heart regeneration ability in humans (Laflamme and Murry, 2011).
For myocardial regeneration, coordinated actions of intrinsic and extrinsic mechanisms are required to cause the dedifferentiation and proliferation of CMs during myocardial regeneration (Foglia and Poss, 2016). Intrinsic mechanisms involve functions of microRNAs (Beauchemin et al., 2015; Rodriguez and Yin, 2019; Yin et al., 2012) and transcription factors, such as Gata4 (Gupta et al., 2013; Kikuchi et al., 2010), Stat3 (Fang et al., 2013) and NF-κB (Karra et al., 2015), which regulate de-differentiation of CMs and their proliferation-competency. Extrinsic factors include extracellular matrix stiffness (Notari et al., 2018), immune cells (Lai et al., 2017; Lai et al., 2019) and secreted growth factors, such as retinoic acid and Neuregulin, derived from the epicardial tissue (Kikuchi 2011, Gemberling et al., 2015). Moreover, insulin-like growth factor, bone morphogenetic proteins, transforming growth factor ß and Hedgehog also regulate CM proliferation (Chablais and Jazwinska, 2012; Choi et al., 2013; Huang et al., 2013; Wu et al., 2016).
When mammalian hearts are injured by infarcts, cell death occurs within the myocardial wall, leading to loss of CMs (Marunouchi and Tanonaka, 2015; Whelan et al., 2010). In an uninjured zebrafish, few apoptotic cells were detected in the myocardial layer (Wang et al., 2011; Yu et al., 2018), which is shown to increase following injury to different degrees depending on the injury protocol used (Chablais et al., 2011; Kikuchi et al., 2011b; Wang et al., 2011; Yu et al., 2018). Although apoptotic cells increase after injury, the majority of apoptotic cells are non-CMs (Chablais et al., 2011; Yu et al., 2018), and levels of CM death in response to injury and during heart regeneration in zebrafish remain unknown.
FGF signaling plays multiple roles during development of the heart (Itoh et al., 2016). In the regenerating zebrafish heart, FGF signaling-dependent epithelial-mesenchymal transition of epicardial cells and their contributions to peri-vascular cells support neo-vascularization in the myocardial layer (Kikuchi et al., 2011a; Lepilina et al., 2006). Similarly, in adult mammalian hearts, FGF signaling regulates angiogenesis in response to cardiac injury (Murakami and Simons, 2008). In addition to regulating the vasculature, FGF signaling plays a role in CM homeostasis in adult mammalian hearts (Sakurai et al., 2013). Specifically, FGF signaling is required for myocardial integrity through maintenance of CM junctions. Perfusion of FGF2 in the isolated adult rat heart protected the heart from subsequent ischemia-reperfusion induced injuries, presumably acting on endothelial cells and CMs (Padua et al., 1995). Pretreatment of cultured CMs with FGF2 protects from doxorubicin-induced cell death (Wang et al., 2013). Furthermore, FGF21, secreted from the liver and adipose tissue, contributes to CM survival after ischemic damage in mice (Liu et al., 2013). Given these roles of FGF signaling in adult mammalian CMs, we revisited roles of FGF signaling during zebrafish heart regeneration. We found a low level of FGF signaling activity in uninjured CMs. The ratio of CMs with active FGF signaling is increased following injury. Our study provides evidence that the FGF-AKT signaling cascade in CMs is necessary for their survival in response to injury. We propose that FGF-AKT signaling maintains CM survival immediately following injury until CM proliferation is induced and regenerative repair of the myocardium begins.
RESULTS AND DISCUSSION
Activation of FGF signaling in cardiomyocytes in response to heart injury
In order to investigate whether FGF signaling is active in CMs, we made use of the Et7 zebrafish line, in which an EGFP reporter cassette is inserted near the dusp6 locus by the sleeping beauty transposon system (Balciunas et al., 2004). A previous characterization of this line indicated that EGFP expression closely mimics the endogenous dusp6 mRNA expression pattern. Dusp6 is an ERK1/2 phosphatase, whose expression is regulated by FGF signaling during development (Kawakami et al., 2003; Li et al., 2007; Tsang et al., 2004). Previous studies showed strong expression of dusp6 in the epicardium and in the endothelial cells of the zebrafish heart (Han et al., 2014; Missinato et al., 2018). Expression of dusp6 was also noted in cardiomyocytes in zebrafish hearts (Missinato et al., 2018). In order to evaluate dusp6-expressing CMs using the Et7 zebrafish, we stained heart sections with ant-Mef2, a nuclear marker of CMs (Beauchemin et al., 2015; Itou et al., 2012b; Marin-Juez et al., 2016; Missinato et al., 2018; Wills et al., 2008), and anti-EGFP antibodies. Although a recent study showed dusp6 mRNA expression in CMs by confocal imaging, the ratio of CMs that express dusp6 remains unknown (Missinato et al., 2018). Therefore, we quantitated Et7-EGFP positive CMs among total CMs without injury and found that approximately 6% of CMs in the apical area are Et7-EGFP positive (Fig. 1A, E). This result is consistent with the recent reports and suggests a basal level of activation of FGF signaling in CMs.
Figure 1. Basal levels of FGF signaling in cardiomyocytes and its activation in response to heart injury.
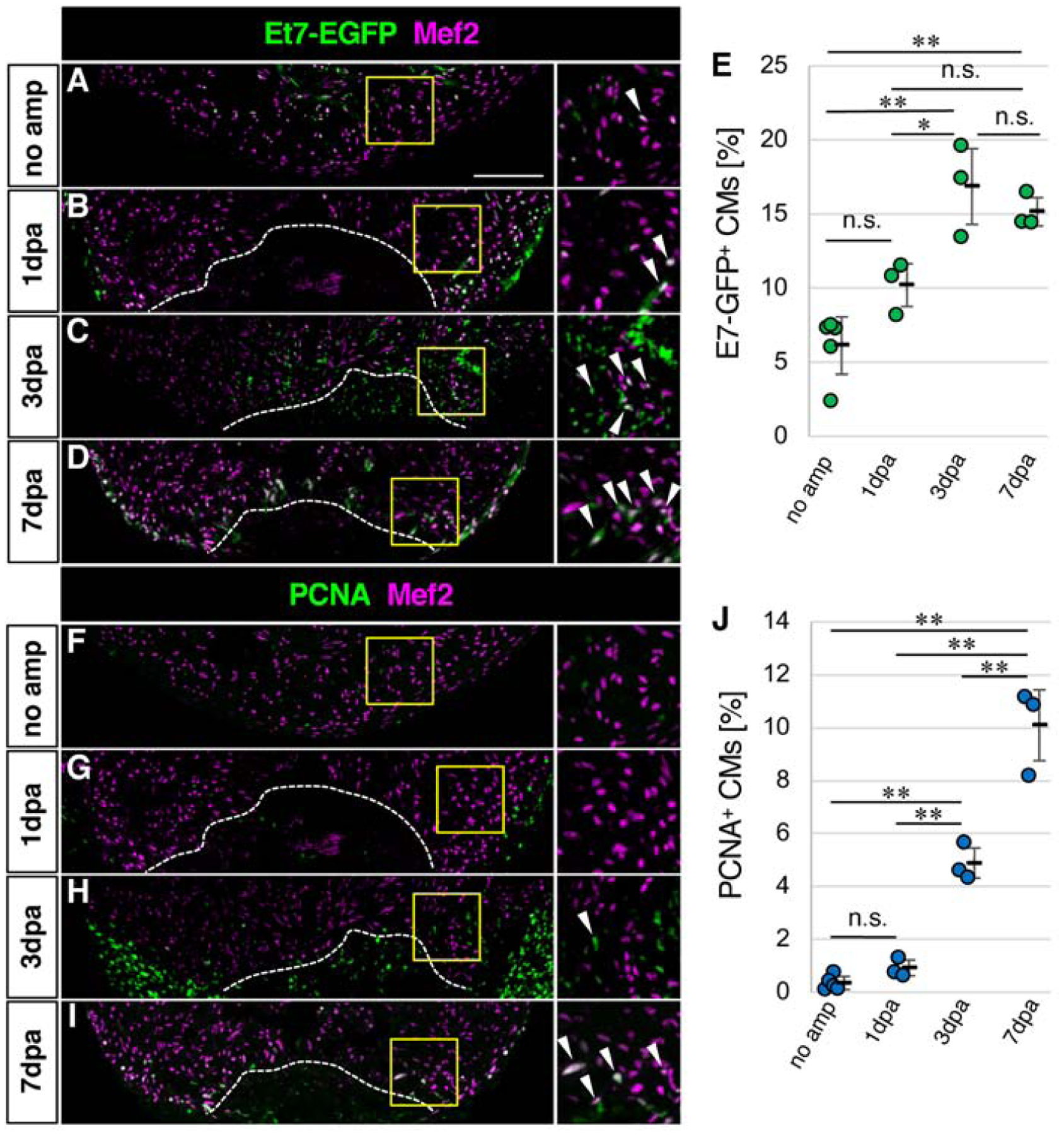
(A-D) Confocal images of the Et7-EGFP and Mef2 signals in the ventricle without amputation
(A) and after amputation (B-D).
(E) Graphic representation of the percentage of the Et7-EGFP-positive CMs among CMs in the apex at indicated time points after ventricular amputation.
(F-I) Confocal images of the PCNA and Mef2 signals in the ventricle without amputation (F) and after amputation (G-I).
(J) Graphic representation of the percentage of the PCNA-positive CMs among CMs in the apex at indicated time points after ventricular amputation.
Dotted lines in B-D and G-H indicate amputation planes. The areas in yellow squares in A-D and F-I are shown on the right side. Arrowheads point to double positive cells.
Scale bar, 100 μm. * p<0.05, ** p<0.01 by one-way ANOVA with Tukey HSD. n.s., not significant (p≥0.05). n=5 for no amputation, and n=3 for 1dpa, 3dpa and 7 dpa in E and J. Each circle in graphs represents an average value for each animal.
Recent studies evaluated upregulation of dusp6 expression in the injured heart by qRT-PCR and Western blotting approaches (Han et al., 2014; Missinato et al., 2018). Given that dusp6 is primarily induced in endothelial cells and epicardial cells after heart injury, it remains unknown whether the ratio of dusp6-expressing CMs changes after heart injury. We next evaluated changes to the ratio of Et7-EGFP-positive CMs in response to apex resection. At one day post amputation (1dpa), the ratio of Et7-EGFP positive CMs became approximately 10% compared to 6% in non-injured hearts (Fig. 1B, E). At 3dpa to 7dpa, when CMs actively proliferate (Fig. 1F–J), the ratio of Et7-EGFP positive CMs increased to approximately 16–17% of CMs in the apex area (Fig. 1C–E). Given that dusp6 is a target of FGF signaling, these data suggest activation of FGF signaling in CMs in response to heart injury.
The ERK pathway is active in endothelial cells
FGF signaling is mediated by several intracellular pathways (Turner and Grose, 2010). The ERK pathway is a major downstream signaling pathway, whose activation causes di-phosphorylation of ERK (dpERK). It has been shown that dpERK-positive cells are primarily located in Dusp6-positive epicardial cells (Han et al., 2014). A recent study demonstrated that dusp6 mutant zebrafish hearts show increased CM proliferation after injury, which provided evidence for a role of Dusp6 in CMs (Missinato et al., 2018). Given that Dusp6 negatively regulates ERK signaling, we examined expression of dpERK in CMs. However, we found less than 1.1% of CMs were dpERK positive without injury (Fig. 2A, D). The ratio of dpERK positive CMs did not increase at 3 or 7dpa (Fig. 2B–D), when CMs actively proliferate (Fig. 1J). Instead, we found that fli1-EGFP-positive endothelial cells were the major cell type with dpERK expression (Fig. 2E–H). These results are consistent with previous reports that demonstrated the requirement of FGF signaling and platelet derived growth factor signaling for re-vascularization of regenerating myocardium (Kim et al., 2010; Lepilina et al., 2006). It has been demonstrated that vascular endothelial growth factor (VEGF) signaling-dependent fast angiogenic re-vascularization after heart injury is essential for heart regeneration in zebrafish (Marin-Juez et al., 2016). Inhibition of VEGF signaling caused reduced re-vascularization and reduced CM proliferation without affecting CM survival. Given that VEGF-ERK signaling promotes angiogenesis during zebrafish development (Shin et al., 2016), upregulation of ERK signaling in endothelial cells after heart injury may contribute to heart regeneration through re-vascularization.
Figure 2. Activation of the ERK pathway in endothelial cells after heart injury.
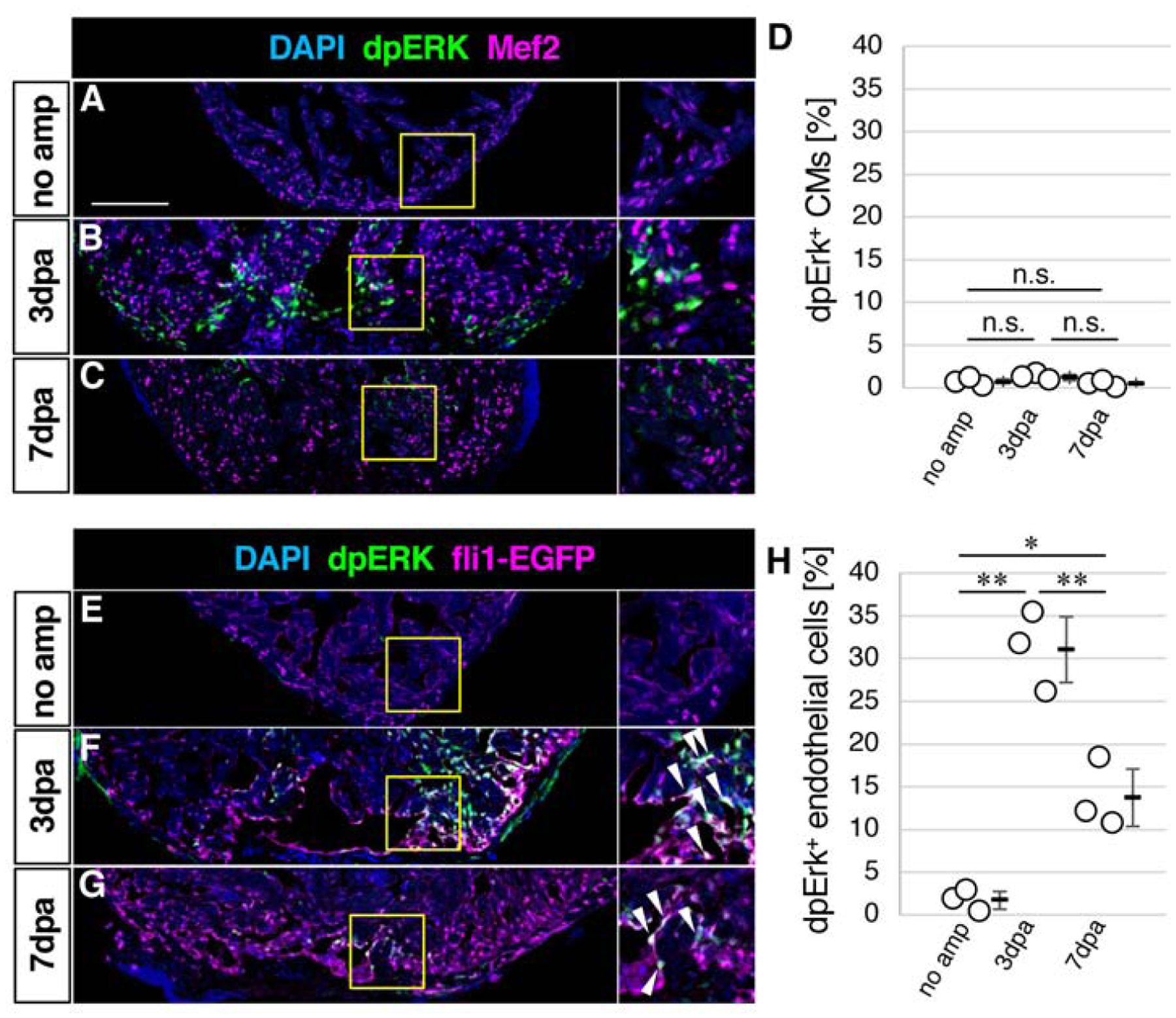
(A-C) Confocal images of the dpERK, Mef2 and DAPI signals in the ventricle without amputation (A) and after amputation (B, C).
(D) Graphic representation of the percentage of the dpERK-positive CMs among CMs in the apex at indicated time points after ventricular amputation.
(E-G) Confocal images of the dpERK, fli1-EGFP and DAPI signals in the ventricle without amputation (E) and after amputation (F, G).
(H) Graphic representation of the percentage of the dpERK-positive endothelial cells among endothelial cells in the apex at indicated time points after ventricular amputation.
The areas in yellow squares in A-C and E-G are shown on the right side. Arrowheads point to double positive cells. Scale bar, 100 μm. * p<0.05, ** p<0.01 by one-way ANOVA with Tukey HSD. n.s., not significant (p≥0.05). n=3 for no amputation, 3dpa and 7 dpa in D and H. Each circle in graphs represents an average value for each animal.
The AKT pathway is active in CMs
Given the lack of dpERK signals in CMs after injury, we turned our attention to the phosphoinositide 3-kinase-AKT pathway, which is another major downstream pathway of FGF signaling. In order to assess whether AKT signaling is active in CMs, we co-stained CMs with anti-Mef2 and anti-phosphoAKT (pAKT), an indicator of activation of AKT signaling. It has been shown that pAKT is present in both the cytoplasm and the nucleus in CMs (Camper-Kirby et al., 2001). Signals in the cytoplasm of CMs are oftentimes difficult to distinguish from those of non-CMs. Therefore, we focused our analysis on nuclear pAKT signals that overlap with the Mef2 signal (Fig. S1). We found that, without injury, approximately 9% of CMs at the apex area are positive for pAKT (Fig. 3A, E), indicating basal levels of activation of AKT signaling in CMs. We next examined whether the ratio of pAKT positive CMs changes after heart injury. At 1dpa, the ratio increased to 15% and stayed around 18–21% from 3dpa to 7 dpa. These results show that CMs have active AKT signaling rather than ERK signaling. The rapid increase of the ratio of pAKT-positive CMs after heart injury also suggests a role of AKT signaling in CMs in response to heart injury.
Figure 3. Activation of the AKT pathway in cardiomyocytes in response to heart injury.
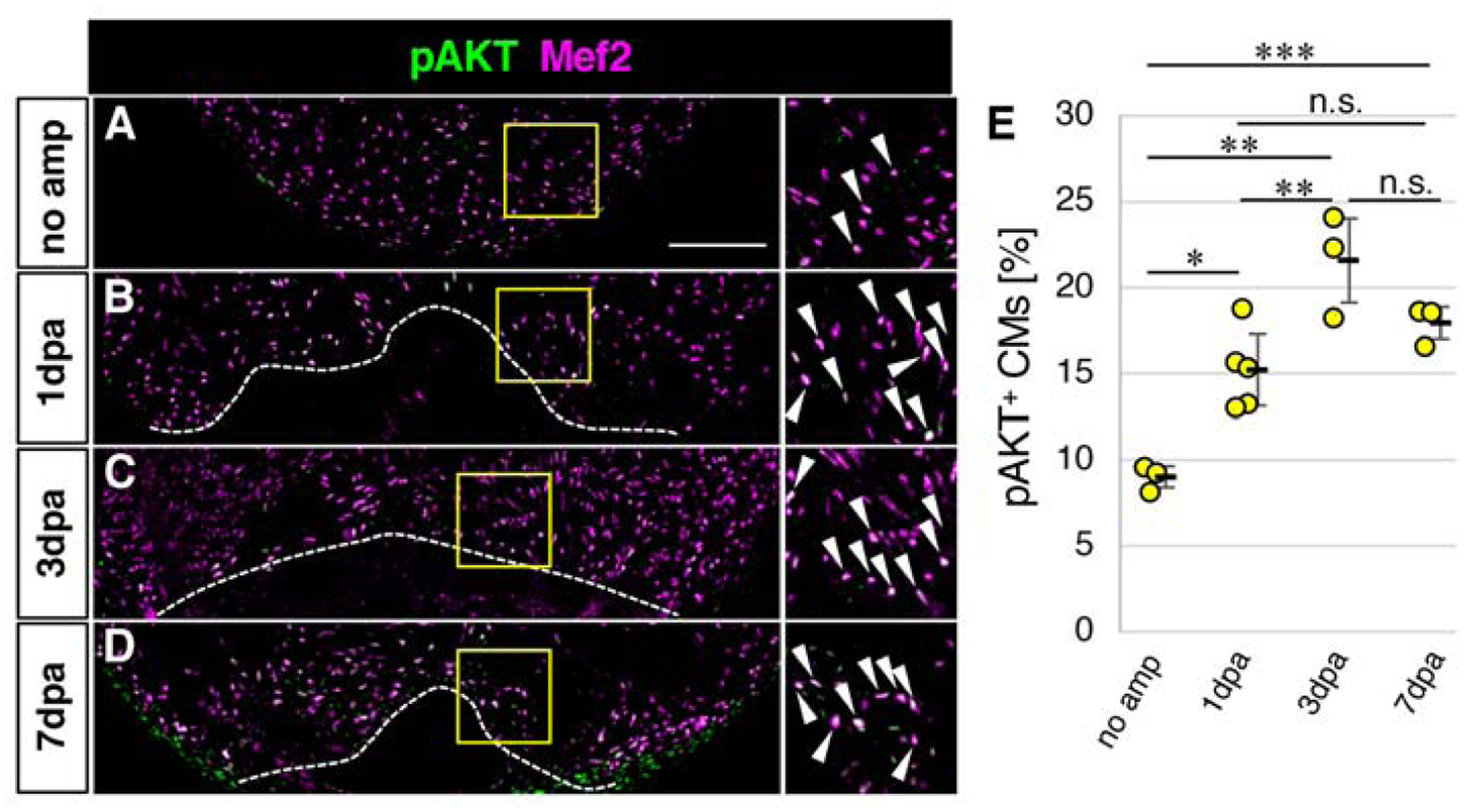
(A-D) Confocal images of the pAKT and Mef2 signals in the ventricle without amputation (A) and after amputation (B-D).
(E) Graphic representation of the percentage of the pAKT-positive CMs among CMs in the apex at indicated time points after ventricular amputation.
Dotted lines in B-D indicate amputation planes. The areas in yellow squares in A-D are shown on the right side. Arrowheads point to double positive cells. Scale bar, 100 μm. n=3 for no amputation, n=5 for 1 dpa, and n=3 for 3dpa and 7 dpa. * p<0.05, ** p<0.01, *** p<0.001 by one-way ANOVA with Tukey HSD. n.s., not significant (p≥0.05). Each circle in E represents an average value for each animal.
We used zebrafish of a range of ages in this study to determine whether the dynamics of AKT signaling activation in CMs are maintained during aging. This approach is consistent with previous zebrafish heart regeneration studies, including our own, which indicated that zebrafish maintain the ability to regenerate the heart throughout their lifetimes (Itou et al., 2012a) . In this study, we compared activation of AKT signaling in CMs in 6 month old fish and 15 month old fish after injury. In both populations, the ratio of pAKT+ CMs peaked at 3dpa (Fig. S2). This result shows that the dynamics of activation of AKT signaling in CMs is similar between young and old fish, consistent with similar myocardial regeneration ability between young and old fish (Itou et al., 2012a).
FGF signaling is required for activation of the AKT pathway and CM survival
The AKT pathway is activated by receptor tyrosine kinase signaling, which includes the FGF pathway and other receptor tyrosine kinase pathways, such as the platelet derived growth factor pathway, the Neuregulin-Erbb pathway and the insulin-like growth factor pathway (Liu et al., 2009). The rapid increase of the ratio of Et7-EGFP-positive CMs and pAKT-positive CMs after injury suggests that FGF signaling activates the AKT pathway in CMs. In order to test this hypothesis, we performed genetic and inducible inhibition of FGF signaling. It has been shown that pulses of heat shock to the Tg(hsp70l:dnfgfr1a-EGFP) zebrafish (hereafter referred to as dnfgfr1 zebrafish) could induce expression of dominant negative FGF receptor 1 and block FGF signaling in adult zebrafish (Lee et al., 2005; Lepilina et al., 2006). We first performed a one hour pulse of heat-shock at 37°C on both dnfgfr1 and wild-type (WT) zebrafish, then harvested the heart after 12 hours without heart injury. This transient heat-shock significantly reduced the ratio of pAKT-positive CMs in the dnfgfr1 zebrafish, compared to WT zebrafish (Fig. 4B, C, N). This result indicates that the basal level of pAKT in CMs is dependent on FGF signaling.
Figure 4. FGF signaling is required for activation of AKT signaling and cardiomyocyte survival.
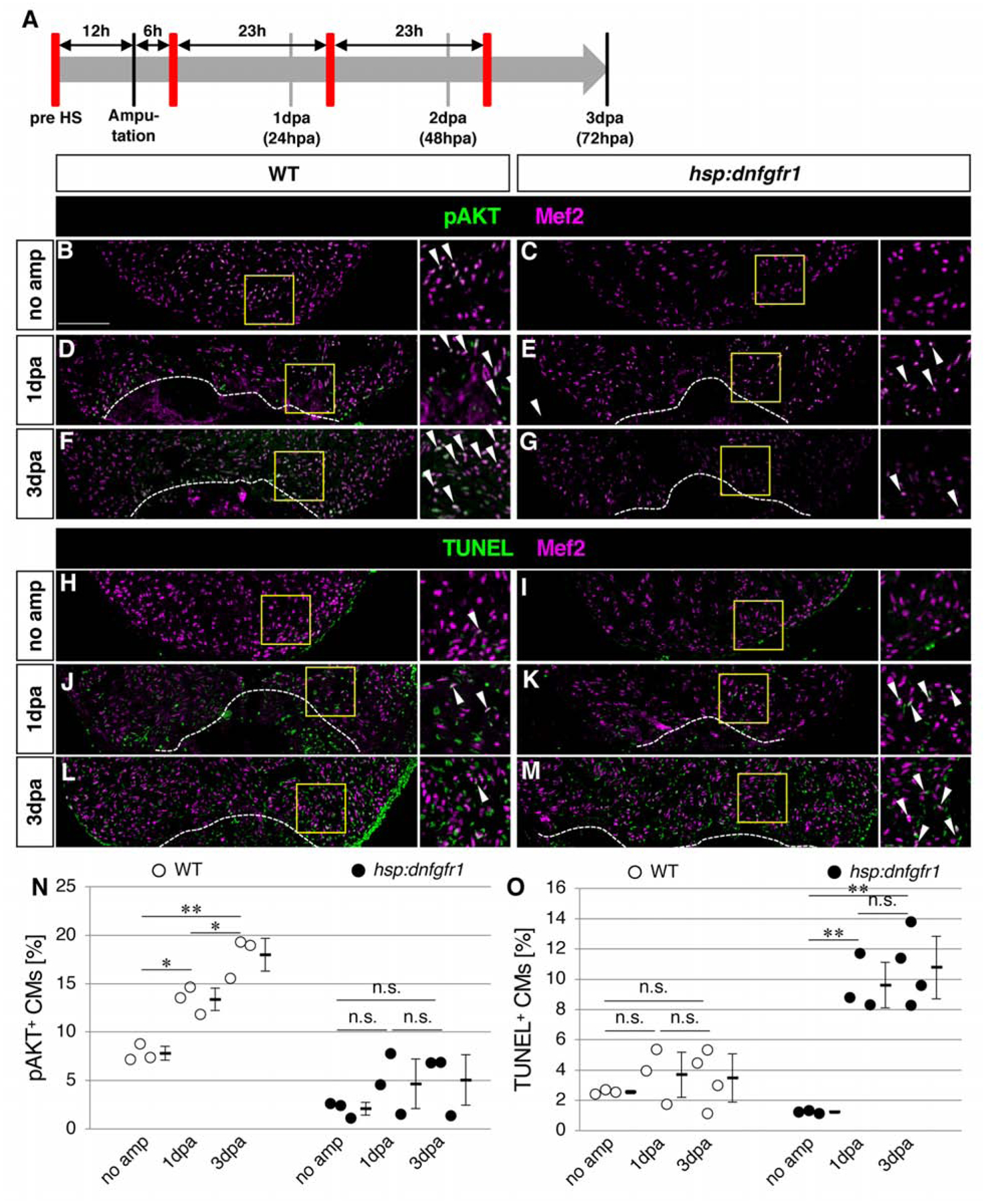
(A) Schematic presentation of experimental design. The red bars represent dnfgfr1 induction by pulses of heat shock.
(B-G) Confocal images of the pAKT and Mef2 signals in the ventricle without amputation (B, C) and after amputation (D-G) in WT (B, D, F) and hsp: dnfgfr1 fish (C, E, G).
(H-M) Confocal images of the Mef2 and TUNEL signals in the ventricle without amputation (H, I) and after amputation (J-M) in WT (H, J, L) and hsp: dnfgfr1 fish (I, K, M).
(N) Graphic presentation of the percentage of the pAKT-positive CMs among CMs in the apex at indicated time points.
(O) Graphic presentation of the percentage of the TUNEL-positive CMs among CMs in the apex at indicated time points.
Dotted lines in D-G and J-M indicate amputation planes. The areas in yellow squares in B-M are shown on the right side. Arrowheads point to double positive cells. Scale bar, 100 μm. * p<0.05, ** p<0.01 by one-way ANOVA with Tukey HSD. n.s., not significant (p≥0.05). n=3 for no amputation, 1 dpa and 3 dpa in both WT and hsp: dnfgfr1 fish in N and O. Each circle in graphs represents an average value for each animal.
Next, we pre-treated zebrafish with a pulse of heat-shock and resected the ventricle. In order to maintain inhibition of FGF signaling, we treated the zebrafish with a daily pulse of heat shock (Fig. 4A). WT hearts exhibited increase in the ratio of pAKT-positive CMs at 1dpa and 3dpa, compared to hearts without amputation; however, FGF signaling-blocked hearts did not show an increase of the ratio of pAKT positive CMs (Fig. 4B–G, N). These data support the idea that FGF signaling activates AKT signaling in CMs in response to injury.
FGF signaling regulates CM survival
CM proliferation elevates at 3 dpa and peaks at 7–14 dpa (Fig. 1J) (Itou et al., 2014). Basal levels of activation of FGF-AKT signaling, as well as rapid activation of this signaling system after ventricular resection, suggest that FGF-AKT signaling in CMs has a role prior to elevation of the ratio of proliferating CMs. One of the well-documented roles of AKT signaling is in cell survival (Sugden, 2003; Turner and Grose, 2010), and the AKT pathway is known to promote CM survival against ischemia-induced injury in mice (Fujio et al., 2000). Therefore, we tested whether the FGF-AKT pathway plays a role in CM survival in zebrafish following injury to the myocardial wall. We heat shock-treated WT and dnfgfr1 zebrafish and assessed cell death with TUNEL assays in combination with anti-Mef2 staining. In the case of mammals, CMs occupy 70% – 80% of the volume of the heart (Anversa et al., 1980; Dammrich and Pfeifer, 1983; Tang et al., 2009). However, it is estimated that more than 50% of cells in the heart are non-CMs (Pinto et al., 2016; Zhou and Pu, 2016). Previous studies combined the TUNEL method and muscle markers, such as myosin heavy chain or tropomyosin to evaluate CM death (Chablais et al., 2011; Yu et al., 2018). CMs are larger than non-CM cells, and it is often difficult to distinguish whether a TUNEL signal is present in CMs or non-CMs that are surrounded by CMs. To correctly distinguish the CMs and non-CMs among apoptotic cells, we used Mef2 as a CM nuclear marker, and co-detected TUNEL signals to evaluate apoptotic CMs. In both WT and dnfgfr1 zebrafish hearts pre-treated with a pulse of heat shock, low levels of CMs death were detected without heart injury (Fig. 4H, I, O) (Yu et al., 2018). Next, we assessed CM death in combination with heart injury and heat-shock induced inhibition of FGF signaling. Ventricular resection in WT zebrafish did not increase CM death at 1dpa or 3dpa (Fig 4H, J, L, O). In contrast, we observed a significant increase of CM death in dnfgfr1 zebrafish at 1dpa and 3dpa (Fig. 4I, K, M, O). These results support the idea that FGF-AKT signaling is necessary for CM survival after heart injury, prior to CM proliferation.
Since FGF signaling plays a role in CM homeostasis in adult mammalian hearts (Sakurai et al., 2013), we also tested whether inhibition of FGF signaling causes CM death without heart injury. We treated WT and dnfgfr zebrafish with 1 hour pulses of heat shock daily for 14 days without heart injury, and assessed CM death. The ratio of TUNEL-positive CMs was higher in dnfgfr zebrafish, compared to WT (Fig. S3). This data suggests that FGF signaling is required for CM homeostasis, and that it has a role outside of post-injury CM regeneration.
AKT signaling is required for CM survival
Our data support the hypothesis that FGF-AKT signaling is required for CM survival. In order to directly test the requirement for AKT signaling, we performed pharmacological inhibition of phosphatidylinositol-3-kinase by LY294002, which inhibits AKT signaling in adult zebrafish (Alvarez et al., 2009). While DMSO-treated control zebrafish exhibited an increased ratio of pAKT+ CMs from 1dpa to 3 dpa, the ratio of pAKT+ CMs did not increase in LY294002-treated zebrafish (Fig. 5A–D, I), confirming that LY294002-treatment was effective in the regenerating heart. We found that LY294002-treated zebrafish exhibited increase in the ratio of TUNEL+CMs from 1dpa to 3dpa, while DMSO-treatment did not induce any change (Fig. 5E–H, J). These results demonstrate that AKT signaling is required for CM survival after heart injury.
Figure 5. Cardiomyocyte survival depends on AKT signaling.
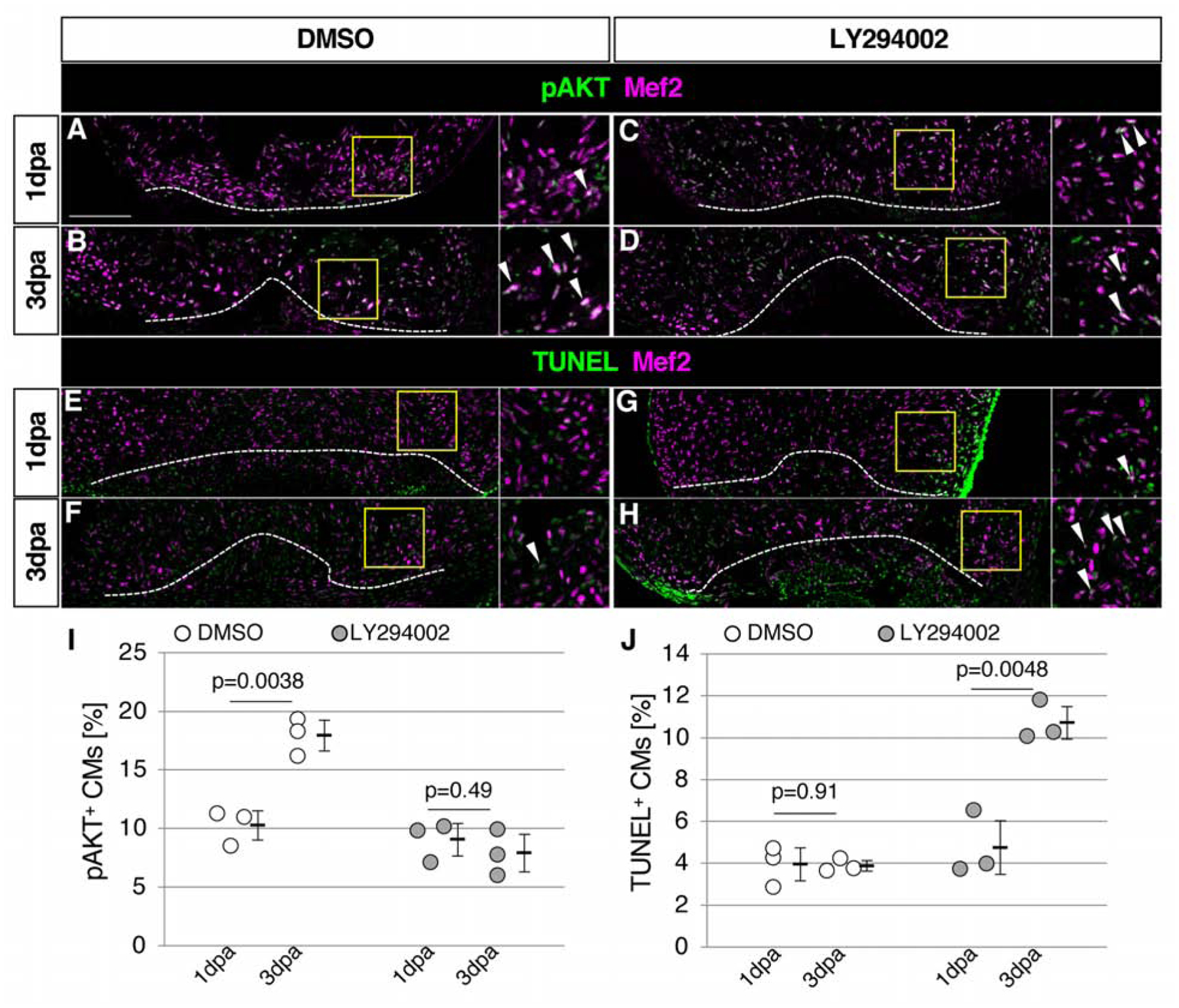
(A-D) Confocal images of the pAKT and Mef2 signals in the ventricle at 1dpa (A, C) and 3dpa (B, D) in DMSO-treated control fish (A, B) and LY294002-treated fish (C, D).
(E-H) Confocal images of the Mef2 and TUNEL signals in the ventricle at 1dpa (E, G) and 3dpa (F, H) in DMSO-treated control fish (E, F) and LY294002-treated fish (G, H).
(I) Graphic presentation of the percentage of the pAKT-positive CMs among CMs in the apex at indicated time points. n=3 for all samples.
(J) Graphic presentation of the percentage of the TUNEL-positive CMs among CMs in the apex at indicated time points.
Dotted lines in A-H indicate amputation planes. The areas in yellow squares in A-H are shown on the right side. Arrowheads point to double positive cells. Scale bar, 100 μm. p values by Student’s t-test are shown in the graph. n=3 for 1 dpa and 3 dpa in both DMSO-treated and LY294002-treated fish in I and J. Each circle in graphs represents an average value for each animal.
The AKT pathway is central to cell survival (Sugden, 2003; Turner and Grose, 2010). In the heart, experiments in mice demonstrated that the AKT pathway contributes to CM survival after ischemic damage (Fujio et al., 2000). The basal levels of FGF signaling, visualized by the Et7-EGFP signals, as well as pAKT levels in CMs, suggest that the FGF-AKT pathway may have a constitutive protective role for CMs in the zebrafish heart. Moreover, the rapid upregulation of the AKT pathway in CMs might protect CMs after trauma. CM proliferation in response to resection is elevated by 3 dpa and increases in the following regeneration phase (Itou et al., 2014; Poss et al., 2002). Dedifferentiation and proliferation of CMs are major driving forces of regeneration of injured myocardium (Jopling et al., 2010; Kikuchi et al., 2010), and sufficient number of CMs may be needed to initiate the regeneration processes. Taken together, our data suggest that CM survival supported by FGF-AKT signaling serves as a prerequisite for the CM proliferation that enables heart regeneration.
MATERIALS AND METHODS
Zebrafish maintenance and heart surgery
Zebrafish were maintained under standard conditions at around 28°C with a 14 h light 10 h dark cycle. Adult zebrafish (6 to 15-months old) were used for experiments. Adult zebrafish were anesthetized and the heart was exposed by manually dissecting the ventral body wall. For resection, we amputated the ventricular apex as previously published (Itou et al., 2012b; Raya et al., 2003). The Et7-EGFP line (mn7-Et) (Balciunas et al., 2004) and the Tg(fli1:EGFP)y1 (referred to as fli1-EGFP) line (Lawson and Weinstein, 2002) have been reported. Care and experimentation were performed in accordance with the Institutional Animal Care and Use Committee of the University of Minnesota.
Inhibition of FGF signaling
The Tg(hsp70l:dn-fgfr1) transgenic fish line (Lee et al., 2005) was used to inhibit FGF signaling. We introduced 37°C heat shock for 1 hour daily to induce expression of dnfgfr1.
Histology and immunostaining
For histological examination, 14 μm thick cryosections were processed for immunofluorescence as previously described (Itou et al., 2014). Briefly, sections were washed with PBS with 0.1 % Triton X-100, and heat-induced antigen retrieval was performed with citrate buffer (pH6.0) by boiling for 40 min, followed by a standard immunofluorescence procedure. The antibodies used in this study are listed in Supplemental Table 1. For detection of pERK and pAKT, after PBS+Triton X-100 washing, the signals were detected and amplified using an Alexa Fluor 488-Tyramide Signal Amplification Kit (Invitrogen, T20912) following the manufacturer’s instructions.
CM proliferation index
Sections around the center of injury were stained with anti-PCNA and anti-Mef2 antibodies. Fluorescent confocal images were obtained using a Zeiss LSM 710 laser scanning microscope system (Carl Zeiss Microscopy), and analyzed by ZEN2009 software (Carl Zeiss Microscopy). The CM proliferation index was defined as the ratio of the total number of PCNA, Mef2-double positive cells in the total Mef2-positive cells. PCNA/Mef2 double positive cells and Mef2-positive cells were manually counted within a defined region (1024 pixel × 256 pixel in 20 × objective lens with 0.6x zoom). Three sections containing the largest injury area were quantified for each heart, and the average value was used as the representative value of each zebrafish. Statistical significance was analyzed by one-way ANOVA with Tukey HSD.
TUNEL assay
After immunostaining, slides were re-fixed with 4% paraformaldehyde/PBS for 50 min and permeabilized by treating with 0.1% sodium citrate/0.1% TritonX-100 on ice for 2 min. Then the In Situ Cell Death Detection Kit, TMR-Red (Roche diagnostics, 12156792910) was used according to the manufacturer’s instructions. The TMR-Red fluorescent signals were detected by confocal imaging.
Assays for Et7-EGFP, dpERK, pAKT or TUNEL-positive CMs, and dpERK-positive endothelial cell assay
Similar to the CM proliferation index, the ratio of the total number of Et7-EGFP, Mef2-double positive cells, dpERK, Mef2-double positive cells, pAKT, Mef2-double positive cells, or TUNEL, Mef2-double positive cells in the total Mef2-positive cells were determined. For pERK positive endothelial cells, the ratio of the total number of pERK, fli1-EGFP-double positive cells in the total fli1-EGFP positive cells were determined. Statistical significance was analyzed by one-way ANOVA with Tukey HSD, except Fig. 5, where we used the Student’s t-test.
Pharmacological inhibition of AKT signaling
After surgery, each zebrafish was maintained in 50 ml system water with 10 μM LY294002 (FUJIFILM Wako Pure Chemical Corporation) or 0.1% DMSO, and the water was refreshed daily. To assist with the penetration of LY294002, the pericardiac cavity was surgically opened daily before changing the water, similar to our previous work (Itou et al., 2012b).
Supplementary Material
Highlights.
Zebrafish myocardium exhibits basal levels of FGF signaling
Heart injury induces activation of the AKT pathway in cardiomyocytes
Blocking FGF signaling in injured hearts causes reduced number of phosphorylated AKT positive cardiomyocytes and elevated cardiomyocyte death
Blocking AKT signaling causes cardiomyocyte death after heart injury
ACKNOWLEDGEMENTS
We thank Dr. Michael O’Connor for sharing his LSM710, Cailin McMahon for editing assistance, Dr. Bhairab Singh for critical reading of the manuscript, Dr. Ken Poss and the Zebrafish International Resource Center for making the Tg(hsp70:dn-fgfr1) line available, and Dr. Steve Ekker for the Et7-EGFP zebrafish line. We also thank the University of Minnesota Zebrafish Core facility for husbandry assistance.
FUNDING
This study was supported by grants from the National Institutes of Health to YK (R01AR064195) and Japan Society for the Promotion of Science KAKENHI Grants to YB (JP17H05768, JP18H02451, JP19H04782). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS STATEMENT
The authors declare no competing interests.
REFERENCES
- Ahuja P, Sdek P, MacLellan WR, 2007. Cardiac myocyte cell cycle control in development, disease, and regeneration. Physiol Rev 87, 521–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez Y, Astudillo O, Jensen L, Reynolds AL, Waghorne N, Brazil DP, Cao Y, O’Connor JJ, Kennedy BN, 2009. Selective inhibition of retinal angiogenesis by targeting PI3 kinase. PLoS One 4, e7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anversa P, Olivetti G, Melissari M, Loud AV, 1980. Stereological measurement of cellular and subcellular hypertrophy and hyperplasia in the papillary muscle of adult rat. J Mol Cell Cardiol 12, 781–795. [DOI] [PubMed] [Google Scholar]
- Balciunas D, Davidson AE, Sivasubbu S, Hermanson SB, Welle Z, Ekker SC, 2004. Enhancer trapping in zebrafish using the Sleeping Beauty transposon. BMC Genomics 5, 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchemin M, Smith A, Yin VP, 2015. Dynamic microRNA-101a and Fosab expression controls zebrafish heart regeneration. Development 142, 4026–4037. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisen J, 2009. Evidence for cardiomyocyte renewal in humans. Science 324, 98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camper-Kirby D, Welch S, Walker A, Shiraishi I, Setchell KD, Schaefer E, Kajstura J, Anversa P, Sussman MA, 2001. Myocardial Akt activation and gender: increased nuclear activity in females versus males. Circulation research 88, 1020–1027. [DOI] [PubMed] [Google Scholar]
- Chablais F, Jazwinska A, 2012. The regenerative capacity of the zebrafish heart is dependent on TGFbeta signaling. Development 139, 1921–1930. [DOI] [PubMed] [Google Scholar]
- Chablais F, Veit J, Rainer G, Jazwinska A, 2011. The zebrafish heart regenerates after cryoinjury-induced myocardial infarction. BMC Dev Biol 11, 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi WY, Gemberling M, Wang J, Holdway JE, Shen MC, Karlstrom RO, Poss KD, 2013. In vivo monitoring of cardiomyocyte proliferation to identify chemical modifiers of heart regeneration. Development 140, 660–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dammrich J, Pfeifer U, 1983. Cardiac hypertrophy in rats after supravalvular aortic constriction. I. Size and number of cardiomyocytes, endothelial and interstitial cells. Virchows Arch B Cell Pathol Incl Mol Pathol 43, 265–286. [DOI] [PubMed] [Google Scholar]
- Fang Y, Gupta V, Karra R, Holdway JE, Kikuchi K, Poss KD, 2013. Translational profiling of cardiomyocytes identifies an early Jak1/Stat3 injury response required for zebrafish heart regeneration. Proc Natl Acad Sci U S A 110, 13416–13421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finegold JA, Asaria P, Francis DP, 2013. Mortality from ischaemic heart disease by country, region, and age: statistics from World Health Organisation and United Nations. Int J Cardiol 168, 934–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foglia MJ, Poss KD, 2016. Building and re-building the heart by cardiomyocyte proliferation. Development 143, 729–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujio Y, Nguyen T, Wencker D, Kitsis RN, Walsh K, 2000. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 101, 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Rosa JM, Martin V, Peralta M, Torres M, Mercader N, 2011. Extensive scar formation and regression during heart regeneration after cryoinjury in zebrafish. Development 138, 1663–1674. [DOI] [PubMed] [Google Scholar]
- Gupta V, Gemberling M, Karra R, Rosenfeld GE, Evans T, Poss KD, 2013. An injury-responsive gata4 program shapes the zebrafish cardiac ventricle. Curr Biol 23, 1221–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han P, Zhou XH, Chang N, Xiao CL, Yan S, Ren H, Yang XZ, Zhang ML, Wu Q, Tang B, Diao JP, Zhu X, Zhang C, Li CY, Cheng H, Xiong JW, 2014. Hydrogen peroxide primes heart regeneration with a derepression mechanism. Cell Res 24, 1091–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Harrison MR, Osorio A, Kim J, Baugh A, Duan C, Sucov HM, Lien CL, 2013. Igf Signaling is Required for Cardiomyocyte Proliferation during Zebrafish Heart Development and Regeneration. PLoS One 8, e67266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh N, Ohta H, Nakayama Y, Konishi M, 2016. Roles of FGF Signals in Heart Development, Health, and Disease. Frontiers in cell and developmental biology 4, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itou J, Akiyama R, Pehoski S, Yu X, Kawakami H, Kawakami Y, 2014. Regenerative responses after mild heart injuries for cardiomyocyte proliferation in zebrafish. Dev Dyn 243, 1477–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itou J, Kawakami H, Burgoyne T, Kawakami Y, 2012a. Life-long preservation of the regenerative capacity in the fin and heart in zebrafish. Biol Open 1, 739–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itou J, Oishi I, Kawakami H, Glass TJ, Richter J, Johnson A, Lund TC, Kawakami Y, 2012b. Migration of cardiomyocytes is essential for heart regeneration in zebrafish. Development 139, 4133–4142. [DOI] [PubMed] [Google Scholar]
- Jopling C, Sleep E, Raya M, Marti M, Raya A, Belmonte JC, 2010. Zebrafish heart regeneration occurs by cardiomyocyte dedifferentiation and proliferation. Nature 464, 606–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karra R, Knecht AK, Kikuchi K, Poss KD, 2015. Myocardial NF-kappaB activation is essential for zebrafish heart regeneration. Proc Natl Acad Sci U S A 112, 13255–13260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami Y, Rodriguez-Leon J, Koth CM, Buscher D, Itoh T, Raya A, Ng JK, Esteban CR, Takahashi S, Henrique D, Schwarz MF, Asahara H, Izpisua Belmonte JC, 2003. MKP3 mediates the cellular response to FGF8 signalling in the vertebrate limb. Nat Cell Biol 5, 513–519. [DOI] [PubMed] [Google Scholar]
- Kikuchi K, Gupta V, Wang J, Holdway JE, Wills AA, Fang Y, Poss KD, 2011a. tcf21+ epicardial cells adopt non-myocardial fates during zebrafish heart development and regeneration. Development 138, 2895–2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Major RJ, Blum N, Dahn RD, Begemann G, Poss KD, 2011b. Retinoic acid production by endocardium and epicardium is an injury response essential for zebrafish heart regeneration. Developmental cell 20, 397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi K, Holdway JE, Werdich AA, Anderson RM, Fang Y, Egnaczyk GF, Evans T, Macrae CA, Stainier DY, Poss KD, 2010. Primary contribution to zebrafish heart regeneration by gata4(+) cardiomyocytes. Nature 464, 601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Wu Q, Zhang Y, Wiens KM, Huang Y, Rubin N, Shimada H, Handin RI, Chao MY, Tuan TL, Starnes VA, Lien CL, 2010. PDGF signaling is required for epicardial function and blood vessel formation in regenerating zebrafish hearts. Proc Natl Acad Sci U S A 107, 17206–17210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE, 2011. Heart regeneration. Nature 473, 326–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Marín-Juez R, Moura PL, Kuenne C, Lai JKH, Tsedeke AT, Guenther S, Looso M, Stainier DY, 2017. Reciprocal analyses in zebrafish and medaka reveal that harnessing the immune response promotes cardiac regeneration. Elife 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Marín-Juez R, Stainier DYR, 2019. Immune responses in cardiac repair and regeneration: a comparative point of view. Cell Mol Life Sci 76, 1365–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM, 2002. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol 248, 307–318. [DOI] [PubMed] [Google Scholar]
- Lee Y, Grill S, Sanchez A, Murphy-Ryan M, Poss KD, 2005. Fgf signaling instructs position-dependent growth rate during zebrafish fin regeneration. Development 132, 5173–5183. [DOI] [PubMed] [Google Scholar]
- Lepilina A, Coon AN, Kikuchi K, Holdway JE, Roberts RW, Burns CG, Poss KD, 2006. A dynamic epicardial injury response supports progenitor cell activity during zebrafish heart regeneration. Cell 127, 607–619. [DOI] [PubMed] [Google Scholar]
- Li C, Scott DA, Hatch E, Tian X, Mansour SL, 2007. Dusp6 (Mkp3) is a negative feedback regulator of FGF-stimulated ERK signaling during mouse development. Development 134, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Cheng H, Roberts TM, Zhao JJ, 2009. Targeting the phosphoinositide 3-kinase pathway in cancer. Nature reviews. Drug discovery 8, 627–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SQ, Roberts D, Kharitonenkov A, Zhang B, Hanson SM, Li YC, Zhang LQ, Wu YH, 2013. Endocrine protection of ischemic myocardium by FGF21 from the liver and adipose tissue. Scientific reports 3, 2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin-Juez R, Marass M, Gauvrit S, Rossi A, Lai SL, Materna SC, Black BL, Stainier DY, 2016. Fast revascularization of the injured area is essential to support zebrafish heart regeneration. Proc Natl Acad Sci U S A 113, 11237–11242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marunouchi T, Tanonaka K, 2015. Cell Death in the Cardiac Myocyte. Biol Pharm Bull 38, 1094–1097. [DOI] [PubMed] [Google Scholar]
- Missinato MA, Saydmohammed M, Zuppo DA, Rao KS, Opie GW, Kuhn B, Tsang M, 2018. Dusp6 attenuates Ras/MAPK signaling to limit zebrafish heart regeneration. Development 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami M, Simons M, 2008. Fibroblast growth factor regulation of neovascularization. Current opinion in hematology 15, 215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notari M, Ventura-Rubio A, Bedford-Guaus SJ, Jorba I, Mulero L, Navajas D, Marti M, Raya A, 2018. The local microenvironment limits the regenerative potential of the mouse neonatal heart. Science advances 4, eaao5553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padua RR, Sethi R, Dhalla NS, Kardami E, 1995. Basic fibroblast growth factor is cardioprotective in ischemia-reperfusion injury. Mol Cell Biochem 143, 129–135. [DOI] [PubMed] [Google Scholar]
- Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD, 2016. Revisiting Cardiac Cellular Composition. Circulation research 118, 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA, 2011. Transient regenerative potential of the neonatal mouse heart. Science 331, 1078–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poss KD, Wilson LG, Keating MT, 2002. Heart regeneration in zebrafish. Science 298, 2188–2190. [DOI] [PubMed] [Google Scholar]
- Raya A, Koth CM, Buscher D, Kawakami Y, Itoh T, Raya RM, Sternik G, Tsai HJ, Rodriguez-Esteban C, Izpisua-Belmonte JC, 2003. Activation of Notch signaling pathway precedes heart regeneration in zebrafish. Proc Natl Acad Sci U S A 100 Suppl 1, 11889–11895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AM, Yin VP, 2019. Emerging Roles for Immune Cells and MicroRNAs in Modulating the Response to Cardiac Injury. Journal of cardiovascular development and disease 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai T, Tsuchida M, Lampe PD, Murakami M, 2013. Cardiomyocyte FGF signaling is required for Cx43 phosphorylation and cardiac gap junction maintenance. Exp Cell Res 319, 2152–2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnabel K, Wu CC, Kurth T, Weidinger G, 2011. Regeneration of cryoinjury induced necrotic heart lesions in zebrafish is associated with epicardial activation and cardiomyocyte proliferation. PLoS One 6, e18503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, Lee RT, 2013. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin M, Beane TJ, Quillien A, Male I, Zhu LJ, Lawson ND, 2016. Vegfa signals through ERK to promote angiogenesis, but not artery differentiation. Development 143, 3796–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden PH, 2003. Ras, Akt, and mechanotransduction in the cardiac myocyte. Circulation research 93, 1179–1192. [DOI] [PubMed] [Google Scholar]
- Tang Y, Nyengaard JR, Andersen JB, Baandrup U, Gundersen HJ, 2009. The application of stereological methods for estimating structural parameters in the human heart. Anatomical record (Hoboken, N.J.: 2007) 292, 1630–1647. [DOI] [PubMed] [Google Scholar]
- Tsang M, Maegawa S, Kiang A, Habas R, Weinberg E, Dawid IB, 2004. A role for MKP3 in axial patterning of the zebrafish embryo. Development 131, 2769–2779. [DOI] [PubMed] [Google Scholar]
- Turner N, Grose R, 2010. Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 10, 116–129. [DOI] [PubMed] [Google Scholar]
- Wang J, Nachtigal MW, Kardami E, Cattini PA, 2013. FGF-2 protects cardiomyocytes from doxorubicin damage via protein kinase C-dependent effects on efflux transporters. Cardiovasc Res 98, 56–63. [DOI] [PubMed] [Google Scholar]
- Wang J, Panakova D, Kikuchi K, Holdway JE, Gemberling M, Burris JS, Singh SP, Dickson AL, Lin YF, Sabeh MK, Werdich AA, Yelon D, Macrae CA, Poss KD, 2011. The regenerative capacity of zebrafish reverses cardiac failure caused by genetic cardiomyocyte depletion. Development 138, 3421–3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan RS, Kaplinskiy V, Kitsis RN, 2010. Cell death in the pathogenesis of heart disease: mechanisms and significance. Annu Rev Physiol 72, 19–44. [DOI] [PubMed] [Google Scholar]
- Wills AA, Holdway JE, Major RJ, Poss KD, 2008. Regulated addition of new myocardial and epicardial cells fosters homeostatic cardiac growth and maintenance in adult zebrafish. Development 135, 183–192. [DOI] [PubMed] [Google Scholar]
- Wu CC, Kruse F, Vasudevarao MD, Junker JP, Zebrowski DC, Fischer K, Noel ES, Grun D, Berezikov E, Engel FB, van Oudenaarden A, Weidinger G, Bakkers J, 2016. Spatially Resolved Genome-wide Transcriptional Profiling Identifies BMP Signaling as Essential Regulator of Zebrafish Cardiomyocyte Regeneration. Developmental cell 36, 36–49. [DOI] [PubMed] [Google Scholar]
- Yin VP, Lepilina A, Smith A, Poss KD, 2012. Regulation of zebrafish heart regeneration by miR-133. Dev Biol 365, 319–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu JK, Sarathchandra P, Chester A, Yacoub M, Brand T, Butcher JT, 2018. Cardiac regeneration following cryoinjury in the adult zebrafish targets a maturation-specific biomechanical remodeling program. Scientific reports 8, 15661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Pu WT, 2016. Recounting Cardiac Cellular Composition. Circulation research 118, 368–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


